Introduction
Hyperuricemia (HUA) is a precursor of gout and is
characterized by a chronic increase in serum uric acid (SUA) levels
(1-3).
HUA is implicated in various systemic disorders and is often
included amongst the diagnostic criteria for metabolic syndrome,
which is a complex disorder of the cardiometabolic system with
potentially lethal systemic and hemodynamic consequences (1-3).
Lifestyle changes, such as a reduction in alcohol consumption and
increase in water intake, as well as drug therapy, including
administration of xanthine oxidase (XOD) inhibitors, such as
allopurinol (AP), can be applied to reduce SUA levels (1,2).
However, lifestyle changes alone are often inadequate to reduce the
SUA levels to physiological levels. Furthermore, drug therapy may
cause various adverse reactions, including severe hypersensitivity
to AP, agranulocytosis and aggravated renal toxicity due to
impaired pyrimidine metabolism (2,4,5). Therefore, there is an urgent need to
develop safe and effective anti-HUA agents.
Daily oral treatment with natural
vanadium-containing Mt. Fuji ground water has been reported to
regulate blood glucose levels in hyperglycemic humans and rats
without severe diabetes mellitus (6). Therefore, a new kind of water-based
formulation to reduce SUA levels may potentially benefit patients
with HUA. In the present study, the effect of lifeceramics
(LC)-treated water (LC water) on SUA concentration and hemorheology
in hyperuricemic rats was assessed. LC is formulated from zeolite
and oyster shells under high temperature and pressure conditions,
and has been reported to possess antibacterial activity (7,8). In our
previous studies, it was shown that sake (alcohol) administration
can induce liver damage and alcoholic hepatitis in rats; however,
the combined treatment with sake and LC did not result in damage
(9,10). To the best of our knowledge, it has
not been determined whether LC water can protect against various
disorders, such as the metabolic syndrome.
In our previous studies, it was shown that
asymptomatic HUA enhances oxidative stress, deteriorated
hemorheological parameters of red blood cells and increased blood
viscosity and coagulation status (3,11). Zhou
et al (12) reported on
oxidative stress and microinflammatory responses in asymptomatic
young patients with primary HUA and suggested that the inflammatory
response is associated with oxidative stress induced by high uric
acid (UA) levels. Furthermore, the protective effects of LC water
against oxidative stress in cultured human cells was reported in
our previous study (13). The aim of
the present study was to examine the effects of LC water on SUA
levels and hemorheological parameters in hyperuricemic rats and
provide a potentially novel lifestyle intervention for the
treatment of patients with HUA.
Materials and methods
Reagents
Potassium oxonate (PO) was purchased from Shanghai
Jinsui Bio-Technology Co., Ltd. The LC particle powder was obtained
from Wedge Co., Ltd. AP was purchased from Hefei Jiulian
Pharmaceutical Co., Ltd. The measurement kits for SUA (cat. no.
20171024SUA) and malondialdehyde (MDA) (cat. no. 20171024MDA)
concentration, and for XOD (cat. no. 20161105XOD) and glutathione
(GSH) (cat. no. 20171024GSH) activities were obtained from Nanjing
Jiancheng Clinical Reagent Co., Ltd. All other reagents were
purchased from Beijing BD Bio-Tech Co., Ltd. (Beijing, China).
Animals and treatment
To prepare LC water, distilled water was mixed with
LC particles and the solution was allowed to stand for 18 h at room
temperature (22±1̊C) to precipitate the large particles of LC
(8). All animals were treated
according to the protocols for animal care approved by the Animal
Ethics Committee of Chengde Medical University (Chengde, China).
These protocols have been described previously (3,11).
Briefly, 60 male Sprague Dawley rats (6-week-old: 180-220 g
weight), purchased from Beijing Vital River Lab Animal Technology
Co. Ltd., were housed in plastic cages of 40x60x80 cm in size (5
rats per cage) and maintained under standard laboratory conditions
on a 12-h light/dark cycle, at a constant ambient temperature of
22±1̊C and relative humidity of 55%. After one week of feeding all
the rats with distilled water, rats were randomly divided into 6
independent groups; Control, PO alone, PO+AP, PO+LC (low), PO+LC
(medium) and PO+LC (high); with 10 rats per a group (Table I).
 | Table ITreatment groups used in the present
study. n=10 per group. |
Table I
Treatment groups used in the present
study. n=10 per group.
| | | | | PO+LC |
|---|
| Reagentsa | Control | PO | PO+AP | Low | Medium | High |
|---|
| PO | - | 250 | 250 | 250 | 250 | 250 |
| AP | - | - | 25 | - | - | - |
| LC | - | - | - | 25 | 50 | 100 |
PO was dissolved in PBS and injected
intraperitoneally every day (250 mg/kg/day) for 5 weeks in all
rats, except for the control group. AP was dissolved in distilled
water. The AP solution and the LC water were administered as
drinking water. Control rats were injected intraperitoneally with
sodium carboxymethyl cellulose dissolved in PBS and administered
distilled water via gavage. The daily consumption of drinking water
was recorded and replaced with freshly prepared water. The mean
value of the consumption volume of drinking water per rat, per day
was 20 ml regardless of the type of water provided (distilled or LC
water) (data not shown).
The treatment regimen of all groups including the 3
groups that received different doses of LC [low (L), medium (M),
high (H)] and with the exception of the group given LC water only,
is shown in Table I and in Fig. S1. Blood was collected from the
abdominal aorta at the end of the 5-week treatment. Serum was
extracted from the blood samples and cryopreserved until use.
Biochemical assays
SUA concentration was measured using the
phosphotungstic acid method; the XOD activity and GSH levels were
determined using colorimetry; and serum MDA concentration was
measured using the thiobarbituric acid method, as previously
described (3,11). All biochemical assays were performed
in our laboratory at a constant ambient temperature of 22±1̊C and
relative humidity of 55%, according to the manufacturer's
protocol.
Measurement of hemorheological
parameters
Blood samples were anticoagulated using 2% heparin,
and the whole blood viscosity of the samples at high (150
s-1), medium (60 s-1) and low (20
s-1) shear rates were measured using a viscometer, as
described previously (3). The
viscosity of the plasma, separated from the anticoagulated blood by
centrifuging at 1,000 x g for 10 min at room temperature (22±1̊C),
was also measured. The erythrocyte deformability index and the
aggregation index were measured using an automatic rheometer, as
described previously (3).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons between multiple groups were performed using a one-way
ANOVA with a post-hoc Tukey's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
SUA concentration
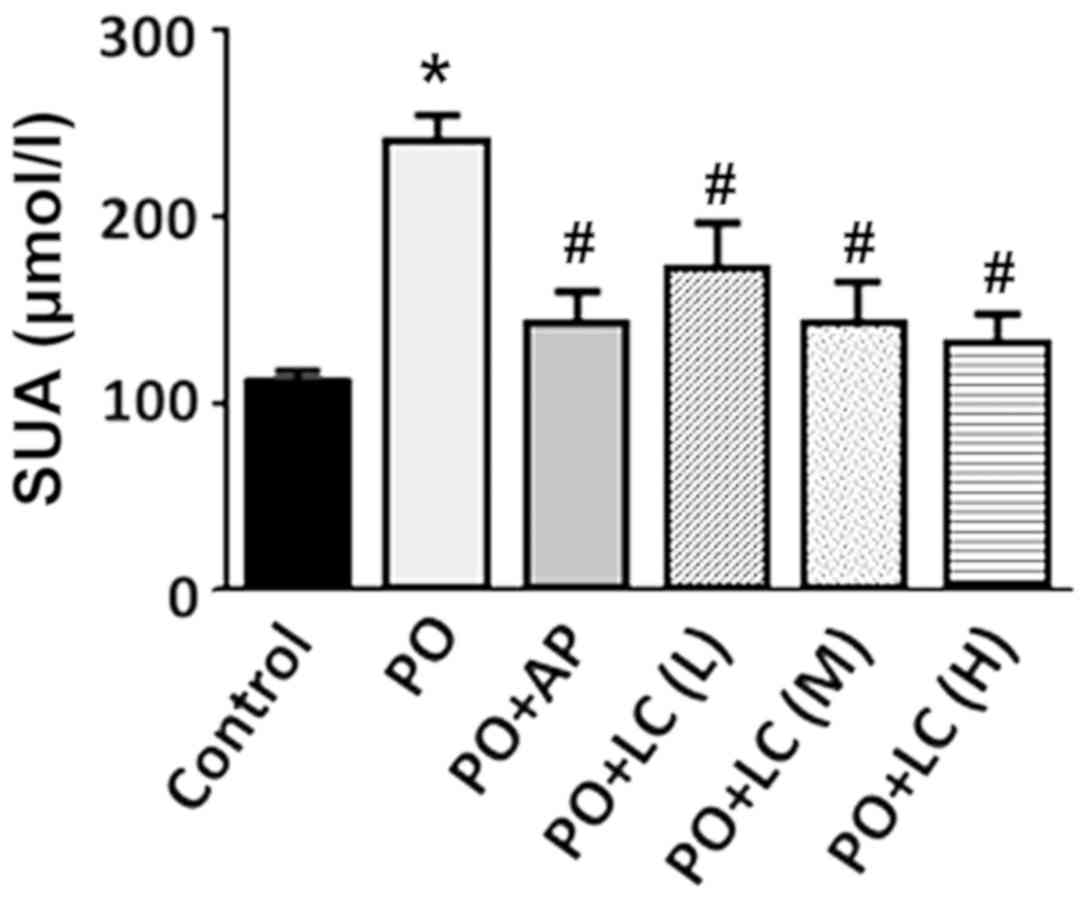
The concentration of SUA in PO-treated rats (PO
rats) was ~2-fold higher compared with the control rats. However,
these levels were reduced when the PO rats were administered AP
(PO+AP) (Fig. 1). Furthermore, the
concentration of SUA in the rats administered with a combination of
PO and varying doses of LC water (PO+LC) was slightly higher
compared with the control rats and lower compared with the PO rats
(Fig. 1). LC (M) and LC (H)
exhibited similar effects compared with that of AP in reducing the
SUA concentration (Fig. 1).
Hemorheological parameters
Plasma and whole blood viscosity of the PO rats were
significantly higher compared with the control rats, and the
viscosity did not differ significantly between rats treated with
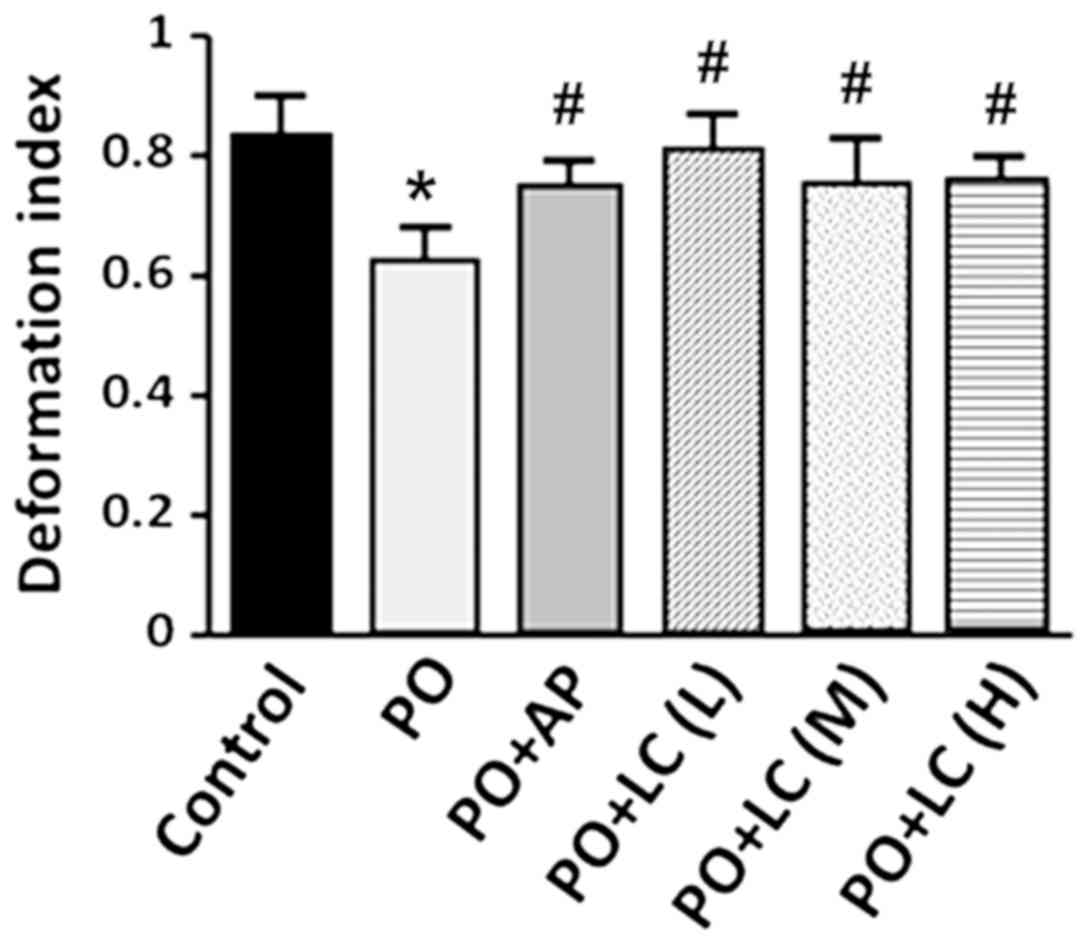
PO, and PO with AP or LC combination (Table II). The erythrocyte deformability
index of the PO rats decreased to ~75% of that observed in the
control rats. However, the erythrocyte deformability index of the
PO+LC rats, as well as that of the PO+AP rats, did not decrease as
much and was significantly higher compared with the PO rats
(Fig. 2). There was no significant
difference observed in the aggregation index among all the
treatment groups (Table II).
 | Table IIHemorheological properties of the rats
in each group. n=10 per group. |
Table II
Hemorheological properties of the rats
in each group. n=10 per group.
| | | | | PO+LC |
|---|
| Hemorheological
property | Control | PO | PO+AP | Low | Medium | High |
|---|
| Plasma viscosity,
mPa·s | 1.14±0.11 |
2.32±0.65a | 2.25±0.13 | 2.10±0.19 | 1.93±0.24 | 2.13±0.26 |
| Whole blood
viscosity, mPa·s |
|
150
s-1 | 4.01±0.27 |
5.59±0.78a | 5.93±0.61 | 6.39±0.91 | 5.49±0.72 | 5.84±0.51 |
|
60
s-1 | 5.55±0.43 |
7.42±1.04a | 8.18±1.06 | 9.18±1.58 | 7.50±0.93 | 7.77±0.48 |
|
20
s-1 | 8.12±0.79 |
10.72±1.69a | 12.33±1.97 | 14.82±3.61 | 11.09±1.66 | 11.31±0.61 |
| Aggregation
index | 2.02±0.06 | 1.91±0.10 | 2.06±0.12 | 2.30±0.33 | 2.01±0.12 | 1.94±0.11 |
Antioxidant capacity
The serum XOD activity in the PO rats was higher
compared with the control rats. The MDA concentration and GSH
activity in the PO rats was slightly but not significantly higher
and lower, respectively, than those in the control rats (Table III). However, the serum XOD
activity in the PO+LC (M) and PO+LC (H) rats decreased to that of
the control rats as did the XOD activity in the PO+AP rats
(Table III). The MDA concentration
in all PO+LC rats was lower compared with the PO rats, but the GSH
activity in the PO+LC (M) rats was not notably higher compared with
the PO rats (Table III).
 | Table IIIEffects of AP and LC water on
oxidative status in serum. n=10 per group. |
Table III
Effects of AP and LC water on
oxidative status in serum. n=10 per group.
| | | | | PO+LC |
|---|
| Factor | Control | PO | PO+AP | Low | Medium | High |
|---|
| Xanthine oxidase,
U/l | 54.41±3.12 |
63.46±9.14a |
52.11±5.92b | 59.96±3.80 |
54.13±6.85b |
53.32±4.39b |
| Malondialdehyde,
nmol/ml | 7.89±0.76 | 8.43±0.92 | 7.22±0.402 |
6.36±0.59b |
5.43±0.44b |
5.40±0.57b |
| Glutathione,
U/ml | 27.96±7.86 | 24.36±9.87 | 28.14±16.57 | 34.24±9.90 |
36.44±6.23b |
38.98±10.00b |
Discussion
In the present study, the increase of SUA in PO rats
was significantly suppressed by administration of LC water as well
as by AP gavage. The PO-untreated and LC water-treated rats did not
show any significant differences in the SUA levels from the control
rats (data not shown). Thus, the results of the present study
suggest that the LC water may have effectively inhibited the
increase in SUA concentration in hyperuricemic rats.
LC is marketed as a food which promotes a healthy
status in humans (8). For a person
weighing 60 kg, daily consumption of LC as food should be 1.6-6.0 g
according to the manufacturer. In the present study, rats consumed
25-100 mg/kg/day (Table I). The
applied PO dosage was in accordance with our previous study
(3). The potential effects of PO
toxicity remains to be determined; however, LC toxicity was
previously evaluated (8). The median
lethal dose of LC administered orally in rats is estimated to be
4.1 g per rat (8). It is possible
that lethality is the result of ileus caused by LC overdose.
However, no other significant side effects have been reported
(8).
UA is an important endogenous antioxidant in the
human body (1,2,14).
However, the ability of UA to scavenge free radicals is limited,
and high concentrations of SUA can affect the redox balance system
and promote oxidative stress injury (1,2,12,15). In
the present study, serum XOD activity in the rats treated with a
combination of PO and LC water was lower compared with PO rats
without LC. XOD is a key enzyme involved in the formation of UA,
and a rate-limiting enzyme for converting hypoxanthine to xanthine,
which is subsequently converted to UA (1,14).
Therefore, the LC water may potentially reduce the concentration of
UA by regulating the activity of XOD.
Recent studies have suggested that HUA may be
directly associated with the hemorheological characteristics
(3,11). A high concentration of SUA can result
in a decrease in erythrocyte deformability, and can increase whole
blood viscosity at different shear rates (3,11). In
the present study, PO-induced HUA significantly decreased
erythrocyte deformation, as previously reported (3,11).
However, the combined administration of PO with LC water
significantly suppressed the decrease of erythrocyte deformation.
Furthermore, serum MDA concentration decreased in the PO+LC rats,
suggesting high oxidative stress conditions in the PO rats and the
possibility of its suppression by LC water. There is the
possibility that the LC water may decrease SUA concentration,
enhance anti-oxidative activity and improve the hemorheological
characteristics.
In our previous study, the anti-oxidative effects of
LC water in cultured human cells were reported (13). Additionally, in the liver of rats
with alcoholism, LC administration reduced MDA content and
increased GSH activity, suggesting the anti-oxidative effects of LC
on alcoholic hepatic injury (9).
Hwang et al (16) also
reported the anti-oxidative properties of water mixed with a
hydrophilic ceramic powder in plant and mammal cell lines. It has
been previously reported that drinking hot spring water is
effective against HUA, possibly due to the pharmacological effects
of the chemicals involved in purine and UA metabolism (17). Thus, drinking LC water may provide
protection against HUA due to the increased anti-oxidative
activity.
The present LC study is based on our established
theory of the ‘human SOS response’, which attempts to explains the
biophysiological mechanism supervising metabolic reactions against
various stressors such as oxidative stress (8), and on the findings regarding the
LC-induced response, which includes regulation of physiological
response to various agents, such as metabolites from foods
(8). The effectiveness of LC has
been explored in various metabolic disorders by using the same
treatment scheme as that of the present study (9,10). In
addition, in hyperglycemic rats, the onset of cataracts is
suppressed by drinking LC water (unpublished results). Similarly,
in hyperlipidemic rats, a decrease in serum triglycerides and low
density lipoprotein is detected in response to drinking LC water
(unpublished results). Interestingly, the levels of triglycerides
in human blood serum tended to decrease after drinking LC water for
30 days (8). Therefore, LC may be a
potential therapeutic for clinical treatment of various
diseases.
Supplementary Material
Figure S1. Flow diagram of the
allocation and treatment of rats. PO, potassium oxonate; AP,
allopurinol; LC, lifeceramics; SCC, sodium carboxymethyl
cellulose.
Acknowledgements
Not applicable.
Funding
This study was supported by the Scientific and
Technological Research of Chengde Medical University (grant nos.
201714 and 201708) (Chengde, China) and the Non-profit
Organization, Chiba Researchers Network for Health Care Promotion
(Chiba, Japan).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KL, XG and JY performed the experiments. FG and XL
prepared and analyzed the data. KL, XT and NS designed the study.
AS and KK analyzed and interpreted the data, and revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Chengde Medical University (Chengde,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Battelli MG, Bortolotti M, Polito L and
Bolognesi A: The role of xanthine oxidoreductase and uric acid in
metabolic syndrome. Biochim Biophys Acta Mol Basis Dis.
1864:2557–2565. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bove M, Cicero AF, Veronesi M and Borghi
C: An evidence-based review on urate-lowering treatments:
Implications for optimal treatment of chronic hyperuricemia. Vasc
Health Risk Manag. 13:23–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li K, Tong X, Yao W, Wang X, Suzuki A and
Suzuki N: Study on hemorheological properties of erythrocytes in
asymptomatic hyperuricemia rat model. Int J Med Res Health Sci.
6:1–7. 2017.
|
|
4
|
Tsuruta Y, Mochizuki T, Moriyama T,
Itabashi M, Takei T, Tsuchiya K and Nitta K: Switching from
allopurinol to febuxostat for the treatment of hyperuricemia and
renal function in patients with chronic kidney disease. Clin
Rheumatol. 33:1643–1648. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Pang M, Fang Y, Chen S, Zhu X, Shan C, Su
J, Yu J, Li B, Yang Y, Chen B, et al: Gypenosides inhibits xanthine
oxidoreductase and ameliorates urate excretion in hyperuricemic
rats induced by high cholesterol and high fat food. Med Sci Monit.
23:1129–1140. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Matsumoto N, Yokokawa A, Ohashi K, Yoshida
K, Hashimoto K and Toda Y: Mechanical properties of β-tricalcium
phosphate ceramics doped with vanadate ions. Phosphorus Res Bull.
24:73–78. 2010.
|
|
7
|
Mori K: Development of ceramic with
powerful sterilization junction from a mixture of baked
oyster-shell and natural zeolite-the background, current status and
outlook. The food industry. 41:48–59. 1998.http://www.wed.co.jp/lifeceramics/.
|
|
8
|
Tong X, Dong M, Kita K, Zhu T, Ma X,
Suzuki A and Suzuki N: Search for food materials that support
healthy conditions, based on the human SOS response theory. Chiba
Med J. 1:15–22. 2018.(In Japanese).
|
|
9
|
Ma X, Li WZ, Liu X, Wang S, Li Y, Dong M
and Suzuki N: Protective effect and mechanism of Lifeceramics on
chronic alcoholic liver injury in rats. Chinese Journal of Clinical
Rational Drug Use. 11:40–42. 2018.(In Chinese).
|
|
10
|
Liu X, Guo S, Ma X, Dong M and Suzuki N:
Protective effect of Lifeceramics on liver of long-term drinking
rats. Journal of Hebei Medical University. 36:253–256. 2015.(In
Chinese).
|
|
11
|
Li K, Li L, Xu S, Tong X and Xie L:
Hemorheology and oxidative stress in rats with asymptomatic
hyperuricemia. J Med Biomech. 32:88–91. 2017.(In Chinese).
|
|
12
|
Zhou Y, Zhao M, Pu Z, Xu G and Li X:
Relationship between oxidative stress and inflammation in
hyperuricemia: Analysis based on asymptomatic young patients with
primary hyperuricemia. Medicine. 97(e13108)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kita K, Sugaya S, Tanaka T, Dong M, Sato C
and Suzuki N: Effects of lifeceramics-treated water on resistance
to oxidative stress in human cells. Food Func. 11:14–19. 2013.(In
Japanese).
|
|
14
|
Ndrepepa G: Uric acid and cardiovascular
disease. Clin Chim Acta. 484:150–163. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu L, Wang L, Liu C, Wang X, Wang C and
Zhang J: Effects of Lishi huoxue forula on blood uric acid and
antioxidant capacity of hyperuricemia rats. World Chinese Med.
12:134–137. 2017.(In Chinese).
|
|
16
|
Hwang SG, Lee HS, Lee BC and Bahng GW:
Effect of antioxidant water on the bioactivities of cells. Int J
Cell Biol. 2017(1917239)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Etani R, Kataoka T, Kanzaki N, Sakoda A,
Tanaka H, Ishimori Y, Mitsunobu F and Yamaoka K: Difference in the
action mechanism of radon inhalation and radon hot spring water
drinking in suppression of hyperuricemia in mice. J Radiat Res.
57:250–257. 2016.PubMed/NCBI View Article : Google Scholar
|