Introduction
Gliomas are a group of malignant brain tumors, and
despite the significant efforts that have been made to develop and
optimize novel therapeutics for treatment of patients with
high-grade glioma, the prognosis of these patients remains poor
(1). Surgery is often incomplete due
to the inaccessibility of the tumor location. Additionally, glioma
cells are often resistant to chemotherapy and radiation (2). Thus, novel treatment strategies are
required. The use of compounds of natural origin may serve as an
alternative method of treatment. The health-promoting and healing
properties of garlic have been used for thousands of years. Garlic
contains several water- and oil-soluble organosulfur compounds
which have conditioning properties, such as antimicrobial,
antithrombotic, antiarthritic and antitumor properties (3). Oil-soluble compounds which include:
Diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide
(DATS) and ajoene are more effective than the water-soluble
compounds when used to prevent or treat cancer. DADS and DATS are
the primary components of garlic oil, which has a yellowish hue,
and is insoluble in water (1).
A number of studies have focused on the evaluation
of the impact of DADS on the human body in health and disease.
These studies have shown that DADS exhibits protective properties
and at the same time target cancer cells (4,5). In
vivo studies have shown that DADS effectively inhibits
carcinogens by modulating the action of cytochrome P450-dependent
monooxygenases, as well as by affecting the activation of phase II
enzymes which are responsible for detoxifying xenobiotics (6). These include glutathione reductase and
glutathione transferase (7).
The anti-proliferative properties of DADS are
related to its ability to decrease the proportion of cells in the
G1 and G2/M phases (8). Furthermore,
several studies have suggested that DADS may induce apoptosis in
several types of cancer cells, and can work as an inhibitor of
histone deacetylase (3,4,9).
Previous studies have suggested that the above mentioned abilities
reduce cell proliferation (10),
angiogenesis, as well as invasion and metastasis in cancer cells
(9). A number of studies have
demonstrated the chemopreventative properties of different forms of
products and compounds derived from garlic, including fresh and
aged garlic extract, as well as garlic oil (11). The anti-cancer properties of garlic
oil is attributed to the presence of organic sulfur compounds in
garlic. The modes of organic sulfur action include its effect on
drug metabolizing enzymes, antioxidant properties and tumor growth
inhibition (12). Therefore, the
consumption of garlic may assist in cancer prevention, while the
use of specific compounds derived from garlic may increase the
therapeutic potential in regard to cancer prevention or even treat
existing cancers. The effectiveness of these compounds has been
demonstrated in several studies on various cancer cell lines
including breast, colon and prostate cancer cells (13); however, its effects have not been
studied on human glioblastoma cells, to the best of our knowledge.
Taking into consideration the above, the aim of the present study
was to determine whether oil-soluble organosulfur compounds derived
from garlic exhibited anti-cancer activity against human glioma
cells. The effect of DADS and garlic oil (which is a combination of
several oil-soluble organosulfur compounds) were assessed on cell
viability and apoptosis induction. The effects were assessed on
four different glioma cell lines of different grades.
Materials and methods
Cell culture
In the present study, four human astrocytoma cell
lines of differing grades were used: CCF-STTG1 (grade IV,
astrocytoma), SW1783 (grade III, astrocytoma), SW1088 (astrocytoma)
and CHLA-03-AA (anaplastic astrocytoma). All cell lines were
purchased from ATCC®. SW1783 and SW1088 cells were grown
as a monolayer in ATCC-formulated Leibovitz's L-15 medium (cat. no.
30-2008; ATCC) supplemented with 10% FBS and 50 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA). These two cell lines were grown at
37˚C in 100% air. For CCF-STTG1 and CHLA-03-AA cells,
ATCC-formulated RPMI-1640 medium (catalog no. 30-2001; ATCC)
supplemented with 10% FBS and 50 µg/ml streptomycin was used. These
cells were grown at 37˚C with 5% CO2. Prior to each
experiment, the cells were detached using 0.25% trypsin with 0.02%
EDTA (Sigma-Aldrich; Merck KGaA).
Chemicals
Garlic oil and DADS was purchased from
Sigma-Aldrich; Merck KGaA (cat. no. 8000-78-0 and SMB00378,
respectively). A range of concentrations of both chemicals were
used in the present study (0.015-150 µg/ml). Both garlic oil and
DADS were diluted in the appropriate culture medium for each cell
line.
MTT assay and determination of the
IC50
The viability of cells was determined using an MTT
assay (Sigma-Aldrich; Merck KGaA) after 24 h of incubation with
different concentrations of garlic oil and DADS (0,015-150 µg/ml).
To assess the effect of garlic oil, concentrations of 15 and 150
µg/ml were used, the results obtained were not included in the
determination of cell viability and IC50, as the
absorbance obtained in those samples was clearly overvalued due to
the presence of the separate oil layer in the suspension.
The MTT assay was used for evaluation of
mitochondrial metabolic function according to the manufacturer's
protocol (Sigma-Aldrich; Merck KGaA). A total of 1x104
cells/well were seeded into 96-well microculture plates. The
absorbance was determined using an Enspire Multiplate reader
spectrophotometer at 570 nm (Perkin Elmer, Inc.). Mitochondrial
metabolic function was expressed as a percentage of the viable
treated cells to the untreated control cells. Based on the
viability values obtained in the MTT assay, the IC50
values for DADS and garlic oil were calculated using GraphPad Prism
version 7.03 (GraphPad Software, Inc.).
Evaluation of apoptosis and
necrosis
Annexin V conjugated to fluorescein isothiocyanate
(FITC) fluorochrome was used for flow cytometry analysis of cells
which were undergoing apoptosis. Staining with Annexin V/FITC was
performed at room temperature for 15 min. The externalization of
phosphatidylserine occurs during the early stages of apoptosis, and
Annexin V/FITC staining can identify apoptosis at an earlier stage
than assays based on nuclear changes, such as DNA fragmentation
(13). Necrotic changes were
identified using propidium iodide (PI), fluorescence of which is
enhanced 20-30-fold upon binding to nucleic acids (13). The analysis was performed using a
commercial FITC Annexin V kit (BioLegend, Inc.; cat. no. 640914).
For evaluation of apoptosis and necrosis, 0.15, 0.75 and 1.5 µg/ml
DADS or garlic oil were used. Analysis was performed using CyFlow
Cube 6 cytometer (Sysmex Europe, GmbH), where FL-1 was used for
Annexin-V-FITC and FL-2 detector was used for necrotic cells (PI
stained) measurements. A representative example of the gating
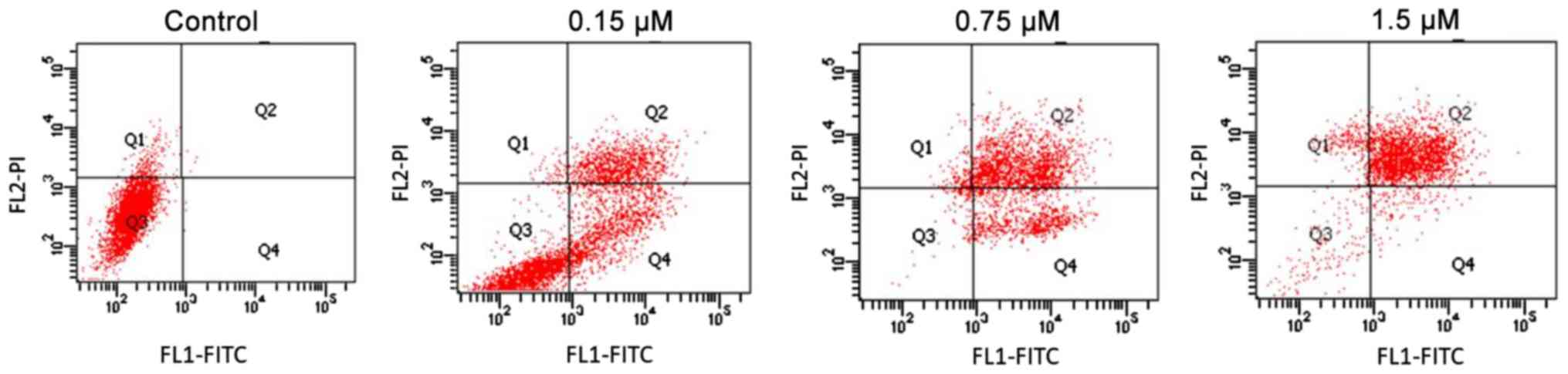
strategy used in each experiment is shown in Fig. 1. In the quadrant gates, Q1, Q2, Q3
and Q4 fields correspond respectively to necrotic, late-apoptotic,
alive cells and early apoptotic cells.
Statistical analysis
Data were analyzed using GraphPad Prism version
7.03. A two-way ANOVA followed by a post-hoc Tukey's test was used
to compare the data were α=0.05. All samples were analyzed in
triplicate. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell viability analysis showed that the highest
assessed DADS concentrations (15 and 150 µg/ml) were cytotoxic for
CCF-STTG1 and CHLA-03-AA cells (Fig.
2). IC50 for CCF-STTG1 and CHLA-03-AA cells were 5.8
and 2.64 µg/ml, respectively (Table
I). For SW1783 and SW1088 cells, DADS was not cytotoxic and
even the highest tested concentration-150 µg/ml, only slightly
reduced cell viability to 88.7% for the SW1783 cell line and to
77.7% for the SW1088 cells (Fig. 2).
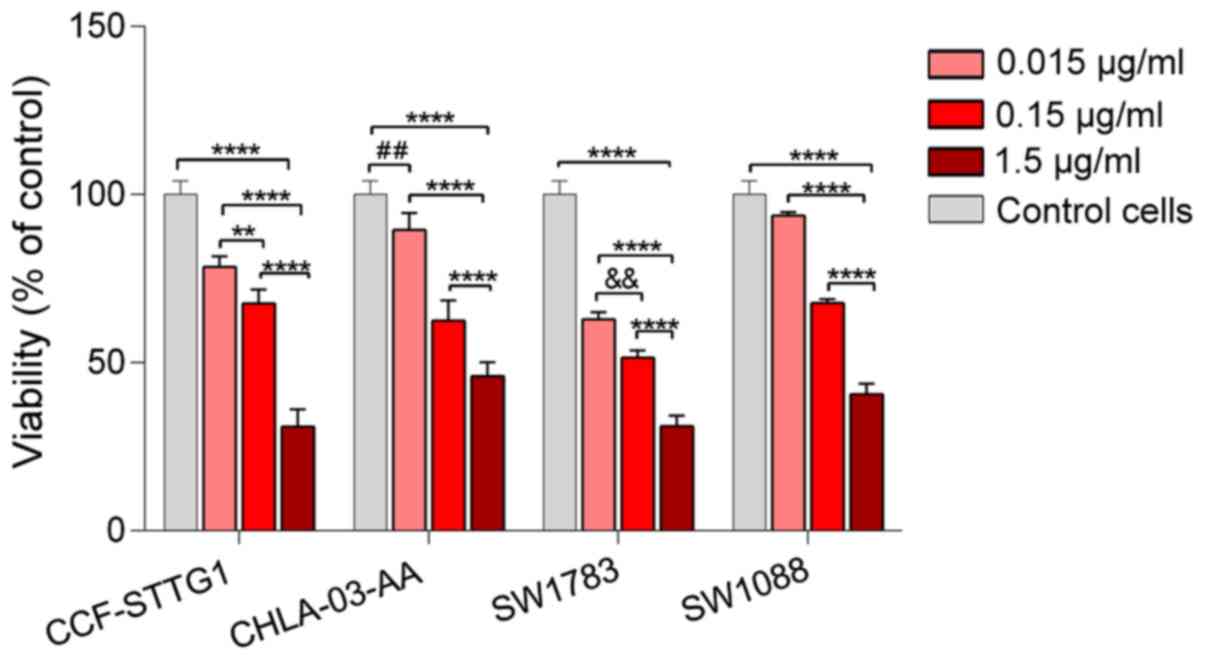
The viability assay for garlic oil was performed using a
concentration range of 0.015 to 1.5 µg/ml due to the presence of a
separate oil layer at the higher tested concentrations. In contrast
to DADS, 1.5 µg/ml garlic oil significantly reduced cell viability
in all tested cell lines to 30.9% in CCF-STTG1, 45.9% in
CHLA-03-AA, 31.1% in SW1783 and 40.6% in SW1088 cells (Fig. 3). The IC50 values were
4.05 µg/ml for CCF-STTG1, 0.072 µg/ml for CHLA-03-AA, 1.11 µg/ml
for SW1783 and 0.15 µg/ml in SW1088 (Table I).
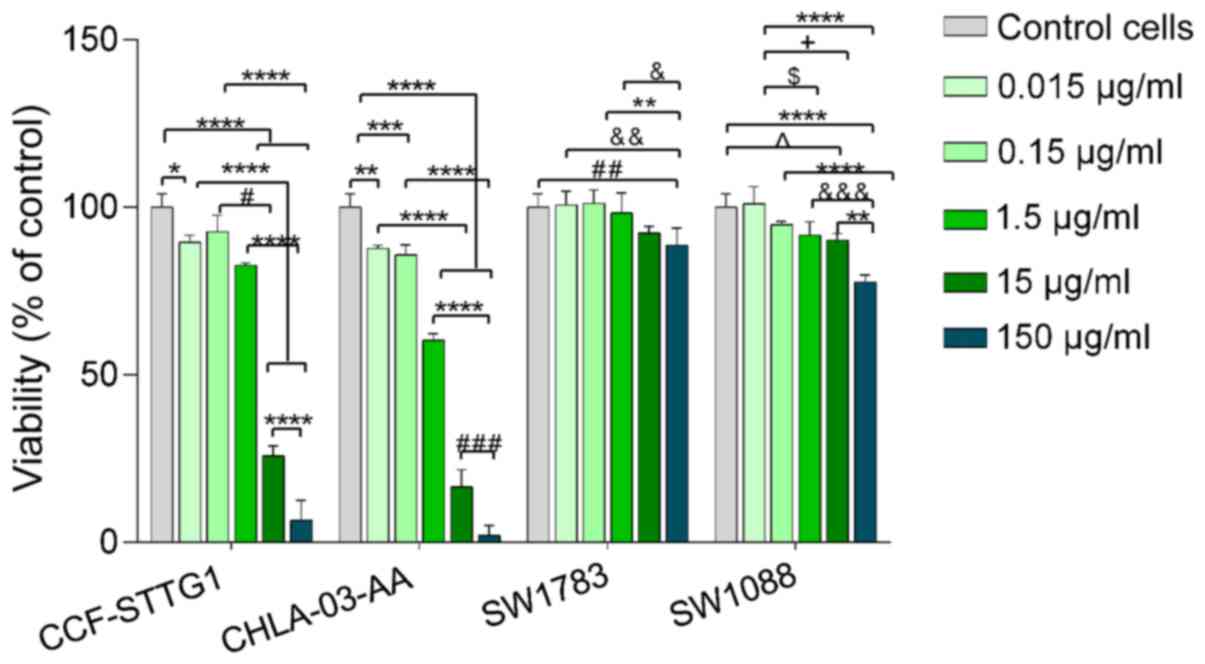 | Figure 2Viability of CCF-STTG1, CHLA-03-AA,
SW1783 and SW1088 cells after 24 h of treatment with increasing
concentrations of DADS. Viability is expressed as a percentage of
the control cells (untreated cells). Data are presented as the mean
± standard deviation of 3 repeats. *P<0.05,
**P<0.01, ***P<0.001,
****P<0.0001. #P<0.05;
##P<0.01, ###P<0.001;
&P<0.05, &&P<0.01,
&&&P<0.001; ^P<0.05;
$P<0.05, +P<0.05. DADS, diallyl
disulfide. |
 | Table IIC50 values of DADS and
garlic oil in glioma cell lines. |
Table I
IC50 values of DADS and
garlic oil in glioma cell lines.
| | IC50,
µg/ml |
|---|
| Cell lines | DADS | Garlic oil |
|---|
| CCF STTG1 | 5.8 | 4.05 |
| SW1783 | Non-toxic | 1.11 |
| SW1088 | Non-toxic | 0.15 |
| CHLA-03-AA | 2.64 | 0.072 |
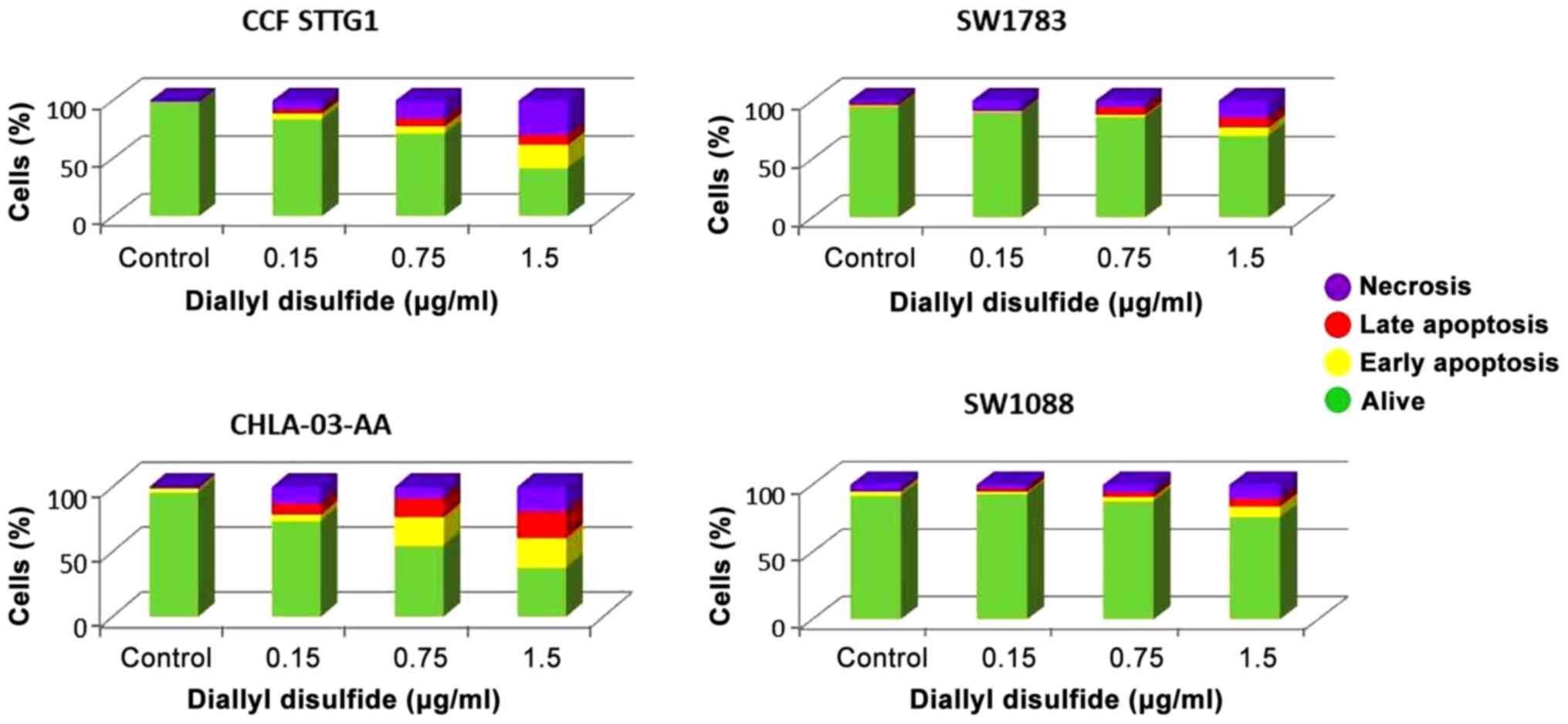
The results of the flow cytometry analysis were
consistent with the viability test. There was a significantly
higher percentage of live cells observed in the DADS-treated
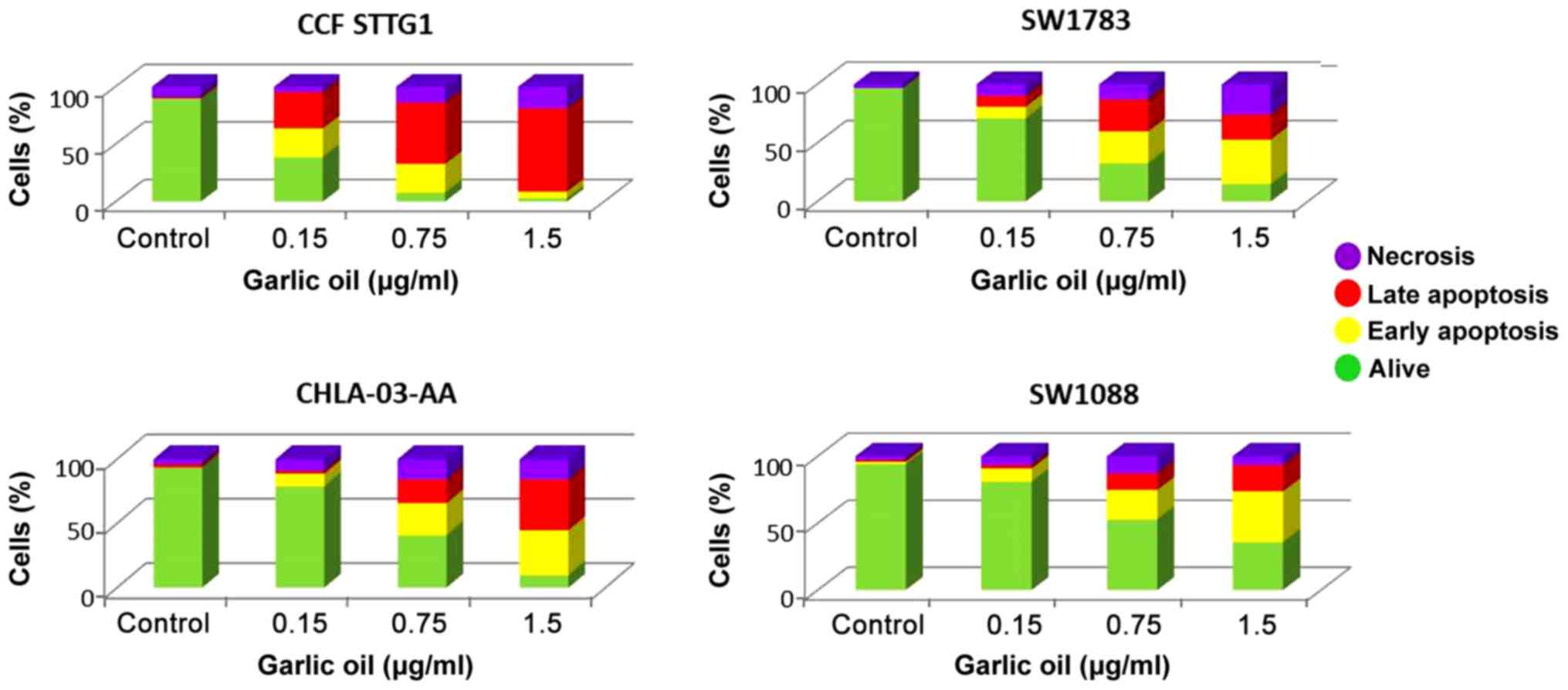
samples for SW1783 and SW1088 cell lines (Fig. 4). For samples treated with 1.5 µg/ml
garlic oil, the percentages of live cells were 3, 10, 15 and 36%
for CCF-STTG1, CHLA-03-AA, SW1783 and SW1088 cells, respectively
(Fig. 5). Flow cytofluorimetric
analyses were performed to determine the proportion of dead cells
using an Annexin V/PI double staining kit. Annexin V signal
detection provides a very sensitive method for detecting cellular
apoptosis, whereas PI is used to detect necrotic or late apoptotic
cells, characterized by the loss of integrity of the plasma and
nuclear membranes (13). Data
generated by flow cytometry was plotted in 2D dot plots, in which
PI was represented vs. Annexin V-FITC. These plots were divided
into four regions corresponding to: i) Viable cells which were
negative to both probes; ii) apoptotic cells which were PI negative
and Annexin-V positive; iii) late apoptotic cells which were PI and
Annexin-V positive; and iv) necrotic cells which were PI positive
and Annexin-V negative. For each condition tested, 10,000 cells
were analyzed, and the percentage of alive, early apoptotic, late
apoptotic and necrotic cells was expressed as a percentage of the
total number of cells assessed. Flow cytometry analysis showed that
DADS induced both, apoptotic and necrotic cell death at a similar
level in the four tested glioma cell lines (Fig. 4). Using 1.5 µg/ml, DADS induced
apoptosis in 28% of CCF-STTG1 cells and necrosis in 30% of these
cells. For SW1783 cells, 15% of the cells were apoptotic and 15% of
the cells were necrotic. For SW1088 cell, 13% of the cells were
apoptotic and 11% were necrotic. In CHLA-03-AA cells, 43% of the
cells were apoptotic cells and 19% of the cells were necrotic.
Garlic oil notably induced apoptotic death in all the tested cell
lines at a low concentration. A dose of 0.75 µg/ml resulted in 78,
43, 54 and 34% proportion of apoptotic cells in the CCF-STTG1,
CHLA-03-AA, SW1783 and SW1088 cells, respectively. At a
concentration of 1.5 µg/ml garlic oil, 78, 74, 59 and 57% of the
CCF-STTG1, CHLA-03-AA, SW1783 and SW1088 cells were apoptotic,
respectively (Fig. 5).
Discussion
The prominent effects of garlic on cell-cycle
arrest, apoptosis and differentiation has been the subject of
several studies (14-16).
At present, there have been no attempts to analyze the effects of
the compounds derived from garlic on glioma of varying degrees of
differentiation, to the best of our knowledge. Hong et al
(17) examined the effect of DAS and
DADS on H460 (p53-wild type) and H1299 (p53-null) non-small cell
lung cancer cells. Both DAS and DADS induced apoptosis in the
non-small cell lung cancer cells, which was correlated with a
marked increase in the protein expression levels of p53 and Bax, as
well as a decrease in Bcl-2 protein expression (17). Nakagawa et al (18) examined the inhibitory effect of DADS
on human estrogen receptor-positive and -negative breast cancer
cell lines. In both these breast cancer cell lines, DADS resulted
in a significant increase in Bax and caspase-3 protein expression,
resulting in apoptotic cell death. In addition, DADS in the
estrogen receptor-negative cells acted synergistically with
eicosapentaenoic acid, which is a tumor cell suppressor. Similarly,
in vivo, DADS resulted in a reduction of tumor weight
compared to the DADS-untreated mice (18).
The toxic effects of DADS and garlic oil were
assessed on four glioma cells in the present study. The results
showed that DADS did not result in cytotoxicity at any of the
assessed concentrations in SW1783 and SW1088 cells. In the
CCF-STTG1 and CHLA-03-AA cells, there was a significant reduction
in cell viability observed after treatment with 15 and 150 µg/ml.
The lack of toxicity at lower doses of DADS and the cytotoxic
effects at higher doses of DADS were consistent with the results of
Koh et al (19). In a
previous study performed on PC-12 cells (cells derived from a
transplantable rat pheochromocytoma), it was shown that the lowest
tested concentration of DADS, 20 µM, exhibited neuroprotective
effects by inducing an increase in Akt kinase activity, triggering
the PI3K/Akt signaling pathway and decreasing the activity of
proapoptotic factors (cytochrome c, caspase 3 and PARP
protein) (20). Lower concentrations
of DADS exhibited cytoprotective potential, whereas 50 µM of DADS
resulted in an increase in the levels of free radicals and lipid
peroxidation of PC-12 cell membranes. Furthermore, 100 µM DADS
inhibited the PI3K/Akt pathway and increased proapoptotic activity
(21). The high degree of
invasiveness of gliomas may be due to aberrant signal transduction
of pathways downstream of PI3K and Akt/PKB kinases, which are
responsible for the regulation of cell proliferation,
differentiation and survival (22-24).
Mutations of genes encoding the above enzymes are commonly observed
in gliomas, and lead to an increase in their activity, resulting in
increased resistance to chemotherapy (25). The absence of a cytotoxic effect on
SW1783 and SW1088 cells following incubation with DADS may be due
to the enhanced resistance exhibited by these cell lines caused by
mutations in the PI3K-Akt/PKB-mTOR signaling pathway gene (26). It is likely that for the CHLA-03-AA
and CCF-STTG1 cells, higher doses of DADS induced cytotoxicity by
decreasing Akt/PKB factor activity, a mechanism that was observed
by Koh et al (19). Apoptosis
inhibition via the Akt/PKT signaling pathway is very complex and
can occur at different levels of the signal transduction pathways
initiating programmed cell death. PI3K/AKT kinase phosphorylates
the pro-apoptotic BAD protein and promotes the binding of BAD to
the cytoplasmic 14-3-3 protein, resulting in inhibition of
apoptosis (22). Das et al
(27) demonstrated that the garlic
compounds DAS, DADS and DATS are effective in inducing apoptosis in
human glioblastoma T98G and U87MG cells. It was suggested that the
compounds present in garlic activate multiple pathways which result
in induction of apoptosis in these cells by increasing the
production of reactive oxygen species (ROS). They have shown that
ROS induces apoptosis via the phosphorylation of p38 MAPK and
activation of the redox-sensitive JNK1 pathway. Production of ROS
in these cells also increased endoplasmic reticulum stress, and
mitochondrial release of cytochrome C and Smac into the cytosol,
which also result in apoptosis (27). Liu et al (28) showed that different variants of the
glutathione S-transferase (GST) genes may affect the pathogenesis
of glioma, and thus may have an impact on the variable responses to
the various types of therapeutic compounds in gliomas with distinct
variants of these genes. The aforementioned differences in GST gene
variants may underlie the disparities observed in DADS-induced
cytotoxicity in the present study. However, multi-center controlled
prospective studies are required to verify this assumption.
Garlic oil contains several active substances that
possess a high therapeutic potential. These include diallyl, allyl
methyl and dimethyl mono- to hexa-sulfides (29). Furthermore, the biological effects of
garlic can be attributed to all of the characteristic organosulfur
compounds that it contains (30).
Thus, it is hypothesized that the substances contained in garlic
oil may amplify the induction of apoptotic cell death by activating
different pathways to DADS, which induced apoptosis only in two
tested cell lines, while garlic oil caused activation of apoptotic
death pathways in all four tested cell lines. Additionally, only
0.75 µg/ml garlic oil was required to induce apoptosis. The
anti-cancer potential of garlic oil remains poorly understood. The
majority of the studies in this field of study have been performed
on blood cancer cell lines (31-33).
Seki et al (31) examined the
effects of garlic oil on HL-60 cells (a human promyelocytic
leukemia cell line). They found that garlic oil markedly reduced
cell proliferation and concluded that it could induced
differentiation of HL-60 cells into granulocytic cells (31). The preliminary results of the present
study are promising, particularly the results obtained for garlic
oil, which induced a significant cytotoxic effect in all tested
astrocytoma cell lines of various degrees of differentiation. In
addition, garlic oil primarily induced activation of apoptosis. It
is crucially important for therapeutic applications used for the
treatment of brain tumors to reduce cell death, where necrotic
death is undesirable due to a resultant strong inflammatory
reaction (34).
The present study has several limitations that
should be addressed in subsequent studies. These include assessing
the mechanism of the induced apoptotic pathway, estimation of
cell-cycle phase distribution, as well as the examination of the
effects of tested compounds on normal human cells. It is
hypothesized that the PI3K-Akt/PKB-mTOR signaling pathway may be
induced following treatment of glioma cells with DADS and garlic
oil. However, it is necessary to assess the mechanisms leading to
apoptosis in astrocytoma cells following treatment with active
substances derived from garlic in more detail. The effects of other
compounds from garlic oil and their antiproliferative properties on
glioma cells should also be addressed. This can be used to identify
and separate the most active substances from the mixture.
Additionally, it would be useful to perform similar research with
normal cells to identify the substances which exhibit selectivity
against malignant cells.
Acknowledgements
Not applicable.
Funding
This work was supported by a grant for Young
Scientists sponsored by Statutory Funds of Wroclaw Medical
University and supported by the Polish Ministry of Science and
Higher Education, (grant no. STM.A040.18.013). Funding for the cell
lines was provided by Dermamed company.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AC and PS designed the study. AC performed the
experiments and acquired the data. AC, JK and JS analyzed and
interpreted the data. AC and JS wrote the first draft. AC, JS and
JK contributed to writing or critical revision of the manuscript.
All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang K, Groom M, Sheridan R, Zhang S and
Block E: Liquid sulfur as a reagent: Synthesis of polysulfanes with
20 or more sulfur atoms with characterization by
UPLC-(Ag+)-coordination ion spray-MS. J Sulfur Chem. 34:55–66.
2013.
|
|
2
|
Osuka S and Van Meir EG: Overcoming
therapeutic resistance in glioblastoma: The way forward. J Clin
Invest. 127:415–426. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Yi L and Su Q: Molecular mechanisms for
the anti-cancer effects of diallyl disulfide. Food Chem Toxicol.
57:362–370. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Druesne N, Pagniez A, Mayeur C, Thomas M,
Cherbuy C, Duée PH, Martel P and Chaumontet C: Repetitive
treatments of colon HT-29 cells with diallyl disulfide induce a
prolonged hyperacetylation of histone H3 K14. Ann NY Acad Sci.
1030:612–621. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang YS, Xie N, Su Q, Su J, Huang C and
Liao QJ: Diallyl disulfide inhibits the proliferation of HT-29
human colon cancer cells by inducing differentially expressed
genes. Mol Med Rep. 4:553–559. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Arunkumar A, Vijayababu MR, Srinivasan N,
Aruldhas MM and Arunakaran J: Garlic compound, diallyl disulfide
induces cell cycle arrest in prostate cancer cell line PC-3. Mol
Cell Biochem. 288:107–113. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Munday R and Munday CM: Relative
activities of organosulfur compounds derived from onions and garlic
in increasing tissue activities of quinone reductase and
glutathione transferase in rat tissues. Nutr Cancer. 40:205–210.
2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ropero S and Esteller M: The role of
histone deacetylases (HDACs) in human cancer. Mol Oncol. 1:19–25.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Myzak MC, Ho E and Dashwood RH: Dietary
agents as histone deacetylase inhibitors. Mol Carcinog. 45:443–446.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Druesne-Pecollo N and Latino-Martel P:
Modulation of histone acetylation by garlic sulfur compounds.
Anticancer Agents Med Chem. 11:254–259. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wlodkowic D, Telford W, Skommer J and
Darzynkiewicz Z: Apoptosis and beyond: Cytometry in studies of
programmed cell death. Methods Cell Biol. 103:55–98.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Thomson M and Ali M: Garlic [Allium
sativum]: A review of its potential use as an anti-cancer
agent. Curr Cancer Drug Targets. 3:67–81. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Petrovic V, Nepal A, Olaisen C, Bachke S,
Hira J, Søgaard CK, Røst LM, Misund K, Andreassen T, Melø TM, et
al: Anti-cancer potential of homemade fresh garlic extract is
related to increased endoplasmic reticulum stress. Nutrients.
10(450)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ariga T and Seki T: Antithrombotic and
anticancer effects of garlic-derived sulfur compounds: A review.
Biofactors. 26:93–103. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Herman-Antosiewicz A and Singh SV:
Checkpoint kinase 1 regulates diallyl trisulfide-induced mitotic
arrest in human prostate cancer cells. J Biol Chem.
280:28519–28528. 2005.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hosono T, Fukao T, Ogihara J, Ito Y, Shiba
H, Seki T and Ariga T: Diallyl trisulfide suppresses the
proliferation and induces apoptosis of human colon cancer cells
through oxidative modification of beta-tubulin. J Biol Chem.
280:41487–41493. 2005.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hong YS, Ham YA, Choi JH and Kim J:
Effects of allyl sulfur compounds and garlic extract on the
expression of Bcl-2, Bax, and p53 in non small cell lung cancer
cell lines. Exp Mol Med. 32:127–134. 2000.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Nakagawa H, Tsuta K, Kiuchi K, Senzaki H,
Tanaka K, Hioki K and Tsubura A: Growth inhibitory effects of
diallyl disulfide on human breast cancer cell lines.
Carcinogenesis. 22:891–897. 2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Koh SH, Kwon H, Park KH, Ko JK, Kim JH,
Hwang MS, Yum YN, Kim OH, Kim J, Kim HT, et al: Protective effect
of diallyl disulfide on oxidative stress-injured neuronally
differentiated PC12 cells. Brain Res Mol Brain Res. 133:176–186.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Borek C: Antioxidant health effects of
aged garlic extract. J Nutr. 131 (3 Suppl):1010S–1015S.
2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pedraza-Chaverri J, González-Orozco AE,
Maldonado PD, Barrera D, Medina-Campos ON and Hernández-Pando R:
Diallyl disulfide ameliorates gentamicin-induced oxidative stress
and nephropathy in rats. Eur J Pharmacol. 473:71–78.
2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cheng CK, Fan QW and Weiss WA: PI3K
signaling in glioma-animal models and therapeutic challenges. Brain
Pathol. 19:112–120. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fan QW, Cheng CK, Nicolaides TP, Hackett
CS, Knight ZA, Shokat KM and Weiss WA: A dual
phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with
blockade of epidermal growth factor receptor in PTEN-mutant glioma.
Cancer Res. 67:7960–7965. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Opel D, Westhoff MA, Bender A, Braun V,
Debatin KM and Fulda S: Phosphatidylinositol 3-kinase inhibition
broadly sensitizes glioblastoma cells to death receptor- and
drug-induced apoptosis. Cancer Res. 68:6271–6280. 2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mueller W, Mizoguchi M, Silen E, D'Amore
K, Nutt CL and Louis DN: Mutations of the PIK3CA gene are rare in
human glioblastoma. Acta Neuropathol. 109:654–655. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee
SH, Zhang J, Signoretti S, Loda M, Roberts TM and Zhao JJ:
Essential roles of PI(3)K-p110beta in cell growth, metabolism and
tumorigenesis. Nature. 454:776–779. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Das A, Banik NL and Ray SK: Garlic
compounds generate reactive oxygen species leading to activation of
stress kinases and cysteine proteases for apoptosis in human
glioblastoma T98G and U87MG cells. Cancer. 110:1083–1095.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu W, Long H, Zhang M, Wang Y, Lu Q, Yuan
H, Qu Q and Qu J: Glutathione S-transferase genes variants and
glioma risk: A case-control and meta-analysis study. J Cancer.
19:4679–4688. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bayan L, Koulivand PH and Gorji A: Garlic:
A review of potential therapeutic effects. Avicenna J Phytomed.
4:1–14. 2014.PubMed/NCBI
|
|
30
|
Omar SH and Al-Wabel NA: Organosulfur
compounds and possible mechanism of garlic in cancer. Saudi Pharm
J. 18:51–58. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Seki T, Tsuji K, Hayato Y, Moritomo T and
Ariga T: Garlic and onion oils inhibit proliferation and induce
differentiation of HL-60 cells. Cancer Lett. 160:29–35.
2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Toledano Medina MÁ, Merinas-Amo T,
Fernández-Bedmar Z, Font R, Del Río-Celestino M, Pérez-Aparicio J,
Moreno-Ortega A, Alonso-Moraga Á and Moreno-Rojas R:
Physicochemical characterization and biological activities of black
and white garlic: In vivo and in vitro assays. Foods.
8(220)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liang R, Huang GS, Wang Z, Chen XQ, Bai
QX, Zhang YQ, Dong BX and Wang WQ: Effects of human bone marrow
stromal cell line (HFCL) on the proliferation, differentiation and
apoptosis of acute myeloid leukemia cell lines U937, HL-60 and
HL-60/VCR. Int J Hematol. 87:152–166. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang Y, Jiang G, Zhang P and Fan J:
Programmed cell death and its role in inflammation. Mil Med Res.
2(12)2015.PubMed/NCBI View Article : Google Scholar
|