Introduction
Chronic kidney disease (CKD), a very common disease,
is considered a growing public health issue, with a notable impact
on the economy and society. CKD progression without effective
treatment may lead to end-stage renal disease (ESRD). The annual
cost of treating ESRD is currently >$15 billion and ~$32 billion
in Japan and the US, respectively (1,2).
Moreover, the number of CKD patients is expected to increase
steadily in several countries, and CKD is considered a risk factor
for cardiovascular diseases (3).
Therefore, new suitable and precision-based diagnostic tools for
the prevention and treatment of CKD, including novel drugs, are
urgently required. Currently, kidney diseases are primarily
diagnosed based on serum creatinine, blood urea nitrogen and
urinary albumin levels. However, these biomarkers are insufficient
to determine the precise pathological state of a patient with CKD,
which can result in heterogeneous outcomes (4,5).
Therefore, a novel diagnostic method is required to achieve
improved CKD diagnosis and management.
Nano-extracellular vesicles (NVs), including
exosomes and microvesicles, are produced by a wide range of cell
types and released into various body fluids such as urine, serum
and saliva (6). Exosomes are
vesicles 30-100 nm in diameter, produced through the fusion between
multivesicular bodies and plasma membranes (6). On the contrary, microvesicles are
100-1,000 nm in diameter, which are released from cells through
direct shedding of plasma membranes (7). Both types of vesicles contain various
components such as cytosol-like proteins and nucleic acids (mRNA,
microRNA and DNA) (7). Urine is a
very useful source of NVs, as it can be obtained easily and
non-invasively, and urinary NVs contain components originating from
cells of all regions of the nephron, including glomeruli and renal
tubules (8). The components of NVs
are considered potential biomarkers for kidney diseases because
they may reflect the physiological and pathophysiological states of
their cells of origin. Recently, several scientists have reported
the practicality of using urinary NVs as a diagnostic tool for
kidney disease, prostate cancer and bladder cancer (8,9).
However, detailed information on how urinary NVs reflect the
physiological and pathological states of the kidney, as well as its
functionality, remains elusive. Accordingly, the clinical
application of urinary NVs for liquid biopsy has not yet been fully
validated.
In the present study, the expression of mRNAs in
urinary NVs from rat models of both glomerular nephritis and
diabetic kidney disease were assessed to investigate their
applicability as a novel diagnostic tool.
Materials and methods
Animals
Male Sprague Dawley (SD), Zucker lean (ZL) and
Zucker diabetic fatty (ZDF) rats were purchased from Charles River
Laboratories, Inc. Prior to the experimental procedures, animals
were acclimatized for at least 5 days and housed under a 12-h
light/dark cycle with ad libitum access to water and
standard chow, CRF-1 (Oriental Yeast Co., Ltd.). For the puromycin
aminonucleoside (PAN)-induced glomerular nephritis model, 73
6-week-old male SD rats received a tail vein injection of 100
mg/kg/5 ml PAN (Sigma-Aldrich; Merck KGaA) in saline buffer. In
addition, 24 saline buffer-injected rats were used as the control.
Prednisolone was administered as a single oral dose of 10 mg/kg/10
ml prednisolone (Shionogi & Co., Ltd.) 1 h prior to PAN
injection using distilled water as the vehicle control. These
animals were kept for 1 week and then were euthanized. For the type
2 diabetes model, 12-week-old male ZL (n=6) and ZDF (n=12) rats
were used at the beginning of the experiment. These animals were
kept for 20 weeks and then were euthanized. All animals were
observed at least once daily for monitoring of health. No
unforeseen deaths of animals occurred in these studies.
Urine collection and measurements of
urinary protein, albumin and creatinine levels
Total urine samples were collected over 24 h from
each animal in the metabolic cages at each time-point. Urine
samples were used immediately or stored at -20˚C until required.
Urinary protein, albumin and creatinine levels were measured using
a Rat Urinary Protein assay kit (Chondrex, Inc.), a LBIS™ Rat
Albumin ELISA kit (FUJIFILM Wako Pure Chemical Corporation) and a
LabAssay™ Creatinine kit (FUJIFILM Wako Pure Chemical Corporation)
respectively, according to the manufacturer's protocol.
Blood collection and blood glucose
measurements
Blood samples were collected from the tail vein at
each time-point. Blood samples were diluted with distilled water
(1:10) and blood glucose was measured using a Glucose Cii Test Wako
kit (FUJIFILM Wako Pure Chemical Corporation) according to the
manufacturer's protocol.
Isolation of NVs
Urinary NVs were isolated from urine samples using
differential centrifugation at 3,000 x g for 10 min at 4˚C. Then,
supernatants were centrifuged at 100,000 x g for 1 h at 4˚C and NVs
were retrieved from the pellets after gently discarding the
supernatants. For nanoparticle tracking analysis (NTA), pellets
were suspended in PBS. NTA was performed using the Nanosight LM20
instrument (Nanosight Ltd.) to analyze the distribution of vesicle
size and the concentration of particles, as previously reported
(10).
mRNA analysis in NVs
For the NVs isolated from the urine of PAN nephritis
model rats, total RNA was purified using the RNeasy micro kit
(QIAGEN, Inc.) according to the manufacturer's protocol. Briefly,
isolated NVs were lysed using lysis buffer containing 1%
β-mercaptoethanol, and total RNA was purified using MinElute spin
columns with on-column DNase digestion (Qiagen, Inc.). RNA was
quantified using a NanoDrop 1000 (Thermo Fisher Scientific, Inc.)
and cDNA was synthesized using a SuperScript™ VILO™ cDNA Synthesis
kit (Thermo Fisher Scientific, Inc.). Reverse
transcription-quantitative (RT-q)PCR was performed using the
Biomark HD system (Fluidigm Corporation) with specific TaqMan Gene
Expression assays for hypoxanthine phosphoribosyltransferase
(Hprt1; Rn01527840_m1), desmin (Rn00574732_m1), aquaporin 1
(Aqp1; Rn00562834_m1), nephrin (Rn00674268_m1), podocin
(Rn00709834_m1), and regulator of calcineurin 1 (Rcan1;
Rn00596606_m1) (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. For analysis of NVs from ZDF and ZL rats,
mRNA was extracted from the isolated NVs using
oligo(dT)-immobilized microplates (Hitachi Chemical Diagnostics,
Inc.) and quantified by RT-qPCR as previously described (11). All mRNA levels were normalized to the
level of Hprt1.
Isolation of glomeruli
Rats were anesthetized using 5% sevoflurane via an
inhalation anesthetic system, and the kidneys were immediately
excised following euthanasia by exsanguination. After removal of
kidney capsules, renal cortexes were minced into very fine
fragments. Glomeruli were collected using standard sieving methods
as previously described (12). Total
RNA was isolated from glomeruli was purified using a RNeasy micro
kit, and RT-qPCR was performed using the Biomark HD system for
specific TaqMan Gene Expression assays as described above.
Immunohistochemistry
Kidneys were collected from euthanized animals.
These samples were immediately fixed in 10% neutralized buffered
formalin for 1 week at room temperature. Fixed tissues were then
embedded in paraffin for immunohistochemical analysis.
Immunostaining of desmin was performed as described previously
(13). Briefly, paraffin sections
were deparaffinized and incubated overnight at 4˚C with an
anti-desmin mouse monoclonal antibody (1:200; cat. no. M0760; Dako;
Agilent Technologies, Inc.), and subsequently incubated for 30 min
at room temperature with horseradish peroxidase-conjugated
anti-mouse IgG goat polyclonal antibody (Nichirei Biosciences
Inc.).
Statistical analysis
Data are presented as mean ± standard error of the
mean unless otherwise stated. A Student's t-test was used for
pairwise comparisons. When comparing >2 groups, statistical
differences were evaluated using a one-way ANOVA followed by a
Dunnett's multiple comparison test. Repeated measure-based
parameters were evaluated using a two-way ANOVA followed by
Bonferroni's correction. P<0.05 was considered to indicate a
statistically significant difference. For correlation analysis,
Pearson correlation coefficients were calculated. All statistical
analyses were performed using GraphPad Prism version 7.04 (GraphPad
Software, Inc.).
Results
Characterization of urinary NVs in the
PAN nephritis rat model
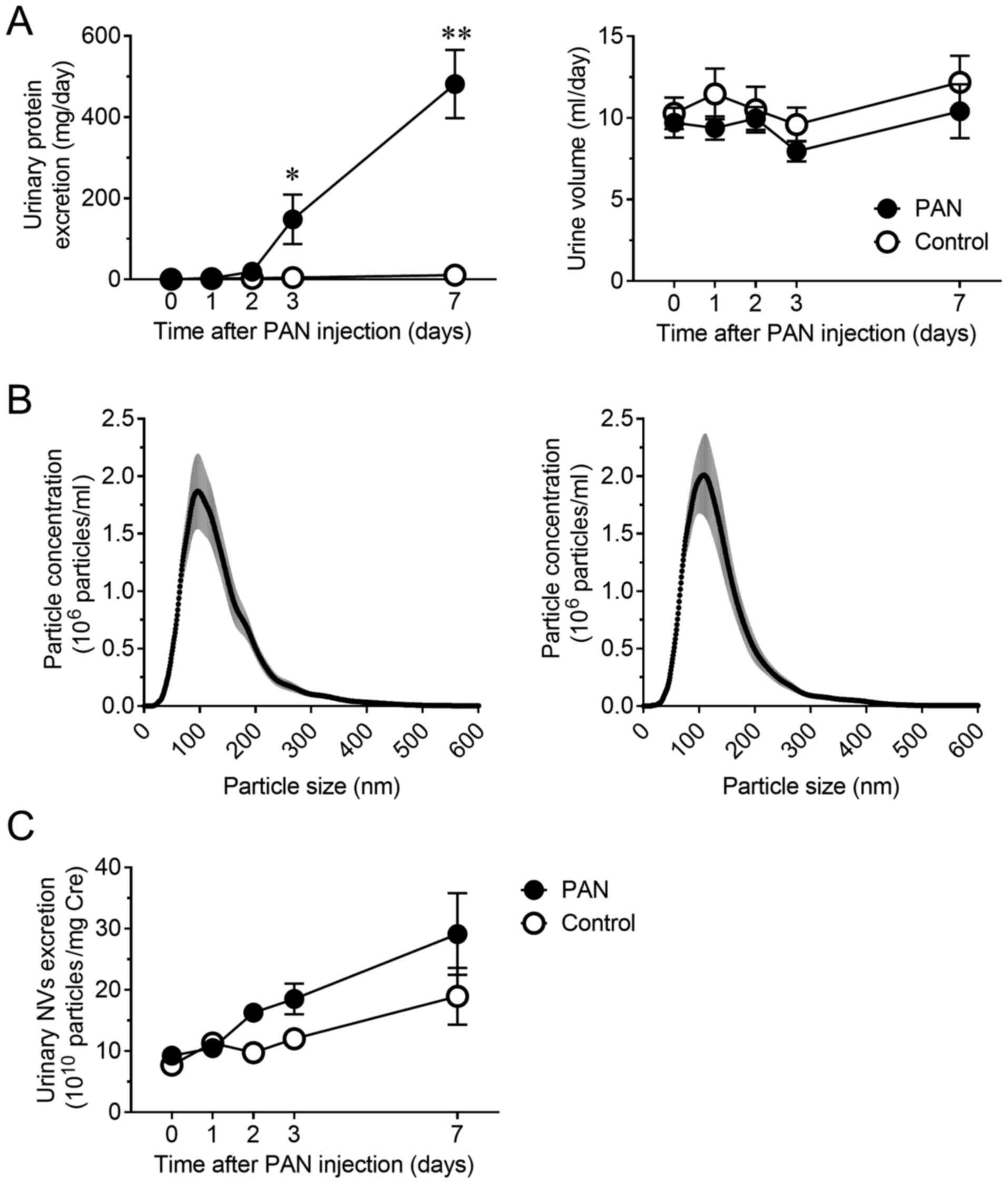
Notable proteinuria developed 3 days after the
induction of PAN nephritis in the rat model. Urinary protein
excretion significantly increased at days 3 and 7 (P<0.05 and
P<0.01, respectively) without a significant change in urine
volume (Fig. 1A). The size
distribution and concentration of NVs in urine were assessed using
NTA to confirm the presence of NVs and compare the vesicle profiles
between normal and diseased animals. As previously reported
(14,15), most of the obtained vesicles were
<200 nm in diameter. The size distribution of NVs was not
altered (Fig. 1B), but the
concentration of urinary NVs was slightly increased in the PAN
nephritis model, even though the difference was not significant
(Fig. 1C).
Changes in mRNA levels of urinary NVs
and glomeruli in the PAN nephritis rat model
Whether urinary NVs could indicate site-specific
damages in glomeruli were next determined. In fact, PAN is an
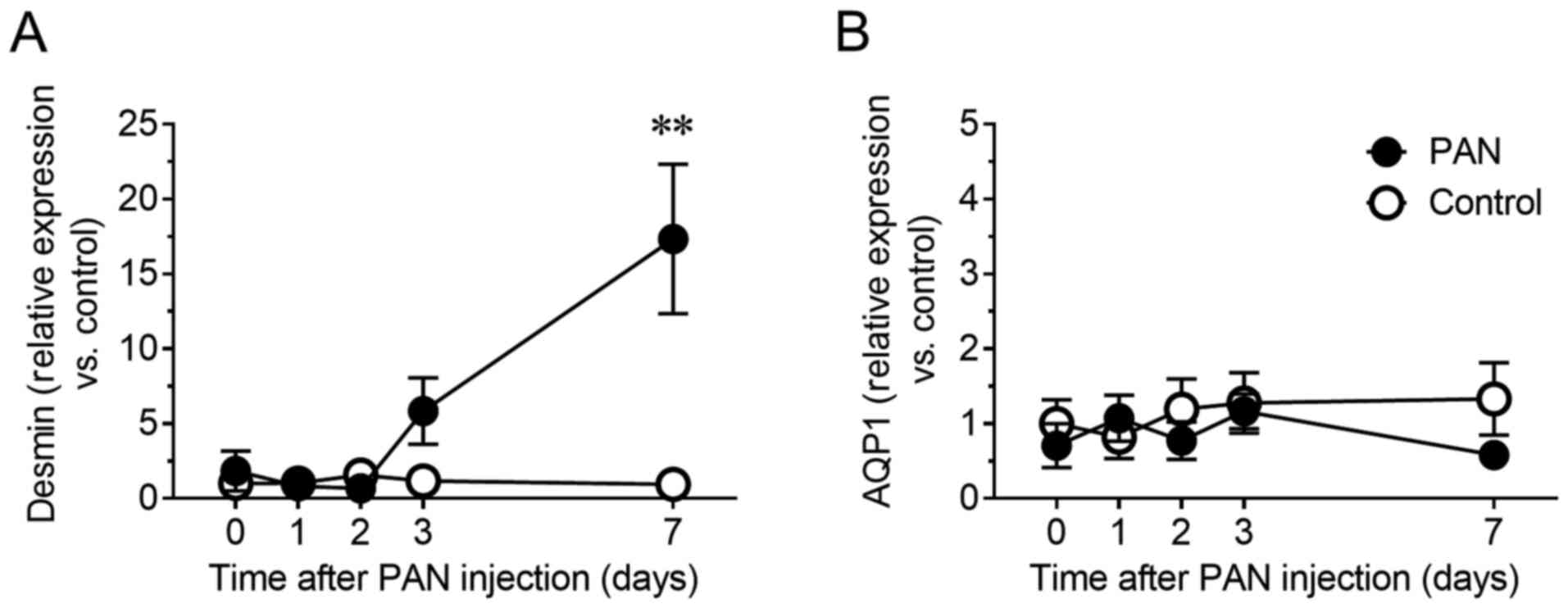
antibiotic well known to cause glomerular specific damage (16). Therefore, the mRNA levels of desmin,
a sensitive biomarker for glomerular injury in rodents (17,18),
were evaluated in urinary NVs. The relative content of desmin mRNA
in urinary NVs of the PAN nephritis model increased 5.8-fold
(P=0.44) on day 3 and 17.3-fold (P<0.01) on day 7 (Fig. 2A). To confirm whether this change
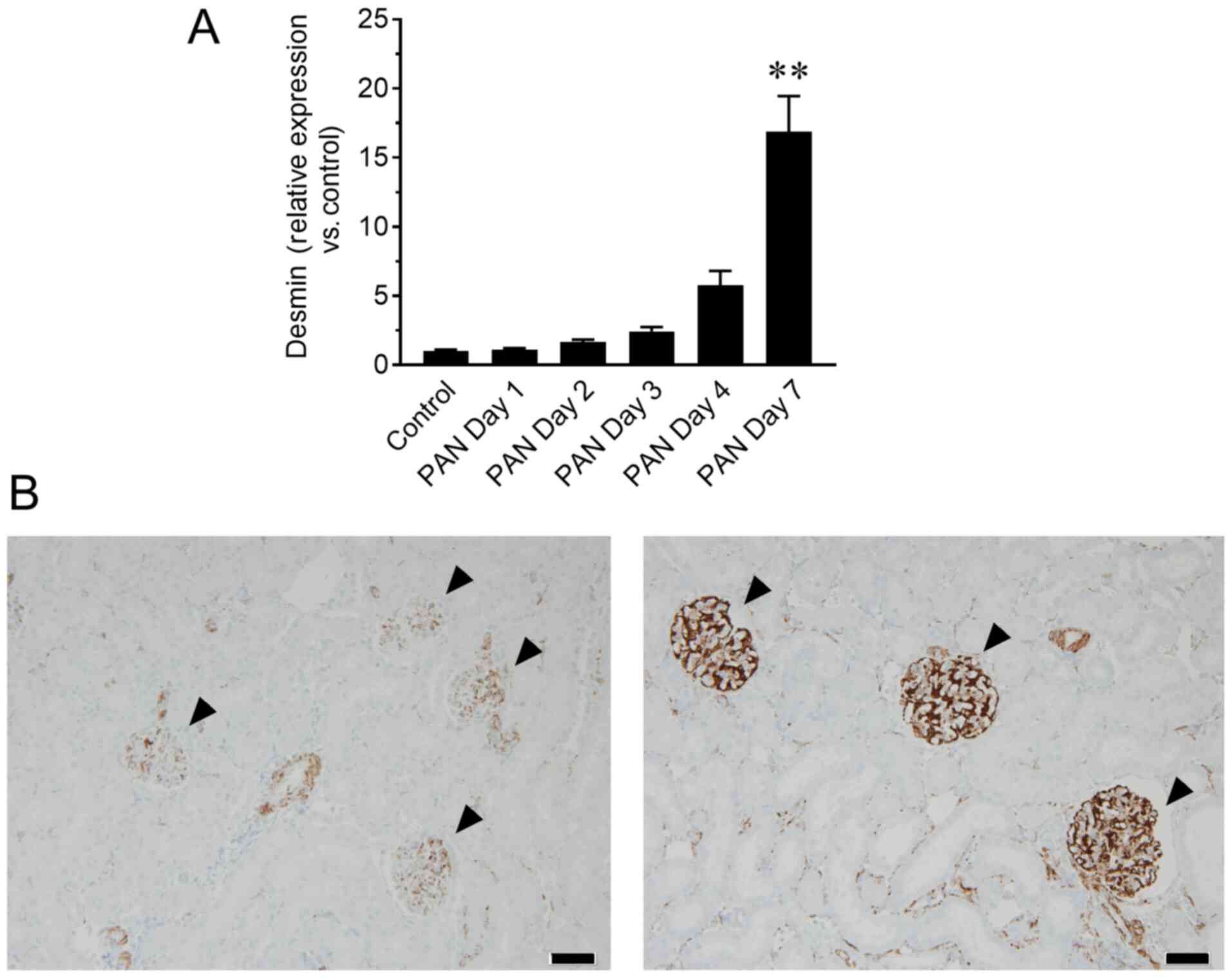
reflected the status of kidney tissue, mRNA and protein levels of
desmin in the glomeruli were assessed by RT-qPCR and
immunohistochemistry, respectively. The mRNA levels of desmin in
glomeruli showed a similar increasing trend to that of urinary NVs,
particularly on day 7 (P<0.01; Fig.
3A), whereas increased desmin immunoreactivity in glomeruli was
observed in the PAN nephritis model (Fig. 3B), corroborating the observations
from urinary NVs. Meanwhile, the mRNA levels of Aqp1, a
proximal tubular marker, in urinary NVs was not altered in the PAN
nephritis model (Fig. 2B).
Analysis of urinary NV mRNAs as
pharmacological biomarkers
To evaluate the potential of mRNAs in urinary NVs as
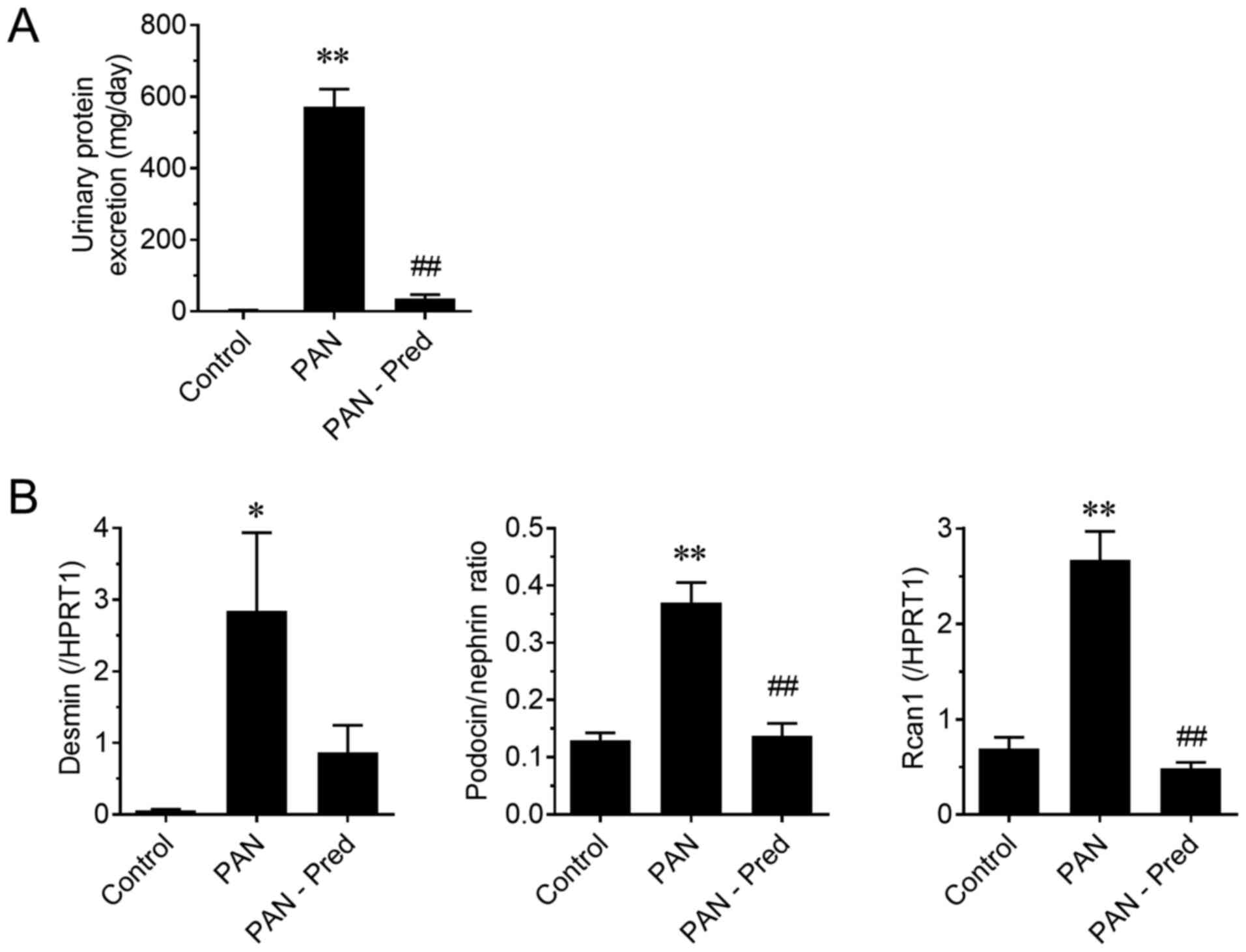
pharmacological biomarkers, the effect of prednisolone on the PAN
nephritis model was assessed. The induced urinary protein excretion
of the PAN nephritis model was significantly mitigated by treatment
with prednisolone (P<0.01; Fig.
4A), confirming previously reported observations (19). Fig. 4B
shows the effect of prednisolone on the mRNA levels of urinary NVs.
The expression of two podocyte injury markers, the mRNA levels of
desmin and the podocin to nephrin ratio (PNR), the latter being a
potential podocyte loss prediction marker reported by Fukuda et
al (20), significantly
increased in the PAN nephritis model (P<0.01), whereas a
decreasing tendency and a significant decrease in the
prednisolone-treated group was observed (P=0.10 and P<0.01,
respectively). The mRNA levels of Rcan1, which is
upregulated during active calcineurin signaling and is therefore
considered a target of immunosuppressants for nephritis treatment
(21), also increased in urinary NVs
of the PAN nephritis model (P<0.01), and it was reversed by
prednisolone treatment (P<0.01). Additionally, the correlations
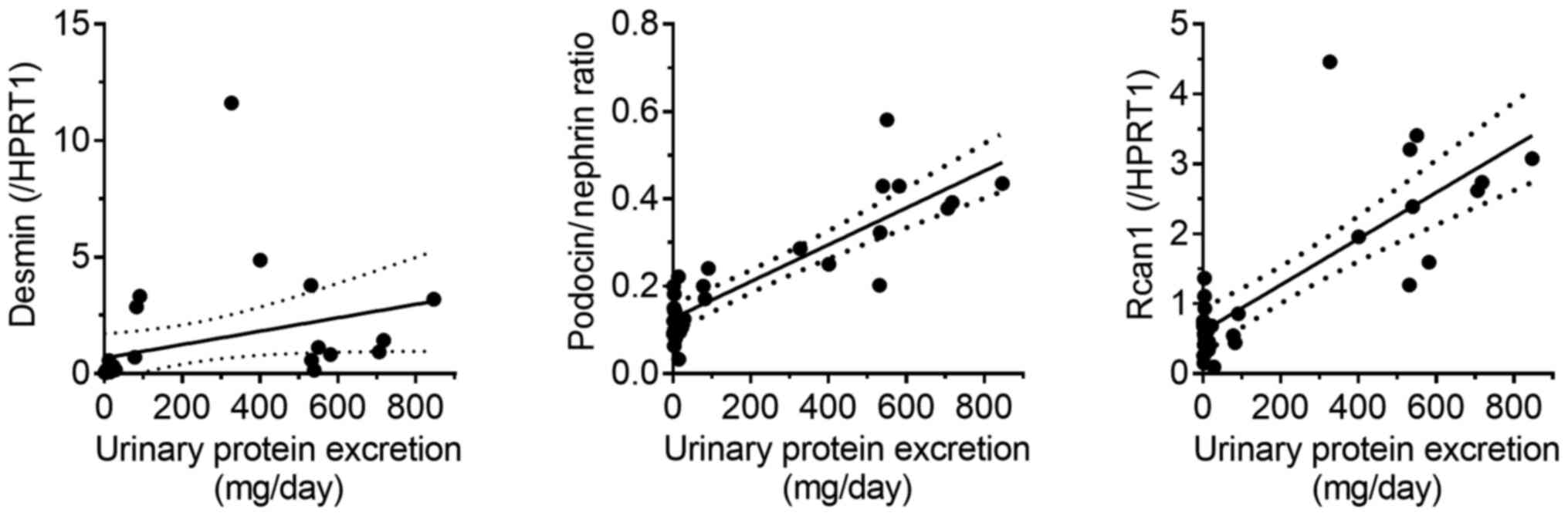
between mRNA levels and disease severity were assessed (Fig. 5). A positive correlation was observed
between PNR and urinary protein excretion, as well as between
Rcan1 levels and urinary protein excretion
(r2=0.75 and 0.64, respectively; P<0.01 in both
cases). A similar but weaker correlation was observed between
desmin mRNA levels and urinary protein excretion
(r2=0.12; P=0.07).
PNR in ZDF the kidney disease
model
The relationship between kidney functionality and
PNR in another type of kidney disease model was assessed. ZDF rats,
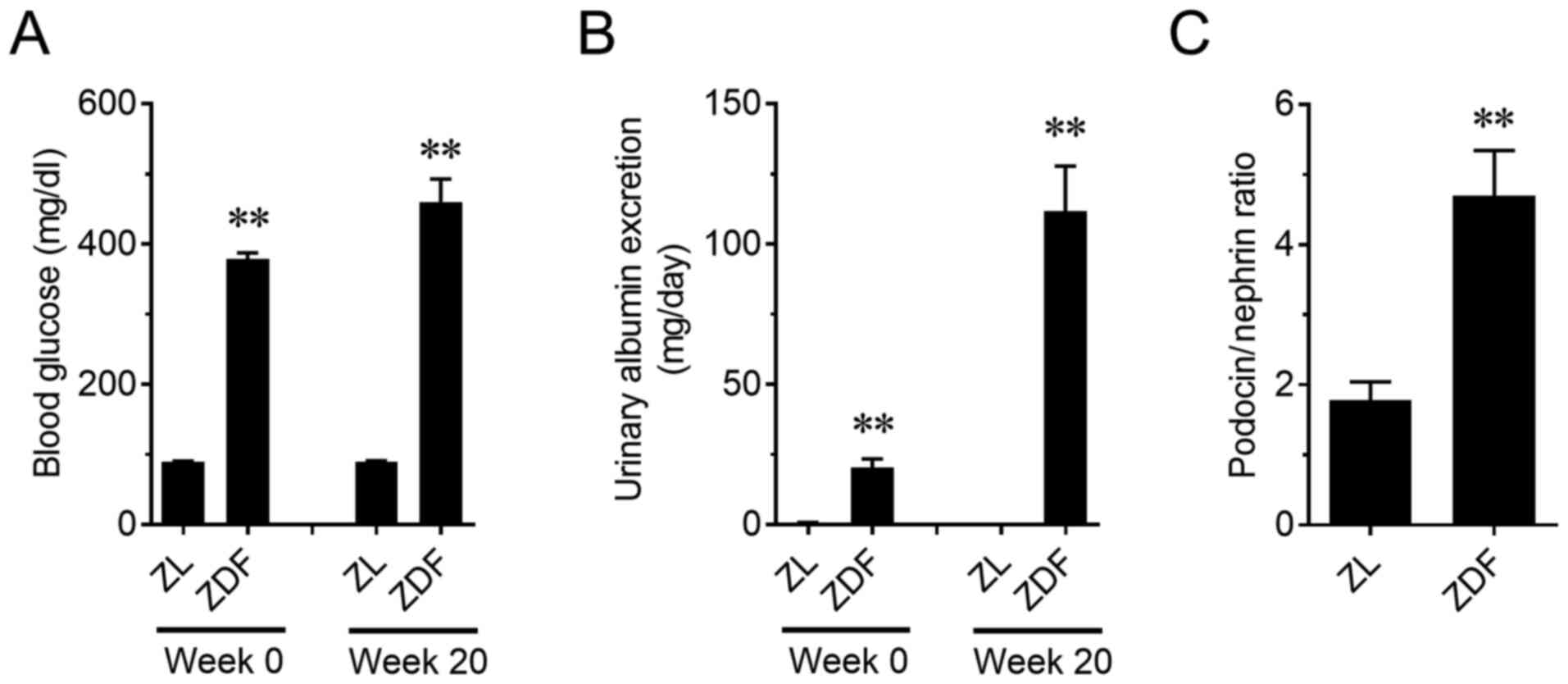
a type 2 diabetes model, showed a significant increase in blood
glucose on weeks 0 and 20 (P<0.01; Fig. 6A). A progressive increase in urinary
albumin excretion was also observed in ZDF rats (P<0.01;
Fig. 6B). In addition, PNR in ZDF
rats on week 20 increased compared to that in the ZL rats
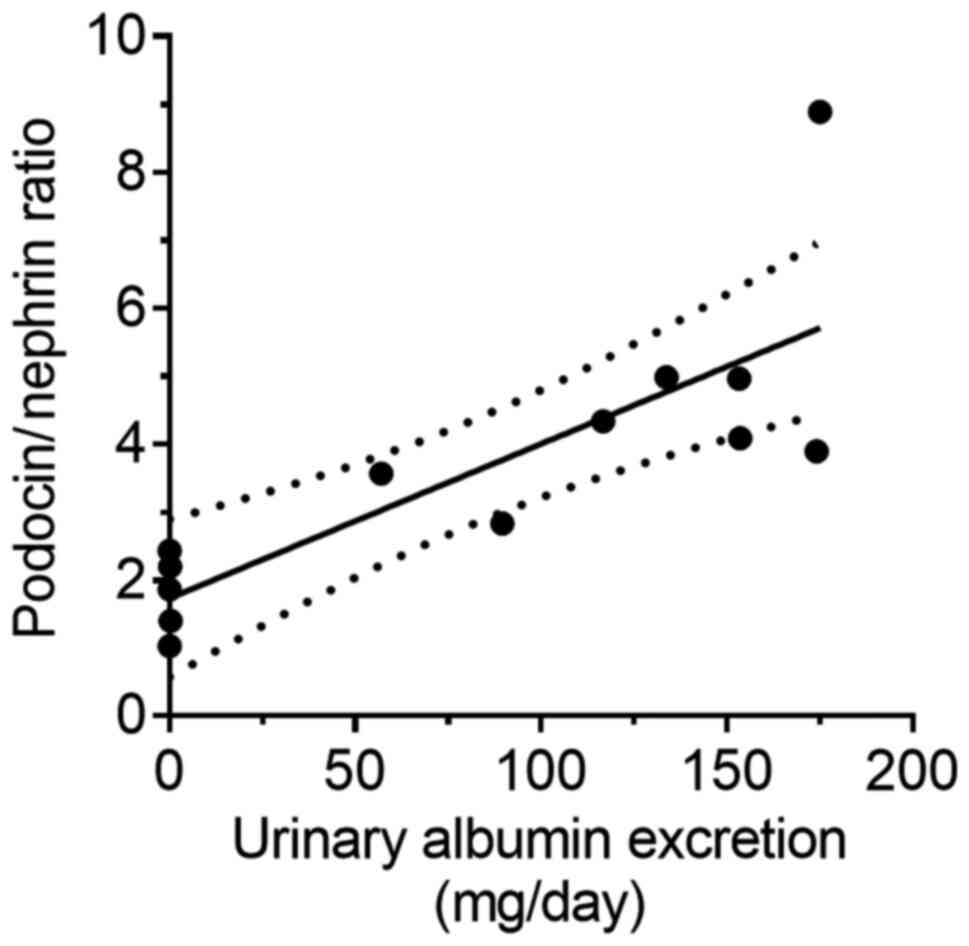
(P<0.01; Fig. 6C). Furthermore,
PNR was positively correlated with urinary albumin excretion,
similar to the PAN nephritis model (r2=0.66; P<0.01;
Fig. 7).
Discussion
Liquid biopsy of blood using NVs has been actively
investigated for oncology, in particular for breast cancer
(22), lung cancer (23) and pancreatic (24) cancer. However, there is relatively
little knowledge regarding the feasibility of liquid biopsy using
NVs as biomarkers for renal disease. Various reports on the
validity of urinary NVs for risk prediction of renal disease have
highlighted urinary exosomal microRNAs as potential biomarkers for
lupus nephritis (25), renal
fibrosis (26,27) and early renal injury in essential
hypertension (28). Moreover, it has
been reported that urinary exosomal Wilms tumor 1 mRNA, which codes
for a podocyte-derived transduction factor, is a candidate
biomarker for diabetic nephropathy (29). Another group also suggested the
potential of using exosomal mRNA levels of C-C motif chemokine
ligand 2 as a diagnostic tool for IgA nephropathy (30). However, it is still unclear whether
changes in the mRNA levels of kidney injury markers in urinary NVs
are linked to the actual status of renal disease. In the present
study, the mRNA levels of desmin and PNR as podocyte injury
markers, as well as the mRNA levels of Rcan1 as a pathogenic
marker, using urinary NVs were assessed, and their applicability as
biomarkers for renal dysfunction and injury were demonstrated.
NVs were isolated by ultracentrifugation. Although
it was reported that the amount and profile of the obtained NVs
should depend on the isolation method (31), the size of the particles isolated in
the present study was similar to that described in previous reports
(14,15), indicating successful NV purification.
No changes were detected in urinary NV excretion or particle size
profiles in the PAN nephritis model. On the contrary, increased
levels of desmin mRNA in urinary NVs reflected similar changes in
glomeruli. However, there was no variation in the mRNA levels of
Aqp1, known as a tubular marker, suggesting that tubules
were not directly injured in the PAN nephritis model. Aqp1
expression has been reported to be decreased in an
ischemia/reperfusion-induced acute kidney injury model in rats and
during kidney transplantation in humans (32), consistent with the podocyte-specific
toxicity of PAN (33). Moreover,
Spanu et al (34) reported
that the mRNA levels of cystatin C in urinary NVs was correlated
with renal cortical expression and urinary cystatin C protein
levels (34). Taking the results of
the present study and those of previous reports together, it is
hypothesized that changes in mRNA levels of urinary NVs reflect
alterations in gene expression in the component cells of the kidney
organ, and thus the analysis of urinary NVs can provide information
on the pathological state of renal tissues in a non-invasive
manner.
The applicability of urinary NVs as pharmacological
biomarkers was also examined. The PNR increased in the PAN
nephritis model, and was significantly decreased upon treatment
with prednisolone. PNR in the urinary sediment was previously
reported as a promising biomarker of podocyte stress in glomeruli
(20,35), since shifts in PNR are thought to be
due to alterations occurring in podocytes. The results of the
present study are consistent with these reports, implying that the
pharmacological effects of a drug for podocyte protection can be
detected by analyzing isolated urinary NVs.
In addition, the mRNA levels of Rcan1 in
urinary NVs were increased in the PAN nephritis model, whereas such
increases were compensated by prednisolone treatment. The
expression of Rcan1 has been reported to be induced through
the activation of calcineurin/nuclear factor of activated T cells
signaling, and the activation of this cascade can cause several
kidney diseases, such as minimal change disease (36) and glomerulosclerosis (37). Calcineurin is known as a target of
cyclosporine and tacrolimus, two common therapeutic agents for
glomerular nephritis (21).
Moreover, Ding et al (38)
reported that calcineurin activity was upregulated by PAN treatment
of podocytes in vitro, and it was suggested that calcineurin
inhibitors protect against podocyte injury in a PAN-induced
nephritis model (39). In addition,
it has been reported that prednisolone and other
glucocorticosteroids decrease the activity of calcineurin (40,41).
Therefore, it is concluded that the levels of urinary NV
Rcan1 can be used as a biomarker to monitor kidney injury in
a non-invasive manner.
Moreover, experiments using ZDF rats, a type 2
diabetes mellitus model, were performed to investigate the
applicability of urinary NV mRNA analysis in another type of kidney
disease. Similar to the PAN nephritis model, ZDF rats display
albuminuria with glomerular and podocyte injuries (42). As observed in the PAN nephritis
model, an increased PNR in urinary NVs was observed, and a
correlation between PNR and urinary albumin excretion was also
observed in ZDF rats. These results suggest that PNR in urinary NVs
can be considered a useful biomarker for renal dysfunction linked
to glomerular injuries, and demonstrates the versatility of urinary
NVs as diagnostic tools in various types of kidney diseases.
In conclusion, the present study showed that changes
in mRNA levels of urinary NVs may serve as reliable predictors of
physiological and pathological alterations in the kidney. Based on
these results, it is suggested that this method should be validated
further and potentially used as a liquid biopsy tool for kidney
disease.
Acknowledgements
The authors are grateful to Professor Akiyoshi
Fukamizu (University of Tsukuba) for meaningful discussions. We
would also like to thank Dr K Kikkawa, D A Umeda, Dr N Shirata, Mr.
T Iguchi (Mitsubishi Tanabe Pharma Corporation) and Dr M Obana
(Osaka university) for their support.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KF and KA designed the study and performed the
experiments, analyzed data as well as drafted and finalized the
manuscript. TM performed experiments and was involved in data
analysis, interpretation of the results and manuscript preparation.
MT and TK performed the experiments. HM was involved in the design
of the study. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the institutional guidelines and approved in advance by the
Committee for Animal Experiments of Mitsubishi Tanabe Pharma
Corporation (approval nos. AJ12-0869, AJ14-0783 and AJ14-0827).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang V, Vilme H, Maciejewski ML and
Boulware LE: The economic burden of chronic kidney disease and
end-stage renal disease. Semin Nephrol. 36:319–330. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Takemoto Y and Naganuma T: Economic issues
of chronic kidney disease and end-stage renal disease. Contrib
Nephrol. 198:87–93. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Glassock RJ, Warnock DG and Delanaye P:
The global burden of chronic kidney disease: Estimates, variability
and pitfalls. Nat Rev Nephrol. 13:104–114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Levin A and Stevens PE: Early detection of
CKD: The benefits, limitations and effects on prognosis. Nat Rev
Nephrol. 7:446–457. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rysz J, Gluba-Brzózka A, Franczyk B,
Jabłonowski Z and Ciałkowska-Rysz A: Novel biomarkers in the
diagnosis of chronic kidney disease and the prediction of its
outcome. Int J Mol Sci. 18(1702)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hessvik NP and Llorente A: Current
knowledge on exosome biogenesis and release. Cell Mol Life Sci.
75:193–208. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Braun F and Muller RU: Urinary
extracellular vesicles as a source of biomarkers reflecting renal
cellular biology in human disease. Methods Cell Biol. 154:43–65.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Palma G, Di Lorenzo VF, Krol S and
Paradiso AV: Urinary exosomal shuttle RNA: Promising cancer
diagnosis biomarkers of lower urinary tract. Int J Biol Markers.
34:101–107. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gardiner C, Ferreira YJ, Dragovic RA,
Redman CW and Sargent IL: Extracellular vesicle sizing and
enumeration by nanoparticle tracking analysis. J Extracell Vesicles
2: 2013.
|
|
11
|
Murakami T, Oakes M, Ogura M, Tovar V,
Yamamoto C and Mitsuhashi M: Development of glomerulus-, tubule-,
and collecting duct-specific mRNA assay in human urinary exosomes
and microvesicles. PLoS One. 9(e109074)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li B, Yao J, Morioka T and Oite T: Nitric
oxide increases albumin permeability of isolated rat glomeruli via
a phosphorylation-dependent mechanism. J Am Soc Nephrol.
12:2616–2624. 2001.PubMed/NCBI
|
|
13
|
Kakimoto T, Okada K, Hirohashi Y, Relator
R, Kawai M, Iguchi T, Fujitaka K, Nishio M, Kato T, Fukunari A and
Utsumi H: Automated image analysis of a glomerular injury marker
desmin in spontaneously diabetic Torii rats treated with losartan.
J Endocrinol. 222:43–51. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Baranyai T, Herczeg K, Onodi Z, Voszka I,
Modos K, Marton N, Nagy G, Mager I, Wood MJ, El Andaloussi S, et
al: Isolation of exosomes from blood plasma: Qualitative and
quantitative comparison of ultracentrifugation and size exclusion
chromatography methods. PLoS One. 10(e0145686)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH,
Chen C, Li H, Li P, Quinn D, Dao M, et al: Isolation of exosomes
from whole blood by integrating acoustics and microfluidics. Proc
Natl Acad Sci USA. 114:10584–10589. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pippin JW, Brinkkoetter PT, Cormack-Aboud
FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM,
Marshall CB, Ohse T and Shankland SJ: Inducible rodent models of
acquired podocyte diseases. Am J Physiol Renal Physiol.
296:F213–F229. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Floege J, Alpers CE, Sage EH, Pritzl P,
Gordon K, Johnson RJ and Couser WG: Markers of complement-dependent
and complement-independent glomerular visceral epithelial cell
injury in vivo. Expression of antiadhesive proteins and
cytoskeletal changes. Lab Invest. 67:486–497. 1992.PubMed/NCBI
|
|
18
|
Hoshi S, Shu Y, Yoshida F, Inagaki T,
Sonoda J, Watanabe T, Nomoto K and Nagata M: Podocyte injury
promotes progressive nephropathy in zucker diabetic fatty rats. Lab
Invest. 82:25–35. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yatsu T, Aoki M and Tanaka A: Effect of
zelandopam, a dopamine D1-like receptor agonist, in puromycin
aminonucleoside nephrosis rats. Eur J Pharmacol. 510:121–126.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fukuda A, Wickman LT, Venkatareddy MP,
Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE and
Wiggins RC: Angiotensin II-dependent persistent podocyte loss from
destabilized glomeruli causes progression of end stage kidney
disease. Kidney Int. 81:40–55. 2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Spurney RF: Non-immunologic actions of
calcineurin inhibitors in proteinuric kidney diseases. Front
Endocrinol (Lausanne). 5(181)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alimirzaie S, Bagherzadeh M and Akbari MR:
Liquid biopsy in breast cancer: A comprehensive review. Clin Genet.
95:643–660. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cui S, Cheng Z, Qin W and Jiang L:
Exosomes as a liquid biopsy for lung cancer. Lung Cancer.
116:46–54. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nuzhat Z, Kinhal V, Sharma S, Rice GE,
Joshi V and Salomon C: Tumour-derived exosomes as a signature of
pancreatic cancer-liquid biopsies as indicators of tumour
progression. Oncotarget. 8:17279–17291. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Perez-Hernandez J, Forner MJ, Pinto C,
Chaves FJ, Cortes R and Redon J: Increased urinary exosomal
MicroRNAs in patients with systemic lupus erythematosus. PLoS One.
10(e0138618)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H,
Chen PS and Liu BC: MicroRNA-29c in urinary exosome/microvesicle as
a biomarker of renal fibrosis. Am J Physiol Renal Physiol.
305:F1220–F1227. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chun-Yan L, Zi-Yi Z, Tian-Lin Y, Yi-Li W,
Bao L, Jiao L and Wei-Jun D: Liquid biopsy biomarkers of renal
interstitial fibrosis based on urinary exosome. Exp Mol Pathol.
105:223–228. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Perez-Hernandez J, Olivares D, Forner MJ,
Ortega A, Solaz E, Martinez F, Chaves FJ, Redon J and Cortes R:
Urinary exosome miR-146a is a potential marker of albuminuria in
essential hypertension. J Transl Med. 16(228)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Abe H, Sakurai A, Ono H, Hayashi S,
Yoshimoto S, Ochi A, Ueda S, Nishimura K, Shibata E, Tamaki M, et
al: Urinary exosomal mRNA of WT1 as diagnostic and prognostic
biomarker for diabetic nephropathy. J Med Invest. 65:208–215.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Feng Y, Lv LL, Wu WJ, Li ZL, Chen J, Ni
HF, Zhou LT, Tang TT, Wang FM, Wang B, et al: Urinary exosomes and
exosomal CCL2 mRNA as biomarkers of active histologic injury in IgA
nephropathy. Am J Pathol. 188:2542–2552. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
He L, Zhu D, Wang J and Wu X: A highly
efficient method for isolating urinary exosomes. Int J Mol Med.
43:83–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sonoda H, Yokota-Ikeda N, Oshikawa S,
Kanno Y, Yoshinaga K, Uchida K, Ueda Y, Kimiya K, Uezono S, Ueda A,
et al: Decreased abundance of urinary exosomal aquaporin-1 in renal
ischemia-reperfusion injury. Am J Physiol Renal Physiol.
297:F1006–F1016. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xia L, Zhou M, Kalhorn TF, Ho HT and Wang
J: Podocyte-specific expression of organic cation transporter PMAT:
Implication in puromycin aminonucleoside nephrotoxicity. Am J
Physiol Renal Physiol. 296:F1307–F1313. 2009.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Spanu S, van Roeyen CR, Denecke B, Floege
J and Muhlfeld AS: Urinary exosomes: A novel means to
non-invasively assess changes in renal gene and protein expression.
PLoS One. 9(e109631)2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fukuda A, Wickman LT, Venkatareddy MP,
Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA and Wiggins RC: Urine
podocin: Nephrin mRNA ratio (PNR) as a podocyte stress biomarker.
Nephrol Dial Transplant. 27:4079–4087. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhang K, Sun W, Zhang L, Xu X, Wang J and
Hong Y: miR-499 ameliorates podocyte injury by targeting
calcineurin in minimal change disease. Am J Nephrol. 47:94–102.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang Y, Jarad G, Tripathi P, Pan M,
Cunningham J, Martin DR, Liapis H, Miner JH and Chen F: Activation
of NFAT signaling in podocytes causes glomerulosclerosis. J Am Soc
Nephrol. 21:1657–1666. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ding F, Li X, Li B, Guo J, Zhang Y and
Ding J: Calpain-mediated cleavage of calcineurin in puromycin
aminonucleoside-induced podocyte injury. PLoS One.
11(e0155504)2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Shen X, Jiang H, Ying M, Xie Z, Li X, Wang
H, Zhao J, Lin C, Wang Y, Feng S, et al: Calcineurin inhibitors
cyclosporin A and tacrolimus protect against podocyte injury
induced by puromycin aminonucleoside in rodent models. Sci Rep.
6(32087)2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sipka S, Szücs K, Szántó S, Kovács I,
Lakos G, Antal-Szalmás P, Szegedi G and Gergely P: Inhibition of
calcineurin activity and protection against cyclosporine A induced
cytotoxicity by prednisolone sodium succinate in human peripheral
mononuclear cells. Immunopharmacology. 48:87–92. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sipka S, Szucs K, Szántó S, Kovács I,
Lakos G, Kiss E, Antal-Szalmás P, Szegedi G and Gergely P:
Glucocorticosteroid dependent decrease in the activity of
calcineurin in the peripheral blood mononuclear cells of patients
with systemic lupus erythematosus. Ann Rheum Dis. 60:380–384.
2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Funk J, Ott V, Herrmann A, Rapp W, Raab S,
Riboulet W, Vandjour A, Hainaut E, Benardeau A, Singer T and
Jacobsen B: Semiautomated quantitative image analysis of glomerular
immunohistochemistry markers desmin, vimentin, podocin,
synaptopodin and WT-1 in acute and chronic rat kidney disease
models. Histochem Cell Biol. 145:315–326. 2016.PubMed/NCBI View Article : Google Scholar
|