Introduction
Histoplasmosis is a mycosis caused by Histoplasma
capsulatum (HC) which can be aggressive under certain
conditions. HC is a spore-forming, dimorphic fungus that has a
hyphal morphology in the natural environment and a yeast-like
morphology in the human host (1). HC
is generally present in the soil, particularly in soil rich in bird
and bat guano; the endemic region for histoplasmosis includes the
Mississippi River basin of the United States and parts of South
America (2,3). Human-to-human transmission via a
pulmonary route has not been reported, to the best of our knowledge
(4). Only a few cases have been
reported in Japan due to the rarity of HC in the natural
environment (5). In Japan, the
majority of the case reports are single cases only, and there are
no publications summarizing the findings of these reports. HC
infects the human lungs via the bronchi and clinical manifestations
most often develop as pulmonary histoplasmosis. Several individuals
have subclinical infections or exhibit mild, cold-like symptoms and
heal spontaneously after 2-3 weeks (6). Infection with HC is characterized
histopathologically by a granulomatous inflammation of the lung,
with the fungal body being entrapped inside the granulomas in the
lung (7). However, in
immunosuppressed patients, such as those with acquired
immunodeficiency syndrome (AIDS), organ transplant recipients and
the elderly, the fungus may disseminate to other organs as visceral
histoplasmosis (8). Sites of onset
are diverse, including the skin, central nervous system and
gastrointestinal (GI) tract. The development of lesions in the GI
tract has been reported in several AIDS patients (9). The frequency of clinically diagnosed GI
histoplasmosis is 3-12% of patients with disseminated
histoplasmosis. GI lesions are found in 70% of disseminated
histoplasmosis cases at autopsy (10). Rapid diagnosis and treatment are
necessary as this infectious disease may be fatal. However, it can
be difficult to diagnose accurately, due to a similarity in
symptoms with bacterial and viral pneumonia, such as fever, chills,
cough and shortness of breath, which can result in delayed
treatment (11). There are several
methods that facilitate diagnosis, including serological tests,
culturing and morphological identification of fungal bodies by
microscopy (8). HC must be
differentiated from several other pathogens as the morphological
findings may be inconclusive; thus, molecular techniques have been
used to aid in identification. This molecular pathological
methodology identifies specific DNA sequences from HC, potentially
from very small amounts of specimen, and this method is the most
effective approach for accurate diagnosis of HC infection (12).
The present report describes the case of a woman who
was immunosuppressed due to an HIV infection, and a duodenal lesion
was incidentally found by upper endoscopy. This report describes
the diagnosis of the duodenal histoplasmosis lesion based on
histopathological and molecular pathological studies.
Case report
Clinical presentation
The patient was a 57-year-old woman who was married
from South America. She was originally healthy and had no relevant
medical or family history. She had a persistent cough accompanied
by sputum for several months; and thus visited the Toyama
University Hospital where severe inflammatory findings in the upper
nasopharynx were noted.
The physical findings on examination in the hospital
were as follows: The patient´s height was 153 cm, weight 43 kg,
having lost 11 kg over the previous 4 months. She complained of
general fatigue and nocturnal fever. Although occasionally troubled
with constipation, she did not have consistent/prolonged complaints
regarding gastrointestinal symptoms. Laboratory findings on
admission showed that serum albumin levels were decreased (3.2
g/dl), and γ-globulin levels (34.7%), serum total protein levels
(8.6 g/dl) and CRP levels were increased (0.75 mg/dl). Peripheral
blood counts indicated normocytic anemia (red blood cell count,
4.05x105/µl; hemoglobin, 11.5 g/dl; hematocrit, 34.3%
cells) with a leukocyte count of 39.8x103/µl. Blood
lymphocyte surface markers, CD4 and CD8, were assessed due to the
persistent symptoms and potentially compromised immunity. The
percentages of CD4 and CD8 T cells were as follows: 4.9%
CD4+CD8-, 80.8%
CD4-CD8+, 0.1% CD4+CD8+
and 14.2% CD4-CD8-. The CD4:CD8 ratio was
0.1, due to markedly decreased proportion of CD4+ T
cells. An HIV antibody test was positive and a quantitative
determination of HIV RNA yielded a value of 1.4x104
copies/ml. Thus, it was concluded that the patient was in an
asymptomatic stage of AIDS, with consequent immunosuppression.
Serological tests for cytomegalovirus and toxoplasma were negative
and β-D glucan was within the normal range. An upper endoscopy was
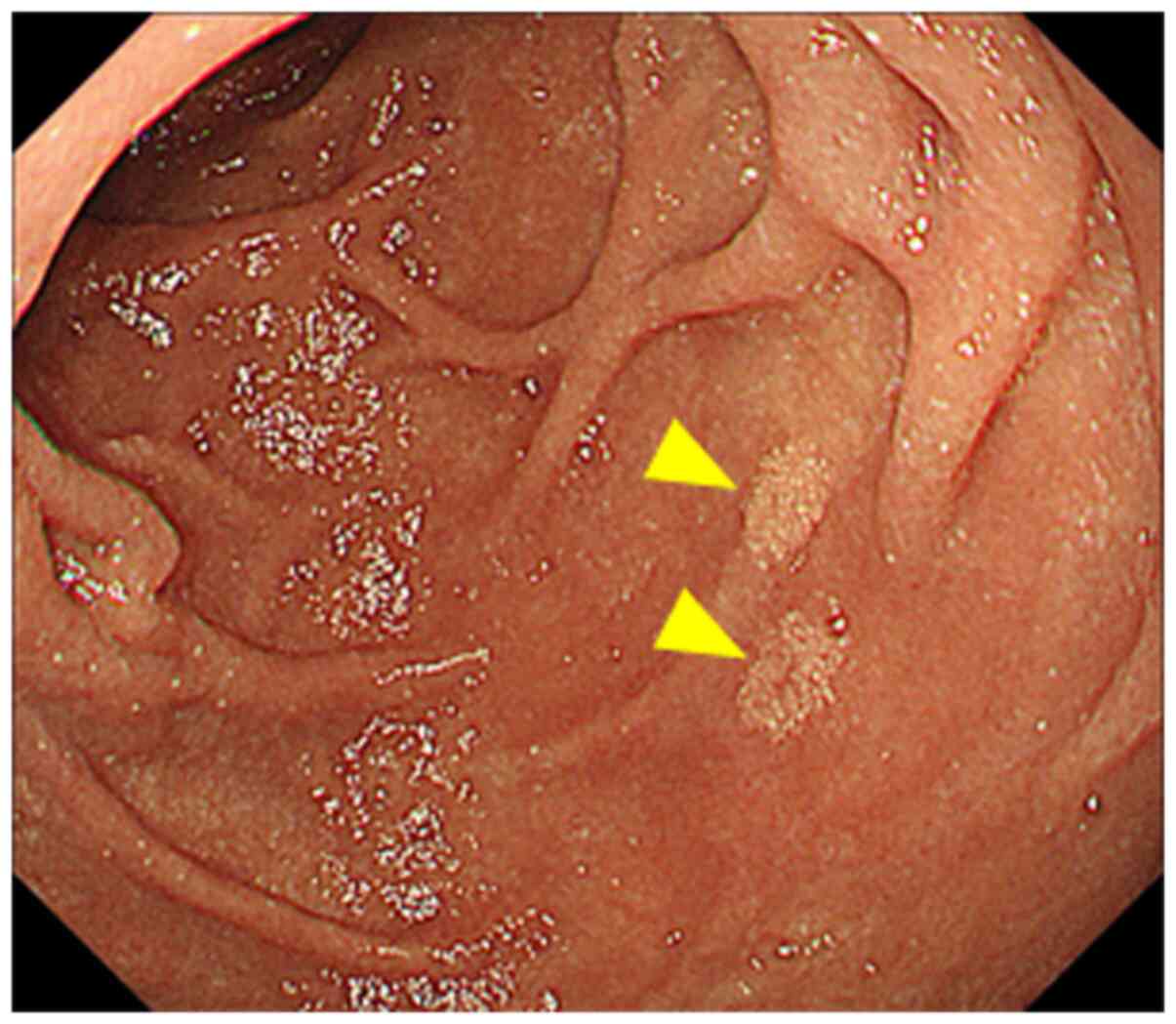
performed and revealed a yellowish punctate and patchy lesion in
the mucosa of the descending portion of the duodenum that was
suspected to be lymphatic dilatation (Fig. 1). A plain chest radiograph showed no
apparent changes in the lungs.
The patient was treated with clarithromycin and
fluconazole, and re-examination by endoscopy and mucosal biopsy 2
months later revealed no abnormalities. There were no lesions in
other organs. She was in a clinically latent state of AIDS with a
low HIV viral load and was treated with a cocktail of anti-HIV
drugs, including EVG/COBI/FTC/TAF for 5 months and a synthetic
antibacterial for >1 year (still being administered).
Pathological findings
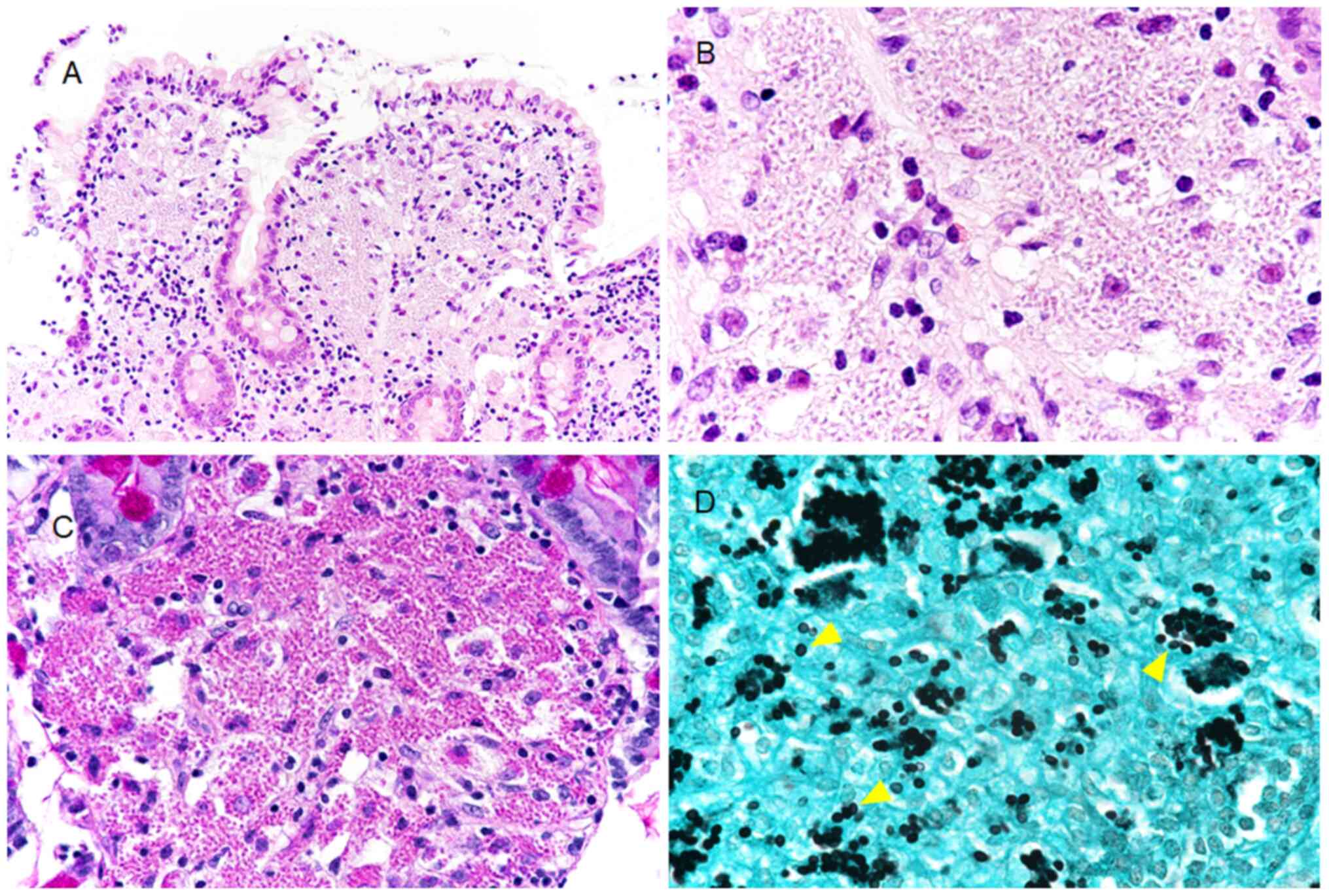
All staining was performed at room temperature for 2
h. An expanded lamina propria with numerous infiltrating foamy
cells was observed in the hematoxylin and eosin (H&E)-stained
sections of the biopsy specimen from the duodenal mucosa. These
foamy cells had several granular bodies with clear halos in the
cytoplasm. Infiltration of the lamina propria by inflammatory cells
other than the foamy cells was relatively rare, and most such cells
were small lymphocytes. Granulomatous changes were not seen in the
lamina propria. Structures suggestive of other infectious organisms
were not found (Fig. 2A and B). Based on histopathological analysis, the
granular bodies were stained positive with periodic acid-Schiff
(PAS) (Fig. 2C). Using Grocott
staining, these bodies were shown to possess a spherical or
drop-like shape, 2-4 µm in diameter, and the central portion was
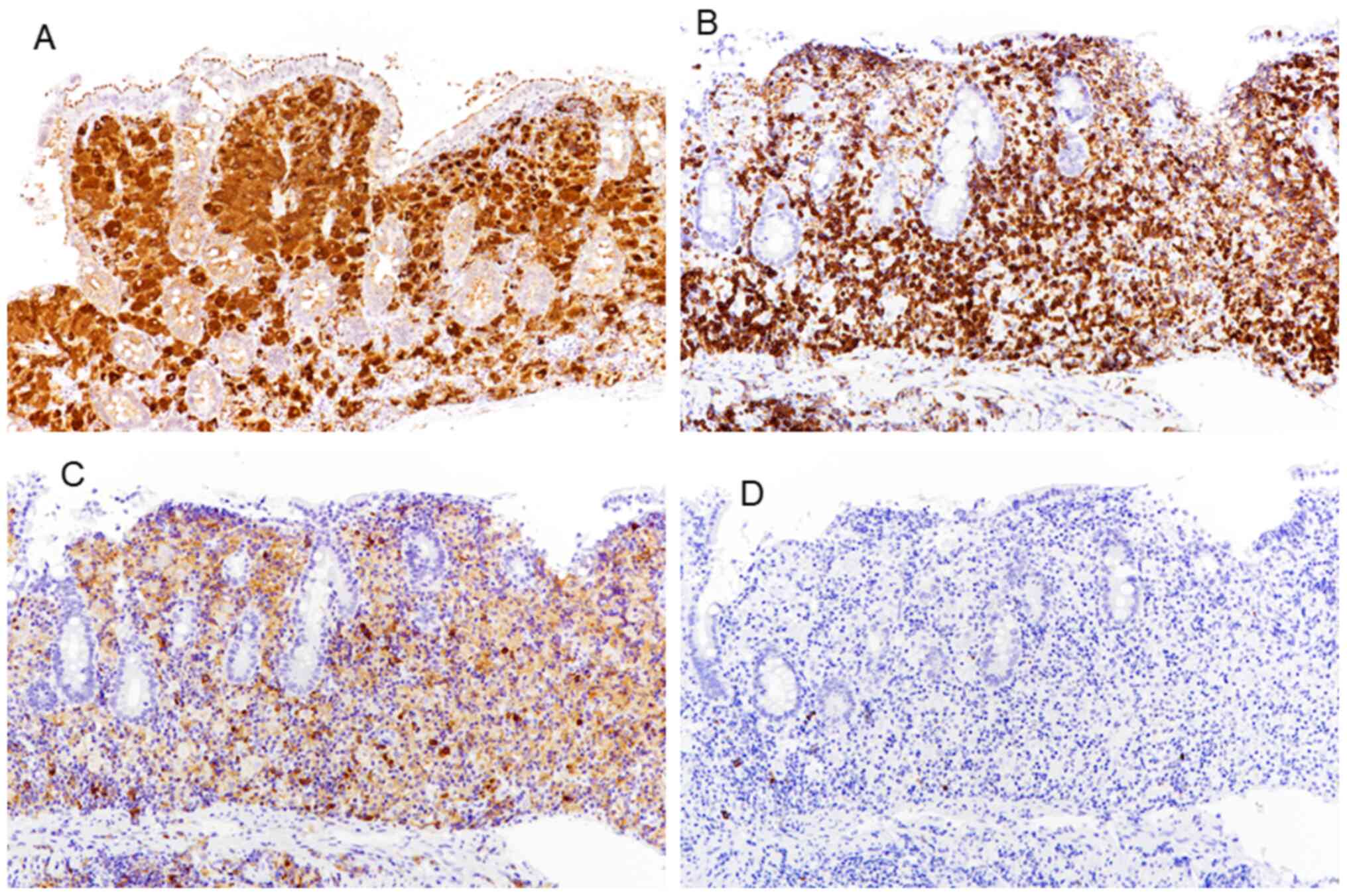
slightly clear, showing a yeast-like form with no hyphae (Fig. 2D). Using immunohistochemistry, it was
shown that the foamy cells were positive for CD68 and were
confirmed to be histiocytes (Fig.
3A). In addition, the lymphoid cells infiltrating the lamina
propria were primarily CD8+ cells, with very few
CD4+ and CD20+ cells (Fig. 3B-D). Based on these findings,
histopathologically, the duodenal mucosal lesion was initially
considered to be a fungal infection with only a yeast-type
morphology. HC was suspected as the most likely due to the
characteristic morphological findings. This was consistent with the
likelihood that cellular immunity in the patient's duodenal mucosa
was compromised by the HIV infection.
Genetics analysis
Molecular pathological tests were performed to
determine whether the fungal yeast-like particles in the foamy
cells were HC. Genomic DNA was extracted from the paraffin sections
of the duodenal mucosa using a QIAamp DNA FFPE Tissue kit according
to the manufacturer's protocol (Qiagen, Inc.). PCR amplification
was performed using universal primers for the fungal internal
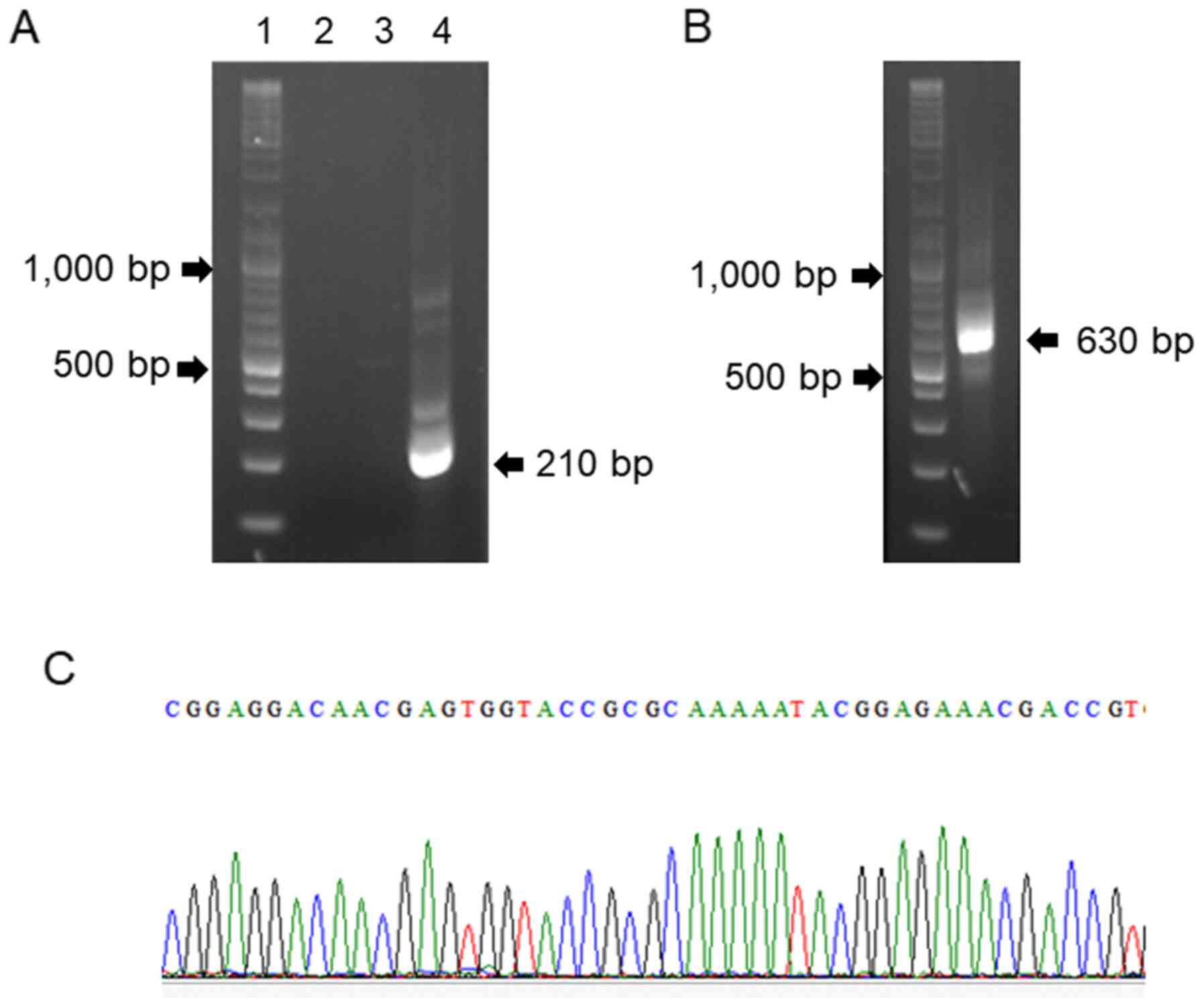
transcribed subunit (ITS) region. No product was amplified in the
first PCR (Fig. 4A), but an
additional run using the same primers yielded an amplicon of 630 bp
(Fig. 4B). The product was cloned
into the pGEM-T Easy vector (Promega Corporation) and subjected to
nucleotide sequencing. In addition, a nested PCR specific for the
HC p100 gene was performed using outer and inner primers. The PCR
product was purified and the sequence was determined on both
strands by direct sequencing (Sanger sequencing) with the inner
primers, HcIII and HcIV. The sequences obtained (Fig. 4C) completely matched those of the HC
ITS region and p100 gene registered in GenBank. The nucleotide
sequence data generated are available in the GenBank databases
under the accession numbers LC523625 and LC517841. The sequences of
the primers used are listed in Table
I. The initial and nested PCR were performed using an AmpliTaq
Gold 360 MasterMix (Thermo Fisher Scientific, Inc.) with the
following thermocycling conditions: 30 cycles of denaturation at
95˚C for 1 min, annealing at 60˚C for 1 min and extension at 72˚C
for 2 min. From these results, the yeast-like particles found in
the foamy cells of the duodenal mucosa were shown to be HC.
 | Table IPrimer sequences for the fungal ITS
region and HC. |
Table I
Primer sequences for the fungal ITS
region and HC.
| | Sequence, 5'-3' |
|---|
| ITS |
|
Forward |
TCCGTAGGTGAACCTGCGG |
|
Reverse |
TCCTCCGCTTATTGATATGC |
| HC p100 |
|
HcI
forward |
GCGTTCCGAGCCTTCCACCTCAAC |
|
HcII
reverse |
ATGTCCCATCGGGCGCCGTGTAGT |
| Inner primers |
|
HcIII
forward |
GAGATCTAGTCGCGGCCAGGTTCA |
|
HcIV
reverse |
AGGAGAGAACTGTATCGGTGGCTT |
Discussion
HC must be differentiated from other pathogens using
morphological and molecular based approaches. These other pathogens
include Candida glabrata, Cryptococcus neoformans,
Talaromyces marneffei, Paracoccidioides brasiliensis,
Sporothrix schenkii and Leishmania donovani, all of
which are very similar to HC (13).
HC is phagocytosed into the cytoplasm of macrophages, whereas the
yeast cells of C. glabrata are rarely found within
macrophages. C. neoformans has a capsule which may be
stained using a polysaccharide stain. As HC is not encapsulated,
these organisms cannot be observed using such staining methods;
however, halos surrounding the fungi can be observed by microscopy
(14). C. neoformans, when
phagocytosed by macrophages, loses its capsule, and thus often
appear small in size. In such a case, the fungal walls can be
visualized by staining using the Fontana-Masson stain (15). T. marneffei may have a
yeast-like form similar to that of HC, but a septum can be found in
these oval-shaped yeast-like cells (13). P. brasiliensis is
characterized by a mariner's wheel, which is formed by a mother
cell surrounded by peripheral daughter yeasts (16,17), and
S. schenkii occasionally presents as an asteroid body
(18). L. donovani is also
similar to HC, but it is negative for the PAS reaction and Grocott
staining (13). L. donovani
is also characterized by its kinetoplast, an organelle which is not
present in HC. Although the kinetoplast is difficult to detect by
H&E staining, it is possible to visualize using Giemsa staining
(14). However, it is difficult to
provide a diagnosis based on morphology alone as there are
relatively few differences between these pathogens.
As detailed above, there are certain characteristic
differential morphological findings which can be used to identify
HC, but definitive diagnosis is difficult in some cases. Thus, it
may occasionally be necessary to base a diagnosis on the results of
molecular techniques. The present case was unequivocally diagnosed
by amplifying a specific genomic region of HC using nested PCR,
followed by direct sequencing. The genomic DNA can be amplified and
visualized readily, even from a small quantity of formalin-fixed,
paraffin-embedded tissues, obtained from an endoscopic duodenal
mucosal biopsy specimen. This is considered to be an extremely
specific diagnostic method for differentiating HC from other
pathogens, including other fungi.
The patient in the present report had not been
abroad for 20 years and may already have been infected with HC when
she came to Japan. Although she was in a clinically latent state of
AIDS, it is likely that her immunocompromised condition led to the
development of histoplasmosis. There are various considerations
regarding the interrelationship between immunodeficiency caused by
HIV infection and the development of histoplasmosis (19). HIV can infect not only
CD4+ T cells but also CD4+ macrophages and
dendritic cells. CD4+ T cells cannot differentiate
following HIV, and a lack of CD4+ T cell helper function
underlies the deficiency of cytotoxic production in patients
infected with HIV (20,21). The phagocytic capacity of macrophages
and the microbicidal ability of neutrophils are all impaired.
CD4+ macrophages infected with HIV may be alive;
however, their microbicidal activity is decreased (22). The number of CD4+ T cells
was markedly reduced and a large number of macrophages
phagocytosing HC were present in the duodenal mucosa of the
patient. These findings support the hypothesis that macrophages are
not destroyed by HIV infection but they may lose their ability to
digest phagocytosed pathogens; accordingly, a large number of HCs
would thus be present in the cytoplasm of these macrophages.
Histoplasmosis is characterized by the formation of
granulomatous inflammatory lesions in the lung (5). However, no typical granulomatous
changes were observed in the duodenal mucosa of this patient, and
it is unclear why no granulomatous lesions were present there. One
possible explanation is that the granuloma-forming activity of
macrophages was decreased and differentiation into Th1 and Th17
cells from CD4+ T cells was impaired due to HIV
infection (23,24).
In conclusion, a case of histoplasmosis that
occurred as the initial lesion in the duodenum of an HIV-infected
patient is reported. It can be difficult to distinguish HC from
other fungi and protozoa based on morphology alone. Therefore, the
final diagnosis of duodenal histoplasmosis should be based on
additional auxiliary techniques, including histopathological
analysis and molecular pathological methods.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the present
study are available from the corresponding author on reasonable
request. The nucleotide sequence data generated are available in
the GenBank databases under the accession numbers LC523625 and
LC517841.
Authors' contributions
SS performed the experiments and wrote the
manuscript. ST, KT, TM, AN, TN and JI contributed to the
pathological analysis. HK performed the molecular analysis. JI
designed the study and wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Comprehensive consent was obtained from the
individual participant for publication of this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Köhler JR, Hube B, Puccia R, Casadevall A
and Perfect JR: Fungi that infect humans. Microbiol Spectr.
5:FUNK-0014–2016. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Diaz JH: Environmental and
wilderness-related risk factors for Histoplasmosis: More than bates
in caves. Wilderness Environ Med. 29:531–540. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bahr NC, Antinori S, Wheat LJ and Sarosi
GA: Histoplasmosis infections worldwide: Thinking outside of the
Ohio River valley. Curr Trop Med Rep. 2:70–80. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Deepe GS: Histoplasma capsulatum
(Histoplasmosis). In: Mandell, Douglas, and Bennett's Principles
and Practice of infectious diseases. Bennett JE, Dolin R and Blaser
MJ (eds). 8th edition. Elsevier Saunders, Philadelphia, PA,
pp2949-2962, 2014.
|
|
5
|
Hatakeyama S, Okamoto K, Ogura K, Sugita C
and Nagi M: Histoplasmosis among HIV-infected patients in Japan: A
case report and literature review. Jpn J Infect Dis. 72:330–333.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kauffman CA: Histoplasmosis: A clinical
and laboratory update. Clin Microbiol Rev. 20:115–132.
2007.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mukhopadhyay S and Doxtader EE: Visibility
of Histoplasma within histiocytes on hematoxylin and eosin
distinguishes disseminated histoplasmosis from other forms of
pulmonary histoplasmosis. Hum Pathol. 44:2346–2352. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zanotti P, Chirico C, Gulletta M,
Ardighieri L, Casari S, Roldan EQ, Izzo I, Pinsi G, Lorenzin G,
Facchetti F, et al: Disseminated histoplasmosis as
AIDS-presentation. Case report and comprehensive review of current
literature. Mediterr J Hematol Infect Dis.
10(e2018040)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sharma R, Lipi L, Gajendra S, Mohapatra I,
Goel RK, Duggal R, Mishra SR and Gautam D: Gastrointestinal
histoplasmosis: A case series. Int J Surg Pathol. 25:592–598.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Psarros G and Kauffman CA: Colonic
histoplasmosis: A difficult diagnostic problem. Gastroenterol
Hepatol (NY). 3:461–463. 2007.PubMed/NCBI
|
|
11
|
Azar MM, Loyd JL, Relich RF, Wheat LJ and
Hage CA: Current concepts in the epidemiology, diagnosis, and
management of histoplasmosis syndromes. Semin Respir Crit Care Med.
41:13–30. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ohno H, Tanabe K, Umeyama T, Kaneko Y,
Yamagoe S and Miyazaki Y: Application of nested PCR for diagnosis
of histoplasmosis. J Infect Chemother. 19:999–1003. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Watanabe M, Hotchi M and Nagasaki M: An
autopsy case of disseminated histoplasmosis probably due to
infection from a renal allograft. Acta Pathol Jpn. 38:769–780.
1988.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Walsh TJ, Larone DH, Schell WA and
Mitchell TG: Histoplasma, blastomyces, coccidioides and other
dimorphic fungi causing systemic mycoses. In: Manual of Clinical
Microbiology. Murray PR, Baron EJ, Jorgensen JH, Pfaller MA and
Yolken RH (eds). Vol 2. 8th edition. ASM Press, Washington, DC,
pp1781-1797, 2003.
|
|
15
|
Wojewoda C and Procop GW: Infections with
Yeast and Yeastlike Fungi. In: Pathology of Infectious Diseases.
Procop GW and Pritt BS (eds). Elsevier, Philadelphia, PA,
pp531-572, 2014.
|
|
16
|
Schmitt BH and Pritt BS: Dematiaceous
fungal infections. In: Pathology of Infectious Diseases. Procop GW
and Pritt BS (eds). Elsevier, Philadelphia, PA, pp516-530,
2014.
|
|
17
|
Headley SA, Pretto-Giordano LG, Di Santis
GW, Gomes LA, Macagnan R, da Nobrega DF, Leite KM, de Alcantara BK,
Itano EN, Alfieri AA, et al: Paracoccidioides
brasiliensis-associated dermatitis and lymphadenitis in a dog.
Mycopathologia. 182:425–434. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang YQ, Xu XG, Zhang M, Jiang P, Zhou
XY, Li ZZ and Zhang MF: Sporotrichosis: Clinical and
histopathological manifestations. Am J Dermatopathol. 33:296–302.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Adenis AA, Aznar C and Couppie P:
Histoplasmosis in HIV-infected patients: a review of new
developments and remaining gaps. Curr Trop Med Rep. 1:119–128.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Abbas AK, Lichtman AH and Pillai S:
Congenital and acquired immunodeficiencies. In: Cellular and
Molecular Immunology. Abbas AK, Lichtman AH and Pillai S (eds). 9th
edition. Elsevier, Philadelphia, PA, pp459-487, 2017.
|
|
21
|
Abbas AK, Lichtman AH and Pillai S:
Immunity to microbes. In: Cellular and Molecular Immunology. Abbas
AK, Lichtman AH and Pillai S (eds). 9th edition. Elsevier,
Philadelphia, PA, pp351-372, 2017.
|
|
22
|
Kumar V, Abbas AK and Aster JC: Diseases
of the immune system. In: Robbins Basic Pathology. Kumar V, Abbas
AK and Aster JC (eds). 10th edition. Elsevier, Philadelphia,
pp121-188, 2017.
|
|
23
|
North RJ and Jung YJ: Immunity to
tuberculosis. Annu Rev Immunol. 22:599–623. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Khader SA and Cooper AM: IL-23 and IL-17
in tuberculosis. Cytokine. 41:79–83. 2008.PubMed/NCBI View Article : Google Scholar
|