Introduction
A number of female reproductive system diseases such
as repeated implantation failure, preeclampsia, endometriosis,
reproductive system malignancy and breast cancer lack tools for
early diagnosis. Although clinical biomarkers such as squamous cell
carcinoma antigen and CA125/199 can be used in the diagnosis of
female reproductive tract tumors, they are of low sensitivity and
specificity (1,2). Therefore, new effective biomarkers are
urgently needed for the diagnosis of these conditions.
Non-coding RNAs have been extensively used in
clinical experiments and are of great potential as biomarkers for
detection of disease (3). Circular
RNAs (circRNAs) are a class of endogenous non-coding RNA and
consist of a covalently closed continuous loop with neither 5'-3'
polarity nor a polyadenylated tail (4,5). Unlike
linear RNAs, circRNAs are protected against the effects of RNA
enzymes due to their lack of free ends; these molecules are thus
more stable compared with linear RNAs (5). With the development of RNA-seq analysis
and bioinformatics technologies, recent studies have reported the
use of circRNAs in the early detection and prognosis of certain
types of cancer, such as gastric cancer (6), hepatocellular carcinoma (7), lung cancer (8), cervical squamous cell carcinoma (CSCC)
and breast cancer (BRCA) (9), as well
as a number of female reproductive system diseases including
repeated implantation failure (10),
preeclampsia (11) and ovarian
endometriosis (12). Considering the
association between hormone-responsive BRCA and the female
reproductive system (13), this
condition was included as a female reproductive system disease in
the present meta-analysis.
The aim of this meta-analysis was to summarize all
circRNAs that have been investigated as diagnostic markers for
female reproductive system diseases and to review their efficiency
as novel diagnostic biomarkers in such diseases. Available data
from published literature were evaluated to determine if circRNAs
may be used as sensitive and specific molecular biomarkers.
Materials amd methods
Search strategy
This systematic meta-analysis was performed in
strict accordance with the guidelines for diagnostic meta-analysis.
Eligible studies published on PubMed (https://www.ncbi.nlm.nih.gov/pubmed), EMBASE
(https://embase.com/), Web of Science (https://www.isiknowledge.com/) and Cochrane Library
(https://www.cochranelibrary.com/) before
February 20, 2019 were selected for meta-analysis. Only studies
published in English were included. No restrictions were applied
for the year of publication or publication status. Databases were
search using the following keywords: ‘Circular RNA’ OR ‘circRNA’
AND ‘endometrial’ OR ‘endometrium’ OR ‘ovarian’ OR ‘ovary’ OR
‘cervical’ OR ‘uterine’ OR ‘uterus’ OR ‘uterine cervix’ OR ‘breast’
OR ‘vagina*’ OR ‘pregnancy’ OR ‘pre-eclampsia’ OR ‘PCOS’ OR
‘placenta previa’ OR ‘gynaecology’ OR ‘obstetrics’ OR ‘genitalia’,
and ‘diagnosis’ OR ‘diagnostic’ OR ‘sensitivity’ OR ‘specificity’
OR ‘receiver operating characteristic curve’ OR ‘ROC’ OR ‘AUC'.
Selection of publications
Two researchers independently reviewed all search
results based on the titles and abstracts; the relevant studies
were included in the full text review. Data extraction was
performed by other researchers. Any disagreement regarding the
inclusion or exclusion of studies were resolved by discussion
involving a third investigator. All studies included in the
meta-analysis met the following criteria: i) Studies that reported
on the diagnostic value of circRNAs for any female reproductive
system diseases type; ii) studies which contained sample,
sensitivity, specificity and AUC data; and iii) studies that
enrolled >30 cases and matched controls. Studies were excluded
as follows: i) Duplicate studies; ii) reviews, letters, conference
abstracts, case reports and articles with insufficient data; iii)
articles studying circRNA in cell lines; and iv) articles published
in languages other than English.
Data extraction and quality
assessment
The following parameters were obtained from all
studies: First author name, publication year, study area, patient
ethnicity, disease type, specimen, sample size, as well as data on
circRNA sensitivity and specificity. The Quality Assessment of
Diagnostic Accuracy Studies-2 (QUADAS-2) tool (14) was used to perform quality assessment
of each included study.
Statistical analysis
All statistical analyses were conducted using the
analytical software RevMan5.3 (The Cochrane Collaboration) and
STATA14.2 (StataCorp LLC). All data such as the number of true
positives (TP), false positives (FP), true negatives (TN) and false
negatives (FN) were extracted from each study to calculate the
pooled sensitivity and specificity, positive likelihood ratio
(PLR), negative likelihood ratio (NLR), diagnostic odds ratio (DOR)
and their 95% confidence intervals (CI), summary receiver operator
characteristic (sROC) curve and area under the curve (AUC). The
data obtained was used to determine the overall performance of
circRNAs in identifying female reproductive system diseases.
P<0.05 was considered to indicate a statistically significant
difference. In addition, heterogeneity across studies was
determined using Cochran's Q and I2 statistics, where
I2>50% indicated the existence of significant
heterogeneity. Meta-regression analysis was utilized to detect the
possible sources of heterogeneity.
Results
Literature search
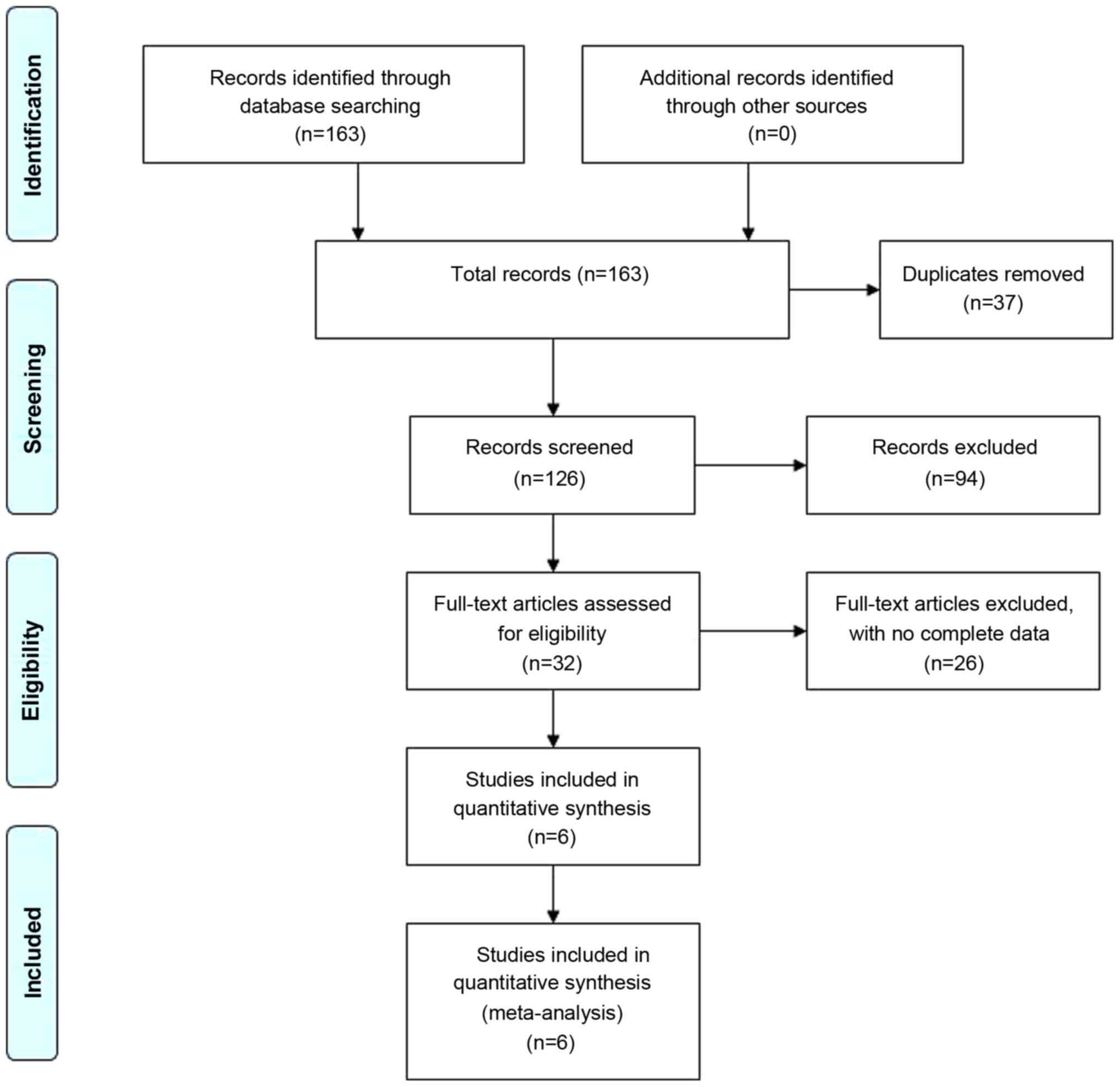
A total of 163 potentially eligible articles were
reviewed in this meta-analysis. The literature search strategy is
depicted as a flow chart in Fig. 1.
Among the 163 studies, 37 were duplicates and 22 were reviews,
letters or conference abstracts. Following screening the titles and
abstracts of the remaining publications, 70 were identified not to
be relevant to the present study. A total of 34 articles were
eligible for full-text review, and 28 articles were excluded due to
incomplete full-texts or incomplete data. Finally, six eligible
studies (each circRNA as a test, a total of 15 tests) were included
in the meta-analysis (15-20).
Study characteristics
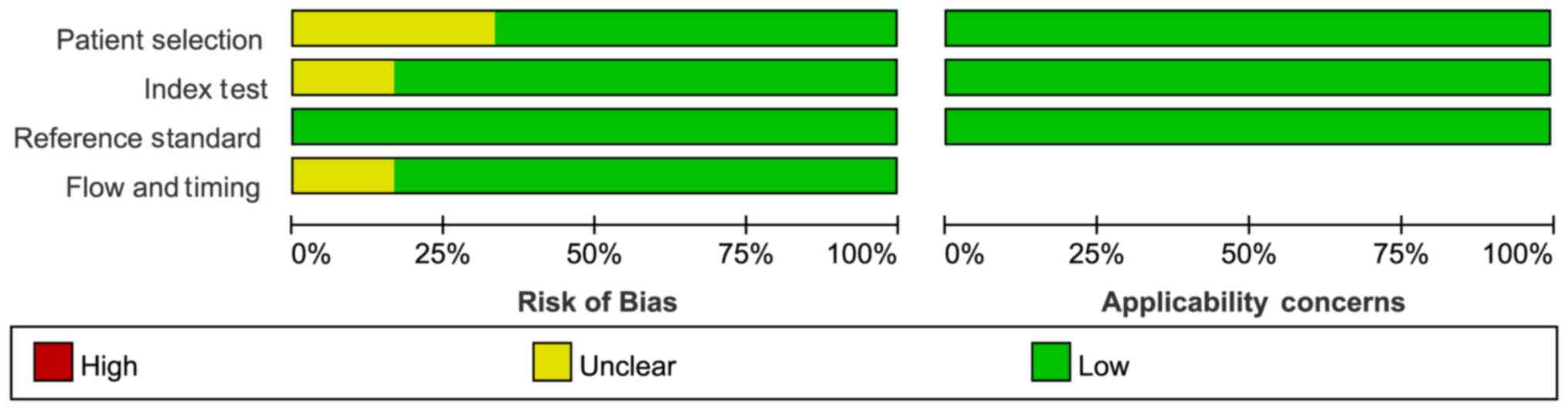
The characteristics of the six included studies with
15 tests are summarized in Table I: A
total of 613 individuals representing three types of disease were
enrolled in the selected studies. These studies were of high
quality based on the QUADAS-2 analysis (Fig. 2).
 | Table ICharacteristics of the included
studies. |
Table I
Characteristics of the included
studies.
| Test (study) no. | Author | Disease type | CircRNA | Specimen | SEN | SPE | TP | FP | FN | TN | Patients, n | Controls, n | Expression | (Refs.) |
|---|
| 1(1) | Lü et al,
2017 | BRCA | hsa_circ_103110 | Tissue | 0.63 | 0.63 | 32 | 19 | 19 | 32 | 51 | 51 | Down | (20) |
| 2(1) | Lü et al,
2017 | BRCA | hsa_circ_104689 | Tissue | 0.57 | 0.55 | 29 | 23 | 22 | 28 | 51 | 51 | Down | (20) |
| 3(1) | Lü et al,
2017 | BRCA | hsa_circ_104821 | Tissue | 0.57 | 0.57 | 29 | 22 | 22 | 29 | 51 | 51 | Down | (20) |
| 4(1) | Lü et al,
2017 | BRCA | hsa_circ_006054 | Tissue | 0.65 | 0.69 | 33 | 16 | 18 | 35 | 51 | 51 | Up | (20) |
| 5(1) | Lü et al,
2017 | BRCA | hsa_circ_100219 | Tissue | 0.69 | 0.71 | 35 | 15 | 16 | 36 | 51 | 51 | Up | (20) |
| 6(1) | Lü et al,
2017 | BRCA |
hsa_circ_406697 | Tissue | 0.63 | 0.63 | 32 | 19 | 19 | 32 | 51 | 51 | Up | (20) |
| 7(2) | Yin et al,
2017 | BRCA |
hsa_circ_0001785 | Plasma | 0.79 | 0.76 | 45 | 4 | 12 | 13 | 57 | 17 | Up | (19) |
| 8(2) | Yin et al,
2017 | BRCA |
hsa_circ_0108942 | Plasma | 0.82 | 0.50 | 45 | 8 | 12 | 9 | 57 | 17 | Up | (19) |
| 9(2) | Yin et al,
2017 | BRCA |
hsa_circ_0068033 | Plasma | 0.73 | 0.58 | 45 | 7 | 12 | 10 | 57 | 17 | Up | (19) |
| 10(3) | Jiang et al,
2018 | Preeclampsia |
hsa_circ_0001855 | Plasma | 0.53 | 0.70 | 19 | 11 | 16 | 24 | 35 | 35 | Up | (18) |
| 11(3) | Jiang et al,
2018 | Preeclampsia |
hsa_circ_0004904 | Plasma | 0.53 | 0.70 | 19 | 11 | 16 | 24 | 35 | 35 | Up | (18) |
| 12(4) | Hu et al,
2018 | Preeclampsia |
hsa_circ_0036877 | Tissue/plasma | 0.85 | 0.73 | 34 | 31 | 6 | 85 | 40 | 116 | Down | (17) |
| 13(5) | Bai et al,
2018 | Preeclampsia |
hsa_circ_0007121 | Tissue/plasma | 0.77 | 0.70 | 23 | 12 | 7 | 29 | 30 | 41 | Down | (16) |
| 14(6) | Wang et al,
2017 | CSCC |
hsa_circ_0101996 | Whole blood | 0.90 | 0.83 | 78 | 9 | 9 | 46 | 87 | 55 | Up | (15) |
| 15(6) | Wang et al,
2017 | CSCC |
hsa_circ_0101119 | Whole blood | 0.70 | 0.93 | 61 | 4 | 26 | 51 | 87 | 55 | Up | (15) |
Meta-analysis
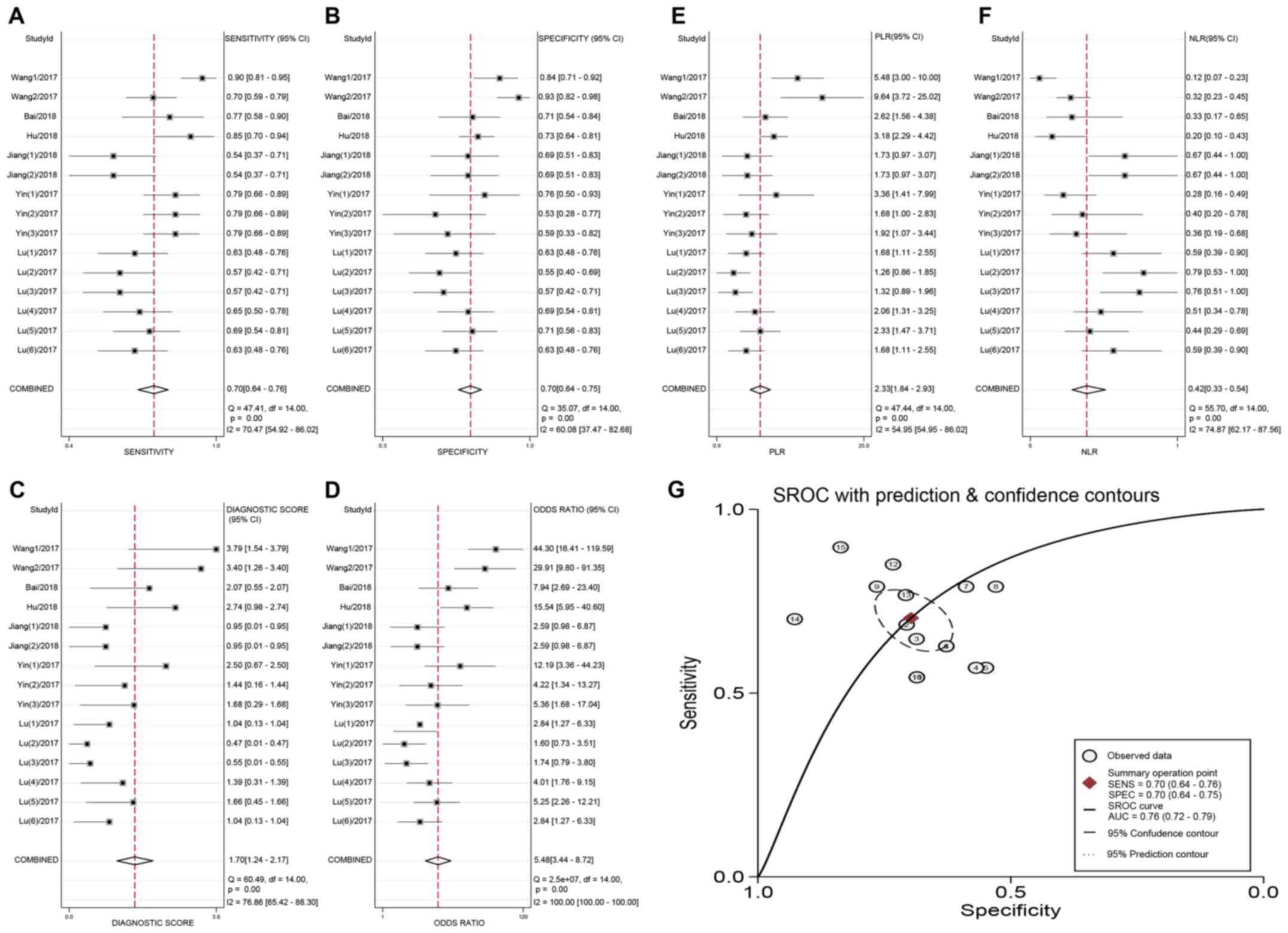
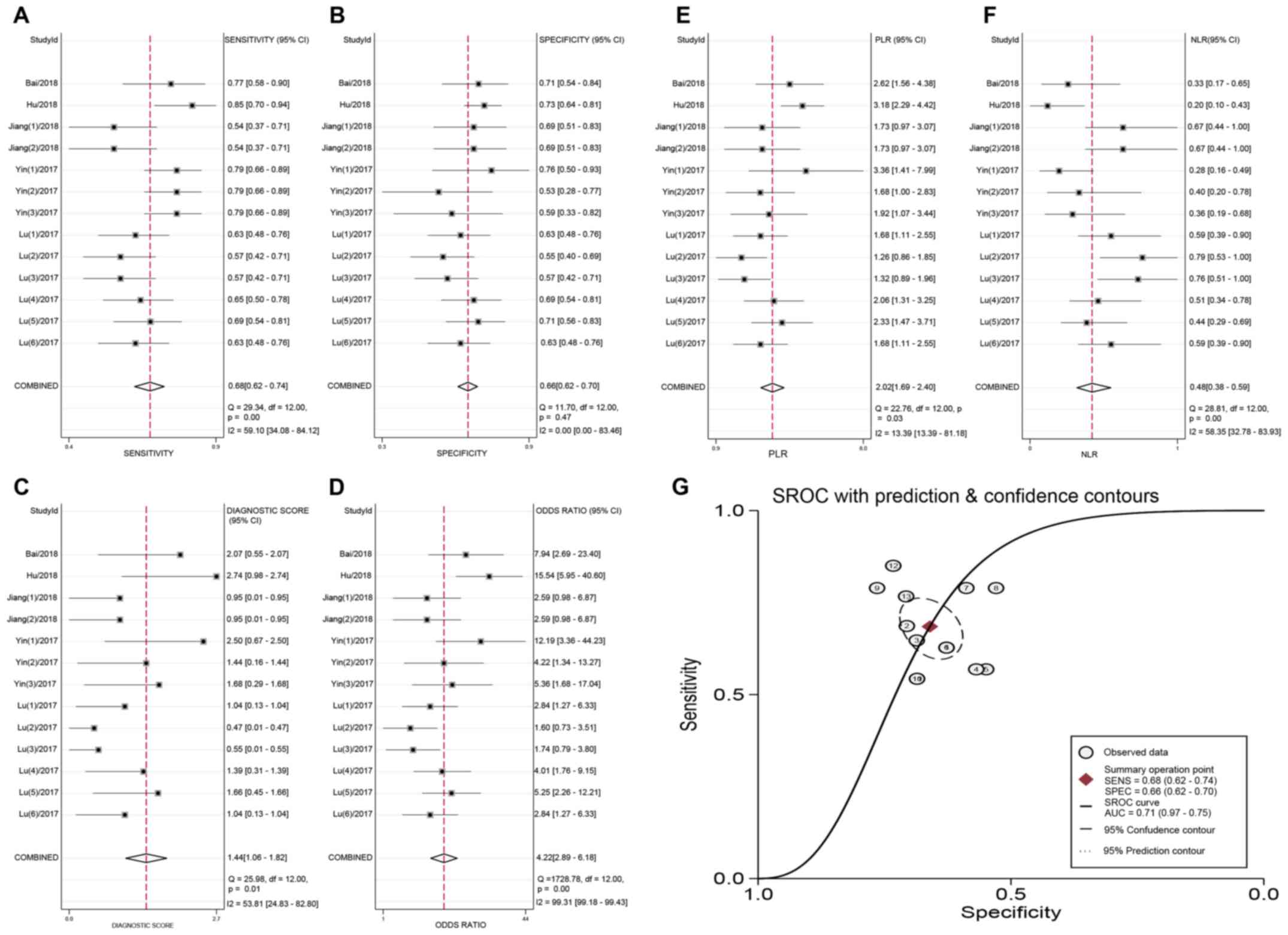
Overall, the detection performance of circRNAs was
as follows: The pooled sensitivity was 0.70 (95% CI, 0.64-0.76;
Q=47.41; P<0.001; I2=70.47%) and the pooled
specificity was 0.70 (95% CI, 0.64-0.75; Q=35.07; P<0.001;
I2=60.08%). The PLR and NLR were 2.33 (Q=47.44; P=0.001;
I2=54.95%) and 0.42 (95% CI, 0.33-0.54; Q=55.70;
P<0.001; I2=74.87%), respectively, and the diagnostic
score was 1.70 (Q=60.49; P<0.001; I2=76.86%). The
pooled DOR was 5.48 (Q=2.5x107; P<0.001;
I2=100.00%). Additionally, the AUC of the sROC curve was
0.76 (95% CI, 0.72-0.79). The relevant forest plots and sROC are
presented in Fig. 3.
Meta-regression analyses
A meta-regression based on disease type (benign or
malignant) and sample size (>100 or ≤100) was used to identify
the possible sources of heterogeneity. The results of the
meta-regression analysis of probable factors suggested that
different sample sizes may increase heterogeneity in pooled
sensitivity (P=0.02), and disease types may result in heterogeneity
in pooled specificity (P=0.04) (Table
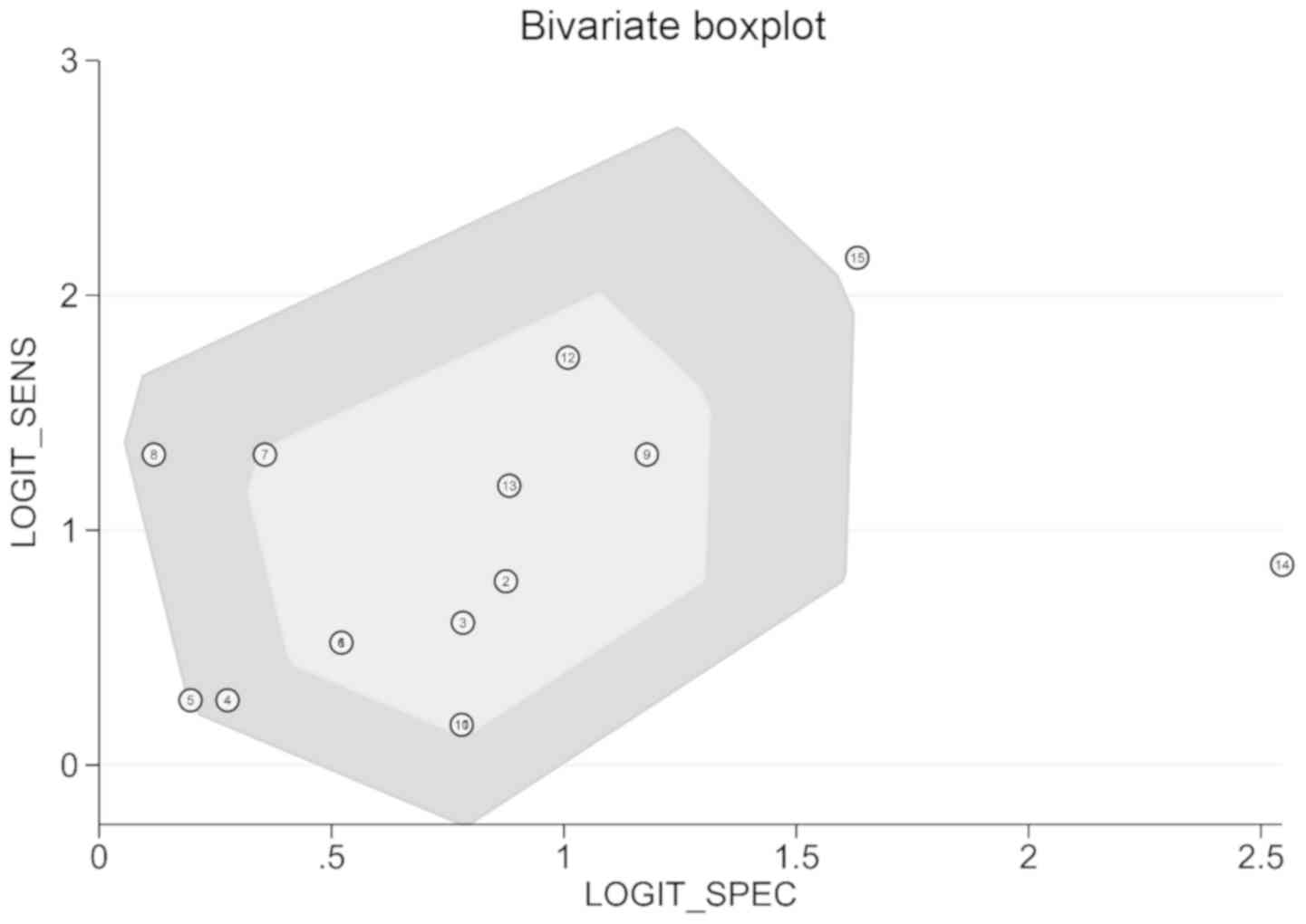
II). The bivariate boxplot demonstrates the heterogeneity of
each study (Fig. 4). Two tests not
included in the boxplot belonged study no. 6 (CSCC group). After
excluding this study, Cochran's Q and I2 were decreased
in the resultant forest plot, which was indicative of improvement
in homogeneity. However, the sensitivity decreased from 0.70 to
0.68, specificity decreased from 0.70 to 0.66, PLR decreased from
2.33 to 2.20, NLR increased from 0.42 to 0.48 and sROC decreased
from 0.76 to 071 (Fig. 5). This
reduction suggested that study 6 (CSCC group) had high sensitivity
and specificity compared with the other studies.
 | Table IIMeta-regression analysis. |
Table II
Meta-regression analysis.
| Parameter | Category | No. of studies | Sensitivity (95%
CI) | P1 | Specificity (95%
CI) | P2 |
|---|
| Disease type | Malignant | 11 | 0.71
(0.64-0.78) | 0.17 | 0.70
(0.63-0.76) | 0.04a |
| | Benign | 4 | 0.68
(0.56-0.81) | | 0.70
(0.60-0.80) | |
| Sample size | ≥100 samples | 9 | 0.70
(0.62-0.77) | 0.02a | 0.70
(0.64-0.77) | 0.08 |
| | <100
samples | 6 | 0.72
(0.63-0.81) | | 0.68
(0.58-0.78) | |
Discussion
CircRNA is a novel category of non-coding RNAs with
a closed circular structure. These specialized structures make
circular RNAs more stable than linear RNAs due to their resistance
to RNA enzymes such as exonuclease and ribonuclease (5). A previous study has demonstrated that
the half-life of mRNAs is only about 10 h, whereas whole circRNAs
have a half-life >48 h (20).
CircRNAs are highly abundant in various human tissues and cell
samples and exhibit highly tissue-specific expression, especially
in hepatocellular, cervical and ovarian carcinoma (21,22). For
these reasons, circRNA are excellent candidate biomarkers for
diagnosing human diseases.
In the present meta-analysis, relevant articles were
screened across four databases, and six relevant studies were
finally included to evaluate the diagnostic value of circRNAs in
diseases of the female reproductive system. To the best of our
knowledge, this is the first meta-analysis performed on this topic.
The pooled sensitivity and specificity were 0.70 and 0.70,
respectively, indicating that circRNAs may be valid diagnostic
markers in female reproductive system diseases. The pooled DOR was
5.48, and the AUC of the sROC curve was 0.76. These results
demonstrated that circRNAs exhibited a moderate diagnostic
performance. Similarly, circRNAs have been reported to possess
prognostic value for female reproductive system tumors, such as
endometrial and epithelial ovarian cancer (23,24), but
no specific diagnostic data was available. Overall, circRNAs may be
appropriate for use as diagnostic biomarkers in female reproductive
system diseases.
However, it should be highlighted that heterogeneity
was present in the current pooled estimates, as the included
studies involved experiments which used whole blood, plasma or
tissues. A meta-regression based on disease type and sample size
was performed; the results demonstrated that the heterogeneity may
arise from the sample size and disease type. The bivariate boxplot
demonstrated that study no. 6 (regarding CSCC) was the source of
heterogeneity. Exclusion of this study resulted in decreased Q and
I2 values, both of which are indicators of disease
heterogeneity. Study no. 6 was the only study on cervical cancer
which utilized whole blood, which may have been the source of
heterogeneity.
The limitations of this meta-analysis should be
taken into consideration. Firstly, all included studies were
authored by Chinese investigators and were on ethnic Chinese
patient samples. Therefore, the overall diagnostic accuracy of
circRNAs may be not be applicable to the general population; future
research regarding circRNAs as biomarkers should be expanded to
multiple countries and ethnicities. Next, the enrolled studies only
included data on those with preeclampsia, CSCC and BRCA. There are
several other female reproductive system diseases that lack early
diagnostic tools including repeated implantation failure and
reproductive system malignancy such as ovarian cancer, the 5-year
survival rate of which is only 30% as a vast majority of patients
present with widespread metastasis (25,26). The
lack of an effective molecular biomarker for diagnosing early stage
ovarian cancer is a key contributor to its overall poor prognosis
(27). Lastly, tissue-extracted
circRNAs may not be the ideal biomarker considering that circRNAs
are also stable and abundant in exosomes (28,29). The
detection of circRNAs from exosomes in plasma or serum may be a
better alternative in diagnosing disease. The results of the
present meta-analysis suggested that circRNAs may serve as a
useful, noninvasive molecular biomarker for clinical practice in
the future. More extensive studies are urgently needed to evaluate
the diagnostic performance of plasma or serum circRNAs in the
context of female reproductive system diseases.
In conclusion, circRNAs possess the potential to
function as diagnostic biomarkers for female reproductive system
diseases. Additional large-scale studies are required to verify the
results of this preliminary study.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Open Project
of The Key Laboratory of Non-coding RNA Transformation Research of
Anhui Higher Education Institution (Wannan Medical College; grant
no. RNA201901).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JD and GN designed the study and prepared the
manuscript with comments from all authors. JD and YL performed data
extraction and quality assessment. JD wrote and revised the
manuscript. NG, QW and LL drafted the manuscript and critically
revised it for important intellectual content. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao S, Mei Y, Wang Y, Zhu J, Zheng G and
Ma R: Levels of CEA, CA153, CA199, CA724 and AFP in nipple
discharge of breast cancer patients. Int J Clin Exp Med.
8:20837–20844. 2015.PubMed/NCBI
|
|
2
|
Wang HF, Li LF, Guo SH, Zeng QY, Ning F,
Liu WL and Zhang G: Evaluation of antibody level against
Fusobacterium nucleatum in the serological diagnosis of colorectal
cancer. Sci Rep. 6(33440)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Beermann J, Piccoli MT, Viereck J and Thum
T: Non-coding RNAs in development and disease: Background,
mechanisms, and therapeutic approaches. Physiol Rev. 96:1297–1325.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Salzman J: Circular RNA expression: Its
potential regulation and function. Trends Genet. 32:309–316.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hsiao KY, Sun HS and Tsai SJ: Circular
RNA-New member of noncoding RNA with novel functions. Exp Biol Med
(Maywood). 242:1136–1141. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shen F, Liu P, Xu Z, Li N, Yi Z, Tie X,
Zhang Y and Gao L: CircRNA_001569 promotes cell proliferation
through absorbing miR-145 in gastric cancer. J Biochem. 165:27–36.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Qu D, Yan B, Xin R and Ma T: A novel
circular RNA hsa_circ_0020123 exerts oncogenic properties through
suppression of miR-144 in non-small cell lung cancer. Am J Cancer
Res. 8:1387–1402. 2018.PubMed/NCBI
|
|
9
|
Zhang HD, Jiang LH, Hou JC, Zhou SY, Zhong
SL, Zhu LP, Wang DD, Yang SJ, He YJ, Mao CF, et al: Circular RNA
hsa_circ_0072995 promotes breast cancer cell migration and invasion
through sponge for miR-30c-2-3p. Epigenomics. 10:1229–1242.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu L, Li L, Ma X, Yue F, Wang Y, Wang L,
Jin P and Zhang X: Altered circular RNA expression in patients with
repeated implantation failure. Cell Physiol Biochem. 44:303–313.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang YG, Yang HL, Long Y and Li WL:
Circular RNA in blood corpuscles combined with plasma protein
factor for early prediction of pre-eclampsia. BJOG. 123:2113–2118.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu XX, Jia SZ, Dai Y, Zhang JJ, Li XY, Shi
JH, Leng JH and Lang JH: Identification of circular RNAs as a novel
biomarker for ovarian endometriosis. Chin Med J (Engl).
131:559–566. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nagini S: Breast Cancer: Current molecular
therapeutic targets and new players. Anticancer Agents Med Chem.
17:152–163. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Whiting PF, Rutjes AW, Westwood ME,
Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA and Bossuyt
PM: QUADAS-2 Group: QUADAS-2: A revised tool for the quality
assessment of diagnostic accuracy studies. Ann Intern Med.
155:529–536. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang YM, Huang LM, Li DR, Shao JH, Xiong
SL, Wang CM and Lu SM: Hsa_circ_0101996 combined with
hsa_circ_0101119 in peripheral whole blood can serve as the
potential biomarkers for human cervical squamous cell carcinoma.
Int J Clin Exp Pathol. 10:11924–11931. 2017.PubMed/NCBI
|
|
16
|
Bai Y, Rao H, Chen W, Luo X, Tong C and Qi
H: Profiles of circular RNAs in human placenta and their potential
roles related to preeclampsia. Biol Reprod. 98:705–712.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu X, Ao J, Li X, Zhang H, Wu J and Cheng
W: Competing endogenous RNA expression profiling in pre-eclampsia
identifies hsa_circ_0036877 as a potential novel blood biomarker
for early pre-eclampsia. Clin Epigenetics. 10(48)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiang M, Lash GE, Zhao X, Long Y, Guo C
and Yang H: CircRNA-0004904, CircRNA-0001855, and PAPP-A: Potential
novel biomarkers for the prediction of preeclampsia. Cell Physiol
Biochem. 46:2576–2586. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yin WB, Yan MG, Fang X, Guo JJ, Xiong W
and Zhang RP: Circulating circular RNA hsa_circ_0001785 acts as a
diagnostic biomarker for breast cancer detection. Clin Chim Acta.
487:363–368. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lü L, Sun J, Shi P, Kong W, Xu K, He B,
Zhang S and Wang J: Identification of circular RNAs as a promising
new class of diagnostic biomarkers for human breast cancer.
Oncotarget. 8:44096–44107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jeck WR and Sharpless NE: Detecting and
characterizing circular RNAs. Nat Biotechnol. 32:453–461.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Gao YL, Zhang MY, Xu B, Han LJ, Lan SF,
Chen J, Dong YJ and Cao LL: Circular RNA expression profiles reveal
that hsa_circ_0018289 is up-regulated in cervical cancer and
promotes the tumorigenesis. Oncotarget. 8:86625–86633.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chen Q, Zhang J, He Y and Wang Y:
hsa_circ_0061140 knockdown reverses FOXM1-mediated cell growth and
metastasis in ovarian cancer through miR-370 sponge activity. Mol
Ther Nucleic Acids. 13:55–63. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen BJ, Byrne FL, Takenaka K, Modesitt
SC, Olzomer EM, Mills JD, Farrell R, Hoehn KL and Janitz M:
Analysis of the circular RNA transcriptome in endometrial cancer.
Oncotarget. 9:5786–5796. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ning L, Long B, Zhang W, Yu M, Wang S, Cao
D, Yang J, Shen K, Huang Y and Lang J: Circular RNA profiling
reveals circEXOC6B and circN4BP2L2 as novel prognostic biomarkers
in epithelial ovarian cancer. Int J Oncol. 53:2637–2646.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bartl T, Schwameis R, Stift A,
Bachleitner-Hofmann T, Reinthaller A, Grimm C and Polterauer S:
Predictive and prognostic implication of bowel resections during
primary cytoreductive surgery in advanced epithelial ovarian
cancer. Int J Gynecol Cancer. 28:1664–1671. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ahmed I, Karedath T, Andrews SS, Al-Azwani
IK, Mohamoud YA, Querleu D, Rafii A and Malek JA: Altered
expression pattern of circular RNAs in primary and metastatic sites
of epithelial ovarian carcinoma. Oncotarget. 7:36366–36381.
2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hang W, Feng Y, Sang Z, Yang Y, Zhu Y,
Huang Q and Xi X: Downregulation of miR-145-5p in cancer cells and
their derived exosomes may contribute to the development of ovarian
cancer by targeting CT. Int J Mol Med. 43:256–266. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hou J, Jiang W, Zhu L, Zhong S, Zhang H,
Li J, Zhou S, Yang S, He Y, Wang D, et al: Circular RNAs and
exosomes in cancer: A mysterious connection. Clin Transl Oncol.
20:1109–1116. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li S, Li Y, Chen B, Zhao J, Yu S, Tang Y,
Zheng Q, Li Y, Wang P, He X and Huang S: ExoRBase: A database of
circRNA, lncRNA and mRNA in human blood exosomes. Nucleic Acids
Res. 46:D106–D112. 2018.PubMed/NCBI View Article : Google Scholar
|