Introduction
Over the past 10-15 years, scientific interest in
microRNAs (miRNA/miRs) and their roles as modern, discriminatory
biomarkers has grown exponentially (1-3).
Now, this small (18-24 nucleotide) non-coding molecule encapsulates
one of the most thriving, sought after areas of research. MiRNAs
have been implicated in several disease processes, particularly in
oncology, with the potential to facilitate diagnosis and predict
disease development earlier than is currently possible (4-7).
Furthermore, miRNAs have been suggested to confer overall
prognosis, survival, drug sensitivity, treatment response and aid
monitoring for disease progression and relapse (1,8-14).
A growing body of literature is additionally emerging on the role
of specific placental-derived miRNAs in pregnancy, particularly
those complicated by processes such as gestational diabetes
(15,16), gestational hypertension (17,18),
pre-eclampsia (19-21)
or congenital abnormalities (22,23).
miRNAs in biofluids, in particular serum and plasma
derived from peripheral blood, are an attractive option for
clinical biomarker development, as they are readily available,
relatively non-invasive to obtain and widely processed in standard
laboratory settings (24-29).
Compared with other potential biomarkers, miRNAs are excellent
candidates, due to their stable expression both in vivo
across a range of tissues and biofluids (26,30-32),
and ex vivo during differing storage conditions, being able
to withstand ~48 h at room temperature or on ice (26,30),
longer-term storage at -80˚C (33,34) and
their ability to undergo multiple (between 4-8) freeze-thaw cycles
(26,30,31).
Furthermore, due to the wide range of downstream miRNA targets,
with each mature miRNA being able to target ~200 mRNAs to exert
their effects (9); their potential
therapeutic capacity is simply vast (35).
In human pregnancy, there are three primary
chromosomal miRNA clusters located on chromosome 19 (C19MC,
miR-371-3) and chromosome 14 (C14MC) which are highly and widely
expressed in placental tissue and as circulating markers, with the
expression profile of these clusters varying during trimesters and
gestational disease processes (21,36).
MiRNAs are released from the trophoblastic layer into the maternal
circulation in various forms, including microvesicle-enveloped, in
apoptotic bodies, exosomally or as protein bound miRNAs (37-39),
in order to avoid digestion by circulating RNAase enzymes (30,40-42).
Several commercially available miRNA isolation kits using serum and
plasma capture the whole biofluid, including the exosomal
component; the latter only representing a subset of the miRNAs
found in this media (43-45).
Although the discovery and validation of miRNA
biomarkers remains at an experimental, pre-clinical stage, there is
a growing body of literature concerning the role of miRNAs in
specific pregnancy-associated conditions. However, there is little
consensus regarding the optimal starting media or justification of
the selection between serum or plasma (15,16,18,46).
Most studies fail to acknowledge the potential differences in miRNA
concentrations and profiles that may emerge when using serum vs.
plasma as the starting biofluid (25-27,33,47-49),
potentially inhibiting their successful applications in a clinical
setting (26,27,47,48).
Furthermore, several studies refer to serum or plasma
interchangeably (25,41), whereas in fact they are quite
different: Serum refers to the cell-free blood component obtained
following the activation of platelets and factors within the
coagulation cascade, whereas plasma refers to the cell-free blood
component obtained prior to the coagulation process, hence
collection tubes contain anticoagulants (EDTA or sodium citrate for
downstream miRNA analysis) which inhibit the coagulation cascade
(50). Heparinised tubes cannot be
used for miRNA analysis as heparin inhibits downstream enzymatic
reactions within the cDNA synthesis and reverse transcription (RT)
steps of quantitative (q)PCR (33,45,49). The
coagulation process itself can significantly alter a sample's miRNA
profile and is a source of variability (for example, coagulation
time and temperature) (27,33,47,49,50),
which cannot be reliably controlled when using serum samples,
leading several researchers to preferentially use plasma.
Additionally, the coagulation process causes cell lysis and
haemolysis, particularly from erythrocytes but also platelets,
releasing RNA and miRNA into the serum, which affects the profile
obtained (27,47,48). It
is essential to acknowledge that haemolysis can also occur in both
serum and plasma samples at the time of venepuncture, hence the
need for standardised techniques to minimise this occurrence,
including the use of larger gauge needles and well-trained
phlebotomists (51). For these
reasons, haemolysis monitoring is a part of the standard quality
control steps of sample preparation, either involving
spectrophotometric or specific miRNA analysis (33,52-54).
Equally, the platelet content of plasma is an important
consideration, given that their rich miRNA content may bias the
determined outcome (24,27,47-49),
highlighting the importance of two centrifugation steps during
sample preparation to generate platelet-poor as opposed to
platelet-rich plasma (7,49,55). One
potential issue faced by miRNA researchers using pre-collected
samples is that the majority of archived blood samples have been
collected as serum.
The optimum starting media is also crucially
dependent upon the patient population under study, for example
whether the individuals are healthy or diseased; pregnant or
non-pregnant. Different starting media may be more appropriate for
specific patient types, which explains the contradiction between
existing studies suggesting that either serum (studies involving
healthy males, females and pregnant women) (25,27) or
plasma (research involving patients with primary liver cancer or
benign liver disease) (25,47) are superior.
To the best of our knowledge, there is only one
comparable study examining the difference in miRNA concentrations
and profiles between serum and plasma derived samples taken from
pregnant women (25). However, this
previous study analysed only three paired serum and plasma samples
at one time point in pregnancy (second trimester) and generated
conflicting outcomes dependent on whether the proportion or
absolute number of detected miRNAs was measured. This is a key
starting point which must be established before miRNA biomarkers
can be reliably pinpointed as hallmarks of gestational disease
states. Therefore, the aim of the present study was to determine
the optimum starting media at three key time points both intra and
post-partum, involving women with an uncomplicated, healthy
pregnancy.
Patients and methods
Sample collection and RNA
isolation
Blood samples were obtained from 10 pregnant women
(median age 29.5 years, range 22-34 years) with uncomplicated,
low-risk pregnancies at the following time points: i) during the
second trimester of pregnancy (18-24 weeks) n=2; ii) during weeks
36-40 of the third trimester n=3; and iii) 6 weeks post-partum,
n=5. At each time point, blood was collected from each patient as
follows: 13.5 ml plasma in 3x4.5 ml sodium citrate vacutainer tubes
(NHS Supply Chain) and 5 ml serum in a 5 ml SST vacutainer tube
(NHS Supply Chain). Standard venepuncture procedures were followed,
according to the National Cancer Institute Early Detection Research
Network, which involved using a 21-gauge needle to minimise
haemolysis (51,56). All low-risk, healthy pregnant women
referred to the antenatal clinic at the Jessop Wing, Sheffield
Teaching Hospitals NHS Foundation Trust were eligible for inclusion
in this study. Pregnant women were excluded from recruitment if: i)
The pregnancy was dated >20 weeks gestation; ii) there was no
foetal heartbeat detected on ultrasound imaging, or iii) there were
known foetal anomalies. All samples were collected in the antenatal
clinic of the Jessop Wing Hospital between January and September
2017.
Following collection, samples were kept upright and
stored on ice (maintained at ~4˚C) to inhibit miRNA degradation by
circulating RNases within the whole blood, and were processed
within 4-6 h. Samples were centrifuged at 1,900 x g for 10 min at
4˚C and the recovered supernatant was aliquoted and immediately
stored at -80˚C. Following gently thawing at room temperature,
plasma samples underwent a second centrifugation step (16,000 x g
for 10 min at 4˚C) to generate platelet deficient plasma. Total RNA
(including miRNA) was extracted from serum and plasma samples using
a prototype version of the Maxwell® Rapid Sample
Concentrator (RSC) miRNA Tissue or Plasma Serum kit (cat. no.
AS1460; Promega Corporation) (57)
with minor modifications to the standard protocol. Specifically,
lyophilised DNase I was resuspended with 275 µl nuclease free
water, mixing gently through inversion. A total of 5 µl Blue Dye
was added to the reconstituted DNase I as a visual indicator,
swirling gently to mix. Aliquots were made and stored at 4˚C for a
few weeks, or at -20˚C for longer storage. Subsequently, 2.5 µl
UniSp2, UniSp4 and UniSp6 (reconstituted according to
manufacturer's instructions; Qiagen RNA spike in kit for RT; cat.
no. 339390; Qiagen, Inc.) (58) was
added to 200 µl binding buffer and mixed thoroughly. A total of 500
µl pre-processed plasma was transferred to a 1.5 ml Eppendorf and
60 µl Proteinase K (Promega Corporation) was added. This was then
combined with the binding buffer and spike-in mix, vortexing for 10
sec. This sample lysate was then incubated at 37˚C for 15 min.
During this time, the Maxwell® RSC cartridges were
prepared by removing their seals and loading them into the RSC deck
tray. An RSC plunger was added to well 8 of each cartridge, 500 µl
elution tubes were loaded into the deck and 60 µl nuclease-free
water was added to each tube. A total of 10 µl reconstituted DNAse
I was added to well 4 (yellow) of the cartridges, before the total
volume of incubated sample lysate was transferred into well 1 of
the cartridges. The Maxwell® RSC Instrument (cat. no.
AS4500: Promega Corporation) (59)
instrument and the ‘RSC miRNA Tissue' method (60) was used to initiate the automated
purification run. Following processing, the eluate was stored at
-80˚C. Extracted RNA was assessed for quality and quantity using an
Agilent Small RNA Chip and 2100 Bioanalyzer (Agilent Technologies
Deutschland GmbH), measuring miRNA percentage and concentration in
pg/µl.
Ethics approval
Ethical approval for the present study (approval no.
16/NE/0292) was obtained from the United Kingdom North East
Newcastle and North Tyneside 1 NHS Research Ethics Committee on
30/08/2016 and The Health Research Authority on 27/09/2016, with
non-substantial amendments approved on 18/10/2018 and 01/05/2019.
Written informed consent was obtained from all patients.
miRNA quantification
cDNA synthesis was performed on the 10 paired serum
and plasma samples (2 replicates per sample; n=20 plasma cDNA, n=20
serum cDNA) using the qScript® microRNA cDNA Synthesis
kit (Quantabio) according to the manufacturer's protocol, with one
minor modification during the initial PolyA tailing reaction,
specifically the addition of 1 µl cel-miR-30-3p and UniSp6 spike-in
mix (Qiagen, Inc.) reconstituted as per manufacturer's protocol. A
total of 6 µl extracted RNA was used in each 10 µl PolyA tailing
reaction. Reverse transcription-quantitative (RT-q)RT-qPCR with a
total reaction volume of 25 µl was performed as follows: 0.5 µl
PerfeCTa Universal Primer within the cDNA synthesis kit
(Quantabio), 12.5 µl ExiLENT SYBR® Green MasterMix
(Exiqon; Qiagen, Inc.), 2 µl cDNA, 9.5 µl nuclease-free water and
0.5 µl of each of the 8 miRNA primers. Two quality control primers
were used to assess the efficiency of RNA extraction (miRCURY LNA
UniSp2 PCR assay) and cDNA synthesis (miRCURY LNA cel-miR-39-3p)
(Qiagen, Inc.), whereas 4 miRNAs were chosen for the comparison
between these starting media; hsa-miR-222-3p, hsa-miR-23a,
hsa-miR-30e-5p and hsa-miR-451a (Integrated DNA Technologies, Inc.)
(Table I). These four miRNAs were
selected as they are known to be consistently and stably detected
in biofluids such as serum and plasma (25,33,47);
facilitating an assessment of their relative quantity.
hsa-let-7i-3p and hsa-miR-148-3p were chosen for normalisation. A
3-step cycling qPCR protocol: 95˚C for 2 min; followed
by 40 cycles of 95˚C for 5 sec, 60˚C for 15 sec and 70˚C for 15 sec
was used with a Bio-Rad CFX96, Real time C1000 Touch Thermal Cycler
(Bio-Rad Laboratories, Inc.). RT-qPCR involved no-template (NTC)
and no-Reverse Transcriptase controls (NRTC).
 | Table IDescription of the 8 miRNA primers
used for reverse transcription-quantitative PCR. |
Table I
Description of the 8 miRNA primers
used for reverse transcription-quantitative PCR.
| miRNA primer | Function | Sequence |
|---|
| UniSp2 | RNA extraction
efficiency | Unavailable |
| cel-miR-39-3p | cDNA synthesis
efficiency |
5'-UCACCGGGUGUAAAUCAGCUUG-3' |
| hsa-miR-451a | Stably expressed
miRNA of interest and detection of haemolysis |
5'-AAACCGUUACCAUUACUGAGUU-3' |
| hsa-miR-23a | Stably expressed
miRNA of interest and detection of haemolysis |
5'-AUCACAUUGCCAGGGAUUUCC-3' |
| hsa-let-7i-3p | Stably expressed
miRNA used for normalisation |
5'-CUGCGCAAGCUACUGCCUUGCU-3' |
| hsa-miR-148-3p | Stably expressed
miRNA used for normalisation |
5'-UCAGUGCAUCACAGAACUUUGU-3' |
| hsa-miR-222-3p | Stably expressed
miRNA of interest |
5'-AGCUACAUCUGGCUACUGGGU-3' |
| hsa-miR-30e-5p | Stably expressed
miRNA of interest |
5'-UGUAAACAUCCUUGACUGGAAG-3' |
Statistical analysis Outliers
For each miRNA analysed, the mean Cq value and
variance within each replicate was calculated. RT-qPCR efficiency
was calculated to be 2.0 and outliers (n=18) were removed
accordingly (Table II).
 | Table IIMethod of identifying RT-qPCR data
outliersa. |
Table II
Method of identifying RT-qPCR data
outliersa.
| Mean Cq of
replicates | Maximal acceptable
variance in Cq replicates |
|---|
| 25 | 0.5 |
| 26 | 0.5 |
| 27 | 0.5 |
| 28 | 0.5 |
| 29 | 0.5 |
| 30 | 0.5 |
| 31 | 0.5 |
| 32 | 0.7 |
| 33 | 0.9 |
| 34 | 1.3 |
| 35 | 1.9 |
Quality control
RNA extraction efficiency, monitored using UniSp2,
was acceptable with Cq values showing consistent values (Δ<3 Cq)
across the dataset (61). Haemolysis
was monitored using ΔCq=mean Cqhsa-miR-23a-mean
Cqhsa-miR-451a, with a ΔCq >5 indicating possible
haemolysis and a ΔCq >7 conferring a high risk of haemolysis
affecting the data obtained (26,33,61).
Samples with a ΔCq >7 were excluded prior to further analysis.
cDNA efficiency was acceptable with consistent values of
cel-miR-39-3p (Δ<2 Cq) across the dataset (61).
Relative expression
The relative expression (ΔCq) of each miRNA
replicate was normalised against the geomean of two normalising
miRNA (hsa-let-7i-3p and hsa-miR-148-3p), both of which are stably
and consistently expressed in serum and plasma samples (33). Standard deviations and confidence
intervals were calculated accordingly (62).
Analysis
Data were assessed for normality (Shapiro-Wilk test)
prior to statistical testing. If the Gaussian distribution was
satisfied, a paired t-test was performed, otherwise a Wilcoxon
matched-pairs signed rank test was used. Statistics were performed
in GraphPad Prism version 8 (GraphPad Software, Inc.). P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Table III
summarises the characteristics of the 10 patients (median age 29.5
years range 22-34 years) that provided paired serum and plasma
samples taken at different time points during their uncomplicated
low-risk pregnancy.
 | Table IIISummary characteristics of recruited
patientsa. |
Table III
Summary characteristics of recruited
patientsa.
| Study time
point | n | Average gestational
age, weeks + days | Range, weeks +
days |
|---|
| Second
trimester | 2 | 18+4 | 17+5-19+1 |
| Third
trimester | 3 | 38+1 | 37+5-39+2 |
| 6 weeks
post-partum | 5 | 6.3 weeks post
delivery | 5-7 weeks |
Bioanalyzer miRNA content
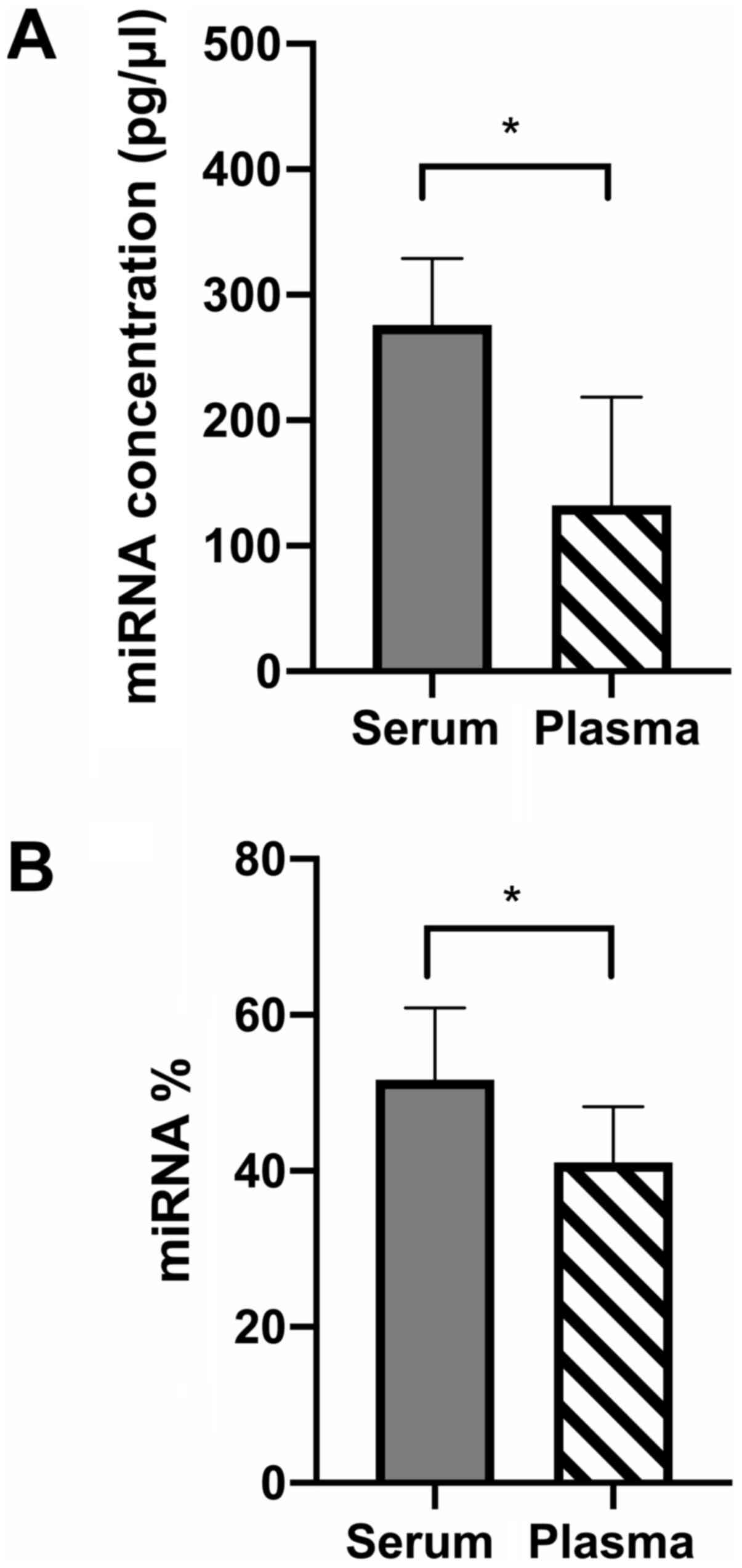
Both the average concentration and percentage of
miRNA was higher within serum compared to plasma samples (Fig. 1).
qPCR quality controls Control
samples
RNA extraction efficiency was deemed efficient with
a ΔCq of UniSp2=2.88 across the dataset. As expected, a Cq result
was not obtained for the NTC or NRTC samples, indicating that
neither the starting serum or plasma samples, or the qPCR reagents
were contaminated.
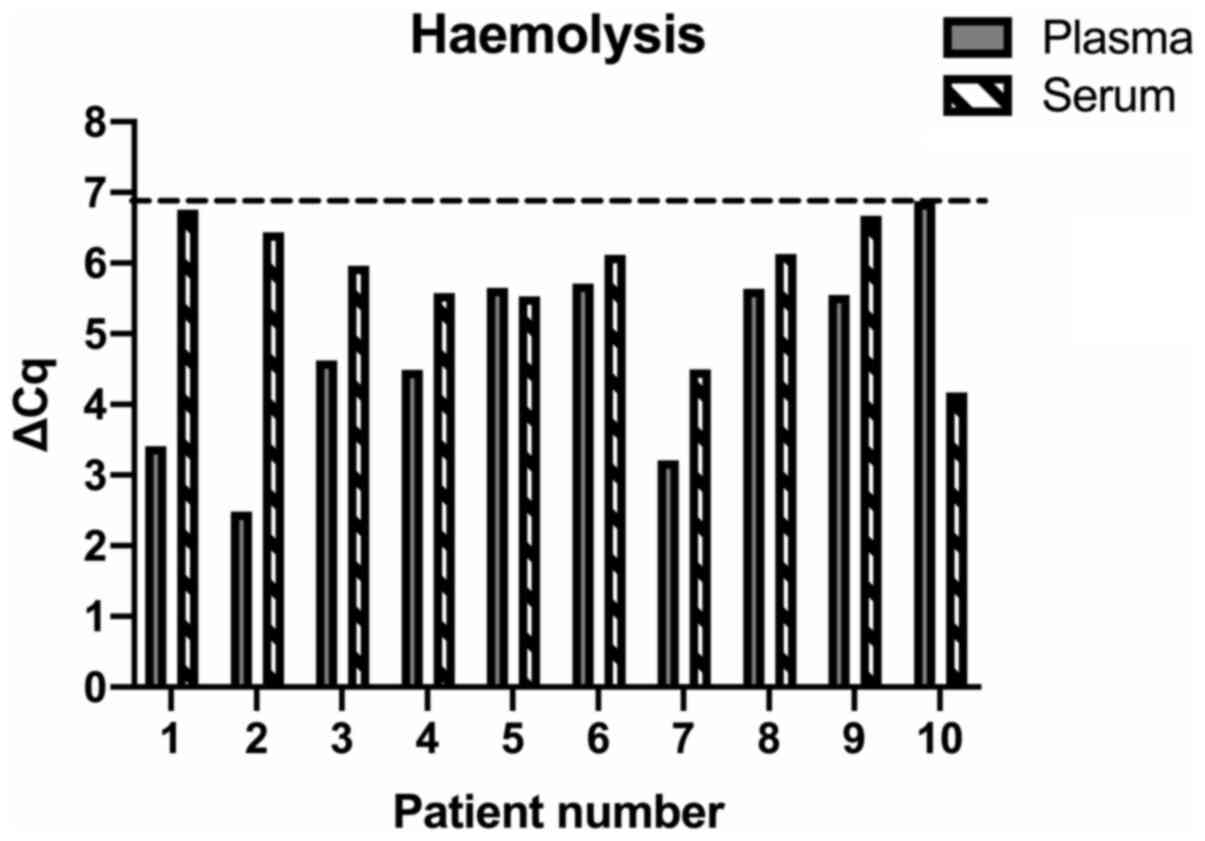
Haemolysis detection
None of the included samples displayed significant
haemolysis (ΔCq=>7 Fig. 2) and
were all retained for downstream analysis.
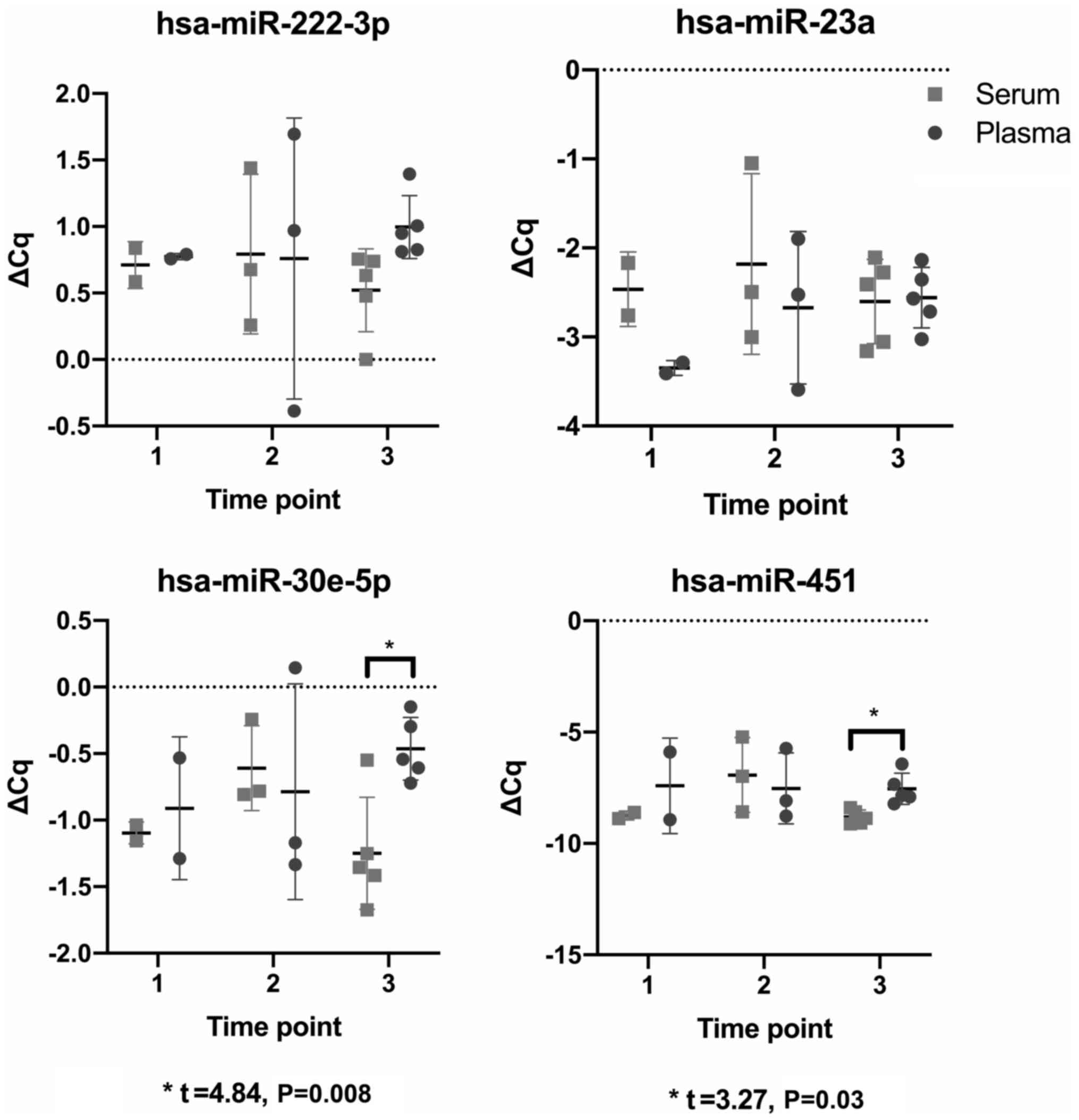
Serum vs. plasma
Using ΔCq values and paired t-tests, comparisons
were made between paired serum and plasma samples taken at each
study time point to determine whether the miRNA profile differed
between media. Only two comparisons were significant; specifically,
the comparison between the paired serum and plasma samples at time
point three, studying hsa-miR-30e-5p (t=4.84, P=0.008) and
hsa-miR-451a (t=3.27, P=0.03) (Fig.
3).
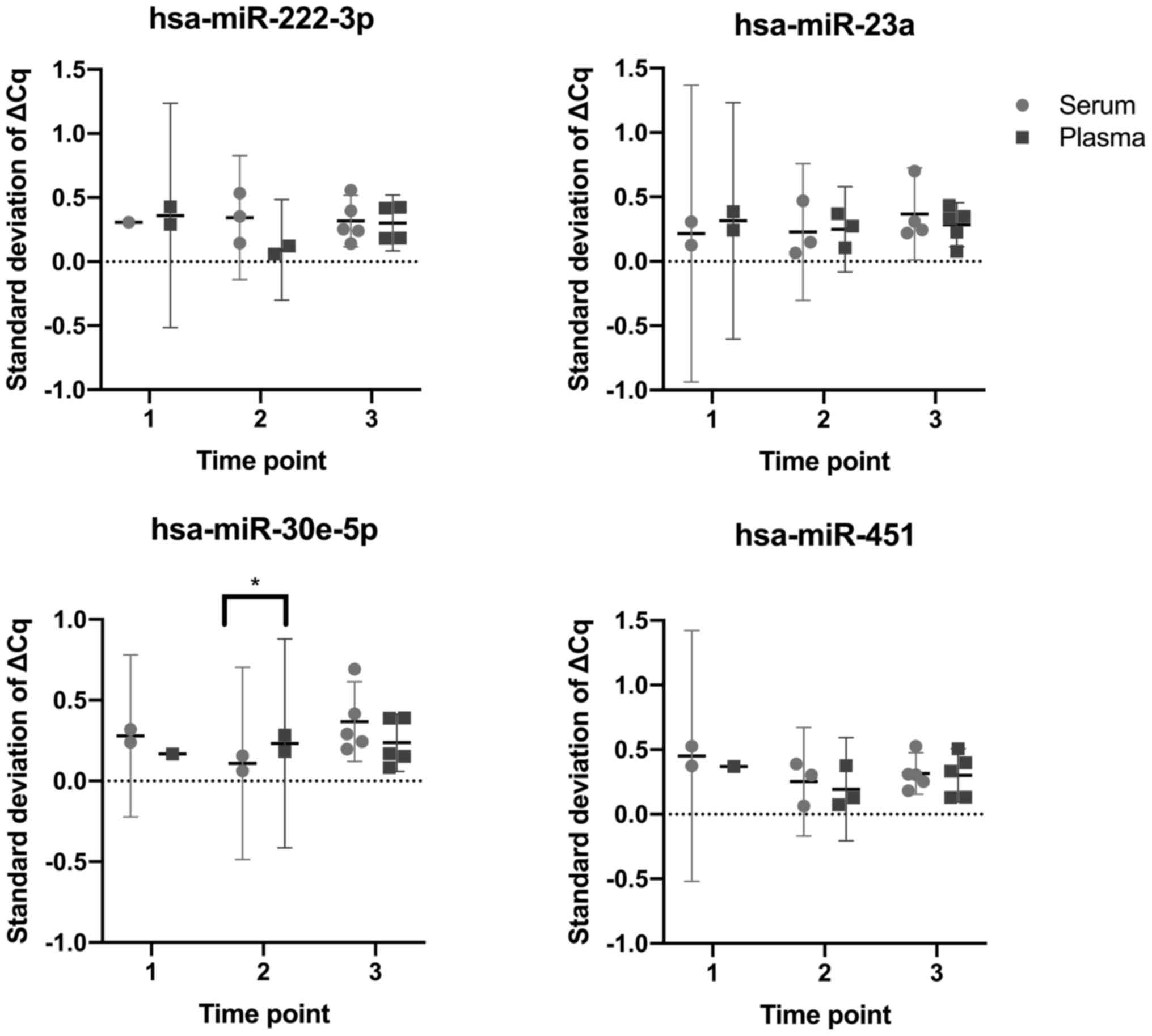
To determine whether serum or plasma samples yielded
more consistent RT-qPCR results, the standard deviations of the ΔCq
values for paired serum and plasma samples taken at each study time
point were compared, analyzing the four miRNAs of interest. Only
one comparison was found to be significant, specifically the
comparison between serum and plasma samples at time point two,
using hsa-miR-30e-5p. All other comparisons revealed no significant
differences (P>0.05; Fig. 4).
Discussion
Selection of the most appropriate starting test
sample for the identification of clinically relevant biomarkers is
essential, and is dependent upon first establishing the underlying
performance of different starting substrates (26,45,47,48). In
the present study. within a cohort of patients with uncomplicated,
low-risk, healthy pregnancies, across the three study time points,
the Bioanalyzer Small RNA chip data revealed the miRNA content of
serum samples to be higher than that of plasma. Dissimilar to the
Bioanalyzer findings, the majority of paired comparisons using
RT-qPCR showed no significant differences between the respective
serum and plasma samples at any of the time points assessed. Only 2
comparisons out of 12 were significant, specifically between paired
serum and plasma samples taken at time point three, using
hsa-miR-30e-5p and hsa-miR-451a. Similarly, when comparing the
consistency of serum vs. plasma samples, only 1 of the 12
comparisons was found to be statistically significant, involving
time point two and hsa-miR-30e-5p.
The only truly comparable previous manuscript
involving a healthy pregnant population both agrees and disagrees
with our findings. This previous study reported a higher proportion
of miRNA in plasma compared to serum, although the absolute number
of miRNAs detected and occurrence of abundant miRNA was higher in
the serum (25) This previous study
included 32 samples (20 serum, 12 plasma), yet only three paired
serum and plasma samples from the same patient were obtained
(25), reducing the generalisability
and reliability of the results obtained, given the known intra and
inter-individual biological variability in the expression of
certain miRNAs (63). Furthermore,
the present study investigated three time points (and as such,
determined whether the optimal starting media changed throughout
pregnancy), as opposed to just one time point (second trimester) in
the previous study (25).
Compared with the previous literature involving
non-pregnant populations, the absence of a significant difference
between serum and plasma samples using RT qPCR is in agreement with
some studies (27,41,47,49,50), but
in disagreement with others (7,25,33,48).
However, direct comparisons cannot truly be made, given the known
differences in miRNA expression profiles between different patient
cohorts in terms of sex and disease states, with the above studies
including lung, breast and colorectal cancer patients (7,41),
healthy male or female subjects (7,27,41,48-50).
It is evident that when using only the existing literature, it is
extremely challenging for researchers to deduce which starting
media they should preferentially use to answer their research
question.
Previous studies have suggested that serum samples
collected and extracted in optimal conditions may display less
variation in the miRNA data than plasma samples (33); however the results of the present
study did not replicate this, showing no significant differences
between the standard deviations of normalised serum and plasma data
based on RT-qPCR.
Regarding the results of the present study, the
discrepancy between quantification techniques is potentially
explained by the uncontrolled and variable release of miRNAs from
erythrocytes and platelets during the coagulation process within
serum samples, which may have contributed to the higher miRNA
levels observed with the Small RNA chip data (27,33,45,47,48,50).
This is corroborated by the higher mean haemolysis levels for serum
samples compared with plasma, with the ΔCq (hsa-miR-23a and
hsa-miR-451a) being 5.78 and 4.76, respectively. The chips used
within the Agilent Bioanalyzer each comprise a network of capillary
channels that separate the sample by means of gel electrophoresis.
The principle is based upon the fact that small fragments migrate
faster than larger ones, using fluorescent dye molecules that
intercalate with the RNA strands. These hybrid molecules are
detected by their fluorescence and translated into gel-like images
(bands) and electropherograms (peaks). Small RNA chips measure RNA
of 6-150 nucleotides in size, and quantify the concentration and
percentage of miRNA within a sample based on the number of
molecules falling within the 4-39 nucleotide region (64); however this pre-defined region for
detection may additionally contain small interfering RNA (20-25
nucleotides in length) or degraded longer RNA molecules including
small nuclear RNA (~100 nucleotides), primitive (pri-miRNA, several
hundred nucleotides) or precursor miRNA (~70 nucleotides) (65), which could falsely elevate the
quantification of miRNA. Dissimilarly, RT-qPCR using specific
primers would only bind and amplify mature miRNA molecules with a
sequence that precisely matches the primer assay, meaning that even
isomers of the miRNA may not be detected (66). This is particularly problematic when
profiling miRNAs, given there are >3,000 known miRNA variants,
most of which differ from the official sequence as denoted on
miRBASE, from which the majority of RT-qPCR primer assays are
designed (67,68). Furthermore, existing studies have
shown key differences between the varying methods of quantifying
RNA and DNA libraries, with electrophoresis-based quantification
techniques (including the Agilent Bioanalyzer) producing higher
concentration estimates (69) or
highly variable quantification results (70) compared with RT-qPCR or the Qubit
fluorometer. Overall, RT-qPCR is suggested to generate more
reliable results, notwithstanding the increased number of
replicates performed using this technique and the high between-run
variability that was noted with the Bioanalyzer in the present
study.
Limitations of the present study include the
relatively small sample size of 10 pregnant patients; however this
is in keeping with the sample sizes of existing literature
(27,48-50)
and is strengthened by the presence of paired samples taken at
exactly the same time points, and being stored and processed using
the same conditions. Despite this, a larger patient cohort would
have been preferable, particularly considering time point i), which
involved only two patients, potentially limiting the
generalisability of the data obtained and capacity to detect a true
difference. Although a significant difference was not observed
between the paired serum and plasma samples, it would have been
preferable to match the baseline characteristics of the patients
more closely, in terms of age, body mass index and the exact
gestation at which the samples were taken, yet previously published
literature have similarly not corrected for these factors (25,27,47).
There is also no consensus on the optimal normalisation strategy
when working with miRNA RT-qPCR data, leading to further
variability and difficulty when comparing with existing studies
(24,71,72). The
approach taken here, to normalise to two stably expressed miRNAs
(hsa-let-7i-3p and hsa-miR-148-3p) across the serum and plasma
samples, is a recognised approach that is preferable to the use of
synthetic spike-ins, such as cel-miR-39-3p. Such synthetic
spike-ins are useful to identify differences in RNA extraction
efficiency, but not to normalise the endogenous miRNA content of
the sample (24,58,73). The
use of two normalising miRNAs was further acceptable for the
present study given that all RNA extractions were performed within
the same batch. However, when this is not the case, and with
greater resources, normalising to 3-5 miRNAs that are stably
expressed within the starting media, or better still, normalising
to the global mean of >90 miRNAs is preferred (33,45,71).
Future work should involve replicating the
experiments using a larger patient population, and using a more
stringent cut-off of ≤5 for the ΔCq for the haemolysis markers on
RT-qPCR, which may reduce the potential to falsely elevate the
miRNA content of serum samples, yet most clinical studies would
accept ΔCq <7 (26,33).
It is clear that the choice of starting media for
ongoing experiments is crucially dependent on three main factors:
i) sample type; ii) the patient cohort under investigation (healthy
vs. disease; pregnant vs. non-pregnant); and iii) the miRNA
quantification technique. As seen in the present study, different
miRNA quantification techniques can produce conflicting information
concerning the optimal starting media, and scientists should be
astutely aware of this when designing studies, particularly those
with a clinical end point. In the healthy pregnant population using
the Agilent Bioanalyzer, the present study showed there to be a
higher miRNA concentration and percentage in serum samples.
However, this was not replicated in the RT-qPCR results, which, due
to the detection of specific miRNAs, is suggested to be more
reliable and less liable to the incorrect inclusion of fragmented
long RNA molecules or miRNAs released during the coagulation
process. Furthermore, RT-qPCR involves a higher number of
replicates, and this increases the reliability of the results,
producing consistent data unlike the high between run variability
that was observed in the present study using the Bioanalyzer. In
view of this, it is concluded that neither serum nor plasma are
superior starting media during the intra or post-partum period in
the healthy pregnant population, and as such, either would be
suitable for studies and downstream analyses investigating the
miRNA profiles or use of clinical biomarkers in this
population.
Acknowledgements
The authors would like to thank Dr Sarah Waite,
Senior Research Technician in the Department of Oncology and
Metabolism (University of Sheffield) for technical assistance with
the laboratory work performed in the present study.
Funding
The present study was supported by funding from
Weston Park Cancer Charity, Sheffield, U.K (grant nos. CA154 and
CA184).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
VLP conceived and designed the study, collected the
data, managed the project, analysed the data and wrote the
manuscript. EG and BM assisted in designing the study, in deciding
the methodology and analysed the data. AP and PRH assisted in
designing the methodology used, supervised the study and reviewed
and edited the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethical approval was obtained from the North East
Newcastle and North Tyneside 1 NHS Research Ethics Committee, UK
(approval no. 16/NE/0292). No patient identifiable information is
presented within the manuscript and thus patient consent was not
required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mishra PJ: MicroRNAs as promising
biomarkers in cancer diagnostics. Biomark Res. 2(19)2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nair VS, Pritchard CC, Tewari M and
Ioannidis JP: Design and analysis for studying micrornas in human
disease: A primer on-omic technologies. Am J Epidemiol.
180:140–152. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hayes J, Peruzzi PP and Lawler S:
MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol
Med. 20:460–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen X, Wang L, Qu J, Guan NN and Li JQ:
Predicting miRNA-disease association based on inductive matrix
completion. Bioinformatics. 34:4256–4265. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Papadaki C, Stratigos M, Markakis G,
Spiliotaki M, Mastrostamatis G, Nikolaou C, Mavroudis D and Agelaki
S: Circulating microRNAs in the early prediction of disease
recurrence in primary breast cancer. Breast Cancer Res.
20(72)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rapado-Gonzalez O, Alvarez-Castro A,
Lopez-Lopez R, Iglesias-Canle J, Suarez-Cunqueiro MM and
Muinelo-Romay L: Circulating microRNAs as promising biomarkers in
colorectal cancer. Cancers (Basel). 11(898)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
van Schooneveld E, Wouters MC, Van der
Auwera I, Peeters DJ, Wildiers H, Van Dam PA, Vergote I, Vermeulen
PB, Dirix LY and Van Laere SJ: Expression profiling of cancerous
and normal breast tissues identifies microRNAs that are
differentially expressed in serum from patients with (metastatic)
breast cancer and healthy volunteers. Breast Cancer Res.
14(R34)2012.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Tan W, Liu B, Qu S, Liang G, Luo W and
Gong C: MicroRNAs and cancer: Key paradigms in molecular therapy.
Oncol Lett. 15:2735–2742. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Macfarlane LA and Murphy PR: MicroRNA:
Biogenesis, function and role in cancer. Curr Genomics. 11:537–561.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang H, Peng R, Wang J, Qin Z and Xue L:
Circulating microRNAs as potential cancer biomarkers: The advantage
and disadvantage. Clin Epigenetics. 10(59)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghasabi M, Mansoori B, Mohammadi A, Duijf
PH, Shomali N, Shirafkan N, Mokhtarzadeh A and Baradaran B:
MicroRNAs in cancer drug resistance: Basic evidence and clinical
applications. J Cell Physiol. 234:2152–2168. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gomes BC, Rueff J and Rodrigues AS:
MicroRNAs and cancer drug resistance. Methods Mol Biol.
1395:137–162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Si W, Shen J, Zheng H and Fan W: The role
and mechanisms of action of microRNAs in cancer drug resistance.
Clin Epigenetics. 11(25)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zhao C, Dong J, Jiang T, Shi Z, Yu B, Zhu
Y, Chen D, Xu J, Huo R, Dai J, et al: Early second-trimester serum
miRNA profiling predicts gestational diabetes mellitus. PLoS One.
6(e23925)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhu Y, Tian F, Li H, Zhou Y, Lu J and Ge
Q: Profiling maternal plasma microRNA expression in early pregnancy
to predict gestational diabetes mellitus. Int J Gynaecol Obstet.
130:49–53. 2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hromadnikova I, Kotlabova K, Ivankova K,
Vedmetskaya Y and Krofta L: Profiling of cardiovascular and
cerebrovascular disease associated microRNA expression in umbilical
cord blood in gestational hypertension, preeclampsia and fetal
growth restriction. Int J Cardiol. 249:402–409. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hromadnikova I, Dvorakova L, Kotlabova K
and Krofta L: The Prediction of gestational hypertension,
preeclampsia and fetal growth restriction via the first trimester
screening of plasma exosomal C19MC microRNAs. Int J Mol Sci.
20(2972)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sheikh AM, Small HY, Currie G and Delles
C: Systematic review of Micro-RNA expression in Pre-eclampsia
identifies a number of common pathways associated with the disease.
PLoS One. 11(e0160808)2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Skalis G, Katsi V, Miliou A, Georgiopoulos
G, Papazachou O, Vamvakou G, Nihoyannopoulos P, Tousoulis D and
Makris T: MicroRNAs in preeclampsia. Microrna. 8:28–35.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cai M, Kolluru GK and Ahmed A: Small
molecule, big prospects: MicroRNA in pregnancy and its
complications. J Pregnancy. 2017(6972732)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu Z, Han S, Hu P, Zhu C, Wang X, Qian L
and Guo X: Potential role of maternal serum microRNAs as a
biomarker for fetal congenital heart defects. Med Hypotheses.
76:424–426. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Duenas A, Exposito A, Aranega A and Franco
D: The role of non-coding RNA in congenital heart diseases. J
Cardiovasc Dev Dis. 6(15)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tay JW, James I, Hughes QW, Tiao JY and
Baker RI: Identification of reference miRNAs in plasma useful for
the study of oestrogen-responsive miRNAs associated with acquired
Protein S deficiency in pregnancy. BMC Res Notes.
10(312)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ge Q, Shen Y, Tian F, Lu J, Bai Y and Lu
Z: Profiling circulating microRNAs in maternal serum and plasma.
Mol Med Rep. 12:3323–3330. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Blondal T, Jensby Nielsen S, Baker A,
Andreasen D, Mouritzen P, Wrang Teilum M and Dahlsveen IK:
Assessing sample and miRNA profile quality in serum and plasma or
other biofluids. Methods. 59 (Suppl):S1–S6. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang K, Yuan Y, Cho JH, McClarty S, Baxter
D and Galas DJ: Comparing the MicroRNA spectrum between serum and
plasma. PLoS One. 7(e41561)2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
He S, Zeng S, Zhou ZW, He ZX and Zhou SF:
Hsa-microRNA-181a is a regulator of a number of cancer genes and a
biomarker for endometrial carcinoma in patients: A bioinformatic
and clinical study and the therapeutic implication. Drug Des Devel
Ther. 9:1103–1175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Creemers EE, Tijsen AJ and Pinto YM:
Circulating microRNAs: Novel biomarkers and extracellular
communicators in cardiovascular disease? Circ Res. 110:483–495.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable Blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gilad S, Meiri E, Yogev Y, Benjamin S,
Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed
N, et al: Serum microRNAs are promising novel biomarkers. PLoS One.
3(e3148)2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kontomanolis EN, Kalagasidou S and
Fasoulakis Z: MicroRNAs as potential serum biomarkers for early
detection of ectopic pregnancy. Cureus. 10(e2344)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Exiqon: Biofluids Guideline: Analysing
microRNAs in liquid biopsies. urihttps://www.gene-quantification.de/exiqon-biofluids-guidelines-2016.pdfsimplehttps://www.gene-quantification.de/exiqon-biofluids-guidelines-2016.pdf.
|
|
34
|
Rounge TB, Lauritzen M, Langseth H, Enerly
E, Lyle R and Gislefoss RE: microRNA biomarker discovery and
high-throughput DNA sequencing are possible using long-term
archived serum samples. Cancer Epidemiol Biomarkers Prev.
24:1381–1387. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kreth S, Hubner M and Hinske LC: MicroRNAs
as clinical biomarkers and therapeutic tools in perioperative
medicine. Anesth Analg. 126:670–681. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Morales-Prieto DM, Ospina-Prieto S,
Chaiwangyen W, Schoenleben M and Markert UR: Pregnancy-associated
miRNA-clusters. J Reprod Immunol. 97:51–61. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bidarimath M, Khalaj K, Wessels JM and
Tayade C: MicroRNAs, immune cells and pregnancy. Cell Mol Immunol.
11:538–547. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Luo SS, Ishibashi O, Ishikawa G, Ishikawa
T, Katayama A, Mishima T, Takizawa T, Shigihara T, Goto T, Izumi A,
et al: Human villous trophoblasts express and secrete
placenta-specific microRNAs into maternal circulation via exosomes.
Biol Reprod. 81:717–729. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mouillet JF, Ouyang Y, Coyne CB and
Sadovsky Y: MicroRNAs in placental health and disease. Am J Obstet
Gynecol. 213 (4 Suppl):S163–S172. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Moreno-Moya JM, Vilella F and Simon C:
MicroRNA: Key gene expression regulators. Fertil Steril.
101:1516–1523. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Cortez MA, Bueso-Ramos C, Ferdin J,
Lopez-Berestein G, Sood AK and Calin GA: MicroRNAs in body
fluids-the mix of hormones and biomarkers. Nat Rev Clin Oncol.
8:467–477. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Qiagen: miRNeasy Serum/Plasma-Handbook for
miRNeasy Serum/Plasma Kit. Journal.
|
|
44
|
Promega: Maxwell RSC miRNA Plasma and
Serum Kit: Instructions for use of product AS1680. urihttps://www.promega.co.uk/products/nucleic-acid-extraction/rna/maxwell-rscmirna-tissue-plasma-serum-kit/?catNum=AS1460#protocolssimplehttps://www.promega.co.uk/products/nucleic-acid-extraction/rna/maxwell-rscmirna-tissue-plasma-serum-kit/?catNum=AS1460#protocols.
|
|
45
|
Exiqon: Profiling of microRNA in blood
(serum/plasma). Guidelines for the miRCURY LNA Universal RT
microRNA PCR system. urihttps://www.gene-quantification.de/microRNA-serum-plasma-guidelines-exiqon.pdfsimplehttps://www.gene-quantification.de/microRNA-serum-plasma-guidelines-exiqon.pdf.
|
|
46
|
Zou P, Luo L, Zhao C, Chen Z, Dong R, Li
N, Wang Y, Wang J, Wang T, Chen M, et al: The serum microRNA
profile of intrahepatic cholestasis of pregnancy: Identification of
novel noninvasive biomarkers. Cell Physiol Biochem. 51:1480–1488.
2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Foye C, Yan IK, David W, Shukla N,
Habboush Y, Chase L, Ryland K, Kesari V and Patel T: Comparison of
miRNA quantitation by Nanostring in serum and plasma samples. PLoS
One. 12(e0189165)2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Max KEA, Bertram K, Akat KM, Bogardus KA,
Li J, Morozov P, Ben-Dov IZ, Li X, Weiss ZR, Azizian A, et al:
Human plasma and serum extracellular small RNA reference profiles
and their clinical utility. Proc Natl Acad Sci USA.
115:E5334–E5343. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Glinge C, Clauss S, Boddum K, Jabbari R,
Jabbari J, Risgaard B, Tomsits P, Hildebrand B, Kääb S, Wakili R,
et al: Stability of circulating Blood-based MicroRNAs-Pre-analytic
methodological considerations. PLoS One.
12(e0167969)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Dufourd T, Robil N, Mallet D, Carcenac C,
Boulet S, Brishoual S, Rabois E, Houeto JL, de la Grange P and
Carnicella S: Plasma or serum? A qualitative study on rodents and
humans using high-throughput microRNA sequencing for circulating
biomarkers. Biol Methods Protoc. 4(bpz006)2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
The Early Detection Research Network
(EDRN): Standard Operating Procedure (SOP) for collection of EDTA
plasma. urihttps://edrn.nci.nih.gov/resources/standard-operating-procedures/standard-operating-procedures/plasma-sop.pdfsimplehttps://edrn.nci.nih.gov/resources/standard-operating-procedures/standard-operating-procedures/plasma-sop.pdf.
|
|
52
|
Qiagen: miRCURY LNA miRNA PCR-Exosomes,
serum/plasma and other biofluid samples handbook. urihttps://www.qiagen.com/fi/resources/resourcedetail?id=7ab5f614-f5d6-4bdc-b22b-246ec3601588&lang=ensimplehttps://www.qiagen.com/fi/resources/resourcedetail?id=7ab5f614-f5d6-4bdc-b22b-246ec3601588&lang=en.
|
|
53
|
Shah JS, Soon PS and Marsh DJ: Comparison
of methodologies to detect low levels of hemolysis in serum for
accurate assessment of serum microRNAs. PLoS One.
11(e0153200)2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kirschner MB, Edelman JB, Kao SCH, Vallely
MP, van Zandwijk N and Reid G: The impact of hemolysis on Cell-free
microRNA biomarkers. Front Genet. 4(94)2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
GmbH T: TechNote TN-01: The impact of
sample type (serum and EDTA-plasma) and platelet contamination on
osteomiR detection.
|
|
56
|
The Early Detection Research Network
(EDRN): Standard Operating Procedure (SOP) For collection of serum.
urihttps://edrn.nci.nih.gov/resources/standard-operating-procedures/standard-operating-procedures/serum-sop.pdfsimplehttps://edrn.nci.nih.gov/resources/standard-operating-procedures/standard-operating-procedures/serum-sop.pdf.
|
|
57
|
Promega: Maxwell® RSC miRNA
Plasma and Serum Kit: Instructions for use of product AS1680.
urihttps://www.promega.co.uk/products/nucleic-acid-extraction/rna/maxwell-rscmirna-tissue-plasma-serum-kit/?catNum=AS1460#protocolssimplehttps://www.promega.co.uk/products/nucleic-acid-extraction/rna/maxwell-rscmirna-tissue-plasma-serum-kit/?catNum=AS1460#protocols.
|
|
58
|
Qiagen: RNA Spike-In Kit, For RT handbook.
urihttps://www.qiagen.com/us/products/discovery-and-translational-research/pcr-qpcr-dpcr/qpcr-assays-and-instruments/mirna-qpcr-assay-and-panels/rna-spike-in-kit-for-rt/?clear=true#resourcessimplehttps://www.qiagen.com/us/products/discovery-and-translational-research/pcr-qpcr-dpcr/qpcr-assays-and-instruments/mirna-qpcr-assay-and-panels/rna-spike-in-kit-for-rt/?clear=true#resources.
|
|
59
|
Promega: Maxwell® RSC
Instrument, AS4500. urihttps://www.promega.co.uk/products/lab-automation/maxwell-instruments/maxwell-rsc-instrument/?catNum=AS4500simplehttps://www.promega.co.uk/products/lab-automation/maxwell-instruments/maxwell-rsc-instrument/?catNum=AS4500.
|
|
60
|
Promega: Maxwell® RSC miRNA
Tissue Kit: Instructions for use of product. urihttps://www.promega.co.uk/products/nucleic-acid-extraction/rna/maxwell-rscmirna-tissue-plasma-serum-kit/?catNum=AS1460#protocolssimplehttps://www.promega.co.uk/products/nucleic-acid-extraction/rna/maxwell-rscmirna-tissue-plasma-serum-kit/?catNum=AS1460#protocols.
|
|
61
|
Exiqon: miRCURY microRNA QC PCR Panel
Instruction Manual 203887-203892. urihttp://www.exiqon.com/ls/Documents/Scientific/QC-PCR-Panel-Manual.pdfsimplehttp://www.exiqon.com/ls/Documents/Scientific/QC-PCR-Panel-Manual.pdf.
|
|
62
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Yoon H, Belmonte KC, Kasten T, Bateman R
and Kim J: Intra- and Inter-individual Variability of microRNA
levels in human cerebrospinal fluid: Critical implications for
biomarker discovery. Sci Rep. 7(12720)2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Agilent: Bioanalyzer Small RNA Analysis.
urihttps://www.agilent.com/en/product/automated-electrophoresis/bioanalyzer-systems/bioanalyzer-rna-kits-reagents/bioanalyzer-small-rna-analysis-228257#supportsimplehttps://www.agilent.com/en/product/automated-electrophoresis/bioanalyzer-systems/bioanalyzer-rna-kits-reagents/bioanalyzer-small-rna-analysis-228257#support.
|
|
65
|
Masotti A, Caputo V, Prudente S and
Bottazzo GF: Analysis of small RNAs with the Agilent 2100
Bioanalyzer. Application note. Agilent Technologies, 2006.
urihttps://www.agilent.com/cs/library/applications/5989-5215EN.pdfsimplehttps://www.agilent.com/cs/library/applications/5989-5215EN.pdf.
|
|
66
|
Magee R, Telonis AG, Cherlin T, Rigoutsos
I and Londin E: Assessment of isomiR discrimination using
commercial qPCR methods. Noncoding RNA Vol. 3(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kuchenbauer F, Morin RD, Argiropoulos B,
Petriv OI, Griffith M, Heuser M, Yung E, Piper J, Delaney A, Prabhu
AL, et al: In-depth characterization of the microRNA transcriptome
in a leukemia progression model. Genome Res. 18:1787–1797.
2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
University of Manchester: miRBase: the
microRNA database. urihttp://www.mirbase.org/simplehttp://www.mirbase.org/.
|
|
69
|
Hussing C, Kampmann ML, Mogensen HS,
Børsting C and Morling N: Quantification of massively parallel
sequencing libraries-a comparative study of eight methods. Sci Rep.
8(1110)2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Garcia-Elias A, Alloza L, Puigdecanet E,
Nonell L, Tajes M, Curado J, Enjuanes C, Díaz O, Bruguera J,
Martí-Almor J, et al: Defining quantification methods and
optimizing protocols for microarray hybridization of circulating
microRNAs. Sci Rep. 8(1110)2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
de Ronde MWJ, Ruijter JM, Lanfear D,
Bayes-Genis A, Kok MGM, Creemers EE, Pinto YM and Pinto-Sietsma SJ:
Practical data handling pipeline improves performance of qPCR-based
circulating miRNA measurements. RNA. 23:811–821. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Hardikar AA, Farr RJ and Joglekar MV:
Circulating microRNAs: Understanding the limits for quantitative
measurement by real-time PCR. J Am Heart Assoc.
3(e000792)2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kroh EM, Parkin RK, Mitchell PS and Tewari
M: Analysis of circulating microRNA biomarkers in plasma and serum
using quantitative reverse transcription-PCR (qRT-PCR). Methods.
50:298–301. 2010.PubMed/NCBI View Article : Google Scholar
|