Introduction
Knee osteoarthritis (KOA) is a chronic disease
characterized by degenerative changes in the articular cartilage,
structural changes in the subchondral bone, and secondary
synovitis. Patients with KOA have a limited range of motion,
experience joint pain, and exhibit gait disturbances, and serious
KOA interferes with daily life (1).
Although radiographic findings do not necessarily correlate with
the symptoms of KOA (2), and a
previous report suggested that degenerative and structural changes
in synovitis and bone marrow lesions are important factors that
cause joint pain and impair the quality of life (3). In a clinical study using MRI, Hill
et al (4) reported that
synovitis in KOA correlates with pain severity, and more recently,
it was reported that in addition to the synovial tissue, fibrotic
changes in the infrapatellar fat pad (IFP) are also involved in the
pathogenesis and mechanism of joint pain in KOA (5).
The IFP is the fatty tissue that fills the space in
the knee joint surrounded by the patellar tendon, proximal tibia,
and femoral condyle at the inferior border of the patella (6). The IFP is in contact with the superior
edge of the patella, the medial patellar retinaculum, and the
lateral patellar retinaculum, and plays an important role in the
smooth movement of knee joints (7).
Furthermore, the IFP is histologically a collagen-rich fibrous
adipose tissue sequential to synovial tissue and is innervated by
branches of the posterior tibial nerve, which are distributed by
nerve fibers with free nerve endings (8). It also acts as a buffer against joint
loading by adjusting the contact pressure on the knee joints during
joint movement (9).
Furthermore, the IFP is known to secrete several
growth factors and cytokines and is reported to affect the
metabolism of articular cartilage and synovial tissue. Distel et
al (10) demonstrated that the
expression of proinflammatory cytokines and IL-6 expression in the
IFP and subcutaneous adipose tissue were elevated in obese
patients, suggesting that cytokine secretion from the IFP may
contribute to the degeneration of articular cartilage. It has also
been reported that lymphocyte infiltration, angiogenesis (11), and an increasing number of cells
expressing monocyte chemoattractant protein-1 (MCP-1) and IL-6 were
observed in the IFP of KOA patients. Thus, the IFP is an important
tissue in the progression of KOA from the perspective of induction
of inflammation in knee joints. Moreover, the IFP is reported to be
involved in the fibrosis of synovial tissues (12), and changes in the mechanical
properties of IFP by fibrosis reduce the buffering capacity against
joint loading (13,14). Therefore, fibrosis of the IFP is a
major cause of functional impairment and pain in KOA patients.
However, the mechanism underlying IFP fibrosis remains unclear.
In general, tissue fibrosis is observed as a
physiological event with the deposition of extracellular matrix
components such as collagen (15).
Cirrhosis of the liver, renal disease, and pulmonary fibrosis are
known to involve severe fibrosis, which is hypothesized to be a
response to chronic inflammation (16,17).
Hypoxia is also considered to be strongly involved in fibrosis in
various adipose tissues in vivo (18). Studies on obese patients have
demonstrated that an increase in the amount of adipose tissue
results in hypoxic conditions in the tissue by delayed
angiogenesis, which activates the transcription factor, HIF-1α, and
causes fibrosis in adipose tissues (19). Thus, HIF-1α may also be a key
molecule in the fibrosis of IFP in the pathogenesis of KOA.
Regarding the relationship between hypoxia and KOA, it was reported
that HIF-1α in articular cartilage and synovial fluid is activated
and is correlated with the severity of KOA (20). In addition, Sotobayashi et al
(21) demonstrated that the
transcriptional activity of HIF-1α in the synovial tissue was
increased in a contracture model of joint immobilization, resulting
in synovial tissue fibrosis. These results strongly suggest that
the increased expression and activation of HIF-1α are important
factors that promote fibrosis in IFP in the pathogenesis of
KOA.
In this study, we focused on HIF-1α and investigated
the direct involvement of HIF-1α in the mechanism of fibrosis of
IFP accompanied by the progression of KOA using an animal model. In
addition, to examine a useful and practical management approach for
KOA, the inhibitory effect of low-intensity pulsed ultrasound
(LIPUS) on HIF-1α activation and fibrosis in IFP was evaluated, as
it has been reported that LIPUS attenuates the fibrosis of synovial
tissues followed by reduced activation of HIF-1α in a joint
contracture model (23,24).
Materials and methods
KOA model
A KOA model was established and LIPUS intervention
was performed on male Wistar rats weighing 300-400 g, obtained from
Japan SLC. Animals were anesthetized with 1.5% isoflurane mixed
with oxygen using inhalation anesthesia and were maintained on the
same concentration of anesthetic throughout the entirety of the
procedure. Animals were then subjected to 0.5% carrageenan
injection into the bilateral knees (25). Animals were sacrificed by
intraperitoneal administration of an overdose of 1-1.5 ml
pentobarbitone (150-200 mg/kg), which amounted to 64.8 mg/ml
pentobarbitone. Death was confirmed by checking for cardiac arrest,
after which the animal was observed for ~5 min. After ensuring that
there were no signs of recovery, tissues were harvested from the
animals.
The unilateral knee joints of the rats were treated
with LIPUS (BR Sonic-Pro, Ito Co., Ltd.). LIPUS was set at a
frequency of 3 MHz and an output power of 120 mW/cm², based on a
previous study, and was performed for 15 min a day, 4 times a week,
every other day (23).
The samples were isolated and used for reverse
transcription-quantitative (RT-q)PCR, ELISA, and histological
analysis.
In addition, the IFP was harvested along with the
synovial tissues after dissection at the inferior pole of the
patella and flipped with the patellar tendon (26).
Ethics statement
All animal procedures were approved by the Ethics
Committee for Animal Experiments of the Morinomiya University of
Medical Sciences (approval no. 2019A001) and performed in
accordance with our institutional guidelines. The animal procedures
were also performed in compliance with the law (no. 105) and
notification (no. 6) of the Japanese government and conducted in
accordance with the guidelines of the National Research Council.
All surgeries were performed under anesthesia, and all efforts were
made to minimize suffering. Signs of significant distress in
animals, such as joint infection, behavioral restriction due to
excessive pain, and avoidance behavior were considered humane
endpoints requiring immediate intervention. However, there were no
cases requiring euthanasia due to observation of a humane endpoint
and, therefore, animals were euthanized only at the end of the
experimental period.
Histological and immunohistochemical
studies
The excised joint was decalcified with Morse's
solution as previously described (27) and fixed with 4% paraformaldehyde in
0.1 M phosphate buffer (pH 7.4) overnight at 4˚C. Then, the excised
joint was processed for routine paraffin embedding. Tissue
cross-sections (5 µm) were rehydrated and stained with immersion in
hematoxylin and eosin (HE) solution for 3-5 min at room temperature
(28).
Toluidine blue staining and collagen staining were
also performed to evaluate the progression of osteoarthritis (OA)
caused by carrageenan and for measuring the OASRI score (29). For toluidine blue staining, tissues
were immersed in the Toluidine blue solution for 15 min at room
temperature. For total collagen staining, sections were rehydrated
and incubated using a Picrosirius Red Stain Kit for 2 h at room
temperature according to the manufacturer's protocol (SR;
Polysciences, Inc.), which stains collagen I and III. The stained
area was measured using ImageJ (National Institutes of Health.
Version 1.48) (21).
For immunohistochemical (IHC) staining, the
anti-RM-4 antibody (cat. no. KT014, Medical Chemistry
Pharmaceutical Inc.) was used to analyze the infiltration of
macrophages in the IFP. IHC staining was performed using the high
polymer HISTOFINE simple stain mouse MAX-PO (Nichirei Bioscience
Inc.) method, as described previously (21). Briefly, 5 µm-thick sections were
deparaffinized, rehydrated before blocking endogenous peroxidase
activity with 3% hydrogen peroxide, and preincubated with 1.5%
blocking reagent (Roche Applied Science) in TBS at room temperature
for 1 h. Diluted primary antibodies (1:500) were then applied to
the sections, and these sections were further incubated at room
temperature for 1 h. Following this, the sections were rinsed twice
with TBS for 5 min each and incubated with HISTOFINE simple stain
mouse MAX-PO (rat) (Nichirei Bioscience Inc.) for 30 min at room
temperature. Peroxidase activity was visualized by treatment with
0.05% diaminobenzidine containing 0.3% hydrogen peroxide. The
sections were rinsed in water, dehydrated, cleared, and
mounted.
RT-qPCR
Total RNA was extracted from the knee capsule,
excluding the cartilage and meniscus. Excised tissues were
homogenized in cold PBS and centrifuged at 20,000 x g for 15 min at
4˚C. Total RNA was extracted from the tissue samples using ISOGEN
II (Nippon Gene) and resuspended in PBS, and its purity was
assessed by spectrophotometry. Only samples with an A260/A280 ratio
in the range of 1.8-2.0 were used. cDNA was synthesized using an
iScript cDNA synthesis kit according to the manufacturer's protocol
(Bio-Rad Laboratories, Inc.). Amplification reactions were
performed using SsoFast EvaGreen Supermix (Bio-Rad Laboratories),
with 100 µm of each primer and 1 µg cDNA in a final volume of 20
µl. Amplification reactions were performed in a MiniOpticon
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.), with
the following amplification protocol: Initial denaturation of 1 min
at 95˚C; followed by 40 cycles of 1 sec at 95˚C and 5 sec at
61-65˚C, with each primer. The expression levels of
hypoxanthine-guanine phosphoribosyltransferase (HRPT) as a
housekeeping gene were used as the internal control, and the
comparative Cq method (2-ΔΔCq) was used to quantify the
gene expression levels (30).
Oligonucleotides were synthesized using Gene Design Inc. (Ibaragi,
Osaka, Japan). The primer sequences used were obtained from
previous studies (21,22,27,31)
and are listed in Table I.
 | Table ISequences of the primers. |
Table I
Sequences of the primers.
| Name | Sequence,
5'-3' | (Refs.) |
|---|
| CTGF | | (21,22) |
|
Forward |
CACCCGGGTTACCAATGACAA | |
|
Reverse |
AGCCCGGTAGGTCTTCACACTG | |
| VEGF | | (21) |
|
Forward |
GCAATGATGAAGCCCTGGAG | |
|
Reverse |
GGTGAGGTTTGATCCGCATG | |
| HIF-1α | | (25) |
|
Forward |
ACCGTGCCCCTACTATGTCG | |
|
Reverse |
GCCTTGTATGGGAGCATTAACTT | |
| HRPT | | (25) |
|
Forward |
TGTTTGTGTCATCAGCGAAAGTG | |
|
Reverse |
ATTCAACTTGCCGCTGTCTTTTA | |
ELISA
Synovial tissue including IFP was removed from the
knee joint. Proteins were extracted from the knee IFP. Extraction
was performed using a Nuclear Extract Kit (Active Motif) according
to the manufacturer's protocol. The binding activity of HIF-1α in
the homogenized tissue was measured using an HIF-1α transcription
factor assay kit, according to the manufacturer's instructions
(cat. no. ab133104, Abcam).
Statistical analysis
All data are presented as the mean ± SEM. The
distribution of the data was analyzed first and subsequently
analyzed using a one-way ANOVA followed by a Tukey-Kramer post-hoc
test or a Kruskal-Wallis followed by a Steel-Dwass post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histological analysis of fibrosis in
IFP
Histological analysis revealed that intra-articular
administration of carrageenan resulted in significant progress in
the pathogenesis of OA, OARSI score: Saline, 0.6±0.55; Car 1 week,
1.8±0.84 (P<0.05 vs. Saline); and Car 2 weeks, 2.4±0.55
(P<0.01 vs. Saline). HE, SR, and IHC staining for macrophages
showed cellular infiltration and fibrotic tissue proliferation in
the adipose tissue of IFP at 1 and 2 weeks after intra-articular
injection of carrageenan, and cellular infiltration and fibrosis
were higher after 2 weeks compared with after 1 week (Fig. 1A).
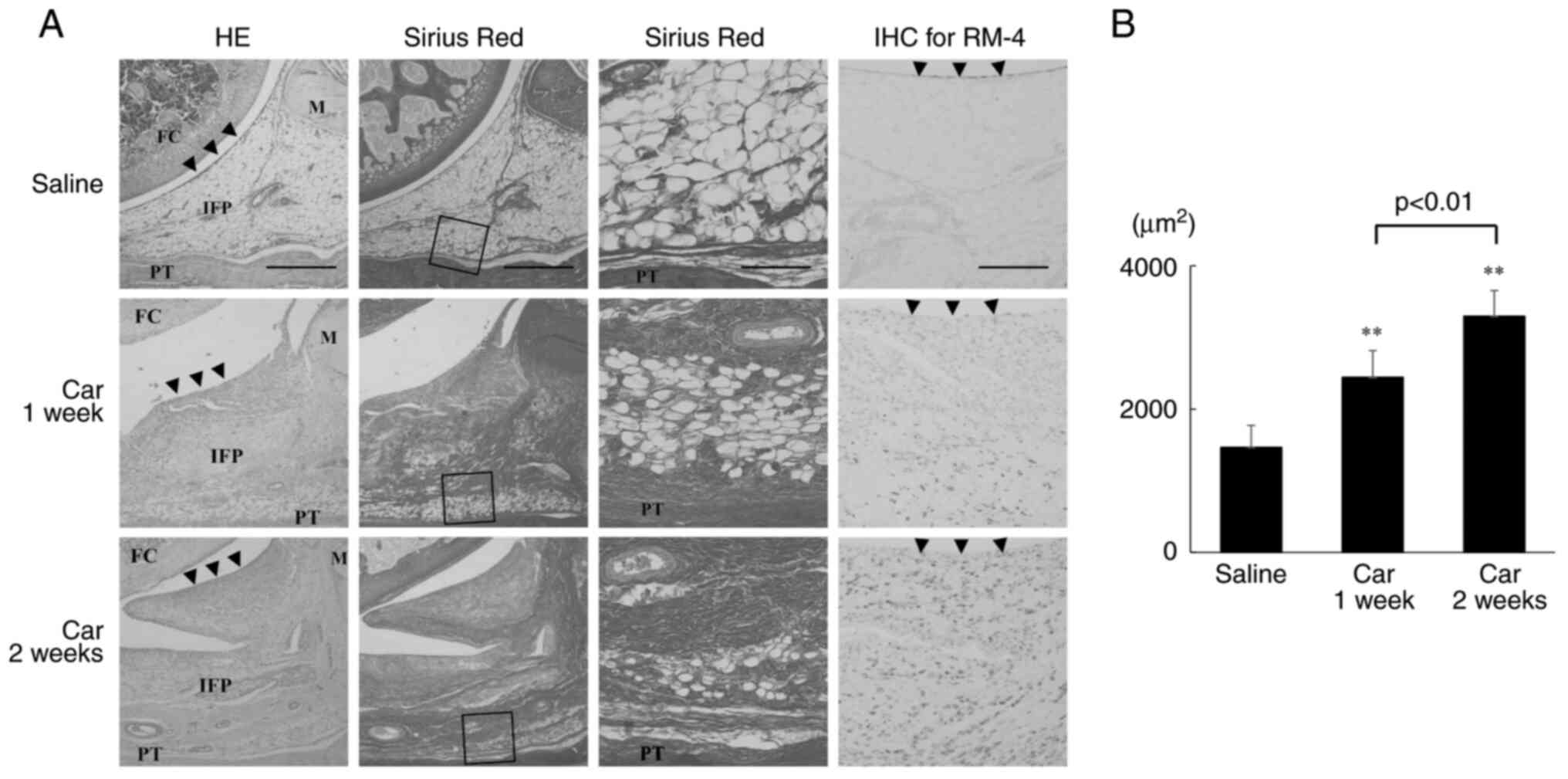 | Figure 1Histological analysis of the IFP. (A)
HE staining (left panels), SR staining (middle-left and
middle-right panels) for analysis of collagen in the fibrosis of
the IFP, and IHC staining for RM-4 (right panels). Left panel:
magnification, x40; scale bar, 500 µm. Middle-left panel:
magnification, x40; scale bar, 500 µm. Middle-right panel:
magnification, x200; scale bar, 100 µm. Right panel, magnification,
x100; scale bar, 200 µm. Arrowheads, synovial membrane. (B)
Quantitative analysis of the volume of collagen in the synovium
measured using ImageJ. **P<0.01 vs. saline. n=5 per
group. Data are presented as the mean ± SEM. HE, hematoxylin and
eosin staining; SR, Sirius Red staining; IHC, immunohistochemical
staining; IFP, infrapatellar fat pad; FC, femoral condyle; M,
meniscus; PT, patella tendon; Saline, a rat knee injected only with
saline; Car 1 week, a rat knee injected 1 week after
intra-articular injection of carrageenan; Car 2 week, a rat knee
injected 2 weeks after intra-articular injection of
carrageenan. |
SR staining, which is specific for type I and III
collagen, demonstrated that significant collagen fibril
proliferation was observed after both 1 and 2 weeks (middle-left
and middle-right panels in Fig.
1A). The area stained was significantly increased 1 and 2 weeks
after treatment with carrageenan compared with that of the saline
group. Furthermore, the stained area was greater after 2 weeks of
treatment with carrageenan compared with after 1 week (Fig. 1B).
Gene expression of fibrosis-related
molecules and HIF-1α in the IFP
We quantitatively analyzed the mRNA expression
levels of CTGF and VEGF, well-known major factors involved in the
mechanisms of fibrosis (32-34),
and found that their expression was significantly increased 1 week
after intra-articular injection of carrageenan compared with the
expression after saline injection and remained high after 2 weeks
(Fig. 2A and B). Moreover, the mRNA expression levels of
HIF-1α, which regulates and induces the expression of CTGF and VEGF
at the genetic level, were higher 1 and 2 weeks after
intra-articular injection of carrageenan compared with that after
saline injection (Fig. 2C).
Furthermore, the transcriptional activity was significantly
increased 2 weeks after the intra-articular injection of
carrageenan compared with that after saline injection (saline,
0.558±0.028; carrageenan, 0.597±0.020; P<0.05).
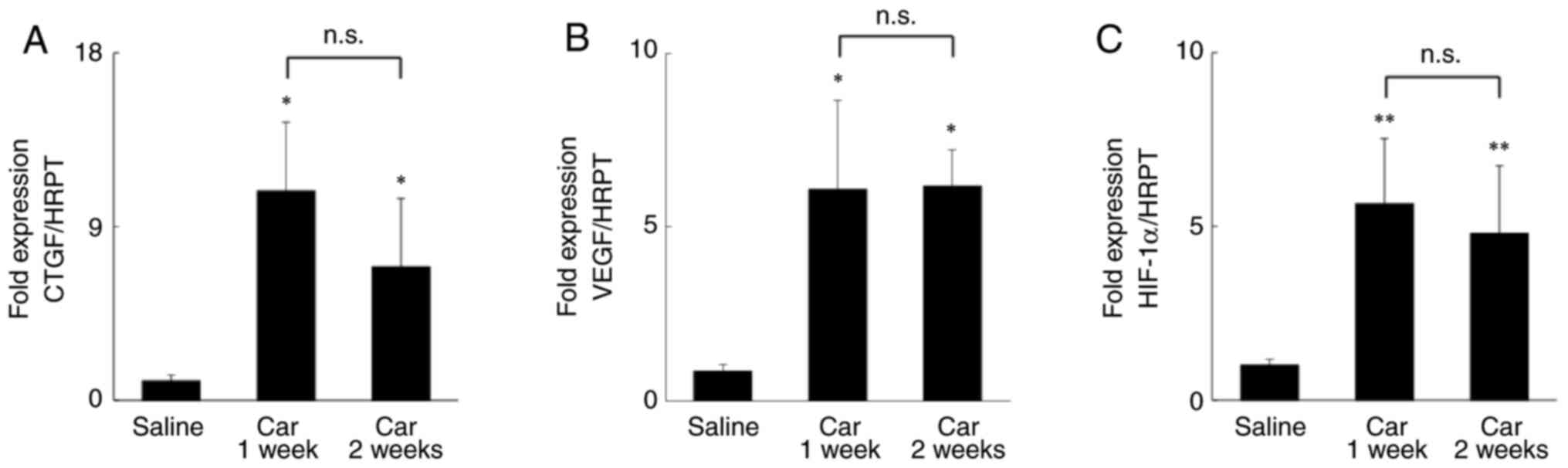 | Figure 2mRNA expression levels of CTGF, VEGF,
and HIF-1α in the IFP of a rat knee. Relative mRNA expressions of
(A) CTGF, (B) VEGF, and (C) HIF-1α. *P<0.05,
**P<0.01 vs. saline. n=5 per group. Data are
presented as the mean ± SEM. CTGF, connective tissue growth factor;
VEGF, vascular endothelial growth factor; Saline, a rat knee
injected only with saline; Car 1 week, a rat knee injected 1 week
after intra-articular injection of carrageenan; Car 2 week, a rat
knee injected 2 weeks after intra-articular injection of
carrageenan. |
Inhibitory effect of LIPUS on fibrosis
in IFP
We also examined the inhibitory effect of LIPUS on
fibrosis in IFP. Histological analysis showed that the area stained
by SR was significantly decreased in IFP treated with LIPUS for 2
weeks after intra-articular injection of carrageenan (Car + LIPUS
group) compared with that of IFP without LIPUS after injection (Car
group) (inside the square box of left panels in Fig. 3A and B), indicating that synovial fibrosis was
attenuated by LIPUS intervention. The HIF-1α mRNA levels were
significantly lower in the Car + LIPUS group in both 1 and 2-week
time points compared with that in the Car group (Fig. 4A). The HIF-1α binding activity was
lower in the Car + LIPUS group compared with the Car group 1 week
after intra-articular injection of carrageenan (Car group,
0.531±0.012; Car + LIPUS group, 0.513±0.011; P<0.05). In
addition, intervention with LIPUS resulted in the inhibition of the
expression of CTGF and VEGF 1 and 2 weeks after injection of
carrageenan (Fig. 4B and C).
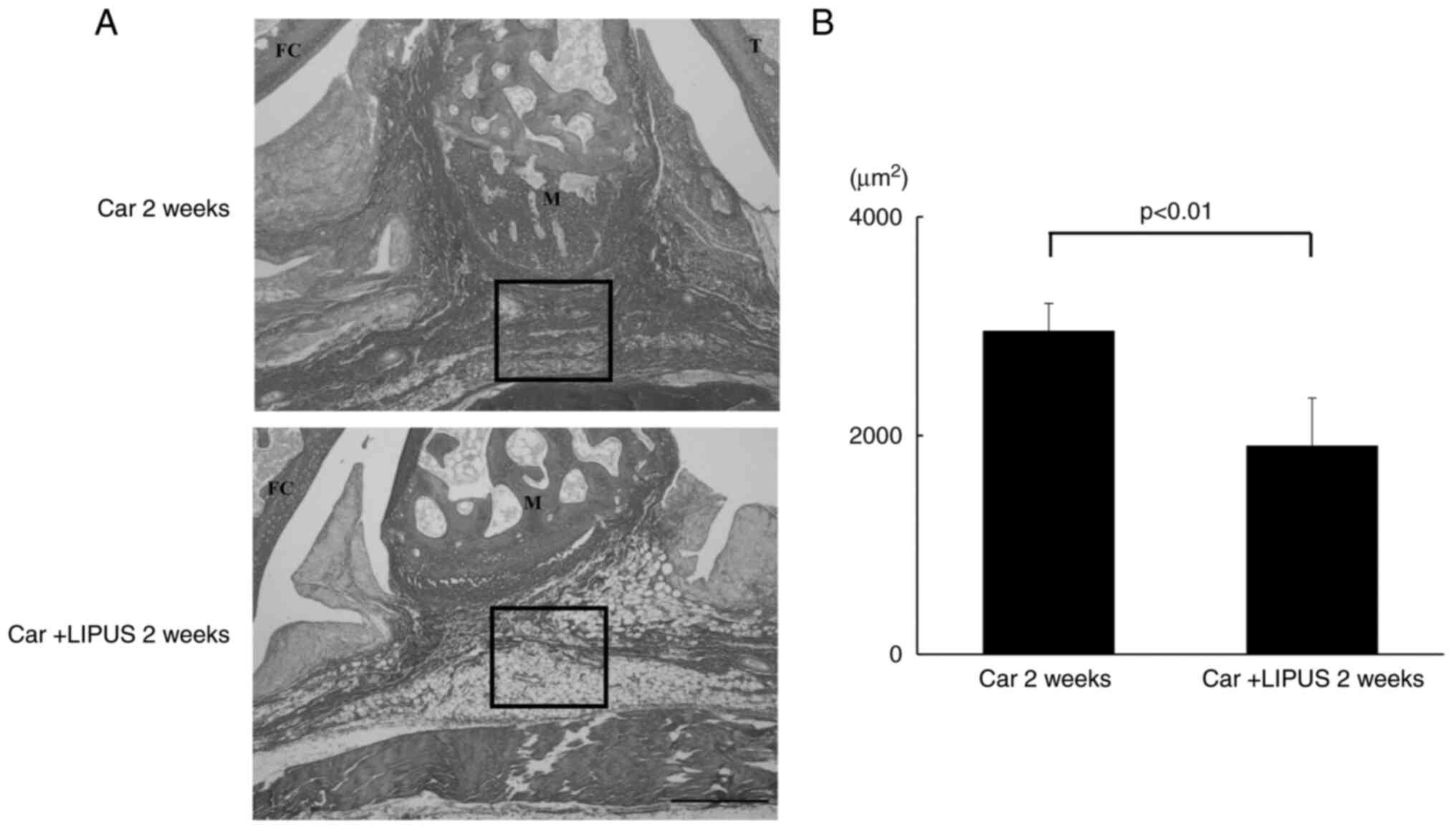 | Figure 3Histological analysis of the effect
of LIPUS on the fibrosis of the IFP assessed by SR staining. (A) SR
staining. magnification, x200; scale bar, 100 µm. Upper panel, Car
2 weeks. Lower panel. (B) Quantitative analysis of the volume of
collagen in the synovium measured using ImageJ. n=5 per group. Data
are presented as the mean ± SEM. LIPUS, low-intensity pulsed
ultrasound; IFP, infrapatellar fat pad; SR, Sirius Red; FC, femoral
condyle; M, meniscus; T, tibia; Car 2 week, a rat knee injected 2
weeks after intra-articular injection of carrageenan; Car + LIPUS 2
weeks, Car + LIPUS 2 weeks, a rat knee treated with LIPUS 2 weeks
after injection of carrageenan. |
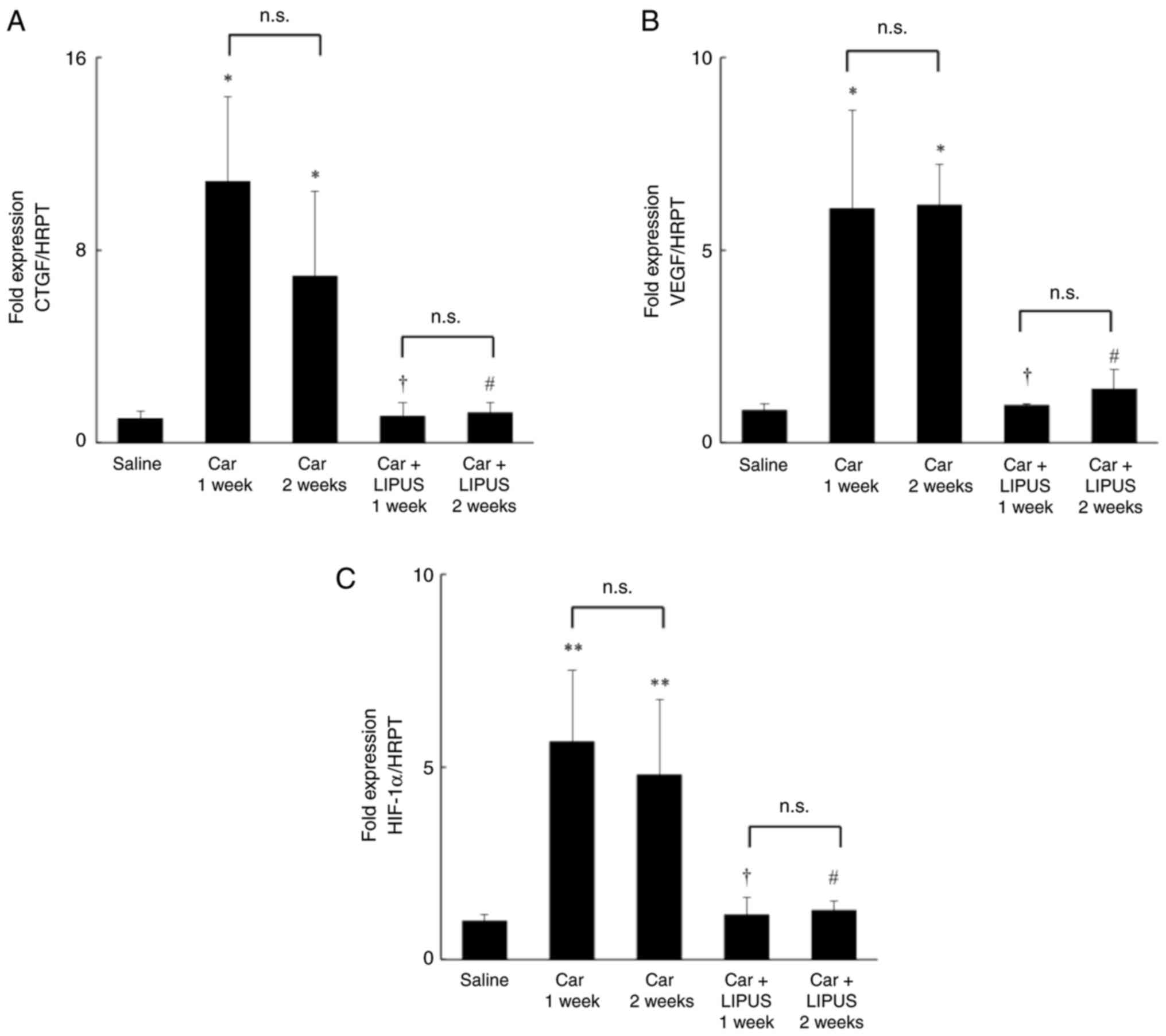 | Figure 4Effect of LIPUS on the mRNA
expression of HIF-1α, CTGF, and VEGF in the IFP of a rat knee.
Relative mRNA expressions levels of (A) CTGF, (B) VEGF, (C) HIF-1α.
Car 1 week, 2 weeks, a rat knee injected at 1 or 2 weeks after
intra-articular carrageenan injection. *P<0.05,
**P<0.01 vs. saline; †P<0.05 vs. Car 1
week; #P<0.05 vs. Car 2 weeks. Data are presented as
the mean ± SEM. LIPUS, low-intensity pulsed ultrasound; IFP,
infrapatellar fat pad; CTGF, connective tissue growth factor; VEGF,
vascular endothelial growth factor; Saline, a rat knee injected
with saline only; Car 1 week, a rat knee injected 1 week after
intra-articular injection of carrageenan; Car 2 week, a rat knee
injected 2 weeks after intra-articular injection of carrageenan;
Car + LIPUS 2 weeks, Car + LIPUS 1 week, a rat knee treated with
LIPUS 1 week after injection of carrageenan; Car + LIPUS 2 weeks,
Car + LIPUS 2 weeks, a rat knee treated with LIPUS 2 weeks after
injection of carrageenan. |
Discussion
The IFP in patients with KOA has been reported to
show hypo-intensity on MRI scans, which is indicative of fibrosis
(35,36), and fibrotic IFP has been
hypothesized to be correlated with the symptoms of KOA and
articular cartilage damage (36).
Fibrosis of IFP has a major impact on the pathogenesis of KOA and
controlling the fibrosis of IFP may be a novel avenue for the
management of KOA, in addition to conventional drug-based and
physical therapy.
Pathological fibrosis induced by inflammation is
widely observed in various diseases such as liver cirrhosis,
nephrosclerosis, and pulmonary fibrosis and is caused by the
activation of myofibroblasts, which play a major role in tissue
repair and induce the production and accumulation of extracellular
matrix components such as collagen (16). TGF-β and various inflammatory
cytokines secreted by innate immune cells such as infiltrated
macrophages are considered to activate myofibroblasts and promote
the production of collagen (37).
The same mechanisms occur in the IFP, which include inflammatory
cell infiltration followed by fibrosis, in carrageenan-induced
animal models of KOA, and in other OA-induced models using ACLT or
monoiodoacetate (38-41).
Furthermore, it has been demonstrated that macrophages migrate into
hypoxic tissues to secrete inflammatory cytokines (42), and HIF-1α, which is a transcription
factor that is activated in response to hypoxic conditions, is
intricately involved in macrophage migration and its functions
(43). These findings suggest that
a hypoxic environment that induces macrophage infiltration via
activation of HIF-1α may contribute to fibrosis in IFP. In the
present study, significant macrophage infiltration was observed in
the IFP of the rat KOA model immediately after carrageenan
administration, suggesting the presence of a hypoxic environment in
the IFP (Fig. 1A). In addition, the
results demonstrated that the expression and activity of HIF-1α
increased after 1 week in the IFP.
Importantly, pathological fibrosis is primarily
caused by fibrosis-associated growth factors such as CTGF and VEGF,
whose expression is regulated by the transactivation of HIF-1α
(32-34).
Sotobayashi et al (21)
demonstrated that HIF-1α activation induced by joint immobilization
promoted the expression of CTGF and VEGF, resulting in the
development of synovial tissue fibrosis that leads to joint
contracture, followed by immobilization and disuse. In addition,
they also provided important results indicating that inhibition of
HIF-1α activity by decoy oligonucleotides attenuated fibrosis of
synovial tissue through suppression of the expression of CTGF and
VEGF. Yabe et al (44)
showed that the expression of HIF-1 was increased in a joint
contracture animal model. These results strongly suggest that the
increased expression and activation of HIF-1α shown in the present
study are involved in the mechanisms of fibrosis in IFP through
upregulation of CTGF and VEGF. In fact, the present study
demonstrated not only increased gene expression and activation of
HIF-1α, but also induced expression of CTGF and VEGF, followed by
accumulation of type I collagen. Therefore, it is hypothesized that
HIF-1α is involved in the fibrosis of IFP in KOA and that
regulation of HIF-1α may serve as a novel therapeutic strategy for
the management of KOA.
Interestingly, in this study, LIPUS was applied to
decrease the expression of HIF-1α based on our previous study
(23), and intervention with LIPUS
inhibited the gene expression levels of fibrosis-related factors
such as CTGF and VEGF, which were suppressed through attenuation of
both gene expression and HIF-1α activation. These results are also
supported by recent studies reporting similar findings regarding
the effect of LIPUS on the activation of HIF-1α and its detailed
mechanisms (23). miR-31-5p, which
regulates the cytoskeleton, was identified as a mechanosensitive
miRNA following LIPUS stimulation, and LIPUS prevented long-term
hypoxia-induced myocardial fibrosis by regulating the HIF-1α/DNA
methyltransferase 3α signaling pathway through the mechanosensitive
protein TRAAK (45,46). These reports suggest that the unique
stimulation of microscopic vibrations by LIPUS directly affects the
regulation of transcriptional activation of HIF-1α via induction of
mechanosensitive molecules. Moreover, LIPUS treatment successfully
decreased the fibrotic lesions in the IFP in the rat model of KOA,
suggesting that LIPUS is an effective device to regulate HIF-1α and
attenuate the fibrotic process of IFP in KOA. In clinical settings,
it has been reported that LIPUS treatment for KOA relieves joint
pain, joint swelling, and a reduction in the limitation of joint
range of motion (47), and our
findings suggest that this phenomenon is mediated by the
antifibrotic effects of LIPUS via the regulation of CTGF, VEGF, and
HIF-1α in the IFP.
The present study aimed to clarify the involvement
of HIF-1α in the development of fibrosis of the IFP in KOA. The IFP
was harvested to investigate gene expression levels and the
transcriptional activity of HIF-1α and related molecules.
Although the present study demonstrated that HIF-1α
participated in the development of fibrosis in the IFP in KOA,
there are several limitations. One of these is the technical
limitation on the collection of IFP samples. The synovial tissue
could not be completely removed from the IFP because of the
continuous histological connection of the IFP with its surrounding
tissues. Therefore, the gene expression and transcriptional
activity may include some synovial tissue responses. Additionally,
although the involvement of HIF-1α in the development of fibrosis
of IFP was investigated, degeneration of the articular cartilage,
another major factor in the pathogenesis of KOA, was not fully
evaluated in this study. In addition, the effects of LIPUS on
HIF-1α activation and degeneration of articular cartilage should be
investigated in the future. Mechanical stress has also been
suggested to be involved in the pathogenesis of KOA. However, given
that carrageenan was used to induce synovitis in this study, the
relationship between HIF-1α and fibrosis of IFP in OA models due to
joint instability, such as destabilization of the medial meniscus
or resection of the ligament, requires further investigation.
Finally, the effect of LIPUS on fibrosis of IFP was evaluated only
2 weeks after LIPUS intervention; lack of data at 1 week is a
limitation of the present study.
In conclusion, the present study using a rat animal
model of KOA demonstrated that activation of HIF-1α, which is
considered to be induced by hypoxic conditions in the IFP, promoted
fibrosis of IFP, followed by upregulation of fibrosis-related
molecules, CTGF and VEGF. Notably, intervention with LIPUS resulted
in attenuation of fibrotic changes in the IFP through reduction of
HIF-1α activity. Our findings reveal a novel therapeutic strategy
for the treatment of KOA, focusing on HIF-1α.
Acknowledgements
We would like to thank Mr Hiroshi Kawanami (Research
Assistant, Graduate School of Health Sciences, Morinomiya
University of Medical Sciences) for the excellent technical
assistance he provided.
Funding
Funding: This work was supported by the Japanese Non-surgical
Orthopedics Society (JNOS) grant (grant no. JNOS202002).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK, HK, MA and SK contributed to the conception and
design of the study, performed the experiments, and contributed to
the acquisition, analysis, and interpretation of data. TK, HK, MA
and SK drafted the manuscript, and revised it critically for
important intellectual content. HK and MA verified all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Morinomiya University of Medical Sciences (grant no. 2019A001).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sellam J and Berenbaum F: The role of
synovitis in pathophysiology and clinical symptoms of
osteoarthritis. Nat Rev Rheumatol. 6:625–635. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Dieppe PA and Lohmander LS: Pathogenesis
and management of pain in osteoarthritis. Lancet. 365:965–973.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hunter DJ and Bierma-Zeinstra S:
Osteoarthritis. Lancet. 393:1745–1759. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hill CL, Gale DG, Chaisson CE, Skinner K,
Kazis L, Gale ME and Felson DT: Knee effusions, popliteal cysts,
and synovial thickening: Association with knee pain in
osteoarthritis. J Rheumatol. 28:1330–1337. 2001.PubMed/NCBI
|
|
5
|
Belluzzi E, Stocco E, Pozzuoli A,
Granzotto M, Porzionato A, Vettor R, De Caro R, Ruggieri P, Ramonda
R, Rossato M, et al: Contribution of infrapatellar fat pad and
synovial membrane to knee osteoarthritis pain. Biomed Res Int.
2019(6390182)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Leese J and Davies DC: An investigation of
the anatomy of the infrapatellar fat pad and its possible
involvement in anterior pain syndrome: A cadaveric study. J Anat.
237:20–28. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Macchi V, Picardi EEE, Fontanella CG,
Porzionato A, Stecco C, Tortorella C, Favero M, Natali A and De
Caro R: The characteristics of the lobular arrangement indicate the
dynamic role played by the infrapatellar fat pad in knee
kinematics. J Anat. 235:80–87. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Macchi V, Stocco E, Stecco C, Belluzzi E,
Favero M, Porzionato A and De Caro R: The infrapatellar fat pad and
the synovial membrane: An anatomo-functional unit. J Anat.
233:146–154. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nakanishi S, Morimoto R, Kitano M,
Kawanishi K, Tanaka A and Kudo S: Difference in movement between
superficial and deep parts of the infrapatellar fat pad during knee
extension. J Funct Morphol Kinesiol. 6(68)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Distel E, Cadoudal T, Durant S, Poignard
A, Chevalier X and Benelli C: The infrapatellar fat pad in knee
osteoarthritis: An important source of interleukin-6 and its
soluble receptor. Arthritis Rheum. 60:3374–3377. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Favero M, El-Hadi H, Belluzzi E, Granzotto
M, Porzionato A, Sarasin G, Rambaldo A, Iacobellis C, Cigolotti A,
Fontanella CG, et al: Infrapatellar fat pad features in
osteoarthritis: A histopathological and molecular study.
Rheumatology (Oxford). 56:1784–1793. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bastiaansen-Jenniskens YM, Wei W, Feijt C,
Waarsing JH, Verhaar JA, Zuurmond AM, Hanemaaijer R, Stoop R and
van Osch GJ: Stimulation of fibrotic processes by the infrapatellar
fat pad in cultured synoviocytes from patients with osteoarthritis:
A possible role for prostaglandin f2α. Arthritis Rheum.
65:2070–2080. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Maculé F, Sastre S, Lasurt S, Sala P,
Segur JM and Mallofré C: Hoffa's fat pad resection in total knee
arthroplasty. Acta Orthop Belg. 71:714–717. 2005.PubMed/NCBI
|
|
14
|
Fontanella CG, Macchi V, Carniel EL, Frigo
A, Porzionato A, Picardi EEE, Favero M, Ruggieri P, de Caro R and
Natali AN: Biomechanical behavior of Hoffa's fat pad in healthy and
osteoarthritic conditions: Histological and mechanical
investigations. Australas Phys Eng Sci Med. 41:657–667.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Henderson NC, Rieder F and Wynn TA:
Fibrosis: From mechanisms to medicines. Nature. 587:555–566.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Wynn TA: Cellular and molecular mechanisms
of fibrosis. J Pathol. 214:199–210. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun K, Tordjman J, Clément K and Scherer
PE: Fibrosis and adipose tissue dysfunction. Cell Metab.
18:470–477. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Halberg N, Khan T, Trujillo ME,
Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV,
Landskroner-Eiger S, Dineen S, Magalang UJ, et al:
Hypoxia-inducible factor 1alpha induces fibrosis and insulin
resistance in white adipose tissue. Mol Cell Biol. 29:4467–4483.
2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Qing L, Lei P, Liu H, Xie J, Wang L, Wen T
and Hu Y: Expression of hypoxia-inducible factor-1α in synovial
fluid and articular cartilage is associated with disease severity
in knee osteoarthritis. Exp Ther Med. 13:63–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sotobayashi D, Kawahata H, Anada N,
Ogihara T, Morishita R and Aoki M: Therapeutic effect of
intra-articular injection of ribbon-type decoy oligonucleotides for
hypoxia inducible factor-1 on joint contracture in an immobilized
knee animal model. J Gene Med. 18:180–192. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Ohtomo S, Nangaku M, Izuhara Y, Takizawa
S, de Strihou CV and Miyata T: Cobalt ameliorates renal injury in
an obese, hypertensive type 2 diabetes rat model. Nephrol Dial
Transplant. 23:1166–1172. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Sotobayashi D and Kawahata H: Beneficial
effect of low-intensity pulsed ultrasound on progression of joint
contracture. J Judo Ther. 27:125–132. 2019.(In Japanese).
|
|
24
|
Itaya N, Yabe Y, Hagiwara Y, Kanazawa K,
Koide M, Sekiguchi T, Yoshida S, Sogi Y, Yano T, Tsuchiya M, et al:
Effects of low-intensity pulsed ultrasound for preventing joint
stiffness in immobilized knee model in rats. Ultrasound Med Biol.
44:1244–1256. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hansra P, Moran EL, Fornasier VL and
Bogoch ER: Carrageenan-induced arthritis in the rat. Inflammation.
24:141–155. 2000.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Clockaerts S, Bastiaansen-Jenniskens YM,
Runhaar J, Van Osch GJ, Van Offel JF, Verhaar JA, De Clerck LS and
Somville J: The infrapatellar fat pad should be considered as an
active osteoarthritic joint tissue: A narrative review.
Osteoarthritis Cartilage. 18:876–882. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang W, Liu Y, Yang C, Qi X, Li S, Liu C
and Li X: Mesoporous bioactive glass combined with graphene oxide
scaffolds for bone repair. Int J Biol Sci. 15:2156–2169.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zarella MD, Breen DE, Plagov A and Garcia
FU: An optimized color transformation for the analysis of digital
images of hematoxylin & eosin stained slides. J Pathol Inform.
6(33)2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Aigner T, Cook JL, Gerwin N, Glasson SS,
Laverty S, Little CB, McIlwraith W and Kraus VB: Histopathology
atlas of animal model systems-overview of guiding principles.
Osteoarthritis Cartilage. 18 (Suppl 3):S2–S6. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Seol D, Choe H, Zheng H, Jang K,
Ramakrishnan PS, Lim TH and Martin JA: Selection of reference genes
for normalization of quantitative real-time PCR in organ culture of
the rat and rabbit intervertebral disc. BMC Res Notes.
4(162)2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Baumann B, Hayashida T, Liang X and
Schnaper HW: Hypoxia-inducible factor-1α promotes
glomerulosclerosis and regulates COL1A2 expression through
interactions with Smad3. Kidney Int. 90:797–808. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Forsythe JA, Jiang BH, Iyer NV, Agani F,
Leung SW, Koos RD and Semenza GL: Activation of vascular
endothelial growth factor gene transcription by hypoxia-inducible
factor 1. Mol Cell Biol. 16:4604–4613. 1996.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Higgins DF, Biju MP, Akai Y, Wutz A,
Johnson RS and Haase VH: Hypoxic induction of Ctgf is directly
mediated by Hif-1. Am J Physiol Renal Physiol. 287:F1223–F1232.
2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Yoon KH, Tak DH, Ko TS, Park SE, Nam J and
Lee SH: Association of fibrosis in the infrapatellar fat pad and
degenerative cartilage change of patellofemoral joint after
anterior cruciate ligament reconstruction. Knee. 24:310–318.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Han W, Aitken D, Zhu Z, Halliday A, Wang
X, Antony B, Cicuttini F, Jones G and Ding C: Hypointense signals
in the infrapatellar fat pad assessed by magnetic resonance imaging
are associated with knee symptoms and structure in older adults: A
cohort study. Arthritis Res Ther. 18(234)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ding N, Wei B, Fu X, Wang C and Wu Y:
Natural products that target the NLRP3 inflammasome to treat
fibrosis. Front Pharmacol. 11(591393)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Takahashi I, Matsuzaki T, Kuroki H and
Hoso M: Induction of osteoarthritis by injecting monosodium
iodoacetate into the patellofemoral joint of an experimental rat
model. PLoS One. 13(e0196625)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Inomata K, Tsuji K, Onuma H, Hoshino T,
Udo M, Akiyama M, Nakagawa Y, Katagiri H, Miyatake K, Sekiya I, et
al: Time course analyses of structural changes in the infrapatellar
fat pad and synovial membrane during inflammation-induced
persistent pain development in rat knee joint. BMC Musculoskelet
Disord. 20(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ekundi-Valentim E, Santos KT, Camargo EA,
Denadai-Souza A, Teixeira SA, Zanoni CI, Grant AD, Wallace J,
Muscará MN and Costa SK: Differing effects of exogenous and
endogenous hydrogen sulphide in carrageenan-induced knee joint
synovitis in the rat. Br J Pharmacol. 159:1463–1474.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ashraf S, Mapp PI, Shahtaheri SM and Walsh
DA: Effects of carrageenan induced synovitis on joint damage and
pain in a rat model of knee osteoarthritis. Osteoarthritis
Cartilage. 26:1369–1378. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Egners A, Erdem M and Cramer T: The
response of macrophages and neutrophils to hypoxia in the context
of cancer and other inflammatory diseases. Mediators Inflamm.
2016(2053646)2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Semba H, Takeda N, Isagawa T, Sugiura Y,
Honda K, Wake M, Miyazawa H, Yamaguchi Y, Miura M, Jenkins DM, et
al: HIF-1α-PDK1 axis-induced active glycolysis plays an essential
role in macrophage migratory capacity. Nat Commun.
7(11635)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yabe Y, Hagiwara Y, Suda H, Ando A, Onoda
Y, Tsuchiya M, Hatori K and Itoi E: Joint immobilization induced
hypoxic and inflammatory conditions in rat knee joints. Connect
Tissue Res. 54:210–217. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhao K, Weng L, Xu T, Yang C, Zhang J, Ni
G, Guo X, Tu J, Zhang D, Sun W and Kong X: Low-intensity pulsed
ultrasound prevents prolonged hypoxia-induced cardiac fibrosis
through HIF-1α/DNMT3a pathway via a TRAAK-dependent manner. Clin
Exp Pharmacol Physiol. 48:1500–1514. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Costa V, Carina V, Conigliaro A, Raimondi
L, De Luca A, Bellavia D, Salamanna F, Setti S, Alessandro R, Fini
M and Giavaresi G: miR-31-5p is a LIPUS-mechanosensitive MicroRNA
that targets HIF-1α signaling and cytoskeletal proteins. Int J Mol
Sci. 20(1569)2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Yang PF, Li D, Zhang SM, Wu Q, Tang J,
Huang LK, Liu W, Xu XD and Chen SR: Efficacy of ultrasound in the
treatment of osteoarthritis of the knee. Orthop Surg. 3:181–187.
2011.PubMed/NCBI View Article : Google Scholar
|


















