Introduction
The body's innate immune system is mediated by
granulocytes, dendritic cells, macrophages, natural killer (NK) and
mast cells, and serves an important role in protecting the body
from infection. Innate immunity is considered the body's first line
of defense against antigenic challenges, with imbalances in this
aspect of the immune system increasing the risk of the development
of cancer, as well as adversely affecting treatment outcomes and
prognosis (1). Chronic inflammation
leads to irreversible changes in tissue remodeling and induces
oxidative stress, resulting in altered protein and DNA structure,
and thus the eventual development of cancer (1). Neutropenia, a common adverse effect of
chemotherapy, is frequently accompanied by fever (febrile
neutropenia) which is assumed to be a manifestation of an
underlying infection, requiring hospitalization and intravenous
administration of antibiotics (2). A
suppressed innate immune system resulting from a chemotherapeutic
regimen may take a long time to recover, particularly for the
recovery of NK cells, which serves an important role in
chemotherapy with their potential for anti-tumor activity (3). Although conventional oncology provides
treatment for chemotherapy-induced neutropenia, this does not
address other components of the innate immune system, and is often
accompanied by significant adverse effects (4).
Several herbal medicinal products have been shown to
exhibit significant effects on the immune system, and may thus be
used to improve the quality of life-associated outcomes in cancer
patients undergoing chemotherapy (5-7).
The botanical formula LCS101 was designed based on the principles
of traditional Chinese medicine (TCM) for the treatment of patients
undergoing chemotherapy for breast cancer. In a randomized,
double-blind, placebo-controlled clinical trial, LCS101 was shown
to significantly reduce anemia and neutropenia in 65 female
patients with locally advanced breast cancer undergoing
anthracycline and taxane-based treatment regimens (8). LCS101 was also shown to exhibit
significant and selective anti-cancer effects, inducing necrosis
and apoptosis in cancer cell lines, whilst protecting non-tumor
cells from the cytotoxic effects of chemotherapy (9). Finally, LCS101 was shown to display
several anti-cancer immune-modulating effects, including
dose-dependent upregulation of NK cell activation and T-cell
proliferation (10).
LCS102 is a botanical compound which is similar to
LCS101 in that it is also based on the principles of TCM, although
it is directed at treating the body's immune response during
chemotherapy. The aim of the present study was to examine the
effects of LCS102 on innate immunity, as well as testing its effect
on chemotherapy.
Materials and methods
Antibodies and reagents
Doxorubicin, Taxol, etoposide and cisplatin were
acquired from Sigma Aldrich; Merck KGaA. Each chemotherapeutic
agent was used in incrementally increased concentrations (from 0 to
3 mg/ml) to 1 mg/ml of the LCS102 preparation (described in detail
below) for 30 min each. RPMI-1640, L-glutamine, FBS, trypsin and
PBS were purchased from Biological Industries. Red blood cell lysis
buffer, Alexa Fluor 488-conjugated mouse anti-human CD56 IgG1κ
(cat. no. 318312; CD56-AF), PE-conjugated mouse anti-human CD69
IgG1κ (cat. no. 310906; CD69-PE), PerCP-conjugated mouse anti-human
CD3 IgG1κ (cat. no. 344814 CD3-PerCP), PE-conjugated mouse IgG1κ
MOPC-21 isotype control antibody, Alexa Fluor 488-conjugated mouse
IgG1κ MOPC-21 isotype control antibody and PerCP-conjugated mouse
IgG1κ MOPC-21 isotype control antibody were all purchased from
BioLegend, Inc. IL-2 was from purchased PeproTech, Inc.
Donor blood samples
The present study was approved by the Sheba Medical
Center Institutional Review Board (Ramat Gan, Israel). For the
present study, 20 volunteers (12 male, 8 female) were asked to
donate blood, 19 of these were healthy donors with no chronic
medical illness or medication use, including drugs with a potential
to alter the immune response; and 1 of the female patients had a
carcinoma of the breast and was undergoing chemotherapy. All
volunteers provided written informed consent, and 5 ml of blood was
drawn from each donor and collected in heparin-treated tubes
(Greiner Bio-One, GmbH). Each tube was inverted several times and
then treated as described below.
Human cell lines
A549 (lung carcinoma), MCF7 (breast adenocarcinoma),
PANC-1 (pancreatic epithelioid carcinoma), U-2 OS (osteosarcoma)
and T24 (bladder transitional cell carcinoma) were purchased from
American Type Culture Collection, and were authenticated using STR
profiling. All cells were cultured in RPMI-1640 with 10% FBS, 2 mM
L-glutamine, 100 µg/ml Pen/Strep (Biological Industries) in a 37˚C
humidified incubator with 5% CO2.
Preparation of LCS102 and LCS101
botanical formulas
LCS102 is comprised of the following herbal
components: Astragalus membranaceus, Poriae cocos, Atractylodes
macrocephala, Ligustrum lucidum, Lycium chinense, Ganoderma
lucidum and Cordyceps sinensis, extracts of which are
manufactured in accordance with good manufacturing practice
guidelines, and imported under license (BARA Herbs, Yokneam,
Israel) in accordance with the regulations of the Israel Ministry
of Health. All batches of the final product were analyzed and
certified to be free of contaminants, such as heavy metals,
microbial contamination, pesticide residues and mycotoxins. A dry
extract powder of the LCS102 and LCS101 formulas (BARA Herbs Ltd)
was dissolved in PBS at a concentration of 100 mg/ml, and incubated
for 30 min with heating and occasional vortexing. The solution was
then centrifuged at 4,300 x g for 5 min, and the supernatant was
filtered through a 0.45 µm Millex PVDF filter (Merck KGaA).
Solubility was estimated by cryophilization and weighing of the
pellet, and was estimated to be ~50%.
Treatment, preparation and staining of
blood samples
Blood samples (100 µl) were plated in U-shaped
96-well plates and treated in triplicate immediately with 0-3 mg/ml
of the botanical extracts. After 48 h of incubation at 37˚C in a 5%
CO2 incubator treated aliquots (50 µl) were stained with
3 µl 1:1:1 CD56-AF/CD69-PE/CD3-PerCP or an isotype-matched control
antibody mix for 30 min at room temperature, lysed with red blood
cell lysis buffer, washed twice with PBS and re-suspended in PBS
for subsequent FACS analysis. In all experiments PBS-treated
samples were used as a negative control, and an IL-2 (20
U/ml)-treated sample (prepared at the same temperature and for the
same duration as the botanical compounds) as the positive
control.
FACS analysis
The samples were analyzed using a BD FACSCalibur
flow cytometer (BD Biosciences), using a three-color protocol with
appropriate compensations. The results were analyzed using WinMDI
version 2.9 (Purdue University Cytometry Laboratories) using the
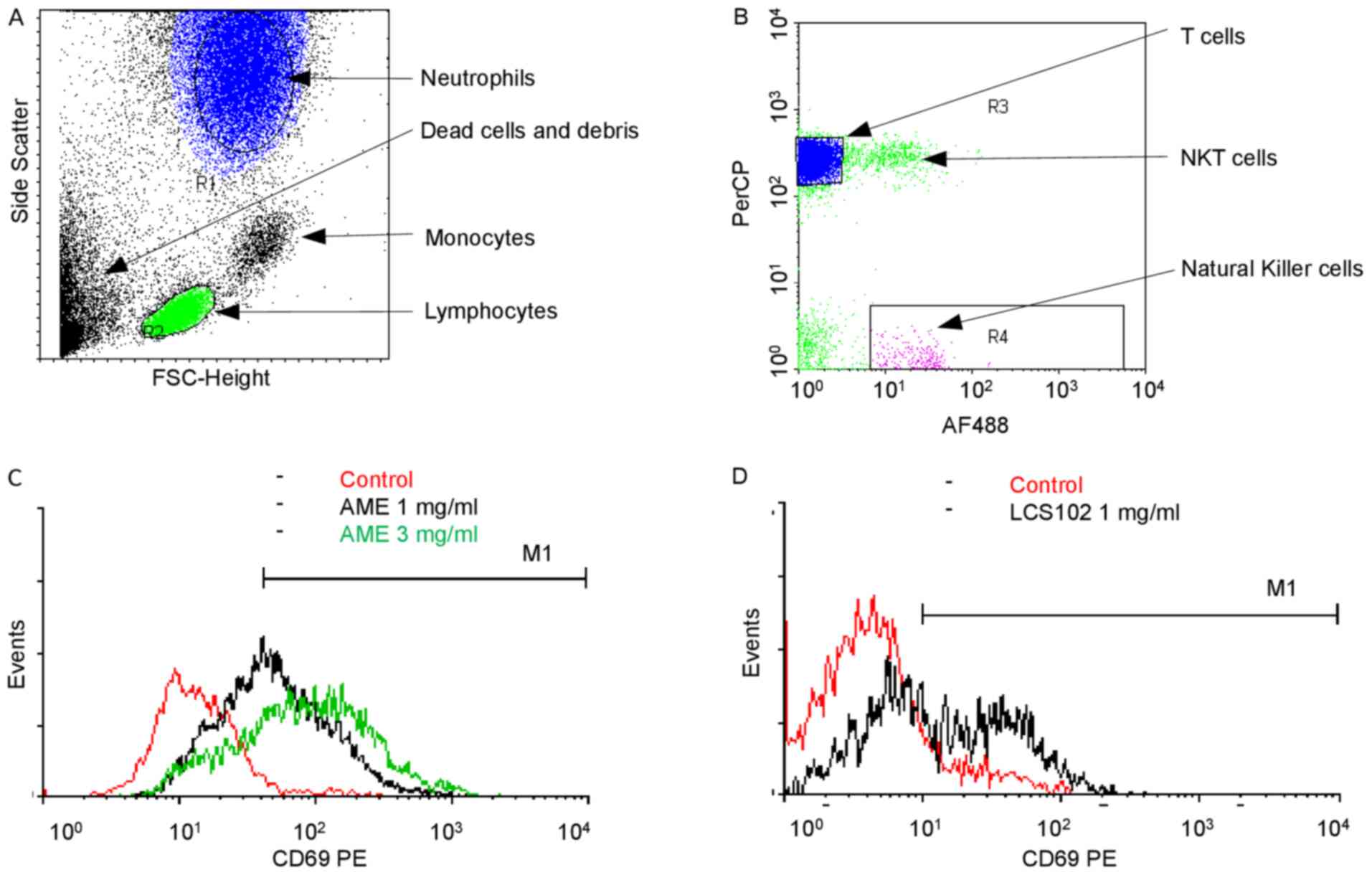
following methodology: First, a forward scatter (FSC)/ side scatter
(SSC) dot plot was used to select neutrophil (R1, blue) and
lymphocyte (R2, green) populations (Fig.
1A). Lymphocytes were further resolved using a
CD3-PerCP/CD56-AF488 logarithmic dot plot (Fig. 1B) to select
CD3-/CD56+ NK cells (R4, purple) population.
Finally, each population was analyzed for CD69 expression on a
CD69-PE logarithmic histogram plot, with appropriate gating
(neutrophils, Fig. 1C; NK cells,
Fig. 1D). An activated population
marker (M1) was set in accordance with the isotype control staining
of each population (data not shown). Due to differences in
self-fluorescence, the settings for the activation markers differ
between NK cells and neutrophils.
Treatment of cancer cell lines and
Sulforodamine B (SRB) viability assay
Sulforodamine B, trichloroacetic acid and acetic
acid were purchased from Sigma Aldrich; Merck KGaA. A total of
3x103 cells were plated per/well in a 96-well plate and
incubated overnight. Subsequently, cells were treated in
triplicates as described above and cultured for 48 h. A SRB
viability test was performed as follows: Cells were fixed for 1 h
on ice with 10% trichloroacetic acid (v/v in RPMI-1640), washed
trice with double distilled water, and dried and stained for 1 h at
room temperature with 0.057% sulforodamine B (w/v in 1% acetic
acid). After staining, the plates were washed three times with 1%
acetic acid and then dried, and 200 µl 10 mM Tris was added to each
well to solubilize the SRB. The absorbance was measured at 570 nm
using an ELISA reader (Biotek Instruments, Inc.). Each experiment
was repeated at least three times.
Statistical analysis
A total of 10 study groups were assessed, each in
triplicate: LCS102, controls, IL-2, AME, AMA, PCAO, LCH, LLU, GLU
and CSI. A total of 45 between-group comparisons were performed,
using a non-parametric Kruskal-Wallis test, with a post-hoc Dunn's
test. The data were analyzed using SPSS version 25 (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
LCS102 activates NK cells and
neutrophils
The effects of the LCS102 formula on neutrophil and
NK cell activation were examined in all of the blood samples.
Samples were treated with 1 mg/ml of LCS102, PBS (negative control)
or IL-2 (positive control) for 48 h, stained with a CD3/CD56/CD69
fluorescent antibody mix and then analyzed using FACS. Neutrophil
populations were gated on an FSC/SSC dot-plot (Fig. 1A), and analyzed on a CD69-PE
histogram (Fig. 1C). NK cells were
first gated on an FSC/SSC dot-plot (Fig.
1A) for lymphocytes, and then on a CD3-PerCP/CD56-AF488
dot-plot for CD3-/CD56+ (NK) cells (Fig. 1B), and analyzed on a CD69-PE
histogram (Fig. 1D).
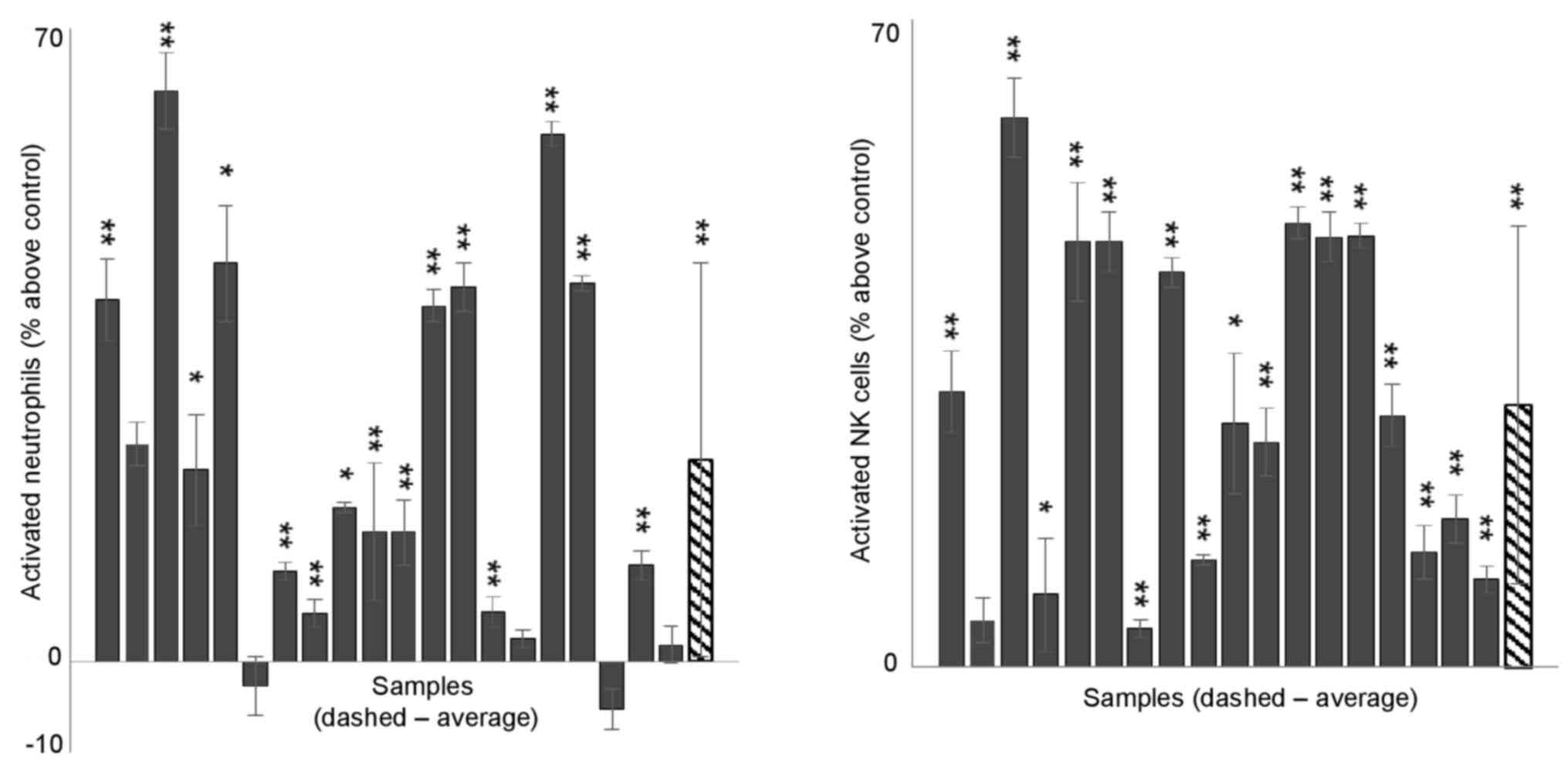
Fig. 2 shows the
percentage of activated NK/neutrophils above/below the negative
control value in each sample, as well as the average value of all
samples for each treatment. LCS102 significantly elevated the
percent of activated neutrophils and NK cells in the majority of
tested blood samples, although the extent of this activation varied
among samples.
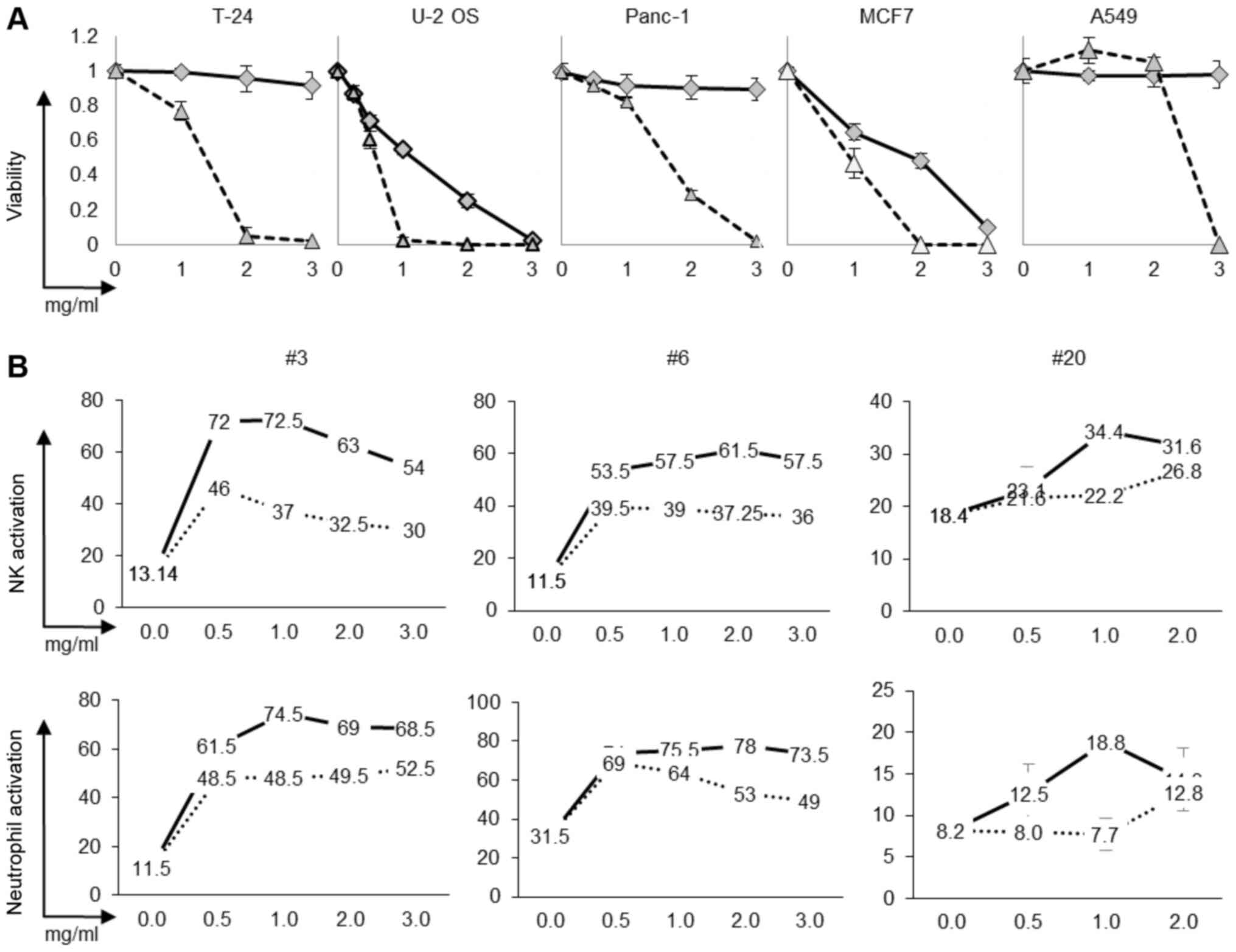
Comparing the cytotoxic and immune
effects of LCS102 to LCS101
The effects of LCS102 on cancer cell survival were
assessed and compared to with the LCS101 formula on a panel of five
human cancer cell lines. As shown in Fig. 3A, LCS102 reduced the proliferation of
two cancer cell lines, U-2 OS and MCF7, although it was less
effective than the anti-cancer effects of LCS101. In order to
address the effects of both formulas on NK and neutrophil
activation, blood samples from three donors were treated with
increasing (0 to 3 mg/ml) concentrations of the compounds. As shown
in Fig. 3B, LCS102 was considerably
more effective in the activation of NK and neutrophils compared
with LCS101.
LCS102 does not reduce the
effectiveness of chemotherapy
Each cell line was exposed to incremental
concentrations of cisplatin (cisPL), paclitaxel (Taxol), etoposide
(VP-16) and doxorubicin (doxo). The addition of 1 mg/ml of LCS102
to each of the chemotherapy-treated cells did not result in reduced
cytotoxic effects for any of the treatments in any of the cancer
cell lines assessed. Additionally, in Panc-1 cells, the addition of
LCS102 to doxorubicin resulted in a synergistic response, which
manifested as increased cell death. This response was also observed
with the addition of LCS102 to 1 µM of cisplatin in T-24 cells
(Fig. 4).
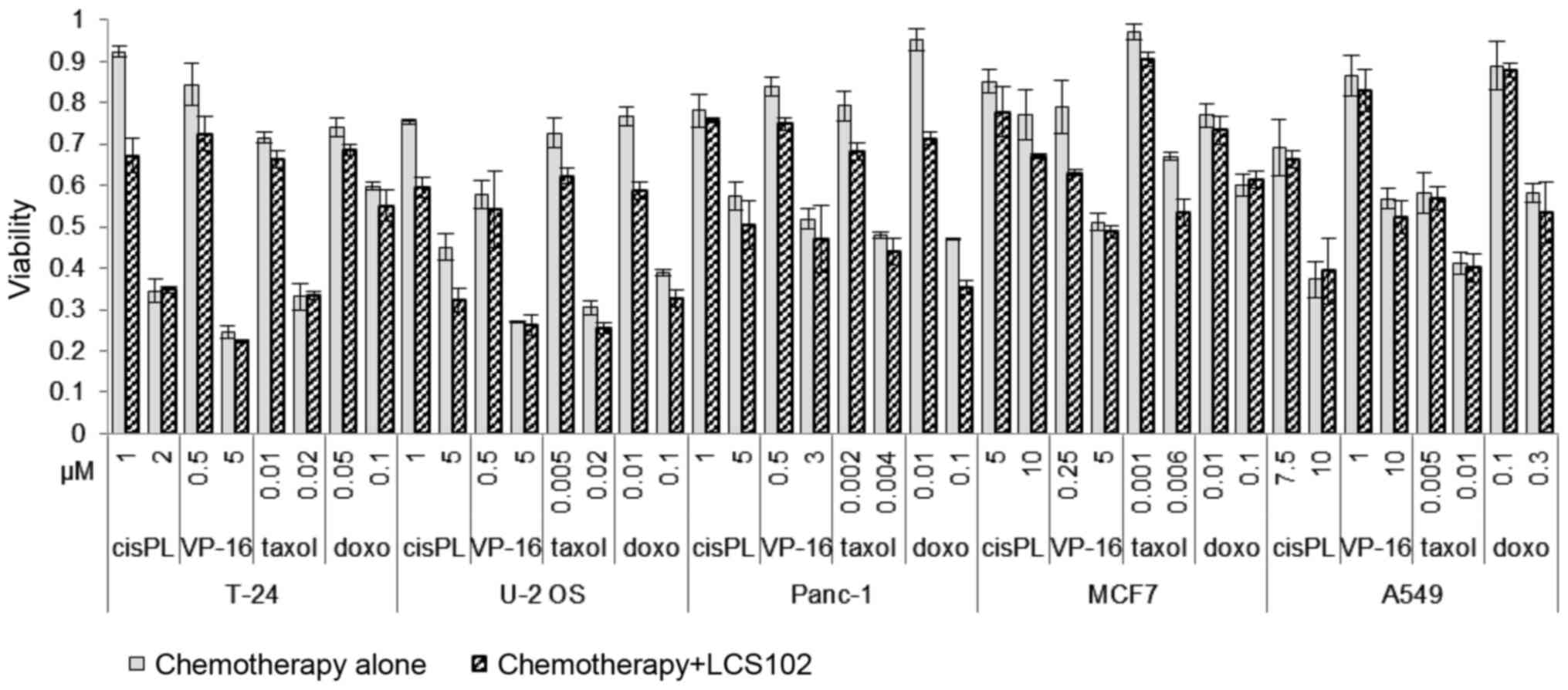 | Figure 4Cytotoxic effects of chemotherapeutic
agents with and without LCS102. A549, T-24, Panc-1, MCF7 and U-2OS
human cancer lines were treated for 48 h with the indicated
concentrations of cisPL), etoposide (VP-16), Taxol and doxorubicin
(doxo), either alone or with 1 mg/ml of LCS102, and the viability
of cells was assessed. Data are presented relative to the
respective untreated control cells. cisPL, cisplatin; VP-16,
etoposide; doxo, doxorubicin. |
Discussion
In the present study, the botanical compound LCS102
was shown to exhibit a number of effects on innate immunity in
blood samples taken from 20 subjects. The botanical formula was
shown to significantly induce activation of neutrophils and NK
cells in the majority of samples studied. Neutrophils are an
important component of innate immunity, and neutropenia can impair
the body's ability to respond to nascent infections, enabling
bacteria and other organisms to multiply and invade the blood and
organs (11). Neutropenia is a major
complication of chemotherapy, and neutropenic fever and infections
have long been a major cause of dose reduction and delays, with
compromised treatment outcomes (12,13). The
use of granulocyte colony-stimulating factor is effective in
reversing this complication, although this treatment is not without
potential short- and long-term side effects, and is not
cost-effective (4).
In previous studies, a number of LCS102 components
have been shown to increase innate immunity, such as the herbs
Astragalus membranaceous and Poriae cocus, which have been shown to
increase macrophage and phagocyte activity (14,15). The
β-glycans of medicinal mushrooms such as Cordyceps sinensis
have been shown to activate leucocytes, and stimulate phagocytic
and cytotoxic activities (16).
Herbal compounds have also been shown to increase NK cell activity,
including LCS102. Astragalus membranaceus has been shown to
stimulate NK-cells in human peripheral lymphocytes, as well as
restore steroid-inhibited NK-cell activity (17). Polysaccharides of Astragalus can
enhance NK cell activity in normal subjects, as well as in systemic
lupus erythematosus patients (18).
Finally, Cordyceps sinensis was shown to increase the
activity of NK cells in cultured rat Kupffer cells (19).
When compared with LCS101, it was shown that LCS102
had a more significant effect on innate immunity. At the same time,
the anti-cancer effects were less significant than those seen with
LCS101. The present study strived to adhere to the TCM-based design
of the two formulas; however, further research is required in order
to verify and improve our understanding of the mechanism of each
product.
In conclusion, the botanical compound LCS102 was
shown to enhance the innate immune response in both healthy human
blood samples, as well as in a sample from a patient with breast
cancer undergoing chemotherapy. The variable response to the
compounds, most pronounced with the effects on NK cell activity,
should be further studied. Finally, the addition of LCS102 did not
reduce the effect of chemotherapeutic drugs on cancer cells, and in
some cell lines, increased the effects. However, the small size of
the sample precludes reaching any conclusions regarding these
findings. Further research is required to examine other aspects of
LCS102, such as the biological effects of the formula and any
potential interactions with chemotherapeutic agents, to improve our
understanding of the potential clinical value of LCS102.
Acknowledgements
Not applicable.
Funding
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZC, YM, RB, TG and NS designed the study. ZC, YM, OM
and NS performed the experiments. ZC, YM, OM, TG, RB and NS
analyzed the data. ZC, YM, OM, TG, RB and NS wrote the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Sheba Medical
Center Institutional Review Board (Ramat Gan, Israel). All
volunteers provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Hughes WT, Armstron D, Bodey GP, Bow EJ,
Brown AE, Calandra T, Feld R, Pizzo PA, Rolston KV, Shenep JL and
Young LS: 2002 Guidelines for the use of antimicrobial agents in
neutropenic patients with cancer. Clin Infet dis. 34:730–751.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Solomayer EF, Feuerer M, Bai L, Umansky V,
Beckhove P, Meyberg GC, Bastert G, Schirrmacher V and Diel IJ:
Influence of adjuvant hormone therapy and chemotherapy on the
immune system analysed in the bone marrow of patients with breast
cancer. Clin Cancer Res. 9:174–180. 2003.PubMed/NCBI
|
|
4
|
Trueman P: Prophylactic G-CSF in patients
with early-stage breast cancer: A health economic review. Br J
Cancer. 101 (Suppl 1):S15–S17. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang M, Liu X, Li J, He L and Tripathy D:
Chinese medicinal herbs to treat the side-effects of chemotherapy
in breast cancer patients. Cochrane Database Syst Rev.
2(CD004921)2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Taixiang W, Munro AJ and Guanjian L:
Chinese medical herbs for chemotherapy side effects in colorectal
cancer patients. Cochrane Database Syst Rev.
1(CD004540)2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen S, Flower A, Ritchie A, Liu J,
Molassiotis A, Yu H and Lewith G: Oral Chinese herbal medicine
(CHM) as an adjuvant treatment during chemotherapy for non-small
cell lung cancer: A systematic review. Lung Cancer. 68:137–145.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yaal-Hahoshen N, Maimon Y,
Siegelmann-Danieli N, Lev-Ari S, Ron IG, Sperber F, Samuels N,
Shoham J and Merimsky O: A prospective, controlled study of the
botanical compound mixture LCS101 for chemotherapy-induced
hematological complications in breast cancer. Oncologist.
16:1197–1202. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cohen Z, Maimon Y, Yoeli-Lerner M, Yang P,
Samuels N and Berger R: Selective anticancer effects and protection
from chemotherapy by the botanical compound LCS101: Implications
for cancer treatment. Int J Oncol. 46:308–316. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rachmut IH, Samuels N, Melnick SJ,
Ramachandran C, Sharabi Y, Pavlovsky A, Maimon Y and Shoham J:
Immunomodulatory effects of the botanical compound LCS101:
Implications for cancer treatment. Onco Targets Ther. 6:437–445.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Crawford J, Dale DC and Lyman GH:
Chemotherapy-induced neutropenia: Risks, consequences, and new
directions for its management. Cancer. 100:228–237. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bodey GP, Buckley M, Sathe YS and
Freireich EJ: Quantitative relationships between circulating
leukocytes and infection in patients with acute leukemia. Ann
Intern Med. 64:328–340. 1966.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Crawford J: Prevention and treatment of
chemotherapy-induced neutropenia. Clin Adv Hematol Oncol.
11:514–517. 2013.PubMed/NCBI
|
|
14
|
Wang J, Tong X, Li P, Cao H and Su W:
Immuno-enhancement effects of Shenqi Fuzheng Injection on
cyclophosphamide-induced immunosuppression in Balb/c mice. J
Ethnopharmacol. 139:788–795. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen X, Zhang L and Cheung PC:
Immunopotentiation and anti-tumor activity of
carboxymethylated-sulfated β-(1→3)-D-glucan from Poria
cocos. Int Immunopharmacol. 10:398–405. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Smiderle FR, Baggio CH, Borato DG,
Santana-Filho AP, Sassaki GL, Iacomini M and Van Griensven LJ:
Anti-inflammatory properties of the medicinal mushroom Cordyceps
militaris might be related to its linear (1→3)-β-D-glucan. PLoS
One. 9(e110266)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bone K and Mills S: Principles and
Practice of Phytotherapy. 2nd edition. Churchill Livingstone,
Edinburgh, 2000.
|
|
18
|
Zhao XZ: Effects of Astragalus
membranaceus and Tripterygium hypoglancum on natural
killer cell activity of peripheral blood mononuclear in systemic
lupus erythematosus. Zhongguo Zhong Xi Yi Jie He Za Zhi.
12:669–671, 645. 1992.PubMed/NCBI(In Chinese).
|
|
19
|
Zhu JS, Halpern GM and Jones K: The
scientific rediscovery of a precious ancient Chinese herbal
regimen: Cordyceps sinensis: part II. J Altern Complement
Med. 4:429–457. 1998.PubMed/NCBI View Article : Google Scholar
|