1. Introduction
Sodium bicarbonate and potassium bicarbonate exert
microbicidal effects against plant bacteria and fungi under
alkaline conditions. In nature, these carbonate solutions dissolve
quickly, making them effective against specific pathogens and small
insects. In contrast, calcium bicarbonate solutions at pH 7.0 do
not exhibit microbicidal activity against bacteria or fungi.
However, when high-voltage electricity is applied to a calcium
bicarbonate solution, mesoscopic crystals (50-500 nm in size) are
formed (1). At pH 12.4, these
crystals, named CAC-717, exert multiple microbicidal effects
against animal and plant pathogens, allowing them to be
characterized as ‘soft’ agricultural chemicals. In another recent
study, the authors reported that CAC-717 efficiently inactivated a
variety of infectious agents, including prions (2).
In the context of the ongoing coronavirus disease 19
(COVID-19) pandemic, the World Health Organization recommends ‘to
ensure that environmental cleaning and disinfection procedures are
followed consistently and correctly. Thoroughly cleaning
environmental surfaces with water and detergent and applying
commonly used hospital-level disinfectants, such as sodium
hypochlorite, are effective and sufficient procedures’ (3). Although some
antiseptics/disinfectants, including ethanol and sodium
hypochlorite, exhibit significant activity against the recently
emerged severe acute respiratory syndrome coronavirus type 2
(SARS-CoV-2), they are harmful to human cells and must be used at
elevated concentrations (4-7).
Furthermore, the presence of organic material significantly reduces
the activity of chlorine-derived compounds (6). In the present review, the effect of
CAC-717 as an environmentally friendly disinfectant against
emerging and recurrent infectious pathogens was described.
2. Calcium bicarbonates
Effects of calcium bicarbonates
Calcium is a major macronutrient for trees and is
used in agricultural fertilizers. It is important for preserving
membrane and cell wall integrity, as well as intracellular
transport (8). At pH 7, calcium
bicarbonate lacks a mesoscopic structure and does not exert a
cytotoxic effect against microorganisms.
Calcium bicarbonate, whose chemical formula is
Ca(HCO3)2, does not refer to an actual solid
compound, as it exists only in dilute aqueous solutions containing
calcium (Ca2+), bicarbonate
(HCO3-), and carbonate
(CO3)2- ions together with dissolved carbon
dioxide (CO2). The relative concentration of these
carbon-containing species depends on the pH, with bicarbonate being
predominant at pH 6.36-10.25 in water. These materials are often
used in chewing gums with protective action on the oral cavity
(9). In clinical practice, calcium
bicarbonate water is used to treat patients with gastroesophageal
reflux disease (10).
Compared to untreated specimens, CAC-717 treatment
has been shown to reduce human norovirus genomic RNA by 3.25
log10 (11). The
virucidal effect of CAC-717 was compared to that of a phosphate
buffer control at pH 12.4 and calcium bicarbonate without the
mesoscopic structure (pH 7.0) (11). A human norovirus (GII.4 Sydney 2012)
(12) was used as a target and was
purified prior to disinfectant treatment. Phosphate buffer at pH
12.4 and calcium bicarbonate at pH 7.0 failed to inactivate human
norovirus (11). Although CAC-717
is an alkaline solution, its pH decreases rapidly (within 1 min) to
8.84 upon contact with human skin (1). Indeed, CAC-717 is classified as
non-irritant (Class 0) and skin sensitization tests conducted in
rabbits according to the Ministry of Health, Labour and Welfare of
Japan Guidelines, Biological Evaluation of Medical Devices-Part 10:
Test for irritation and skin sensitization (ISO 10993-10, July 2,
2006) have not revealed any harmful properties (1). Furthermore, rabbit eye toxicity tests
using the OECD Guidelines for Testing Chemicals no. 405: Acute Eye
Irritation/Corrosion, performed under the same animal welfare
requirements (ISO 10993-2, July 2, 2006) confirmed CAC-717
biosafety (1). Concentrated CAC-717
exhibited cytotoxicity in cultivated cell lines (11); but even this effect was lost after a
1:2 dilution in phosphate buffer.
Preparation of calcium bicarbonate
with a mesoscopic structure
To obtain CAC-717, an electric field is applied to
mineral water containing calcium bicarbonate (1,2,13,14).
According to the Japanese patent No. 5778328, CAC-717 (Food and
Drug Administration/USA Regulation no. 880.6890 Class 1
disinfectant) is produced by applying a voltage of 2x104
V for 48 h using Teflon-coated electrostatic-field electrodes
(2). The resulting material has a
pH of approximately 12.4 and contains 6.9 mM calcium bicarbonate
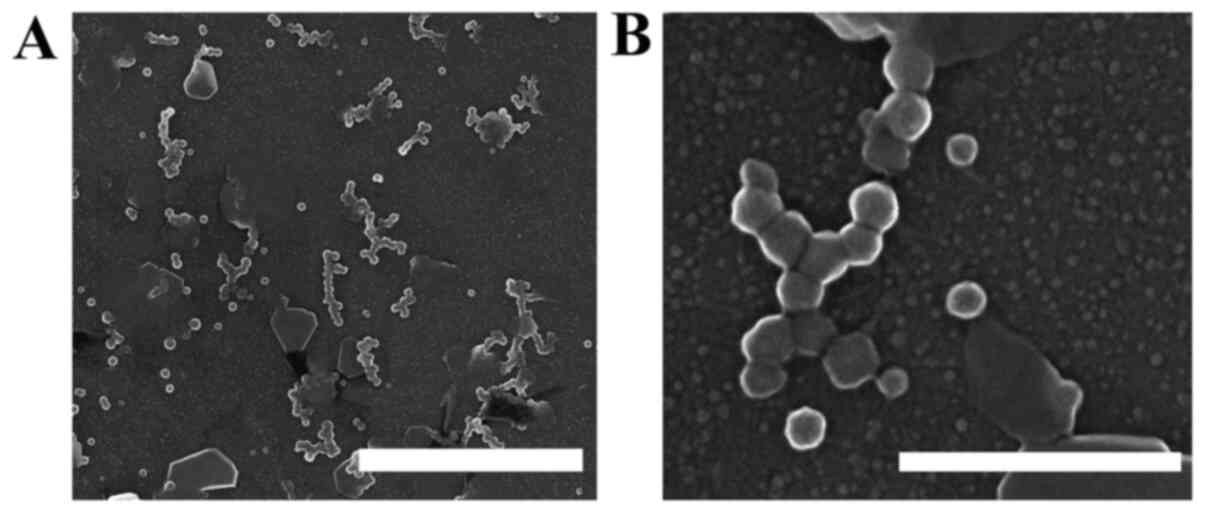
particles (81,120 mg/l) with a mesoscopic structure (50-500 nm)
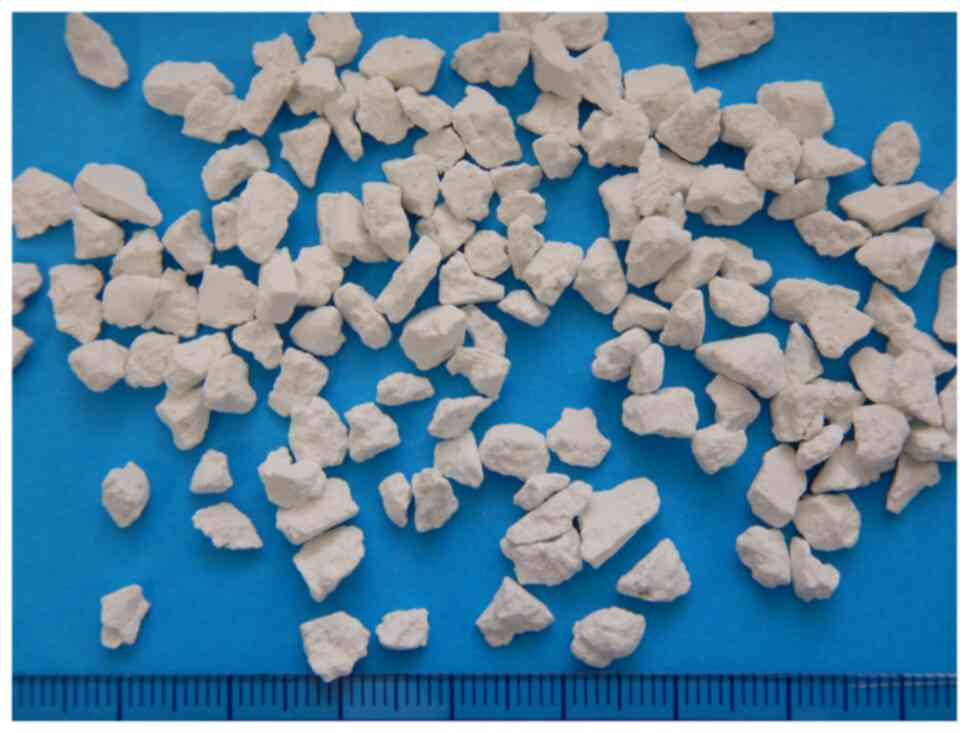
that can be observed under an electron microscope (Fig. 1). CAC-717 is adsorbed onto a ceramic
surface and air-dried for storage in the form of CAC-717 stones
(Fig. 2). Prior to use, the CAC-717
stones are placed in fresh distilled water, calcium bicarbonate is
dissolved, and CAC-717 is reconstituted. Reconstituted CAC-717 has
the same microbicidal properties as original CAC-717.
The CAC-717 suspension, which is a colorless
disinfectant, can be sprayed and dried on metal or plastic
surfaces, resulting in a white powder coating (1). Scanning electron microscopy samples
were obtained from a CAC-717 suspension after drying on a glass
slide and used for quality control assessment, together with pH and
calcium bicarbonate content measurements (1).
3. Effect of mesoscopic calcium bicarbonate
particles on animal and plant pathogens
Bacteria
CAC-717 has been shown to be effective in
inactivating plant pathogens (14).
Black rot is a disease affecting cabbage and other cruciferous
plant leaves, and is caused by Xanthomonas campestris pv.
campestris (Xcc), a gram-negative seed-borne
bacterium (15). The number of
viable Xcc cells after treatment with CAC-717 was
determined. An Xcc suspension (8.22 log10
colony-forming units/ml) was incubated with an equal volume of
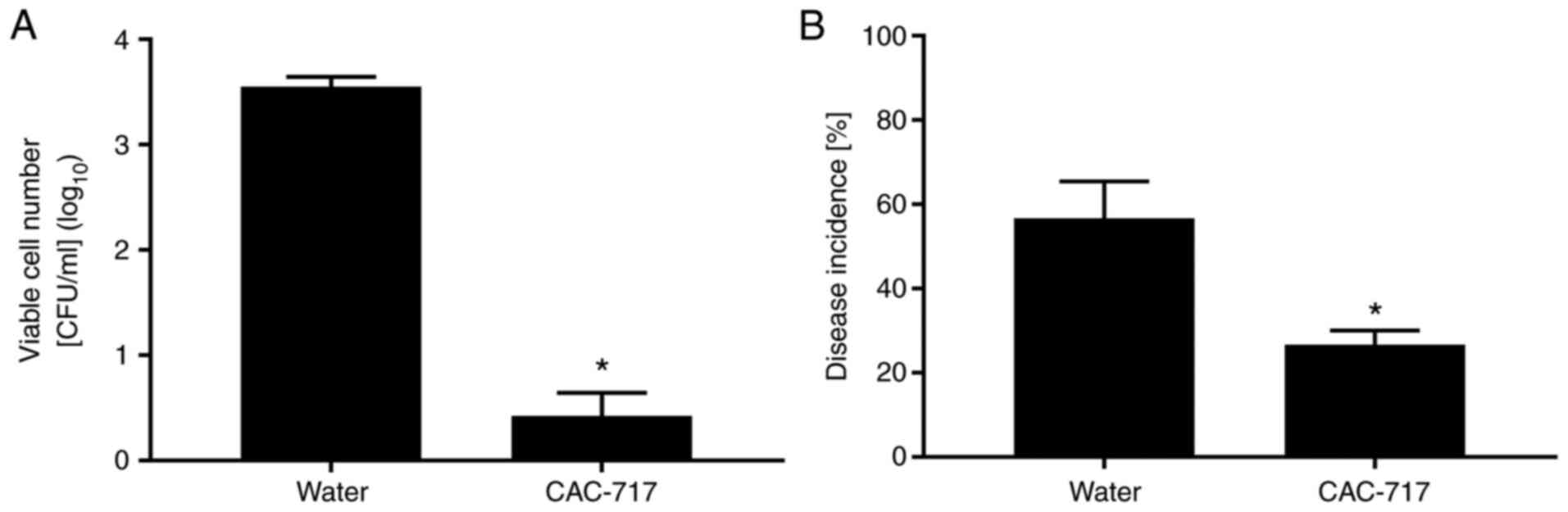
CAC-717 at 25˚C for different periods of time (Table I). CAC-717 treatment caused a
decrease in the number of Xcc cells after 0.5 min (Fig. 3A). In control experiments, no
significant reduction in the number of Xcc cells was
observed after treatment with distilled water at 25˚C; whereas hot
water (50˚C) treatment significantly decreased the number of
Xcc cells (14). In test
samples, CAC-717 caused a low incidence of black rot, with only
26.77±3.33% of seeds exhibiting signs of disease after incubation
at 25˚C for 5 days, compared with 56.67±8.82% in the distilled
water group (Fig. 3B). No
significant difference in the germination rate and plant stem
length was detected between distilled water (25˚C) and CAC-717
treatment after cultivation for 5 days.
 | Table IAntimicrobial, antiviral, and
prion-inhibiting effects of CAC-717. |
Table I
Antimicrobial, antiviral, and
prion-inhibiting effects of CAC-717.
| | Pathogen titer | |
|---|
| Pathogen | Duration of
treatment (min) | Untreated | CAC-717 | (Refs.) |
|---|
| Xanthomonas
campestris pv. campestris | 0.5 | 8.22
log10 CFU/ml | 5.63
log10 CFU/ml | (15) |
| Escherichia
coli | 2 |
1.52x109±0.35x109
CFU/ml |
7.50x106±2.50x106
CFU/ml | (14) |
| Salmonella
enterica | 2 |
2.14x107±0.12x107
CFU/ml | Undetectable | (14) |
| Murine
norovirus | 1 | 5.98
log10 TCID50/ml | Undetectable | (12) |
| Feline
calicivirus | 2 |
7.26x105±2.70x105
TCID50/ml | <10
TCID50/ml | (14) |
| Influenza
virus | 15 | 6.58
log10 TCID50/ml | <1.50
log10 TCID50/ml | (1) |
| Scrapie
prion | 60 | 9.95
log10 PMCA50/ml | 5.20
log10 PMCA50/ml | (2) |
CAC-717 displays a bactericidal effect against
Escherichia coli (E. coli) and Salmonella
enterica (S. enterica) (13) (Table
I). Following treatment with CAC-717 for 2 min, viable E.
coli cells decreased by approximately 3.00-2.00
log10 and S. enterica by more than 7.00
log10, indicating that some bacteria are more sensitive
than others to CAC-717. When using sodium hypochlorite (4 ppm) as a
fungicide for chicken eggs mixed with S. enterica, the
average multiple endpoint D value was 0.195 min (16). In contrast, CAC-717 displayed an
average multiple-endpoint D value of 0.080 min, indicating a
more powerful bactericidal effect than sodium hypochlorite.
Prions
Extreme autoclaving conditions (134˚C, 18 min) are
required to inactivate prions, although some prions retain
transmissibility even after dry-heating at 400˚C (16). Alternatively, prions are inactivated
upon treatment with sodium dodecyl sulfate, sodium hydroxide, and
sodium hypochlorite (17), but
these chemicals are generally impractical due to their corrosive
properties.
In another recent study, the authors reported that
CAC-717 inactivated prions (2)
(Table I). Western blotting was
used for scrapie prion (PrPSc) analysis (18). To examine the seeding activity of
PrPSc, protein misfolding cyclic amplification (PMCA)
was used, which mimics the in vivo reaction and amplifies
PrPSc in vitro (19). Western blotting and PMCA were
applied to follow proteinase K-resistant PrPSc, while a
transgenic mouse bioassay was used to test the transmissibility of
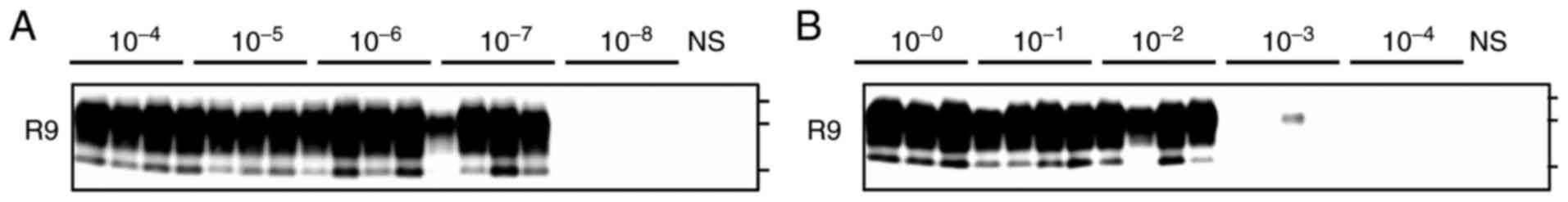
prions. Western blot analysis showed that the levels of proteinase
K-resistant PrPSc in lysates obtained from
prion-infected N2a cells were markedly reduced after CAC-717
treatment. Next, PMCA was used to assess the conversion activity of
any remaining PrPSc after CAC-717 treatment. The seeding
activity of PrPSc was also clearly reduced in
CAC-717-treated samples (2)
(Table I). In addition, mice
injected with CAC-717-treated samples survived longer than those
injected with PBS-treated controls. These findings suggest that
CAC-717 reduced both PrPSc conversion activity and prion
transmissibility (Fig. 4).
Further studies are required to determine the
mechanism by which CAC-717 inactivates prions. Given that CAC-717
does not cause irritation and is not corrosive, it offers an
alternative to other prion disinfectants such as hypochlorous acid
(20); however, detailed comparison
with the latter is still needed.
Animal viruses
Recently, CAC-717 was tested against
SARS-CoV-2(21). CAC-717 exhibited
a strong virucidal effect against all the examined mutant forms of
SARS-CoV-2 isolated in Japan. Viral infectivity decreased by 3.8 to
4.2 log10 within 15 sec (Table II), implying a virucidal activity
similar to that of 80% ethanol. This strong microbicidal effect of
CAC-717 has been linked to its elevated alkalinity, which may be
due to the relative decrease in H+ and concomitant
increase in OH-. However, given that SARS-CoV-2 retains
its infectivity for up to 30 sec at pH 12.4 (pH-adjusted Dulbecco's
modified Eagle's medium) (21), it
is more likely that an unidentified mechanism controls the
virucidal activity of CAC-717. SARS-CoV-2 is known for the
emergence of numerous strains. In addition to the conventional
original virus (WK-521), the virucidal activity of CAC-717 was
confirmed against α, β, γ, and δ variants, with the
log10 reduction in infectivity ranging from 3.6 to 4.4
(Table II). The effectiveness of
CAC-717 in the presence of organic matter was analyzed to confirm
its beneficial properties in the general environment (21). The tissue culture infectious dose of
the virus mixed with 5% bovine serum albumin was 104.8
and the virucidal effect of CAC-717 corresponded to >4.3
log10 (21). A lower
virucidal effect of sodium hypochlorite has been observed in the
presence of organic material; whereas the value obtained for
CAC-717 indicates only a minor reduction. Numerous disinfectants
have been used for COVID-19 control; hence, their impact on human
health and the environment should be taken into account. As CAC-717
is harmless and does not cause skin or eye toxicity in rabbits
(1), its future use as an
anti-COVID-19 disinfectant is less problematic. Moreover, because
the calcium bicarbonate component of CAC-717 is derived from plant
material, it is non-flammable and can be used in a variety of
applications for which ethanol is not suitable (21).
 | Table IIVirucidal efficacy of CAC-717 against
SARS-CoV-2. |
Table II
Virucidal efficacy of CAC-717 against
SARS-CoV-2.
| | Viral titer
(TCID50/ml) |
|---|
| Strain | Variant type | Distilled
water | CAC-717 |
|---|
|
SARS-CoV-2/WK-521 | Original | 4.9 | ≤0.6 |
|
SARS-CoV-2/KH-1/2021 | Original | 5.0 | ≤0.6 |
|
hCoV-19/Japan/QK002/2020 | α | 4.2 | ≤0.6 |
|
hCoV-19/Japan/TY8-612/2021 | β | 4.8 | ≤0.6 |
|
hCoV-19/Japan/TY7-501/2021 | γ | 4.4 | ≤0.7 |
|
SARS-CoV-2/KH-25/2021 | δ | 4.9 | ≤0.6 |
In addition to SARS-CoV-2, a recent study showed
that CAC-717 possessed virucidal activity against 22 different
types of DNA or RNA viruses. Destruction of viral nucleic acids in
RNA viruses has been observed (22), but the exact effect of CAC-717
against viral DNA remains to be determined (22). In nature, human norovirus is
typically associated with the fecal matrix. To investigate the
microbiological properties of CAC-717, four fecal specimens were
evaluated. Compared to the untreated specimen, CAC-717 resulted in
log10 reductions of 1.36, 2.78, 1.64, and 3.52 in the
GI.2, GII.4 Sydney 2012, GII.5, and GII.7 specimens, respectively
(Table I). Thus, CAC-717 could
successfully inactivate human norovirus under clinical conditions.
A similar effect was reported with heat treatment, but not with
1,000-ppm sodium hypochlorite in a stool suspension (except for
human norovirus GII.17) (11) or
70% (v/v) ethanol.
CAC-717 has been proven effective against influenza
virus (1) and feline calicivirus
(FCV) (19) (Table I). To investigate the mechanistic
effect of CAC-717 against viruses, real-time PCR revealed a
progressive decline in the integrity of the FCV genome with
increasing CAC-717 treatment time, as opposed to only intact RNA
from untreated FCV samples (13).
Hypochlorous acid solution is also effective against
several viruses (23,24); however, it is less stable in the
presence of ultraviolet irradiation or in contact with air or
organic compounds. Consequently, hypochlorous acid must be stored
under cool and dark conditions to maintain its microbicidal
activity (23).
Recently, concentrated bioshell calcium oxide
(BiSCaO) water has been reported as an effective skin disinfectant
(7,25). Although BiSCaO is strongly alkaline
(pH 12.8), the pH of BiSCaO water decreased to 8.5 within 5 min
after spraying on the back skin of hairless rats. The generation of
CaCO3 following the interaction between Ca2+
ions in BiSCaO water and CO2 in air was suspected to be
the cause of this rapid drop in pH (25). This finding echoes the rapid
decrease in pH of CAC-717 when in contact with rabbit skin
(1).
4. Conclusion
The SARS-CoV-2 pandemic has caused both a massive
healthcare crisis and enormous economic damage worldwide. With
increasing hygiene and safety challenges, disinfection with
electrolyzed water is of potential significance in the clinical
field as a means to cut off the route of viral transmission
(21,26). CAC-717 has strong antimicrobial
efficacy without associated irritation (21), highlighting potential applications
in the food and medical sectors for disinfecting hard surfaces, in
addition to its established anti-parasitic role in agriculture.
After the discovery that pangolins could harbor
SARS-CoV-2-related coronaviruses, Chinese authorities banned the
trade and sale of these animals. Currently, it is too early to
evaluate the overall impact of SARS-CoV-2 on wild animal management
and conservation. A recent study by a Japanese group identified a
bat sarbecovirus loosely related to SARS-CoV-2(27). Future research efforts should focus
on the likely origins of novel pathogens, along with the
development of vaccines and drugs (28,29).
SARS-CoV-2 RNA was detected in secondary-treated wastewater samples
when COVID-19 cases peaked in the community (30). The present study suggests that, when
dried CAC-717 stones are placed in fresh water, calcium bicarbonate
is dissolved and CAC-717 is reconstituted. Existing evidence
suggests that reconstituted CAC-717 retains the same microbicidal
activity as original CAC-717. These CAC-717 stones may be useful
for eliminating SARS-CoV-2 from the environment, including sewage
and running water. Daily cleaning and disinfection of surfaces in
hospitals are essential to limit the spread of infection (31). The US Environmental Protection
Agency recommends the use of disinfectants with hypochlorite acid
as the active ingredient for disinfection of surfaces to combat
SARS-CoV-2(31); however the mild
toxicity of this agent calls for the use of alternatives such as
CAC-717.
This review highlights the latest developments and
new perspectives related to CAC-717, especially its application in
clinical fields. In the future, CAC-717 may be applied on the
surface of numerous materials as a safe and environmentally
friendly antiseptic/disinfectant. Further studies and validation in
other model systems are required to better understand its mechanism
of action and untapped potential.
Acknowledgements
Not applicable.
Funding
Funding: ORIX Corp. and Santa Mineral Co., Ltd. provided
financial support for the study and the preparation of this
article.
Availability of data and materials
Not applicable.
Authors' contributions
TO, AS, MH, KF, and RO conceptualized the present
study. TO wrote the original draft. TO, AS, YI, TY, MH, KS, TH, HS,
and KF wrote, reviewed and edited the final manuscript. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
KF and RO are employed by the Mineral Activation
Technical Research Center and Santa Mineral Co., Ltd.,
respectively. The remaining coauthors have no competing interests
related to the contents of this article.
References
|
1
|
Nakashima R, Kawamoto M, Miyazaki S,
Onishi R, Furusaki K, Osaki M, Kirisawa R, Sakudo A and Onodera T:
Evaluation of calcium hydrogen carbonate mesoscopic crystals as a
disinfectant for influenza A viruses. J Vet Med Sci. 79:939–942.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sakudo A, Iwamaru Y, Furusaki K, Haritani
M, Onishi R, Imamura M, Yokoyama T, Yoshikawa Y and Onodera T:
Inactivation of scrapie prions by the electrically charged
disinfectant CAC-717. Pathogens. 9(536)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kampf G, Todt D, Pfaender S and Steinmann
E: Persistence of coronaviruses on inanimate surfaces and their
inactivation with biocidal agents. J Hosp Infect. 104:246–251.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wilson JR, Mills JG, Prather ID and
Dimitrijevich SD: A toxicity index of skin and wound cleansers used
on in vitro fibroblasts and keratinocytes. Adv Skin Wound Care.
18:373–378. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
McCauley RL, Linares HA, Pelligrini V,
Herndon DN, Robson MC and Heggers JP: In vitro toxicity of topical
antimicrobial agents to human fibroblasts. J Surg Res. 46:267–274.
1989.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kinoda J, Ishihara M, Hattori H, Nakamura
S, Fukuda K and Yokoe H: Cytotoxicity of silver nanoparticle and
chitin-nanofiber sheet composites caused by oxidative stress.
Naonmaterials (Basel). 6(189)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ishihara M, Hata Y, Hiruma S, Takayama T,
Nakamura S, Sato Y, Ando N, Fukuda K, Murakami K and Yokoe H:
Safety of concentrated bioshell calcium oxide water application for
surface and skin disinfections against pathogenic microbes.
Molecules. 25(4502)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Percival GC and Haynes I: The influence of
calcium sprays to reduce fungicide inputs against apple scab
[Venturia inaequalis (Cook) G. Wint.]. Arboric Urban For.
35:263–270. 2009.
|
|
9
|
Ly KA, Milgrom P and Rothen M: The
potential of dental-protective chewing gum in oral health
interventions. J Am Dent Assoc. 139:553–563. 2008.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Grassi M, Fraioli A, Pappalardo G, Messina
B, Belardinelli L and Guadalaxara A: Alkalizing activity of a
calcium-bicarbonate-containing water, evaluated for pH, in patients
with gastroesophageal reflux. Clin Ter. 143:131–136.
1993.PubMed/NCBI(In Italian).
|
|
11
|
Shimakura H, Gen-Nagata F, Haritani M,
Furusaki K, Kato Y, Yamashita-Kawanishi N, Le DT, Tsuzuki M, Tohya
Y, Kyuwa S, et al: Inactivation of human norovirus and its
surrogate by the disinfectant consisting of calcium hydrogen
carbonate mesoscopic crystals. FEMS Microbiol Lett.
366(fnz235)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
van Beek J, de Graaf M, Al-Hello H, Allen
DJ, Ambert-Balay K, Botteldoorn N, Brytting M, Buesa J, Cabrerizo
M, Chan M, et al: Molecular surveillance of norovirus, 2005-16: An
epidemiological analysis of data collected from the NoroNet
network. Lancet Infect Dis. 18:545–553. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sakudo A, Yamashiro R, Haritani M,
Furusaki K, Onishi R and Onodera T: Inactivation of non-enveloped
viruses and bacteria by an electrically charged disinfectant
containing meso-structure nanoparticles via modification of the
genome. Int J Nanomedicine. 15:1387–1395. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sakudo A, Haritani M, Furusaki K, Onishi R
and Onodera T: Electrically charged disinfectant containing calcium
hydrogen carbonate mesoscopic crystals as a potential measure to
control Xanthomonas campestris pv. campestris on
cabbage seeds. Microorganisms. 8(1606)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Williams PH: Black rot: A continuing
threat to world crucifiers. Plant Dis. 64:736–742. 1980.
|
|
16
|
Hines JD, McKelvey PJ and Bodnaruk PW:
Inappropriate use of D-values for determining biocidal activity of
various antimicrobials. J Food Sci. 76:M8–M11. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsuura Y, Ishikawa Y, Murayama Y,
Yokoyama T, Somerville RA, Kitamoto T and Mohri S: Eliminating
transmissibility of bovine spongiform encephalopathy by dry-heat
treatment. J Gen Virol. 101:136–142. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Onodera T, Ikeda T, Muramatsu Y and
Shinagawa M: Isolation of scrapie agent from the placenta of sheep
with natural scrapie in Japan. Microbiol Immunol. 37:311–316.
1993.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Giaccone G and Moda F: PMCA applications
for prion detection in peripheral tissues of patients with variant
Creutzfeldt-Jakob disease. Biomolecules. 10(405)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hughson AG, Race B, Kraus A, Sangare LR,
Robins L, Groveman BR, Saijo E, Phillips K, Contreras L, Dhaliwal
V, et al: Inactivation of prions and amyloid seeds with
hypochlorous acid. PLoS Pathog. 12(e1005914)2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yokoyama T, Nishimura T, Uwamono Y, Kosaki
K, Frusaki K, Onishi R, Onodera T, Haritani M, Sugiura K, Kirisawa
R and Hasegawa A: Virucidal effect of the mesoscopic structure of
CAC-717 against severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2). Microorganisms. 9(2096)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kirisawa R, Kato R, Furusaki K and Onodera
T: Universal activity of calcium biocarbonate mesoscopic crystals
that provides an effective and biosafe disinfectant.
Microorganisms. 10(262)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ishihara M, Murakami K, Fukuda K, Nakamura
S, Kuwabara M, Hattori H, Fujita M, Kiyosawa T and Yokoe H:
Stability of weakly acidic hypochlorous acid solution with
microbicidal activity. Biocontrol Sci. 22:223–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hatanaka N, Yasugi M, Sato T, Mukamoto M
and Yamazaki S: Hypoclorous acid solution is a potent antiviral
agent against SARS-CoV-2. J Appl Microbiol. 132:1496–1502.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nakamura S, Ishihara M, Sato Y, Takayama
T, Hiruma S, Ando N, Fukuda K, Murakami K and Yokoe H: Concentrated
bioshell calcium oxide (BiSCaO) water kills pathogenic microbes:
Characterization and activity. Molecules. 25(3001)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yan P, Daliri EB and Oh DH: New clinical
applications of electrolyzed water: A review. Microorganisms.
9(136)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Murakami S, Kitamura T, Suzuki J, Sato R,
Aoi T, Fujii M, Matsugo H, Kamiki H, Ishida H, Takenaka-Uema A, et
al: Detection and characterization of bat sabecovirus
phylogenetically related to SARS-CoV-2, Japan. Emerg Infect Dis.
26:3025–3029. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Haramoto E, Malla B, Thakali O and
Kitajima M: First environmental surveillance for the presence of
SARS-CoV-2 RNA in wastewater and river water in Japan. Sci Total
Environ. 737(140405)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Boyce JM: Modern technologies for
improving cleaning and disinfection of environmental surfaces in
hospitals. Antimicrob Resist Infect Control. 5(10)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Samara F, Badran R and Dalibalta S: Are
disinfectants for the prevention and control of COVID-19 safe?
Health Secur. 18:496–498. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Petrillo F, Chianese A, De Bernado M,
Zannella C, Galdiero M, Reibaldi M, Avitabile T, Boccia G, Galdiero
M, Rosa N and Franci G: Inhibitory effect of ophthalmic solutions
against SARS-CoV-2: A preventive action to block the viral
transmission? Microorganisms. 9(1550)2021.PubMed/NCBI View Article : Google Scholar
|