A feature of the present review is the systemized
presentation of cancer treatment directions aimed at macrophages,
the process of their polarization, and their therapeutic use. Three
primary strategies are described: i) Drugs that are able to
modulate the activity of TAMs; ii) designed carriers for targeted
drug delivery to macrophages, TAMs or specific pro-tumor M2-TAMs;
and iii) the use of macrophages to target the tumor. At present,
research regarding TAMs is largely focused on an increased interest
in the search for markers characterizing functionally different
subpopulations of macrophages associated with tumor progression and
the effectiveness of chemotherapy, which may result in
identification of potential targets for treatment (13-18).
The simplified dichotomous classification of M1/M2 provides a
conceptual basis for describing the polarization of macrophages and
the identification of polarizing stimuli (19-24).
The high plasticity of macrophages with respect to changes in their
polarization under the influence of various microenvironmental
conditions opens up prospects for the directed differentiation of
macrophages into an antitumor M1 phenotype or blocking of M2
polarization.
The objectives of the present review were to assess
the state of TAM research and to evaluate the possible use of TAMs
in cancer therapy.
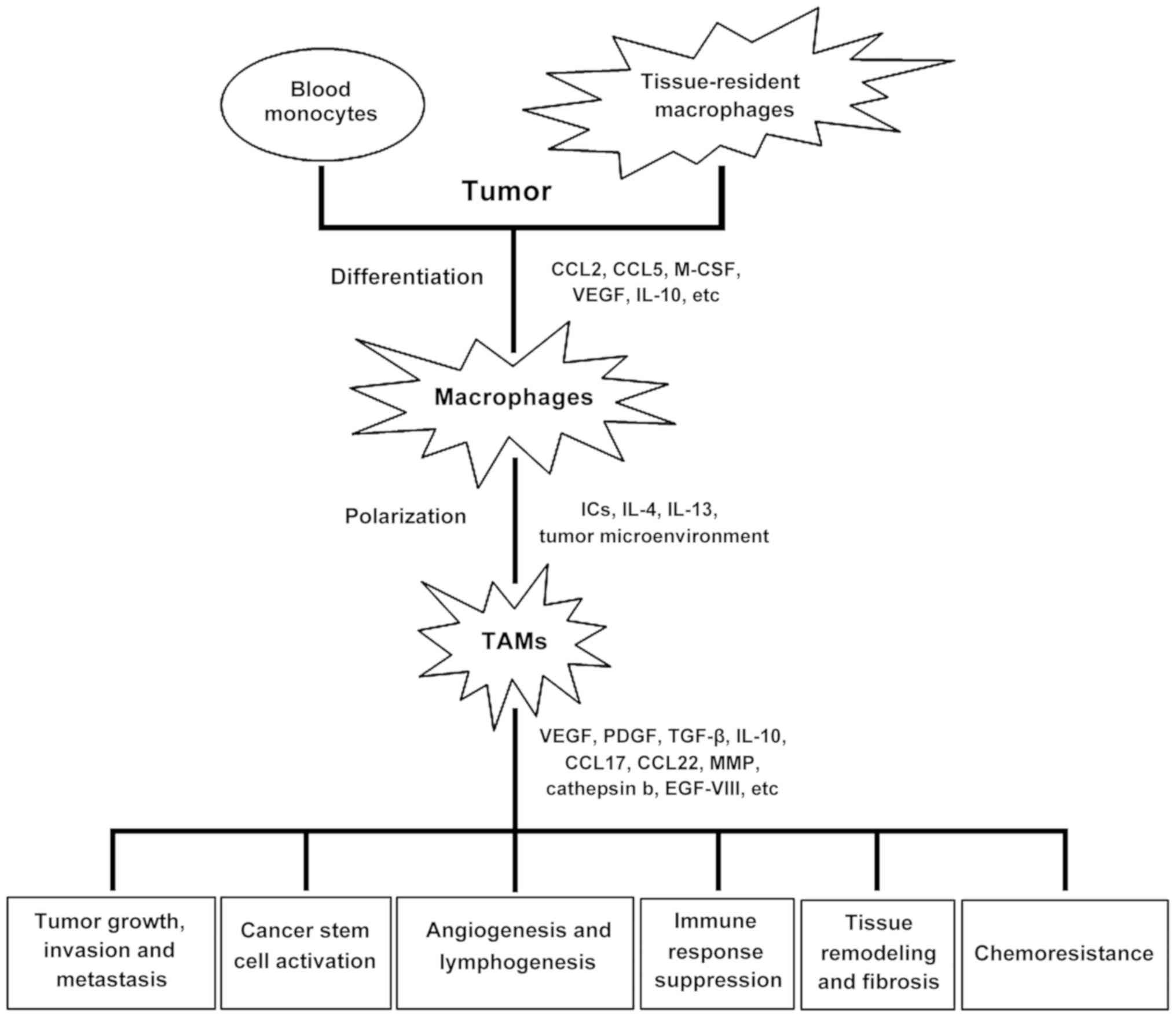
The process of tumorigenesis in the body begins to
affect the tumor microenvironment, including macrophages. Blood
monocytes penetrate the tumor, and differentiate into macrophages
with an anti-inflammatory phenotype in response to signaling
molecules produced by the tumor, such as interleukin (IL)-4, IL-10
and transforming growth factor (TGF)-β. These signals suppress
antitumor immunity, and stimulate the development of new blood
vessels and thus tumor growth and metastasis (17). The role of TAMs in tumor progression
are illustrated in Fig. 1.
TAMs have attracted substantial attention for the
past 30 years (from the time when the concept of a macrophage
dichotomy was advanced) (25,26).
TAMs are classed as type II-activated macrophages (M2). Stein et
al (27) first characterized
TAMs as alternatively activated macrophages in 1992. Data on TAM
markers and TAM-suppressing factors subsequently accumulated in
further studies (28-31).
The M2 population is highly heterogeneous (32,33).
Macrophages with the M2 phenotype serve an important role in the
process of tumorigenesis by suppressing the immune response,
remodeling the extracellular matrix and stimulating angiogenesis
(26). M2 macrophages are
characterized by the expression of specific receptors, such as
arginase-1, mannose receptor (CD206), CD163, CD209, FIZZ1 and Ym1/2
(22,29).
Macrophages with the M1 phenotype (classically
activated macrophages) express bactericide molecules and receptors
(34). Macrophages acquire the M1
phenotype in response to endogenous inflammatory stimuli, such as
the Th1-associated cytokine interferon-γ, or exogenous stimuli,
such as lipopolysaccharides (23,35). M1
macrophages produce pro-inflammatory cytokines and thereby
stimulate the inflammatory response (36). A total of 5,598 publications on TAMs
were available on PubMed as of July 10, 2020. The annual number of
such publications increased from 51 in 2007 to 660 in 2019.
Macrophages are intricately involved in the immune
response, and thus serve a protective role. They participate in the
clearance of cellular debris and iron processing, degradation of
dead cells and foreign material, response to infection,
immunomodulation and modulation of inflammatory processes,
angiogenesis, and facilitating wound healing (20,22,23).
Furthermore, macrophages serve an important in organ development,
and in tissue turnover and regeneration (37,38).
Adverse reactions are also often caused by macrophages and are
associated with their M1/M2 polarization. M1 macrophages serve
critical roles in innate host defense and in the killing of tumor
cells. Therefore, they are considered as antitumor macrophages. M2
macrophages tend to exert an immunosuppressive phenotype, favoring
tissue repair, and tumor promotion. Thus, they are considered as
pro-tumorigenic macrophages. The expression of inhibitory cytokines
in tumor cells or macrophages provides a mechanism of resistance to
anticancer therapy. Hence, a therapeutic strategy targeting
macrophages or macrophage-derived cytokines may be a promising and
effective method for targeting tumorigenesis (39).
TAMs are an important component of the tumor
microenvironment, which affects tumor growth, tumor angiogenesis,
immunity suppression, metastasis and chemoresistance. TAMs
substantially affect the clinical efficacy of these drugs and drug
resistance. For example, TAMs release chemoprotective factors, such
as cathepsin b and milk-fat globule EGF-VIII, which promotes
tumorigenicity of cancer stem cells and induces anticancer drug
resistance. Furthermore, drugs targeting TAMs have been shown to
exhibit promising results for potential use in anticancer therapy
(40). The role that macrophages
serve in carcinogenesis has been the focus of several studies,
including systematic reviews (41-43).
M1 macrophages promote tumor elimination, whereas M2
macrophages facilitate carcinogenesis (44). As demonstrated over half a century
ago, M1 macrophages are capable of killing and eliminating cancer
cells in accordance with their primary physiological function, the
elimination of foreign and harmful substances (45). M1 cells initiate cytokine production
in the tumor microenvironment and facilitate cancer cell
destruction by recruiting pro-immunostimulatory leukocytes and
phagocytizing tumor cells (46,47). M2
macrophages serve a leading role in tumor spread (48). M2 macrophages have a notable effect
on tumor development in both the primary and metastatic foci. Their
effects are associated with basement membrane degradation,
angiogenesis and general immunosuppression (49,50).
Macrophages have been shown to be present not only in the M1 or M2
states in the tumor microenvironment, but also in transitional
states, and the role of the transitional states in tumorigenesis
remains poorly understood (51). The
elimination of all macrophage populations regardless of the
polarization state may provide a potentially effective approach to
therapy as both primary and metastatic tumorigenesis is reduced as
a result (52).
The activation of macrophages is widely regarded as
polarization in the direction of the M1 or M2 states. However, the
M2 activation state includes heterogeneous and functionally
distinct macrophages. Studies on the existence of macrophages of
the M2a, M2b, M2c and M2d phenotypes make it possible to specify a
number of aspects regarding the nature of the immune response
(Table I).
M2a and M2b phenotype macrophages typically exhibit
anti-inflammatory activity. Macrophages of the M2c phenotype are
very similar to M1 macrophages, with the exception of high
(increased) IL-10 expression as opposed to pro-inflammatory
cytokines (53). Wang et al
(54) isolated the M2d phenotype,
characterized by decreased secretion of IL-12 and increased
secretion of IL-10. M2d macrophages are common in the tumor
microenvironment. It is hypothesized that M2d macrophages are
induced following stimulation with Toll-like receptor agonists and
adenosine, and/or tumor-related factors. Isolation of subtypes of
macrophages of the M2 family may facilitate the possibility of
their targeted therapeutic use for treatment of tumors.
The presence of macrophages in primary tumors is
associated with a poor prognosis (56-59),
with colorectal cancer serving as the only exception (60). M1 and M2 macrophages present in the
tumor microenvironment have been the focus of an increasing number
of studies (20,28,51).
Although the causal associations have not yet been established, the
available body of research highlight the possibility of novel
therapeutic strategies that are aimed at eliminating macrophages or
altering the macrophage phenotype (61).
Since increased TAM infiltration is associated with
a poor prognosis and therapeutic failure in cancer, TAM
reprogramming toward the anticancer M1 phenotype and TAM
suppression may provide promising strategies for the treatment of
cancer (62).
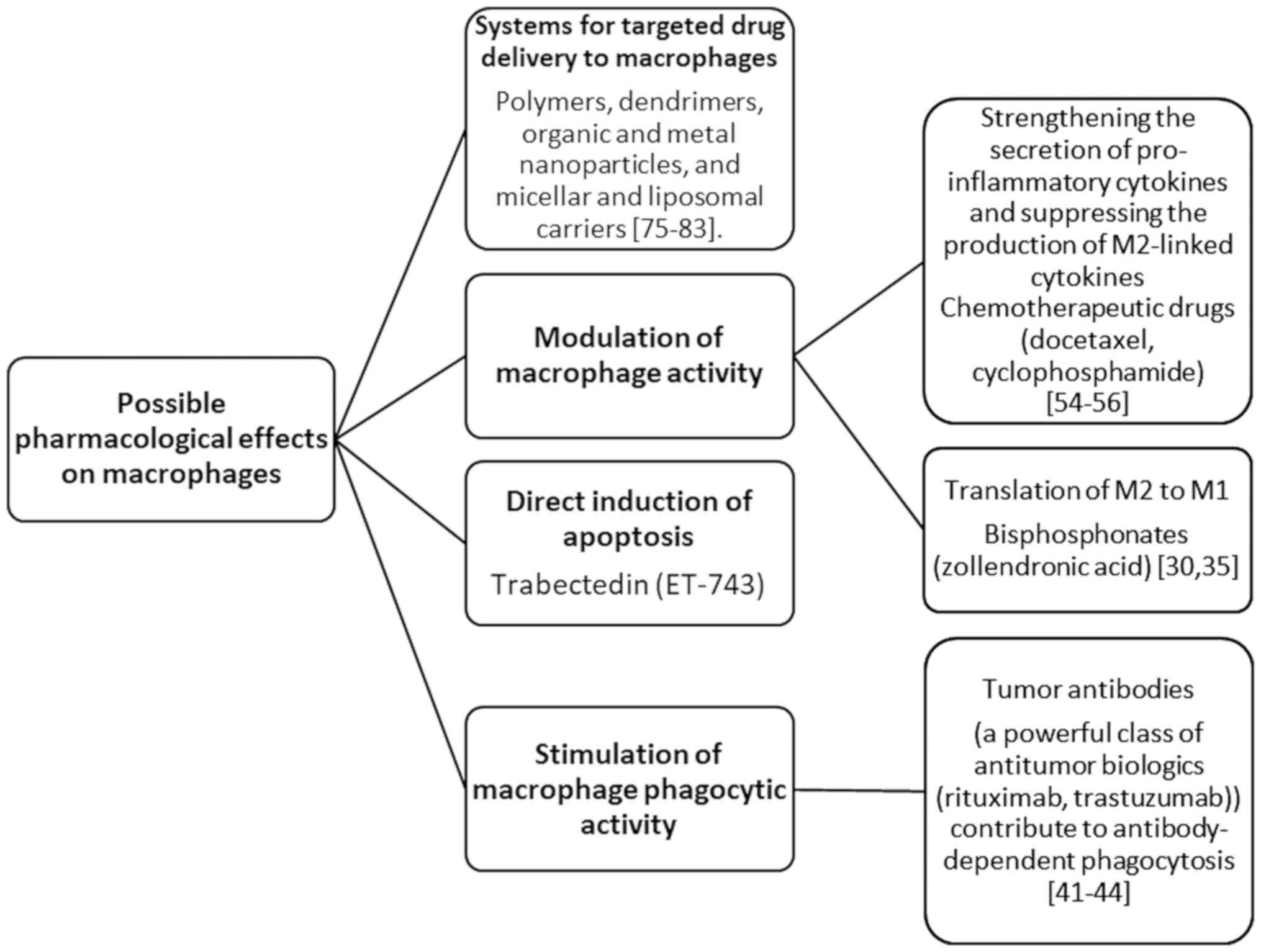
Based on the literature search performed for the
present review, three macrophage-related strategies of cancer
therapy are speculated. These strategies involve drugs that
modulate TAM activity; engineered carriers for targeted drug
delivery to macrophages, TAMs, or specific pro-tumoral M2-TAMs; and
macrophage self-targeting to the tumor.
Bisphosphonates modulate macrophage activity and are
used in the treatment of bone tissue disorders, such as
osteoporosis and bone metastases in cancer. A previous preclinical
study using a mouse model of breast tumors suggested that an extra
skeletal therapeutic effect is additionally exerted by
bisphosphonates (63).
Zoledronic acid, which is a medication used in the
treatment of cancer, has been shown to revert macrophage
polarization from the M2 to the M1 phenotype, thus inhibiting
spontaneous breast carcinogenesis (64). Zoledronic acid acts as a potent
inhibitor of farnesyl pyrophosphate synthase, which is a key enzyme
of the mevalonate pathway. By inhibiting farnesyl pyrophosphate
synthase, zoledronic acid prevents the prenylation of small
G-proteins, such as Ras, Rho and Rap1A, which are necessary for
cancer cell adhesion, migration and invasion. It has been shown
that zoledronic acid binds primarily with microcalcifications
present in breast tumors and is then phagocytized by TAMs, leading
to apoptosis and M2-to-M1 transformation. It has been demonstrated
in vivo that zoledronic acid inhibits the production of the
proangiogenic factor, matrix metalloproteinase, and triggers the
TAM transition from the pro-tumoral M2 phenotype to the antitumor
M1 phenotype (65).
Another study demonstrated that zoledronic acid
combined with ultrasonic treatment was significantly more effective
than zoledronic acid alone (P<0.01). The B02 tumor size in mice
treated with zoledronic acid and ultrasound was 42% lower
(P<0.002) compared with mice treated with bisphosphonate alone
(68). Bisphosphonates are
administered in liposomes or attached to nanoparticles to improve
their pharmacokinetics, reduce the side-effects and to alter their
biodistribution (65). Liposomal
bisphosphonate forms are capable of inducing the M2-to-M1
phenotypic transition (69).
Thus, studies on bisphosphonates used alone or in
combination with anticancer drugs or physicochemical methods for
the treatment of tumorigenesis are promising fields of research,
and highlight possibility of developing novel therapeutic
strategies (70-74).
The phagocytosis of foreign bodies, apoptotic cells
and cancer cells, and the stimulation of adaptive immunity by
presenting the antigens of assimilated materials, are two important
innate immune functions of macrophages (75). Tumor-specific antibodies are a class
of potent biopharmaceuticals, which act by directly inhibiting the
transmission of survival signals, mediating antibody-dependent cell
cytotoxicity of natural killer cells, inducing complement-dependent
cytotoxicity via the activation of the complement cascade, and thus
promoting antibody-dependent cell phagocytosis by macrophages
(76). Studies have indicated that
monoclonal antibodies approved as anticancer drugs, such as
rituximab and trastuzumab, exert their therapeutic effects mostly
through antibody-dependent cell phagocytosis (77,78). In
spite of their potential to stimulate tumor cell invasion, TAMs are
capable of tumor cell phagocytosis in the presence of target
antibodies (79,80).
Thus, to improve the therapeutic strategy based on
stimulating antibody-dependent cell phagocytosis, the Fc fragments
of antibodies should be engineered to increase their interaction
with receptors on macrophages (81).
Although IgG class antibodies are typically used to design
antibody-based therapeutics, the therapeutic potential of other
antibody isotypes (IgA and IgE) has been the subject of several
preclinical studies, where monocytes/macrophages also serve an
important role in affecting the functions of antibody-dependent
cell cytotoxicity and antibody-dependent cell phagocytosis
(82,83).
Chemotherapeutic drugs are also considered potential
means with which to modulate macrophages. A number of anticancer
chemotherapeutics exert their pharmacological effects on non-tumor
cell populations, although additional studies are required in the
field, as the current literature is limited to preliminary results
from in vitro experiments (84-89)
In particular, trabectedin and lurbinectedin (a second-generation
analog) are efficient in eliminating TAMs (84,85).
Trabectedin mechanically interacts with the TRAIL-R2
ligand-receptor, and induces tumor necrosis factor-related
apoptosis of mononuclear phagocytes by causing receptor clustering
and subsequent caspase 8-dependent apoptosis activation (86).
In addition to exerting cytotoxic effects, certain
chemotherapeutics modulate the macrophage response to the tumor
(15,87). A previous study using mouse models of
fibrosarcoma and breast tumors demonstrated that docetaxel promotes
target cell polarization to macrophages with an antitumor M1
phenotype (88). Cyclophosphamide
treatment facilitates macrophage infiltration, increases the
secretion of proinflammatory cytokines (IL-6 and IL-12) and
suppresses the production of pro-tumoral M2-associated cytokines
(IL-4, IL-10 and IL-13) (89,90). As
a mechanism of self-protection against chemotherapy, chemoresistant
cancer cells secrete IL-34, which increases their survival and
promotes the polarization of TAMs towards an M2 phenotype to
further facilitate an immunosuppressive environment (91). Thus, a combination of chemotherapy
and immunotherapy may be more efficient in inducing tumor
regression.
Systems for targeted drug delivery to TAMs are
associated with the second strategy of the macrophage-related
therapy of cancer. After establishing the positive effects of a
drug, the next focus of research should be to determine strategies
with which to selectively deliver the drugs to TAMs with minimal
side-effects on healthy cells (92-97).
Phagocytic activity is extremely high in
macrophages. Microparticles and nanoparticles are efficiently
phagocytized by macrophages. However, the rate of phagocytosis is
influenced by certain properties of micro- and nanoparticles, such
as the shape, size, contact angle and surface charge (98-100).
Liposomes are captured by macrophages more rapidly and in greater
quantities when their size is increased to >100 nm, particularly
when 1-3 µm in size. A decrease in liposome size to <100 nm
similarly increases their capture by macrophages (101). The composition and structure of
particles also affects their capture by macrophages (102,103).
Particles with highly positive or highly negative ζ
potentials are captured with improved efficiency by macrophages
compared with particles having a nearly neutral ζ potential.
Spherical particles are captured more efficiently than cylindrical
particles (104).
There is still no universal method available to
ensure specific molecular targeting to TAMs. In 2013, Cieslewicz
et al (105) reported the
discovery of the so-called M2pep peptide sequence. The M2pep
preferentially binds to mouse M2 cells, including TAMs, and
exhibits a low affinity for other cells. Confocal visualization
revealed that M2pep accumulated in TAMs in vivo after being
injected into the tail vein of mice. The injection of M2pep with a
pro-apoptotic peptide into the tail vein increased mouse mortality,
and selectively reduced the M2-like TAM population. The study by
Cieslewicz et al (105) was
among the first to describe a molecular targeting construct for
mouse TAMs, supporting the targeted approach to cancer therapy.
Cancer immunotherapy aimed at selectively modulating
M2-like TAMs and enabling the reversal of the M2-to-M1 ratio is a
promising therapeutic strategy. In 2017, Ngambenjawong and Pun
(106) reported the construction of
a high-avidity macrophage-selective drug delivery platform on the
basis of M2 macrophage-targeting peptides (M2pep) grafted on
poly(N-(2-hydroxypropyl)methacrylamide). Furthermore,
polymer-grafted M2pep exhibited increased serum stability in
addition to increased M2 macrophage-selective toxicity (106).
A targeted system was constructed using a copolymer
of hyaluronic acid with poly(lactic acid) and poly(glycolic acid).
The copolymer was assembled together with the anticancer drug,
SN38, in an aqueous phase, and the nanoparticle surface was then
coated with methoxypoly(ethylene glycol)-b-poly(histamine
methacrylamide) via hydrophobic association to improve the colloid
stability both in vitro and in vivo. When the
nanoparticles arrived into the tumor microenvironment, which is
acidic, the coating was detached from the nanoparticle surface as a
result of the c harge transition of the poly(histamine
methacrylamide) blocks from a neutral hydrophobic to a positively
charged hydrophilic state through an acid-acid state, which was
induced by the protonation of the imidazole groups in the tumor
microenvironment (an acidic medium). The exposure of the
nanoparticle shell led to an increased uptake of nanoparticles by
CD44-expressing tumor cells, including cancer cells and TAMs
(107).
Nanosystems can rationally be designed to attain
multivalent states and, when necessary, multifunctional entities
with multiplex and/or enhanced biological activity. Nanosystems
engineered to contain macrophage-specific targeting fragments and
loaded or associated with drugs are promising options for
modulating or even eliminating pro-tumoral macrophages in
vivo (108). Engineered
nanosystems include polymers, dendrimers, organic and metal
nanoparticles, and micellar and liposomal carriers (109-117).
Taking advantage of the ability of macrophages to
target and migrate to the tumor is the third promising strategy
involving the use of macrophages. Macrophages have attracted
substantial interest as carriers for drug delivery. This is due to
their ability to target the tumor, their high phagocytic activity
toward drug-loaded nanoparticles and their capability to directly
kill cancer cells (118). For
example, peritoneal macrophages can be loaded with drugs, typically
included in nanoparticles or liposomes, and can then be transferred
back to animals or patients (119,120).
Another approach that takes advantage of macrophage self-targeting
to the tumor is the in vivo administration of nanoparticles
of a suitable size with a specific ligand to allow nanoparticle
uptake by macrophages or TAMs, and subsequent prolonged release
(121-123).
The long-term survival of the macrophage host is limited by the
toxicity of the drug. It is thus advisable to use systems for drug
delivery to decrease the acute toxicity to the macrophage carrier.
When the drug is not toxic to macrophages, a proper formulation
ensures the prolonged release of the loaded drug from macrophages
for at least two weeks, as demonstrated by Dou et al
(124,125) for indinavir associated with
nanoparticles.
With the appropriate strategy for nanoparticle
encapsulation to ensure intracellular stability, biological
preparations, such as proteins, can be loaded in macrophages
(126,127). Chang et al (101) demonstrated that the size of
nanoparticles internalized in macrophages may substantially affect
their macrophage uptake. Smaller nanoparticles (30-50 nm) exhibited
increased macrophage uptake compared with larger nanoparticles
(100-500 nm), but this reduced the macrophage migration velocity at
the same time. Nanoparticles with a size of 100 nm were shown to
provide a good balance between efficient drug loading and
macrophage migration (101).
To investigate the pharmacological activity of
carriers captured by macrophages, macrophages have been loaded with
temperature-sensitive liposomes for inducible release (121), nanosized silica-gold nanoshells for
photothermal therapy (120) and
iron oxide nanoparticles (119,128,129).
The reduction of TAM survival is generally
considered to improve the therapeutic effects of anticancer
therapy. The direct induction of apoptosis with chemical or
synthetic substances provides an efficient strategy with which to
eliminate TAMs. Trabectedin (ET-743) is an anticancer drug used in
the treatment of platinum-sensitive soft-tissue sarcomas. The drug
causes selective TAM depletion in cancer patients by activating the
extrinsic apoptotic pathway through TRAIL receptors. As trabectedin
directly affects monocyte/macrophage-mediated host defense in
addition to targeting TAMs (85),
designing TAM-specific agents may result in a reduction of
side-effects.
Reviewing TAM-related literature revealed that the
number of publications in this field has increased in number over
the past three years, highlighting novel possibilities for the use
of a combination of immunotherapy and chemotherapy to treat cancer.
However, there are several issues which warrant further
investigation. These issues include: Attaining a deeper
understanding of the process of M1/M2 macrophage polarization,
macrophage characteristics at intermediate polarization steps, and
their role in tumorigenesis; the conditions that are necessary for
transitions between the M1 and M2 macrophage phenotypes and the
signals that this process is dependent on from the
microenvironment; the cause-and-effect relationships between the
quantity and quality of macrophages, and the prognosis and outcome
of the pathological process; modulation of macrophages and
stimulation of their phagocytic activity with drugs; development of
suitable and safe targeted vector-based systems for drug delivery
to macrophages; and the development of targeted drug delivery
systems with macrophages as carriers, thus potentially combining
chemotherapy and immunotherapy.
Not applicable.
No funding was received.
Not applicable.
OVZ, IVM, TFK, EVA and SAR were all involved in
drafting and revising the manuscript. All authors read and approved
the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mitra AK, Agrahari V, Mandal A, Cholkar K,
Natarajan C, Shah S, Joseph M, Trinh HM, Vaishya R, Yang X, et al:
Novel delivery approaches for cancer therapeutics. J Control
Release. 219:248–268. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Talekar M, Tran TH and Amiji M:
Translational nano-medicines: Targeted therapeutic delivery for
cancer and inflammatory diseases. AAPS J. 17:813–827.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
You W and Henneberg M: Cancer incidence
increasing globally: The role of relaxed natural selection. Evol
Appl. 11:140–152. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang Z, Zhou L, Xie N, Nice EC, Zhang T,
Cui Y and Huang C: Overcoming cancer therapeutic bottleneck by drug
repurposing. Signal Transduct Target Ther. 5(113)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
World Health Organization (WHO): Cancer:
Key facts. WHO, Geneva, 2018. https://www.who.int/news-room/fact-sheets/detail/cancer.
Accessed September 12, 2018.
|
|
10
|
Lewis LD: Cancer pharmacotherapy: 21st
century ‘magic bullets’ and changing paradigms. Br J Clin
Pharmacol. 62:1–4. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Samadi AK, Bilsland A, Georgakilas AG,
Amedei A, Amin A, Bishayee A, Azmi AS, Lokeshwar BL, Grue B, Panis
C, et al: A multi-targeted approach to suppress tumor-promoting
inflammation. Semin Cancer Biol. 35 (Suppl):S151–S184.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shaked Y: The pro-tumorigenic host
response to cancer therapies. Nat Rev Cancer. 19:667–685.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guo Q, Jin Z, Yuan Y, Liu R, Xu T, Wei H,
Xu X, He S, Chen S, Shi Z, et al: Corrigendum to ‘New mechanisms of
tumor-associated macrophages on promoting tumor progression: Recent
research advances and potential targets for tumor immunotherapy’. J
Immunol Res. 2018(6728474)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Larionova I, Cherdyntseva N, Liu T,
Patysheva M, Rakina M and Kzhyshkowska J: Interaction of
tumor-associated macrophages and cancer chemotherapy.
Oncoimmunology. 8(1596004)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mantovani A, Marchesi F, Malesci A, Laghi
L and Allavena P: Tumour-associated macrophages as treatment
targets in oncology. Nat Rev Clin Oncol. 14:399–416.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nielsen SR and Schmid MC: Macrophages as
key drivers of cancer progression and metastasis. Mediators
Inflamm. 2017(9624760)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Prill S, Rebstock J, Tennemann A, Körfer
J, Sönnichsen R, Thieme R, Gockel I, Lyros O, Monecke A, Wittekind
C, et al: Tumor-associated macrophages and individual
chemo-susceptibility are influenced by iron chelation in human
slice cultures of gastric cancer. Oncotarget. 10:4731–4742.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lampiasi N, Russo R and Zito F: The
alternative faces of macrophage generate osteoclasts. Biomed Res
Int. 2016(9089610)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin Y, Xu J and Lan H: Tumor-associated
macrophages in tumor metastasis: Biological roles and clinical
therapeutic applications. J Hematol Oncol. 12(76)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Porta C, Riboldi E, Ippolito A and Sica A:
Molecular and epigenetic basis of macrophage polarized activation.
Semin Immunol. 27:237–248. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schliefsteiner C, Peinhaupt M, Kopp S,
Lögl J, Lang-Olip I, Hiden U, Heinemann A, Desoye G and Wadsack C:
Human placental hofbauer cells maintain an anti-inflammatory M2
phenotype despite the presence of gestational diabetes mellitus.
Front. Immunol. 8(888)2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yao Y, Xu XH and Jin L: Macrophage
polarization in physiological and pathological pregnancy. Front
Immunol. 10(792)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mantovani A, Bottazzi B, Colotta F,
Sozzani S and Ruco L: The origin and function of tumor-associated
macrophages. Immunol Today. 13:265–270. 1992.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mantovani A and Locati M: Tumor-associated
macrophages as a paradigm of macrophage plasticity, diversity, and
polarization: Lessons and open questions. Arterioscler Thromb Vasc
Biol. 33:1478–1483. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Stein M, Keshav S, Harris N and Gordon S:
Interleukin 4 potently enhances murine macrophage mannose receptor
activity: A marker of alternative immunologic macrophage
activation. J Exp Med. 176:287–292. 1992.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sawa-Wejksza K and Kandefer-Szerszeń M:
Tumor-associated macrophages as target for antitumor therapy. Arch
Immunol Ther Exp (Warsz). 66:97–111. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhou J, Tang Z, Gao S, Li C, Feng Y and
Zhou X: Tumor-Associated Macrophages: Recent insights and
therapies. Front Oncol. 10(188)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gratchev A, Guillot P, Hakiy N, Politz O,
Orfanos CE, Schledzewski K and Goerdt S: Alternatively activated
macrophages differentially express fibronectin and its splice
variants and the extracellular matrix protein betaIG-H3. Scand J
Immunol. 53:386–392. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Gratchev A, Kzhyshkowska J, Kannookadan S,
Ochsenreiter M, Popova A, Yu X, Mamidi S, Stonehouse-Usselmann E,
Muller-Molinet I, Gooi L and Goerdt S: Activation of a
TGF-beta-specific multistep gene expression program in mature
macrophages requires glucocorticoid-mediated surface expression of
TGFbeta receptor II. J Immunol. 180:6553–6565. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Goerdt S, Politz O, Schledzewski K, Birk
R, Gratchev A, Guillot P, Hakiy N, Klemke CD, Dippel E, Kodelja V
and Orfanos CE: Alternative versus classical activation of
macrophages. Pathobiology. 67:222–226. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Glass CK and Natoli G: Molecular control
of activation and priming in macrophages. Nat Immunol. 17:26–33.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Van Ginderachter JA, Movahedi K,
Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G and De
Baetselier P: Classical and alternative activation of mononuclear
phagocytes: Picking the best of both worlds for tumor promotion.
Immunobiology. 211:487–501. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Osborn O and Olefsky JM: The cellular and
signaling networks linking the immune system and metabolism in
disease. Nat Med. 18:363–374. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rőszer T: Understanding the Mysterious M2
macrophage through activation markers and effector mechanisms.
Mediators Inflamm. 2015(816460)2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tamura R, Tanaka T, Yamamoto Y, Akasaki Y
and Sasaki H: Dual role of macrophage in tumor immunity.
Immunotherapy. 10:899–909. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Jinushi M and Komohara Y: Tumor-associated
macrophages as an emerging target against tumors: Creating a new
path from bench to bedside. Biochim Biophys Acta. 1855:123–1230.
2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wynn TA, Chawla A and Pollard JW:
Macrophage biology in development, homeostasis and disease. Nature.
496:445–455. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ginhoux F and Jung S: Monocytes and
macrophages: Developmental pathways and tissue homeostasis. Nat Rev
Immunol. 14:392–404. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lewis CE, Harney AS and Pollard JW: The
multifaceted role of perivascular macrophages in tumors. Cancer
Cell. 30:18–25. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Ngambenjawong CH, Heather H and Suzie H:
Progress in tumor-associated macrophage (TAM)-targeted
therapeutics. Adv Drug Deliv Rev. 114:206–221. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Evans R and Alexander P: Cooperation of
immune lymphoid cells with macrophages in tumour immunity. Nature.
228:620–622. 1970.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen Y, Song Y, Du W, Gong L, Chang H and
Zou Z: Tumor-associated macrophages: An accomplice in solid tumor
progression. J Biomed Sci. 26(78)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jeannin P, Paolini L, Adam C and Delneste
Y: The roles of CSFs on the functional polarization of
tumor-associated macrophages. FEBS J. 285:680–699. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang HW and Joyce JA: Alternative
activation of tumor-associated macrophages by IL-4: Priming for
protumoral functions. Cell Cycle. 9:4824–4835. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Caux C, Ramos RN, Prendergast GC,
Bendriss-Vermare N and Ménétrier-Caux C: A milestone review on how
macrophages affect tumor growth. Cancer Res. 76:6439–6442.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Allavena P, Sica A, Solinas G, Porta C and
Mantovani A: The inflammatory micro-environment in tumor
progression: The role of tumor-associated macrophages. Crit Rev
Oncol Hematol. 66:1–9. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Ostuni R, Kratochvill F, Murray PJ and
Natoli G: Macrophages and cancer: From mechanisms to therapeutic
implications. Trends Immunol. 36:229–239. 2017.
|
|
53
|
Kreider T, Anthony RM, Urban JF Jr and
Gause WC: Alternatively activated macrophages in helminth
infections. Curr Opin Immunol. 19:448–453. 2007.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Wang Q, Ni H, Lan L, Wei X, Xiang R and
Wang Y: Fra-1 pro-tooncogene regulates IL-6 expression in
macrophages and promotes the generation of M2d macrophages. Cell
Res. 20:701–712. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Quail DF and Joyce JA: Molecular pathways:
Deciphering mechanisms of resistance to macrophage-targeted
therapies. Clin Cancer Res. 23:876–884. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yuan ZY, Luo RZ, Peng RJ, Wang SS and Xue
C: High infiltration of tumor-associated macrophages in
triple-negative breast cancer is associated with a higher risk of
distant metastasis. Onco Targets Ther. 7:1475–1480. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
He Y, Zhang M, Wu X, Sun X, Xu T, He Q and
Di W: High MUC2 expression in ovarian cancer is inversely
associated with the M1/M2 ratio of tumor-associated macrophages and
patient survival time. PLoS One. 8(e79769)2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ding P, Wang W, Wang J, Yang Z and Xue L:
Expression of tumor-associated macrophage in progression of human
glioma. Cell Biochem Biophys. 70:1625–1631. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Pantano F, Berti P, Guida FM, Perrone G,
Vincenzi B, Amato MM, Righi D, Dell'Aquila E, Graziano F, Catalano
V, et al: The role of macrophages polarization in predicting
prognosis of radically resected gastric cancer patients. J Cell Mol
Med. 17:1415–1421. 2013.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ruffell B, Affara NI and Coussens LM:
Differential macrophage programming in the tumor microenvironment.
Trends Immunol. 33:119–126. 2012.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C,
Lin F, Liao H, You Z and Liu L: Prognostic impact of
tumor-associated macrophage infiltration in non-small cell lung
cancer: A systemic review and meta-analysis. Oncotarget.
7:34217–34228. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
icroenvironment. Cancers (Basel). 6:1670–1690. 2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Junankar S, Shay G, Jurczyluk J, Ali N,
Down J, Pocock N, Parker A, Nguyen A, Sun S, Kashemirov B, et al:
Real-time intravital imaging establishes tumor-associated
macrophages as the extraskeletal target of bisphosphonate action in
cancer. Cancer Discov. 5:35–42. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Coscia M, Quaglino E, Iezzi M, Curcio C,
Pantaleoni F, Riganti C, Holen I, Monkkonen H, Boccadoro M, Forni
G, et al: Zoledronic acid repolarizes tumour-associated macrophages
and inhibits mammary carcinogenesis by targeting the mevalonate
pathway. J Cell Mol Med. 14:2803–2815. 2010.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Rogers TL and Holen I: Tumour macrophages
as potential targets of bisphosphonates. J Transl Med.
9(177)2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Rogers TL, Wind N, Hughes R, Nutter F,
Brown HK, Vasiliadou I, Ottewell PD and Holen I: Macrophages as
potential targets for zoledronic acid outside the skeleton-evidence
from in vitro and in vivo models. Cell Oncol (Dordr). 36:505–514.
2013.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ali N, Jurczyluk J, Shay G, Tnimov Z,
Alexandrov K, Munoz MA, Skinner OP, Pavlos NJ and Rogers MJ: A
highly sensitive prenylation assay reveals in vivo effects of
bisphosphonate drug on the Rab prenylome of macrophages outside the
skeleton. Small GTPases. 6:202–211. 2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Tardoski S, Ngo J, Gineyts E, Roux JP,
Clézardin PH and Melodelima D: Low-intensity continuous ultrasound
triggers effective bisphosphonate anticancer activity in breast
cancer. Sci Rep. 5(16354)2015.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Sousa S, Auriola S, Mönkkönen J and Mааttа
J: Liposome encapsulated zoledronate favours M1-like behaviour in
murine macrophages cultured with soluble factors from breast cancer
cells. BMC Cancer. 15(4)2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Hiroshima Y, Maawy A, Hassanein MK, Menen
R, Momiyama M, Murakami T, Miwa S, Yamamoto M, Uehara F, Yano S, et
al: The tumor-educated-macrophage increase of malignancy of human
pancreatic cancer is prevented by zoledronic acid. PLoS One.
9(e103382)2014.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Esser AK, Schmieder AH, Ross MH, Xiang J,
Su X, Cui G, Zhang H, Yang X, Allen JS, Williams T, et al:
Dual-therapy with αvβ3-targeted Sn2 lipase-labile
fumagillin-prodrug nanoparticles and zoledronic acid in the Vx2
rabbit tumor model. Nanomedicine. 12:201–211. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zekri J, Mansour M and Karim SM: The
anti-tumour effects of zoledronic acid. J Bone Oncol. 3:25–35.
2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kopecka J, Porto S, Lusa S, Gazzano E,
Salzano G, Pinzòn-Daza ML, Giordano A, Desiderio V, Ghigo D, De
Rosa G, et al: Zoledronic acid-encapsulating self-assembling
nanoparticles and doxorubicin: A combinatorial approach to overcome
simultaneously chemoresistance and immunoresistance in breast
tumors. Oncotarget. 7:20753–20772. 2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Fowler DW, Copier J, Dalgleish AG and
Bodman-Smith MD: Zoledronic acid renders human M1 and M2
macrophages susceptible to Vδ2+ γδ T cell cytotoxicity
in a perforin-dependent manner. Cancer Immunol Immunother.
66:1205–1215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lavin Y and Merad M: Macrophages:
Gatekeepers of tissue integrity. Cancer Immunol Res. 1:201–209.
2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gul N and van Egmond M: Antibody-dependent
phagocytosis of tumor cells by macrophages: A potent effector
mechanism of monoclonal antibody. Therapy of cancer. Cancer Res.
75:5008–5013. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Tipton TR, Roghanian A, Oldham RJ, Carter
MJ, Cox KL, Mockridge CI, French RR, Dahal LN, Duriez PJ,
Hargreaves PG, et al: Antigenic modulation limits the effector cell
mechanisms employed by type I anti-CD20 monoclonal antibodies.
Blood. 125:1901–1909. 2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Shi Y, Fan X, Deng H, Brezski RJ, Rycyzyn
M, Jordan RE, Strohl WR, Zou Q, Zhang N and An Z: Trastuzumab
triggers phagocytic killing of high HER2 cancer cells in vitro and
in vivo by interaction with Fcgamma receptors on macrophages. J
Immunol. 194:4379–4386. 2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Grugan KD, McCabe FL, Kinder M, Greenplate
AR, Harman BC, Ekert JE, van Rooijen N, Anderson GM, Nemeth JA,
Strohl WR, et al: Tumor-associated macrophages promote invasion
while retaining Fc-dependent anti-tumor function. J Immunol.
189:5457–5466. 2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Taylor RP and Lindorfer MA: Analyses of
CD20 monoclonal antibody-mediated tumor cell killing mechanisms:
Rational design of dosing strategies. Mol Pharmacol. 86:485–491.
2014.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Richards JO, Karki S, Lazar GA, Chen H,
Dang W and Desjarlais JR: Optimization of antibody binding to
FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol
Cancer Ther. 7:2517–2527. 2008.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Brandsma AM, Ten Broeke T, Nederend M,
Meulenbroek LA, van Tetering G, Meyer S, Jansen JH, Beltrán
Buitrago MA, Nagelkerke SQ, Németh I, et al: Simultaneous Targeting
of FcgammaRs and FcalphaRI enhances tumor cell killing. Cancer
Immunol Res. 3:1316–1324. 2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Josephs DH, Bax HJ, Dodev T, Georgouli M,
Nakamura M, Pellizzari G, Saul L, Karagiannis P, Cheung A, Herraiz
C, et al: Anti-folate receptor-α IgE but not IgG recruits
macrophages to attack tumors via TNF-α/MCP-1 signaling. Cancer Res.
77:1127–1141. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Cespedes MV, Guillen MJ, Lopez-Casas PP,
Sarno F, Gallardo A, Álamo P, Cuevas C, Hidalgo M, Galmarini CM,
Allavena P, et al: Lurbinectedin induces depletion of
tumor-associated macrophages, an essential component of its in vivo
synergism with gemcitabine, in pancreatic adenocarcinoma mouse
models. Dis Model Mech. 9:1461–1471. 2016.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Germano G, Frapolli R, Belgiovine C,
Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M,
Pasqualini F, et al: Role of macrophage targeting in the antitumor
activity of trabectedin. Cancer Cell. 23:249–262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Liguori M, Buracchi C, Pasqualini F,
Bergomas F, Pesce S, Sironi M, Grizzi F, Mantovani A, Belgiovine C
and Allavena P: Functional TRAIL receptors in monocytes and
tumor-associated macrophages: A possible targeting pathway in the
tumor microenvironment. Oncotarget. 7:41662–41676. 2016.PubMed/NCBI View Article : Google Scholar
|
|
87
|
De Palma M and Lewis CE: Macrophage
regulation of tumor responses to anticancer therapies. Cancer Cell.
23:277–286. 2013.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Kodumudi KN, Woan K, Gilvary DL, Sahakian
E, Wei S and Djeu JY: A novel chemoimmunomodulating property of
docetaxel: Suppression of myeloid-derived suppressor cells in tumor
bearers. Clin Cancer Res. 16:4583–4594. 2010.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Guerriero JL, Ditsworth D, Catanzaro JM,
Sabino G, Furie MB, Kew RR, Crawford HC and Zong WX: DNA alkylating
therapy induces tumor regression through an HMGB1-mediated
activation of innate immunity. J Immunol. 186:3517–3526.
2011.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Bryniarski K, Szczepanik M, Ptak M,
Zemelka M and Ptak W: Influence of cyclophosphamide and its
metabolic products on the activity of peritoneal macrophages in
mice. Pharmacol Rep. 61:550–557. 2009.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Baghdadi M, Wada H, Nakanishi S, Abe H,
Han N, Putra WE, Endo D, Watari H, Sakuragi N, Hida Y, et al:
Chemotherapy-Induced IL34 enhances immunosuppression by
tumor-associated macrophages and mediates survival of
chemoresistant lung cancer cells. Cancer Res. 76:6030–6042.
2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhou Y and Dai Z: New Strategies in the
design of nanomedicines to oppose uptake by the mononuclear
phagocyte system and enhance cancer therapeutic efficacy. Chem
Asian J. 13:3333–3340. 2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Niu M, Naguib YW, Aldayel AM, Shi YC,
Hursting SD, Hersh MA and Cui Z: Biodistribution and in vivo
activities of tumor-associated macrophage-targeting nanoparticles
incorporated with doxorubicin. Mol Pharm. 11:4425–4436.
2014.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Ngambenjawong C, Cieslewicz M, Schellinger
JG and Pun SH: Synthesis and evaluation of multivalent M2pep
peptides for targeting alternatively activated M2macrophages. J
Control Release. 224:103–111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Silva VL and Al-Jamal WT: Exploiting the
cancer niche: Tumor-associated macrophages and hypoxia as promising
synergistic targets for Nano-based therapy. J Control Release.
253:82–96. 2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Andón FT, Digifico E, Maeda A, Erreni M,
Mantovani A, Alonso MJ and Allavena P: Targeting tumor associated
macrophages: The new challenge for nanomedicine. Semin Immunol.
34:103–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Li M, Zhang F, Su Y, Zhou J and Wang W:
Nanoparticles designed to regulate tumor microenvironment for
cancer therapy. Life Sci. 201:37–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Tabata Y and Ikada Y: Effect of the size
and surface charge of polymer microspheres on their phagocytosis by
macrophage. Biomaterials. 9:356–362. 1988.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Champion JA and Mitragotri S: Role of
target geometry in phagocytosis. Proc Natl Acad Sci USA.
103:4930–4934. 2006.PubMed/NCBI View Article : Google Scholar
|
|
100
|
He C, Hu Y, Yin L, Tang C and Yin C:
Effects of particle size and surface charge on cellular uptake and
biodistribution of polymeric nanoparticles. Biomaterials.
31:3657–3666. 2010.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Chang YN, Guo H, Li J, Song Y, Zhang M,
Jin J, Xing G and Zhao Y: Adjusting the balance between effective
loading and vector migration of macrophage vehicles to deliver
nanoparticles. PLoS One. 8(e76024)2013.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Yu SS, Lau CM, Thomas SN, Jerome WG, Maron
DJ, Dickerson JH, Hubbell JA and Giorgio TD: Size- and
charge-dependent nonspecific uptake of PEGylated nanoparticles by
macrophages. Int J Nanomedicine. 7:799–813. 2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Herd H, Daum N, Jones AT, Huwer H,
Ghandehari H and Lehr CM: Nanoparticle geometry and surface
orientation influence mode of cellular uptake. ACS Nano.
7:1961–1973. 2013.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Li Z, Sun L, Zhang Y, Dove AP, O'Reilly RK
and Chen G: Shape effect of Glyco-nanoparticles on macrophage
cellular uptake and immune response. ACS Macro Lett. 5:1059–1064.
2016.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Cieslewicz M, Tang J, Yu JL, Cao H,
Zavaljevski M, Motoyama K, Lieber A, Raines EW and Pun SH: Targeted
delivery of proapoptotic peptides to tumor-associated macrophages
improves survival. Proc Natl Acad Sci USA. 110:15919–15924.
2013.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Ngambenjawong C and Pun SH: Multivalent
polymers displaying M2 macrophage-targeting peptides improve target
binding avidity and serum stability. ACS Biomater Sci Eng.
3:2050–2053. 2017.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Huang WC, Chen SH, Chiang WH, Huang CW, Lo
CL, Chern CS and Chiu HC: Tumor microenvironment-responsive
nanoparticle delivery of chemotherapy for enhanced selective
cellular uptake and transportation within tumor. Biomacromolecules.
17:3883–3892. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Poupot R, Goursat C and Fruchon S:
Multivalent nanosystems: Targeting monocytes/macrophages. Int J
Nanomedicine. 13:5511–5521. 2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Cupaioli FA, Zucca FA, Boraschi D and
Zecca L: Engineered nanoparticles How brain friendly is this new
guest? Prog Neurobiol. 119-120:20–38. 2014.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Costa A, Sarmento B and Seabra V:
Mannose-functionalized solid lipid nanoparticles are effective in
targeting alveolar macrophages. Eur J Pharm Sci. 114:103–113.
2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Sarwar HS, Ashraf S, Akhtar S, Sohail MF,
Hussain SZ, Rafay M, Yasinzai M, Hussain I and Shahnaz G:
Mannosylated thiolated polyethylenimine nanoparticles for the
enhanced efficacy of antimonial drug against Leishmaniasis.
Nanomedicine (Lond). 13:25–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Fallarini S, Paoletti T, Battaglini CO,
Ronchi P, Lay L, Bonomi R, Jha S, Mancin F, Scrimin P and Lombardi
G: Factors affecting T cell responses induced by fully synthetic
glyco-gold-nanoparticles. Nanoscale. 5:390–400. 2013.PubMed/NCBI View Article : Google Scholar
|
|
113
|
He H, Yuan Q, Bie J, Wallace RL, Yannie
PJ, Wang J, Lancina MG III, Zolotarskaya OY, Korzun W, Yang H and
Ghosh S: Development of mannose functionalized dendrimeric
nanoparticles for targeted delivery to macrophages: Use of this
platform to modulate atherosclerosis. Transl Res. 193:13–30.
2018.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Sun X, Li W, Zhang X, Qi M, Zhang Z, Zhang
XE and Cui Z: In vivo targeting and imaging of atherosclerosis
using multifunctional virus-like particles of Simian Virus. Nano
Lett. 16:6164–6171. 2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Zhu S, Niu M, O'Mary H and Cui Z:
Targeting of tumor-associated macrophages made possible by
PEG-sheddable, mannose-modified nanoparticles. Mol Pharm.
10:3525–3530. 2013.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Qian Y, Qiao S, Dai Y, Xu G, Dai B, Lu L,
Yu X, Luo Q and Zhang Z: Molecular-targeted immunotherapeutic
strategy for melanoma via dual-targeting nanoparticles delivering
small interfering RNA to tumor-associated macrophages. ACS Nano.
11:9536–9549. 2017.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Lamanna G, Russier J, Dumortier H and
Bianco A: Enhancement of anti-inflammatory drug activity by
multivalent adamantane-based dendrons. Biomaterials. 33:5610–5617.
2012.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Lee S, Kivimae S, Dolor A and Szoka FC:
Macrophage-based cell therapies: The long and winding road. J
Control Release. 240:527–540. 2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Choi J, Kim HY, Ju EJ, Jung J, Park J,
Chung HK, Lee JS, Lee JS, Park HJ, Song SY, et al: Use of
macrophages to deliver therapeutic and imaging contrast agents to
tumors. Biomaterials. 33:4195–4203. 2012.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Madsen SJ, Baek SK, Makkouk AR, Krasieva T
and Hirschberg H: Macrophages as cell-based delivery systems for
nanoshells in photothermal therapy. Ann Biomed Eng. 40:507–515.
2012.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Ikehara Y, Niwa T, Biao L, Ikehara SK,
Ohashi N, Kobayashi T, Shimizu Y, Kojima N and Nakanishi H: A
carbohydrate recognition-based drug delivery and controlled release
system using intraperitoneal macrophages as a cellular vehicle.
Cancer Res. 66:8740–8748. 2006.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Miller MA, Zheng YR, Gadde S, Pfirschke C,
Zope H, Engblom C, Kohler RH, Iwamoto Y, Yang KS, Askevold B, et
al: Tumour-associated macrophages act as a slow-release reservoir
of nano-therapeutic Pt(IV) pro-drug. Nat Commun.
6(8692)2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Tanei T, Leonard F, Liu X, Alexander JF,
Saito Y, Ferrari M, Godin B and Yokoi K: Redirecting transport of
nanoparticle albumin-bound paclitaxel to macrophages enhances
therapeutic efficacy against liver metastases. Cancer Res.
76:429–439. 2016.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Dou H, Destache CJ, Morehead JR, Mosley
RL, Boska MD, Kingsley J, Gorantla S, Poluektova L, Nelson JA,
Chaubal M, et al: Development of a macrophage-based nanoparticle
platform for antiretroviral drug delivery. Blood. 108:2827–2835.
2006.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Dou H, Grotepas CB, McMillan JM, Destache
CJ, Chaubal M, Werling J, Kipp J, Rabinow B and Gendelman HE:
Macrophage delivery of nanoformulated antiretroviral drug to the
brain in a murine model of neuroAIDS. J Immunol. 183:661–669.
2009.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Zhao Y, Haney MJ, Klyachko NL, Li S, Booth
SL, Higginbotham SM, Jones J, Zimmerman MC, Mosley RL, Kabanov AV,
et al: Polyelectrolyte complex optimization for macrophage delivery
of redox enzyme nanoparticles. Nanomedicine (Lond). 6:25–42.
2011.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Klyachko NL, Haney MJ, Zhao Y, Manickam
DS, Mahajan V, Suresh P, Hingtgen SD, Mosley RL, Gendelman HE,
Kabanov AV and Batrakova EV: Macrophages offer a paradigm switch
for CNS delivery of therapeutic proteins. Nanomedicine (Lond).
9:1403–1422. 2014.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Muthana M, Kennerley AJ, Hughes R, Fagnano
E, Richardson J, Paul M, Murdoch C, Wright F, Payne C, Lythgoe MF,
et al: Directing cell therapy to anatomic target sites in vivo with
magnetic resonance targeting. Nat Commun. 6(8009)2015.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Han J, Zhen J, Du Nguyen V, Go G, Choi Y,
Ko SY, Park JO and Park S: Hybrid-actuating macrophage-based
microrobots for active cancer therapy. Sci Rep.
6(28717)2016.PubMed/NCBI View Article : Google Scholar
|