1. Introduction
Human papillomavirus (HPV) is a small DNA virus that
infects the basal keratinocytes of the squamous epithelium through
micro-wounds and abrasions in the skin and mucosa (1). To date, >150 HPV genotypes have been
identified, of which ~40 infect the mucosa (mucosal HPV types)
(1). Mucosal HPV types that infect
the anogenital mucosa have also been shown to also infect the
mucosa of the oral cavity (2).
Smoking and sexual behavior have been recognized as predominant
risk factors for oral HPV infection (3).
Several studies have been performed to investigate
the association between HPV prevalence and periodontal disease
(4-15).
Periodontal diseases such as gingivitis and periodontitis are
polymicrobial infectious diseases that affect epithelial tissue
(the gingiva), tooth-supporting connective tissue (the periodontal
ligament) and alveolar bone. Periodontal disease is a major cause
of tooth loss, and is caused by interactions between
periodontopathic bacteria, host immune responses and environmental
factors (for example, smoking) (16). Periodontopathic bacteria such
as Treponema denticola, Tannerella forsythia and
Porphyromonas gingivalis serve vital roles in the
pathogenesis of periodontal disease (17). Importantly, periodontal disease
causes destruction of the crevicular epithelium (16), which may increase the opportunity for
HPV to infect basal cells in the epithelium.
Oral virome analyses have revealed that several
human viruses are stable members of the microbiota, and oral
viruses display a sex-specific prevalence (18). Previous research has demonstrated
that, together with periodontal disease-related bacteria, the
herpes virus serves a significant role in the pathogenesis of
periodontal disease (19). However,
it remains to be elucidated whether oral HPV is implicated in
periodontitis and the virulence of periodontopathic bacteria. In
the present review, the findings of recent studies of oral HPV
prevalence in relation to periodontitis are discussed, as well as
periodontopathic bacteria and periodontal herpes virus.
2. Association between oral HPV and
periodontitis
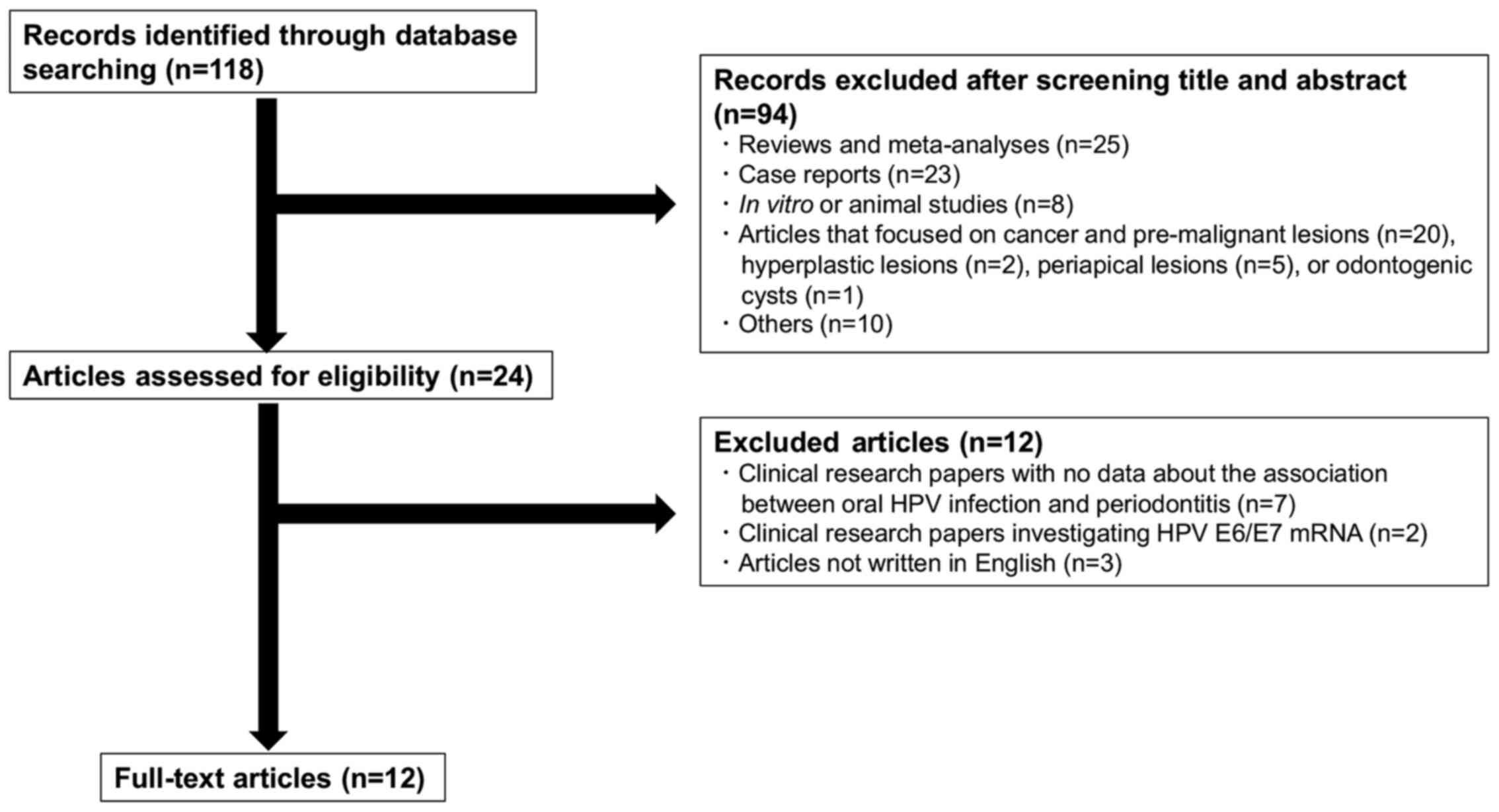
The PubMed search engine was used to search papers
using the following key words: ‘(HPV OR human papillomavirus) AND
(periodontitis OR periodontal disease)’, yielding 118 papers
published between June 1980 and October 2020. After reviewing the
titles and abstracts for relevance, reviews and meta-analyses
(n=25), case reports (n=23), in vitro or animal studies
(n=8), articles that focused on cancer and pre-malignant lesions
(n=20), hyperplastic lesions (n=2), periapical lesions (n=5) or
odontogenic cysts (n=1), as well as other articles, including news
(n=3), editorials and commentaries (n=3), letters to editors (n=1),
congress (n=1), questionnaire surveys (n=1) or a demand study
(n=1), were all excluded (Fig. 1).
Additionally, clinical research papers with no data regarding the
association between oral HPV infection and periodontitis (n=7),
clinical research papers investigating HPV E6/E7 mRNA rather than
HPV DNA (n=2), and articles not written in English (n=3) were
excluded. This resulted in 12 original papers that investigated the
association between oral HPV DNA prevalence and periodontal disease
(4-15).
Table I presents a summary of the
studies included in the present literature review. Of the 12 papers
that reported the association between oral HPV DNA and periodontal
disease, periodontal tissue samples were used in 5 studies
(4,6,7,9,11), oral
swab samples were used in one study (8), oral rinse samples were used in 5
studies (10,12-15)
and crevicular fluid samples were used in one study (5). A nucleic acid amplification assay (for
example, PCR) was the most common detection method for HPV DNA and
was employed in 11 of the 12 studies (5-15).
Nucleic acid hybridization assays (such as southern blotting) were
used for detection of HPV DNA (4,6).
 | Table ISummary of studies included in the
present literature review. |
Table I
Summary of studies included in the
present literature review.
| Author, year | Country | Subjects | Sample | Detection
method | HPV DNA positive
rate, % (positive n/total n) | Refs. |
|---|
| Madinier et
al, 1992 | France | 6 patients with
adult periodontitis and 2 patients with rapidly progressive
periodontitis | Gingival
tissues | Southern blot | Percentages of HPV
positivity were 16.7% (1/6) in patients with adult periodontitis
and 50% (1/2) in patients with rapidly progressive
periodontitis | (4) |
| Parra and Slots,
1996 | USA | 30 patients with
advanced periodontitis | Crevicular fluid
samples | PCR | 16.7% (5/30) | (5) |
| Hormia et
al, 2005 | Finland | 38 individuals with
clinically diagnosed periodontal disease | Gingival
tissues | PCR, southern
blot | 25.8% (8/31) | (6) |
| Horewicz et
al, 2010 | Brazil | 56 systemically
healthy adults with chronic periodontitis | Paraffin blocks of
gingival tissues | PCR for HPV16 DNA
detection | 0% (0/56) | (7) |
| Fuster-Rossello
et al, 2014 | Argentina | 11 women with HPV-
associated gynecological diseases and periodontitis | Oral swab
samples | PCR | Unknown | (8) |
| Jacob et al,
2014 | India | 67 systemically
healthy participants with periodontitis | Gingival
tissues | PCR for HPV16 DNA
detection | 0% (0/67) | (9) |
| Wiener et
al, 2015 | USA | Participants with
periodontal disease from the National Health and Nutrition
Examination Survey data from between 2009 and 2012 | Oral rinse
samples | PCR | 10.5%
(309/2945) | (10) |
| Baez et al,
2016 | Brazil | 74 kidney
transplanted or non-transplanted patients with gingivitis and/or
periodontitis | Gingivitis and/or
periodontitis tissues | PCR | 41.9% (31/74) | (11) |
| Sun et al,
2017 | Australia | 89 participants
with periodontitis | Oral rinse
samples | PCR for HPV16 DNA
detection | 3.4% (3/89) | (12) |
| Ortiz et al,
2018 | Puerto Rico | Participants of the
San Juan Overweight Adults Longitudinal Study between 2014 and
2016 | Oral rinse
samples | PCR | Percentages of HPV
positivity were 5.3% in patients with mild/moderate periodontitis
and 11.3% in patients with severe periodontitis | (13) |
| Ortiz et al,
2018 | Puerto Rico | Participants of the
San Juan Overweight Adults Longitudinal Study between 2011 and
2013 | Oral rinse
samples | PCR | Percentages of HPV
positivity were 4.4% (13/297) in patients with none/mild
periodontitis, 4.1% (12/290) in those with moderate periodontitis,
and 11.5% (17/148) in those with severe periodontitis | (14) |
| McDaniel et
al, 2020 | USA | Participants of the
National Health and Nutrition Examination Surveys from between 2011
and 2012 as well as 2013 and 2014 | Oral rinse
samples | PCR | Not determined | (15) |
To assess the prevalence of oral HPV DNA using
periodontal tissue samples, HPV DNA was detected in adult
periodontitis and rapidly progressive periodontitis using gingival
papilla specimens and southern blotting (4). Carcinogenic HPV DNA was detected in 26%
of the gingival tissues obtained from patients with periodontitis
by PCR (6). Furthermore, HPV DNA was
visualized in the nucleus of the junctional epithelium using in
situ hybridization (6). These
results indicate that local periodontal inflammation may provide an
opportunity for HPV to infect epithelial basal cells. Additionally,
a higher HPV DNA positive rate was found in gingivitis and/or
periodontitis biopsy samples from kidney transplant patients
compared with those from non-transplanted patients (11). Conversely, Horewicz et al
(7) reported that HPV16 DNA was not
detected by PCR in any paraffin embedded-gingival tissues (chronic
periodontitis, gingivitis or healthy periodontium) of Brazilian
patients with good general health. Furthermore, HPV16 DNA was not
detected by PCR in the marginal periodontium of systemically
healthy patients, excluding pregnant women, patients with
uncontrolled systemic diseases and smokers (9). It is speculated that the prevalence of
HPV DNA is low in normal periodontal tissues. Patients with general
good health maintain an effective local immune system which may
provide a defense against HPV, and may explain the absence of HPV
in periodontal tissues.
Oral swab samples from the external gingival
epithelium and the internal gingival epithelium (the periodontal
pocket epithelium) were used to detect HPV DNA by PCR in women with
HPV-associated gynecological diseases, and 13.3% of external
gingival epithelium samples and 16.7% of internal gingival
epithelium were HPV DNA positive (8). However, there was no positive
correlation between HPV DNA and the incidence and severity of
periodontitis (8). The relationship
between oral HPV DNA and oral health status (for example, plaque
accumulation and bleeding in the gingival sulcus) was investigated
using oral swab samples in individuals with no history of HPV
vaccination (20). The detection
rate for high risk type HPV was greater in the individuals with a
high gingival bleeding index compared with those with a low index
(20). A significant independent
association was found between oral HPV DNA detection and plaque
accumulation or gingival sulcus bleeding after adjustment for age
and sex (20). Bleeding of the
ulcerated epithelial surface of the periodontal pocket is
considered a significant indicator of periodontal inflammation
caused by periodontal pathogens (21). Thus, it is likely that oral HPV
infection is significantly associated with periodontal
inflammation.
Oral rinse samples were employed in 5 previous
studies to investigate oral HPV prevalence (10,12-15).
HPV DNA detection and genotyping were performed using oral rinse
samples in 740 Hispanic adults (13). The prevalence of oral HPV DNA was
significantly higher amongst individuals with severe periodontitis
(11.3%) than those with mild or moderate periodontitis (5.3%) or no
periodontitis (2.6%) (13). The same
group also reported a significant association between oral HPV
infection and the severity of periodontitis in Hispanic
participants of the San Juan Overweight Adults Longitudinal Study
between 2011 and 2013(14). Sun
et al (12) investigated the
relationship between oral HPV16 DNA and periodontal health status
(including presence of bleeding on probing, dental calculus and
periodontal pockets) by PCR, using oral rinse samples in patients
at a dental school clinic. The HPV16 DNA detection rate was higher
in individuals without periodontal disease (5.3%) than in those
with periodontal disease (3.4%); however, no significant
association was found between the oral HPV16 DNA detection rate and
periodontal health status (12).
Wiener et al (10) performed
a study based on the National Health and Nutrition Examination
Survey data and revealed that 58.7% of HPV positive participants
had periodontitis, whereas 38.7% of HPV negative participants had
periodontitis (10). A significant
association was found between the presence of oral HPV DNA and
periodontitis (10). However, no
independent association was found between oral HPV DNA and
periodontitis after adjustment for clinical factors such as sex,
ethnicity, education, age, income to poverty ratio, smoking,
alcohol use and number of lifetime sexual partners (10). Additionally, McDaniel et al
(15) performed a study based on the
National Health and Nutrition Examination Survey data between 2011
and 2012 as well as 2013 and 2014, and reported that the median
predicted oral HPV prevalence rates were higher in individuals with
periodontitis than in those without periodontitis amongst
non-HPV-vaccinated individuals (15).
Gingival crevicular fluid contains not only serum
and blood cells, but also periodontal epithelial cells and
subgingival plaque (22). Therefore,
gingival crevicular fluid can be used to investigate the
localization of HPV in periodontal pockets. Parra and Slots
(5) investigated the prevalence of
HPV DNA by PCR using gingival crevicular fluid in patients with
advanced periodontitis or gingivitis. HPV DNA was detected in 16.7%
of patients with advanced periodontitis, but was not detected in
patients with gingivitis (5). In our
previous study, the presence of HPV16 DNA in gingival crevicular
fluid collected by inserting paper points into periodontal pockets
was investigated in middle-aged and older Japanese individuals
(23). Of the 89 participants, four
women (4.5%) were HPV16 DNA-positive, but no men exhibited HPV16
DNA positivity (23). Postmenopausal
women were more likely to be infected with HPV in the cervix
because of sex-hormone-related immunosuppression (24). Female sex hormones, such as
progesterone, enhances the regulatory response to HPV16 virus-like
particles in peripheral blood mononuclear cells (25). Therefore, postmenopausal women may be
more susceptible to oral HPV infection as well as cervical HPV
infection. It is thus considered that factors specific to women,
such as reduced levels of sex hormones, may increase the risk of
HPV infection in periodontal pockets.
The oral HPV detection rate may be affected by
differences in sample detection methods. Oral rinse samples contain
a mix of saliva, bacteria, epithelial cells and blood cells derived
from various sites in the oral cavity. Furthermore, contamination
of the pharynx by gargling may have affected the positive rate of
HPV DNA in oral rinse samples because tonsillar tissue acts as a
reservoir for microorganisms due to its specific anatomical and
histological structure (26). It is
thus considered that sampling methods, such as gingival tissue
biopsies and crevicular fluid samples may be more appropriate to
determine the presence of periodontal disease-related HPV.
Collectively, the evidence suggests that oral HPV
infection may be associated with periodontitis. A recent systematic
review and meta-analysis revealed a positive relationship between
oral HPV infection and periodontitis, although the certainty of the
evidence is low (27). It is
important to consider the effect of clinical factors contributing
to oral HPV infection (age, sex, smoking, immunosuppressive
condition and vaccination) on HPV DNA prevalence to clarify the
presence of periodontitis-related HPV. Additionally, sampling
methods should be carefully chosen to directly detect HPV DNA in
periodontal tissues. Further studies are required to demonstrate
the presence of HPV in periodontal tissues and clarify the
biological role of HPV in periodontitis.
3. Association between oral HPV and
periodontopathic bacteria
Our previous study revealed that increased HPV16 E6
viral copy numbers were associated with an increased number of oral
bacteria in hospital patients, which suggests that poor oral
hygiene may be related to oral HPV infection and viral replication
(28). Additionally, the HPV16 DNA
positivity of gingival crevicular fluid was significantly
associated with the prevalence of Treponema denticola and
Fusobacterium nucleatum (23). It is thus considered that oral HPV
prevalence is related to the presence of periodontal bacteria.
Analysis of the microbiome shows a strong association between the
diversity of the vaginal microbiota and HPV infection (29). Vaginal HPV infection and its
persistent infection are characterized by a reduced abundance of
vaginal Lactobacillus spp. (29). A significant relationship between
carcinogenic HPV infection and the Prevotella genus was
found in the vagina of HIV-negative participants (30). These observations suggest that
changes in the microbiome may potentially facilitate vaginal HPV
infection. The composition of the oral microbiome has been shown to
reflect differences in the periodontal health condition (31). Prevotella- and
Veillonella-dominant oral microbiomes were associated with
the active phase of periodontitis amongst Japanese individuals
(31). Conversely,
Neisseria-, Haemophilus-, Aggregatibacter- and
Porphyromonas-abundant oral microbiomes reflected healthy
periodontal tissue (31). The
relationship between the oral microbiome and oral HPV infection has
not been fully elucidated, limited by the small number of studies
and small sample sizes in these studies (32,33). The
association between the composition of the bacterial microbiota and
HPV DNA in the oral cavity was investigated in 39 Finnish women
(32). Unclassified
Bifidobacteriaceae and Finegoldia genera were
significantly dominant, but the Haemophilus genus was less
numerous in HPV positive cases than in HPV negative cases (32). Capnocytophaga ochracea was
more abundant in HPV16 DNA positive periodontal granulation tissue
than in HPV negative tissues in Indians (33). It has been reported that the oral
viral community is significantly characterized according to the sex
of the host (34), which indicates
that human sex hormones may affect the composition of oral viromes.
Furthermore, it is hypothesized that aging may be a significant
factor affecting the composition of oral viromes as a result of
declining immune function. Therefore, it is necessary to consider
the effect of sex and age when evaluating the association between
oral HPV and periodontopathic bacteria. Further analysis of
microbial communities may provide greater insight into the
relationship between HPV and specific oral bacteria.
4. Association between herpes virus and
periodontitis
It is clear that the herpes virus is notably
associated with periodontitis. Herpes viruses such as herpes
simplex virus (HSV), human cytomegalovirus (HCMV) and Epstein-Barr
virus (EBV) were detected with a wide range of positive percentages
in gingivitis and chronic periodontitis (35-39).
In two previous independent meta-analyses, it was suggested that
oral EBV and HCMV are significantly associated with periodontitis
(35,37). According to a review by Slots
(38), the median prevalence of HSV,
HCMV and EBV was 45, 40 and 32% in chronic periodontitis and 12, 3
and 7% in healthy periodontal tissue, respectively (38). Co-infection of herpes virus and
periodontal disease-related bacteria induces the risk of
periodontitis (40,41). Herpes virus-induced local
proinflammatory cytokines in the presence of immunosuppression may
contribute to periodontitis (38).
Proinflammatory cytokines released by HCMV-infected gingival
fibroblasts serve an important role in attracting cytotoxic T-cells
and natural killer cells (41).
Attachment of Actinobacillus actinomycetemcomitans to
periodontal epithelial cells can be enhanced by HCMV (42). These results highlight the important
role of herpes virus in periodontitis.
5. Association between oral HPV and herpes
virus
Concurrent infection of the herpes virus and HPV was
found in individuals with advanced periodontitis (5). The prevalence of both HPV DNA and EBV
DNA in gingivitis and periodontitis tissue biopsies was 25% in
kidney transplant patients, but 0% in non-transplanted patients
(11). Kidney transplant patients
receiving immunosuppressive therapy exhibited high HPV and EBV DNA
positivity, which indicates that immunosuppressive conditions may
elicit susceptibility to concurrent viral infection in the
periodontium.
Several in vitro studies have been performed
to investigate the biological relationship between HPV and the
herpes virus (43-45).
HSV facilitated integration and amplification of the HPV genome in
HPV18 DNA-transfected cervical cancer cells (43). Co-expression of EBV latent membrane
protein-1 and HPV16 E6 induced malignant transformation in primary
mouse embryonic fibroblasts through NF-κB signaling (44). HPV can increase EBV genome stability
and lytic reactivation of EBV in oral keratinocytes (45), which indicates that HPV promotes the
pathogenicity of oral EBV. Collectively, these results suggest that
HPV and the herpes virus induce adverse oncogenic events.
6. Association of smoking with oral HPV
infection and periodontitis
Smoking causes the destruction of periodontal tissue
through microcirculatory dysfunction and impairment of host immune
systems (46). Therefore, smoking is
a significant risk factor for periodontal disease (47). Notably, smoking is thought to cause
dysbiosis of the periodontal microbiome (48), which suggests that the
smoking-induced imbalance in the microbiome is detrimental to
periodontal health. Several studies have demonstrated that smoking
is a major risk factor for oral HPV infection (49-54).
Our previous meta-analysis showed the association between oral HPV
infection and smoking (3). Current
smoking was a significant risk factor for oral HPV infection
(3). Furthermore, smoking increased
the duration of high-risk HPV infection in the oral cavity
(55). These results suggest that
smoking is a major risk factor for both oral HPV infection and
periodontitis. It is thus speculated that HPV tends to infect the
smoking-deteriorated periodontal tissue due to the suppression of
the host defense by smoking.
Chronic inflammation in periodontitis contributes to
the development of several types of cancer caused by carcinogens,
such as ROS, produced by activated inflammatory cells in response
to periodontal pathogens (56-59).
Periodontopathic bacteria are reported to be a risk factor for oral
squamous cell carcinomas (60,61).
Furthermore, a history of periodontitis is importantly associated
with the HPV status of patients with oral cavity cancer (62,63).
Therefore, it is hypothesized that periodontitis can increase the
possibility of adverse oncogenic events independently or
cooperatively with oncogenic HPV.
HPV and smoking are thought to interact in
carcinogenesis in the following manner. Smoking upregulates the
number of HPV genome copies and promotes integration of viral
genomes into the host genome in HPV-infected cells (64). Next, the HPV oncoproteins E6 and E7
inhibit p53 function, which results in accumulation of chromosomal
instability and loss of cell cycle control (64). Finally, HPV-induced immortalization
and tobacco smoke-associated DNA damage cause carcinogenesis
(64). Therefore, persistent
carcinogenic HPV infections induced through smoking may contribute
to the development of HPV-related oral cavity cancer.
7. Conclusion
HPV localizes to inflammatory periodontal tissue and
is thought to infect basal keratinocytes in the ulcerated gingival
sulcus epithelium. Inflammatory periodontal pockets serve a
significant role as a reservoir for HPV. Although the interactions
between HPV and periodontopathic bacteria remain unclear, oral HPV
infection may be associated with a characteristic oral microbiome.
Smoking induces destruction of periodontal tissue, and HPV then
tends to infect periodontal tissue due to the smoking-induced
suppression of the host defense. Carcinogenic HPV and periodontitis
are likely to contribute to the development of oral cavity cancers.
However, to date, oral HPV E6/E7 expression (transcriptionally
active HPV) has not been fully investigated in individuals with
periodontitis. Collectively, the available literature suggests that
oral HPV may be associated with periodontitis. To clarify the
association between oral HPV and periodontitis, the effects of
clinical factors contributing to oral HPV DNA prevalence should be
considered. Additionally, methods of sampling that can directly
detect HPV DNA in inflammatory periodontal tissues should be
further investigated.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
HS contributed to the conception of the study and
wrote the manuscript. MS and KO aided in writing the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
2
|
Kay P, Meehan K and Williamson AL: The use
of nested polymerase chain reaction and restriction fragment length
polymorphism for the detection and typing of mucosal human
papillomaviruses in samples containing low copy numbers of viral
DNA. J Virol Methods. 105:159–170. 2002.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shigeishi H and Sugiyama M: Risk factors
for oral human papillomavirus infection in healthy individuals: A
systematic review and meta-analysis. J Clin Med Res. 8:721–729.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Madinier I, Doglio A, Cagnon L, Lefèbvre
JC and Monteil RA: Southern blot detection of human
papillomaviruses (HPVs) DNA sequences in gingival tissues. J
Periodontol. 63:667–673. 1992.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Parra B and Slots J: Detection of human
viruses in periodontal pockets using polymerase chain reaction.
Oral Microbiol Immunol. 11:289–293. 1996.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hormia M, Willberg J, Ruokonen H and
Syrjänen S: Marginal periodontium as a potential reservoir of human
papillomavirus in oral mucosa. J Periodontol. 76:358–363.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Horewicz VV, Feres M, Rapp GE, Yasuda V
and Cury PR: Human papillomavirus-16 prevalence in gingival tissue
and its association with periodontal destruction: A case-control
study. J Periodontol. 81:562–568. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fuster-Rossello L, Ribotta E, Cuffini C
and Fuster-Juan M: Human papilloma virus in oral mucosa and its
association with periodontal status of gynecologically infected
women. Acta Odontol Latinoam. 27:82–88. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jacob A, Janam P and Babu Vijayamma JM:
Prevalence of human papilloma virus in marginal periodontium and
its association with periodontitis: A cross sectional study. J
Indian Soc Periodontol. 18:447–450. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wiener RC, Sambamoorthi U and Jurevic RJ:
Association of periodontitis and human papillomavirus in oral rinse
specimens: Results from the National Health and Nutrition Survey
2009-2012. J Am Dent Assoc. 146:382–389. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Baez CF, Savassi-Ribas F, Rocha WM,
Almeida SG, Gonçalves MT, Guimarães MA, Cavalcanti SM and Varella
RB: Association of Epstein-Barr virus (EBV) but not human
papillomavirus (HPV) with gingivitis and/or periodontitis in
transplanted individuals. Rev Inst Med Trop São Paulo.
58(58)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sun CX, Bennett N, Tran P, Tang KD, Lim Y,
Frazer I, Samaranayake L and Punyadeera C: A pilot study into the
association between oral health status and human papillomavirus-16
infection. Diagnostics (Basel). 7(E11)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ortiz AP, González D, Vivaldi-Oliver J,
Castañeda M, Rivera V, Díaz E, Centeno H, Muñoz C, Palefsky J,
Joshipura K, et al: Periodontitis and oral human papillomavirus
infection among Hispanic adults. Papillomavirus Res. 5:128–133.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ortiz AP, González D, Ramos J, Muñoz C,
Reyes JC and Pérez CM: Association of marijuana use with oral HPV
infection and periodontitis among Hispanic adults: Implications for
oral cancer prevention. J Periodontol. 89:540–548. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
McDaniel JT, Davis JM, McDermott RJ,
Maxfield I and Kapatamoyo K: Predicted prevalence of oral human
papillomavirus (HPV) by periodontitis status and HPV vaccination
status. J Public Health Dent. 80:132–139. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kwon T, Lamster IB and Levin L: Current
concepts in the management of periodontitis. Int Dent J: Dec 5,
2020 (Epub ahead of print). doi: 10.1111/idj.12630.
|
|
17
|
Socransky SS, Haffajee AD, Cugini MA,
Smith C and Kent RL Jr: Microbial complexes in subgingival plaque.
J Clin Periodontol. 25:134–144. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Abeles SR, Robles-Sikisaka R, Ly M, Lum
AG, Salzman J, Boehm TK and Pride DT: Human oral viruses are
personal, persistent and gender-consistent. ISME J. 8:1753–1767.
2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Slots J: Human viruses in periodontitis.
Periodontol 2000. 53:89–110. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dalla Torre D, Burtscher D, Sölder E,
Rasse M and Puelacher W: The correlation between the quality of
oral hygiene and oral HPV infection in adults: A prospective
cross-sectional study. Clin Oral Investig. 23:179–185.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Checchi L, Montevecchi M, Checchi V and
Zappulla F: The relationship between bleeding on probing and
subgingival deposits. An endoscopical evaluation. Open Dent J.
3:154–160. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Khurshid Z, Mali M, Naseem M, Najeeb S and
Zafar MS: Human gingival crevicular fluids (GCF) proteomics: An
overview. Dent J (Basel). 5(E12)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shigeishi H, Murodumi H, Ohta K and
Sugiyama M: Detection of HPV16 E6 DNA in periodontal pockets of
middle-aged and older people. Oral Sci Int. 18:50–55. 2021.
|
|
24
|
Smith EM, Johnson SR, Ritchie JM,
Feddersen D, Wang D, Turek LP and Haugen TH: Persistent HPV
infection in postmenopausal age women. Int J Gynaecol Obstet.
87:131–137. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Marks MA, Gravitt PE, Burk RD, Studentsov
Y, Farzadegan H and Klein SL: Progesterone and 17beta-estradiol
enhance regulatory responses to human papillomavirus type 16
virus-like particles in peripheral blood mononuclear cells from
healthy women. Clin Vaccine Immunol. 17:609–617. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Faraji F, Zaidi M, Fakhry C and Gaykalova
DA: Molecular mechanisms of human papillomavirus-related
carcinogenesis in head and neck cancer. Microbes Infect.
19:464–475. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ali A, Lassi ZS, Kapellas K, Jamieson L
and Rumbold AR: A systematic review and meta-analysis of the
association between periodontitis and oral high-risk human
papillomavirus infection. J Public Health (Oxf): Sep 11, 2020 (Epub
ahead of print). doi: 10.1093/pubmed/fdaa156.
|
|
28
|
Shigeishi H, Sugiyama M, Ohta K, Yokoyama
S, Sakuma M, Murozumi H, Kato H and Takechi M: High HPV16 E6 viral
load in the oral cavity is associated with an increased number of
bacteria: A preliminary study. Biomed Rep. 8:59–64. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mitra A, MacIntyre DA, Marchesi JR, Lee
YS, Bennett PR and Kyrgiou M: The vaginal microbiota, human
papillomavirus infection and cervical intraepithelial neoplasia:
What do we know and where are we going next? Microbiome.
4(58)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dareng EO, Ma B, Famooto AO, Adebamowo SN,
Offiong RA, Olaniyan O, Dakum PS, Wheeler CM, Fadrosh D, Yang H, et
al: Prevalent high-risk HPV infection and vaginal microbiota in
Nigerian women. Epidemiol Infect. 144:123–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Takeshita T, Nakano Y, Kumagai T, Yasui M,
Kamio N, Shibata Y, Shiota S and Yamashita Y: The ecological
proportion of indigenous bacterial populations in saliva is
correlated with oral health status. ISME J. 3:65–78.
2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tuominen H, Rautava S, Syrjänen S, Collado
MC and Rautava J: HPV infection and bacterial microbiota in the
placenta, uterine cervix and oral mucosa. Sci Rep.
8(9787)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chowdhry R, Singh N, Sahu DK, Tripathi RK,
Mishra A, Singh A, Mukerjee I, Lal N, Bhatt MLB and Kant R:
Dysbiosis and variation in predicted functions of the granulation
tissue microbiome in HPV positive and negative severe chronic
periodontitis. Biomed Res Int. 2019(8163591)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Abeles SR and Pride DT: Molecular bases
and role of viruses in the human microbiome. J Mol Biol.
426:3892–3906. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gao Z, Lv J and Wang M: Epstein-Barr virus
is associated with periodontal diseases: A meta-analysis based on
21 case-control studies. Medicine (Baltimore).
96(e5980)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rodrigues PM, Teixeira AL, Kustner EC and
Medeiros R: Are herpes virus associated to aggressive
periodontitis? A review of literature. J Oral Maxillofac Pathol.
19:348–355. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Botero JE, Rodríguez-Medina C,
Jaramillo-Echeverry A and Contreras A: Association between human
cytomegalovirus and periodontitis: A systematic review and
meta-analysis. J Periodontal Res. 55:551–558. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Slots J: Periodontal herpesviruses:
Prevalence, pathogenicity, systemic risk. Periodontol 2000.
69:28–45. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhu C, Li F, Wong MC, Feng XP, Lu HX and
Xu W: Association between herpesviruses and chronic periodontitis:
A meta-analysis based on case-control studies. PLoS One.
10(e0144319)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Imbronito AV, Okuda OS, Maria de Freitas
N, Moreira Lotufo RF and Nunes FD: Detection of herpesviruses and
periodontal pathogens in subgingival plaque of patients with
chronic periodontitis, generalized aggressive periodontitis, or
gingivitis. J Periodontol. 79:2313–2321. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Botero JE, Parra B, Jaramillo A and
Contreras A: Subgingival human cytomegalovirus correlates with
increased clinical periodontal parameters and bacterial coinfection
in periodontitis. J Periodontol. 78:2303–2310. 2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Teughels W, Sliepen I, Quirynen M, Haake
SK, Van Eldere J, Fives-Taylor P and Van Ranst M: Human
cytomegalovirus enhances A. actinomycetemcomitans adherence to
cells. J Dent Res. 86:175–180. 2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Hara Y, Kimoto T, Okuno Y and Minekawa Y:
Effect of herpes simplex virus on the DNA of human papillomavirus
18. J Med Virol. 53:4–12. 1997.PubMed/NCBI
|
|
44
|
Shimabuku T, Tamanaha A, Kitamura B,
Tanabe Y, Tawata N, Ikehara F, Arakaki K and Kinjo T: Dual
expression of Epstein-Barr virus, latent membrane protein-1 and
human papillomavirus-16 E6 transform primary mouse embryonic
fibroblasts through NF-κB signaling. Int J Clin Exp Pathol.
7:1920–1934. 2014.PubMed/NCBI
|
|
45
|
Makielski KR, Lee D, Lorenz LD, Nawandar
DM, Chiu YF, Kenney SC and Lambert PF: Human papillomavirus
promotes Epstein-Barr virus maintenance and lytic reactivation in
immortalized oral keratinocytes. Virology. 495:52–62.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ojima M and Hanioka T: Destructive effects
of smoking on molecular and genetic factors of periodontal disease.
Tob Induc Dis. 8(4)2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Mullally BH: The influence of tobacco
smoking on the onset of periodontitis in young persons. Tob Induc
Dis. 2:53–65. 2004.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hanioka T, Morita M, Yamamoto T, Inagaki
K, Wang PL, Ito H, Morozumi T, Takeshita T, Suzuki N, Shigeishi H,
et al: Smoking and periodontal microorganisms. Jpn Dent Sci Rev.
55:88–94. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Antonsson A, Cornford M, Perry S, Davis M,
Dunne MP and Whiteman DC: Prevalence and risk factors for oral HPV
infection in young Australians. PLoS One. 9(e91761)2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Cook RL, Thompson EL, Kelso NE, Friary J,
Hosford J, Barkley P, Dodd VJ, Abrahamsen M, Ajinkya S, Obesso PD,
et al: Sexual behaviors and other risk factors for oral human
papillomavirus infections in young women. Sex Transm Dis.
41:486–492. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Dahlstrom KR, Burchell AN, Ramanakumar AV,
Rodrigues A, Tellier PP, Hanley J, Coutlée F and Franco EL: Sexual
transmission of oral human papillomavirus infection among men.
Cancer Epidemiol Biomarkers Prev. 23:2959–2964. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Hang D, Liu F, Liu M, He Z, Sun M, Liu Y,
Li J, Pan Y, Ning T, Guo C, et al: Oral human papillomavirus
infection and its risk factors among 5,410 healthy adults in China,
2009-2011. Cancer Epidemiol Biomarkers Prev. 23:2101–2110.
2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Lang Kuhs KA, Gonzalez P, Struijk L,
Castro F, Hildesheim A, van Doorn LJ, Rodriguez AC, Schiffman M,
Quint W, Lowy DR, et al: Costa Rica Vaccine Trial Group: Prevalence
of and risk factors for oral human papillomavirus among young women
in Costa Rica. J Infect Dis. 208:1643–1652. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Gillison ML, Broutian T, Pickard RK, Tong
ZY, Xiao W, Kahle L, Graubard BI and Chaturvedi AK: Prevalence of
oral HPV infection in the United States, 2009-2010. JAMA.
307:693–703. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kero K, Rautava J, Syrjänen K, Willberg J,
Grenman S and Syrjänen S: Smoking increases oral HPV persistence
among men: 7-year follow-up study. Eur J Clin Microbiol Infect Dis.
33:123–133. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Michaud DS, Joshipura K, Giovannucci E and
Fuchs CS: A prospective study of periodontal disease and pancreatic
cancer in US male health professionals. J Natl Cancer Inst.
99:171–175. 2007.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Meyer MS, Joshipura K, Giovannucci E and
Michaud DS: A review of the relationship between tooth loss,
periodontal disease, and cancer. Cancer Causes Control. 19:895–907.
2008.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Chrysanthakopoulos NA: Periodontal disease
- cancer association and the specific role of periodontal disease
in lung cancer pathogenesis. Mathews J Dentistry. 3(018)2018.
|
|
59
|
Hirschfeld J, White PC, Milward MR, Cooper
PR and Chapple IL: Modulation of neutrophil extracellular trap and
reactive oxygen species release by periodontal bacteria. Infect
Immun. 85:e00297–e17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gholizadeh P, Eslami H, Yousefi M,
Asgharzadeh M, Aghazadeh M and Kafil HS: Role of oral microbiome on
oral cancers, a review. Biomed Pharmacother. 84:552–558.
2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mager DL, Haffajee AD, Devlin PM, Norris
CM, Posner MR and Goodson JM: The salivary microbiota as a
diagnostic indicator of oral cancer: A descriptive, non-randomized
study of cancer-free and oral squamous cell carcinoma subjects. J
Transl Med. 3(27)2005.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tezal M, Sullivan Nasca M, Stoler DL,
Melendy T, Hyland A, Smaldino PJ, Rigual NR and Loree TR: Chronic
periodontitis-human papillomavirus synergy in base of tongue
cancers. Arch Otolaryngol Head Neck Surg. 135:391–396.
2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Tezal M, Scannapieco FA, Wactawski-Wende
J, Hyland A, Marshall JR, Rigual NR and Stoler DL: Local
inflammation and human papillomavirus status of head and neck
cancers. Arch Otolaryngol Head Neck Surg. 138:669–675.
2012.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Wei L, Griego AM, Chu M and Ozbun MA:
Tobacco exposure results in increased E6 and E7 oncogene
expression, DNA damage and mutation rates in cells maintaining
episomal human papillomavirus 16 genomes. Carcinogenesis.
35:2373–2381. 2014.PubMed/NCBI View Article : Google Scholar
|