Introduction
L-carnitine
(4-N-trimethylammonium-3-hydroxybutyric acid), the
biologically active form of carnitine, is synthesized from the
essential amino acids, methionine and lysine in certain organs,
including the liver (1). Serum
L-carnitine concentration varies according to the tissue type
(2), nutritional status (3,4) and the
quality of the foodstuff (5).
L-carnitine regulates the cellular process of generating energy by
transferring acyl groups from the cytoplasm to the mitochondrial
matrix for β-oxidation (6).
L-carnitine deficiency has been demonstrated to reduce the
availability of energy in the liver and is associated with
impairments in various aspects of energy metabolism, including
utilization and accumulation of carbohydrate and lipids in liver
cirrhosis (7,8). Several studies have described the
ability of L-carnitine to reduce serum ammonia concentration in
clinical trials (9,10). L-carnitine supplementation also
reduces the rate of loss of muscle mass (11) and muscle cramps (12) in patients with liver cirrhosis.
Intravenous L-carnitine prevents the recurrence of overt hepatic
encephalopathy in patients with decompensated cirrhosis (13). Recently, a meta-analysis showed that
L-carnitine administration produces favorable effects based on the
levels of biochemical markers in patients with cirrhosis (14). Furthermore, its adverse effects are
minimal, but may include abdominal pain, nausea, diarrhea and a
fishy smell emanating for the patient (15). To the best of our knowledge, there
are no studies which have addressed the effect of L-carnitine on
health-related quality of life in patients with cirrhosis. The aim
of the present study was to evaluate the effects of L-carnitine on
chronic fatigue in patients with cirrhosis through the use of
self-report questionnaires.
Materials and methods
Patients
The present study was designed as a clinical trial
to prospectively assess the effect of L-carnitine supplementation
on chronic fatigue in patients with cirrhosis. The diagnosis of
cirrhosis was based on histological examination of biopsy
specimens, transient elastography, laboratory tests or imaging
findings. Exclusion criteria were evidence of hepatocellular
carcinoma, human immunodeficiency virus infection, pregnancy or
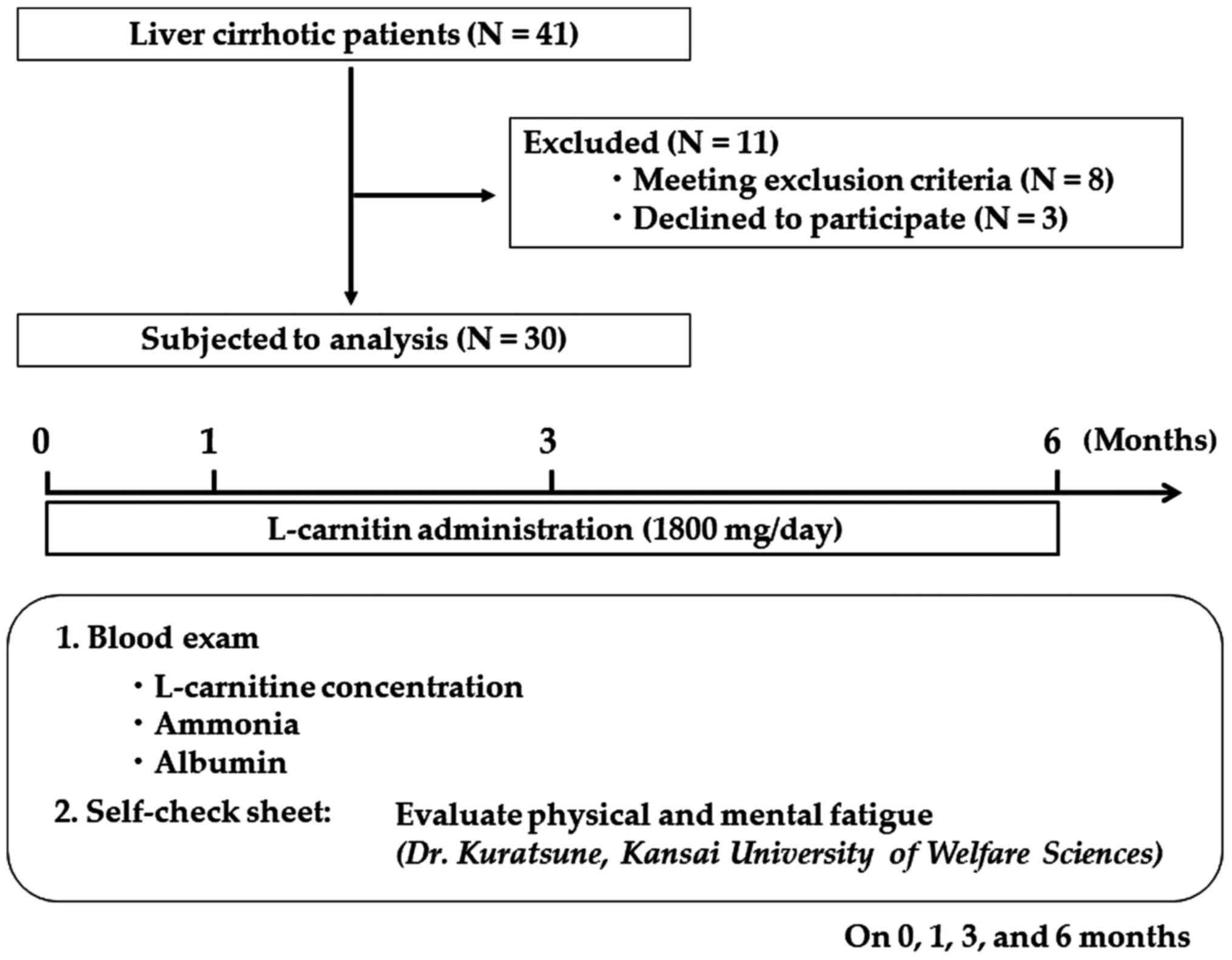
severe cardiac, pulmonary, or renal diseases. A total of 41
patients with cirrhosis who visited the Department of
Gastroenterology in Bellland General Hospital (Osaka, Japan), were
asked to participate between April and September 2013 (Fig. 1). Of the 41 patients, 8 patients were
excluded as they met at least one of the exclusion criteria, and 3
patients refused to participate; thus, a total of 30 patients were
enrolled in the present study. The recruited cohort consisted of 30
patients (median age 69 years; age range, 43-78
years) with 19 (63.3%) men and 11 (36.7%) women. Patients
took 600 mg (as two 300 mg tablets) of levocarnitine chloride
(L-carnitine; Otsuka Pharmaceutical Co., Ltd.) three times a day
(total daily dose, 1,800 mg) for 6 months. Blood samples were
collected before treatment and 1, 3 and 6 months after L-carnitine
supplementation was initiated, to measure the concentrations of
several biochemical parameters, free and total carnitine, and
acylcarnitine levels. Multiple factors affect the accuracy of
ammonia levels. The patients were told not to exercise or smoke
cigarettes 12 h before the fasting blood test was performed. The
level of fasting venous ammonia was measured. The blood samples
were immediately placed on ice after collection and was centrifuged
within 15 min of collection, as described previously (16). None of the patients recruited in the
final cohort were prescribed either cyanide, carbamazepine,
valproic acid or iron, which can cause secondary hyperammonemia as
a result of disruption of mitochondrial pathways (17,18). The
initial version of Child-Pugh score, included two continuous
variables (bilirubin and albumin) and three discrete (quantitative)
variables (ascites, encephalopathy and nutritional status)
(19). The selection of these five
variables, as well as the cut-off values for bilirubin and albumin
were empirical. The five variables and their respective cut-off
values were arranged so as to define three distinct groups of
increasing severity (A, B and C). Model For End-Stage Liver Disease
(MELD) scores had been originally created with the aim of
predicting survival after transjugular intrahepatic portosystemic
shunt. The score (the ancestor of the current MELD score), derived
from a survival function, was calculated as follows: R=0.957
Loge(creatinine [mg/dl])+0.378 Loge(bilirubin
[mg/dl])+1.120 loge (Prothrombin Time-International Normalized
Ratio)+0.643 (cause of cirrhosis). Before and after L-carnitine
supplementation, patients were asked to complete the Chronic
Fatigue Questionnaire (20). The
severity of fatigue was reflected in self-reported ratings, based
on daily activities and was represented by the performance status
score (21,22). Informed consent was obtained from all
participants before the initiation of the study. The present study
was approved by the Human Ethics Review Committee of Bellland
General Hospital (approval no. 2015-0004) and was performed in
accordance with the Declaration of Helsinki (23). Subjects were monitored for safety and
tolerance of L-carnitine during the course of the study.
Measurement of oxidative stress
markers [d-reactive oxygen metabolites (ROMs)] and antioxidant
capacity [biological antioxidant potential (BAP)]
Measurement of oxidative stress markers was
performed by measuring oxidative stress in vivo with simple
and accurate tests, d-ROMs-derived compounds and BAP test. The
collected samples were immediately centrifuged at 3,000 x g at 4˚C
for 10 min to separate the serum. The separated samples were stored
in a freezer at -20˚C until further use. Before the assay was
initiated, serum levels of d-ROMs and BAP were measured using
commercial kits (Diacron International: Cat. nos. AT3274 and
BR4873, respectively) on an Olympus AU640 (Olympus, Japan)
according to manufacturer's protocol (24). The results of the d-ROMs analysis are
expressed in Carratelli units (U.Carr), where 1 U.Carr corresponds
to 0.8 mg/l H2O2. The results of the BAP
analysis are expressed in µmol/l of the reduced ferric ions
(25,26). Antioxidant/oxidant (BAP/dROMs) ratio
was calculated to express relative antioxidant capacity.
Chronic Fatigue Questionnaire
Patients' health status was quantified using the
Chronic Fatigue Questionnaire at four different time points: Before
treatment, and 1, 3 and 6 months after the initiation of treatment.
All patients enrolled in the present study completed the Chronic
Fatigue Questionnaire, a 20-item self-report questionnaire used to
examine physical and mental functioning, originally produced by
Kuratsune et al (27) and
Mizuno et al (28). All items
were scored from 0-4; the physical health subscale and the mental
health subscale each had 10 items. The physical and mental health
scores reflected the mental and physical fitness performance,
respectively. To calculate the total score, the scores of each item
were added; thus the total score ranged from 0-40 points, with
higher scores indicating greater fatigue and/or greater effects of
fatigue on quality of life.
Statistical analysis
Numerical variables were calculated as the mean ±
standard deviations. Quantitative parameters were compared using a
one-way ANOVA with a post-hoc Tukey's tests. Spearman's rank
correlation analysis was used to determine correlations. P<0.05
was considered to indicate a statistically significant difference.
All statistical analyses were performed using SPSS version 20.0
(IBM, Corp.).
Results
Patient characteristics
The characteristics of the patients are listed in
Table I. The recruited cohort
consisted of 30 patients. The cause of liver disease was hepatitis
C viral infection in 14 patients, hepatitis B viral infection in 2
patients, alcohol abuse in 9 patients, and other disorders,
including primary biliary cholangitis, autoimmune hepatitis and
idiopathic causes in 5 patients. The number of patients with
Child-Pugh classifications (19) of
A, B and C were 18, 10 and 2, respectively. The median serum level
of ammonia was 91.0±52.0 µg/dl, and that of albumin was 3.3±0.5
g/dl. No adverse reactions attributable to L-carnitine
administration were observed.
 | Table IClinical and demographic
characteristics of patients with cirrhosis. |
Table I
Clinical and demographic
characteristics of patients with cirrhosis.
|
Characteristics | Valuea |
|---|
| Age, years | 71.0±8.4 |
| Sex,
male/female | 19/11 |
| Etiology,
HBV/HCV/Alcohol/Others | 2/14/9/5 |
| Child-Pugh
classification, A/B/C | 10/18/2 |
| Aspartate
transaminase, IU/l | 39.5±21.1 |
| Alanine
aminotransferase, IU/l | 40.2±18.5 |
| Alkaline
phosphatase, IU/l | 230±102 |
| γ-glutamyl
transpeptidase, U/l | 31±30 |
| Total bilirubin,
mg/dl | 1.5±0.8 |
| Blood urea
nitrogen, mg/dl | 13±10 |
| Total cholesterol,
mg/dl | 165±39 |
| Triglyceride,
mg/dl | 90±52 |
| Albumin, g/dl | 3.3±0.5 |
| Platelets,
x104/µl | 11.4±5.5 |
| Prothrombin time,
% | 71.9±15.1 |
| Ammonia, µg/dl | 91±52 |
| Total carnitine,
µmol/l | 66.5±12.8 |
| Free carnitine,
µmol/l | 52.4±12.1 |
| Acylcarnitine,
µmol/l | 14.1±3.7 |
Effects of 6-month of L-carnitine
supplementation on serum levels of total carnitine, free carnitine
and acylcarnitine
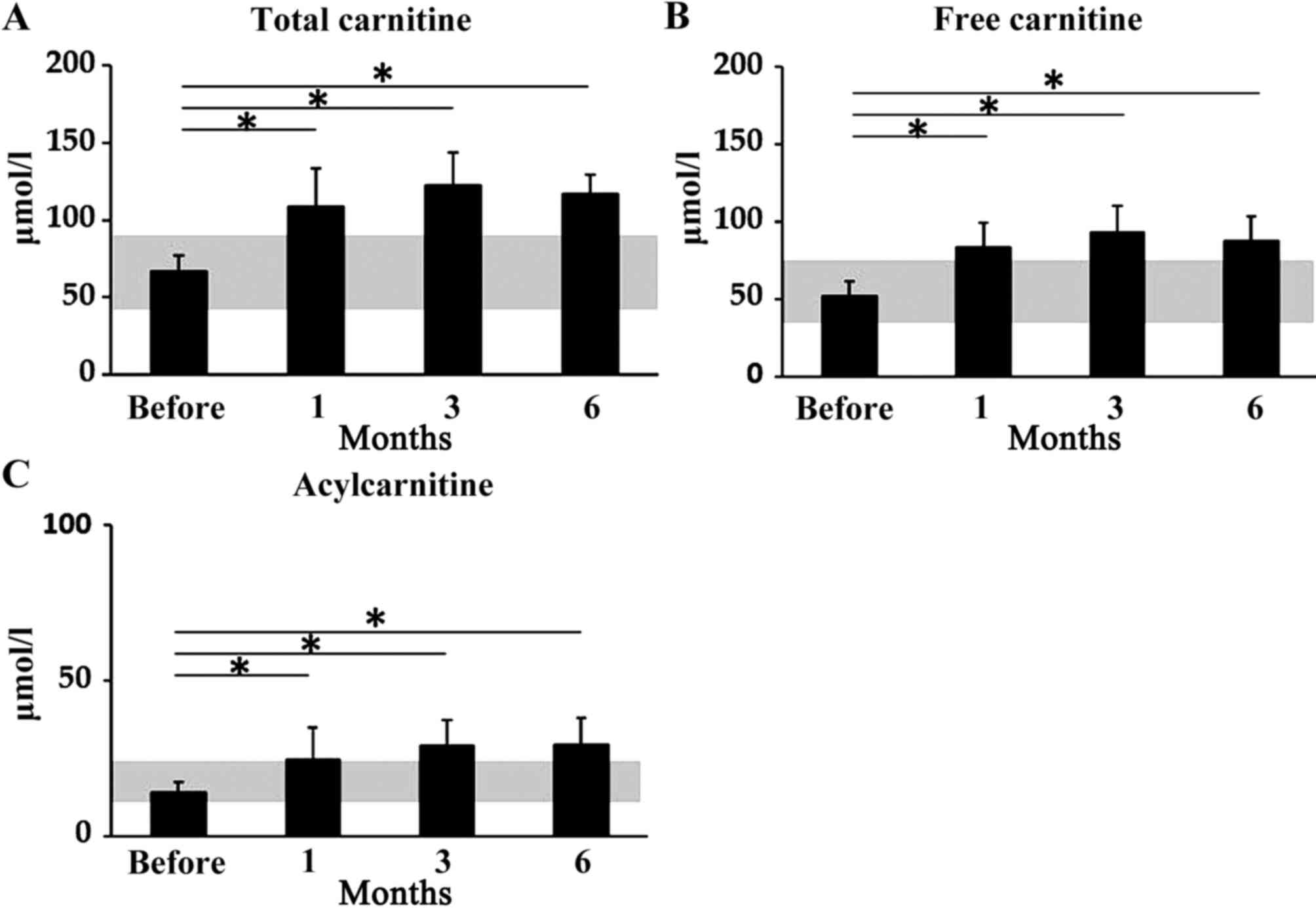
The levels of total carnitine, free carnitine and
acylcarnitine were significantly higher 1, 3 and 6 months after
therapy initiation compared with before treatment was initiated.
The mean serum levels of total carnitine were 66.1±10.5 µmol/l
before treatment, 108.2±25.2 µmol/l after 1 month of treatment
(P<0.05), 122.0±21.2 µmol/l after 3 months (P<0.05) and
117.2±12.2 µmol/l after 6 months (P<0.05; Fig. 2A). The mean serum levels of free
carnitine were 52.4±9.1 µmol/l before treatment, 83.8±15.3 µmol/l
after 1 month (P<0.05), 93.2±17.1 µmol/l after 3 months
(P<0.05) and 87.5±16.2 µmol/l after 6 months (P<0.05;
Fig. 2B). Mean serum levels of
acylcarnitine were 14.1±3.1 µmol/l before treatment, 24.5±10.5
µmol/l after 1 month (P<0.05), 29.1±8.3 µmol/l after 3 months
(P<0.05) and 29.4±8.5 µmol/l after 6 months (P<0.05; Fig. 2C).
Effects of 6-month L-carnitine
supplementation on serum levels of albumin and ammonia
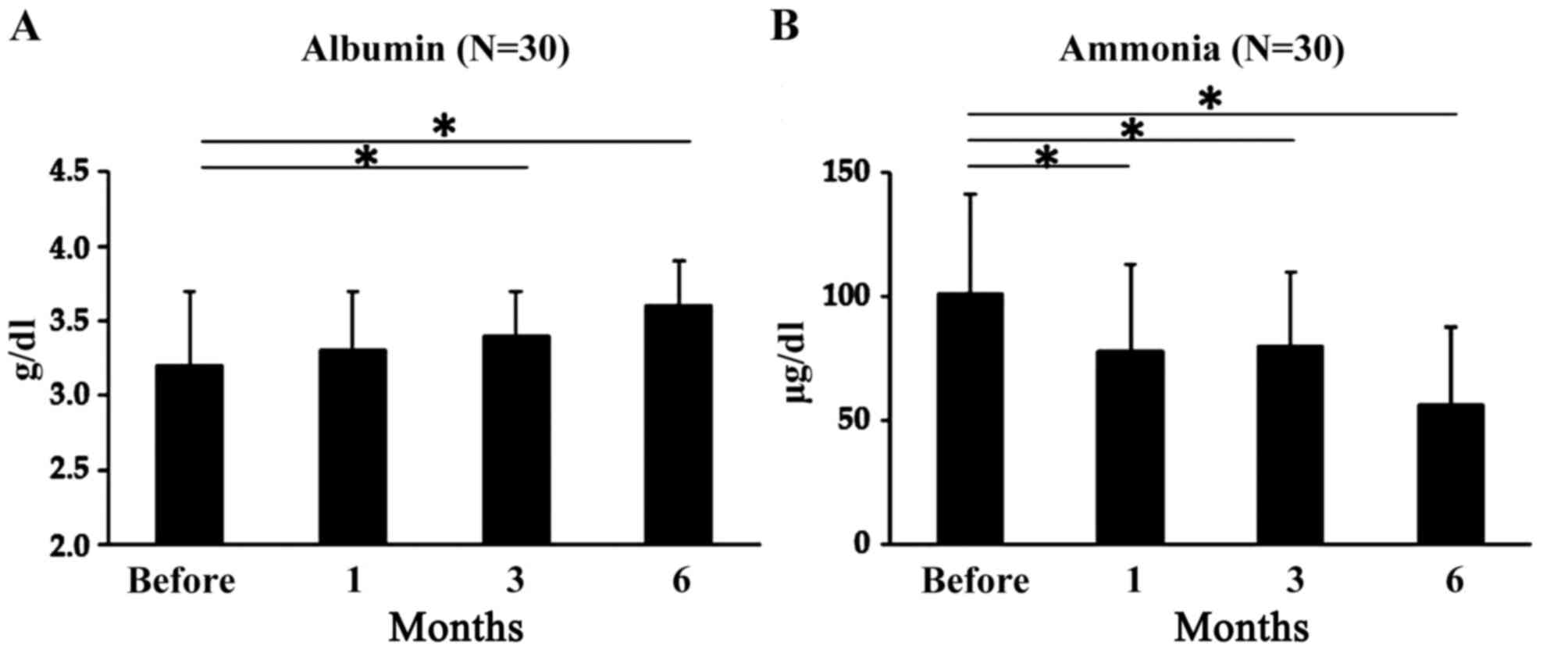
Serum albumin levels did not change significantly 1
month after L-carnitine therapy was initiated, but they were
significantly higher after 3 and 6 months of supplementation
compared with before treatment initiation. Mean serum levels were
3.2±0.5 µg/dl before treatment, 3.3±0.4 µg/dl after 1 month
(P=0.78), 3.4±0.3 µg/dl after 3 months (P<0.05) and 3.6±0.3
µg/dl after 6 months (P<0.05; Fig.
3A). In all patients, serum ammonia levels did not change
significantly 1 month after L-carnitine therapy was initiated, but
they were significantly higher 3 and 6 months after therapy
initiation compared with before treatment initiation. Mean serum
ammonia levels were 101±40 µg/dl before treatment, 78±35 µg/dl
after 1 month (P<0.05), 80±30 µg/dl after 3 months (P<0.05)
and 56.5±31 µg/dl after 6 months (P<0.05; Fig. 3B).
Effects of 6-month L-carnitine
supplementation on Child-Pugh and MELD scores
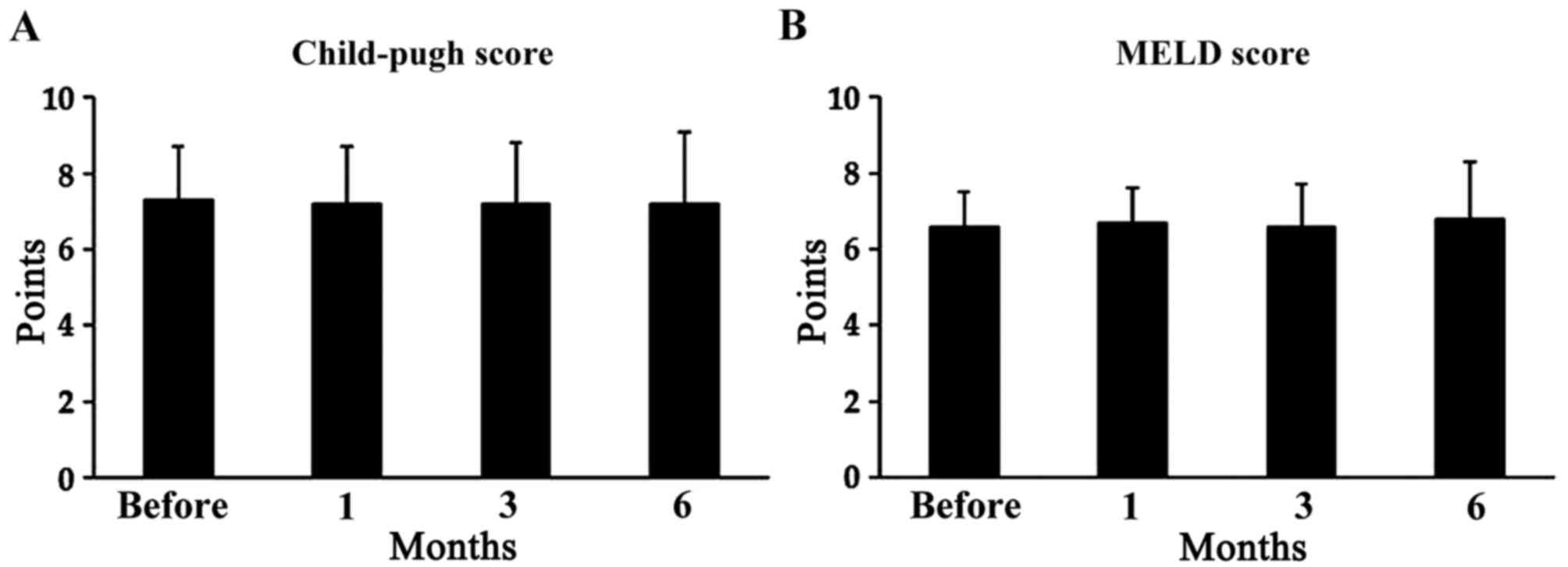
L-carnitine supplementation did not have any
significant effect on Child-Pugh or MELD scores. The Child-Pugh
score was 7.3±1.4 before treatment, 7.2±1.5 after 1 month (P=0.58),
7.2±1.6 after 3 months (P=0.38) and 7.2±1.9 after 6 months (P=0.32;
Fig. 4A). The MELD score was 6.6±0.9
before treatment, 6.7±0.9 after 1 month (P=0.32), 6.6±1.1 after 3
months (P=0.74) and 6.8±1.5 after 6 months (P=0.65; Fig. 4B).
Effect of L-carnitine supplementation
on chronic fatigue
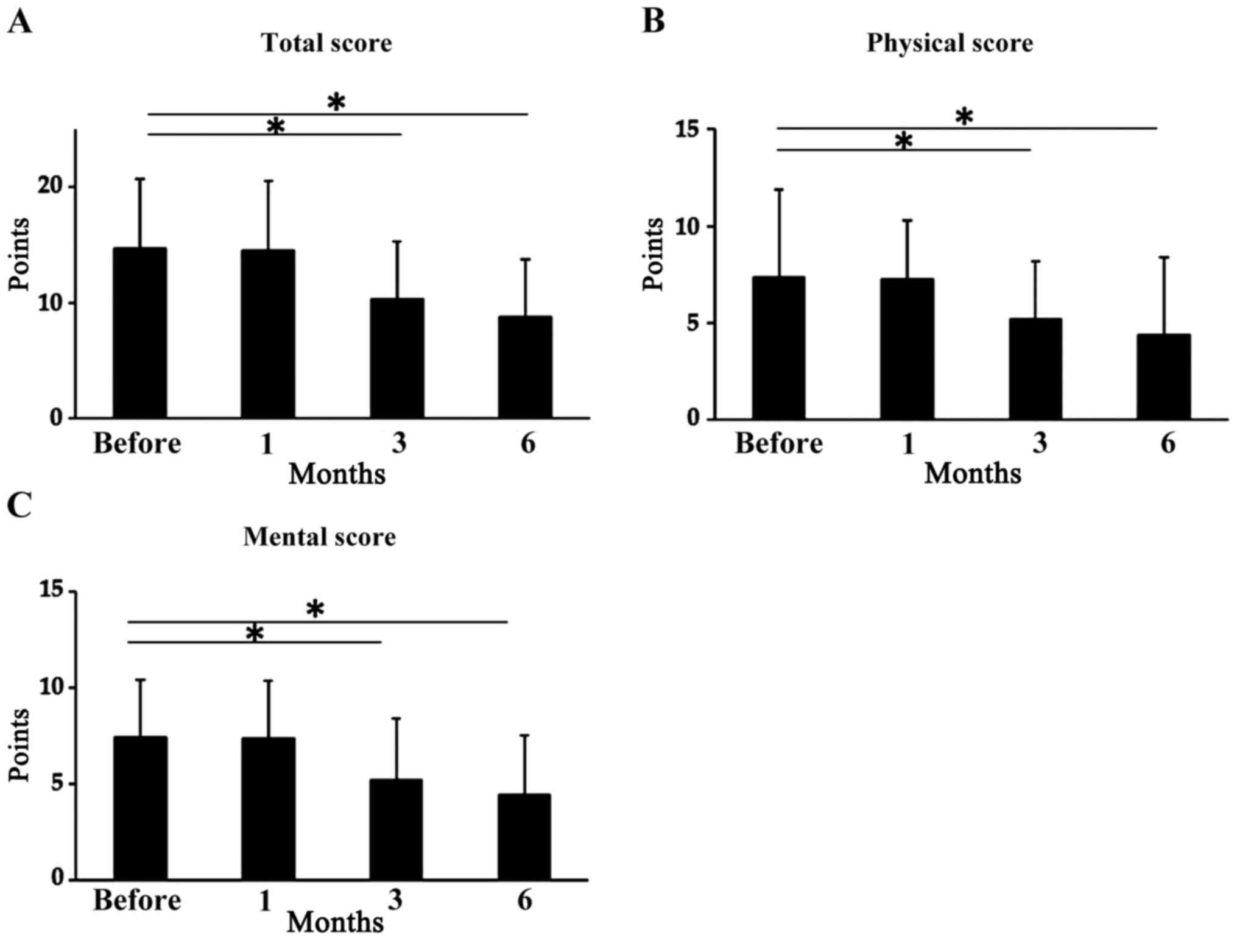
The total scores on the Chronic Fatigue
Questionnaire before treatment and after 1 month of L-carnitine
supplementation did not differ significantly, but were
significantly decreased after 3 and 6 months. The mean total scores
were 14.7±6.0 before treatment, 14.5±6.0 after 1 month (P>0.05),
10.3±5.0 points after 3 months (P<0.05) and 8.74±5.0 after 6
months (P<0.05; Fig. 5A). On the
physical subscale, scores were significantly decreased 3 and 6
months after therapy initiation; mean scores were 7.37±4.5 points
before treatment, 7.27±3.0 points after 1 month (P>0.05),
5.17±3.0 after 3 months (P<0.05) and 4.37±4.0 after 6 months
(P<0.05; Fig. 5B). On the mental
subscale, the scores were significantly decreased 3 and 6 months
after therapy initiation; mean scores were 7.42±3.0 before
treatment, 7.37±3.0 points after 1 month (P>0.05), 5.22±3.2
after 3 months (P<0.05) and 4.43±3.1 after 6 months (P<0.05;
Fig. 5C).
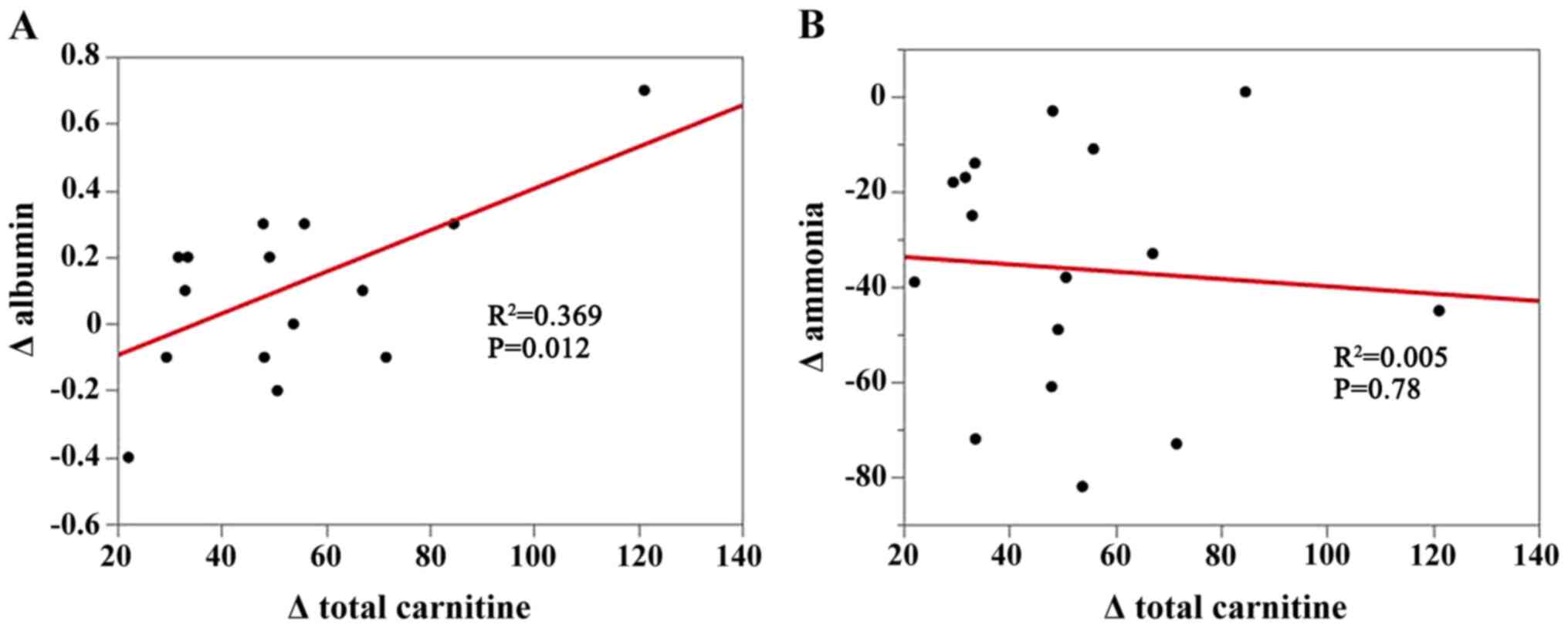
Correlation of changes in total
carnitine levels with changes in serum albumin levels and changes
in serum ammonia levels
Changes in serum carnitine concentrations were
positively correlated with changes in serum albumin levels
(R2=0.369; P=0.012), but not with changes in serum
ammonia levels (R2=0.005; P=0.78; Fig. 6A and B).
Effects of 6-month L-carnitine
supplementation on the d-ROMs/BAP ratio
L-carnitine supplementation did not significantly
affect the serum d-ROM levels after 1 month; however, serum d-ROM
levels were significantly lower 3 and 6 months after therapy
initiation compared with before the treatment. Mean levels were
246.1±46.2 U.Carr before treatment, 225.2±50.6 U.Carr after 1 month
(P=0.67), 210.9±20.3 U.Carr after 3 months (P<0.05) and
205.8±30.5 U.Carr after 6 months (P<0.05; Fig. 7A). However, L-carnitine
supplementation did not have any significant effect on serum BAP
levels. Mean levels were 2,100±322 µmol/l before treatment,
2,095±310 µmol/l after 1 month (P=0.52), 2,152±390 µmol/l after 3
months (P=0.43) and 2,120±385 µmol/l after 6 months (P=0.28;
Fig. 7B). There was no significant
effect of L-carnitine supplementation after 1 month on the
d-ROMs/BAP ratio, but the d-ROMs/BAP ratio was significantly higher
3 and 6 months after therapy initiation compared with before
treatment initiation. The ratio of d-ROMs/BAP was 8.5±2.0 before
treatment, 9.3±2.2 after 1 month (P=0.21), 10.2±1.9 µg/dl after 3
months (P<0.05) and 10.3±1.5 after 6 months (P<0.05; Fig. 7C).
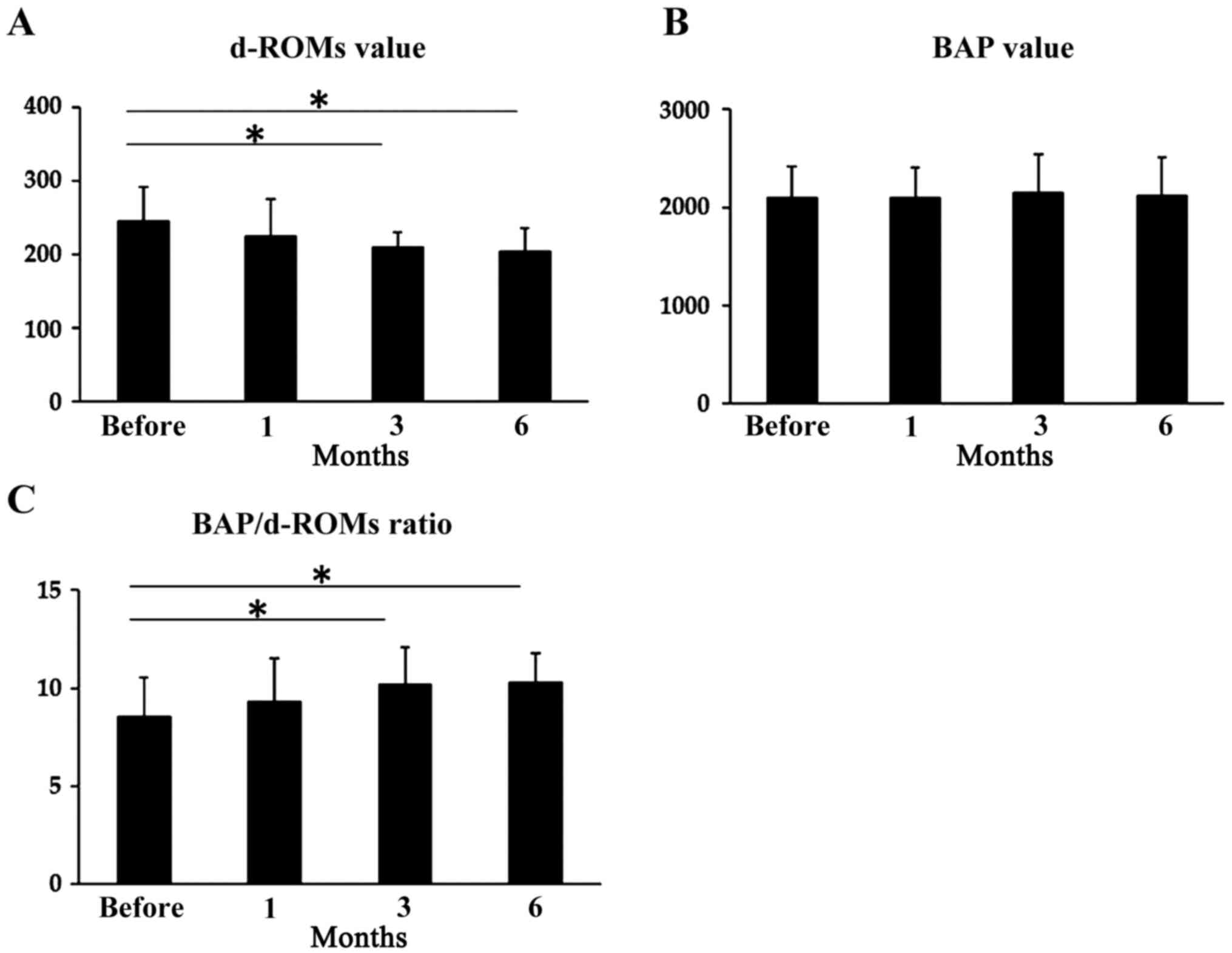 | Figure 7Changes in d-ROM levels, BAP levels
and the BAP/d-ROMs ratio during L-carnitine supplementation in
patients with cirrhosis. (A) Serum d-ROM levels were significantly
lower after 3 months (210.9±20.3 U.Carr) and 6 months (205.8±30.5
U.Carr) of L-carnitine supplementation compared with before
treatment (225.2±50.6 U.Carr). In contrast, there was no
significant effect of L-carnitine supplementation for 1 month on
serum albumin levels (246.1±46.2 U.Carr). (B) L-carnitine
supplementation did not have any significant effect on serum BAP
levels: 2,100±322 µmol/l before treatment and 2,095±310 µmol/l
after 1 month, 2,152±390 µmol/l after 3 months and 2,120±385 µmol/l
after 6 months. (C) The d-ROMs/BAP ratio was significantly higher
after 3 and 6 months of therapy: 8.5±2.0 before treatment, 9.3±2.2
after 1 month, 10.2±1.9 after 3 months and 10.3±1.5 after 6 months.
*P<0.05. d-ROM, d-reactive oxygen metabolite; BAP,
biological antioxidant potential. |
Discussion
L-carnitine is a nutrient prerequisite for energy
generation in vital organs, including the liver, kidneys and brain
(29,30). Carnitine deficiency occurs in various
disorders, such as malnutrition, liver cirrhosis, endocrine
disorders and end-stage renal disease treated with hemodialysis,
all of which are considered to affect health-related quality of
life (31,32). To the best of our knowledge, the
present study is the first prospective study to show that 1,800
mg/day L-carnitine supplementation for 6 months exhibited favorable
effects on chronic fatigue in patients with cirrhosis. L-carnitine
increased carnitine profiles, serum albumin levels, the ratio of
BAP/d-ROM and decreased serum ammonia levels. L-carnitine increases
carnitine palmitoyl transferase activity, which reflects
mitochondrial fatty acid oxidation resulting in increased
acylcarnitine levels (33). As
changes in serum carnitine concentrations were positively
correlated with changes in serum albumin levels, but not with
changes in serum ammonia levels, this suggests that L-carnitine
ameliorated chronic fatigue by attenuating oxidative stress.
A meta-analysis revealed that supplemental intake of
4,000 mg L-carnitine increases serum albumin levels and ameliorates
serum ammonia levels in patients with hepatic encephalopathy
(14). L-carnitine supplementation
reduced the levels of ammonia in patients with cirrhosis with
physiological blood carnitine levels (34), suggesting that increased quantities
of carnitine is required for patients with cirrhosis compared with
the healthy individuals, and that patients may have exhibited a
relative carnitine deficiency. In contrast, 600 mg oral L-carnitine
daily was reported to counteract the reduction in serum albumin
levels 1 week after transarterial chemoembolization (TACE) in
patients with cirrhosis (35). These
findings suggest that higher doses of L-carnitine supplementation
may not be necessary for producing the therapeutic benefits as the
homeostatic control of serum L-carnitine levels is tight and subtle
(15). In the present study, it was
shown that both Child-Pugh and MELD scores did not change
significantly during the 6-month follow-up. However, L-carnitine
supplementation maintains the levels of branched-chain amino acids
for albumin synthesis, and this has been shown to improve
Child-Pugh scores following TACE (35). The discrepancy may be partly
explained by the differences in patient characteristics between the
two studies. A systematic review and meta-analysis revealed
beneficial effects of L-carnitine on serum albumin levels in
patients with cirrhosis (14).
However, L-carnitine did not increase the serum albumin levels in
patients with nonalcoholic steatohepatitis, whose serum albumin
levels were already relatively high (36). These data reinforce the fact that
L-carnitine improves serum albumin levels in patients with
cirrhosis, which is frequently complicated by hypoalbuminemia
(37).
The complication of minimal encephalopathy may cause
mental disorders in patients with cirrhosis. Administration of
acetyl-L-carnitine, an acetylated form of L-carnitine improved the
mental status to normal levels and resolved hyperammonemia in a
patient on chronic hemodialysis who exhibited elevated serum
ammonia levels and altered mental status (38). With acetyl-L-carnitine, a significant
decrease in serum ammonia levels was observed in patients with
cirrhosis, along with a significant improvement in mental function
(39). Patients with hepatic
encephalopathy treated with L-carnitine showed significant
improvements with regard to mental fatigue (40). An intensity-dependent relationship
was found between ammonia levels and exercise, which suggested that
ammonia accumulation exhibits a pivotal role in both physical and
mental fatigue (41). Malaguarnera
et al (42) demonstrated that
in patients with minimal hepatic encephalopathy, acetyl-L-carnitine
improved the quality of life by reducing serum albumin levels.
However, L-carnitine did not exert considerable effects on ammonia
levels 3 months after L-carnitine supplementation (42). The causes of differences in serum
carnitine profiles between the studies are unclear. However, one
possible explanation is the differences in the causes of liver
cirrhosis and in the functional reserve capacities in patients
(43,44). Furthermore, in the present study,
changes in total carnitine levels were not significantly correlated
with changes in ammonia levels. These findings suggest that the
favorable effects of L-carnitine on chronic fatigue in patients
with cirrhosis cannot be attributed to improvements in serum
ammonia levels.
The mechanism underlying the favorable effects of
L-carnitine on chronic fatigue remain unclear. L-carnitine
supplementation increases albumin levels in patients with
cirrhosis. Patients receiving L-carnitine treatment showed
improvements in daily activity performance levels and the
performance efficiency of high-intensity muscular exercise, through
the regulation of mitochondrial homeostasis (45). More recent evidence has suggested a
close association between chronic fatigue and oxidative stress
(46). In the present study, it was
shown that L-carnitine supplementation increased the BAP/d-ROM
ratio with serum albumin levels in patients with cirrhosis. Albumin
is involved in several bioactive functions, such as antioxidant
activity (47). Furthermore, serum
albumin reportedly decreased oxidative stress by inhibiting the
activation of nicotinamide adenine dinucleotide phosphate oxidase
in human vascular smooth muscle (48). In addition, L-carnitine
supplementation augmented fat oxidation and energy expenditure
during prolonged exercise, which therefore spares glycogen stores
and delays the onset of fatigue (39). These findings suggest that
L-carnitine supplementation can potentially ameliorate chronic
fatigue by attenuating oxidative stress (49). However, the exact mechanisms by which
L-carnitine ameliorates chronic fatigue requires further
investigation.
Several limitations associated with the present
study must be mentioned. First, it was not possible to compare
patients who received L-carnitine treatment with patients who did
not. Second, the relationship between physical function and
skeletal muscle mass were not assessed, which may explain the
effect of L-carnitine on sarcopenia. Furthermore, the sample size
was relatively small. A future study with a larger cohort is
required to confirm the results of the present study. However, the
present study is the first prospective clinical trial in which the
beneficial effects of L-carnitine supplementation on physical and
mental functional abilities in patients with cirrhosis and chronic
fatigue were assessed through the use of a self-report
questionnaire. The results showed that improvement in carnitine
profiles were accompanied by increases in serum albumin levels, but
not changes in serum ammonia levels. This suggests that L-carnitine
ameliorates chronic fatigue by attenuating oxidative stress.
L-carnitine alleviated chronic fatigue and increased serum albumin
levels, which are recognized markers of antioxidant status in
patients with cirrhosis. Nevertheless, larger studies are required
to evaluate the effects of carnitine on health-related quality of
life in patients with cirrhosis and investigate the exact
mechanisms by which L-carnitine ameliorates chronic fatigue.
Acknowledgements
The authors would like to thank Ms Nakai (Nara
Medical University) for assistance in collecting the clinical
data.
Funding
This study was partially supported by Grants-in-Aid
for Scientific Research from Nara Medical University (grant no.
15K08077).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SSat, MF, SSai, HK, KKa, HT, NS, YS, KKi, KM, TAk,
NH and TAn performed the data analysis. AM, HY and TN made
substantial contributions to the conception and design of the
study, as well as analysis and interpretation of the data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all participants
before the initiation of the study. This study was approved by the
Human Ethics Review Committee of Bellland General Hospital (Osaka,
Japan), (approval no. 2015-0004) and was performed in accordance
with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oney-Birol S: Exogenous L-carnitine
promotes plant growth and cell division by mitigating genotoxic
damage of salt stress. Sci Rep. 9(17229)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rinaudo MT, Curto M, Bruno R, Piccinini M
and Marino C: Acid soluble, short chain esterified and free
carnitine in the liver, heart, muscle and brain of pre and post
hatched chicks. Int J Biochem. 23:59–65. 1991.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Khan L and Bamji MS: Tissue carnitine
deficiency due to dietary lysine dificiency: Triglyceride
accumulation and concomitant impairment in fatty acid oxidation. J
Nutr. 109:24–31. 1979.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rebouche CJ: Carnitine function and
requirements during the life cycle. FASEB J. 6:3379–3386.
1992.PubMed/NCBI
|
|
5
|
Cimmino A, Andolfi A, Zonno MC, Troise C,
Santini A, Tuzi A, Vurro M, Ash G and Evidente A: Phomentrioloxin:
A phytotoxic pentasubstituted geranylcyclohexentriol produced by
Phomopsis sp., a potential mycoherbicide for Carthamus lanatus
Biocontrol. J Nat Prod. 75:1130–1137. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Longo N, Frigeni M and Pasquali M:
Carnitine transport and fatty acid oxidation. Biochim Biophys Acta.
1863:2422–2435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ringseis R, Keller J and Eder K: Role of
carnitine in the regulation of glucose homeostasis and insulin
sensitivity: Evidence from in vivo and in vitro studies with
carnitine supplementation and carnitine deficiency. Eur J Nutr.
51:1–18. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Irvine KM, Ratnasekera I, Powell EE and
Hume DA: Causes and consequences of innate immune dysfunction in
cirrhosis. Front Immunol. 10(293)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Malaguarnera M, Vacante M, Motta M,
Giordano M, Malaguarnera G, Bella R, Nunnari G, Rampello L and
Pennisi G: Acetyl-L-carnitine improves cognitive functions in
severe hepatic encephalopathy: A randomized and controlled clinical
trial. Metab Brain Dis. 26:281–289. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cecere A, Ciaramella F, Tancredi L, Romano
C and Gattoni A: Efficacy of L-carnitine in reducing
hyperammonaemia and improving neuropsychological test performance
in patients with hepatic cirrhosis: Results of a randomised trial.
Clin Drug Investig. 22 (Suppl 1):S7–S14. 2002.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hiramatsu A, Aikata H, Uchikawa S, Ohya K,
Kodama K, Nishida Y, Daijo K, Osawa M, Teraoka Y, Honda F, et al:
Levocarnitine use is associated with improvement in sarcopenia in
patients with liver cirrhosis. Hepatol Commun. 3:348–355.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nakanishi H, Kurosaki M, Tsuchiya K,
Nakakuki N, Takada H, Matsuda S, Gondo K, Asano Y, Hattori N,
Tamaki N, et al: L-carnitine reduces muscle cramps in patients with
cirrhosis. Clin Gastroenterol Hepatol. 13:1540–1543.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tajiri K, Futsukaichi Y, Kobayashi S,
Yasumura S, Takahara T, Minemura M and Sugiyama T: L-carnitine for
the treatment of overt hepatic encephalopathy in patients with
advanced liver cirrhosis. J Nutr Sci Vitaminol (Tokyo). 64:321–328.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Abbasnezhad A, Choghakhori R, Kashkooli S,
Alipour M, Asbaghi O and Mohammadi R: Effect of L-carnitine on
liver enzymes and biochemical factors in hepatic encephalopathy: A
systematic review and meta-analysis. J Gastroenterol Hepatol.
34:2062–2070. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shang R, Sun Z and Li H: Effective dosing
of L-carnitine in the secondary prevention of cardiovascular
disease: A systematic review and meta-analysis. BMC Cardiovasc
Disord. 14(88)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Howanitz JH, Howanitz PJ, Skrodzki CA and
Iwanski JA: Influences of specimen processing and storage
conditions on results for plasma ammonia. Clin Chem. 30:906–908.
1984.PubMed/NCBI
|
|
17
|
Bajaj JS, Cordoba J, Mullen KD, Amodio P,
Shawcross DL, Butterworth RF and Morgan MY: International Society
for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN). Review
article: The design of clinical trials in hepatic encephalopathy-an
International Society for Hepatic Encephalopathy and Nitrogen
Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther.
33:739–747. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Michalak A, Rose C, Butterworth J and
Butterworth RF: Neuroactive amino acids and glutamate (NMDA)
receptors in frontal cortex of rats with experimental acute liver
failure. Hepatology. 24:908–913. 1996.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Peng Y, Qi X and Guo X: Child-pugh versus
MELD score for the assessment of prognosis in liver cirrhosis: A
systematic review and meta-analysis of observational studies.
Medicine (Baltimore). 95(e2877)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tanaka S, Kuratsune H, Hidaka Y, Hakariya
Y, Tatsumi KI, Takano T, Kanakura Y and Amino N: Autoantibodies
against muscarinic cholinergic receptor in chronic fatigue
syndrome. Int J Mol Med. 12:225–230. 2003.PubMed/NCBI
|
|
21
|
Okada T, Tanaka M, Kuratsune H, Watanabe Y
and Sadato N: Mechanisms underlying fatigue: A voxel-based
morphometric study of chronic fatigue syndrome. BMC Neurol.
4(14)2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Matsuda Y, Matsui T, Kataoka K, Fukada R,
Fukuda S, Kuratsune H, Tajima S, Yamaguti K, Kato YH and Kiriike N:
A two-year follow-up study of chronic fatigue syndrome comorbid
with psychiatric disorders. Psychiatry Clin Neurosci. 63:365–373.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
General Assembly of the World Medical
Association. World Medical Association Declaration of Helsinki:
Ethical principles for medical research involving human subjects. J
Am Coll Dent. 81:14–18. 2014.PubMed/NCBI
|
|
24
|
Morimoto M, Hashimoto T, Tsuda Y, Kitaoka
T and Kyotani S: Evaluation of oxidative stress and antioxidant
capacity in healthy children. J Chin Med Assoc. 82:651–654.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Celi P, Sullivan M and Evans D: The
stability of the reactive oxygen metabolites (d-ROMs) and
biological antioxidant potential (BAP) tests on stored horse blood.
Vet J. 183:217–218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bergami R, Maranesi M, Marchetti M,
Sangiorgi Z and Tolomelli B: Influence of dietary n-3
polyunsaturated fatty acids on plasma lipemic effect of vitamin B6
deficiency. Int J Vitam Nutr Res. 69:315–321. 1999.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kuratsune H, Yamaguti K, Lindh G, Evengård
B, Hagberg G, Matsumura K, Iwase M, Onoe H, Takahashi M, Machii T,
Kanakura Y, et al: Brain regions involved in fatigue sensation:
Reduced acetylcarnitine uptake into the brain. Neuroimage.
17:1256–1265. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mizuno K, Tanaka M, Nozaki S, Mizuma H,
Ataka S, Tahara T, Sugino T, Shirai T, Kajimoto Y, Kuratsune H,
Kajimoto O and Watanabe Y: Antifatigue effects of coenzyme Q10
during physical fatigue. Nutrition. 24:293–299. 2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Malaguarnera M: Carnitine derivatives:
Clinical usefulness. Curr Opin Gastroenterol. 28:166–176.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kendler BS: Carnitine: An overview of its
role in preventive medicine. Prev Med. 15:373–390. 1986.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Malaguarnera M: Acetyl-L-carnitine in
hepatic encephalopathy. Metab Brain Dis. 28:193–199.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gonwa TA and Wadei HM: Kidney disease in
the setting of liver failure: Core curriculum. Am J Kidney Dis
2013. 62:1198–1212. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Flanagan JL, Simmons PA, Vehige J, Willcox
MD and Garrett Q: Role of carnitine in disease. Nutr Metab (Lond).
7(30)2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shiraki M, Shimizu M, Moriwaki H, Okita K
and Koike K: Carnitine dynamics and their effects on hyperammonemia
in cirrhotic Japanese patients. Hepatol Res. 47:321–327.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hassan A, Tsuda Y, Asai A, Yokohama K,
Nakamura K, Sujishi T, Ohama H, Tsuchimoto Y, Fukunishi S, Abdelaal
UM, Arafa UA, et al: Effects of oral L-carnitine on liver functions
after transarterial chemoembolization in intermediate-stage HCC
patients. Mediators Inflamm. 2015(608216)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Malaguarnera M, Gargante MP, Russo C,
Antic T, Vacante M, Malaguarnera M, Avitabile T, Li Volti G and
Galvano F: L-carnitine supplementation to diet: A new tool in
treatment of nonalcoholic steatohepatitis-a randomized and
controlled clinical trial. Am J Gastroenterol. 105:1338–1345.
2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Soeters PB, Wolfe RR and Shenkin A:
Hypoalbuminemia: Pathogenesis and clinical significance. JPEN J
Parenter Enteral Nutr. 43:181–193. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
DaVanzo WJ and Ullian ME: L-carnitine
administration reverses acute mental status changes in a chronic
hemodialysis patient with hepatitis C infection. Clin Nephrol.
57:402–405. 2002.PubMed/NCBI
|
|
39
|
Stephens FB, Constantin-Teodosiu D and
Greenhaff PL: New insights concerning the role of carnitine in the
regulation of fuel metabolism in skeletal muscle. J Physiol.
581:431–444. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Malaguarnera M, Gargante MP, Cristaldi E,
Vacante M, Risino C, Cammalleri L, Pennisi G and Rampello L:
Acetyl-L-carnitine treatment in minimal hepatic encephalopathy. Dig
Dis Sci. 53:3018–3025. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Malaguarnera M, Vacante M, Giordano M,
Pennisi G, Bella R, Rampello L, Malaguarnera M, Li Volti G and
Galvano F: Oral acetyl-L-carnitine therapy reduces fatigue in overt
hepatic encephalopathy: A randomized, double-blind,
placebo-controlled study. Am J Clin Nutr. 93:799–808.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Malaguarnera M, Bella R, Vacante M,
Giordano M, Malaguarnera G, Gargante MP, Motta M, Mistretta A,
Rampello L and Pennisi G: Acetyl-L-carnitine reduces depression and
improves quality of life in patients with minimal hepatic
encephalopathy. Scand J Gastroenterol. 46:750–759. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Amodio P, Angeli P, Merkel C, Menon F and
Gatta A: Plasma carnitine levels in liver cirrhosis: Relationship
with nutritional status and liver damage. J Clin Chem Clin Biochem.
28:619–626. 1990.PubMed/NCBI View Article : Google Scholar
|
|
44
|
D'Arienzo A, Mattera D, Ambrogio G,
Duranti L, Zeuli L and Mazzacca G: Hypercarnitinemia in cirrhosis.
Hepatology. 5(343)1985.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gimenes AC, Bravo DM, Napolis LM, Mello
MT, Oliveira AS, Neder JA and Nery LE: Effect of L-carnitine on
exercise performance in patients with mitochondrial myopathy. Braz
J Med Biol Res. 48:354–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee JS, Kim HG, Lee DS and Son CG:
Oxidative stress is a convincing contributor to idiopathic chronic
fatigue. Sci Rep. 8(12890)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sitar ME, Aydin S and Cakatay U: Human
serum albumin and its relation with oxidative stress. Clin Lab.
59:945–952. 2013.PubMed/NCBI
|
|
48
|
Kinoshita H, Watanabe K, Azma T, Feng GG,
Akahori T, Hayashi H, Sato M, Fujiwara Y and Wakatsuki A: Human
serum albumin and oxidative stress in preeclamptic women and the
mechanism of albumin for stress reduction. Heliyon.
3(e00369)2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Malaguarnera M, Pistone G, Astuto M,
Vecchio I, Raffaele R, Lo Giudice E and Rampello L: Effects of
L-acetylcarnitine on cirrhotic patients with hepatic coma:
Randomized double-blind, placebo-controlled trial. Dig Dis Sci.
51:2242–2247. 2006.PubMed/NCBI View Article : Google Scholar
|