Introduction
Dermatomyositis (DM) is a systemic autoimmune
disorder with high mortality (50-70% mortality rate) (1). It is characterized by progressive
proximal skeletal muscle weakness, Gottron papules, heliotrope
rash, and histopathological changes in cutaneous rashes (including
calcinosis and ulceration) (2-4).
Furthermore, this disease is always associated with extramuscular
manifestations such as interstitial lung disease and malignancies,
which are a major cause of death (5-7).
The development of suitable animal models of DM pathogenesis is of
relevance for improving our understanding of the pathophysiology
and improving therapeutic options.
The mechanisms of DM include autoimmune responses
(8) and calciphylaxis (9). Humoral and cell-mediated responses are
involved in the pathogenesis of DM. DM is the result of complement
5b-9 (C5b-9) deposition in endomysial vasculature, leading to
inflammation of the microvasculature, which in turn results in
muscle atrophy (10). However, the
autoantibodies targeting endothelial cell antigens have not been
identified (11). In patients with
DM, the immune cells in perimysial and perivascular infiltrates
comprise mainly CD4+ T cells (12). The skin lesions also show
perivascular inflammation with CD4+ T cells in the
dermis (13). Cell-mediated immune
responses also play a leading role in DM (14). Moreover, slight calciphylaxis in DM
was demonstrated as the precipitating factor for the generation of
calcified nodules in cutaneous arterioles (15).
In the present study, a novel DM model in rats was
established using allogeneic plasma membrane protein-induced
autoimmune injury, followed by toxin-induced calciphylaxis
(16). The membrane proteins were
extracted from the skeletal muscle and adjacent subcutaneous
connective tissue of normal rats, then emulsified with complete
Freund's adjuvant (CFA) to prepare membrane antigen. The rats
received membrane antigen sensitization and challenge to induce
autoimmune injury. Cutaneous calciphylaxis was then induced with
dihydrotestosterone (DHT), iron-dextrin (Fe-Dex), polymyxin (PMX),
either alone or in combination, as previous study described
(9). Clinical manifestations, serum
levels of specific antibodies and cytokines, and pathological
changes were detected to evaluate the establishment of the DM
model.
Materials and methods
Animals and materials
A total of 60 female SD rats (7 weeks old, weighting
160±10 g) were obtained from Animal Centre of the Chinese Academy
of Medical Sciences. Animals were maintained in a temperature
(25.0±0.2˚C) and humidity (45%±5%) controlled specific pathogen
free laboratory, under a 12-light/dark cycle, fed with standard
rodent pellets and allowed free access to filtered water. The
animals were acclimatized for three days. All experimental
procedures were approved by the Ethics Committee of the Institute
of Medicinal Plant Development, Chinese Academy of Medical Sciences
and Peking Union Medical College (approval no.
SLXD-20170705016).
DHT (cat. no. C10033864), Fe-Dex (cat. no.
50807A-1), PMX (cat. no. 1405-20-5) and CFA (cat. no. 1001646446)
were obtained from MilliporeSigma. A Tissue Membrane Protein
Extraction Kit (cat. no. KGP3100-2) was obtained from Nanjing
KeyGen Biotech Co., Ltd. The ELISA kits used for the detection of
rat anti-histidyl tRNA synthetase (Jo-1) antibody (cat. no.
QS49908), anti-melanoma differentiation-associated protein 5 (MDA5)
antibody (cat. no. QS49938), tumor necrosis factor-α (TNF-α; cat.
no. QS41721), and interferon-γ (IFN-γ; cat. no. QS44104) were
purchased from Beijing Qisong Biotechnology Co., Ltd. Among all the
rats, 12 rats were used as donors for allogeneic plasma membrane
antigen preparation.
Allogeneic plasma membrane antigen
preparation
The plasma membrane antigen preparation was modified
from a method described previously (17) under aseptic conditions. Briefly,
membrane proteins were obtained from 12 wild-type female SD rats.
The rats were euthanized under anesthesia by intraperitoneal
injection of sodium pentobarbital (50 mg/kg). After the blood was
taken from abdominal aorta, normal saline (20 ml/rat) was perfused
to eliminate remaining blood. Death was confirmed when the
spontaneous breathing ceased. The trapezius, gastrocnemius, and
their nearby subcutaneous connective tissue were then collected.
The tissue samples were rinsed with pre-cooled phosphate buffer
saline, and adipose tissue was removed. The samples were then cut
to small pieces (0.2x0.2x0.2 mm) and homogenized in lysis buffer by
sonication(power 500W, pulses of 15 sec on 10 sec off, with 30-40
cycles). The membrane protein was extracted from the samples using
a membrane protein extraction kit, according to the manufacturer's
instructions. The extracted samples were quantified using a BCA
assay and adjusted to the concentration of 10.0 mg/ml. Lastly, the
membrane protein (10 mg/ml) was emulsified with an equal volume of
CFA.
Experimental design
The rats were randomly divided into 6 groups
(n=8/group), including a control group and five immunized groups.
Rats in all five immunized groups received 10 mg/kg membrane
antigen subcutaneously once weekly from d01 to
d28 for sensitization immunization. A total of 14 days
after the last sensitization immunization, these rats received
membrane antigen challenge immunization with subcutaneous
administration once weekly from d42 to d63.
Rats in the control group were subcutaneously administered normal
saline (1.00 ml/kg). At d77, four groups of
antigen-immunized rats received 10 mg/kg DHT intragastrically, 10
mg/kg Fe-Dex intraperitoneally and 20 mg/kg PMX administration, or
a combination of these three toxins (DHT10 mg/kg + 10 mg/kg Fe-Dex
+ 20 mg/kg PMX) to mimic calciphylaxis, respectively. At
d84, the severity of DM manifestations in each rat was
scored according to the assessment method described in Lennon et
al (18). At d91,
rats were euthanized by exsanguination under anesthesia with
intraperitoneal injection of sodium pentobarbital (50 mg/kg), and
were immobilized in the supine position with spontaneous breathing,
the bloods were collected, and skin and skeletal muscles were
collected from the limbs for further analysis (Fig. 1).
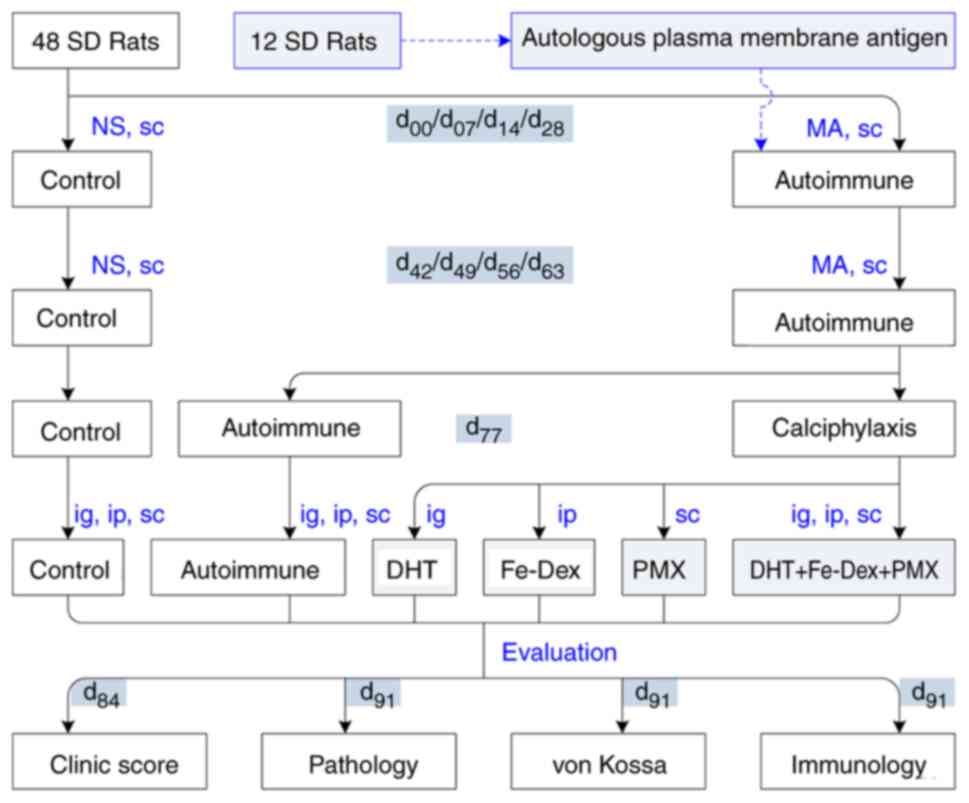 | Figure 1Experimental procedure. 48 SD rats
were randomly divided into autoimmune and control groups. The
control group received NS sc. Fourteen days weekly MA sensitization
for 4 consecutive weeks from d00, the rats in the five
autoimmune groups were challenged weekly with plasma MA sc for 4
consecutive weeks from d42. The rats in the
calciphylaxis group were divided into DHT, Fe-Dex, PMX and DHT +
Fe-Dex + PMX groups at d77, the DHT group was
administered DHT (10 mg/kg ig). The Fe-Dex group was administered
Fe-Dex (10 mg/kg ip). The PMX group was given PMX (20 mg/kg sc).
The DHT + Fe-Dex + PMX group received all three toxins (DHT10 mg/kg
+ 10 mg/kg Fe-Dex + 20 mg/kg PMX). Clinical manifestations in each
experimental animal were scored on d84 according to the
assessment of dermatomyositis symptoms. Pathological features were
examined using hematoxylin and eosin staining, cutaneous
calciphylaxis was evaluated using von Kossa staining, and
autoimmune injuries were evaluated based on the serum levels of
antibodies and cytokines at d91. NS, normal saline; MA,
membrane antigen; sc, subcutaneously; ig, intragastrically; ip,
intraperitoneally; DHT, dihydrotestosterone; Fe-Dex, iron-dextrin;
PMX, polymyxin. |
DM clinical score
DM manifestation in each animal was scored as
described by assessment of muscle weakness as described by Lennon
et al (18), to mimic that
used climactically for patients. The scoring criteria are shown in
Table I.
 | Table IManifestation score in rats with
dermatomyositis. |
Table I
Manifestation score in rats with
dermatomyositis.
| Score | Symptom |
|---|
| 0 | No definite
weakness |
| 1 | Weak grip or cry
with fatigability, edema of limbs |
| 2 | Hunched posture
with head down, movements uncoordinated, and forelimb digits
flexed |
| 3 | Severe generalized
weakness, tremulous, and moribund |
Pathological observation
The skin and skeletal muscle tissue samples were
fixed in PBS-buffered 10% formalin. Following paraffin embedding,
the tissue sections (6 µm) were stained with hematoxylin and eosin
(H&E) or von Kossa using standard protocols. Briefly, the HE
staining procedures were as follows: Slides were deparaffinized
using xylene, hydrated using a gradient of alcohols solutions (100,
95 and 80%) followed by distilled water. Secondly, the slides were
stained with hematoxylin solution for 5 min, rinsed with tap water,
differentiated using 10% acetic acid in 95% alcohol for 1 min and
rinsed with tap water. Finally, the slides were stained with eosin
Y solution for 2~3 min, rinsed with 80% alcohol, dehydrated using a
gradient of alcohols solutions (95, 100%), and then cleared with
xylene. All these steps were performed at room temperature. Images
were acquired using the Digital Pathology system (KFBIO), and
pathological changes were quantified using Image Pro-Plus 7.0.1
(Media Cybernetics).
To quantify T lymphocyte infiltration, the
lymphocyte infiltration areas (TA) and observed areas (OA) were
measured under x20 magnification in skeletal muscle slides. The
volume ratio of infiltrated T lymphocytes (ITL)was calculated using
the formula: [(TA/OA)3/2x100%] (14).
To quantify calcified nodules, the skeletal muscle
tissue sections were stained at room temperature using the von
Kossa method standard protocols. Briefly, after deparaffinizing and
dehydrating, the slide was treated with 5% silver nitrate solution
for 30 min, exposed to incandescent light for 60 min, and then
treated with 5% sodium thiosulfate for 1 min. Finally, the slides
were washed and stained with eosin for 10 min to made the plasma
exhibit thin red. Calcified nodules area (CA) in arteriole and
arteriole areas (AA) were analyzed under x40 magnification. The
volume ratio of calcified nodules in arteriole was calculated using
the formula: [(CA/AA)3/2x100%] (15).
Antibody and cytokine measurement
At d14, one rat was randomly selected
from the antigen-immunized rats, and the blood was obtained from
its tail vein (1 ml) (19). The
blood sample was centrifuged at 3,000 x g for 15 min at 4˚C, and
the serum was collected and stored at -80˚C until analysis. The
specific antibodies against plasma membrane antigen were detected
using the agar immunodiffusion method. Briefly, an agar plate was
prepared, and holes (diameter, 3 mm) were drilled into the agar ~5
mm apart. The prepared allogeneic plasma membrane antigen from the
aforementioned donor animals or serum was added to the antigen well
or sample well, respectively, and the plates were incubated at 37˚C
for 72 h. A sample well was filled with normal saline as a negative
control. Lastly, the plates were stained with Coomassie brilliant
blue to display the immunocomplexes. Images were acquired using a
digital camera (Canon, Inc.).
The serum levels of antibodies (anti-Jo-1 and
anti-MDA5 antibodies) or cytokines (TNF-α and IFN-γ) from all rats
were measured by ELISA using the double-antigen (‘antibody
sandwich’) method according to the manufacturer's protocols
(11). The concentration of
pre-coated rat Jo-1 or MDA5 antigen was 1.5 or 2.5 µg/ml,
respectively. The concentration of pre-coated anti-TNF-α or
anti-IFN-γ antibody was both 2.0 µg/ml. The optical density at 450
nm was measured using a microplate reader (M1000; Tecan Group,
Ltd.), and the concentrations were calculated by converting the
optical density using a standard curve.
Statistical analysis
The data are presented as means ± SD. The
differences between groups were analyzed with SPSS 16.0 software
(SPSS, Inc.) with non-parametrical test using a Kruskal-Wallis'
test followed by a Dunn's post-hoc test. Correlation analysis
between two data was performed using Spearman's correlation
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Mimic clinical score in rats with
DM
The clinical score in DM rats showed that 2 weeks
after the antigen immunization challenge, rats that received
membrane antigen immunization without the toxins presented local
cutaneous swelling and enlarged lymph nodes caused by the injected
skin drainage. Following toxin administration to induce
calciphylaxis (DHT, Fe-Dex, PMX or all three combined), rats
exhibited marked weakness, ear hyperemia, and limb edema,
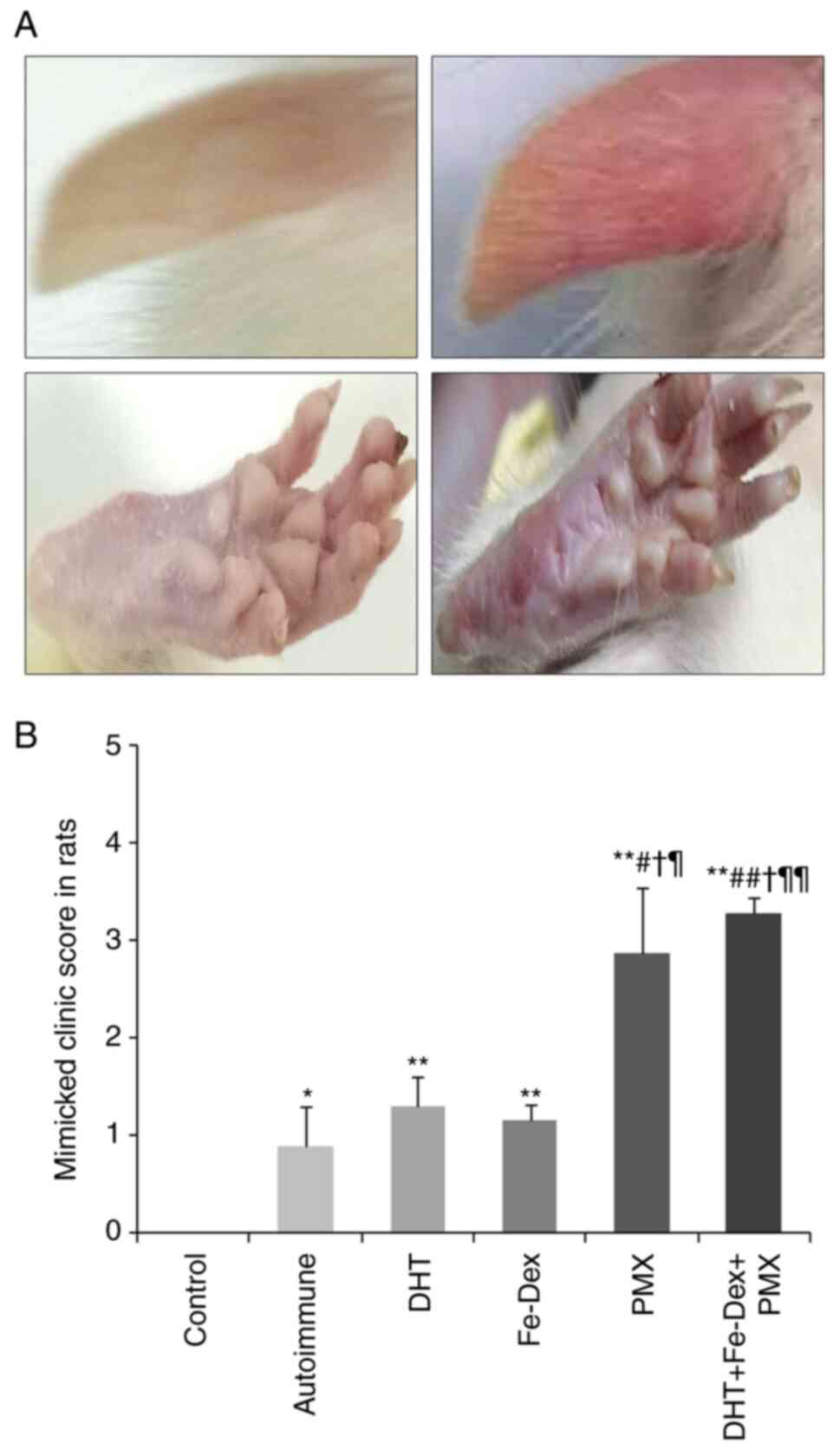
especially in the PMX and DHT + Fe-Dex + PMX groups (Fig. 2A). The clinical scores in the PMT
and DHT + Fe-Dex + PMX groups were higher than those in the
antigen-immunized, DHT, and Fe-Dex groups (Fig. 2B). These results demonstrated that
the lesions in rats that received with PTM and DHT + Fe-Dex + PMX
were most similar to the clinical manifestations seen in
patients.
Pathological changes of skins and
skeletal muscles in rats with DM
To evaluate autoimmune injury, the pathological
changes in the skin and skeletal muscles of DM model rats were
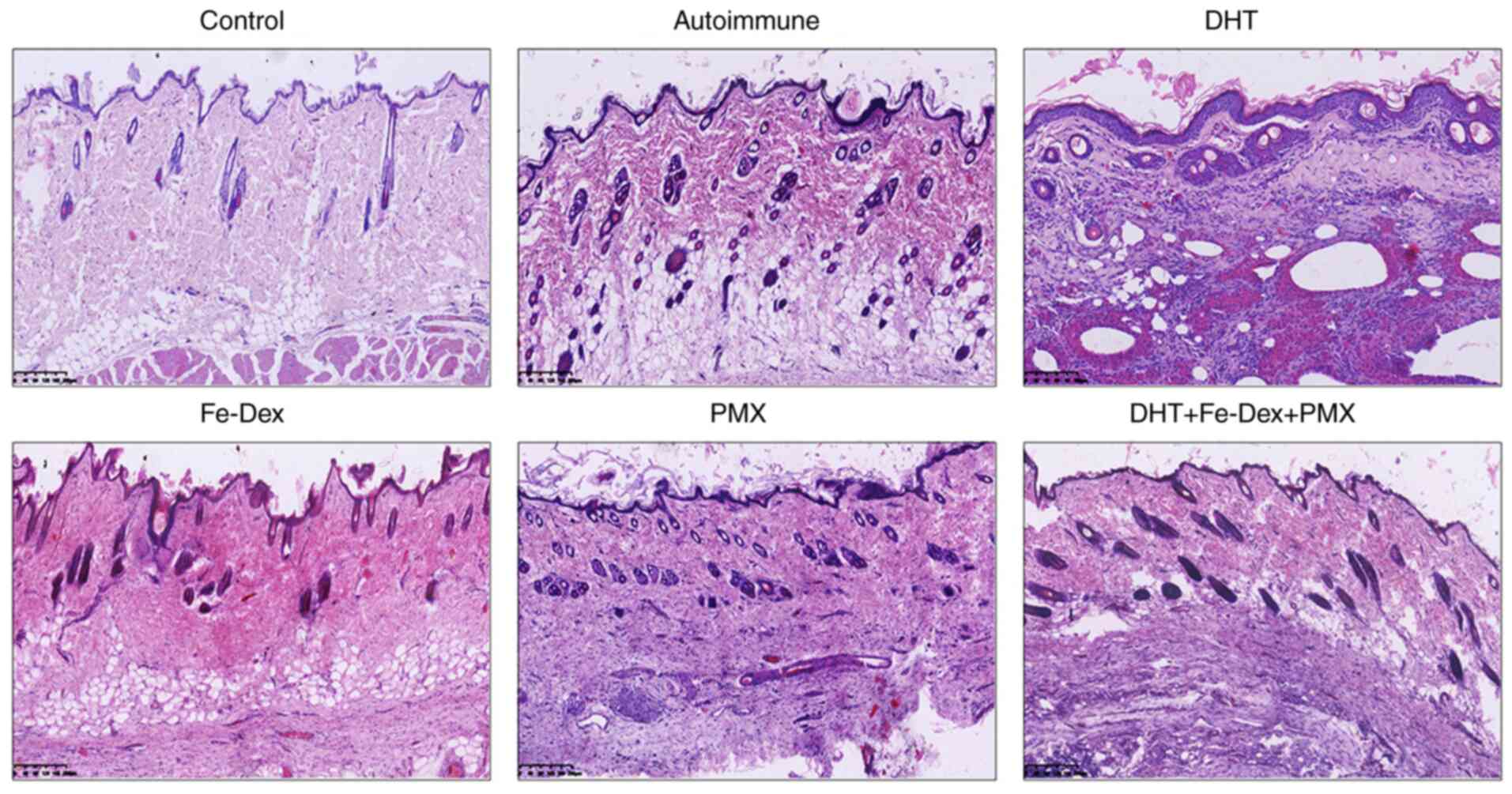
observed using HE staining. In the skin tissue sections, rats in
all five autoimmune groups showed dyskeratotic keratinocytes,
basement membrane thickening, lymphocytes and few neutrophils
infiltrated in the dermis. These changes were obvious in Fe-Dex and
DHT + Fe-Dex + PMX groups (Fig.
3).
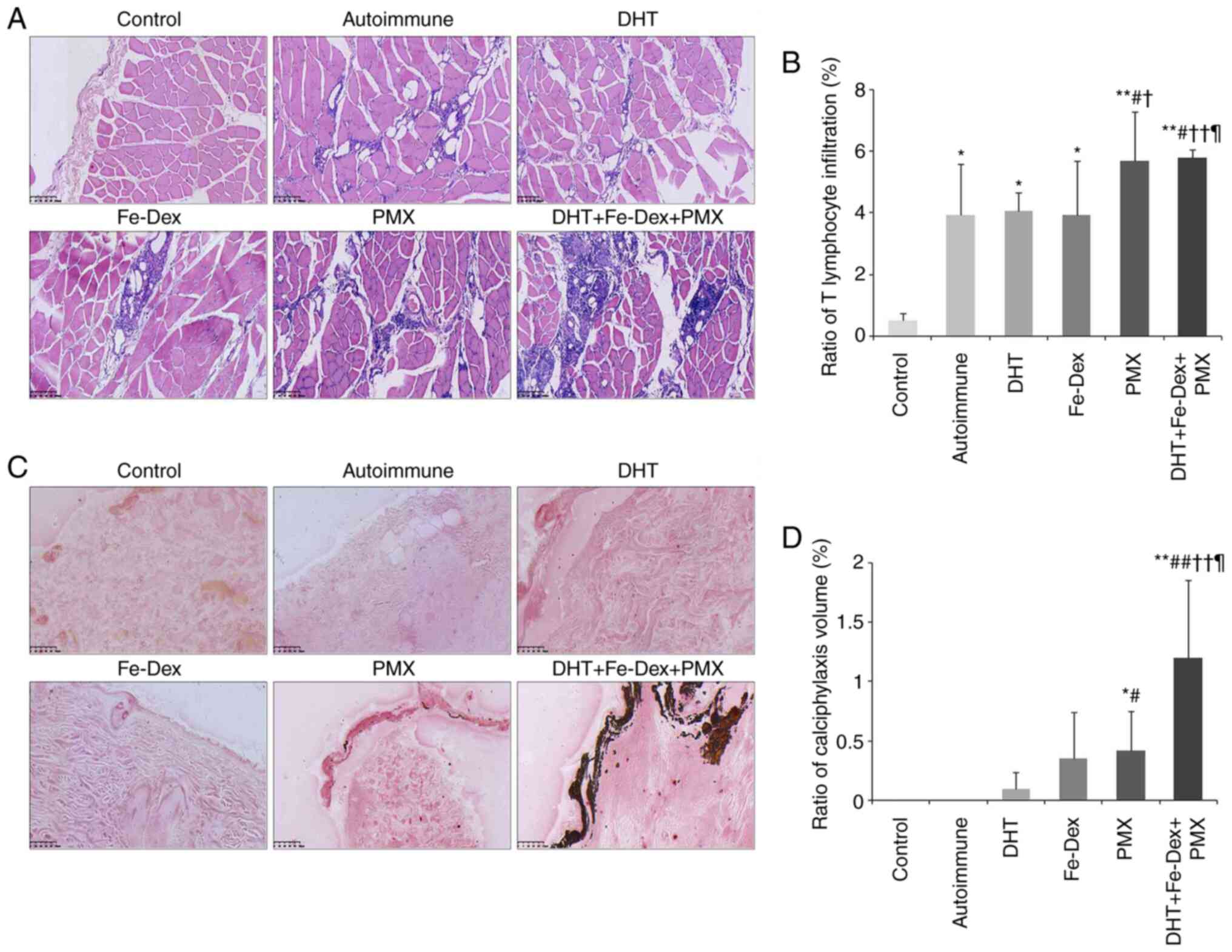
In the skeletal muscle tissue, compared with the
control group, all the autoimmune groups showed edema and necrotic
of muscle fibers, perifascicular atrophy, as well as perifascicular
and perivascular lymphocytes infiltration (Fig. 4A). The morphometry results showed
that the volume ratio of ITL was significantly increased in all the
autoimmune groups. Moreover, the ITL volume ratio in the DHT +
Fe-Dex + PMX group was higher than those in the antigen-immunized
(P<0.05), DHT (P<0.01) and Fe-Dex (P<0.05) groups
(Fig. 4B). This indicated that
antigen immunization followed by DHT + Fe-Dex + PMX administration
may represent the suitable model for DM.
Cutaneous calciphylaxis in rats with
dermatomyositis
Calciphylaxis was analyzed following calcified
nodule staining using the von Kossa method. The calcified nodules
stained as black deposits, which were most apparent in the DHT +
Fe-Dex + PMX group (Fig. 4C). The
severity of cutaneous calciphylaxis was measured as the volume
ratio of calcified nodules in arterioles. Compared with the
control, the calciphylaxis ratio in the PMX (P<0.05) and DHT +
Fe-Dex + PMX groups was significantly increased (P<0.01). The
rats in the DHT + Fe-Dex + PMX group had the highest ratio of
calcified nodules in arterioles (Fig.
4D).
Changes in autoimmune antibody and
cytokine levels in rats with DM
Autoimmune injury was demonstrated to have occurred
based on the production of special antigens. Positivity for
antibodies against plasma membrane antigens, as well as anti-Jo-1
antibody, anti-MDA5 antibody, TNF-α and IFN-γ were used to evaluate
autoimmune injury.
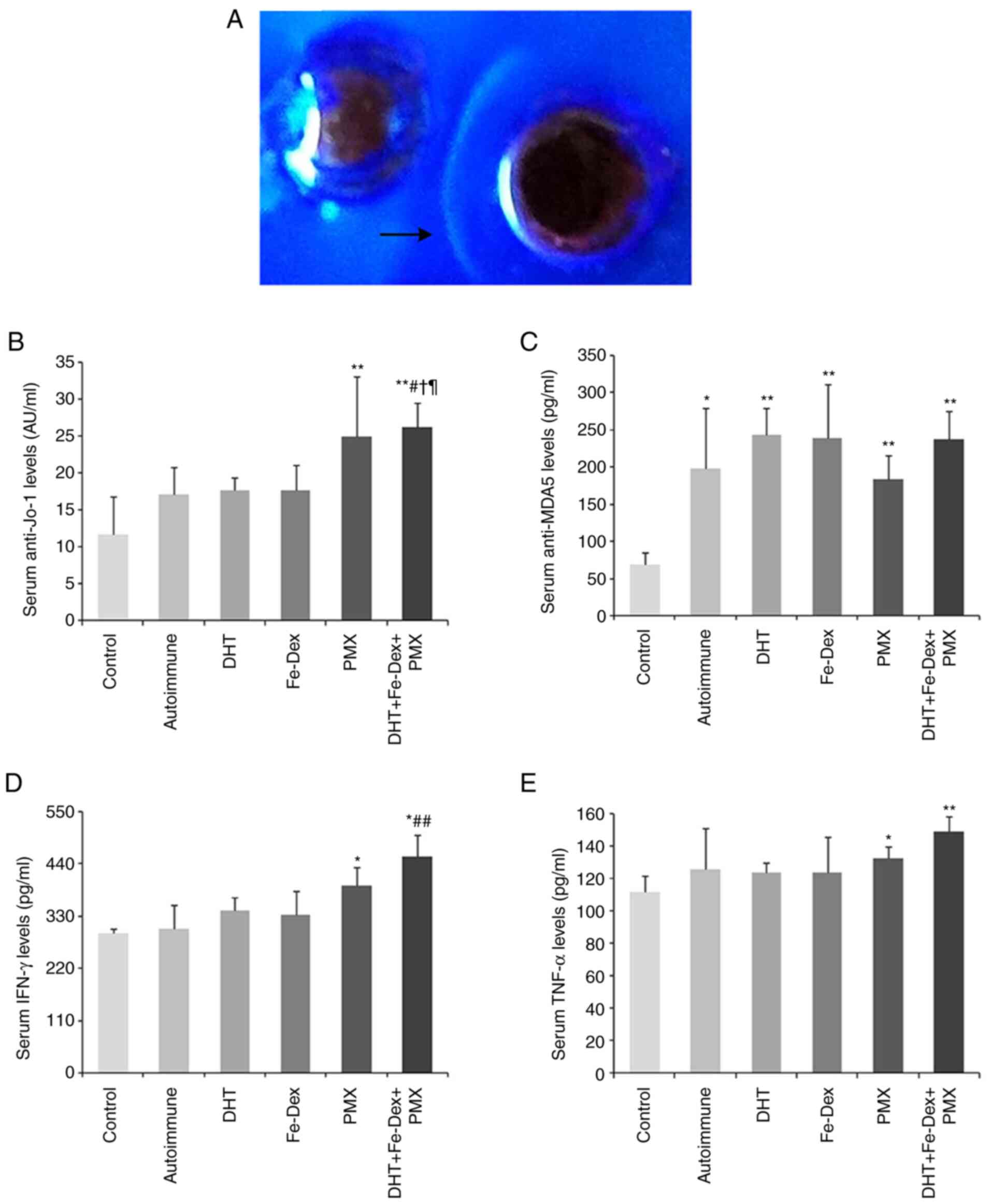
In the agar immunodiffusion assay, of one randomly
selected rats from the antigen-immunized animals, positive
precipitate line was observed, which indicated production of
specific antigens against membrane antigens (Fig. 5A).
Anti-MDA5 and anti-Jo-1 antibodies are important
biomarkers for DM diagnosis (20).
The levels of anti-Jo-1 antibody in the PMX and DHT + Fe-Dex + PMX
groups increased significantly compared with those in the control
(P<0.01). Among the four calciphylaxis groups, anti-Jo-1
antibody levels in DHT + Fe-Dex + PMX group was greater than those
in the antigen-immunized (P<0.05), DHT (P<0.05), and Fe-Dex
(P<0.05) groups (Fig. 5B). The
serum anti-MDA5 antibody levels in all five autoimmune groups were
increased significantly compared with those of the control
(Fig. 5C).
As markers for the severity and prognosis of DM
(21), the levels of IFN-γ and
TNF-α were measured. IFN-γ is the signature cytokine of T helper 1
cells, and its levels increased in patients with DM in parallel
with the progression of muscle weakness (22). In the present study, IFN-γ levels
increased significantly in the PMX (P<0.05) and DHT + Fe-Dex +
PMX (P<0.05) groups compared with the control group. Among all
autoimmune groups, IFN-γ levels in the DHT + Fe-Dex + PMX group
were the highest (Fig. 5D). TNF-α
directly regulates muscle metabolism and regeneration (23). The levels of TNF-α significantly
increased in the PMX (P<0.05) and DHT + Fe-Dex + PMX (P<0.01)
groups compared with the control group (Fig. 5E).
Calciphylaxis aggravates autoimmune
injury in rats with DM
Correlation analysis was performed (Table II). The results indicated that the
clinical scores correlated with indicators of autoimmune injury
(IFN-γ, ITL, TNF-α, anti-Jo-1 and anti-MDA5 antibody) and cutaneous
calciphylaxis. Moreover, cutaneous calciphylaxis was also
correlated with indicators of autoimmune injuries (IFN-γ, ITL,
TNF-α, and anti-Jo-1 antibody).
 | Table IICorrelation analysis between score
and autoimmune injury, or calciphylaxis and autoimmune injury. |
Table II
Correlation analysis between score
and autoimmune injury, or calciphylaxis and autoimmune injury.
| Parameters | Spearman's
coefficient | P-values |
|---|
| Score | | |
|
IFN-γ | 0.823 |
<0.001b |
|
ITL | 0.730 |
<0.001b |
|
TNF-α | 0.595 |
<0.001b |
|
Anti-Jo-1 | 0.663 |
<0.001b |
|
Anti-MDA5 | 0.442 | 0.008a |
|
ACN | 0.760 |
<0.001b |
| ACN | | |
|
IFN-γ | 0.673 |
<0.001b |
|
TNF-α | 0.433 | 0.009a |
|
ITL | 0.495 | 0.002a |
|
Anti-Jo-1 | 0.480 | 0.004a |
Discussion
DM is a serious systemic autoimmune disease
characterized by skin rashes and progressive muscle weakness
(3). However, there are no specific
treatments for DM (24). The lack
of suitable animal model constrains novel drug discovery.
Autoimmune injuries with slight cutaneous calciphylaxis are
important factors involved in DM pathogenesis (3). In the present study, a rat model of DM
was established using allogeneic plasma membrane antigen
immunization and toxin-induced cutaneous calciphylaxis. The
interventions used were optimized to replicate the lesions observed
in patients with DM.
According to the pathophysiology of human DM, four
aspects were designed as the evaluating system of a novel DM model
for developing targeted therapies, i.e., muscle weakness,
pathological features of heterogenic autoimmune injury, slight
cutaneous calciphylaxis, and the molecules that are involved in
autoimmune injuries.
According to the diagnostic criteria, the DM model
developed in the present study mimicked the main clinical
manifestations of the disease, such as muscle weakness and
characteristic cutaneous hyperemia. Muscle weakness gradually
aggravated during this experimental process. The cutaneous
hyperemia or edema in ear or limb skins appeared consequently. Rats
receiving PMX alone or DHT, Fe-Dex and PMX combined showed obvious
clinical manifestations, which started at 30 min after
administration of the toxins and lasted until the endpoint of the
experimental procedure.
The general pathological features of DM include
inflammation of muscles, skin, and blood vessels (25). The gross feature of human DM has
been described as red or purple rashes in affected skin, fixed
locations (chest, face, neck, upper back, shoulders, ankles, and
knees) appearing as a sunburn, being possible rough, dry, and
scaly, leading to calcium deposits under the skin, forming nodules
(26). These inflammatory damages
occurring in muscle tissue result in pain, weakness, and atrophy
(27). The microscopic
characteristics of human DM have been described as an
autoimmune-mediated inflammation in skeletal muscle and its nearby
subcutaneous connective tissue, which was observed as necrosis and
phagocytosis, regeneration, perifascicular atrophy, internal
nuclei, fiber vacuolation, fiber diameter variation, mononuclear
cell infiltration (lymphocyte, macrophage and plasma cell) in
perimysial and endomysial connective tissue (11,28).
In the present study, focusing on the autoimmune injuries in the DM
rats, perifascicular fiber atrophy, necrosis of muscle fiber,
degenerative changes in intramuscular capillaries, and T lymphocyte
infiltration were examined. The analysis showed that increased
ratios of infiltrated T lymphocytes were detectable in this
model.
Cutaneous calciphylaxis is a systemic process
characterized with arterial calcification, microthrombosis, and
fibrointimal hyperplasia in arterioles, even leading to ischemia
and necrosis (29). The rats that
were administered all three toxins combined exhibited numerous
arteriole calcified nodules, those in the PMX exhibited few
calcified nodules, and those in the DHT or Fe-Dex groups only had
scattered calcified nodules. The inflammatory cells may release
hydrogen ions leading to an acidic environment, then resulting in
phosphate dissociation (30). When
high levels of calcium-phosphate products form, calcific deposition
occurs in arterioles, which is described as calcified nodules
(31,32) are considered predisposing factors in
DM pathogenesis (9). Autoimmune
injury sensitizes the animals to toxin-induced calciphylaxis
(33). In the present study,
calcified nodules in arterioles in cutaneous tissue positively
correlated with indicators of autoimmune injury, such as IFN-γ
level, TNF-α level, T lymphocyte infiltration, and anti-Jo-1
antibody levels. Slight calciphylaxis also aggravates the clinical
manifestations and autoimmune injuries (lymphocyte infiltration and
cytokines). The morphometry analysis showed an increased ratio of
calcified nodules in arterioles, which demonstrated a slight degree
of cutaneous calciphylaxis in this model.
In the present study, autoimmune injury was induced
with plasma membrane antigen that extracted from the skeletal
muscle and its nearby cutaneous tissues. Autoimmune injury consists
of tissue damage resulting from immunological mechanisms; the four
types of hypersensitivity always play roles in the pathogenesis of
autoimmune disorders: i) Type I-immunoglobulin (Ig) E and mast
cells; ii) type II-IgM, IgG and complement; iii) type III-IgG
immunocomplexes; and type IV T lymphocytes and macrophages
(34-36).
In the present study, 14 days after membrane antigen stimulation,
positive precipitation lines were observed in agar immunodiffusion
assays carried out in randomly selected rats. However, this assay
was not performed in all autoimmune rats, which is the limitation
of this study.
Furthermore, the presence of antibodies specific for
autologous plasma membrane antigens were found in the sera of
autoimmune rats, suggesting possible antibody-dependent damage. In
addition, type-II or -III hypersensitivity may be involved in the
injury mechanism in the antigen-sensitized rats. Finally, it was
demonstrated that type II hypersensitivity-associated mechanisms
taking part in the antibody-dependent damage in this DM model,
which was confirmed based on the increased levels of serum unique
antibodies (Jo-1/MDA5) and the cellular swelling of muscle fibers
(2,37-39).
Antibody-independent damage, associated with type-IV
hypersensitivity, was confirmed in this DM model, which was
evidenced by the pathological characteristics of the skeletal
muscle and the increased levels of serum cytokines (TNF-α/IFN-γ)
(22,40-42).
Each biomarker in this process positively correlated with the
clinical score or the pathological changes. The pathogenesis of DM,
initiated by cellular immune injuries, and the increased levels of
specific antibodies in the sera were the results of autoimmune
lesions, which were consistent with the diagnosis of DM patients in
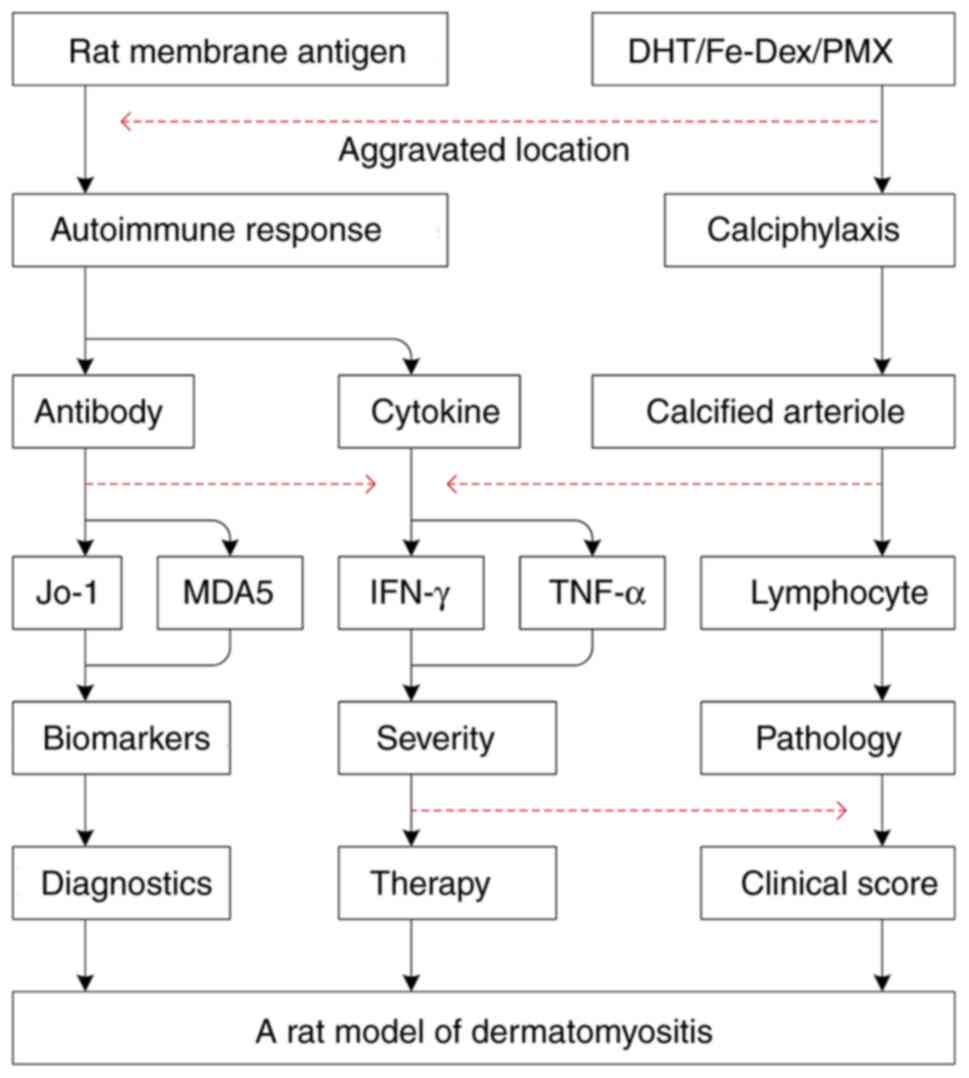
clinics (Fig. 6).
In conclusion, a novel DM model in rats was
developed from both of the autoimmune injuries induced with
autologous plasma membrane antigen and toxin-induced cutaneous
calciphylaxis. The autoimmune injury mimicked the leading
mechanisms in DM pathogenesis, and the slight cutaneous
calciphylaxis was a precipitating factor that aggravated the
observed lesions. In this model, there is a wide observational
window that could satisfy experimental studies, with a fixed
interval between antigen immunization and cutaneous calciphylaxis
(from 6 to 13 weeks). This model may prove useful for the
evaluation of the mechanisms of DM pathogenesis and may facilitate
the development of novel DM treatment methods.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by The National Natural Science
Foundation of China (grant nos. 81541082 and 81673674 and The
Chinese Academy of Medical Sciences Medical and Health Science and
Technology Innovation Project (grant no. 2018-I2M-AI-015).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DC designed the study. DC, XZ and LL drafted and
revised the manuscript. XZ and LL performed the experiments and
analyzed the data. SG, JL, SL and HW performed the experiments. All
authors have read and approved the final manuscript. DC, XZ and LL
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This retrospective study was approved by The Ethics
Committee of the Institute of Medicinal Plant Development, Chinese
Academy of Medical Sciences & Peking Union Medical College
(approval no. SLXD-20170705016).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Santo AH, Souza JM, Pinheiro CE, Souza DC
and Sato EI: Trends in dermatomyositis- and polymyositis-related
mortality in the state of Sao Paulo, Brazil, 1985-2007: Multiple
cause-of-death analysis. BMC Public Health. 10(597)2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Johnson C, Connors GR, Oaks J, Han S,
Truong A, Richardson B, Lechtzin N, Mammen AL, Casciola-Rosen L,
Christopher-Stine L and Danoff SK: Clinical and pathologic
differences in interstitial lung disease based on antisynthetase
antibody type. Respir Med. 108:1542–1548. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
DeWane ME, Waldman R and Lu J:
Dermatomyositis: Clinical features and pathogenesis. J Am Acad
Dermatol. 82:267–281. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Smith ES, Hallman JR, DeLuca AM,
Goldenberg G, Jorizzo JL and Sangueza OP: Dermatomyositis: A
clinicopathological study of 40 patients. Am J Dermatopathol.
31:61–67. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vermaak E, Shaddick G and McHugh NJ:
Mortality In Polymyositis and Dermatomyositis: A Single Centre
Study. Arthritis Rheum-Us 65: S878-S878, 2013. https://www.webofscience.com/wos/alldb/full-record/WOS:000325359205007.
|
|
6
|
Yang WM and Chen JJ: Advances in
biomarkers for dermatomyositis. Clin Chim Acta. 482:172–177.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun KY, Fan Y, Wang YX, Zhong YJ and Wang
GF: Prevalence of interstitial lung disease in polymyositis and
dermatomyositis: A meta-analysis from 2000 to 2020. Semin Arthritis
Rheum. 51:175–191. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kao L, Chung L and Fiorentino DF:
Pathogenesis of dermatomyositis: Role of cytokines and interferon.
Curr Rheumatol Rep. 13:225–232. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Selye H, Gentile G and Jean P: An
experimental model of ‘dermatomyositis’ induced by calciphylaxis.
Can Med Assoc J. 85:770–776. 1961.PubMed/NCBI
|
|
10
|
Aljabban J, Syed S, Rohr M, Weisleder N,
McElhanon KE, Hasan L, Safeer L, Hoffman K, Aljabban N, Mukhtar M,
et al: Investigating genetic drivers of dermatomyositis
pathogenesis using meta-analysis. Heliyon. 6(e04866)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luo YB and Mastaglia FL: Dermatomyositis,
polymyositis and immune-mediated necrotising myopathies. Biochim
Biophys Acta. 1852:622–632. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Okiyama N, Ichimura Y, Shobo M, Tanaka R,
Kubota N, Saito A, Ishitsuka Y, Watanabe R, Fujisawa Y, Nakamura Y,
et al: Immune response to dermatomyositis-specific autoantigen,
transcriptional intermediary factor 1γ can result in experimental
myositis. Ann Rheum Dis. 80:1201–1208. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Iaccarino L, Ghirardello A, Bettio S, Zen
M, Gatto M, Punzi L and Doria A: The clinical features, diagnosis
and classification of dermatomyositis. J Autoimmun. 48-49:122–127.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shimojima Y, Matsuda M, Ishii W, et al:
Phenotypic analysis of lymphocytes using flow cytometry in
dermatomyositis with and without interstitial pneumonia. In:
Proceeding of the 8th International Congress of Neuroimmunology:
209-212, 2006. https://www.webofscience.com/wos/alldb/full-record/WOS:000251732700043.
|
|
15
|
Cassius C, Moguelet P, Monfort JB, Fessi
H, Michel PA, Boulahia G, Cury K, Frances C and Senet P:
Calciphylaxis in haemodialysed patients: Diagnostic value of
calcifications in cutaneous biopsy. Br J Dermatol. 178:292–293.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nemoto H, Bhopale MK, Constantinescu CS,
Schotland D and Rostami A: Skeletal muscle myosin is the
autoantigen for experimental autoimmune myositis. Exp Mol Pathol.
74:238–243. 2003.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kang J, Zhang HY, Feng GD, Feng DY and Jia
HG: Development of an improved animal model of experimental
autoimmune myositis. Int J Clin Exp Pathol. 8:14457–14464.
2015.PubMed/NCBI
|
|
18
|
Lennon VA, Lindstrom JM and Seybold ME:
Experimental autoimmune myasthenia: A model of myasthenia gravis in
rats and guinea pigs. J Exp Med. 141:1365–1375. 1975.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abbas AK and Lichtman AH: Basic
immunology: Functions and disorders of the immune system. Saunders,
Philadelphia, PA, 2004.
|
|
20
|
Bodoki L, Nagy-Vincze M, Griger Z,
Betteridge Z, Szollosi L and Danko K: Four dermatomyositis-specific
autoantibodies-anti-TIF1ү, anti-NXP2, anti-SAE and anti-MDA5-in
adult and juvenile patients with idiopathic inflammatory myopathies
in a Hungarian cohort. Autoimmun Rev. 13:1211–1219. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ishikawa Y, Iwata S, Hanami K, Nawata A,
Zhang M, Yamagata K, Hirata S, Sakata K, Todoroki Y, Nakano K, et
al: Relevance of interferon-gamma in pathogenesis of
life-threatening rapidly progressive interstitial lung disease in
patients with dermatomyositis. Arthritis Res Ther.
20(240)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Giris M, Durmus H, Yetimler B, Tasli H,
Parman Y and Tuzun E: Elevated IL-4 and IFN-ү levels in muscle
tissue of patients with dermatomyositis. In Vivo. 31:657–660.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shinjo SK, de Souza FH and de Moraes JC:
Dermatomyositis and polymyositis: From immunopathology to
immunotherapy (immunobiologics). Rev Bras Reumatol. 53:101–110.
2013.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
24
|
Sasaki H and Kohsaka H: Current diagnosis
and treatment of polymyositis and dermatomyositis. Mod Rheumatol.
28:913–921. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang SH, Chang C and Lian ZX: Polymyositis
and dermatomyositis-challenges in diagnosis and management. J
Transl Autoimmun. 2(100018)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Dalakas MC and Hohlfeld R: Polymyositis
and dermatomyositis. Lancet. 362:971–982. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miller FW, Rider LG, Plotz PH, Isenberg DA
and Oddis CV: Diagnostic criteria for polymyositis and
dermatomyositis. Lancet. 362:1762–1763. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Goebels N, Michaelis D, Engelhardt M,
Huber S, Bender A, Pongratz D, Johnson MA, Wekerle H, Tschopp J,
Jenne D and Hohlfeld R: Differential expression of perforin in
muscle-infiltrating T cells in polymyositis and dermatomyositis. J
Clin Invest. 97:2905–2910. 1996.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nigwekar SU, Kroshinsky D, Nazarian RM,
Goverman J, Malhotra R, Jackson VA, Kamdar MM, Steele DJ and
Thadhani RI: Calciphylaxis: Risk factors, diagnosis, and treatment.
Am J Kidney Dis. 66:133–146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhou Q, Neubauer J, Kern JS, Grotz W, Walz
G and Huber TB: Calciphylaxis. Lancet. 383(1067)2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Aghagolzadeh P, Bachtler M, Bijarnia R,
Jackson C, Smith ER, Odermatt A, Radpour R and Pasch A:
Calcification of vascular smooth muscle cells is induced by
secondary calciprotein particles and enhanced by tumor necrosis
factor-α. Atherosclerosis. 251:404–414. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mathur RV, Shortland JR and el-Nahas AM:
Calciphylaxis. Postgrad Med J. 77:557–561. 2001.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Romero-Díaz J, Vargas-Vóracková F,
Kimura-Hayama E, Cortázar-Benítez LF, Gijón-Mitre R, Criales S,
Cabiedes-Contreras J, Iñiguez-Rodríguez Mdel R, Lara-García EA,
Núñez-Alvarez C, et al: Systemic lupus erythematosus risk factors
for coronary artery calcifications. Rheumatology (Oxford).
51:110–119. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Iammatteo M, Keskin T and Jerschow E:
Evaluation of periprocedural hypersensitivity reactions. Ann
Allergy Asthma Immunol. 119:349–355.e2. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Castells M: Diagnosis and management of
anaphylaxis in precision medicine. J Allergy Clin Immunol.
140:321–333. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Javed B, Padfield P, Sperrin M, Simpson A
and Mills ENC: A protocol for a systematic review to identify
allergenic tree nuts and the molecules responsible for their
allergenic properties. Food Chem Toxicol. 106:411–416.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dourmishev LA, Dourmishev AL and Schwartz
RA: Dermatomyositis: An association of gingival telangiectases and
anti Jo-1 antibody in the adult. Acta Dermatovenerol Alp Pannonica
Adriat. 16:67–72. 2007.PubMed/NCBI
|
|
38
|
Sontheimer RD: MDA5 autoantibody-another
indicator of clinical diversity in dermatomyositis. Ann Transl Med.
5(160)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hoshino K, Muro Y, Sugiura K, Tomita Y,
Nakashima R and Mimori T: Anti-MDA5 and anti-TIF1-gamma antibodies
have clinical significance for patients with dermatomyositis.
Rheumatology (Oxford). 49:1726–1733. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Arshanapalli A, Shah M, Veerula V and
Somani AK: The role of type I interferons and other cytokines in
dermatomyositis. Cytokine. 73:319–325. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Brunasso AM, Aberer W and Massone C: New
onset of dermatomyositis/polymyositis during anti-TNF-α therapies:
A systematic literature review. ScientificWorldJournal.
2014(179180)2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mamyrova G, O'Hanlon TP, Sillers L, Malley
K, James-Newton L, Parks CG, Cooper GS, Pandey JP, Miller FW and
Rider LG: Childhood Myositis Heterogeneity Collaborative Study
Group. Cytokine gene polymorphisms as risk and severity factors for
juvenile dermatomyositis. Arthritis Rheum. 58:3941–3950.
2008.PubMed/NCBI View Article : Google Scholar
|