Introduction
Glycyrrhiza uralensis, also known as Guolao,
Lingtong, Sweet grass and Lolium, is the dried root and rhizome of
Glycyrrhiza uralensis Fisch., Glycyrrhiza inflata Bat
or Glycyrrhiza glabra L. Glycyrrhiza uralensis, and is
traditionally used as a root and a rhizome. However, its aerial
parts account for over one-third of the plant (1). Proper shoot trimming promotes the
accumulation of active substances in the roots and rhizomes
(2). A total of 61 flavonoids and 7
phenolic components have been isolated from the aerial part of
Glycyrrhiza uralensis (3).
Flavonoids possess antitumor, anti-AIDS, anti-ulcer,
anti-inflammatory, anti-aging and other beneficial pharmacological
properties (4). A total of 23
flavonoids have been identified by Bo et al, Soheila et
al and Zhang et al (5-7).
The incidence of prostatitis has been increasing
since 1995(8), and chronic
nonbacterial prostatitis (CNP) accounts for 90-95% of all
prostatitis cases (9), with
clinical symptoms including pelvic pain, frequent, urgent, painful
micturition and increased nocturnal urination. In our previous
study, it was reported that Glycyrrhiza uralensis exhibited
therapeutic efficacy against CNP in rats (10). However, the effective parts of the
aerial parts of Glycyrrhiza uralensis against CNP have not
been identified, and the pharmacodynamic compounds and mechanisms
remain unclear. Therefore, comparison of anti-CNP activity based on
the extraction and enrichment of different parts of the aerial part
of Glycyrrhiza uralensis, as well as determining the
anti-CNP active components of the aerial part of Glycyrrhiza
uralensis and their molecular mechanisms are vital.
Network pharmacology is based on the multi-layer
disease-gene-drug network, and is used to predict drug targets and
increase the efficiency of drug discovery (11). The concept of this research method
is based on the view that traditional Chinese medicine (TCM) exerts
improved overall therapeutic efficacy compared to the sum of its
parts (12). Numerous studies have
studied the interface between TCM and network pharmacology, and
several methods have emerged to integrate the study of genes
associated with disease and target information prediction, with the
active components of TCM (13-16).
The present study applied network pharmacology
(17) to obtain 23 flavonoids from
the aerial part of Glycyrrhiza uralensis, identified CNP
targets, and combined the biological target with the network to
determine the modes of action and pathways of the anti-CNP
constituents. This study provides insights into the
pharmacokinetics of these ingredients with anti-CNP activity. The
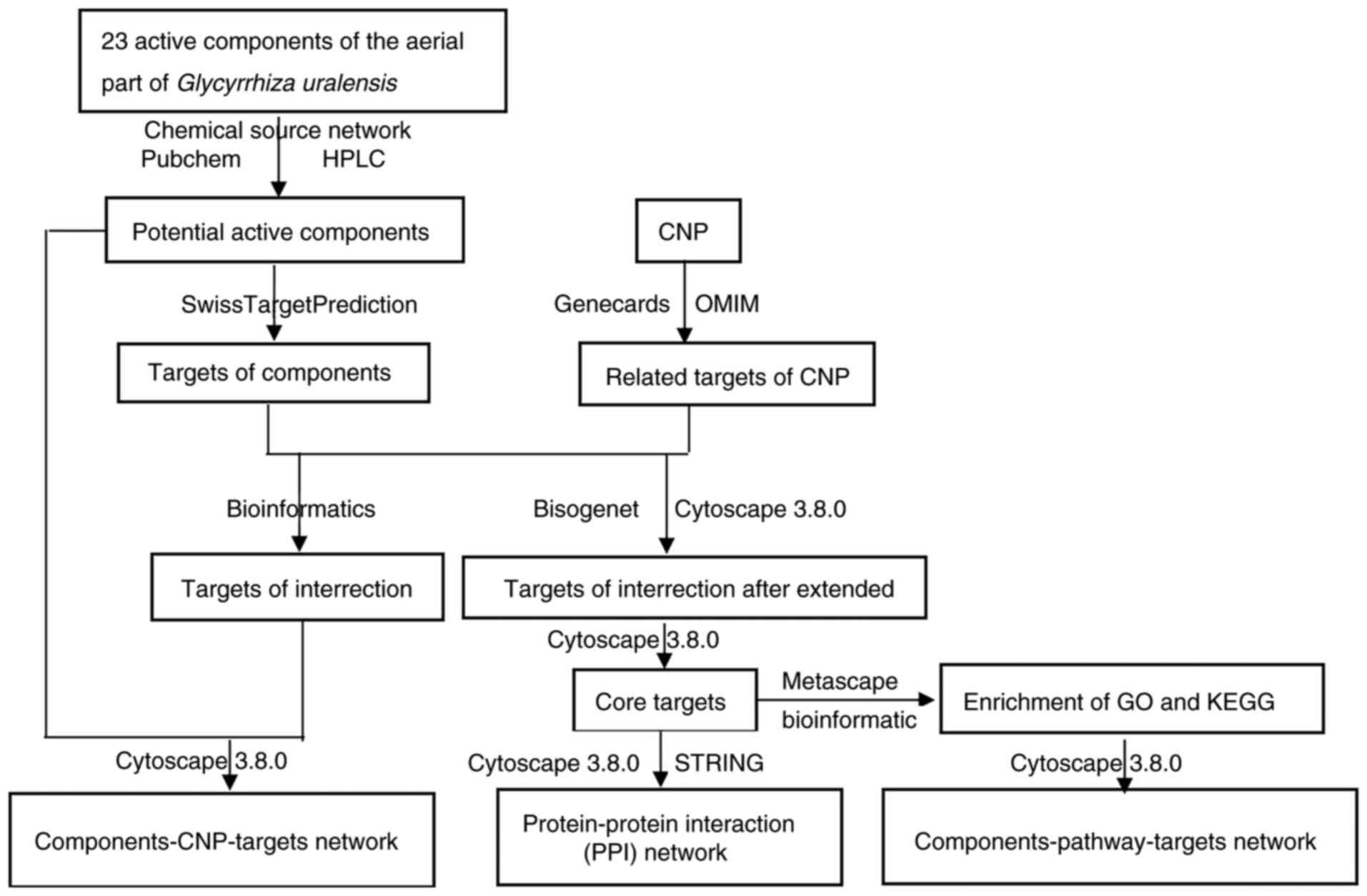
design of the network pharmacology study is illustrated in Fig. 1.
Materials and methods
Preparation of extracts from the
aerial part of Glycyrrhiza uralensis
The original materials of the aerial part of
Glycyrrhiza uralensis included the stems, leaves and fruit
pods; the stem:leaf weight ratio was ~4:1. The materials were
collected from Hedong Township, (Guazhou, Jiuquan, Gansu) in
September 2016. The plants were 7 years old and cultivated for
licorice production. Their aerial parts were identified by
Professor Wang Wenquan of the Beijing University of Traditional
Chinese Medicine (Beijing, China).
Laboratory animals and feeding
conditions
Healthy male Sprague-Dawley (SD) rats (180-200 g; 8
weeks old; animal certificate no. SLXD-20200902021), were purchased
from Beijing Weitong Lihua Experimental Animal Technology Co.,
Ltd.; the rats were fed a regular diet and had free access to
drinking water. All rats were handled according to the National
Guidelines for the Care and Use of Laboratory Animals, and all
animal experiments were approved by the Animal Ethics Committee of
the Chinese Academy of Medical Sciences and Institute of Medicinal
Plant Development (approval no. SLXD-20200902021). For humane
endpoints, the principle of minimal injury and trade-off was
followed to minimize pain and meet the needs of the experiment.
Euthanasia was performed when a wound did not heal after the
construction of a prostatitis model in rats.
Experimental instruments and
equipment
The following apparatus was used in the present
study: Multiskan FC Enzyme Marker (Thermo Fisher Scientific, Inc.);
UltiMate-3000 HPLC (Thermo Fisher Scientific, Inc.), Agilent SB-C18
column (250x4.6 mm, 5 µm) (Agilent Technologies, Inc.), N6000
ultraviolet spectrophotometer (Shanghai Yuke Instruments), FA 2004N
analytical balance (Shanghai Yuke Instruments); RE-52 rotary
evaporator (Shanghai Yarong Biochemistry Instrument Factory); 101-2
AB electrothermal blast dryer (Tianjin Tester Instrument Co.,
Ltd.); JJ-12J dehydrator (Wuhan Junjie Electronics Co., Ltd.);
JB-P5 (Wuhan Junjie Electronics Co., Ltd.); RM2016
histopathological slicer (Shanghai Leica Instrument Co., Ltd.);
JB-L5 freezing platform (Wuhan Junjie Electronics Co., Ltd.); KD-P
stand (Zhejiang Jinhua Cody Equipment Co., Ltd.); DHG-9140A oven
(Shanghai Huitai Instrument Manufacturing Co., Ltd.); 10212432C
slides and coverslips (Jiangsu Shitai Experimental Equipment Co.,
Ltd.); NIKON ECLIPSE CI Orthopedic Optical Microscope (Nikon
Corporation); NIKON DS-U3 imaging system (Nikon Corporation);
MM823LA6-NS microwave oven (Midea Microwave Appliance Manufacturing
Co., Ltd.); WD-9405A decolorization shaker (Beijing 61 Instrument
Factory); TYXH-II vortex mixer (Tianyue Electronics); GT1001
composition pen (Gene Tech); and NIKON ECLIPSE TI-SR positive
fluorescence microscope (Nikon Corporation). Other equipment
included surgical scissors, tweezers, curved needles, surgical
sutures and syringes.
Reagents and chemicals
The following reagents and test kits were used:
Carrageenan (Beijing Solarbio Science and Technology Co., Ltd.;
cat. no. 1202 A053); sodium cellulose (Tianjin Fuchen Chemical
Reagent Factory); chloral hydrate (Tianjin Fuchen Chemical Reagent
Factory); prostaglandin E2 (PGE2; cat. no. F2944-B), NF-κB (cat.
no. F8592-A), nitric oxide (NO; cat. no. A012-1-1), induced NO
synthase (iNOS; cat. no. A014-1-2), prostate-specific antigen (PSA;
cat. no. F3343-B) and malondialdehyde (MDA; cat. no. A003-1-2)
(Nanjing Institute of Bioengineering or Beijing Baioske Biomedical
Technology Co., Ltd.); IL-1β (cat. no. GB11113), TGF-β (cat. no.
GB11179), connective tissue growth factor (cat. no. 23936-1-AP),
monocyte chemoattractant protein-1 (MCP-1; cat. no. GB11199), TNF-α
(cat. no. GB13188-2), α-smooth muscle actin (α-SMA; cat. no.
GB13044) all from Wuhan Seville Biotechnology Co., Ltd. or Thermo
Fisher Scientific, Inc.; G1203EDTA (pH 9.0) antigen repair solution
(Wuhan Google Biotechnology Co., Ltd.); G0002PBS buffer (Wuhan
Google Biotechnology Co., Ltd.); EDTA (pH 8.0) antigen repair
solution (Wuhan Google Biotechnology Co., Ltd.); G1202 citric acid
(pH 6.0) antigen repair solution (Wuhan Google Biotechnology Co.,
Ltd.); AR1010 normal rabbit serum (Boster Biological Technology);
G1004 hematoxylin dye solution (Wuhan Google Biotechnology Co.,
Ltd.); K5007 II (Dako; Agilent Technologies, Inc.); K5007
histochemical kit DAB chromogenic agent (Dako; Agilent
Technologies, Inc.); acetonitrile (chromatographic purity),
methanol (chromatographic purity) (Thermo Fisher Scientific, Inc.);
formic acid (Beijing Chemical Plant); and Qianlukang (Zhejiang
Kangenbei Pharmaceutical Co., Ltd.; cat. no. Z33020479; lot no.
190306).
Test substance preparation
For water extraction, 4.2 kg stem and leaf powder
was decocted twice for 2 h at 100˚C and was then concentrated. A
total of 1 g extract was equivalent to 6.33 g raw product. For
ethanol extraction, 17.8 kg stem and leaf powder was extracted
twice for 17 min in 70% ethanol at 70˚C and was then concentrated.
A total of 1 g extract was equivalent to 7.8 g raw product.
HPLC analysis of the components in the
test substance
The chromatographic conditions were as follows:
Agilent SB-C18 column (250x4.6 mm, 5 µm), acetonitrile as the
mobile phase A, and 0.5% formic acid-water as the mobile phase B.
The elution gradient is indicated in Table I. The column temperature was set at
30˚C with a flow rate of 0.8 ml/min. The detection wavelength was
280 nm, and the injection volume was 10 µl.
 | Table IFlow phase gradient elution
conditions. |
Table I
Flow phase gradient elution
conditions.
| Time, min | Acetonitrile,
% | Formic acid-water,
% |
|---|
| 0-5 | 10→15 | 90→85 |
| 5-22 | 15→22 | 85→78 |
| 22-40 | 22→40 | 78→60 |
| 40-52 | 40→50 | 60→50 |
| 52-76 | 50→85 | 50→15 |
| 76-77 | 85→10 | 15→90 |
| 77-87 | 10→10 | 90→90 |
Sample solutions were prepared by accurately
weighing 1 g sample powder in a conical flask, adding 10 ml 70%
(v/v) ethanol, weighing the mixture, ultrasonicating (100 W) for 30
min, cooling it, reweighing, making up the lost volume by topping
it up with 70% (v/v) ethanol, shaking by hand, filtering and
collecting the filtrate.
Pharmacodynamics study
A total of 40 clean, healthy male SD rats (180-200
g; 8 weeks) were used in the present study. To produce the CNP
model, the rats were anesthetized using 10% chloral hydrate (300
mg/kg) by intraperitoneal injection. The rats were considered
completely anesthetized when the rats' paws were pressed hard and
the rats did not exhibit a response. No symptoms of peritonitis
were observed in the rats after anesthesia; thus, the skin on the
middle of the lower abdomen was disinfected and an incision of ~2
cm was aseptically made. Then, 0.1 ml carrageenan in saline was
injected into the rats in the treatment group, while an equal
volume of saline was injected into the rats in the control group.
The incisions were closed with sutures, and the wounds were
disinfected with iodine. After producing the model, the rats were
then treated accordingly. A total of 24 rats with induced
prostatitis were randomly divided into three different treatment
groups: Water extract (1,000 mg/kg), ethanol extract (1,000 mg/kg)
and Qianzhikang (586 mg/kg) groups; each group consisted of 8 rats.
Rats in the five treatment groups were administered the drugs once
daily. The blank control consisted of 8 sham-operated rats. The
control and model rats received 0.7% CMC-Na (20 ml/kg) by
continuous gavage for 30 days via gastric administration. In our
previous study, 1,000 mg/kg of licorice was determined to induce
anti-CNP activity. The dose administered to each rodent was
obtained by flavonoid conversion.
A total of 30 days later, the rats (~300 g) were
anesthetized. Then, 10 ml blood was collected from each rat's
abdominal aorta. After collecting the blood, each rat was
euthanized by cervical vertebrae dislocation; rats were considered
dead after the heartbeat ceased. The organs were collected
immediately after confirmation of death.
Detection of body weight and organs of
rats
By day 30, rat hair, diet, activity, body weight,
visceral lesions and visceral index (combination of prostate index,
thymus gland index, cardiac index, kidney index, adrenal index and
spleen index) were observed and measured. The visceral index as a
percentage was calculated as: [weight of the viscera (g)/weight of
the body (g)] x 100.
Multigroup comparisons of the means were carried out
using a one-way ANOVA with a post hoc Duncan's test.
Assessment of the prostate tissue in
rats Hematoxylin and eosin (HE) staining of prostate tissue
Patients with CNP show glandular atrophy, glandular
epithelial cell shedding, necrosis, interstitial lymphocytic
infiltration, tissue fibrosis hyperplasia and other lesions
(18). HE staining can be used to
observe tissue structure, inflammatory cell infiltration and to
analyze the pathological morphology of prostate tissue.
The prostate tissue was fixed in 4% paraformaldehyde
for 30 h at room temperature. After dressing the fixed prostate
tissue with a scalpel, the tissue was dehydrated with anaerobic
ethanol, paraffin embedded, sliced to 4-6 µm. The steps were as
follows i) Sections were deparaffinized in xylene for 5-10 min. ii)
Sections were moved into a mixture of xylene and pure alcohol (1:1)
for about 5 min. iii) Sections were hydrated in 100, 95, 85 and 70%
alcohol solutions for 2-5 min. iv) Tissues were stained with
hematoxylin for 5-15 min. v) Excess dye was washed and sections
were placed in 0.5-1% hydrochloride alcohol (70% alcohol prepared)
for 10 sec. vi) Sections were rinsed with running water for 15-30
min. vii) Sections were stained with 0.1-0.5% eosin for 1-5 min.
viii) Sections were dehydrated with 70, 85, 95 and 100% alcohol at
all levels for 2-3 min. ix) Sections were cleared using xylene
(secondary) for ~10 min. x) Sealing, excess xylene around the
section was wiped without allowing the fixative to dry, and then
the slide was placed quickly in an appropriate amount of neutral
gum, to allow the cover glass to be sealed. All of the above steps
were performed at room temperature. The nucleus was stained blue,
and the cytoplasm was red.
Observation and evaluation methods of
HE slices
After HE staining of the prostate tissues, the
pathological changes of the tissue were observed under a light
microscope (magnification, x400), and the morphology of prostate
glands, infiltration of inflammatory cells in prostate stroma and
hyperplasia of fibrous tissue were recorded. Based on previous
findings (19), the pathological
changes of tissues were graded and the scoring criteria were
established as follows: i) Morphology of glands scoring: 2 points,
the prostate gland was complete, glandular epithelial cells
arranged neatly, folded into the lumen to form a fold; 4 points,
glandular epithelium was damaged, and glandular epithelial folds
were reduced; and 6 points, the glandular epithelium was severely
damaged and deformed, and the glandular epithelium folds were
reduced or absent. ii) Degree of infiltration of inflammatory
cells: 2 points, occasional inflammatory cell infiltration in the
glandular interstitium; 4 points, moderate inflammatory cell
infiltration in the glandular stroma; and 6 points, extensive
inflammatory cell infiltration in the glandular stroma. iii) Degree
of fibrous tissue hyperplasia: 2 points, occasional fibrous tissue
hyperplasia; 4 points, moderate fibrous tissue hyperplasia; and 6
points, extensive fibrous tissue hyperplasia.
Masson staining of prostate
tissue
Prostate tissue in patients with CNP is often
accompanied by a large number of fibrous connective hyperplasia and
a hard texture (20). Masson
staining can dye the fibrous tissue blue, which is used to analyze
the degree of fibrosis and hyperplasia of prostate tissue.
After dissecting the rats, the prostate tissue was
taken and immediately fixed as described above. After the fixed
prostate tissue was obtained with a scalpel, it was dehydrated,
embedded in paraffin and sliced (4 µm thick). The following
procedures were then performed: Sections were washed with distilled
water and nucleus was stained with Weigert's hematoxylin (BIOSS,
Beijing; cat. no. S0082-2) for 5-10 min, then washed with distilled
water. Tissues were next stained with Ponceau de xylidine-acid
fuchsin mixture (Gurr) for 5-10 min. Then sections were immersed in
2% aqueous glacial acetic acid solution for 2 min. Sections were
differentiated with an aqueous solution of 1% phosphate-molybdate
acid for 3-5 min. Then they were directly stained with light green
SF yellowish (BIOSS, Beijing; cat. no. D10419) for 5 min and
immersed for 2 min in aqueous 0.2% glacial acetic acid. Excess dye
was wiped using 95% alcohol, then dehydrated using anhydrous
alcohol and cleared using xylene, followed by sealing using the
neutral gum, as described above. All of the above steps were
performed at room temperature. The collagen fibers, mucus and
cartilage were stained blue, muscle fibers, cellulose and red blood
cells were stained red, and the nucleus was stained blue-black.
Image Pro-Plus 6.0 (Media Cybernetics) analysis
software was used for quantitative analysis. A total of 5
non-overlapping fields were randomly selected for each slice. The
degree of fibrosis in the prostate tissue was analyzed by
calculating the area of positive staining (area) and the cumulative
optical density (IOD). The larger the area of fibrosis and the IOD
were, the higher the degree of fibrosis and hyperplasia in the
prostate tissue was.
IHC staining of prostate tissue
The expression of inflammatory and fibrotic factors
in the prostate tissue of patients with CNP is increased (21,22).
IHC staining was used to analyze the distribution and content of
certain inflammatory factors qualitatively and quantitatively in
the tissue sections using specific antibodies (Rabbit antibodies).
After dehydration of paraffin sections, sections were boiled in
EDTA Antigen Repair Buffer (pH 9.0), and subsequently, the dewaxed
sections were placed in repair fluid for 2 min. The sections were
allowed to cool passively to room temperature. Slides were removed
and washed with PBS three times, 5 min per wash. Sections were
soaked in 3% H2O2 for 20 min and washed with
PBS three times, 5 min prewash. Tissues were next incubated with
rabbit serum (cat. no. AR1010; Boster) or 10 min at room
temperature. Next, tissues were incubated with the primary
antibodies: MCP-1 antibody (cat. no. 45071; Signalway Antibody LLC;
1:100), TNF-α Monoclonal antibody (cat. no. 44073; Signalway
Antibody LLC; 1:100), TGF-β antibody (cat. no. 5559-100; BioVision,
Inc.; 1:200), α-SMA Monoclonal Antibody (cat. no. 40482; Signalway
Antibody; 1:200)] all diluted in PBS (cat. no. G0002; Cellway) at
37˚C for 1-2 h, followed by incubation with the goat anti-rabbit
IgG secondary antibodies (cat. nos. ZI215-1 or ZS402-2; ZOMANBIO;
1:1,000) at 37˚C for 10-30 min, and the corresponding inflammatory
factors in the tissue were dyed brownish/yellow to analyze the
expression intensity (IOD value) of inflammatory factors in
prostate tissue.
Selection of observation
indicators
Rat hair, diet, activity, body weight, visceral
lesions and the viscera index were measured and observed during the
pharmacological experiments. The viscera index comprised the
prostate and thymus indices. Additionally, the expression levels of
the serum detection factors MCP-1, TNF-α, PGE2 and NF-κB were
determined.
To observe and evaluate the hair, diet, activity,
body weight, organ lesion and organ index of rats during the
pharmacological experiments, and to evaluate the prostate gland
morphology, inflammatory factor infiltration, fibrosis hyperplasia,
expression of inflammatory factor and fibrotic factor using HE
staining, Masson staining and IHC staining, the expression of
MCP-1, TNF-α, PGE2 and NF-κB in rat serum was measured. By
analyzing the effect of each administration group on the expression
of serum inflammatory factors, the anti-CNP activity of different
extraction and separation components on the aerial part of
Glycyrrhiza uralensis was evaluated, and the basis of
pharmacodynamics was determined.
Methods of staining IHC sections
The prostate tissue was fixed in 4% paraformaldehyde
for >24 h at room temperature. After the fixed prostate tissue
was repaired with a scalpel, the prostate tissue was dehydrated
with distilled water for 1 h, embedded in paraffin and sliced into
4 µm thick sections. After dewaxing, antigen retrieval was
performed using EDTA antigen repair buffer (pH 9.0) and sections
were placed in a boiling water bath. After boiling for 15 min,
tissues were allowed to cool passively to room temperature. The
endogenous peroxidases were quenched using 3% hydrogen peroxide
solution. The primary and secondary antibodies as well as DAB
chromogenic solution were added, and the nucleus was re-stained
with Harris hematoxylin for 3-15 min at room temperature. The
slices were dehydrated with distilled water for 1 h, cleared until
they were transparent, dried and sealed using neutral gum. The
positive expression in tissue appeared brownish/yellow.
Evaluation method of IHC sections
IHC staining can dye the positively expressing
tissue brownish/yellow. Image Pro-Plus software was used for
quantitative analysis. A total of 5 non-overlapping fields of
vision were randomly selected for each slice. The expression of
related factors was analyzed by calculating the IOD of the positive
staining area. The greater the IOD value, the greater the
expression of the associated factors, which helps to determine the
therapeutic effect of each drug on CNP.
Serum sampling and detection of serum
inflammatory factor expression in rats
A total of 24 h after the final treatment, blood
samples were obtained from the abdominal aorta, placed at 25±1˚C
for 0.5 h, and centrifuged using a frozen centrifuge at 1,006 x g
for 10 min; the supernatant was obtained (serum). Detection of
inflammatory factors was performed according to the kit's
instructions. The absorbance A was detected at 450 nm, and the
content of each factor was, then, calculated.
Network pharmacology analysis Target
prediction of the 23 components in the aerial part of Glycyrrhiza
uralensis
All structural formulae, based on the components of
the aerial part of Glycyrrhiza uralensis determined
previously (7), were introduced
into SwissADME (swissadme.ch/) to screen for 17
chemical components (excluding Schaftoside, Puerarin, Vicenin-2,
Isoschaftoside, Isoquercitrin, Isoliquiritin; Table II) that would exhibit
gastrointestinal absorption and drug-like efficacy; at least two
‘yes’ in the drug-like efficacy predictions and gIabsortion
absorption was considered ‘High’. The levels of the 6 components
(Schaftoside, Puerarin, Vicenin-2, Isoschaftoside, Isoquercitrin
and Isoliquiritin) were higher than those for the 17 chemical
components selected in SwissADME. Hence, the structures of the 23
components were imported into SwissTargetPrediction (swisstargetprediction.ch/). Homo sapiens
was used as the research object and all other parameters were left
as the system defaults. The chemical components corresponding to
human target proteins and their corresponding genes were noted and
their targets were predicted.
 | Table IIThe 23 chemical components in the
aerial parts of Glycyrrhiza uralensis. |
Table II
The 23 chemical components in the
aerial parts of Glycyrrhiza uralensis.
| No. | CAS | Name | Chemical
formula | Type |
|---|
| MOL 1 | 491-70-3 | Luteolin |
C15H10O6 | Flavone |
| MOL 2 | 480-41-1 | Naringenin |
C15H12O5 | Flavone |
| MOL 3 | 446-72-0 | Genistein |
C15H10O5 | Isoflavone |
| MOL 4 | 520-34-3 | Diosmetin |
C16H12O6 | Flavone |
| MOL 5 | 480-19-3 | Isorhamnetin |
C16H12O7 | Flavone |
| MOL 6 | 480-39-7 | Pinocembrin |
C15H12O4 | Flavonone |
| MOL 7 | 548-83-4 | Galangin |
C15H10O5 | Flavonol |
| MOL 8 | 116709-70-7 |
Glycyrrhisoflavone |
C20H18O6 | Prenylated
Isoflavone |
| MOL 9 | 51225-30-0 | Wighteone |
C20H18O5 | Prenylated
Flavonoid |
| MOL 10 | 109605-79-0 | Topazolin |
C21H20O6 | Prenylated
Flavonoid |
| MOL 11 | 486-66-8 | Daidzein |
C15H10O4 | Isoflavone |
| MOL 12 | 34221-41-5 | Retrochalcone |
C16H14O4 | Chalcone |
| MOL 13 | 485-72-3 | Formononetin |
C16H12O4 | Isoflavone |
| MOL 14 | 51938-32-0 | Schaftoside |
C26H28O14 | Flavonoid
Dioxide |
| MOL 15 | 3681-99-0 | Puerarin |
C21H20O9 | Isoflavone
Oxyglycoside |
| MOL 16 | 23666-13-9 | Vicenin-2 |
C27H30O15 | Flavonoid Double
Carbon Glycoside |
| MOL 17 | 52012-29-0 | Isoschaftoside |
C26H28O14 | Flavonoid
Dioxide |
| MOL 18 | 21637-25-2 | Isoquercitrin |
C21H20O12 | Flavonol
Glycoside |
| MOL 19 | 5041-81-6 | Isoliquiritin |
C21H22O9 | Chalcone Oxide |
| MOL 20 | 63631-41-4 | Arvensan |
C17H18O4 | Benzopyrone |
| MOL 21 | 520-18-3 | Kaempferol |
C15H10O6 | Flavone |
| MOL 22 | 139163-15-8 | Uralenol |
C20H18O7 | Prenylflavonol |
| MOL 23 | 126716-36-7 | Gancaonin I |
C21H22O5 | Prenylated
Flavonoid |
CNP target prediction
CNP-related targets with ‘Chronic Nonbacterial
Prostatitis’ were searched for in Genecards (genecards.org/) and in Gene Map in OMIM (omim.org/). The data obtained in Genecards and Gene
Map were merged using Excel2016 (Microsoft Corporation). Data for
the coinciding gene entries including the corresponding protein
names and gene entry IDs were eliminated from the Excel spreadsheet
to obtain data for genes and target proteins associated with
CNP.
Construction of the
component-CNP-target gene network
A Venn diagram was plotted in Bioinformatics
(bioinformatics.psb.Ugent.be/webtools/Venn/) to
obtain potential CNP-associated target genes for the 23 active
compounds. The network was generated using Cytoscape version
3.8.0(23) to illustrate the
complex relationships amongst the genes, aerial part components and
CNP. In the network, compounds, genes and CNP were represented by
nodes, whereas their interactions were indicated by edges. The
value for each node was calculated and represented as the number of
edges linked to it. In this manner, the major nodes in the network
were characterized. The probability that a component was a key
ingredient in CNP treatment increased with node value.
Construction of the protein-protein
interaction (PPI) networks
The targets of the chemical components and CNP were
uploaded into the Cytoscape plug-in Bisogenet (24) to expand the target. The intersecting
targets of the expanded chemical components and CNP were selected.
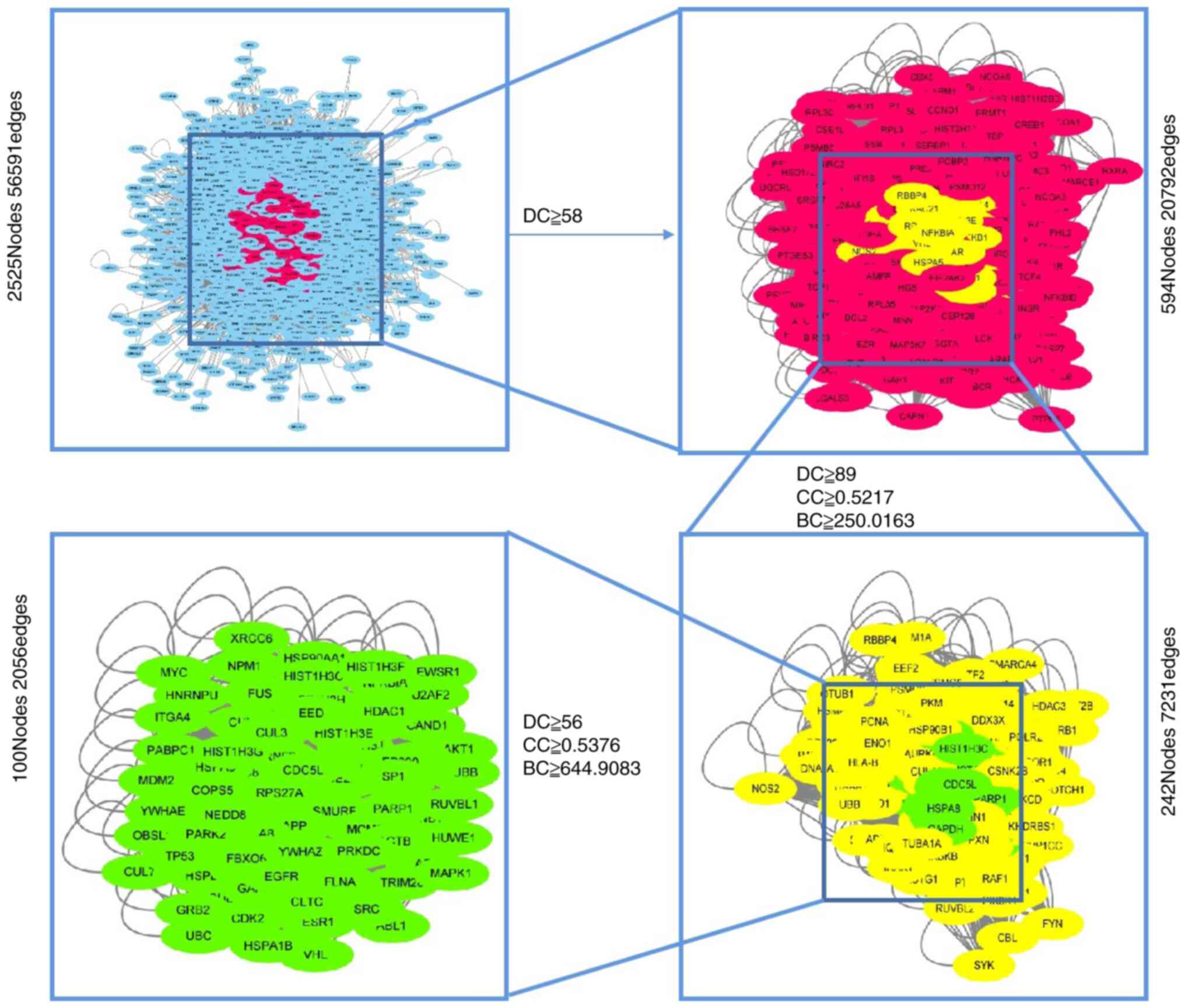
Filtration of the ‘Degree’ median was performed twice to obtain
network 1 whereas network 2 was obtained via a single filtration of
the ‘Degree’, ‘Closeness’ and ‘Betweenness’ medians in network 1.
Network 3 was obtained using a single filtration of the ‘Degree’,
‘Closeness’ and ‘Betweenness’ medians in network 2, and included
100 nodes and 2,056 edges. The screening flow chart is shown in
Fig. 2. The 100 nodes were regarded
as core targets and placed in Search Tool for the Retrieval of
Interacting Genes/Proteins (STRING; tring-db.org/cgi/input.pl) to build the PPI
interaction network. Cytoscape was used to construct and visualize
the PPI network. ‘Degree’ refers to the number of nodal connections
in the entire network and reflects the interactions amongst nodes.
The ‘Degree’ value was proportional to the core target
importance.
Gene ontology (GO) and kyoto
encyclopedia of genes and genomes (KEGG) pathway enrichment
analyses
Metascape (metascape.org/gp/index.html#/main/step1) was used to
process the data and visualize the results of the GO (25,26)
enrichment and KEGG (27) pathway
analyses. GO enrichment included biological processes (BP),
molecular functions (MF) and cellular components (CC). KEGG
enrichment identified the potential biological pathways and
functions associated with the target. The minimum overlap was set
to 3 and the P-value cutoff was set to 0. A minimum value of 1.5
indicated significant enrichment. The first 20 entries were
selected, and the results were visualized using a Venn diagram
(bioinformatics.psb.ugent.be/webtools/Venn/).
Construction of the chemical
component-pathway-target network
The top 20 pathways were selected according to the
number of genes on the KEGG pathways, corresponding to the 23
chemical components of the licorice aboveground part to the core
targets, to build a chemical component-pathway-target network.
Statistical analysis
Data analysis was performed using SAS version 9.0
(University of North Carolina), Origin 2018 64 Bit (American
OriginLab Corporation) and Excel 2016. A Kolmogorov-Smirnov test
was used to determine the normality of distribution, and variance
analysis was assessed using a one-way ANOVA with a post hoc Tukey's
test. The HE staining score was analyzed using a non-parametric
Kruskal-Wallis test with a Dunn's post hoc test, and the data are
presented as box plots of the median and interquartile range.
Multiple comparisons of the means in pharmacological experiments
were performed using a one-way ANOVA with a post hoc Tukey's test.
Each group of results consisted of 8 repeats. P<0.05 was
considered to indicate a statistically significant difference.
Therefore, it is advised to reanalyze these data
using non-parametric tests such as Kruskal-Wallis test with a
Dunn's post hoc test, which do not assume that the analyzed data
are continuous, and to also present the data as box plots of the
median and interquartile range.
Results
Analysis of pharmacological
experiments Weight changes in rats
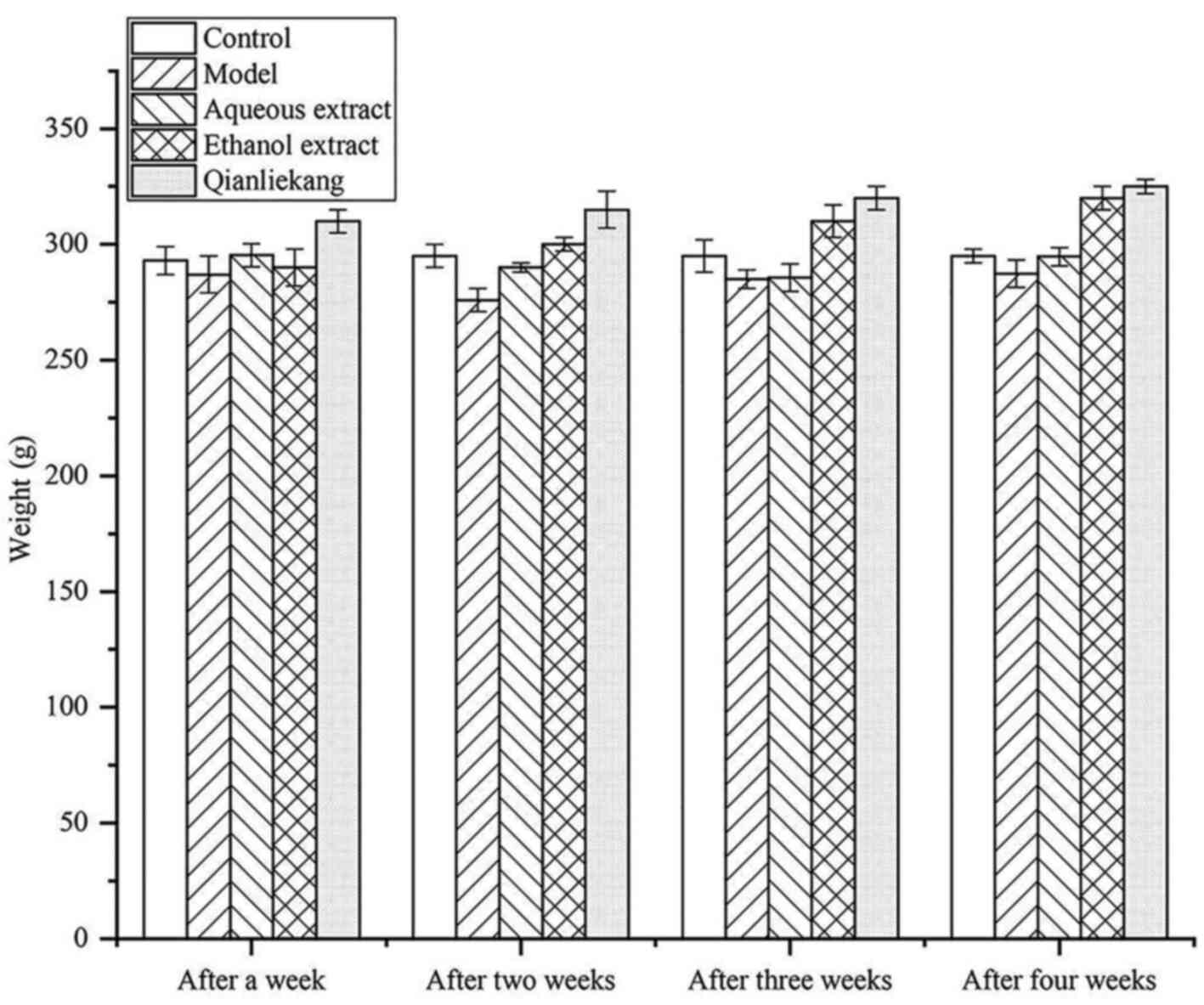
Changes in rat body weights are illustrated in
Fig. 3. The average body weight of
rats in each group was similar after 1 week of treatment with no
significant differences compared with the control group. After 2
weeks of treatment, the body weight in the model group was slightly
lower than that in the sham group, but not significantly different.
After 4 weeks of treatment, the body weight in the ethanol
extraction group was slightly lower than that in the other groups,
but not significantly different.
These results showed that there was no significant
difference in body weight amongst the groups during treatment,
indicating that the drug did not affect the weight of the rats.
Effects of the different extraction
and enrichment parts on viscera anatomy
The rats in the sham group were irritable, excited,
hirsute and exhibited weight loss, with a red, glossy prostate
tissue surfaces and soft, elastic glandular tissue. The prostate
glands in the model group were red and swollen. There were no
abnormal lesions in any other organ.
Effects of the different extraction
and enrichment parts on the viscera index
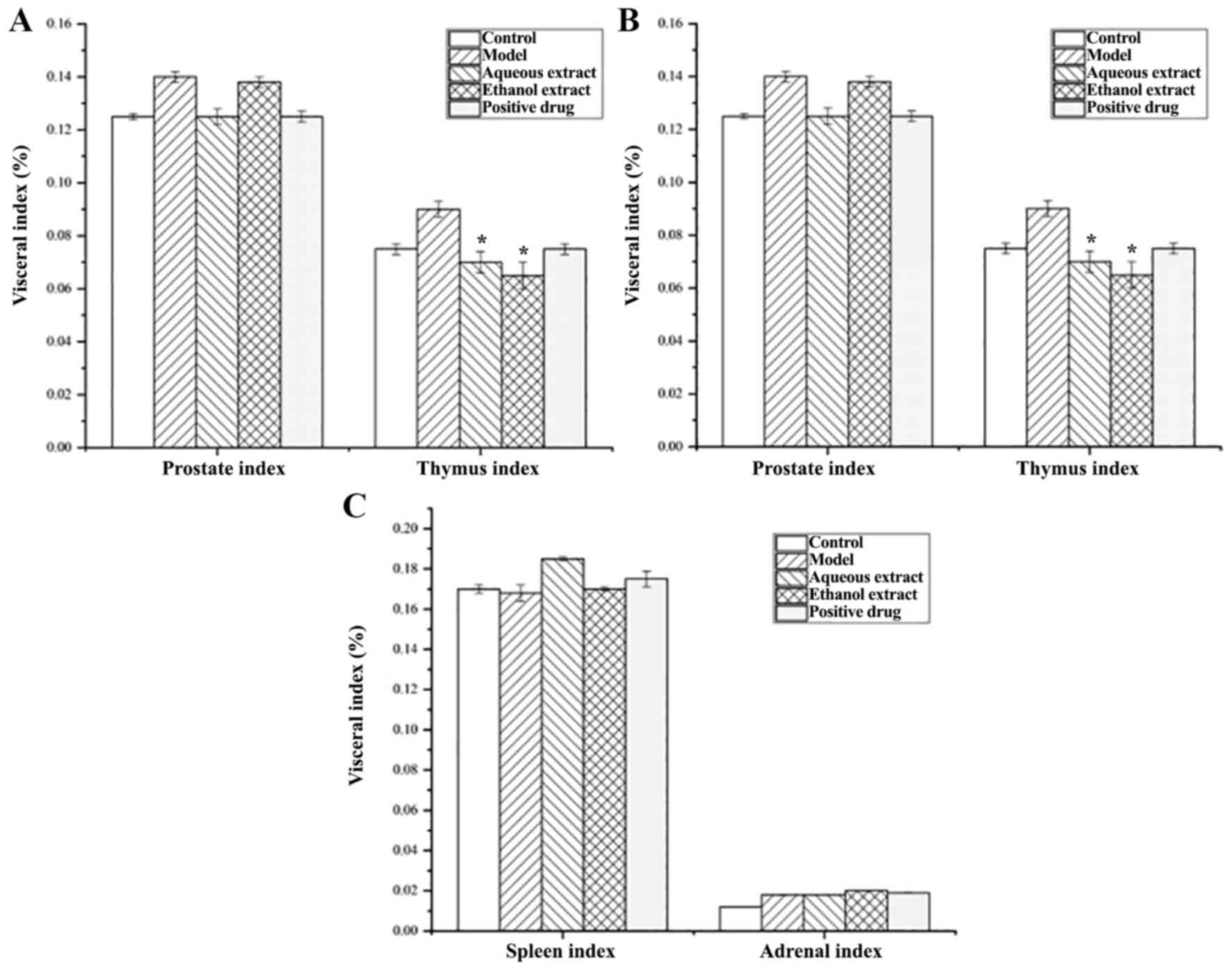
Fig. 4 shows that
the model group had a slightly higher prostate index than that of
the sham group, but the difference was not significant. The
prostate index decreased in the water extraction groups, and the
decrease was greater than that of the Qianluikang group, but not
significantly different compared with the model group. Furthermore,
the model group had a slightly higher thymus index than the sham
operation group, but it was not significant. The thymus index of
the water and ethanol extraction groups were significantly lower
than that of the model group (all P<0.05).
In terms of renal index, there was no significant
differences amongst the water extraction and Qianliekang groups
compared with the model group, which was abnormally high; there was
no significant difference between the cardiac, spleen and adrenal
indices.
These results show that the organs most likely to be
affected by CNP were the prostate and thymus; the water extraction
groups showed a decreasing trend of prostate index and thymus index
compared with the model group.
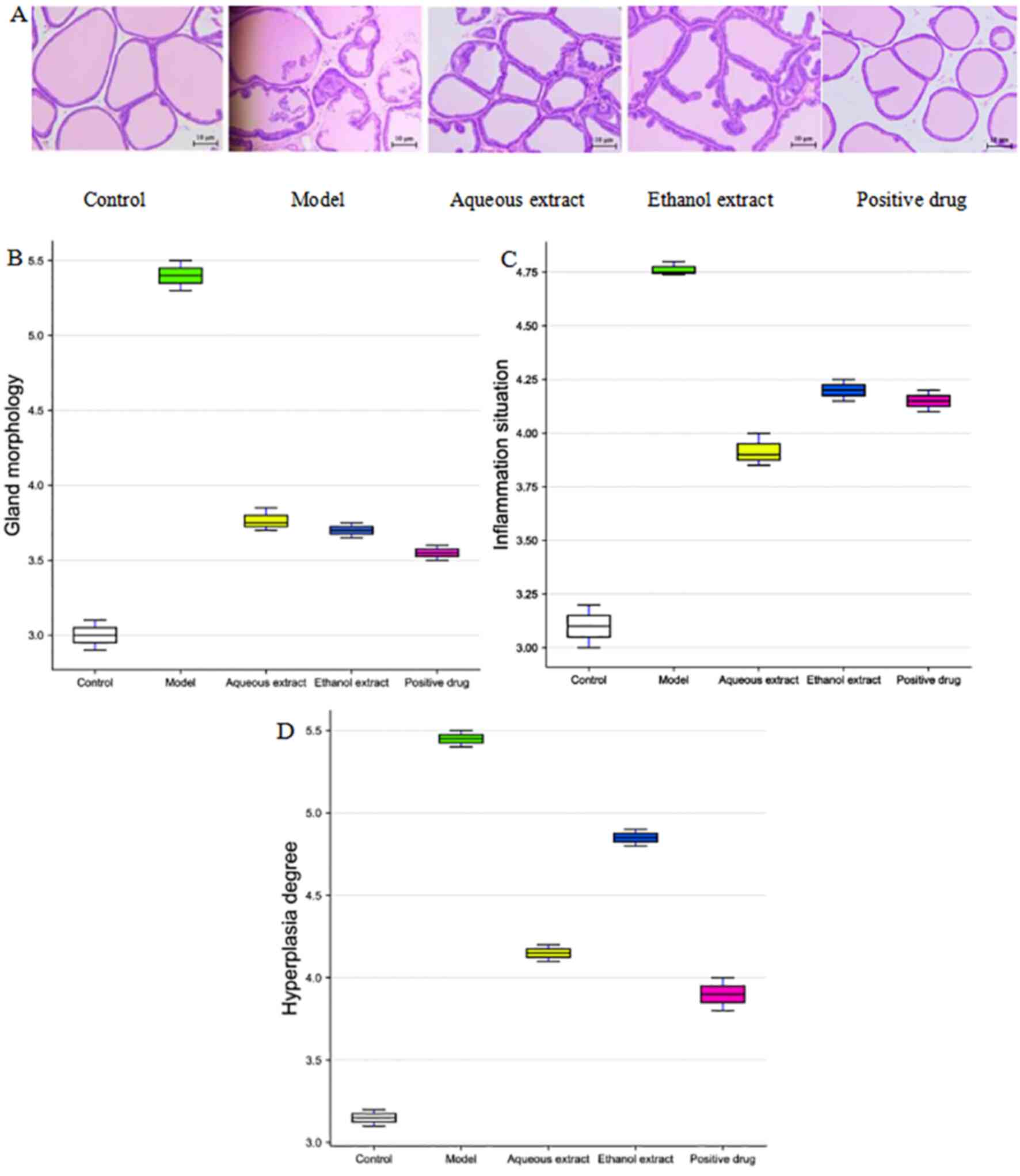
Pathological morphology of prostate
tissue
The HE-stained pathological sections of the rat
prostate tissues are shown in Fig.
5A. The prostate gland of rats in the sham group was intact
without atrophy, and the single-layer cell structure of the
glandular epithelium was also intact. There was no inflammatory
cell infiltration, no fibrous tissue hyperplasia and abundant
secretion in the glandular space. In the model group, the prostate
gland of rats was severely deformed, the gland was atrophied and
the gland epithelium was exfoliated and necrotic. There was
extensive inflammatory cell infiltration in the gland stroma, and
the secretions in the gland cavity were reduced, whilst some were
colorless. The prostate glands of rats in the water and alcohol
extraction groups were slightly deformed, significant inflammatory
cell infiltration was observed and the secretions in the glands
were slightly reduced. In summary, the inflammatory cells in the
glandular space of the water, alcohol and Qianliekang groups were
reduced to varying degrees, and the most obvious reductions were
observed in the water groups.
The HE staining scores of the prostate tissue in the
rats are shown in Fig. 5B-D. The
results showed that compared with the sham group, the scores of
gland morphological deformation, inflammatory cell infiltration and
fibrous tissue hyperplasia in the model group were increased. In
terms of prostate gland morphology in rats, compared with the model
group, the scores of each administration group were decreased, and
the decrease in the alcohol extraction group was the most
significant. In terms of inflammatory cell infiltration in the
prostate tissue of rats, compared with the model group, the scores
of each administration group were reduced to varying degrees;
amongst these, the decrease in the water extraction group was the
most notable, and was lower than that in the Qianliekang group. In
terms of fibrosis and hyperplasia of prostate tissue in rats,
compared with the model group, each administration group exhibited
differing degrees of reduction; amongst them, the decrease in the
water extraction group was the most notable, and was lower than
that in the Qianliekang group.
Thus, the aqueous extract, ethanol extract and
Qianliekang groups all tended to improve the morphology of prostate
gland in rats. The water extraction exhibited the most potent
mitigation on the hyperplasia of prostate tissue in rats.
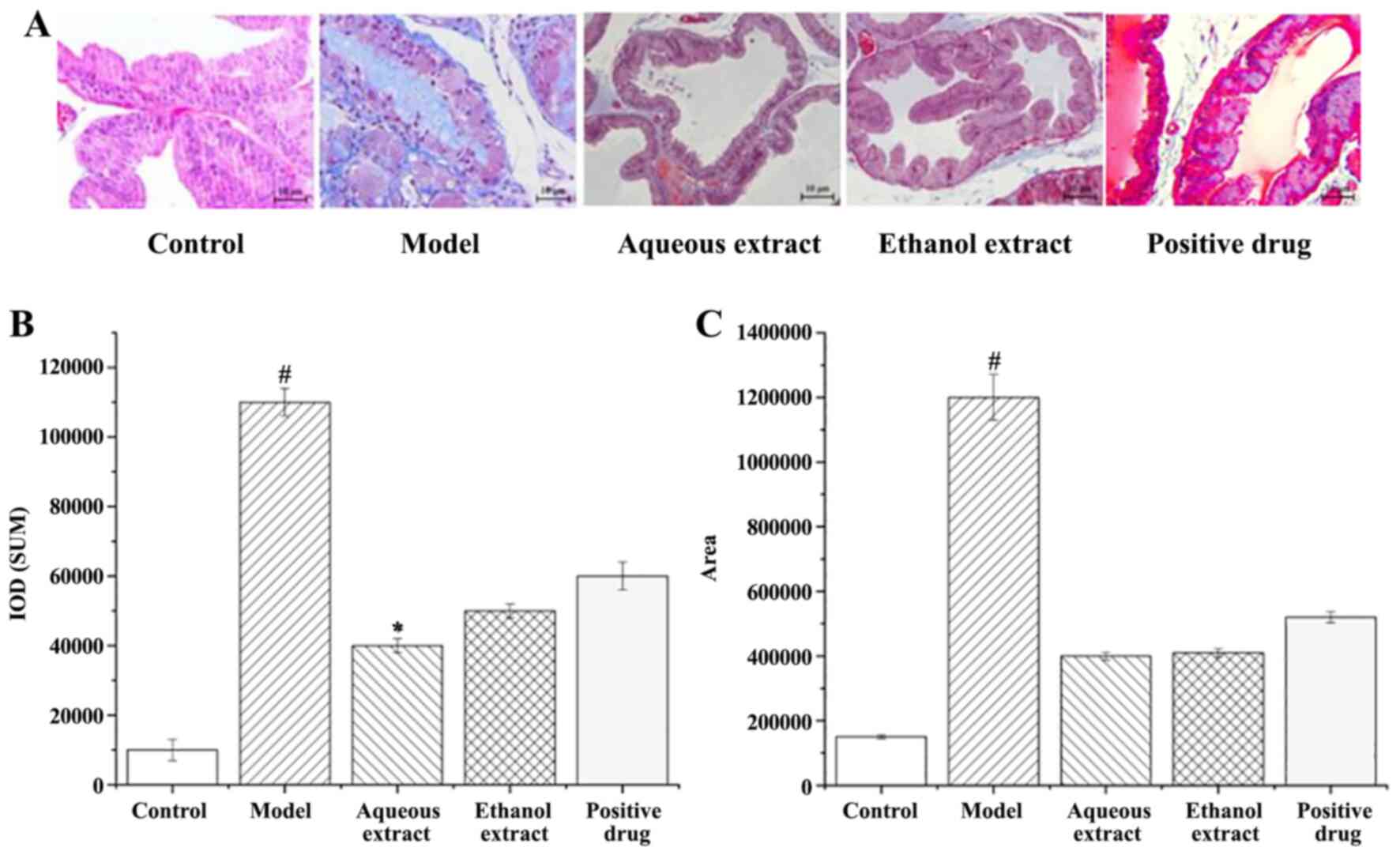
Analysis of the degree of fibrosis in prostate
tissue. Masson stained sections of rat prostate tissue are shown in
Fig. 6A. There was no obvious
hyperplasia in the sham group. The blue area of the prostate tissue
in the model group was visible and large, and fibrosis was
observed. Compared with the model group, the blue area in each
administration group was significantly reduced, and that in the
water extraction group is the most significantly decreased.
The calculation of Masson staining IOD and area of
fibrosis hyperplasia in rat prostate tissues are shown in Fig. 6B and C; the larger the value, the greater the
degree of fibrosis hyperplasia. Hyperplasia of prostate tissue in
the model group was serious, and significantly higher than that in
the sham operation group (P<0.05). i) In terms of the IOD of
prostate tissues in rats, compared with the model group, each
treatment reduced it by varying degrees, amongst them, the water
extraction groups resulted in significant decreases (P<0.05).
ii) Compared with the model group, the area of fibrosis hyperplasia
in the prostate tissues of rats in each administration group was
decreased to varying degrees (P<0.05).
In summary, the degree of prostatic fibrosis and
hyperplasia in the administration group decreased to varying
degrees, and the water extraction group showed the best
results.
Analysis of expression intensity of
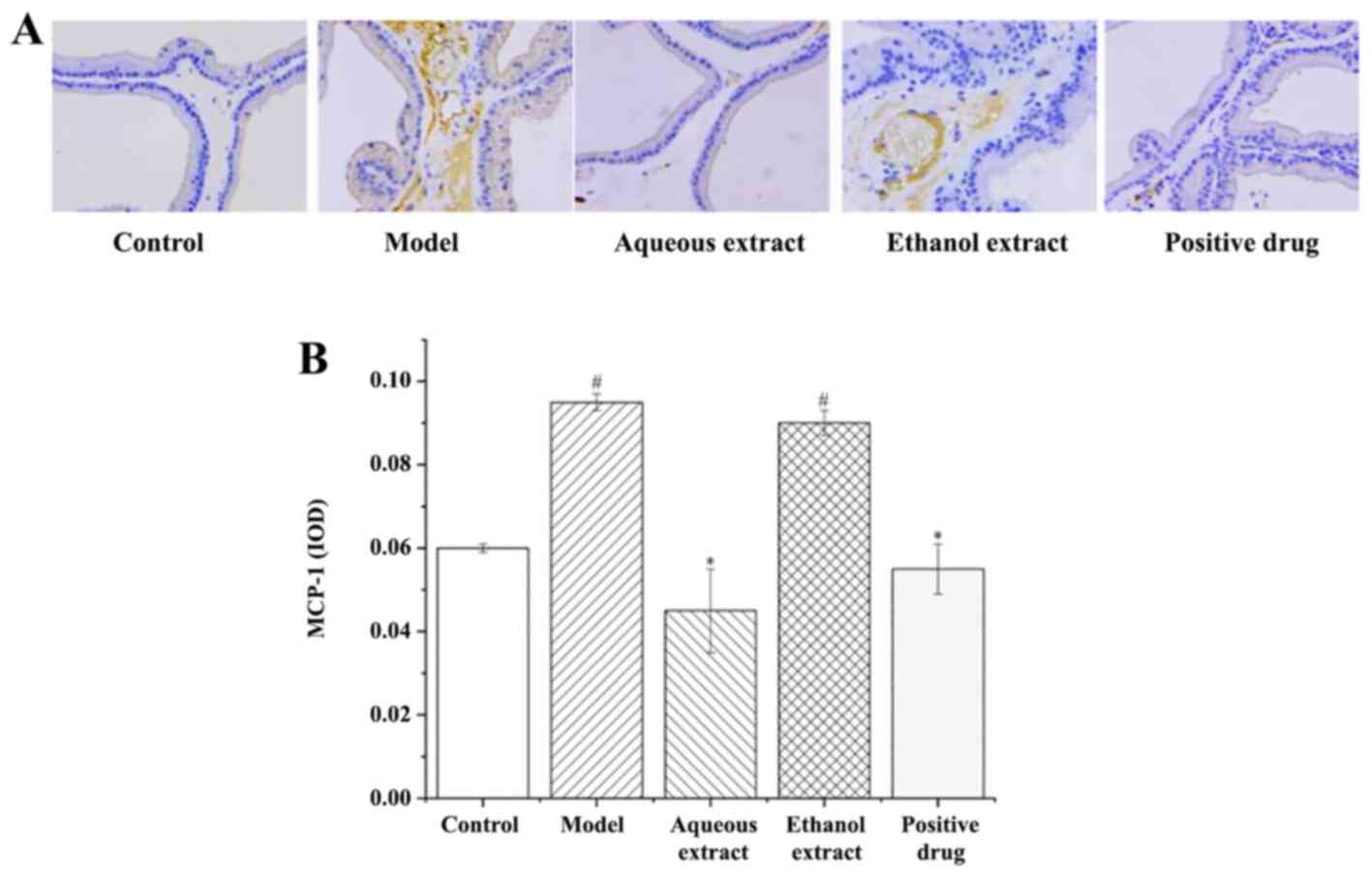
inflammatory factors in prostate tissues MCP-1 expression in
prostate tissues
The results of MCP-1 expression in rat prostate
tissues (IHC staining sections) are shown in Fig. 7A. The expression of MCP-1 in each
group was as follows: i) Sham group, there was no brown granules in
the cytoplasm and no MCP-1 positive expression; ii) model group, a
large area of brown granules was distributed in the cytoplasm,
MCP-1 positive expression was notably present; iii) ethanol
extraction, the presence of the brownish yellow granules in the
cytoplasm was significantly reduced compared with the model group,
with some positive expression of MCP-1; iv) water extraction and
Qianliekang groups, there were only a few brownish yellow granules
present in the cytoplasm, and the positive expression of MCP-1 was
significantly lower than that in the model group.
The results of analysis of MCP-1 expression in rat
prostate tissues are shown in Fig.
7B. The results showed that: i) The average optical density of
MCP-1 in the model group was significantly higher than that in the
sham group (P<0.05); ii) compared with the model group, the
expression of MCP-1 in each administration group showed a
decreasing trend, and the water extraction and Qianliekang groups
were significantly lower (P<0.05); iii) the expression levels of
MCP-1 in the water extraction group were lower than those in the
Qianliekang group, and the expression levels of MCP-1 in the water
extraction group were the lowest.
In summary, the water extract and Qianliekang groups
could significantly reduce the expression of MCP-1 in rat prostate
tissues, and the water extract group showed the best results.
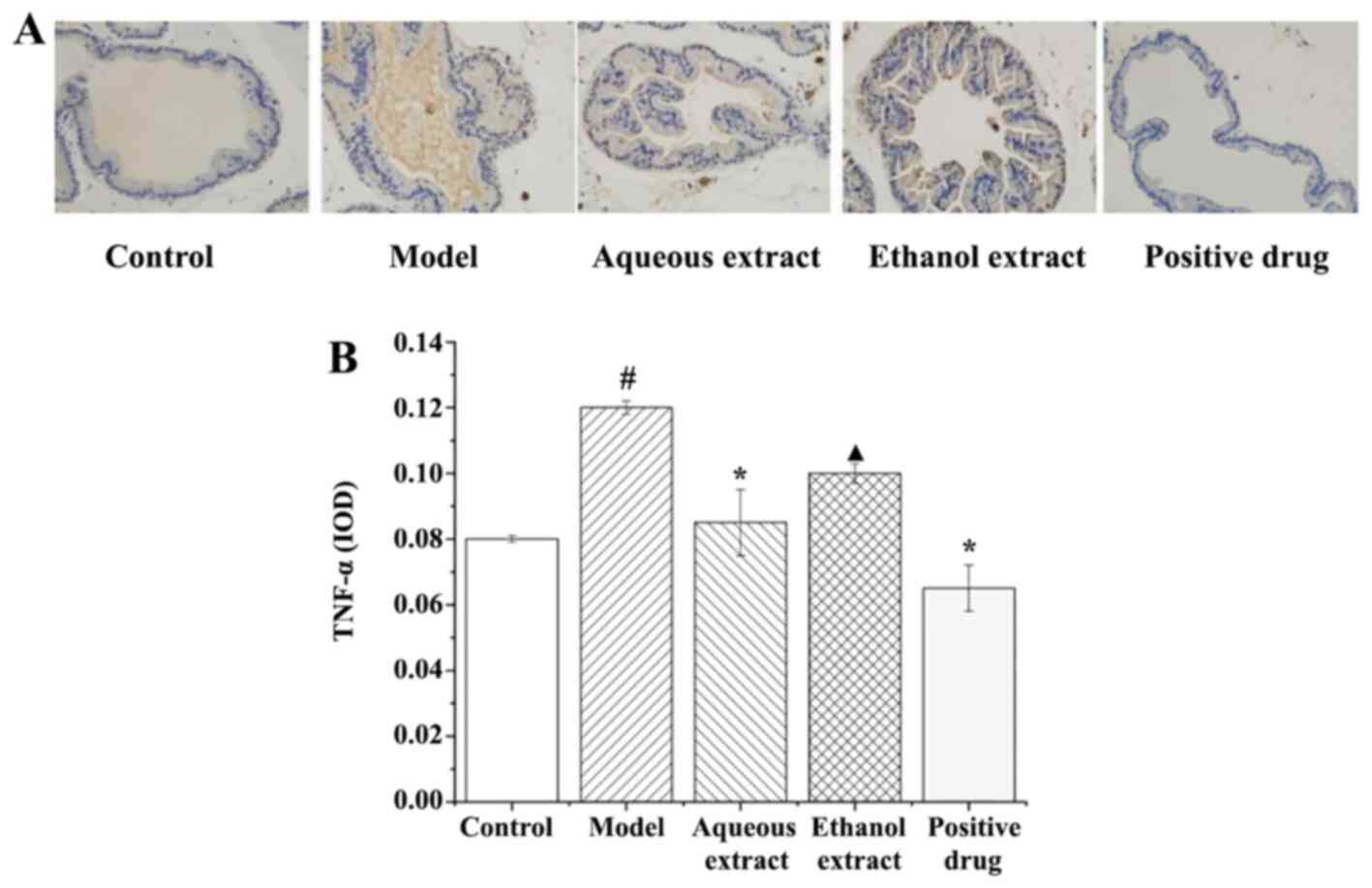
TNF-α expression in prostate
tissues
The results of TNF-α expression in rat prostate
tissues (IHC staining sections) are shown in Fig. 8A, and they clearly illustrate that
the expression of TNF-α in each group exhibited the following
characteristics: i) in the sham group, there was no brownish yellow
granules in the cytoplasm, and no positive expression of TNF-α; ii)
model group, a large area of brownish yellow granules was
distributed in the cytoplasm, and the positive expression of TNF-α
was notably present; iii) ethanol extract group, there were some
brown granules in the cytoplasm, and TNF-α positive expression was
strong; iv) water extraction and Qianliekang groups, there were
only a few brownish yellow granules in the cytoplasm, and the
positive expression of TNF-α was significantly lower than that in
the model group.
The analysis results of TNF-α expression in rat
prostate tissues are shown in Fig.
8B. The results showed that: i) The average optical density of
TNF-α in the model group was significantly higher than that in the
sham group (P<0.05); ii) compared with the model group, the
expression levels of TNF-α in each administration group showed a
decreasing trend, and the expression levels of TNF-α in the water
extraction and Qianliekang groups was significantly decreased
(P<0.05); iii) the expression levels of TNF-α in the ethanol
extraction group were significantly higher than those in the
Qianliekang group (P<0.05).
In summary, water extract and Qianliekang could
significantly reduce the expression of TNF-α in rat prostate
tissues, and Qianliekang showed the best results.
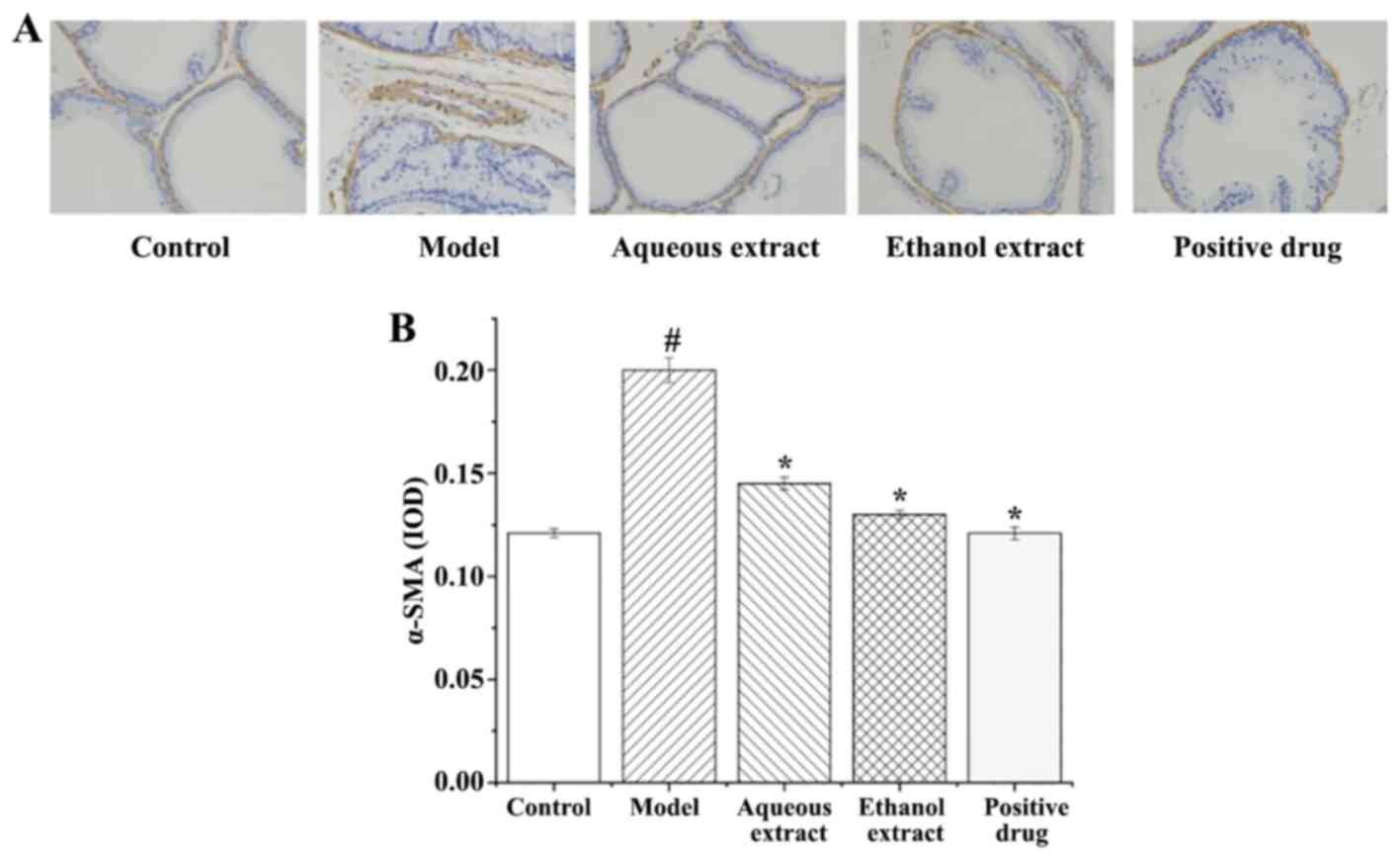
α-SMA expression in prostate
tissues
α-SMA expression in rat prostate tissues (IHC
staining sections) are shown in Fig.
9A. The expression of α-SMA in each group exhibited the
following characteristics: i) Sham group, there were only a few
brownish yellow granules in the smooth muscle, with less positive
expression of α-SMA; ii) in the model group, smooth muscle
exhibited large areas of brown particles, α-SMA positive expression
was notably present; iii) the brownish yellow granules in the
smooth muscle of each group were reduced to varying degrees, and
the positive expression of α-SMA was significantly lower than that
in the model group.
The analysis of α-SMA expression in the rat prostate
tissues are shown in Fig. 9B. The
results showed that: i) Expression of α-SMA in the model group was
significantly higher than that in the sham group (P<0.05); ii)
compared with the model group, the expression of α-SMA in each
group was significantly decreased.
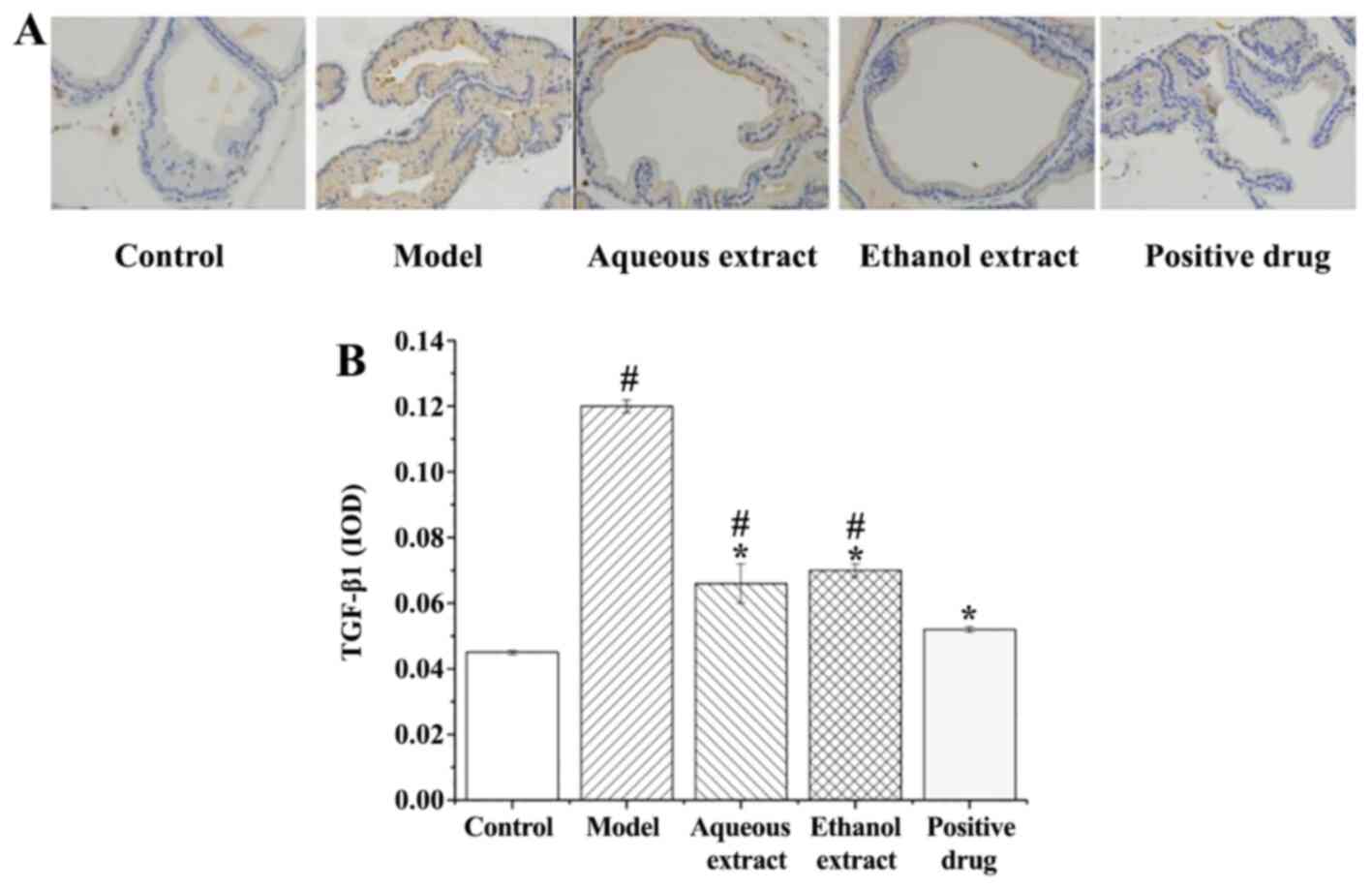
TGF-β1 expression in prostate
tissues
TGF-β1 expression in rat prostate tissues (IHC
staining sections) are shown in Fig.
10A. TGF-β1 expression in each group exhibited the following
characteristics: i) Sham group, there were only a few brownish
granules in the glands, and less positive expression of TGF-β1; ii)
model group, large area of brownish yellow granules distributed in
the glands, and positive expression of TGF-β1 was notably present;
iii) water and ethanol extraction groups, the number of brownish
yellow granules in the cytoplasm was decreased compared with the
model group, with some positive expression of TGF-β1; iv)
Qianliekang group, only a few brownish granules in the cytoplasm,
TGF-β1 positive expression was significantly lower than the model
group.
The analysis of TGF-β1 expression in the prostate
tissues of rats is shown in Fig.
10B. The results showed that: i) TGF-β1 expression in the model
group was significantly higher than that in the sham group
(P<0.05); ii) compared with the model group, the expression
levels of TGF-β1 in each group were significantly lower.
Thus, each concoction/drug significantly reduced the
expression of TGF-β1 in rat prostate tissues, and the water group
exhibited the most prominent effect.
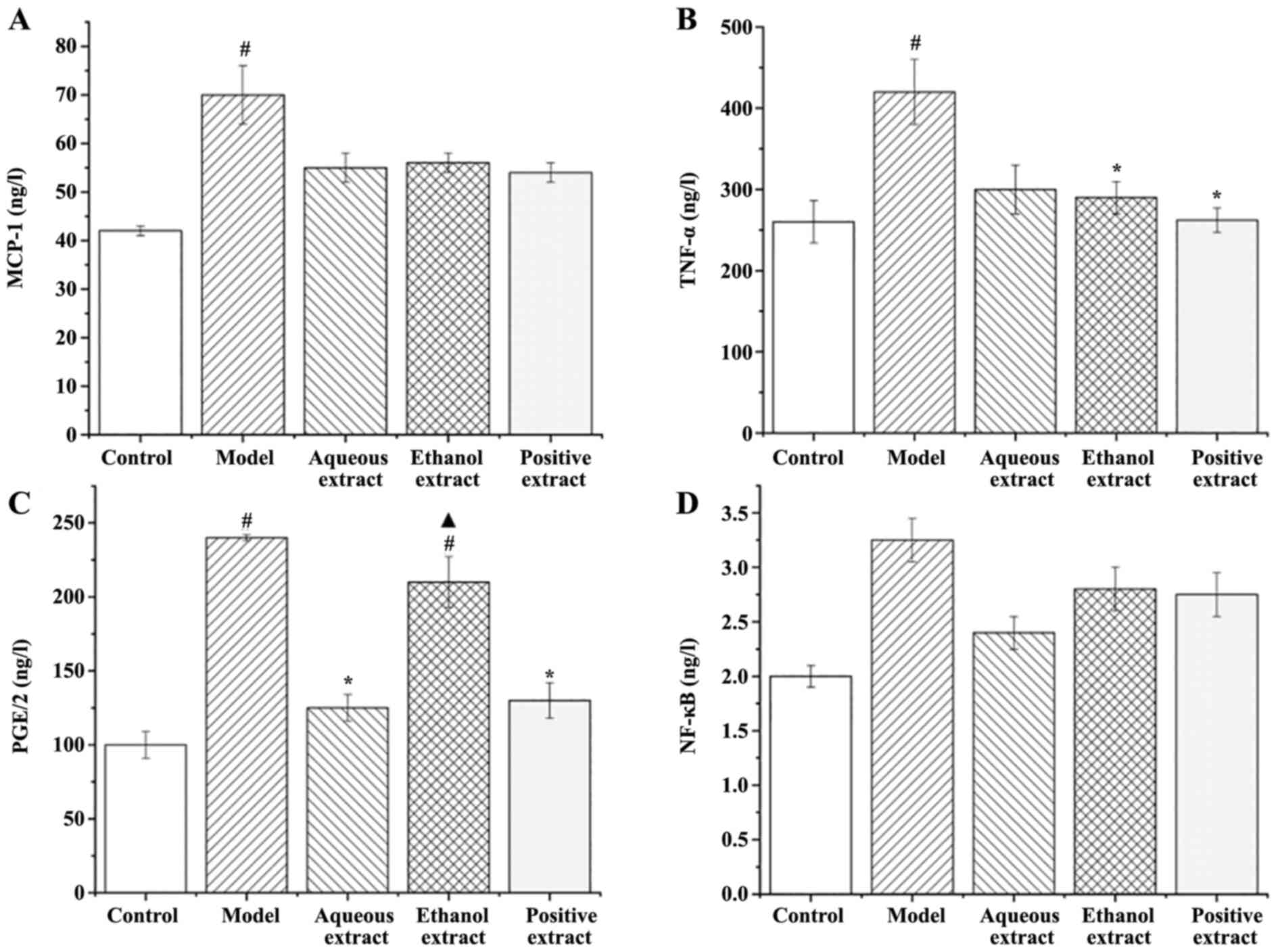
Effects of different extraction parts
on serum inflammatory factor expression in rats Expression of
MCP-1
The expression of MCP-1 in the serum of rats in each
group is shown in Fig. 11A: i)
Expression of MCP-1 in the serum of rats in the model group was
significantly higher than that in the sham group (P<0.05); ii)
compared with the model group, the expression levels of MCP-1 in
each administration group showed a decreasing trend.
Expression of TNF-α in serum
The expression of TNF-α in the serum of rats in each
group is shown in Fig. 11B: i)
Expression of TNF-α in the serum of rats in the model group was
significantly higher than that in sham group (P<0.05); ii)
compared with the model group, the expression of TNF-α in each
administration group was decreased to varying degrees, and the
decrease observed in the ethanol extraction and Qianliekang groups
was significant (P<0.05).
In summary, the ethanol extract and Qianliekang
groups significantly reduced the expression of serum TNF-α in CNP
rats.
Expression of serum PGE2
The expression levels of PGE2 in the serum of rats
in each group is shown in Fig.
11C: i) The expression levels of PGE2 in the serum of rats in
the model group were significantly higher than that in the sham
operation group (P<0.05); ii) compared with the model group, the
PGE2 expression levels in the water extraction and Qianliekang
groups were significantly decreased (P<0.05), and the PGE2
expression levels in the ethanol extraction group was also
decreased, but not significantly compared with the model group;
iii) the expression of PGE2 in the ethanol extract group was
significantly higher than that in the Qianliekang group
(P<0.05). There was no significant difference in the expression
of PGE2 between the water extraction group and Qianliekang group,
although it was lowest in the Qianliekang group. In summary, water
extract could significantly reduce the serum PGE2 expression of CNP
rats.
Expression of NF-κB in serum
The expression of NF-κB in the serum of rats in each
group is shown in Fig. 11D: i) The
expression of NF-κB in the serum of rats in the model group was
slightly higher than that in the sham group, but the difference was
not significant; ii) compared with the model group, the expression
levels of NF-κB in each administration group decreased to varying
degrees, but the decrease was not significant.
Network pharmacology analysis
Prediction of the targets of the 23 components of the aerial part
of Glycyrrhiza uralensis
A total of 17 chemical components with strong
gastrointestinal absorption and drug-like properties were
identified, all of which were either flavonoids or flavonoid
glycosides. Compositional analysis of the components in the test
substance showed that this plant material contained high levels of
six flavonoid glycosides These six components were part of the 23
components previously determined to be present in the aerial part
of Glycyrrhiza uralensis; their gastrointestinal absorption
and drug-like properties were poor, but their levels were high in
the subjects (Fig. 7). The
structural formulae of the 23 components were introduced into
SwissTargetPrediction to obtain relevant targets. The 23 chemical
components had 501 predicted targets.
CNP target prediction
A total of 137 potential CNP targets were screened
in Genecards and OMIM using the term ‘Chronic Nonbacterial
Prostatitis’.
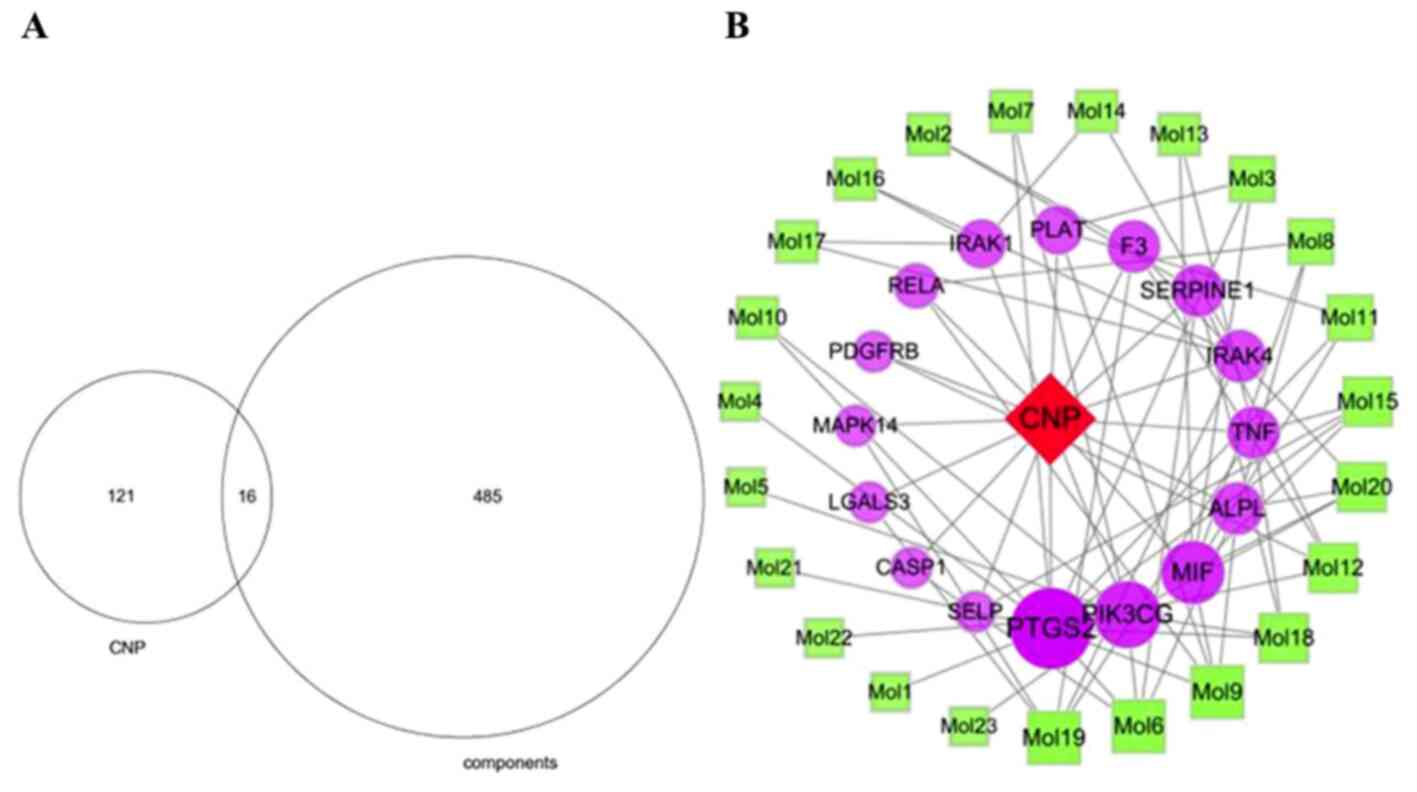
Construction of the
component-CNP-target gene network
The 137 CNP targets were compared with the 501
component targets to obtain 16 common targets (Fig. 12A). The component-CNP-target
network contained 40 nodes including one disease, 23 components, 16
genes and 76 edges (Fig. 12B). The
green squares represent the chemical components, the purple circles
represent the target genes, and the red diamond represents CNP.
Importance is portrayed proportional to node size. The degree value
of a node represents the number of other nodes connected to it in
the network. Analysis of the nodes with large degree values
screened using network topology indicated that nodes with
relatively more chemical components or targets play pivotal roles
in the entire network and may be key chemical components or
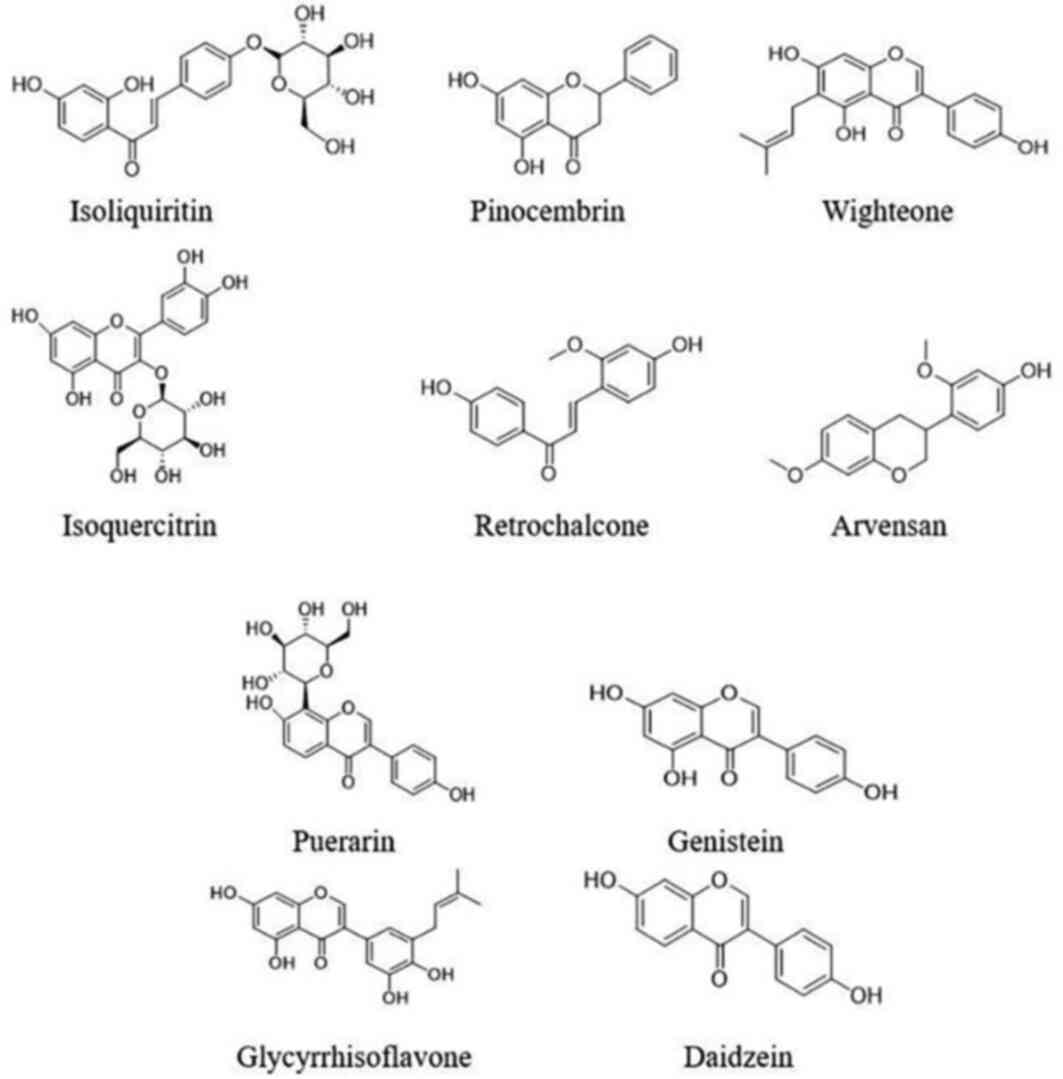
targets. The top 10 components were CAS5041-81-6 (Isoliquiritin; 5
nodes), CAS480-39-7 (Pinocembrin; 5 nodes), CAS51225-30-0
(Wighteone; 5 nodes), CAS21637-25-2 (Isoquercitrin; 4 nodes),
CAS34221-41-5 (Retrochalcone; 4 nodes), CAS63631-41-4 (Arvensan; 4
nodes), CAS3681-99-0 (Puerarin; 3 nodes), CAS446-72-0 (Genistein; 3
nodes), CAS116709-70-7 (Glycyrrhisoflavone; 3 nodes) and
CAS486-66-8 (Daidzein; 3 nodes). Details of these components are
shown in Table III and Fig. 13. The top three targets were PTGS2,
PIK3CG and MIF, which interacted with 12, 8 and 7 chemical
components, respectively.
 | Table IIICore active components and their
topological properties. |
Table III
Core active components and their
topological properties.
| No. | CAS | Name | Chemical
formula | Degree | Type |
|---|
| 1 | 5041-81-6 | Isoliquiritin |
C21H22O9 | 5 | Chalcone Oxide |
| 2 | 480-39-7 | Pinocembrin |
C15H12O4 | 5 | Flavonone |
| 3 | 51225-30-0 | Wighteone |
C20H18O5 | 5 | Prenylated
Flavonoid |
| 4 | 21637-25-2 | Isoquercitrin |
C21H20O12 | 4 | Flavonol
Glycoside |
| 5 | 34221-41-5 | Retrochalcone |
C16H14O4 | 4 | Chalcone |
| 6 | 63631-41-4 | Arvensan |
C17H18O4 | 4 | Benzopyrone |
| 7 | 3681-99-0 | Puerarin |
C21H20O9 | 4 | Isoflavone
Oxyglycoside |
| 8 | 446-72-0 | Genistein |
C15H10O5 | 3 | Isoflavone |
| 9 | 116709-70-7 |
Glycyrrhisoflavone |
C20H18O6 | 3 | Prenylated
Isoflavone |
| 10 | 486-66-8 | Daidzein |
C15H10O4 | 3 | Isoflavone |
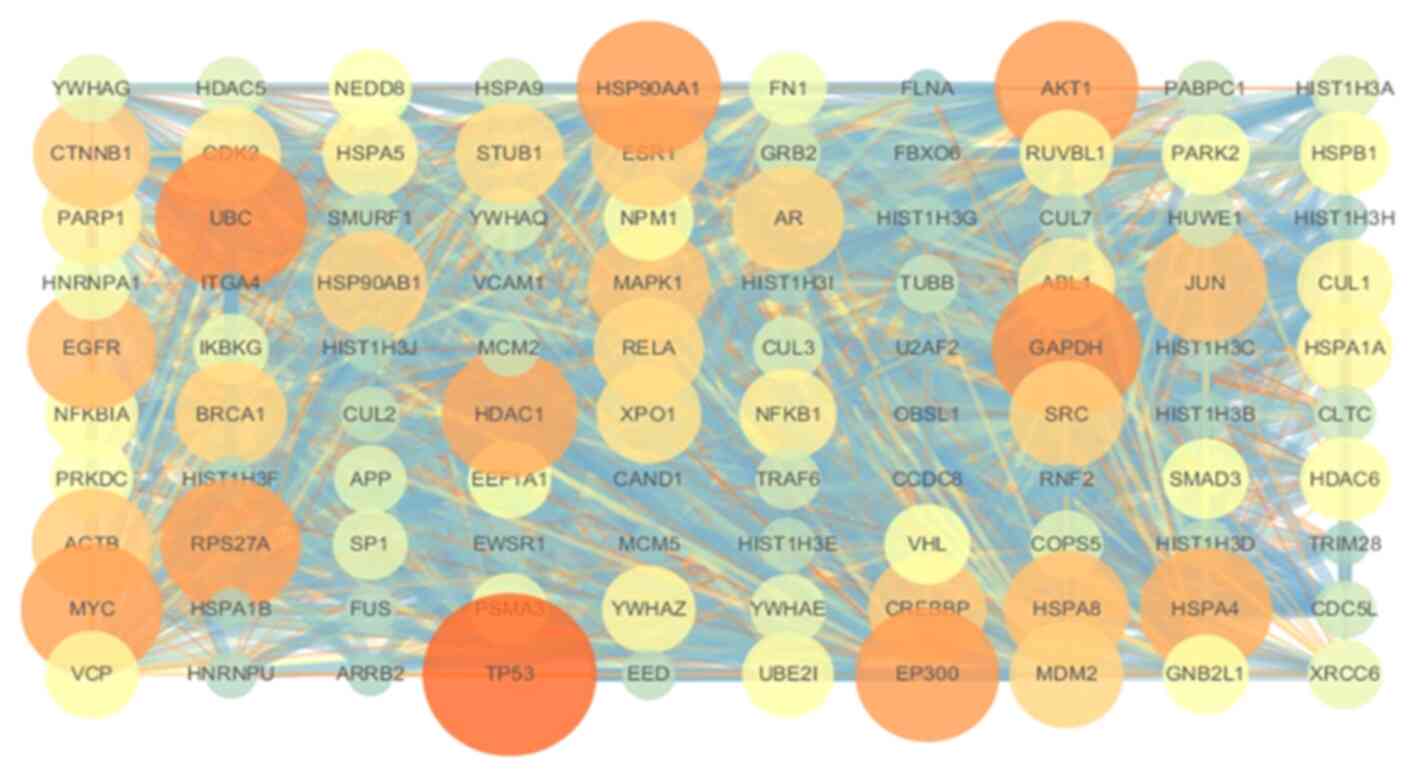
Construction of the PPI networks
A total of 100 core targets were screened using
Cytoscape. The PPI network (Fig.
14) showed strong associations between the targets and the
complex interlaced networks. The network contained 100 nodes, which
were the core targets of the aerial part of Glycyrrhiza
uralensis in CNP treatment. TP53, AKT1, EP300, EGFR, HDAC1 and
MYC were the hub genes.
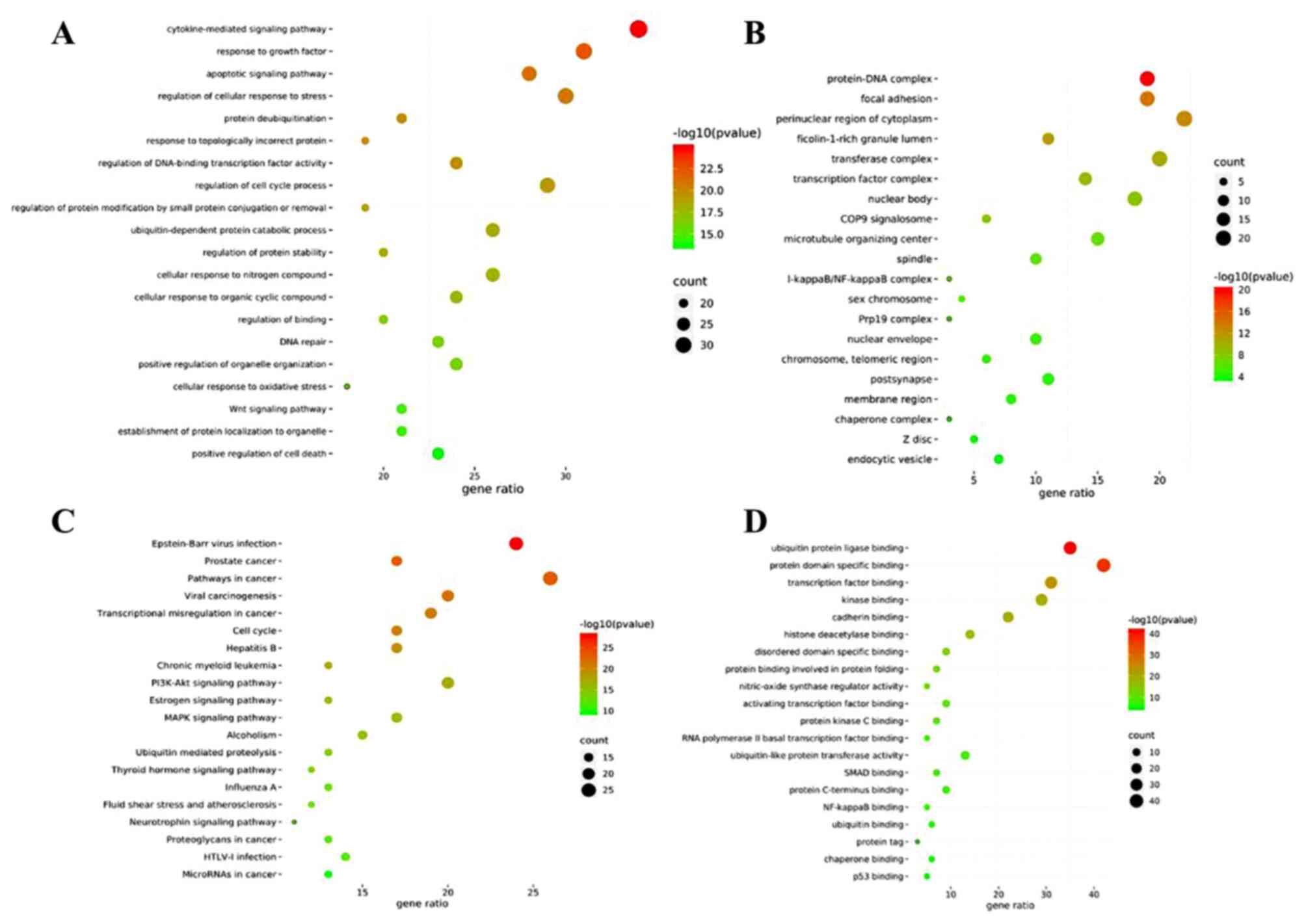
GO and KEGG enrichment analyses
GO enrichment was performed to identify the gene
intersections. A total of 1,392 top-ranking biological process
terms were selected, including the cytokine-mediated signaling
pathway, response to growth factor, regulation of cellular response
to stress, regulation of cell cycle process and apoptotic signaling
pathway (Fig. 15A). The molecular
function terms consisted of protein domain specific, ubiquitin
protein ligase, transcription factor, kinase and cadherin binding,
amongst others (Fig. 15B).
Enrichment analysis of the cellular components (Fig. 15C) showed that the targets were the
perinuclear cytoplasmic region, transferase complex, protein-DNA
complex, focal adhesion and nuclear body, amongst others.
KEGG pathway enrichment was performed to cluster the
major effects associated with CNP. The top 20 ranking pathways were
screened out (P<0.01; Fig.
15D). The main pathways were involved in cancer, including
prostate cancer, estrogen, PI3K-Akt and MAPK signaling. The genes
involved in these pathways are shown in Table IV.
 | Table IVPrimary pathway targets. |
Table IV
Primary pathway targets.
| Pathway in | Primary
targets |
|---|
| Cancer | ABL1, AKT1, AR,
CDK2, CREBBP, EGFR, CTNNB1, EP300 |
| Prostate
cancer | CDK2, CREBBP,
EP300, AKT1, EGFR, MAPK1 |
| Viral
carcinogenesis | CDK2, CREBBP,
EP300, GRB2, JUN, MDM2, NFKBIA, MAPK1 |
| Estrogen
signaling | AKT1, EGFR, ESR1,
GRB2, HSPA1A, HSPA1B, HSPA8, HSP90AA1 |
| Thyroid hormone
signaling | ACTB, AKT1, CREBBP,
CTNNB1, EP300, ESR1, HDAC1 |
| Epstein-Barr virus
infection | AKT1, CDK2, CREBBP,
EP300, HDAC1, HSPA1A, HSPA1B, HSPA8 |
| PI3K-Akt
signaling | AKT1, BRCA1, CDK2,
EGFR, FN1, HSP90AA1, HSP90AB1 |
| MAPK signaling | AKT1, ARRB2, EGFR,
FLNA, GRB2, HSPA1, AHSPA1B, HSPA8 |
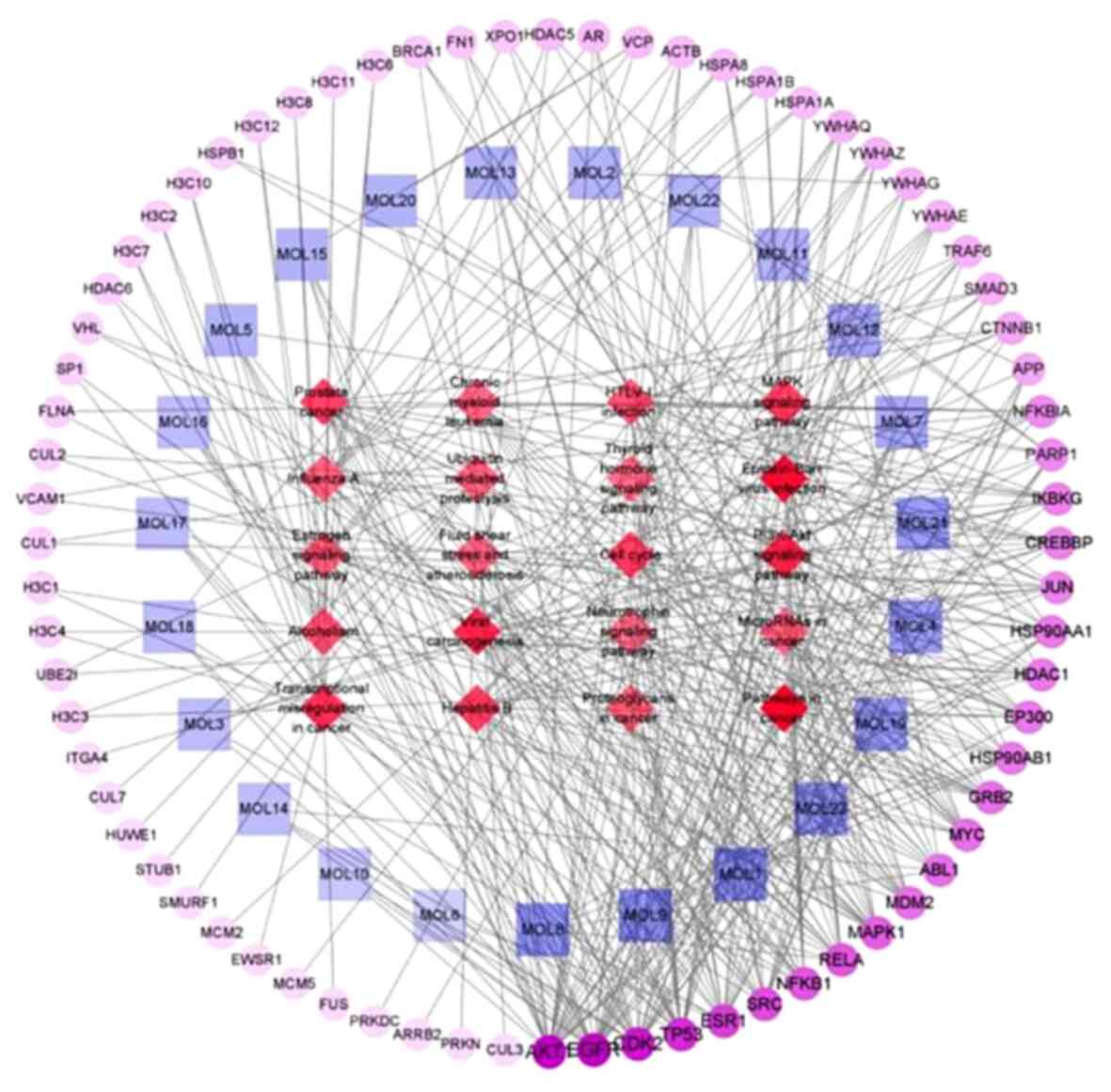
Construction of the chemical
component-pathway-target network
As shown in Fig.
16, the network showed that the components connected to the
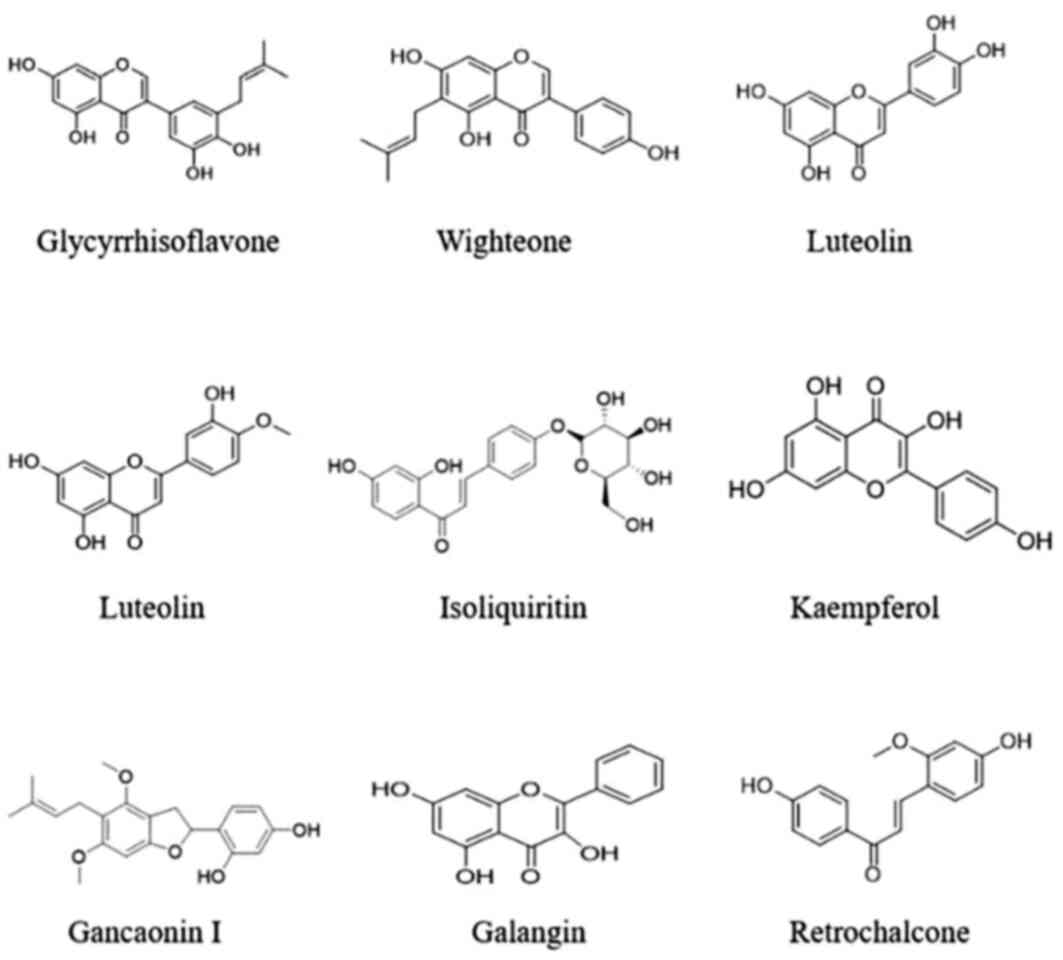
core target genes were glycyrrhisoflavone, wighteone, luteolin,
diosmetin, isoliquiritin, Kaempferol, Gancaonin I, Galangin and
Retrochalcone. Hence, these compounds may play major roles in the
aerial part of Glycyrrhiza uralensis. Details of these top 9
components are shown in Table V and
Fig. 17. Protein kinase-associated
receptors (AKT1 and MAPK1), cyclin-dependent kinase 2,
inflammation-associated receptors (RELA, NFKB1 and HDAC1),
cancer-associated receptors (TP53), growth factor-associated
receptors (EGFR), and hormone metabolism-associated estrogen
receptor 1 (ESR1) were nodal proteins in the entire network, and
were identified by measuring the pathway-component-target degree
value.
 | Table VCore active components and their
topological properties. |
Table V
Core active components and their
topological properties.
| No. | CAS | Name | Chemical
formula | Degree | Type |
|---|
| 1 | 116709-70-7 |
Glycyrrhisoflavone |
C20H18O6 | 8 | Prenylated
Isoflavone |
| 2 | 51225-30-0 | Wighteone |
C20H18O5 | 8 | Prenylated
Flavonoid |
| 3 | 491-70-3 | Luteolin |
C15H10O6 | 8 | Flavone |
| 4 | 520-34-3 | Diosmetin |
C16H12O6 | 7 | Flavone |
| 5 | 5041-81-6 | Isoliquiritin |
C21H22O9 | 7 | Chalcone Oxide |
| 6 | 520-18-3 | Kaempferol |
C15H10O6 | 7 | Flavone |
| 7 | 126716-36-7 | Gancaonin I |
C21H22O5 | 7 | Prenylated
Flavonoid |
| 8 | 548-83-4 | Galangin |
C15H10O5 | 6 | Flavonol |
| 9 | 34221-41-5 | Retrochalcone |
C16H14O4 | 6 | Chalcone |
Discussion
At present, the root of the licorice ground biomass
accounts for a large proportion (approximately one-third) of the
total licorice amount harvested. Annually, ~50,000 tons of licorice
roots are produced; however, the ground part is not well utilized
(1).
Dong et al (29) demonstrated that the total content of
flavonoids found in licorice ground biomass is >4X that of the
licorice root and rhizome. The majority of the flavonoids are
isoflavone rhizones and dihydroflavonoids in the licorice root and
rhizome, with no repetitive composition of Ural licorice rhizoid
and root and rhizome (30).
Flavonoids have various pharmacological activities, such as free
radical antioxidant (31),
anti-tumor (32), anti-viral
(32), anti-inflammatory (33), anti-inflammatory and analgesic
activity (34), cardio and
cerebrovascular protective properties, as well as a
liver-preserving effect (35).
Hence the aerial part of licorice has notable clinical prospects.
Siracusa et al (36) studied
the anti-inflammatory activity of different extraction sites of
licorice leaves and found the ethyl acetate extraction site to be
the best. Fan (1) concluded that
the main flavonoids in ethyl acetate extract of licorice include 21
isoflavones, 17 dihydroisoflavones (alcohol), 5 dihydroflavonoids,
4 orangones, 6 rosewood (alkene) and 4 Charketones. The primary
anti-inflammatory active parts of flavonoids are the side and
lipophilic components.
In the male reproductive system, there is a
blood-testicular barrier and a blood-epididymal barrier, which
protects the sperm, and the blood-prostate barrier also limits drug
entry (37). Thus, the
concentration of drugs in the prostate tissue is lower than that in
the plasma, and hence the therapeutic effect is poor, whereas
substances with strong lipophilicity can easily penetrate the
blood-prostate barrier. The above ground liposoluble substances of
Glycyrrhiza uralensis showed a good effect on reducing the
prostate index, but the underlaying mechanism remains to
determined.
Combining pharmacological experiments and network
pharmacology analysis, research found that the MCP-1 signal channel
was involved in breast, prostate, colorectal, pancreatic, bladder
and esophageal cancer. Lin et al (38) demonstrated that MCP-1 activates the
PI3K/Akt signal channel by mediating mTORC1 activation, thus
stimulating the proliferation and metastasis of prostate cell
strains, such as PC3 and VCaP, which inhibits autophagy-mediated
death of prostate cancer cells. Guyon et al (39) found that MCP-1 could also induce
small gliocytes to produce TNF-α, which can promote the production
of T lymphocytes, in-turn promoting the pathogenesis of
inflammation and thus participating in the development of disease
via induction of NF-κB. MAPK is an important transmitter of signals
from the cell surface to the nucleus, and the p38-MAPK pathway is
one of the best studied pathways in this family. Deng et al
(40) found that parasitic extracts
simultaneously achieved anti-inflammatory and anti-oxidative stress
through the regulation of the MAPK and NF-kB pathways.
TNF-α plays an important role in the activation of
multiple inflammatory factors and increases vascular permeability
and accelerates the release and aggregation of various inflammatory
factors, including prostaglandin-E2 (PGE-2) (41). Regulation of TNF-α is closely
related to the expression of the NF-κB. Certain extracellular
stimulatory signals activate NF-κB in vivo, thereby
enhancing the transcription of the TNF-α gene and promoting TNF-α
expression (42). TNF-α can then
reactivate NF-κB via a positive feedback mechanism, which not only
increases TNF-α secretion, but also induces the production and
release of other pro-inflammatory cytokines, such as IL-1 and IL-8,
resulting in a series of cascade responses leading to further
amplification of the initial inflammatory signal (43).
PGE-2 is prevalent in tissues in vivo, and is
one of the metabolites of arachidonic acid (AA), where COX-2
transforms AA into multiple species by catalyzing epoxy
oxygenation. Prostaglandin (PG) substances, which then serve a
peroxidase function, convert PGG2 to PGH2, which under various
synthases are converted to multiple peanut acid, such as PGE-2,
which are finally passed downstream through different G protein
receptors (44). Various studies
have shown that PGE2 is an important inflammatory medium that can
directly result in the increase in tissue vascular permeability and
promote the secretion of inflammatory factors, such as IL-6 in
relevant tissues (45). COX-2 is a
rate-limiting enzyme for PGE-2 synthesis, and serves an important
role in regulating the activity of PGE-2(46).
NF-κB is a transcription factor that belongs to the
Rel family, which is involved in regulating the transcription of
genes involved in immunity and inflammation (47). The results of pharmacological
experiments showed that water and alcohol may counter CNP by
regulating the oxidative stress response, body immune response and
hormones, and this appeared to more prominent with water group.
Network pharmacological analysis showed that anthocyanins,
Kaempferol, Retrochalcone, Glycyrrhisoflavone, arvensan and
wighteone can regulate inflammation by regulating the proportion of
hormones. Wighteone can regulate inflammation by managing
inflammatory factors, and kaempferol can regulate inflammation by
managing immunity.
Analysis of the pharmacological network showed that
the aerial part of Glycyrrhiza uralensis reduced the
inflammatory response in CNP via multiple mechanisms. The
inflammatory response is attenuated by regulating inflammatory
factor secretion. Abnormal inflammatory cytokine (such as TNF-α,
IL-1, IL-2, IL-6, IL-8, IL-10, IL-1β, COX-2 and IFN-γ) levels have
been reported in the semen and prostate fluid of patients with
chronic prostatitis (48).
Proinflammatory cytokines enhance inflammatory cell infiltration
and adhesion to prostate gland epithelial cells, and stimulate
abnormal cellular hypertrophy, dysregulate glandular secretion and
promote apoptosis (49). These
factors are closely associated with inflammatory signaling
pathways. Activated TNF-α binds to its receptors, TNFR1 and TNFR2,
in the cell membrane and upregulates several genes in the MAPK
pathway, which regulates NF-κB expression; the NF-κB signaling
pathway is important in the inflammatory process (50). Fan et al (52) found that NF-κB was upregulated in
patients with CNP and induced the secretion of various
proinflammatory factors and aggravated inflammation. PI3K-AKT is
upstream of the NF-κB signaling pathway. Activation of PI3K-AKT
signaling can induce NF-κB, which in-turn induces inflammation. The
majority of flavonoids exhibit an anti-inflammatory effect. The
diagram of the chemical component-pathway-target network showed
that wighteone is associated with NFKB1, and may reduce
inflammation by regulating proinflammatory factor secretion.
RELA, which is associated with glycyrrhisoflavone
and wighteone, is an inflammatory receptor. Glycyrrhisoflavone and
wighteone can attenuate the inflammatory response by regulating the
pathways associated with inflammation. Huang et al (53) reported that kaempferol significantly
downregulated THP-1 in the MAPK pathway induced by
lipopolysaccharides in a human monocytic leukemia cell line.
Kaempferol suppressed inflammatory factors, such as
macrophage-derived chemokine (MDC), IFN-induced protein-10 (IP-10)
and IL-8. Panathur et al (54) found that repressing the
transcription of multiple inflammation-related genes decreased the
occurrence and impeded the progression of inflammatory responses.
HDAC1 is a type III histone deacetylase, an important PPI target,
and a part of the chemical component-pathway-target network, which
downregulates several inflammation-related genes by deacetylating
histones, NF-κB and activated protein 1. Hence, it regulates the
occurrence and progression of the inflammatory response. Analysis
of the chemical component-pathway-target network revealed that
isoliquiritin and retrochalcone are associated with HDAC1, and may
also attenuate inflammation by preventing the transcription of
inflammation-related genes.
Zhuo et al (55) reported that oxidative stress is the
primary cause of cancer and inflammation, amongst other diseases.
In prostatitis, numerous inflammatory factors are released in
response to local inflammatory reactions. In turn, excess free
radicals are generated, peroxidation occurs, high levels of ROS
form in the prostate microenvironment; thus, antioxidant enzymes,
such as SOD, CAT and GSH-Px are inactivated, and MDA production
increases. When cellular ROS and reactive nitrogen species (RNS)
are produced faster than they are removed, they accumulate in large
quantities and cause oxidative stress (56). Schaftoside inhibits the release of
NO and various proinflammatory factors through the NF-κB, MAPKs and
Nrf2-Keapl pathways; in this manner, it reduces the inflammatory
response (57).
Of the top 20 pathways enriched in KEGG, six were
associated with cancer. Prostatitis is closely related to cancer
and studies have shown that prostate cancer is often accompanied by
chronic inflammation (58).
Popovics et al (59) found
that chronic prostatitis and prostate cancer are associated with
EGFR expression, whereas the analysis in the present study showed
that EGFR was the primary target in the chemical
component-pathway-target network. Herein, it was shown that 19
chemical components, including luteolin, isorhamnetin, galangin,
glycyrrhisoflavone, wighteone, and retrochalcone were related to
EFGR. These components may regulate inflammation through the cancer
signaling pathway. IL-6 is associated with chronic inflammation in
prostate cancer and regulates prostate cancer cell proliferation
and apoptosis via the JAK, MAPK and PI3-K signaling pathways
(60). Luteolin sensitizes cancer
cells to cytotoxic drugs, regulates the secretion of inflammatory
factors, and suppresses cell survival pathways, such as P13K and
NF-κB.
Chen et al (61) showed that tumor-suppressing gene
upregulation induces inflammatory cytokine expression. The PPI
network showed that TP53 had a maximum degree value in inflamed
prostate cells. In contrast, TP53 was present only at low levels in
normal prostate cells. When cells are under stress, relative TP53
expression and activity increase and tumor cell production is
inhibited (62). Chen et al
(61) demonstrated that the ∆Np63
in the TP53 family regulates gene clusters associated with cellular
proliferation, survival, adhesion and inflammation. ∆Np63
overexpression may upregulate target genes and inflammatory
cytokines. Thus, cancer is closely associated with inflammation.
The chemical component-pathway-target network demonstrated that
puerarin is associated with TP53, and may attenuate inflammation by
preventing TP53 upregulation and regulating inflammatory factor
expression.
Normal prostate growth and development is closely
associated with the levels and ratios of testosterone, estradiol
(E2) and other sex hormones. Studies have reported that endogenous
estrogen induces chronic prostate inflammation and precancerous
lesions in mice. The imbalance in the sex hormone ratio that occurs
in aging men may promote prostate epithelial cell proliferation and
differentiation, modulate vascularization, and trigger certain
diseases. Isoflavones and coumarins are phytoestrogens that
structurally resemble E2(63).
Zheng et al (64) reported
that puerarin regulates estrogen receptor (ER)α encoded by estrogen
receptor 1 (ESR1). Puerarin hinders uterine growth in combination
with E2. Guo et al (65)
showed that kaempferol is estrogen-like and activates the ER. The
chemical component-pathway-target network analysis performed in the
present study showed that isoliquiritin, pinocembrin, wighteone,
retrochalcone, arvensan, puerarin, glycyrrhisoflavone, luteolin,
diosmetin and kaempferol are associated with ESR1. Hence, these
chemical components may reduce inflammation by regulating sex
hormone ratios.
A previous study have demonstrated that prostatitis
is closely associated with abnormal autoimmunity (66). In this disease state, IgG, IgA, IgM
and secretory immunoglobulin A levels are significantly increased,
whereas the levels of immunosuppressive factors are significantly
decreased. Thus, prostatitis occurrence enhances immunity (67). The gene recombinant protein-UBC had
a second-degree value in the PPI network and inhibited macrophage
activation, the primary response when the mammalian innate immune
system is induced. Macrophages secrete the proinflammatory factors
TNF-α and IL-6. During inflammation, recombinant protein-UBC may
suppress immune system activation by inhibiting macrophages
(68). T lymphocytes are important
components of cellular immunity, and CD4+ are helper T
lymphocytes (69). Lin et al
(70) found that prostatitis
symptoms may be mitigated by reducing inflammatory CD4+
infiltration. Mu et al (71)
reported that kaempferol is a natural immunosuppressant that lowers
the incidence of autoimmune diseases and organ transplant rejection
caused by excessive T lymphocyte activation and proliferation.
The analyses herein suggested that the anti-CNP
mechanism of the aerial part of Glycyrrhiza uralensis may
involve repression of the inflammatory response via reduction of
MDC, interferon-IP-10, IL-8 and other inflammatory factors in the
MAPK, PI3-K and other signaling pathways affected by components
such as wighteone and glycyrrhisoflavone. Inflammation may be
attenuated by downregulating inflammation-related genes via
components such as isoliquiritin and retrochalcone, and it may also
be alleviated by decreasing ROS and RNS generation via components
such as schaftoside and kaempferol. Chronic prostatitis is
associated with EGFR and PDGFR expression, and therefore, prostate
cancer. A total of 19 chemical constituents, including luteolin,
isorhamnetin, galangin, glycyrrhisoflavone, wighteone and
kaempferol are associated with growth factor-related receptors,
such as EFGR. Chronic inflammation in prostate cancer may be
attenuated by inhibiting cell survival pathways, such as P13K,
NF-κB and XIAP, and enhancing cancer cell sensitivity to cytotoxic
drugs, such as luteolin. Inflammation may be alleviated by
modulating the sex hormone ratio and preventing prostate epithelial
cell proliferation, differentiation and prostaglandin-promoted
angiogenesis. Isoliquiritin, pinocembrin, wighteone, retrochalcone,
arvensan, puerarin, glycyrrhisoflavone, luteolin, diosmetin and
kaempferol are associated with the estrogen receptor, ESR1 and may
reduce inflammation by regulating the sex hormone ratio. Prostate
inflammation is also alleviated by inhibiting immune enhancement
caused by excessive T lymphocyte activation and proliferation via
substances such as kaempferol.
The results of the present study showed that
comparison of different extraction of Glycyrrhiza uralensis,
the trend in the ability of water group to reduce prostate index
was the most notable, and was better than that of Qianliekang. The
liposoluble substances of the aerial part Glycyrrhiza
uralensis can easily penetrate the blood-prostate barrier and
play a role in inhibiting prostatic hyperplasia. In the comparison
experiments of the activities of different extraction parts, water
and alcohol extraction groups exhibited an anti-CNP role. The
chemical components were analyzed, and water extraction and alcohol
extraction were primarily composed of flavonoids and some saponins.
It was preliminarily speculated that the components of the aerial
part of Glycyrrhiza uralensis that played a role in
inhibiting inflammatory factors was primarily the flavonoids. Water
extraction and alcohol extraction showed a good inhibitory effect
on the pathological changes of prostate tissue and the expression
of inflammatory factors and fibrotic factors in CNP rats, but no
difference was confirmed when compared with positive drugs. Amongst
these, the effect of water extraction on reducing the infiltration
of inflammatory factors was slightly stronger than that of alcohol
extraction. Overall, the effect of water on the chronic prostatitis
rats was significant, and the chemical components responsible were
primarily the liposoluble flavonoids.
In conclusion, the aerial part of Glycyrrhiza
uralensis exhibits anti-CNP properties, which is exerted in a
multi-component, multi-target and multi-pathway manner. It reduced
the production of proinflammatory factors, downregulated
inflammation-related genes, reduced the oxidative stress response,
suppressed the cell survival pathway, modulated sex hormone ratios,
impeded immune enhancement and decreased the occurrence of
inflammation. The present study proposed and tested a novel method
of exploring the anti-CNP components in the aerial part of
Glycyrrhiza uralensis and their modes of action, and
provided a theoretical reference for the development of innovative
anti-CNP drugs.
Acknowledgements
Not applicable.
Funding
This study was supported by the Institute of Medicinal Plant
Development, Chinese Academy of Medical Sciences, and Peking Union
Medical College, Beijing (grant no. 100193) and the National Key
R&D Program of China (grant no. 2018YFC1706500).
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
HL designed the study, performed the experiments,
analyzed the data, performed the literature review and drafted the
manuscript. LZ performed the experiments. GC conceived the study
and performed the literature search. JC and WW assisted in the
design of the study, and in the acquisition, analysis and
interpretation of data. All the authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
All rats were handled according to the National
Guidelines for the Care and Use of Laboratory Animals, and all
animal experiments were approved by the Animal Ethics Committee of
the Chinese Academy of Medical Sciences and Institute of Medicinal
Plant Development (approval no. SLXD-20200902021).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jiang L, Akram W, Luo B, Hu S, Faruque MO,
Ahmad S, Yasin NA, Khan WU, Ahmad A, Shikov AN, et al: Metabolomic
and pharmacologic insights of aerial and underground parts of
Glycyrrhiza uralensis fisch ex DC. for maximum utilization
of medicinal resources. Front Pharmacol. 12(658670)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guo ZJ and Ma CJ: An overview of studies
on flavonoids in the aerial part of Glycyrrhiza uralensis.
Aerosp Med. 16:62–64. 2005.
|
|
3
|
Li SD, Fu L, Lu Q and Qiu C: Study on the
flavononoids in the leaves of Glycyrrhiza uralensis fisch. J
Jilin Agric Univ. 18:35–37. 1996.(In Chinese).
|
|
4
|
Cui YR, Chen P, Liu JH, Liu T and Zheng
QS: Structure-activity relationship of flavonoids from Radix
Glycyrrhiza. Shizhen Guo Yi Guo Yao. 21:3041–3043. 2010.(In
Chinese).
|
|
5
|
Bo YY, Zhang YH, Yang L and Cui J: Study
on different extraction methods and antioxidant activities of total
flavones from the aerial parts of Glycyrrhiza uralensis. J
Tradit Chin Med. 39:145–151. 2020.(In Chinese).
|
|
6
|
Soheila JM, Jesus FC and Beatriz C:
Anti-inflammatory effects of flavonoids. Food Chem.
299(125124)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang L, Kang XF, Li LH, Cui J and Wang
WQ: Analysis of flavonoids in the aerial parts of Glycyrrhiza
uralensis by HPLC-MS. J Liaoning Univ Tradit Chin Med.
20:48–51. 2018.
|
|
8
|
Zhu YX and Xu NG: Clinical treatment of
chronic nonbacterial prostatitis of kidney-yang deficiency type by
acupuncture of Sanhuang points. Zhen Ci Yan Jiu. 44:443–445.
2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
9
|
Yuan B: Clinical observation of integrated
traditional Chinese and western medicine on chronic nonbacterial
prostatitis of damp-heat stagnation syndrome. Shanxi J Tradit Chin
Med. 36:25–27. 2020.(In Chinese).
|
|
10
|
Zhang L, Zhao ZH, Yu BL and Cui J: Effect
of the aerial parts of Glycyrrhiza uralensis on chronic
prostatitis rats. Chin Tradit Pat Med. 41:1407–1410. 2019.(In
Chinese).
|
|
11
|
Qiu ZK, Liu ZT, Pang JL, Wu HB, Liu X,
Yang ZM, Li X and Chen JS: A network pharmacology study with
molecular docking to investigate the possibility of licorice
against posttraumatic stress disorder. Metab Brain Dis: Aug 21,
2021 (Epub ahead of print).
|
|
12
|
Liu J, Liu J, Tong X, Peng W, Wei S, Sun
T, Wang Y, Zhang B and Li W: Network pharmacology prediction and
molecular docking-based strategy to discover the potential
pharmacological mechanism of Huai Hua San against ulcerative
colitis. Drug Des Devel Ther. 15:3255–3276. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chen XL, Tang C, Xiao QL, Pang ZH, Zhou
DD, Xu J, Wang Q, Zhao YX and Zhu QY: Mechanism of Fei-Xian formula
in the treatment of pulmonary fibrosis on the basis of network
pharmacology analysis combined with molecular docking validation.
Evid Based Complement Alternat Med. 2021(6658395)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hu M, Zhong Y, Xiao W, Wang Y, Tang T,
Wang S, Cui H, Li T and Luo J: Deciphering the therapeutic
mechanisms of Wuzi Ershen decoction in treating
oligoasthenozoospermia through the network pharmacology approach.
Evid Based Complement Alternat Med. 2021(5591844)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qi X, Xu H, Zhang P, Chen G, Chen Z, Fang
C and Lin L: Investigating the mechanism of scutellariae barbata
herba in the treatment of colorectal cancer by network pharmacology
and molecular docking. Evid Based Complement Alternat Med.
2021(3905367)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu ZH and Sun XB: Network pharmacology:
New opportunity for the modernization of traditional Chinese
medicine. Yao Xue Xue Bao. 47:696–703. 2012.PubMed/NCBI(In Chinese).
|
|
17
|
Ma SJ, Zhao MM, Chang MY, Wang RM, Yu Y
and Zhang Y: Network pharmacology of astragalus in the treatment of
chronic nephritis. World J Integr Tradit West Med. 15:1467–1472,
1479. 2020.
|
|
18
|
Cao Z, Sloper DT and Nakamura N:
Identification of altered proteins in the plasma of rats with
chronic prostatic inflammation induced by estradiol benzoate and
sex hormones. ACS Omega. 6:14361–14370. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhang L, Zhao Z, Yu B, Cui J, Hou J and
Wang W: Effect of the aerial part of Glycyrrhiza uralensis
on rats with chronic prostatitis. Chin Tradit Pat Med.
41:1407–1410. 2019.(In Chinese).
|
|
20
|
Holt JD, Garrett WA, McCurry TK and
Teichman JM: Common questions about chronic prostatitis. Am Fam
Physician. 93:290–296. 2016.PubMed/NCBI
|
|
21
|
Yang C, Yao J and Chen T: The effect of
Qianliekang tablets on the clinical efficacy, immune function, and
inflammatory factor levels in the prostatic fluid of elderly
chronic prostatitis patients. Am J Transl Res. 13:7363–7369.
2021.PubMed/NCBI
|
|
22
|
Zeng H, He Y, Yu Y, Zhang J, Zeng X, Gong
F, Liu Q and Yang B: Resveratrol improves prostate fibrosis during
progression of urinary dysfunction in chronic prostatitis by mast
cell suppression. Mol Med Rep. 17:918–924. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fan Y, Liu W, Jin Y, Hou X, Zhang X, Pan
H, Lu H and Guo X: Integrated molecular docking with network
pharmacology to reveal the molecular mechanism of simiao powder in
the treatment of acute gouty arthritis. Evid Based Complement
Alternat Med. 2021(5570968)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The gene
ontology consortium. Nat Genet. 25:25–29. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
The Gene Ontology Consortium. The gene
ontology resource: 20 years and still GOing strong. Nucleic Acids
Res. 47 (D1):D330–D338. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kanehisa M: Post-genome informatics. 1st
edition. Oxford University Press, Oxford, 2000.
|
|
28
|
Li HL, Kang XF, Hou JL, Wang WQ and Wu HH:
Study on purification process of total flavonoids in part of water
extract from licorice. Journal of Liaoning University of TCM.
18:60–63. 2016.
|
|
29
|
Dong Y, Zhao M, Zhao T, Feng M, Chen H,
Zhuang M and Lin L: Bioactive profiles, antioxidant activities,
nitrite scavenging capacities and protective effects on
H2O2-injured PC12 cells of Glycyrrhiza glabra L. leaf and
root extracts. Molecules. 19:9101–9113. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shakeri A, Akhtari J, Soheili V,
Taghizadeh SF, Sahebkar A, Shaddel R and Asili J: Identification
and biological activity of the volatile compounds of Glycyrrhiza
triphylla Fisch. & C.A.Mey. Microb Pathog. 109:39–44.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang YT, Chi ZL, Wang SM, Wu HQ, Qin KM
and Li WD: Research progress on licorice flavonoids and their
antitumor activities. Chin J New Drugs. 26:1532–1537. 2017.(In
Chinese).
|
|
32
|
Ma FX, Xue PF, Wang YY, Wang YN and Xue
SY: Research progress of serum pharmacochemistry of traditional
Chinese medicine. China Journal of Chinese Materia Medica.
42:1265–1270. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lu T, Yang J, Gao X, Chen P, Du F, Sun Y,
Wang F, Xu F, Shang H, Huang Y, et al: Plasma and urinary tanshinol
from Salvia miltiorrhiza (Danshen) can be used as
pharmacokinetic markers for cardiotonic pills, a cardiovascular
herbal medicine. Drug Metab Dispos. 36:1578–1586. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cheng T, Sheng T, Yi Y, Zhang T and Han H:
Metabolism profiles of icariin in rats using ultra-high performance
liquid chromatography coupled with quadrupole time-of-flight tandem
mass spectrometry and in vitro enzymatic study. J Chromatogr B
Analyt Technol Biomed Life Sci. 1033-1034:353–360. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li HL, Kang XF, Hou JL, Wang WQ and Wu HH:
Purification of total flavonoids of water extract from the aerial
part of licorice. J Liaoning Univ Tradit Chin Med. 18:60–63.
2016.(In Chinese).
|
|
36
|
Siracusa L, Saija A, Cristani M, Cimino F,
D'Arrigo M, Trombetta D, Rao F and Ruberto G: Phytocomplexes from
liquorice (Glycyrrhiza glabra L.) leaves-chemical
characterization and evaluation of their antioxidant,
anti-genotoxic and anti-inflammatory activity. Fitoterapia.
82:546–556. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Urióstegui-Acosta M, Tello-Mora P,
Solís-Heredia MJ, Ortega-Olvera JM, Piña-Guzmán B, Martín-Tapia D,
González-Mariscal L and Quintanilla-Vega B: Methyl parathion causes
genetic damage in sperm and disrupts the permeability of the
blood-testis barrier by an oxidant mechanism in mice. Toxicology.
438(152463)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lin TH, Liu HH, Tsai TH, Chen CC, Hsieh
TF, Lee SS, Lee YJ, Chen WC and Tang CH: CCL2 increases αvβ3
integrin expression and subsequently promotes prostate cancer
migration. Biochim Biophys Acta. 1830:4917–4927. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Guyon A, Skrzydelski D, De Giry I, Rovère
C, Conductier G, Trocello JM, Daugé V, Kitabgi P, Rostène W, Nahon
JL, et al: Long term exposure to the chemokine CCL2 activates the
nigrostriatal dopamine system: A novel mechanism for the control of
dopamine release. Neuroscience. 162:1072–1080. 2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Deng JS, Chi CS, Huang SS, Shie PH, Lin TH
and Huang GJ: Antioxidant, analgesic, and anti-inflammatory
activities of the ethanolic extracts of Taxillus
liquidambaricola. J Ethnopharmacol. 137:1161–1171.
2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Haas S and Straub RH: Disruption of
rhythms of molecular clocks in primary synovial fibroblasts of
patients with osteoarthritis and rheumatoid arthritis, role of
IL-1β/TNF. Arthritis Res Ther. 14(R122)2012.PubMed/NCBI View
Article : Google Scholar
|
|
42
|
Jing SH, Gao X, Yu B and Qiao H: Raf
kinase inhibitor protein (RKIP) inhibits tumor necrosis factor-α
(TNF-α) induced adhesion molecules expression in vascular smooth
muscle bells by suppressing (nuclear transcription factor-κB
(NF-kappaB) pathway. Med Sci Monit. 23:4789–4797. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Shindo S, Kumagai T, Shirawachi S, Takeda
K and Shiba H: Semaphorin3A released from human dental pulp cells
inhibits the increase in interleukin-6 and CXC chemokine ligand 10
production induced by tumor necrosis factor-α through suppression
of nuclear factor-κB activation. Cell Biol Int. 45:238–244.
2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Armstrong JM, Boura AL, Hamberg M and
Samuelsson B: A comparison of the vasodepressor effects of the
cyclic effects of the cyclic endoperoxides PGG, and PGH2 with those
of PGD2 and PGE2 in hypertensive and normotensive rats. Eur J
Pharmacol. 39:251–258. 1976.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kawada M, Inoue H, Ohba S, Yoshida J,
Masuda T, Yamasaki M, Usami I, Sakamoto S, Abe H, Watanabe T, et
al: Stromal cells positively and negatively modulate the growth of
cancer cells: Stimulation via the PGE2-TNFα-IL-6 pathway and
inhibition via secreted GAPDH-E-cadherin interaction. PLoS One.
10(e119415)2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Greenhough A, Wallam CA, Hicks DJ,
Moorghen M, Williams AC and Paraskeva C: The proapoptotic BH3-only
protein Bim is downregulated in a subset of colorectal cancers and
is repressed by antiapoptotic COX-2/PGE2 signalling in
colorectal adenoma cells. Oncogene. 29:3398–3410. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Tian D, Ling S, Chen G, Li Y, Liu J, Ferid
M and Bian K: Hypertensive nephropathy treatment by
heart-protecting musk pill: a study of anti-inflammatory therapy
for target organ damage of hypertension. Int J Gen Med. 4:131–139.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang X, Hai CX, Liang X, Yu SX, Zhang W
and Li YL: The protective effects of acanthopanax senticosus harms
aqueous extracts against oxidative stress: Role of Nrf2 and
antioxidant enzymes. J Ethnopharmacol. 127:424–432. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Guo JJ and Zhou Y: Research progress in
detection methods of chronic prostatitis. Med Recapit.
21:1268–1270. 2015.
|
|
50
|
Yang J: Changes and significance of NE,
pH, IL-1β, TNF-α and PGE2 in EPS patients with type III
prostatitis. J Clin Pathol Res. 38:2583–2588. 2018.
|
|
51
|
Scotece M, Conde J, Abella V, López V,
Francisco V, Ruiz C, Campos V, Lago F, Gomez R, Pino J and Gualillo
O: Oleocanthal inhibits catabolic and inflammatory mediators in
LPS-activated human primary osteoarthritis (OA) chondrocytes
through MAPKs/NF-κB pathways. Cell Physiol Biochem. 49:2414–2426.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fan G, Jiang X, Wu X, Fordjour PA, Miao L,
Zhang H, Zhu Y and Gao X: Anti-inflammatory activity of tanshinone
IIA in LPS-stimulated RAW264.7 macrophages via miRNAs and
TLR4-NF-κB pathway. Inflammation. 39:375–384. 2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Huang CH, Jan RL, Kuo CH, Chu YT, Wang WL,
Lee MS, Chen HN and Hung CH: Natural flavone kaempferol suppresses
chemokines expression in human monocyte THP-1 cells through MAPK
pathways. J Food Sci. 75:H254–H259. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Panathur N, Dalimba U, Koushik PV, Alvala
M, Yogeeswari P, Sriram D and Kumar V: Identification and
characterization of novel indole based small molecules as
anticancer agents through SIRT1 inhibition. Eur J Med Chem.
69:125–138. 2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhuo MQ, Luo Z, Xu YH, Li DD, Pan YX and
Wu K: Functional analysis of promoters from three subtypes of the
PI3K family and their roles in the regulation of lipid metabolism
by insulin in yellow catfish Pelteobagrus fulvidraco. Int J
Mol Sci. 19(265)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Zhang YW, Shao DY, Shi JL and Zhu J: A
review on biological activities of kaempferol. Chin Bull Life Sci.
29:400–405. 2017.
|
|
57
|
Ďuračková Z: Some current insights into
oxidetive stress. Physiol Res. 59:459–469. 2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Porcaro AB, Tafuri A, Novella G, Sebben M,
Mariotto A, Inverardi D, Corsi P, Processali T, Pirozzi M, Amigoni
N, et al: Inverse association of prostatic chronic inflammation
among prostate cancer tumor grade groups: Retrospective study of
738 consecutive cases elected to a first random biopsy set. Urol
Int. 100:456–462. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Popovics P, Cai R, Sha W, Rick FG and
Schally AV: Growth hormone-releasing hormone antagonists reduce
prostatic enlargement and inflammation in carrageenan-induced
chronic prostatitis. Prostate. 78:970–980. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Nguyen DP, Li J and Tewari AK:
Inflammation and prostate cancer: The role of interleukin 6 (IL-6).
BJU Int. 113:986–992. 2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen Z, Zhang JL and Yang XP: The research
progression of tumor suppressor TP53 and the new family members
TP63 and TP73. J Inner Mongolia Med Univ. 35:57–62. 2013.
|
|
62
|
Ma ZM, Tian YJ, Wang ZP and Zhai ZX:
Updated roles of SIRT1 in prostate diseases. Zhonghua Nan Ke Xue.
22:356–360. 2016.PubMed/NCBI(In Chinese).
|
|
63
|
Ma J, Liu Y, Sun LN and Liu C: Clinical
observation of serum testosterone and estradiol in patients with
prostatitis and healthy people. Chin J Hum Sexuality. 26:25–26.
2017.(In Chinese).
|
|
64
|
Zheng GL, Zhang XY, Zheng JW, Meng QC and
Zheng DS: Estrogen-like activity of total isoflavones of puerarin
and pueraria. J Chin Med Mater. 566–568. 2002.PubMed/NCBI(In Chinese).
|
|
65
|
Guo AJ, Choi RC, Zheng KY, Chen VP, Dong
TT, Wang ZT, Vollmer G, Lau DT and Tsim KW: Kaempferol as a
flavonoid induces osteoblastic differentiation via estrogen
receptor signaling. Chin Med. 7(10)2012.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhou XH, Han L, Zhou ZH, Liu ZD, Yang JX,
Lv YW and You CL: Morphological and molecular biological
peculiarities of the experimental automimune prostatitis rat model.
Zhonghua Nan Ke Xue. 11:290–295. 2005.PubMed/NCBI(In Chinese).
|
|
67
|
John H, Maake C, Barghorn A, Zbinden R,
Hauri D and Joller-Jemelka HI: Immunological alterations in the
ejaculate of chronic prostatitis patients: clues for autoimmunity.
Andrologia. 35:294–299. 2003.PubMed/NCBI
|
|
68
|
Zong A, Cao H and Wang F: Anticancer
polysaccharides from natural resources: A review of recent
research. Carbohydr Polym. 90:1395–1410. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Wei C, Liu C and Wang H: Effects of
suplatast tosilate on CD4+ T cells and IL-6 in prostate tissue of
rat model with chronic bacterial prostatitis. Chin J Androl.
23:10–13. 2009.
|
|
70
|
Lin SJ, Chou FJ, Lin CY, Chang HC, Yeh S
and Chang C: New therapy with ASC-J9® to suppress the
prostatitis via altering the cytokine CCL2 signals. Oncotarget.
7:66769–66775. 2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Mu JJ, Zeng YY, Huang XY, Zhao XH and Song
B: Effects of Kaempferol on activation, proliferation and cell
cycle of mouse T lymphocytes in vitro. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 25:1106–1108. 2009.PubMed/NCBI(In Chinese).
|
|
72
|
Tang L, Gao XH, Zhao B, Luo JR, Shi XY, Ge
R, Ban SR and Li QS: Design and synthesis of new disubstituted
benzoxazolone derivatives that act as iNOS inhibitors with potent
anti-inflammatory activity against LPS-induced acute lung injury.
(ALI). Bioorg Med Chem. 28(115733)2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Takahashi M, Khan MSI, Makino R, Cline MA
and Tachibana T: Role of nitric oxide on zymosan-induced inhibition
of crop emptying in chicks. Comp Biochem Physiol A Mol Integr
Physiol. 261(111057)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Costales MG, Alam MS, Cavanaugh C and
Williams KM: Extracellular adenosine produced by
ecto-5'-nucleotidase (CD73) regulates macrophage pro-inflammatory
responses, nitric oxide production, and favors Salmonella
persistence. Nitric Oxide. 72:7–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Scailteux LM, Capelle V, Balusson F, Oger
E, Vincendeau S, Mathieu R and Chapron A: Changes in prostate
cancer screening practice by blood PSA testing between 2011 and.
2017, a French population-based study. Curr Med Res Opin.
37:1435–1441. 2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Huang BX, Su HC and Sun FK: Compound
ciprofloxacin suppository combined with ningbitai and yunnan baiyao
for histological prostatitis with PSA elevation. Zhonghua Nan Ke
Xue. 18:986–990. 2012.PubMed/NCBI(In Chinese).
|
|
77
|
Brkljača BN, Gotić J, Šuran J, Brozić D,
Klobučar K, Bojanić K and Vrbanac Z: Effect of prolonged submaximal
exercise on serum oxidative stress biomarkers (d-ROMs, MDA, BAP)
and oxidative stress index in endurance horses. BMC Vet Res.
14(216)2018.PubMed/NCBI View Article : Google Scholar
|