Craniocerebral injury (CBI) is a heterogeneous group
of non-congenital tissue damages caused by a sudden mechanical
force, which results in neurological, neuropsychological and
psychiatric dysfunctions (1,2).
Currently, CBI is one of the leading causes of death in addition to
cardiovascular disease and cancer (3). A histogram of age-related CBI shows a
bimodal distribution with the first and second maximums for
subjects aged 15 and >75 years old, respectively (4). CBI is more common amongst men than
among women, showing a ratio between 1.9:1-2.8:1 (4-8).
Current clinical diagnosis of CBI is based on a
number of methods, including the Glasgow Coma Scale (GCS) (9), the measurement of cerebral perfusion
pressure, the Transcranial Doppler Test (TCD) and analysis of the
levels of biomarkers, allowing quantitative evaluation and
treatment monitoring of brain tissue damage (10,11).
Amongst a variety of biomarkers of CBI, the serum
levels of S-100B reflects the degree of posttraumatic brain damage
(10,12-30).
S-100B is secreted primarily by astrocytes in the cerebral cortex
(31,32) and is present in large quantities in
astroglial cells (33). It also
plays an essential role in cell growth metabolism (34). S-100B secretion triggers autocrine
and paracrine effects in glial cells, microgel cells and neurons
(35). Furthermore, S-100B
stimulates neuronal growth in nanomolar and picomolar
concentrations (36), and induces
apoptosis in micromolar concentrations (37).
TCD spectral analysis is used to determine the
velocity of blood flow through the maximum systolic velocity, the
end-diastolic velocity and the time-averaged mean maximum velocity
(Vmean) in insolated blood vessels (38). The clinical practicality of
Vmean values has been confirmed in the treatment of
severe cases of traumatic brain injury (TBI) (39).
Given the importance of precise prognostic methods
for the diagnosis and effectiveness of TBI treatment, advances in
the quality of the early diagnosis of CBI are essential. Therefore,
the time-related changes in S-100B protein levels and the
Vmean, as well as the associations between these
parameters in patients diagnosed with severe CBI as defined by a
GCS score ≤8 were investigated in the present study.
All experiments and methods were performed following
relevant guidelines and regulations (40). The Bioethics Committee of the
Postgraduate Education Medical Center (Warsaw, Poland) approved the
experimental protocols (approval no. 501-2-1-20-49/04). Informed
consent was obtained from all subjects or their guardians, and a
parent/legal guardian of subjects under 18 years of age.
The present study included patients with severe CBI
(GCS score ≤8) admitted to the Department of Neurosurgery and
Trauma of the Nervous System at the Medical Center of Postgraduate
Education (Warsaw, Poland).
The total number of patients included in the study
was 60, consisting of 51 patients in the unfavorable group and 9
patients in the favorable group. The mean age ± standard deviation
were 48.41±15.24 years (age range, 19-73 years) and 47.20±16.64
years (age range, 14-75 years) in the unfavorable and favorable
groups, respectively.
The GCS score was calculated at admission using an
internal encoded GCS calculator (41,42)
written in Python (Python Software Foundation; python.org/).
According to the European Consortium of Brain Injury
Guidelines, all patients were subjected to the standard diagnostic
and therapeutic protocols (43). In
patients for whom poor ventilation was suspected, a gasometric
examination, using a CDI™ blood parameter monitoring system 500,
was performed to optimize pCO2 (range, 30-40 mmHg).
Furthermore, hematocrit and hemoglobin levels were maintained at
30-40% and 12-14 g/dl, respectively.
On discharge from the Department of Neurosurgery,
the patient's health was evaluated using the traditional Glasgow
outcome scale (GOS) (44), which is
comprised of the following five categories: 1, death; 2, persistent
vegetative state; 3, severe disability; 4, moderate disability; and
5, low disability. For this study, patients with a GOS ≥4 and GOS
<4 were classified as ‘favorable’ and ‘unfavorable’,
respectively.
The inclusion criteria, based on an analysis of GOS
as described above, resulted in a study group of 60 patients (48
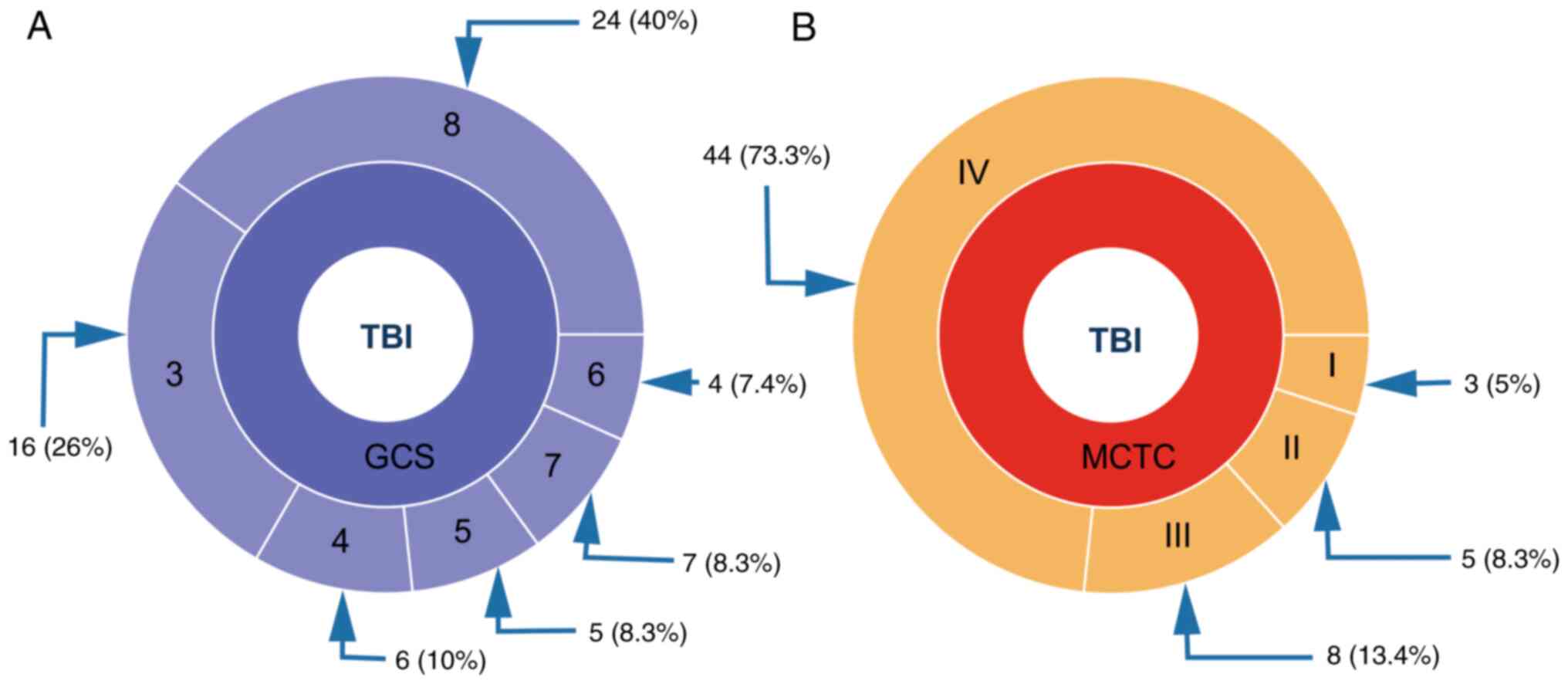
men and 12 women). The clinical description of the study group
after admission to the hospital according to the GCS and Marshall
(MCTC) classification (45) is
presented in Fig. 1.
The S-100B protein levels were measured in 5 ml
venous blood samples collected from patients upon admission to the
hospital. Subsequent blood samples were collected at 24-h intervals
for 96 h. After clotting and centrifugation for 10 min at 2,000 x g
at 4˚C, blood samples were stored for further use at -22˚C. The
S-100B protein concentration was measured using a Anti-S-100
antibody kit (S1-61; cat. no. sc-53438; Santa Cruz Biotechnology,
Inc.) according to the manufacturer's protocol (Liaison Sangtec
100; Sangtec Ltd.). The Sangtec 100 kit uses three different
monoclonal antibodies (SMST12, SMSK 25 and SMSK 28) directed
against the β-chains of the S-100B homodimer, and has a wide
detection range (0.02-30 µg/l). Protein concentration was measured
using a LIAISON analyzer (DiaSorin) calibrated with a freeze-dried
Sangtec 100 Cal (Low/High) calibrator. The sensitivity threshold
for this test was 0.02 µg/l.
Given the repeated nature of the data and the
mortality of the patients, the data was censored to balance the
factorial ANOVA model. Post hoc analysis was performed using the
estimated marginal means (emmeans) procedure. The velocity of
time-dependent changes in a specific parameter is defined by the
slope (tangent) of a line obtained from connected means at
consecutive measurement times. P<0.01 was considered to indicate
a statistically significant difference. All analyses were performed
in R (51).
This study was carried out using two groups of
patients stratified by the GOS score (52) at discharge; patients were classified
into either an unfavorable (GOS score <4) and favorable (GOS
score ≥4) group. No significant differences in age were found
between the groups. Fig. 1 shows a
general description of the severity of craniotrauma in patients
assessed using the GOS and MCTC scores (53).
The reference range obtained from a healthy patients
reference group consisting of 40 healthy volunteers [22 men and 18
women; 47.0±14.77 (age range, 21-80)] for the S-100B levels used in
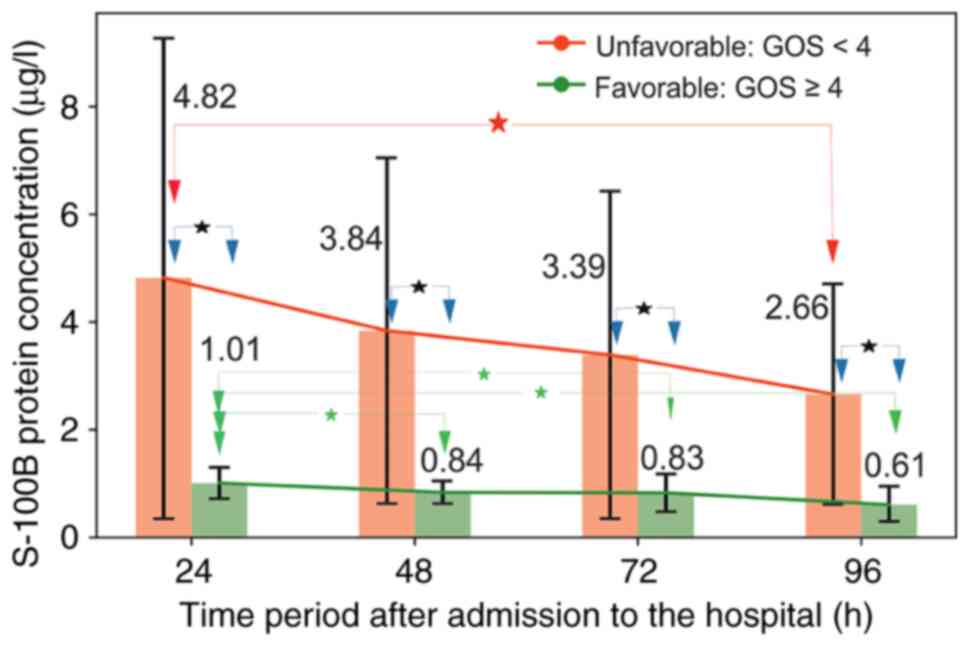
this study was 0.05-0.23 µg/l. A graphical and numerical
representation of the changes in S-100B levels is shown in Fig. 2 and Table I. The results showed that the
patients in the unfavorable group had higher levels of S-100B than
those in the favorable group at all measured time points. No
statistically significant time-dependent differences, defined by
the lack of an overlay between specific confidence intervals, in
S-100B concentrations were found within the unfavorable group.
However, a significant decrease in serum S-100B protein levels was
found between measurements at 24 vs. 48 h, 24 vs. 72 h and 24 vs.
96 h in the favorable group. The difference in the S-100B decrease
velocity between the two groups showed a relative decrease equal to
5.4, with velocities of VS-100B_U=-0.03 µg/l/h and
VS-100B_F=-0.006 µg/l/h for the unfavorable and
favorable groups, respectively.
Briefly, this study introduced and validated a novel
concept for predicting a treatment outcome in patients in a
favorable and unfavorable group using an amalgam of physical and
biochemical parameters collected during the initial hospitalization
stage, encompassing the first 4 days after hospital admission.
Over the past 20 years, studies have focused on
patient treatment outcomes following a TBI (64-66).
Mercier et al (56)
summarized 41 reports on the applicability of correlations between
TBI and the S-100B levels in patients with severe TBI for long-term
prognosis. However, the report described unfavorable treatment
results for increased levels of S-100B protein. The present study
improved on predicting treatment outcomes based on a combination of
physical and biochemical parameters collected during the initial
stage of hospitalization.
The therapeutic outcomes of treatment were validated
against the GOS, which was evaluated on the 4th day of
hospitalization. Patients with a GOS score <4 or GOS score ≥4
were stratified into unfavorable and favorable groups,
respectively. The mean age of the study sample was 48.73±16.3
years, which is slightly higher than previously reported (67,68).
However, no significant differences were determined were found
between the age distribution of the present study and those of Jain
et al (67) and Jennett
(68) using a Student's t-test. The
mortality rate of the patients in the current study was 26.6%,
which is higher than previously reported (69). The reasons for these differences are
unknown.
The reference range for the normal concentration of
S-100B established in the present study was 0.05-0.23 µg/l. The
lower limit of the reference range was similar to that shown in
previous studies. However, the upper limit was twice as high
(70). In addition, the upper
reference limit obtained in the present study was twice the value
reported for patients with an isolated head injury for whom CT
scans for mild TBI were negative (71,72).
Furthermore, an apparent discrepancy was found between the
pathological levels of S-100B reported in this study (>0.235
µg/l) and those of a previous study (>0.5 µg/l) (73).
The present study is amongst only a few to report
the association between time-dependent changes in S-100B levels and
the outcome of TBI treatment (74-76).
A striking discrepancy was observed between the data in the present
study and those reported by Gyorgy et al (74), where it was previously shown that
there was no correlation between the severity of TBI and serum
S-100B levels. Additionally, the study by Gyorgy et al
(74) also showed an increase in
the S-100B levels between 7-72 h after injury, whereas the current
study showed a pronounced decrease in the S-100B levels. However, a
comparison of the results of this study with those reported by
Shakeri et al (16),
revealed a lack of statistical differences between S-100B protein
levels stratified into favorable and unfavorable groups in both
studies. The observed difference between this and the latter study
may be due to different analytic techniques, such as the use of
monoclonal antibodies in the present study and ELISA in the ins the
study by Gyorgy et al (74).
Furthermore, the present study employed advanced techniques for the
analysis of nonparametric data, such as one-way aligned rank
transformation for nonparametric factorial ANOVA and bootstrap
analysis. Such an approach allowed for identification of the subtle
differences between S-100B levels and the mean cerebral blood flow
velocity between the favorable and unfavorable outcome groups.
The results of the present study also corroborate
with those of previous studies (79-82),
which showed that the initial concentration of S-100B is of
paramount importance for the prediction of the outcome of TBI. In
addition, the present study also substantiated the previous
findings relating to the clinical significance of S-100B levels up
to 3 days after hospital admission (83-89).
Cerebral flow dynamics determined by TCD examination
has been one of the most popular neurosurgical diagnostic tools
since the 1980s (90,91). This technique allows for analysis of
abnormalities in cerebral circulation in patients with
craniocerebral trauma (91-95).
The impairment of cerebral flow reflected in Vmean is of
paramount importance for determining the treatment outcomes of
patients with severe craniocerebral trauma (96,97).
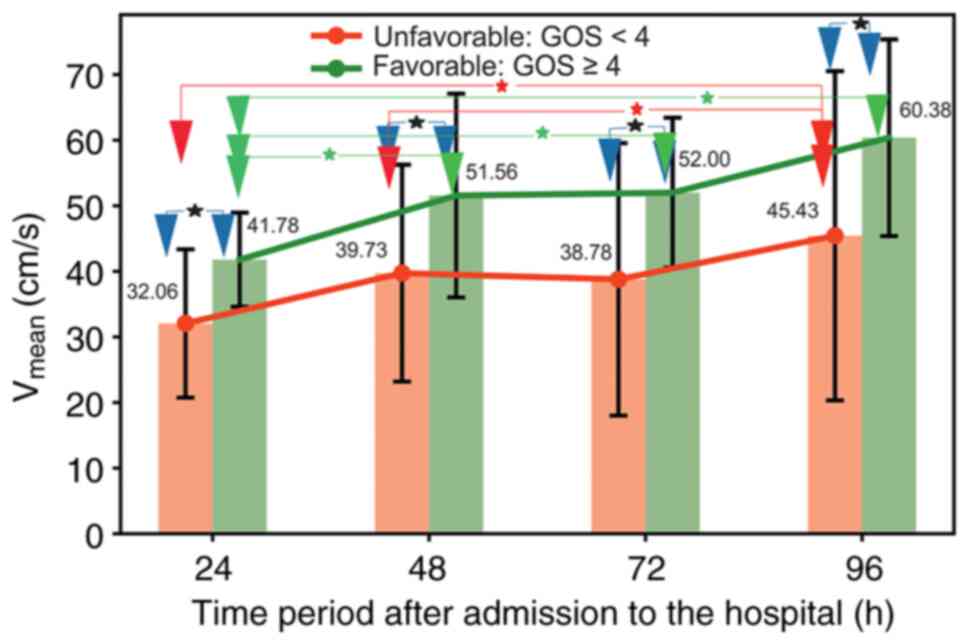
In the present study, an abnormal Vmean level was
defined by values <30.9 cm/s, which is in agreement with
previous reports (39,98).
The present study showed that in the successful
group, the majority of the patients exhibited a Vmean
>30.9 cm/s (the threshold value defining the healthy subjects)
during the first 24 h of hospitalization. However, in the
unfavorable group, the number of patients with Vmean
>30.9 cm/s was notably lower. This observation indicates the
direct applicability of Vmean for predicting treatment
outcomes amongst patients with severe CBI.
Analysis of the relationship between the parameters
that define the unfavorable treatment outcomes led to the following
observations. A significant time-dependent decrease in S-100B
levels (a negative velocity equivalent to 0.03 µg/l/h) was
associated with a statistically significant increase in
Vmean levels (a positive velocity of 0.26 cm/sec/h). A
favorable outcome was defined by the lack of changes in S-100B
levels and a time-dependent increase in Vmean with a
velocity of 0.18 cm/sec/h.
In conclusion, the present study is the first to
report on the associations between S-100B protein levels and
Vmean to predict patient treatment outcomes in those who
have suffered a TBI or CBI, to the best of our knowledge. It was
established that within the first 4 days of hospitalization, a
constant level of S-100B protein even slightly above the normal
range, associated with an increase in Vmean, was a
predictor of successful treatment outcomes. Moreover, following the
conclusion of the study by Thelin et al (22), which suggested that S100B could be
used as a versatile screening, monitoring and prediction tool in
the management of TBI patients, the present study revealed that
serum concentration of S100B itself was of limited use in
predicting TBI outcomes. However, additional studies are required
to validate this observation and to obtain the appropriate
statistical power. Moreover, the limited clinical applicability of
currently studied CBI markers indicates the need for the continuous
search for other markers, which exhibit improved specificity and
sensitivity in a clinical environment.
Not applicable.
Funding: The research reported in this publication was supported
by the Commission for Scientific Research of the Medical Center for
Postgraduate Education in Warsaw Research (grant no.
501-2-1-20-49/04).
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
SD collected and analyzed the data. SD, MZ, AZ and
RT collected the data and wrote the manuscript. RT analyzed the
data. All authors have read and approved the final manuscript. SD,
RT and MZ have seen and confirmed the authenticity of the raw
data.
The Bioethics Committee of the Postgraduate
Education Medical Center in Warsaw (Warsaw, Poland) approved the
experimental protocols (approval no. 501-2-1-20-49/04). Informed
consent was obtained from all subjects or their guardians and a
parent/legal guardian of subjects under 18 years of age.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Seletz E: Craniocerebral injuries. Calif
Med. 84:292–294. 1956.PubMed/NCBI
|
|
2
|
Romodanov AP and Pedachenko EG: Features
of the clinical manifestation of contusion of the cerebral
hemisphere in patients with hypertension. Zh Vopr Neirokhir Im N N
Burdenko. 3:3–6. 1983.PubMed/NCBI(In Russian).
|
|
3
|
Dewan MC, Rattani A, Gupta S, Baticulon
RE, Hung YC, Punchak M, Agrawal A, Adeleye AO, Shrime MG, Rubiano
AM, et al: Estimating the global incidence of traumatic brain
injury. J Neurosurg: April 1, 2018 (Epub ahead of print). doi:
10.3171/2017.10.JNS17352.
|
|
4
|
Toccalino D, Colantonio A and Chan V:
Update on the epidemiology of work-related traumatic brain injury:
A systematic review and meta-analysis. Occup Environ Med.
78:769–776. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lasry O, Liu EY, Powell GA, Ruel-Laliberte
J, Marcoux J and Buckeridge DL: Epidemiology of recurrent traumatic
brain injury in the general population: A systematic review.
Neurology. 89:2198–2209. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bruns J Jr and Hauser WA: The epidemiology
of traumatic brain injury: A review. Epilepsia. 44:2–10.
2003.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Masson F, Thicoipe M, Aye P, Mokni T,
Senjean P, Schmitt V, Dessalles PH, Cazaugade M and Labadens P:
Aquitaine Group for Severe Brain Injuries Study. Epidemiology of
severe brain injuries: A prospective population-based study. J
Trauma. 51:481–489. 2001.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Maegele M, Engel D, Bouillon B, Lefering
R, Fach H, Raum M, Buchheister B, Schaefer U, Klug N and Neugebauer
E: Incidence and outcome of traumatic brain injury in an urban area
in Western Europe over 10 years. Eur Surg Res. 39:372–379.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Teasdale G and Jennett B: Assessment of
coma and impaired consciousness. A practical scale. Lancet.
2:81–84. 1974.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dadas A, Washington J, Diaz-Arrastia R and
Janigro D: Biomarkers in traumatic brain injury (TBI): A review.
Neuropsychiatr Dis Treat. 14:2989–3000. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gan ZS, Stein SC, Swanson R, Guan S,
Garcia L, Mehta D and Smith DH: Blood biomarkers for traumatic
brain injury: A quantitative assessment of diagnostic and
prognostic accuracy. Front Neurol. 10(446)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Korfias S, Stranjalis G, Papadimitriou A,
Psachoulia C, Daskalakis G, Antsaklis A and Sakas DE: Serum S-100B
protein as a biochemical marker of brain injury: A review of
current concepts. Curr Med Chem. 13:3719–3731. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Harris A, Keuler S, Kerska A and Rogatzki
M: Serum protein S100B, a biomarker for head injury or skeletal
muscle damage? FASEB J. 31:lb123. 2017.
|
|
14
|
Savola O, Pyhtinen J, Leino TK, Siitonen
S, Niemelä O and Hillbom M: Effects of head and extracranial
injuries on serum protein S100B levels in trauma patients. J
Trauma. 56:1229–1234. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rodríguez-Rodríguez A, Egea-Guerrero JJ,
Gordillo-Escobar E, Enamorado-Enamorado J, Hernández-García C, Ruiz
de Azúa-López Z, Vilches-Arenas Á, Guerrero JM and Murillo-Cabezas
F: S100B and Neuron-specific Enolase as mortality predictors in
patients with severe traumatic brain injury. Neurol Res.
38:130–137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shakeri M, Mahdkhah A and Panahi F: S100B
protein as a Post-traumatic biomarker for prediction of brain death
in association with patient outcomes. Arch Trauma Res. 2:76–80.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Pfortmueller CA, Drexel C,
Krähenmann-Müller S, Leichtle AB, Fiedler GM, Lindner G and
Exadaktylos AK: S-100 B Concentrations are a predictor of decreased
survival in patients with major trauma, independently of head
injury. PLoS One. 11(e0152822)2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rodríguez-Rodríguez A, Egea-Guerrero JJ,
León-Justel A, Gordillo-Escobar E, Revuelto-Rey J, Vilches-Arenas
A, Carrillo-Vico A, Domínguez-Roldán JM, Murillo-Cabezas F and
Guerrero JM: Role of S100B protein in urine and serum as an early
predictor of mortality after severe traumatic brain injury in
adults. Clin Chim Acta. 414:228–233. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Duda I, Krzych Ł, Jędrzejowska-Szypułka H
and Lewin-Kowalik J: Serum levels of the S100B protein and
neuron-specific enolase are associated with mortality in critically
ill patients. Acta Biochim Pol. 64:647–652. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Olivecrona M, Rodling-Wahlström M, Naredi
S and Koskinen LO: S-100B and neuron specific enolase are poor
outcome predictors in severe traumatic brain injury treated by an
intracranial pressure targeted therapy. J Neurol Neurosurg
Psychiatry. 80:1241–1247. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Goyal A, Failla MD, Niyonkuru C, Amin K,
Fabio A, Berger RP and Wagner AK: S100b as a Prognostic biomarker
in outcome prediction for patients with severe traumatic brain
injury. J Neurotrauma. 30:946–957. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Thelin EP, Nelson DW and Bellander BM: A
review of the clinical utility of serum S100B protein levels in the
assessment of traumatic brain injury. Acta Neurochir (Wien).
159:209–225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Beyer H, Biberthaler P and Bogner-Flatz V:
Chapter 10-S100 biomarkers in patients with traumatic brain injury.
In: Biomarkers for Traumatic Brain Injury. Wu AHB and Peacock WF
(eds). Academic Press, Cambridge, MA, pp155-167, 2020.
|
|
24
|
Michetti F, Corvino V, Geloso MC, Lattanzi
W, Bernardini C, Serpero L and Gazzolo D: The S100B protein in
biological fluids: More than a lifelong biomarker of brain
distress. J Neurochem. 120:644–659. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rodriguez A, Cervera E, Macchia G, Mendoza
X, Martínez W, Pérez M, Sanjuán H and Villalba H: Utility of S-100B
as a potential tool for Neuromonitoring and prediction of
neuroworsening in acute phase of traumatic brain injury. Panam J
Trauma Crit Care Emerg Surg. 9:105–113. 2020.
|
|
26
|
Czeiter E, Amrein K, Gravesteijn BY, Lecky
F, Menon DK, Mondello S, Newcombe VFJ, Richter S, Steyerberg EW,
Vyvere TV, et al: Blood biomarkers on admission in acute traumatic
brain injury: Relations to severity, CT findings and care path in
the CENTER-TBI study. EBioMedicine. 56(102785)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cata JP, Abdelmalak B and Farag E:
Neurological biomarkers in the perioperative period. Br J Anaesth.
107:844–858. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ballesteros MA, Rubio-Lopez MI, San Martín
M, Padilla A, López-Hoyos M, Llorca J and Miñambres E: Serum levels
of S100B from jugular bulb as a biomarker of poor prognosis in
patients with severe acute brain injury. J Neurol Sci. 385:109–114.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim HJ, Tsao JW and Stanfill AG: The
current state of biomarkers of mild traumatic brain injury. JCI
Insight. 3(e97105)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Oris C, Pereira B, Durif J, Simon-Pimmel
J, Castellani C, Manzano S, Sapin V and Bouvier D: The biomarker
S100B and mild traumatic brain injury: A Meta-analysis. Pediatrics.
141(e20180037)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tiu SC, Chan WY, Heizmann CW, Schäfer BW,
Shu SY and Yew DT: Differential expression of S100B and S100A6(1)
in the human fetal and aged cerebral cortex. Brain Res Dev Brain
Res. 119:159–168. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hagmeyer S, Romão MA, Cristóvão JS,
Vilella A, Zoli M, Gomes CM and Grabrucker AM: Distribution and
relative abundance of S100 proteins in the brain of the APP23
Alzheimer's disease model mice. Front Neurosci.
13(640)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tateishi N, Shimoda T, Yada N, Shinagawa R
and Kagamiishi Y: S100B: Astrocyte specific protein. Nihon Shinkei
Seishin Yakurigaku Zasshi. 26:11–16. 2006.PubMed/NCBI(In Japanese).
|
|
34
|
Donato R, Cannon BR, Sorci G, Riuzzi F,
Hsu K, Weber DJ and Geczy CL: Functions of S100 proteins. Curr Mol
Med. 13:24–57. 2013.PubMed/NCBI
|
|
35
|
Sorci G, Riuzzi F, Arcuri C, Tubaro C,
Bianchi R, Giambanco I and Donato R: S100B protein in tissue
development, repair and regeneration. World J Biol Chem. 4:1–12.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nishiyama H, Knopfel T, Endo S and Itohara
S: Glial protein S100B modulates long-term neuronal synaptic
plasticity. Proc Natl Acad Sci USA. 99:4037–4042. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Scotto C, Deloulme JC, Rousseau D, Chambaz
E and Baudier J: Calcium and S100B regulation of p53-dependent cell
growth arrest and apoptosis. Mol Cell Biol. 18:4272–4281.
1998.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tegeler C and Ratanakorn D: Physics and
principles. In: Transcranial Doppler Ultrasonography. Babikian V
and Wechsler L (eds). Butterworth-Heinemann, Waltham, MA, pp3-11,
1999.
|
|
39
|
Ract C, Le Moigno S, Bruder N and Vigué B:
Transcranial Doppler ultrasound goal-directed therapy for the early
management of severe traumatic brain injury. Intensive Care Med.
33:645–651. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
World Medical Association. World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Murray GD, Butcher I, McHugh GS, Lu J,
Mushkudiani NA, Maas AI, Marmarou A and Steyerberg EW:
Multivariable prognostic analysis in traumatic brain injury:
Results from the IMPACT study. J Neurotrauma. 24:329–337.
2007.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Steyerberg EW, Mushkudiani N, Perel P,
Butcher I, Lu J, McHugh GS, Murray GD, Marmarou A, Roberts I,
Habbema JD and Maas AI: Predicting outcome after traumatic brain
injury: Development and international validation of prognostic
scores based on admission characteristics. PLoS Med.
5(e165)2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Maas AI, Dearden M, Teasdale GM, Braakman
R, Cohadon F, Iannotti F, Karimi A, Lapierre F, Murray G, Ohman J,
et al: EBIC-guidelines for management of severe head injury in
adults. European brain injury consortium. Acta Neurochir (Wien).
139:286–294. 1997.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Salottolo K, Carrick M, Stewart Levy A,
Morgan BC, Slone DS and Bar-Or D: The epidemiology, prognosis, and
trends of severe traumatic brain injury with presenting Glasgow
Coma Scale of 3. J Crit Care. 38:197–201. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mahadewa TGB, Golden N, Saputra A and
Ryalino C: Modified revised Trauma-Marshall score as a proposed
tool in predicting the outcome of moderate and severe traumatic
brain injury. Open Access Emerg Med. 10:135–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Naqvi J, Yap KH, Ahmad G and Ghosh J:
Transcranial Doppler ultrasound: A review of the physical
principles and major applications in critical care. Int J Vasc Med.
2013(629378)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
D'Andrea A, Conte M, Cavallaro M,
Scarafile R, Riegler L, Cocchia R, Pezzullo E, Carbone A, Natale F,
Santoro G, et al: Transcranial Doppler ultrasonography: From
methodology to major clinical applications. World J Cardiol.
8:383–400. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Saculinggan M and Balase EA: Empirical
power comparison of goodness of fit tests for normality in the
presence of outliers. J Phys Conf Series. 435(012041)2013.
|
|
49
|
Chernick MR, González-Manteiga W,
Crujeiras RM and Barrios EB: Bootstrap methods. In: International
Encyclopedia of Statistical Science. Lovric M (ed). Springer,
Berlin, Heidelberg, pp169-174, 2011.
|
|
50
|
Kay M and Wobbrock J: ARTool: Aligned Rank
Transform for Nonparametric Factorial ANOVAs. R package version
0.10.0. https://zenodo.org/record/44586#.YfzdrWCxVTY.
Accessed January 11, 2016.
|
|
51
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing,
Vienna, 2010.
|
|
52
|
Jennett B and Bond M: Assessment of
outcome after severe brain damage. Lancet. 1:480–484.
1975.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Marshall LF, Marshall SB, Klauber MR, Van
Berkum Clark M, Eisenberg H, Jane JA, Luerssen TG, Marmarou A and
Foulkes MA: The diagnosis of head injury requires a classification
based on computed axial tomography. J Neurotrauma. 9 (Suppl
1):S287–S292. 1992.PubMed/NCBI
|
|
54
|
Pace MC, Cicciarella G, Barbato E, Maisto
M, Passavanti MB, Gazzerro G, Barbarisi M and Aurilio C: Severe
traumatic brain injury: Management and prognosis. Minerva
Anestesiol. 72:235–242. 2006.PubMed/NCBI(In English, Italian).
|
|
55
|
Maas AI and Steyerberg EW: Monitoring
prognosis in severe traumatic brain injury. Crit Care.
18(150)2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mercier E, Boutin A, Lauzier F, Fergusson
DA, Simard JF, Zarychanski R, Moore L, McIntyre LA, Archambault P,
Lamontagne F, et al: Predictive value of S-100β protein for
prognosis in patients with moderate and severe traumatic brain
injury: Systematic review and meta-analysis. BMJ.
346(f1757)2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lingsma H, Andriessen TM, Haitsema I, Horn
J, van der Naalt J, Franschman G, Maas AI, Vos PE and Steyerberg
EW: Prognosis in moderate and severe traumatic brain injury:
External validation of the IMPACT models and the role of
extracranial injuries. J Trauma Acute Care Surg. 74:639–646.
2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Roozenbeek B, Lingsma HF, Lecky FE, Lu J,
Weir J, Butcher I, McHugh GS, Murray GD, Perel P, Maas AI, et al:
Prediction of outcome after moderate and severe traumatic brain
injury: External validation of the International Mission on
Prognosis and Analysis of Clinical Trials (IMPACT) and Corticoid
Randomisation after significant head injury (CRASH) prognostic
models. Crit Care Med. 40:1609–1617. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Jiang JY, Gao GY, Li WP, Yu MK and Zhu C:
Early indicators of prognosis in 846 cases of severe traumatic
brain injury. J Neurotrauma. 19:869–874. 2002.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Myburgh JA, Cooper DJ, Finfer SR,
Venkatesh B, Jones D, Higgins A, Bishop N and Higlett T:
Australasian Traumatic Brain Injury Study (ATBIS) Investigators for
the Australian; New Zealand Intensive Care Society Clinical Trials
Group. Epidemiology and 12-month outcomes from traumatic brain
injury in Australia and New Zealand. J Trauma. 64:854–862.
2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Thornhill S, Teasdale GM, Murray GD,
McEwen J, Roy CW and Penny KI: Disability in young people and
adults one year after head injury: Prospective cohort study. BMJ.
320:1631–1635. 2000.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Xydakis MS, Ling GS, Mulligan LP, Olsen CH
and Dorlac WC: Epidemiologic aspects of traumatic brain injury in
acute combat casualties at a major military medical center: A
cohort study. Ann Neurol. 72:673–681. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kraus JF and McArthur DL: Epidemiologic
aspects of brain injury. Neurol Clin. 14:435–450. 1996.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mamelak AN, Pitts LH and Damron S:
Predicting survival from head trauma 24 hours after injury: A
practical method with therapeutic implications. J Trauma. 41:91–99.
1996.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Lieberman JD, Pasquale MD, Garcia R,
Cipolle MD, Mark Li P and Wasser TE: Use of admission Glasgow Coma
Score, pupil size, and pupil reactivity to determine outcome for
trauma patients. J Trauma. 55:437–443. 2003.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Combes P, Fauvage B, Colonna M, Passagia
JG, Chirossel JP and Jacquot C: Severe head injuries: An outcome
prediction and survival analysis. Intensive Care Med. 22:1391–1395.
1996.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Jain S, Dharap SB and Gore MA: Early
prediction of outcome in very severe closed head injury. Injury.
39:598–603. 2008.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Jennett B: Epidemiology of head injury. J
Neurol Neurosurg Psychiatry. 60:362–369. 1996.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gerber LM, Chiu YL, Carney N, Hartl R and
Ghajar J: Marked reduction in mortality in patients with severe
traumatic brain injury. J Neurosurg. 119:1583–1590. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Wiesmann M, Missler U, Gottmann D and
Gehring S: Plasma S-100b protein concentration in healthy adults is
age- and sex-independent. Clin Chem. 44:1056–1058. 1998.PubMed/NCBI
|
|
71
|
Biberthaler P, Mussack T, Wiedemann E,
Kanz KG, Koelsch M, Gippner-Steppert C and Jochum M: Evaluation of
S-100b as a specific marker for neuronal damage due to minor head
trauma. World J Surg. 25:93–97. 2001.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Pandor A, Goodacre S, Harnan S, Holmes M,
Pickering A, Fitzgerald P, Rees A and Stevenson M: Diagnostic
management strategies for adults and children with minor head
injury: A systematic review and an economic evaluation. Health
Technol Assess. 15:1–202. 2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Raabe A and Seifert V: Protein S-100B as a
serum marker of brain damage in severe head injury: Preliminary
results. Neurosurg Rev. 23:136–138. 2000.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gyorgy A, Ling G, Wingo D, Walker J, Tong
L, Parks S, Januszkiewicz A, Baumann R and Agoston DV:
Time-dependent changes in serum biomarker levels after blast
traumatic brain injury. J Neurotrauma. 28:1121–1126.
2011.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Gyorgy A, Ling G, Wingo D, Walker J, Tong
L, Parks S, Januszkiewicz A, Baumann R and Agoston DV:
Time-dependent changes in serum biomarker levels after blast
traumatic brain injury. J Neurotrauma. 28:1121–1126.
2011.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Dadas A, Washington J, Marchi N and
Janigro D: Improving the clinical management of traumatic brain
injury through the pharmacokinetic modeling of peripheral blood
biomarkers. Fluids Barriers CNS. 13(21)2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Hu J, Ferreira A and Van Eldik LJ:
S100beta induces neuronal cell death through nitric oxide release
from astrocytes. J Neurochem. 69:2294–2301. 1997.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Koppal T, Lam AG, Guo L and Van Eldik LJ:
S100B proteins that lack one or both cysteine residues can induce
inflammatory responses in astrocytes and microglia. Neurochem Int.
39:401–407. 2001.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Woertgen C, Rothoerl RD and Brawanski A:
Early S-100B serum level correlates to quality of life in patients
after severe head injury. Brain Inj. 16:807–816. 2002.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Raabe A, Kopetsch O, Woszczyk A, Lang J,
Gerlach R, Zimmermann M and Seifert V: Serum S-100B protein as a
molecular marker in severe traumatic brain injury. Restor Neurol
Neurosci. 21:159–169. 2003.PubMed/NCBI
|
|
81
|
Müller K, Townend W, Biasca N, Undén J,
Waterloo K, Romner B and Ingebrigtsen T: S100B serum level predicts
computed tomography findings after minor head injury. J Trauma.
62:1452–1456. 2007.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Gonzćlez-Mao MC, Repáraz-Andrade A, Del
Campo-Pérez V, Alvarez-García E, Vara-Perez C and Andrade-Olivié
MA: Model predicting survival/exitus after traumatic brain injury:
Biomarker S100B 24h. Clin Lab. 57:587–597. 2011.PubMed/NCBI
|
|
83
|
Petzold A, Green AJ, Keir G, Fairley S,
Kitchen N, Smith M and Thompson EJ: Role of serum S100B as an early
predictor of high intracranial pressure and mortality in brain
injury: A pilot study. Crit Care Med. 30:2705–2710. 2002.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Romner B, Ingebrigtsen T, Kongstad P and
Børgesen SE: Traumatic brain damage: Serum S-100 protein
measurements related to neuroradiological findings. J Neurotrauma.
17:641–647. 2000.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Murillo-Cabezas F, Muñoz-Sánchez MA,
Rincón-Ferrari MD, Martín-Rodríguez JF, Amaya-Villar R,
García-Gómez S and León-Carrión J: The prognostic value of the
temporal course of S100beta protein in post-acute severe brain
injury: A prospective and observational study. Brain Inj.
24:609–619. 2010.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Watt SE, Shores EA, Baguley IJ, Dorsch N
and Fearnside MR: Protein S-100 and neuropsychological functioning
following severe traumatic brain injury. Brain Inj. 20:1007–1017.
2006.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Herrmann M, Curio N, Jost S, Grubich C,
Ebert AD, Fork ML and Synowitz H: Release of biochemical markers of
damage to neuronal and glial brain tissue is associated with short
and long term neuropsychological outcome after traumatic brain
injury. J Neurol Neurosurg Psychiatry. 70:95–100. 2001.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Pelinka LE, Toegel E, Mauritz W and Redl
H: Serum S 100 B: A marker of brain damage in traumatic brain
injury with and without multiple trauma. Shock. 19:195–200.
2003.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Thelin EP, Johannesson L, Nelson D and
Bellander BM: S100B is an important outcome predictor in traumatic
brain injury. J Neurotrauma. 30:519–528. 2013.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Aaslid R, Lindegaard KF, Sorteberg W and
Nornes H: Cerebral autoregulation dynamics in humans. Stroke.
20:45–52. 1989.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Aaslid R, Markwalder TM and Nornes H:
Noninvasive transcranial Doppler ultrasound recording of flow
velocity in basal cerebral arteries. J Neurosurg. 57:769–774.
1982.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Chan KH, Dearden NM and Miller JD:
Transcranial Doppler-sonography in severe head injury. Acta
Neurochir Suppl (Wien). 59:81–85. 1993.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Fatima N, Shuaib A, Chughtai TS, Ayyad A
and Saqqur M: The role of transcranial Doppler in traumatic brain
injury: A systemic review and Meta-analysis. Asian J Neurosurg.
14:626–633. 2019.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Jarus-Dziedzic K, Zub W and Czernicki Z:
The application of transcranial Doppler sonography in the
evaluation of cerebral flow disturbances after head trauma. Neurol
Neurochir Pol. 33:151–167. 1999.PubMed/NCBI(In Polish).
|
|
95
|
Weber M, Grolimund P and Seiler RW:
Evaluation of posttraumatic cerebral blood flow velocities by
transcranial Doppler ultrasonography. Neurosurgery. 27:106–112.
1990.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Chan KH, Miller JD and Dearden NM:
Intracranial blood flow velocity after head injury: Relationship to
severity of injury, time, neurological status and outcome. J Neurol
Neurosurg Psychiatry. 55:787–791. 1992.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kinoshita K: Traumatic brain injury:
Pathophysiology for neurocritical care. J Intensive Care. 4:29.
2016.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Chang T, Li L, Yang Y, Li M, Qu Y and Gao
L: Transcranial Doppler ultrasonography for the management of
severe traumatic brain injury after Decompressive Craniectomy.
World Neurosurgery. 126:e116–e124. 2019.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Frontera JA, Fernandez A, Schmidt JM,
Claassen J, Wartenberg KE, Badjatia N, Connolly ES and Mayer SA:
Defining vasospasm after subarachnoid hemorrhage: What is the most
clinically relevant definition? Stroke. 40:1963–1968.
2009.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Tsivgoulis G, Alexandrov AV and Sloan MA:
Advances in transcranial Doppler ultrasonography. Curr Neurol
Neurosci Rep. 9:46–54. 2009.PubMed/NCBI View Article : Google Scholar
|