Introduction
Peripheral nerve injury (PNI) is a serious chronic
disease that can result in motor and sensory function impairments.
Furthermore, PNI causes the death of motor neurons in the spinal
cord and sensory neurons in dorsal root ganglia (DRG), which can
result in reduced motor and sensory recovery (1,2). PNI
consequently induces axonal demyelination and degeneration, and
this can result in the absence of reinnervation to the target
organs leading to more severe morphological changes, such as
muscular atrophy (3,4). Therefore, faster long-distance
regeneration of nerves is required for improvement of functional
recovery. Following PNI, oxidative stress is increased at the site
of the injured nerve and this plays a crucial role in exacerbating
damage and hindering nerve reformation (5). Free radical molecules, such as
reactive oxygen species, destroy numerous molecules in the cell,
resulting in mitochondrial dysfunction, lipid peroxidation and
eventually cellular apoptosis (6).
Nelumbo nucifera (N. nucifera), also
known as lotus or Bualuang in Thai, is an aquatic plant in the
family Nelumbonaceae, and it is widely cultivated in Asian
countries (7). All the parts of
N. nucifera are used in traditional herbal medicines and
have been reported to exhibit several beneficial pharmacological
effects, including antimicrobial, anticancer and anti-inflammatory
activity (8,9). Extracts of N. nucifera seeds
can increase superoxide dismutase and catalase levels, which
results in increased free radical scavenging activity in rat
kidneys and liver (10). A study in
a diabetic rat model showed that extracts from lotus leaves could
decrease diabetes-induced nephropathy (11). Aporphine alkaloids extracted from
N. nucifera flowers were shown to promote neurite outgrowth
in PC-12 cells (12). In addition,
it has been shown that the major component in lotus flower oil is
palmitic acid, which possesses antioxidant activity and can
increase the phosphorylation of ERK and can increase cellular
proliferation (13,14).
Since oxidative stress is increased following PNI,
and free radicals play a role in inhibiting axonal regeneration and
increasing cellular apoptosis following nerve injury, the aim of
the present study was to investigate the effects of lotus flower
oil on neurite outgrowth and nerve regeneration in an in
vivo model of PNI.
Materials and methods
Gas chromatography-mass spectrometry
analysis of lotus flower oil
Whole lotus flowers were used for extraction using
an absolute extraction method from Tropicalife Co., Ltd., according
to the manufacturer's protocol. The organic compounds of the lotus
essential oil (LEO) were analyzed using GC-MS by Scientific and
Technological Instruments Center of Mae Fah Luang University,
Thailand. The operating conditions were as follows: The capillary
column (HP-5 ms) was used with a flow rate of 1 ml/min. The gas
chromatograph oven was set at 40-240˚C. Hexane was used as the
solvent. The temperature of the injector and detector were set at
250 and 230˚C, respectively. The mass spectrometer was set to
determine a molecular weight range of 20-300 Dalton. The organic
compounds were identified by comparing their retention times and
mass spectra with the data from the National Institute of Standards
and Technology Mass Spectra database (NIST08). The relative
quantity of each compound was presented by area %.
Antioxidant activity of LEO using the
DPPH method
LEO antioxidant activity was evaluated by assessing
the radical scavenging effect of the stable DPPH free radical.
According to the method described by Kumaran et al (15), 50 µl LEO at various concentrations
(5, 10, 20, 40, 80, 160, 320 and 640 µg/ml) was added to 200 µl 0.1
mM DPPH-ethanol solution in a 96-well plate. After 30 min of
incubation in the dark at 25˚C, the absorbance was determined at
520 nm using a Cytation5 microplate reader (BioTek Instruments,
Inc.) and the DPPH radical scavenging activity was calculated as
the IC50 using the formula: % inhibition =
[(Acontrol -
Asample)/Acontrol]x100; where
Acontrol is the absorbance of DPPH without sample and
Asample is the absorbance of DPPH with LEO. The
experiment was performed three times for each sample.
DRG cell culture and treatment
The dilution of essential oil in ethanol was
performed as described by Shinomiya et al (16) was used. Briefly, LEO was diluted 50%
(v/v) in 70% ethanol before treatment. The final concentration of
ethanol was 0.01% in RPMI medium. DRG cell culture was performed as
described by Hausott et al (17). Briefly, male Wistar rats were
sacrificed using CO2 at a volume displacement rate of
30-70% of the chamber per min for 5 min.
Death was confirmed by cardiac arrest, the spinal
cord was exposed and ~45 DRGs were collected in cold RPMI medium
(Invitrogen; Thermo Fisher Scientific, Inc.). Thereafter, DRGs were
incubated in collagenase type I (5,000 U/ml, Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h at 37˚C followed by 0.25%
trypsin/EDTA (Invitrogen; Thermo Fisher Scientific, Inc.) for 15
min at 37˚C. DRGs were next washed 3 times in RPMI medium
containing 10% horse serum and 5% fetal bovine serum
(Sigma-Aldrich; Merck KGaA), and then transferred to culture medium
(RPMI supplemented with B27; Invitrogen; Thermo Fisher Scientific,
Inc.) and dissociated using fire-polished Pasteur pipettes. The
cell suspension was seeded onto plastic dishes coated with
poly-D-lysine hydrobromide (100 µg/ml, Sigma-Aldrich; Merck KGaA)
at 37˚C overnight and then coated with laminin (5 µg/ml;
Sigma-Aldrich ; Merck KGaA) at 37˚C for 4 h. A total of 1 h after
seeding the medium was changed and the cells were treated with
0.15, 0.3 or 0.6 µl/ml LEO in RPMI medium supplemented with B27
supplement and an antibiotic-antimycotic (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were incubated in a humidified incubator
at 37˚C with 5% CO2 for 24 h. The DRG neuron cultures
were performed three times.
Sciatic nerve crush model
Male Wistar rats with an initial weight of 200-220 g
(6-8 weeks old) were obtained from Nomura Siam International Co.,
Ltd. The rats were housed in a temperature (25±2˚C) and humidity
(35-60%) controlled room with a 12 h light/dark cycle, and provided
ad libitum access to food and water. The rats were randomly
divided into a control, sham, sciatic nerve injury (SNI), SNI + 50
mg/kg LEO or SNI + 100 mg/kg LEO group (6 rats/group). To induce
SNI, the rats were anesthetized using 50 mg/kg intraperitoneal
injection of sodium pentobarbital. The nerve crush procedure was
performed on the left hind limb. An incision was made in the middle
of the thigh, and the muscles were carefully incised at the
intermuscular septum without cutting the muscle fibers to expose
the sciatic nerve. The nerve was clamped at 1 cm proximal to the
bifurcation using artery forceps (straight 12 cm for 30 sec. The
sham rats underwent the same surgery without clamping of the nerve.
Thereafter, the skin was sutured with a nylon suture to close the
wound without stitching up muscles to avoid muscle damage (18,19).
After surgery, all rats were an intraperitoneal injection of 10
mg/kg meloxicam to provide pain relief and reduce inflammation for
3 consecutive days.
Foot withdrawal test
Thermal stimulation was applied to determine
recovery of sensory function on days 7, 14, 21 and 28 after surgery
(20). Prior to the test, the rats
were placed on a non-heated plate for 10 min. Subsequently, the paw
withdrawal latency (PWL) in response to a heat stimulus (50˚C)
using a hot plate at the plantar side of the hind paw was recorded.
The rats were not allowed contact with the heated plate for more
than 20 sec. PWL was recorded on the left paw three times and the
time interval between tests was 5 min. The experiment was repeated
three times with each rat.
Walking track analysis
The recovery of motor function was assessed on days
7, 14, 21 and 28 after surgery using walking tract analysis.
Briefly, the hind paws of trained rats were pressed down onto a
stamp pad soaked with water-soluble black ink. The rats were
allowed to walk along a confined box (30x90x20 cm), which was
placed on a white paper (21x90 cm). The footprints were recorded to
calculate the sciatic functional index (SFI) as described by Bain
et al (21). The experiment
was repeated three times with each rat.
Immunocytochemistry and histological
analysis
After 24 h, DRG neurons were fixed with 4%
paraformaldehyde at 25˚C for 30 min. Cells were washed three times
with PBS and permeabilized with 0.5% Triton X-100 (Sigma-Aldrich;
Merck KGaA) at 25˚C for 5 min, and blocked with 10% goat serum
(Thermo Fisher Scientific, Inc.) in PBS at 25˚C for 30 min. Cells
were incubated with primary antibodies against neuron specific
β-III tubulin (Thermo Fisher Scientific, Inc.; cat. no. MA1-118;
1:1,000) and phospho- (p-)ERK (Cell Signaling Technology, Inc.;
cat. no. 3179; 1:250) at 4˚C overnight. Secondary antibodies
(Alexa-594 chicken anti-mouse; cat. no. A-21201) for β-III tubulin
staining and Alexa-488 goat anti-rabbit (cat. no. A32731) for p-ERK
staining; both at 1:1,000; Invitrogen; Thermo Fisher Scientific,
Inc.) were applied for 30 min at 25˚C. Immunofluorescence labeled
neurons were observed using an inverted fluorescence microscope
(Zeiss Axiovert 100, Carl Zeiss AG; magnification, x20 objective
lens). p-ERK fluorescence intensity measurements and neurite
outgrowth were determined using Metamorph© version 7.
Evaluation of the average fluorescence intensity was performed
after background subtraction. The longest neurite from the cell
body to the growth cone was measured and taken as the maximal
distance (22).
Histological analysis was performed on day 28 after
nerve injury. The rats were sacrificed using CO2 (30-70%
volume displacement rate for 5 min), the sciatic nerve and L4-L6
DRG were fixed in 4% PFA at 4˚C for 48 h followed by 15% sucrose in
PBS for 24 h and then 30% sucrose in PBS for 48 h. Sections were
cut (20 µm thick) using a cryostat (Leica Microsystems, Inc.,
CM1950) for hematoxylin & eosin (H&E) (Sigma-Aldrich; Merck
KGaA) staining. The sections were stained for 10 min with
hematoxylin followed by 20 sec with eosin both at 25˚C. DRG neurons
were counted in area of 80,000 µm2 using a 40x objective
lens, and axon diameters were measured under a 100x oil immersion
lens. Tissue morphology was determined using the ECLIPSE Ni-U |
Upright Microscopes (Nikon Corporation) and analyzed using NIS
Elements imaging software version 5 (Nikon Corporation). All
histological experiments were performed three times.
Statistical analysis
Data are presented as the mean ± SD for DPPH
analysis or SEM for DRG cell culture, tissue morphological analysis
and behavioral tests. A one-way ANOVA followed by a Tukey's
post-hoc test was used to analyze the data in GraphPad Prism
version 9 (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Composition analysis and assessment of
the free radical scavenging activity of LEO
There were three primary compounds that individually
accounted for >10% of the total content of LEO: Palmitic acid
ethyl ester (25.12%), linoleic acid ethyl ester (18.17%) and methyl
8,11,14-heptadecatrienoate (10.45%) (Table I). DPPH is widely used to evaluate
radical scavenging activity (23-25).
Ascorbic acid and Trolox (both from Sigma-Aldrich; Merck KGaA) were
used as positive controls antioxidants. LEO had ability to inhibit
DPPH radical activity with an IC50 value of 29.01±2.93
µg/ml (Table II).
 | Table IGas chromatography-mass spectrometry
analysis of lotus essential oil. |
Table I
Gas chromatography-mass spectrometry
analysis of lotus essential oil.
| Compound name | Retention time,
min | Area, % |
|---|
|
(6Z,9E)-Heptadeca-6,9-diene | 29.46 | 2.62 |
| Palmitic acid
methyl ester | 34.66 | 2.79 |
| Palmitic acid ethyl
ester | 35.40 | 25.12 |
| Heneicosane | 36.28 | 7.26 |
| Linoleic acid ethyl
ester | 36.93 | 18.17 |
| Methyl
8,11,14-heptadecatrienoate | 37.07 | 10.45 |
| Methyl
2-methylhexadecanoate | 37.23 | 7.09 |
| Hexadecane | 38.12 | 2.78 |
 | Table IIDPPH radical inhibition by lotus
essential oil. |
Table II
DPPH radical inhibition by lotus
essential oil.
| Test samples | IC50 ±
SD, µg/ml |
|---|
| Lotus essential
oil | 29.01±2.93 |
| Ascorbic acid | 1.37±0.21 |
| Trolox | 2.89±0.54 |
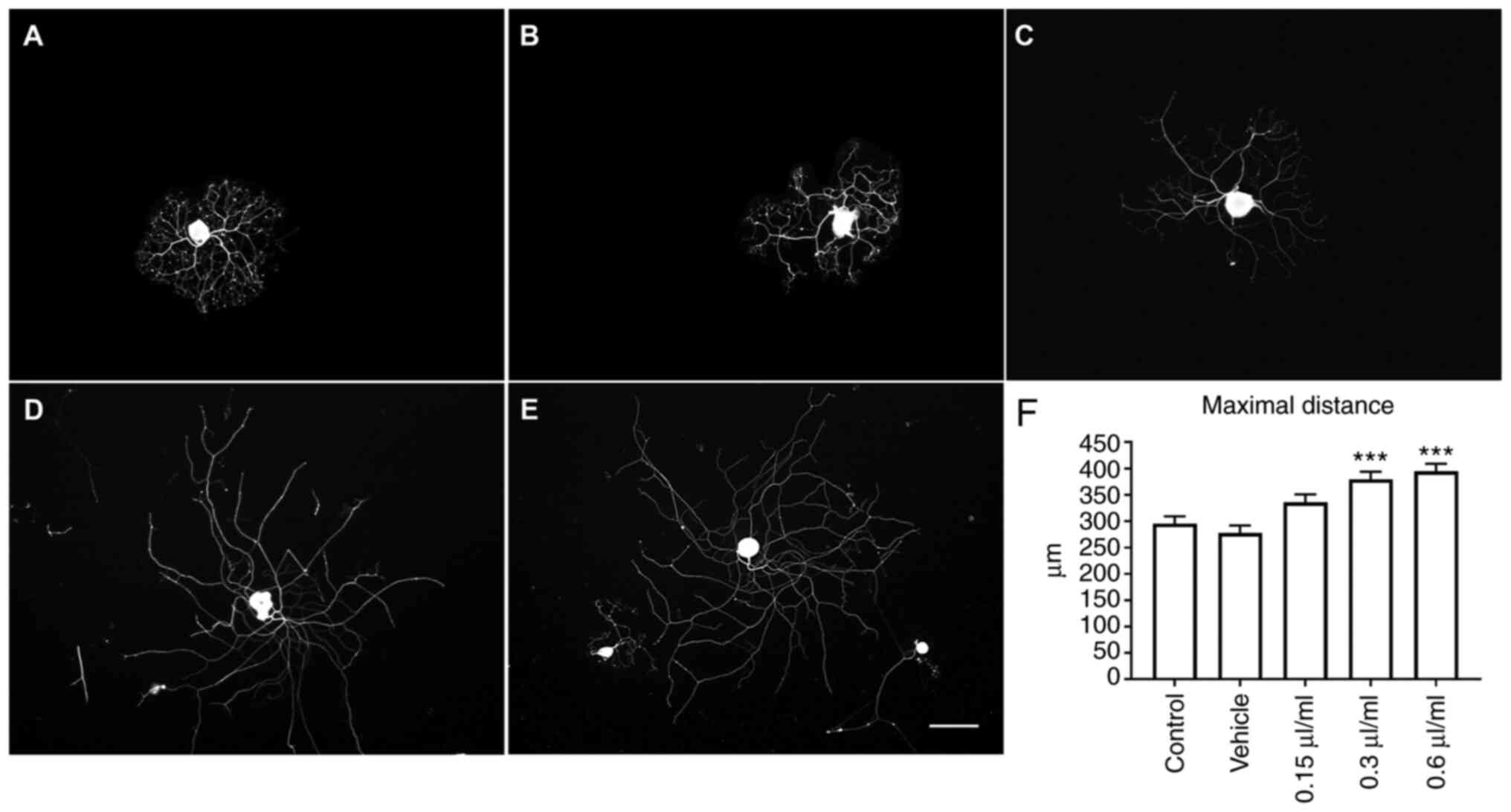
LEO promotes neurite elongation and
ERK phosphorylation
DRG neurons were cultured and treated with various
concentration of LEO. The length of the longest neurite of DRG
neurons was measured as the maximal distance to indicate the effect
of LEO on axon elongation. Neurite outgrowth was observed after 24
h of treatment, LEO at concentrations of 0.3 and 0.6 µl/ml showed a
significant increase in maximal distance when compared with the
negative control and vehicle control groups (both P<0.001,
Fig. 1). LEO-mediated promotion of
neurite outgrowth may be mediated via ERK activation. Elevation of
the p-ERK/ERK ratio plays a crucial role in axonal elongation in
vivo (22). Phosphorylation of
ERK was investigated by immunofluorescence staining of DRG neurons
after 24 h of LEO treatment. Average fluorescence intensity of the
p-ERK/ERK ratio in DRG neurons treated with 0.3 or 0.6 µl/ml LEO
was significantly higher compared with the negative control and
vehicle control groups (P<0.01 and P<0.001, respectively;
Fig. 2).
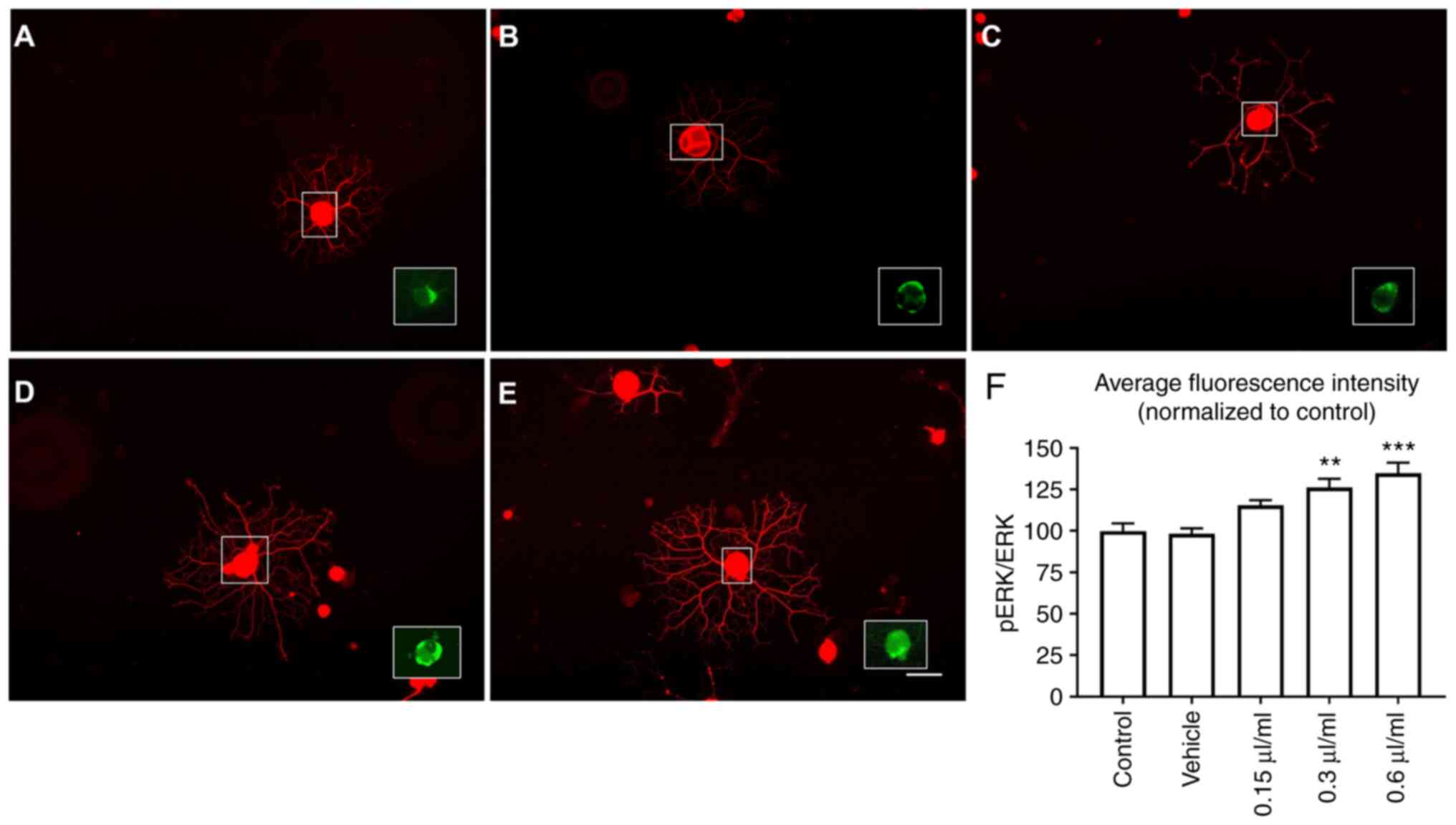 | Figure 2Effect of LEO on ERK activation, DRG
neurons were treated with LEO for 24 h. When treated with 0.3 or
0.6 µl/ml, DRG neurons exhibited a significant increase in the
ratio of p-ERK/ERK fluorescence. (A) Control, (B) vehicle, (C) 0.15
µl/ml LEO, (D) 0.3 µl/ml LEO and (E) 0.6 µl/ml LEO. (F)
Quantitative analysis of p-ERK/ERK fluorescence intensity. Data are
presented as the mean ± SEM of three independent experiments. Scale
bar, 100 µm. **P<0.01, ***P<0.001 vs.
control and vehicle groups. LEO, lotus essential oil; DRG, dorsal
root ganglion; p-, phospho-. |
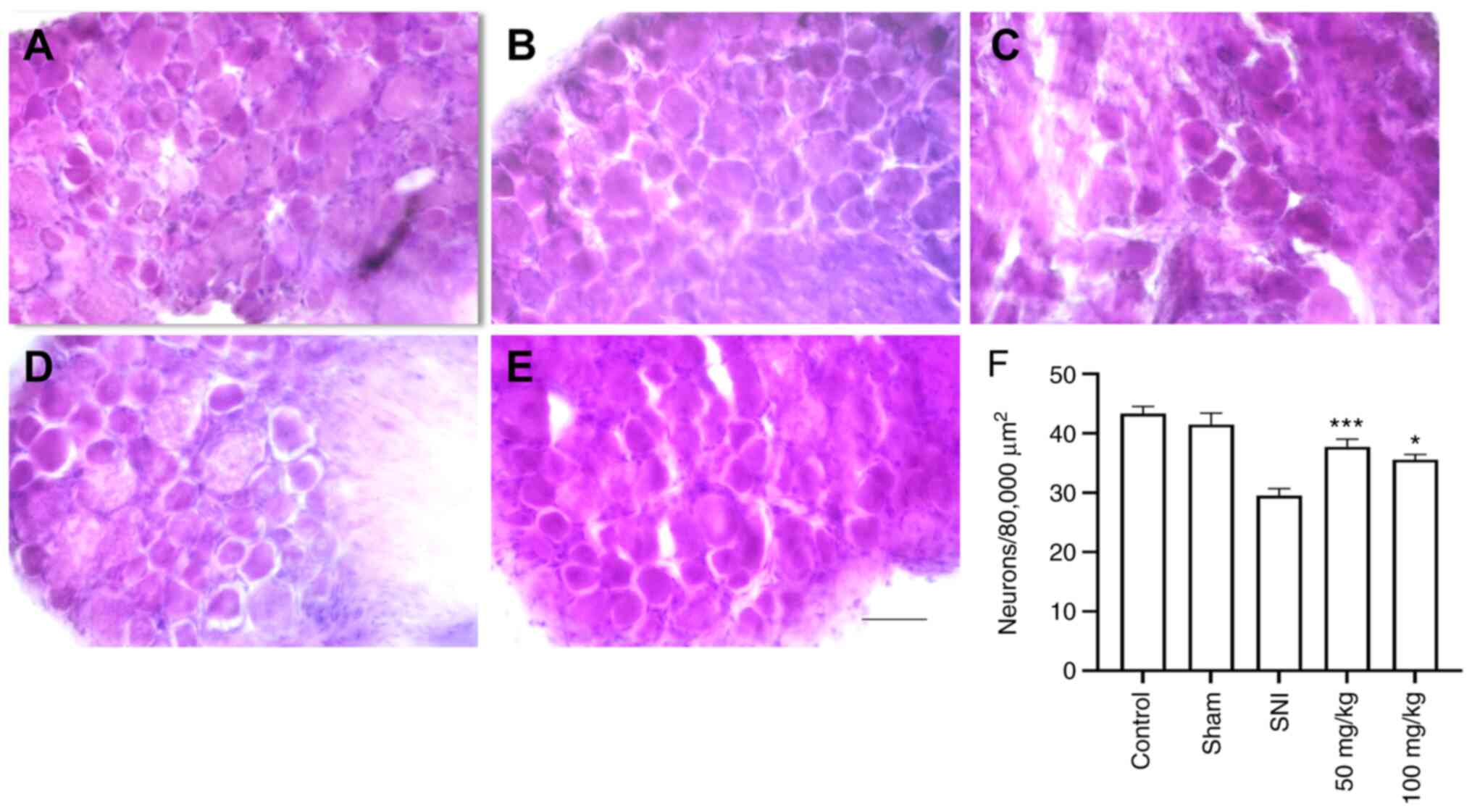
Histological analysis of DRG and
sciatic nerve after crush injury
To study the effect of LEO on PNI, sciatic nerve and
L4-L6 DRG were cut and stained with H&E. After 28 days of SNI,
injured rats orally administered 50 or 100 mg/kg LEO showed a
significant increase in the number of DRG neurons compared with the
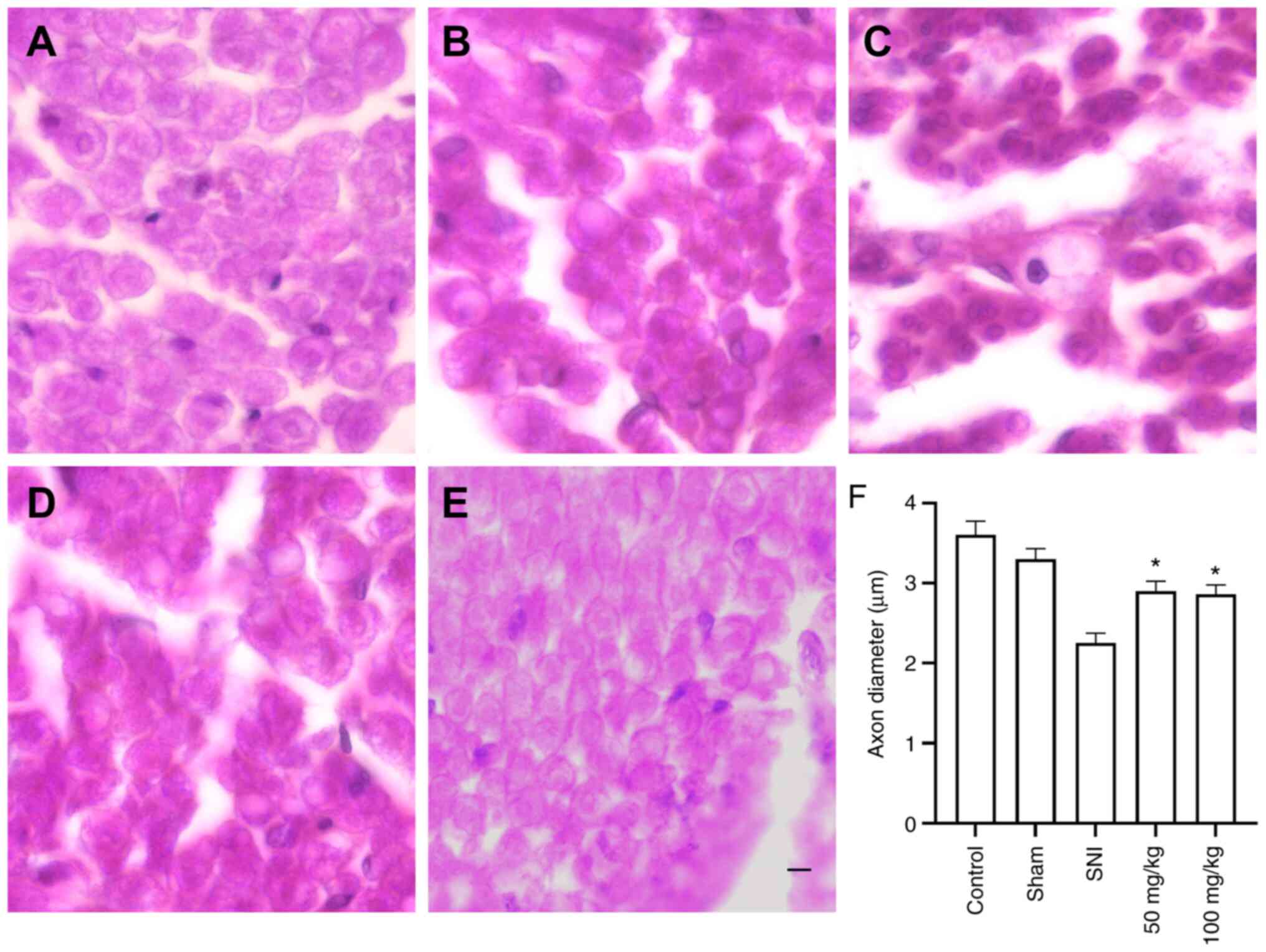
SNI group (P<0.001 and P<0.05, respectively; Fig. 3). Axon diameters were measured to
assess the effect of LEO on nerve regeneration. On day 28 after
SNI, axon diameters were decreased in all injured animals. However,
oral administration of 50 or 100 mg/kg LEO significantly increased
axon diameter when compared to the SNI group (P<0.05, Fig. 4).
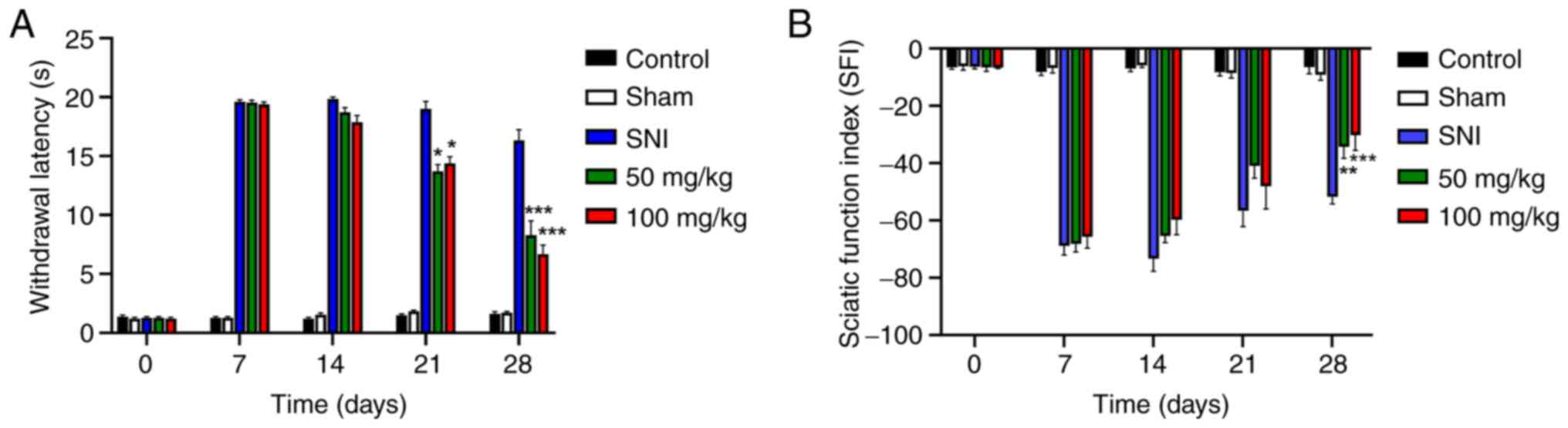
LEO promotes functional recovery after
SNI
The in vitro and in vivo data
indicated that LEO enhanced neurite elongation, and promoted DRG
neuron survival and nerve regeneration. Therefore, whether oral
administration of LEO exerted a therapeutic effect on sensory and
motor function recovery was next assessed in vivo. Prior to
establishment of the SNI model, all animals were tested
behaviorally to obtain baseline values of locomotor function using
walking tract analysis and sensory function using a
thermo-withdrawal test. The baseline values did not differ between
animals. After SNI, injured animals exhibited sensory and motor
function deficits. Behaviors were assessed over a 4-week period.
Sensory function recovery was improved in mice treated with 50 or
100 mg/kg LEO by day 21 after SNI (P<0.05). The differences
became more pronounced by day 28 (P<0.001) (Fig. 5A).
Motor function recovery was assessed by walking
tract analysis. The SFI gradually improved with time. Significant
differences between SNI alone vs. SNI + 50 or 100 mg/kg LEO were
observed on week 4 after lesion (P<0.01 and P<0.001,
respectively; Fig. 5B). Overall,
the results indicated that administration of LEO improved sensory
and motor functions in a rat model of SNI, and this corresponded
with enhanced nerve regeneration.
Discussion
Local upregulation of free radicals is observed
following PNI (26). Increases in
the levels of free radical molecules has been shown to attenuate
recovery of nerve function after injury (27). The levels of free radical molecules
have been reported to increase after injury to the DRG and the
sciatic nerve (28). The increase
in the free radical levels also plays a role in inducing nerve
degeneration and interferes with the regeneration process of the
injured nerve (29). In this study,
the effect of LEO, the crude extract from lotus flower, on nerve
functional recovery after injury was assessed. The results from the
GC-MS analysis showed three major components of lotus flower oil,
including palmitic acid, linoleic acid and methyl
8,11,14-heptadecatrienoate. A previous study reported that palmitic
acid is a fatty acid that exhibits antioxidant activity (30). To confirm whether LEO exhibited
radical scavenging activity, the DPPH method was used, and the
results showed that LEO exhibited potent DPPH radical scavenging
activity with an IC50 value of 29.01±2.93 µg/ml. A
previous study reported that the antioxidant and DPPH radical
scavenging activities of lotus extract could prevent oxidative
stress-induced neuronal death in the central nervous system
(15). Faster axon elongation and
nerve re-innervation are required in nerve recovery after injury
(31). The results of the present
study showed that, treatment of DRG neurons with LEO resulted in
increased axonal distance growth compared with the control and
vehicle-treated groups. LEO induced neurite outgrowth and
elongation in vitro, and this was likely associated with the
upregulation of ERK activation, as shown through increased ERK
phosphorylation. Stimulation of ERK activation has been reported to
promote neurite outgrowth in vitro (32) and axon regrowth after nerve
transection (33); thus, these are
important processes for nerve regeneration. Lu et al
(34) reported that the
accumulation of antioxidant molecules at the site of the injured
nerve resulted in an increase in the ratio of p-ERK/ERK, and this
could improve axon regeneration.
A notable finding of the present study was that LEO
enhanced neurite outgrowth and elongation, both of which are
essential processes for nerve repair and functional recovery. Thus
the effect of LEO on functional recovery of nerves was assessed in
the sciatic nerve crush model. After sciatic nerve injury, the
animals exhibited motor and sensory neuron death as a result of
impaired nerve regeneration (35).
A previous study reported that the recovery of nerve sensory
function following PNI could be defined by the number of cells that
survived within the DRG (36). Α
decrease in DRG neurons after PNI can reduce sensory functional
recovery (37). The results of the
present study showed that administration of LEO could prevent DRG
neurons from death after sciatic nerve lesion. Animals treated with
50 and 100 mg/kg LEO showed an increase in the number of DRG
neurons compared with the animals in the control group. A previous
study showed that antioxidants from the lotus plant could protect
neurons from death following acute nerve injury (38). The neuroprotective effect of LEO on
DRG neurons was likely associated with the antioxidant effect and
increased phosphorylation of ERK activation (34). The rescue of sensory function was
evaluated using thermal stimulation. Rats with sciatic nerve lesion
exhibited an increased foot withdrawal threshold. By day 21 of LEO
treatment, sensory function was improved in the animals treated
with LEO compared with the untreated SNI animals. Of note, the
improvement in sensory function continually increased with the
treatment period. This result also correlates with the higher
numbers of neurons in the DRG.
In the present study, it was shown that treatment
with LEO could improve locomotor functional recovery by day 28
after sciatic nerve crush. Axon regrowth and remyelination are
required for reinnervation of target tissues, and these are
associated with functional motor recovery (39). Remyelination after injury to the
peripheral nerve is possible and required to connect the nerve to
the skin or other target tissues (40). The results of the present study
showed that the axon diameters were increased in the LEO treated
groups compared with the SNI group. Since free radicals and
oxidative stress have been reported to damage the peripheral nerve
and delay its functional recovery (41), increased axon diameters in LEO
treated groups may be the result of the antioxidant activity of
lotus flower oil. Upregulation of antioxidant enzymes, such as
glutathione reductase, can increase the axon diameter and density
after SNI. Therefore, antioxidative systems are also involved in
the recovery process after nerve lesions (42). Thus, the ability of LEO to increase
radical scavenging activity, neurite elongation activity and
functional recovery of the nerve may have been achieved through the
synergistic action of different bioactive components. However, the
effects of the primary components of LEO individually have not been
tested on neurite elongation and functional nerve recovery,
therefore, this is a limitation of the present study and the
subject of a future study. Further experiments are required to
determine the individual effects of the constituents of LEO
separately in the PNI model. LEO is a rich source of several
long-chain fatty acids; however, the effect of these fatty acids on
functional nerve recovery have not been assessed. Therefore,
additional experiments are to study the effects of these long-chain
fatty acids, such as stearic acid, oleic acid and linolenic acid on
PNI. Antioxidants have been shown to promote nerve recovery after
injury. Ascorbyl palmitate, a fat-soluble form of ascorbic acid
with potent antioxidant activity, should be assessed future
experiments of sciatic nerve injury. Moreover, LEO can promote
nerve regeneration and functional recovery after crush injury, and
this may be due to the effects of LEO on the expression of
proteins. Thus, the expression levels of proteins related to
remyelination, such as myelin protein zero and peripheral myelin
protein 2, should be assessed to better understand the effect of
LEO on the mechanism of nerve remyelination.
In summary, injury to the peripheral nerve results
in motor and sensory functional impairment, and administration of
LEO accelerated functional nerve recovery. Lotus, a plant that
possesses antioxidant activity, can prevent sensory neurons from
death and promote an increase in axon diameter. Based on the
results of the present study, LEO may be a potential beneficial
therapeutic option for the management of PNI. However, the effect
of LEO on the activity and levels of endogenous antioxidant enzymes
remains unclear. Therefore, additional studies are required to
investigate the effect of LEO on endogenous antioxidant enzyme
activities after nerve injury.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported in part by funding from the
School of Medical Sciences, University of Phayao (Phayao, Thailand;
grant no. MS 201017).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ST and NS wrote the manuscript, analyzed the data
and designed the study. RK collected the data. All authors have
read and approved the final manuscript. ST, NS and RK confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of University of Phayao (Phayao, Thailand;
approval no. 630104008).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Kinugasa T, Ozaki S, Hamanaka S and Kudo
N: The effects of sciatic nerve axotomy on spinal motoneurons in
neonatal Bax-deficient mice. Neurosci Res. 44:439–446.
2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shi TJ, Hua XY, Lu X, Malkmus S, Kinney J,
Holmberg K, Wirz S, Ceccatelli S, Yaksh T, Bartfai T and Hökfelt T:
Sensory neuronal phenotype in galanin receptor 2 knockout mice:
Focus on dorsal root ganglion neurone development and pain
behaviour. Eur J Neurosci. 23:627–636. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zachodne DL and Ho T: Endoneural
microenvironment and acute nerve crush injury in the rat sciatic
nerve. Brain Res. 535:43–48. 1990.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rasul A, Al-shawi AA, Malik SA, Anwar H,
Rasool B, Razzaq A, Aziz N, Kamran SKS, Sarfraz I, Shabbir A, et
al: Neurada procumbens promotes functions regain in a mouse model
of mechanically induced sciatic nerve injury. Pak J Pharm Sci. 32
(Suppl 4):S1761–S1766. 2019.PubMed/NCBI
|
|
5
|
Dong L, Li R, Li D, Wang B, Lu Y, Li P, Yu
F, Jin Y, Ni X, Wu Y, et al: FGF10 enhances peripheral nerve
regeneration via the preactivation of the PI3K/Akt
signaling-mediated antioxidant response. Front Pharmacol.
16(1224)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang CH, Wu SB, Wu YT and Wei YH:
Oxidative stressresponse elicited by mitochondrial dysfunction:
Implication in thepathophysiology of aging. Exp Biol Med (Maywood).
238:450–460. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ono Y, Hattori E, Fukaya Y, Imai S and
Ohizumi Y: Anti-obesity effect of Nelumbo nucifera leaves extract
in mice and rats. J Ethnopharmacol. 106:238–244. 2006.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Park E, Kim GD, Go MS, Kwon D, Jung IK,
Auh JH and Kim JH: Anti-inflammatory effects of Nelumbo leaf
extracts and identification of their metabolites. Nutr Res Pract.
11:265–274. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liu CP, Tsai WJ, Lin YL, Liao JF, Chen CF
and Kuo YC: The extracts from Nelumbo nucifera suppress cell cycle
progression, cytokine genes expression, and cell proliferation in
human peripheral blood mononuclear cells. Life Sci. 75:699–716.
2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rai S, Wahile A, Mukherjee K, Saha BP and
Mukherjee PK: Antioxidant activity of Nelumbo nucifera (sacred
lotus) seeds. J Ethnopharmacol. 104:322–327. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Chen HW, Yang MY, Hung TW, Chang YC and
Wang CJ: Nelumbo nucifera leaves extract attenuate the pathological
progression of diabetic nephropathy in high-fat diet-fed and
streptozotocin-induced diabetic rats. J Food Drug Anal. 27:736–748.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yano M, Nakashima S, Oda Y, Nakamura S and
Matsuda H: BBB-permeable aporphine-type alkaloids in Nelumbo
nucifera flowers with accelerative effects on neurite outgrowth in
PC-12 cells. J Nat Med. 74:212–218. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jeon S, Kim NH, Koo BS, Kim JY and Lee AY:
Lotus (Nelumbo nuficera) flower essential oil increased
melanogenesis in normal human melanocytes. Exp Mol Med. 41:517–525.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schevzov G, Kee AJ, Wang B, Sequeira VB,
Hook J, Coombes JD, Lucas CA, Stehn JR, Musgrove EA, Cretu A, et
al: Regulation of cell proliferation by ERK and signal-dependent
nuclear translocation of ERK is dependent on Tm5NM1-containing
actin filaments. Mol Biol Cell. 26:2475–2490. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kumaran A, Ho CC and Hwang LS: Protective
effect of Nelumbo nucifera extracts on beta amyloid protein induced
apoptosis in PC12 cells, in vitro model of Alzheimer's disease. J
Food Drug Anal. 26:172–181. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shinomiya M, Kawamura K, Tanida E, Nagoshi
M, Motoda H, Kasanami Y, Hiragami F and Kano Y: Neurite outgrowth
of PC12 mutant cells induced by orange oil and d-limonene via the
p38 MAPK pathway. Acta Med Okayama. 66:111–118. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hausott B, Vallant N, Auer M, Yang L, Dai
F, Brand-Saberi B and Klimaschewski L: Sprouty2 down-regulation
promotes axon growth by adult sensory neurons. Mol Cell Neurosci.
42:328–340. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ramli D, Aziz I, Mohamad M, Abdulahi D and
Sanusi J: The changes in rats with sciatic nerve crush injury
supplemented with evening primrose oil: Behavioural, morphologic,
and morphometric analysis. Evid Based Complement Alternat Med.
2017(3476407)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sriraksa N, Kongsui R, Thongrong S,
Duangjai A and Hawiset T: Effect of Azadirachta Indica flower
extract on functional recovery of sciatic nerve crush injury in rat
models of DM. Exp Ther Med. 17:541–550. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Menéndez L, Lastra A, Hidalgo A and
Baamonde A: Unilateral hot plate test: A simple and sensitive
method for detecting central and peripheral hyperalgesia in mice. J
Neurosci Methods. 113:91–97. 2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bain JR, Mackinnon SE and Hunter DA:
Functional evaluation of complete sciatic, peroneal, and posterior
tibial nerve lesions in the rat. Plast Reconstr Surg. 83:129–138.
1989.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Marvaldi L, Thongrong S, Kozłowska A,
Irschick R, Pritz CO, Bäumer B, Ronchi G, Geuna S, Hausott B and
Klimaschewski L: Enhanced axon outgrowth and improved long-distance
axon regeneration in sprouty2 deficient mice. Dev Neurobiol.
75:217–231. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang D, Wang Q, Ke L, Jiang J and Ying T:
Antioxidant activities of various extracts of lotus (Nelumbo
nuficera Gaertn) rhizome. Asia Pac J Clin Nutr. 16 (Suppl
1):S158–S163. 2007.PubMed/NCBI
|
|
24
|
Gangwar M, Gautam MK, Sharma AK, Tripathi
YB, Goel RK and Nath G: Antioxidant capacity and radical scavenging
effect of polyphenol rich Mallotus philippenensis fruit extract on
human erythrocytes: An in vitro study. ScientificWorldJournal.
2014(279451)2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Huang B, Ban X, He J, Tong J, Tian J and
Wang YL: Comparative analysis of essential oil components and
antioxidant activity of extracts of Nelumbo nucifera from various
areas of China. J Agric Food Chem. 58:441–448. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Varija D, Kumar KP, Reddy KP and Reddy VK:
Prolonged constriction of sciatic nerve affecting oxidative
stressors & antioxidant enzymes in rat. Indian J Med Res.
129:587–592. 2009.PubMed/NCBI
|
|
27
|
Zhang L, Johnson D and Johnson JA:
Deletion of Nrf2 impairs functional recovery, reduces clearance of
myelin debris and decreases axonal remyelination after peripheral
nerve injury. Neurobiol Dis. 54:329–338. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saray A, Apan A and Kisa U: Free
radical-induced damage in experimental peripheral nerve injection
injury. J Reconstr Microsurg. 19:401–406. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hervera A, De Virgiliis F, Palmisano I,
Zhou L, Tantardini E, Kong G, Hutson T, Danzi MC, Perry RB, Santos
CXC, et al: Reactive oxygen species regulate axonal regeneration
through the release of exosomal NADPH oxidase 2 complexes into
injured axons. Nat Cell Biol. 20:307–319. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tyagi T and Mala A: Phytochemical and
GC-MS analysis of bioactive constituents in the ethanolic of Pistia
stratiotes L and Eichhornia crassipes (Mart.) solms. J Pharmacogn
Phytochem. 6:195–206. 2017.
|
|
31
|
Jungnickel J, Haase K, Konitzer J, Timmer
M and Grothe C: Faster nerve regeneration after sciatic nerve
injury in mice over-expressing basic fibroblast growth factor. J
Neurobiol. 66:940–948. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X, Wang Z, Yao Y, Li J, Zhang X, Li
C, Cheng Y, Ding G, Liu L and Ding Z: Essential role of ERK
activation in neurite outgrowth induced by α-lipoic acid. Biochim
Biophys Acta. 1813:827–838. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chierzi S, Ratto GM, Verma P and Fawcett
JW: The ability of axons to regenerate their growth cones depends
on axonal type and age, and is regulated by calcium, cAMP and ERK.
Eur J Neurosci. 21:2051–2062. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu Y, Li R, Zhu J, Wu Y, Li D, Dong L, Li
Y, Wen X, Yu F, Zhang H, et al: Fibroblast growth factor 21
facilitates peripheral nerve regeneration through suppressing
oxidative damage and autophagic cell death. J Cell Mol Med.
23:497–511. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kemp SW, Chiang CD, Liu EH, Wood MD,
Willand MP, Gordon T and Borschel GH: Characterization of neuronal
death and functional deficits following nerve injury during the
early postnatal developmental period in rats. Dev Neurosci.
37:66–77. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hart AM, Terenghi G and Wiberg M: Neuronal
death after peripheral nerve injury and experimental strategies for
neuroprotection. Neurol Res. 30:999–1011. 2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
McKay Hart A, Brannstrom T, Wiberg M and
Terenghi G: Primary sensory neurons and satellite cells after
peripheral axotomy in the adult rat: Timecourse of cell death and
elimination. Exp Brain Res. 142:308–318. 2002.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xiong W, MacColl Garfinkel AE, Li Y,
Benowitz LI and Cepko CL: NRF2 promotes neuronal survival in
neurodegeneration and acute nerve damage. J Clin Invest.
125:1433–1445. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
39
|
Chen P, Piao X and Bonaldo P: Role of
macrophages in Wallerian degeneration and axonal regeneration after
peripheral nerve injury. Acta Neuropathol. 130:605–618.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Fatemi MJ, Foroutan KS, Ashtiani AK,
Mansoori MJ, Vaghardoost R, Pedram S, Hosseinpolli A, Rajabi F and
Mousavi SJ: Comparison of divided sciatic nerve growth within
dermis, venous and nerve graft conduit in rat. J Res Med Sci.
15:208–213. 2010.PubMed/NCBI
|
|
41
|
Levy D, Kubes P and Zochodne DW: Delayed
peripheral nerve degeneration, regeneration, and pain in mice
lacking inducible nitric oxide synthase. J Neuropathol Exp Neurol.
60:411–421. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Renno WM, Benov L and Khan KM: Possible
role of antioxidative capacity of (-)-epigallocatechin-3-gallate
treatment in morphological and neurobehavioral recovery after
sciatic nerve crush injury. J Neurosurg Spine. 27:593–613.
2017.PubMed/NCBI View Article : Google Scholar
|