Introduction
Chronic myeloid leukemia (CML) is a malignancy of
the blood and bone marrow that affects children and adults. An
abnormal chromosomal translocation known as t(9;22) results in the
establishment of the Philadelphia chromosome, which contains the
BCR-ABL gene, culminating in the development of this syndrome
(1). Due to the introduction of
imatinib, a tyrosine kinase inhibitor (TKI), CML patients now
benefit from treatment (2).
However, imatinib resistance has developed as a significant issue.
At least four generations of TKIs have been produced and
demonstrated to have therapeutic efficacy (3). However, not all TKI resistance issues
have been resolved.
The mechanism of resistance has been the subject of
numerous studies. Alterations in the BCR-ABL sequences are
one of the most common explanations (3). Additionally, several resistance
mechanisms, such as BCR-ABL genomic amplification, are
considered (4). Focusing on
preventing BCR-ABL activation is quite efficient, as imatinib has
demonstrated, but may not be the optimal option. Combining reagents
with distinct mechanisms of action may be the solution to the
problem of drug resistance (5).
Traditional medicine, which focuses on plants that
have medicinal effects, has attracted criticism from a wide range
of scientists. Herbal medicine has been shown to be effective
against cancer in a vast number of trials, however the specific
mechanism by which they work remains a mystery to scientists to
date. It could be a combination of acts with varying degrees of
variance (6). However, due to their
long history of use in the community, most herbal medicine is safe
for humans, with a few exceptions (7). As such, it represents a potential
source of effective therapeutic reagents for human use.
Artemisia vulgaris (A. vulgaris) is a
significant medicinal plant species belonging to the genus
Artemisia. It is most well-known for its volatile oils. Due
of the chemical and biological richness of the genus
Artemisia, it has garnered considerable interest. The
discovery and isolation of the promising antimalarial medication
artemisinin is one of the beneficial applications of A.
vulgaris (8,9). A. vulgaris has a long history
of being used to cure human illness. This medicinal plant is
anti-malarial, anti-inflammatory, anti-hypertensive, antioxidant,
anticancer, immunomodulatory, liver-protective, antispasmodic, and
anti-infection (9).
In previous research, the authors demonstrated that
the methanol extract of A. vulgaris (AVM) has the ability to
inhibit the viability of CML cells (10). The aim of present study was to
determine and clarify the mechanism of action of AVM. BCR/ABL
activation is present in >90% of CML cases (11). Thus, cells expressing different
forms of BCR/ABL were recruited for the present study, including
K562 [human wild-type (WT)] or TCCY-T315I [human imatinib-resistant
(IR)] and the Ba/F3-(T315I/E279K/Y253H) (mouse BCR/ABL point
mutation-tranfected cells).
Materials and methods
Plant materials and standard
extraction preparation
A. vulgaris was gathered in the southern
region of Vietnam (Bay Nui-An Giang). As mentioned in a previous
study, A. vulgaris was identified by herbalists at the
Traditional Medical Center in Tinh-Bien, An-Giang, Vietnam (voucher
no. BNAG-2017-0102) (10).
Drying of the samples was carried out in a dry oven
at a temperature of 40˚C until they were completely dry. Using a
blender, dry samples were ground to a fine powder. The samples were
then dissolved in methanol [1:10 (w/v)]. The powder and methanol
mixture was continuously swirled at room temperature for four days
before being filtered through a Whatman filter paper. To get crude
methanol extracts, filtrates were evaporated at 40˚C in a vacuum.
The powder was then weighed and diluted in methanol yielding stock
solutions of plant extracts (200 mg/ml). Subsequently, the solution
was divided into aliquots and stored at 20˚C until needed. The
crude methanol extract was saturated in water and then partially
fractionated with n-hexane (to generate the n-hexane fraction),
chloroform (to generate the chloroform fraction), ethyl acetate
solvent (to generate the ethyl acetate fraction), and finally
distilled water to reach the aqueous fraction.
Cell lines, culture conditions
Professor Yuko Sato (University of Tokyo, Tokyo,
Japan) provided the human leukemia cell lines TCCY-T315I, K562, and
Mus musculus (B cells) Ba/F3 cells; the Ba/F3 cells with E279K,
Y253H and T315I were created by the authors as previously described
(12). A humidified incubator (5%
CO2 at 37˚C) was used to grow Vero cells (ATCC CCL- 81™;
American Type Culture Collection) in DMEM and other cells
(TCCY-T315I, K562, Ba/F3-E279K/Y253H/T315I) in RPMI-1640 medium
(both from Sigma-Aldrich; Merck KGaA) supplied with 10%
heat-inactivated fetal bovine serum (Thermo Fisher Scientific,
Inc.) and 100 IU/ml penicillin and 0.1 mg/ml streptomycin.
Western blot analysis
At a density of 1x105 cells/ml, the cells
(K562 and TCCY-T315I) were plated onto a 10-cm dish with varied AVM
concentrations (25, 50 or 100 µg/ml). Cells were collected and
washed twice with PBS (-) after the specified incubation durations
(8, 16 or 24 h). On ice for 30 min, the cells were dissolved in a
protein lysis buffer
(Na2H2P2O7 10 mM, NaF
50 mM, EDTA 5 mM, Na3VO4 1 mM, HEPES 5 mM,
phenylmethylsulfonyl fluoride 1 mM, Triton X-100 0.01%, NaCl 150
mM, and aprotinin 75 µg/ml). Total protein cell lysates were then
obtained after centrifugation at 15,000 x g for 10 min at and 4˚C.
A total of 20 µg of total protein samples (determined by BCA
protein assay kit) were placed into wells and separated through
polyacrylamide gel electrophoresis (12.5%) and electroblotting onto
a Hypond-P membrane (Amersham; Cytiva). Subsequently, 5% skim milk
buffer was used to block the membrane for 1 h at room temperature.
Antibodies were employed to probe the membrane after washing, and
antibody binding was detected using enhanced chemiluminescence ECL
(Amersham; Cytiva). The following primary antibodies were used:
anti-c-Abl (cat. no. sc-23; dilution 1:500; Santa Cruz
Biotechnology, Inc.), anti-actin (cat. no. A2066; 1:1,000;
Sigma-Aldrich; Merck KGaA), caspase-3 (1:1,000; cat. no. 9662),
phosphorylated (p)-p44/42 MAPK (Thr202/Tyr204; 1:1,000; cat. no.
9101S), and AKT (1:1,000; cat. no. 9272; all from Cell Signaling
Technology, Inc.), and anti-PARP (1:1,000; cat. no. 016-16831;
FUJIFILM Wako Pure Chemical Corporation). The primary antibodies
were incubated for 1 h at room temperature or overnight at 4˚C.
Subsequently, the membranes were washed for 15 min, twice, and
incubated with horseradish peroxidase (HRP)-conjugated secondary
antibody [1:1,000 anti-mouse IgG HRP (cat. no. sc-2031) or
anti-rabbit IgG HRP (cat. no. sc-2317)] provided by Santa Cruz
Biotechnology, Inc. for 1 h at room temperature.
Cell viability
Cell viability was assessed using the trypan blue
dye exclusion test on suspension cells, as previously described
(13). The half maximal inhibitory
concentration (IC50) was determined by plotting a graph
between the various concentrations of AVM (6.25, 12.5, 25, 50 and
100 µg/ml) and the percentage of inhibition in cell viability. The
ratio of the IC50 value for Vero cells to the
IC50 value for cancer cell lines (K562, TCCY-T315I,
Ba/F3-T315I/Y253H/E279K) was used to construct the selectivity
index (SI). The SI values imply that the plant extracts kill
leukemia cells preferentially, rather than being non-selective
cytotoxic extracts. SI values >3 were considered to be highly
selective for cancer cells (14).
Morphological changes in AVM-treated
cells
Cells (K562, TCCY-T315I, Ba/F3-T315I/Y253H/E279K)
were seeded at a density of 1x105 cells/well in six-well
culture plates, overnight. Subsequently, the cells were treated
with various concentrations of AVM (50 and 100 µg/ml) and
maintained at 37˚C with 5% CO2 for 72 h. The cells that
were not treated acted as a control. A 10-fold
magnification-inverted light microscope was used to detect the
morphological changes in the cells.
Detection of DNA fragmentation
TCCY-T315I cells were treated with or without 50
µg/ml AVM for 3 days. Subsequently, the cells were harvested and
total genomic DNA was extracted using a standard procedure. A total
of 10 µg genomic DNA from each sample was blotted and
electrophoresed on a 1.2% agarose gel for the DNA fragmentation
experiment. UV light was used to detect DNA fragmentation.
Statistical analysis
Data were compiled from three independent
experiments and were presented as the mean ± SEM. Data were
compared using unpaired Student's t-test or one-way ANOVA with
Tukey's post hoc test. GraphPad Prism version 8.3.0 (GraphPad
Software, Inc.) was used to perform statistical analysis. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
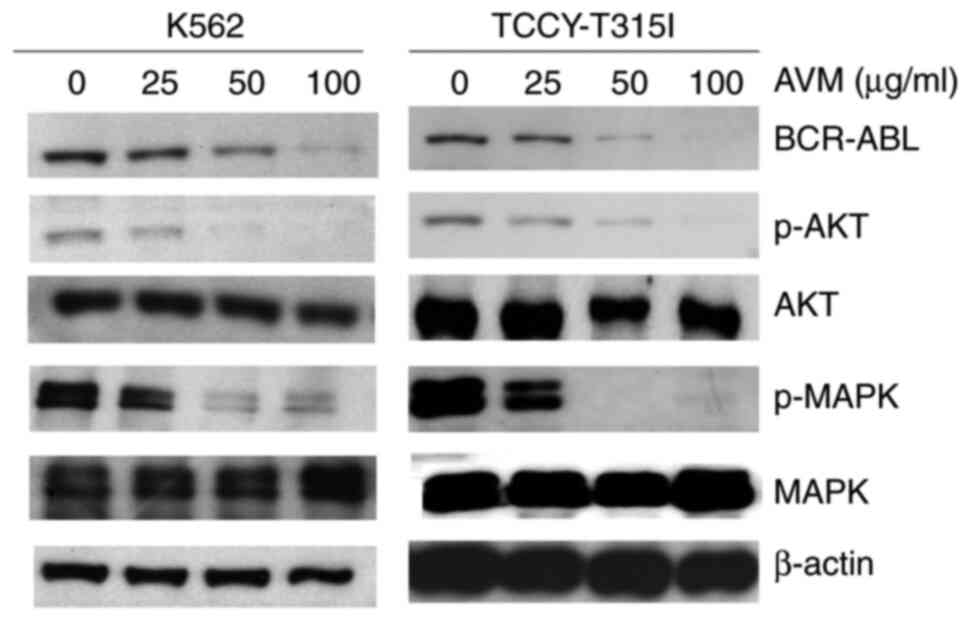
AVM suppresses the expression of
BCR-ABL
BCR/ABL activation is present in >90% of CML
cases (15). Thus, it was
investigated whether treatment with AVM had any effect on BCR-ABL
expression. For 24 h, K562 and TCCY-T315I cells were treated with
varying concentrations of the AVM extract. BCR-ABL1 is ~210 kDa in
size (11). As illustrated in the
western blot results of Fig. 1,
BCR-ABL expression was dose-dependently decreased. Additionally,
downstream signaling pathways of BCR/ABL, including as AKT and
MAPK, were impacted, consistent with BCR-ABL inhibition (Fig. 1). These results indicated that the
antileukemic activity of AVM may be mediated by disruption of the
BCR/ABL signaling cascade.
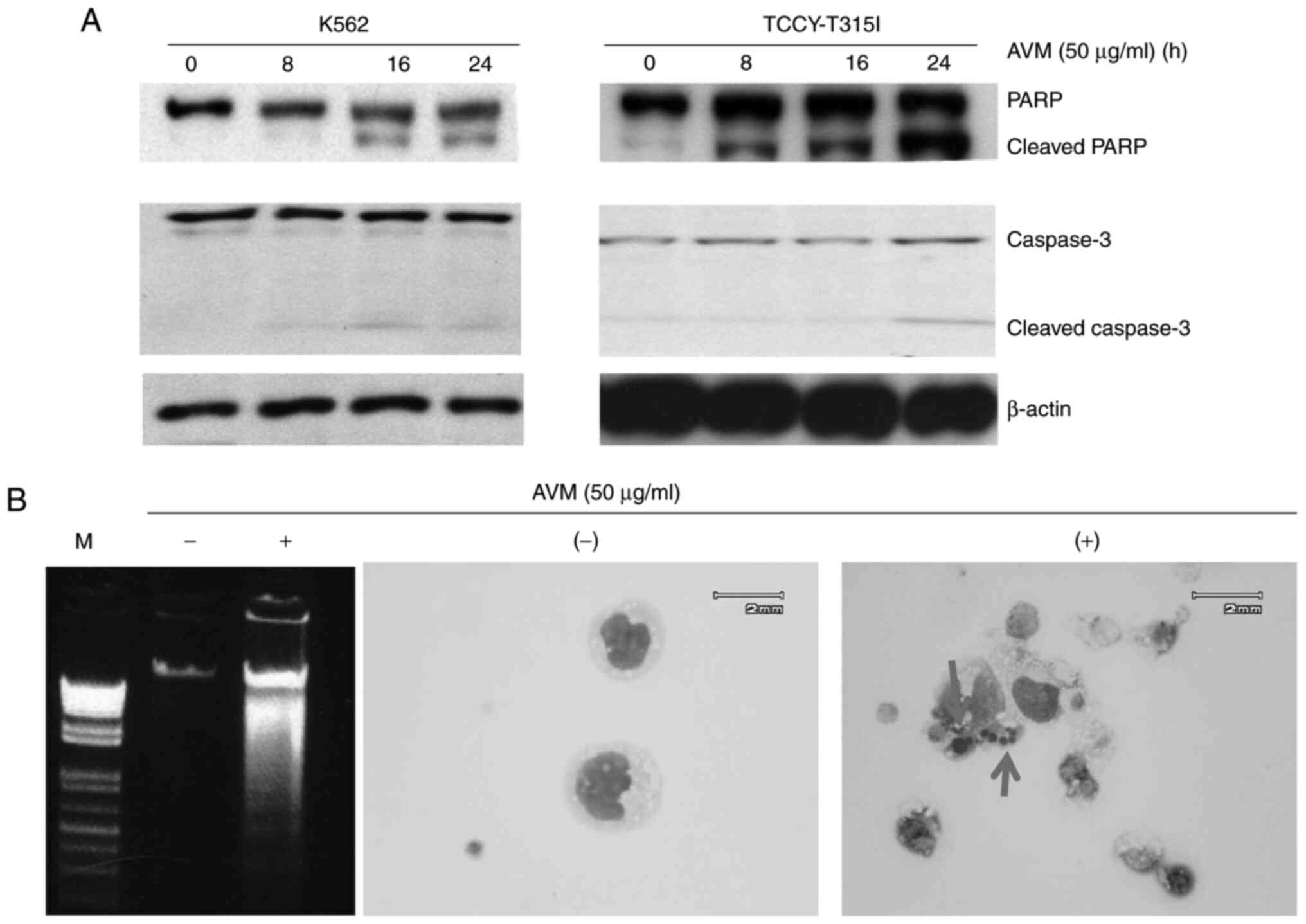
Cell apoptosis is induced by AVM
The purpose of the following experiments was to
determine whether treatment of cells with the AVM extract induces
apoptotic biomarkers such as caspase activation, DNA fragmentation,
and an intact nucleus in cells. K562 and TCCY-T315I cells were
co-cultured with 50 µg/ml of AVM for 24 h. Subsequently, western
blotting was performed to determine the effect of AVM on molecule
activation of the caspase pathway. It was observed that PARP and
caspase-3 molecules were cleaved (Fig.
2A), indicating that apoptosis occurred in leukemia cells
following AVM treatment. Moreover, the total DNA was extracted and
run through electrophoresis, and the results showed the
fragmentation of DNA (Fig. 2B, left
panel). To further demonstrate apoptosis, the nucleus of cells was
stained; as revealed in Fig. 2B
(right panel), the nucleus was broken in cells treated with AVM. In
comparison, control cells retained their nucleus (untreated with
AVM). Taken together, apoptosis was the form of cell death produced
by the AVM extract.
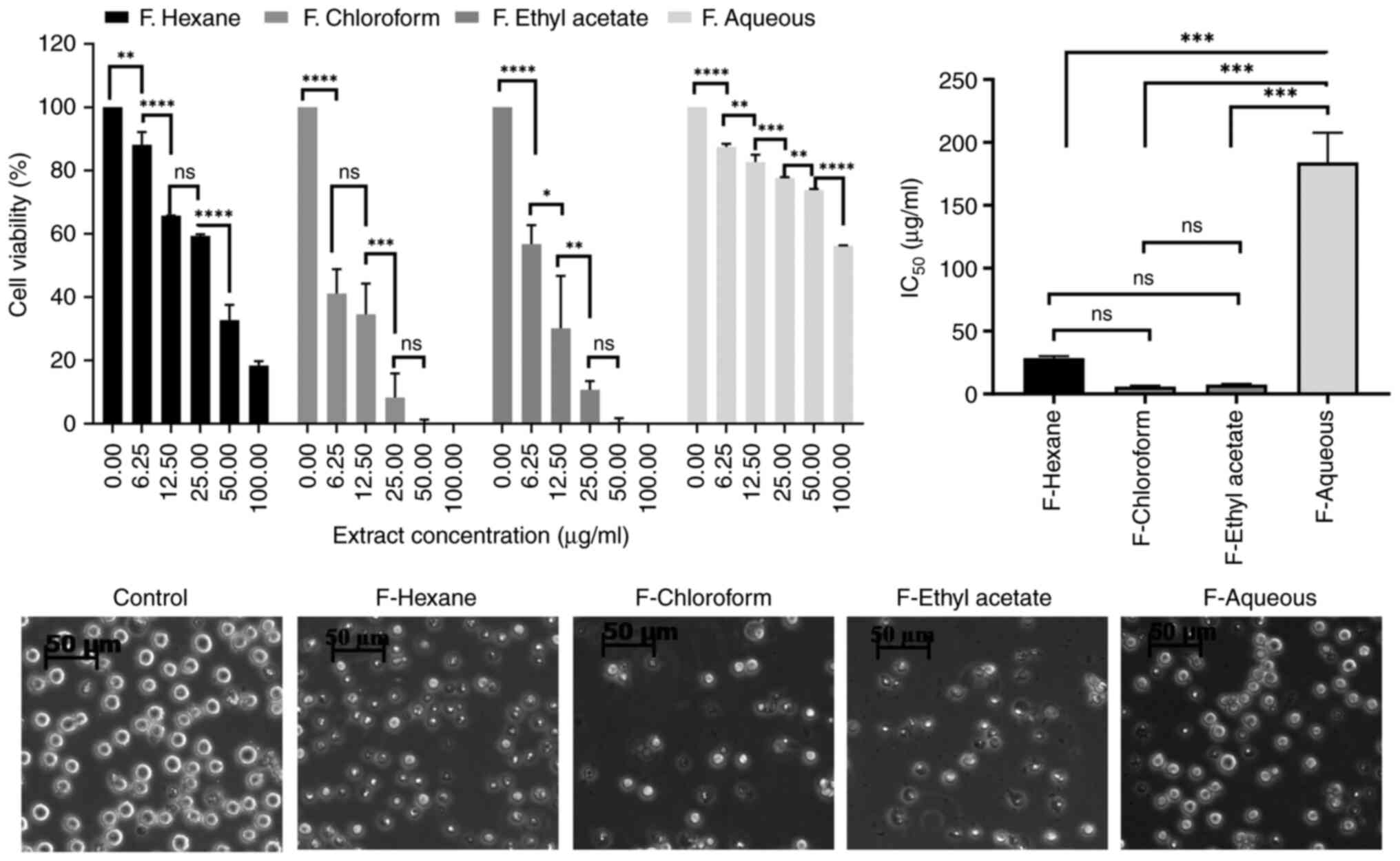
Chloroform and ethyl acetate fractions
of the AVM extract cause the main anti-leukemic effect
Since the AVM crude extract contains numerous
different components, it was dissolved into solvents ranging from
non-polar (n-hexane) to low polarity (chloroform), medium polarity
(ethyl acetate), and strong polarity (water), and generated
fractions, which were then assessed for toxicity to K562 cell
viablity. Interestingly, it was determined that the chloroform and
ethyl acetate fractions suppressed the viability of K562 cells very
effectively, whereas the aqueous fraction did not (Fig. 3).
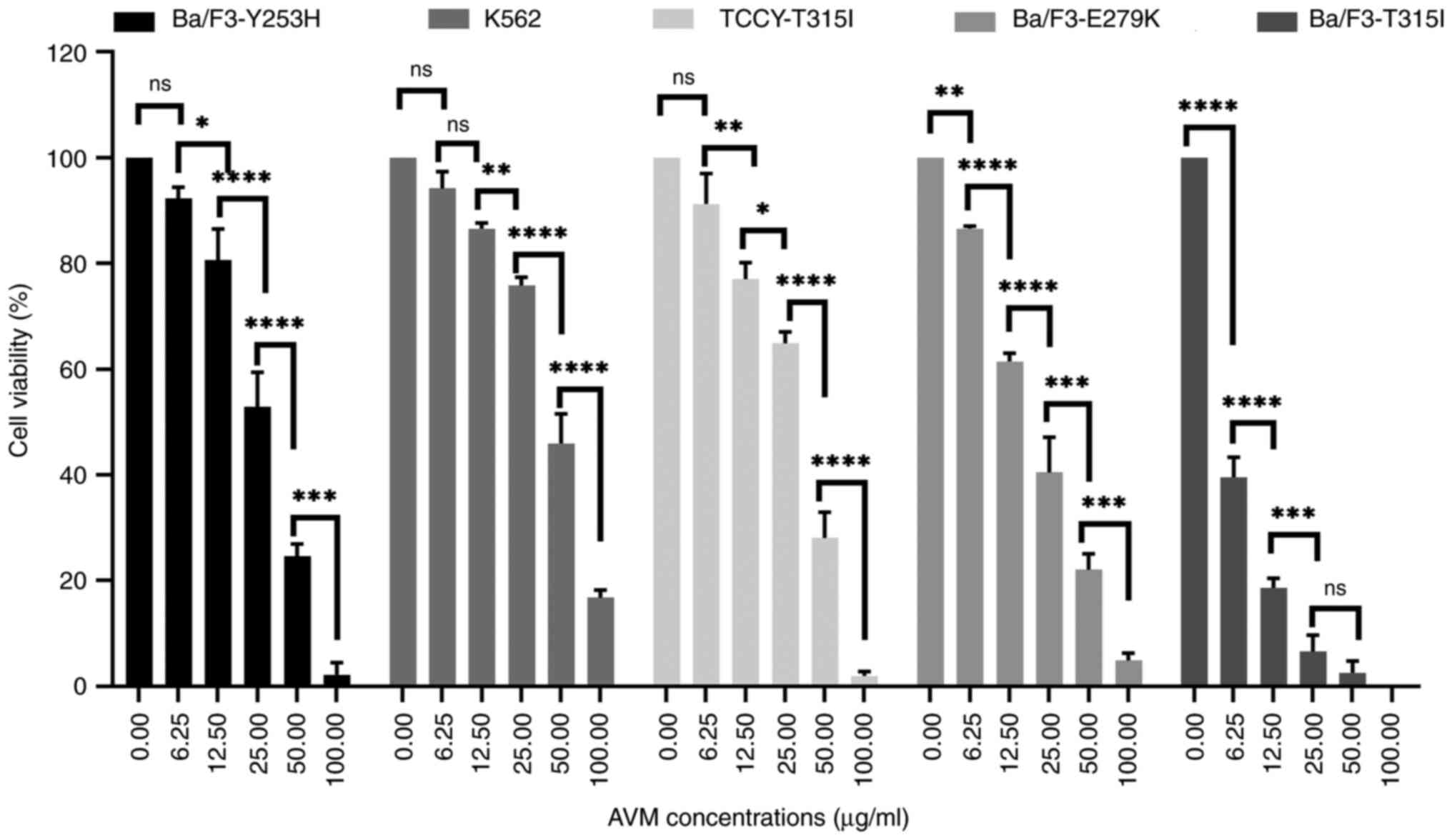
Potential of AVM to overcome
imatinib-resistance
Despite the marked success of imatinib in improving
survival rates (16), resistance
development continues to be a challenge. The mechanism of
resistance most frequently described is point mutations in the
BCR-ABL1 gene. Numerous studies indicate that BCR-ABL point
mutations are detected at a rate of 12-63% in imatinib-resistant
patients with CML. Over 90 different mutations have been found, but
~2/3 of mutated cases have amino acid substitutions in T315, Y253,
E255, M351, G250, F359, and H396 (17,18).
In this next experiment, cells with BCR/ABL point mutations
including human TCCY-T315I and stable murine-transfected Ba/F3
cells with BCR/ABL point mutations including T315I, Y253H, and
E279K, were used.
It was first verified whether AVM affected the
viability of imatinib-resistant cells. The viability rate of the
cells was determined using a trypan blue test. The results
demonstrated that the AVM extract inhibited the viability of K562
and TCCY-T315I cells (Fig. 4),
indicating that the AVM extract could suppress the viability of
human leukemic cells. Similar results were obtained with the murine
Ba/F3-(T315I/Y253H/E279K). This finding is significant because it
suggests that AVM may be able to overcome imatinib resistance,
allowing it to be used in the future as a new tyrosine kinase
inhibitor.
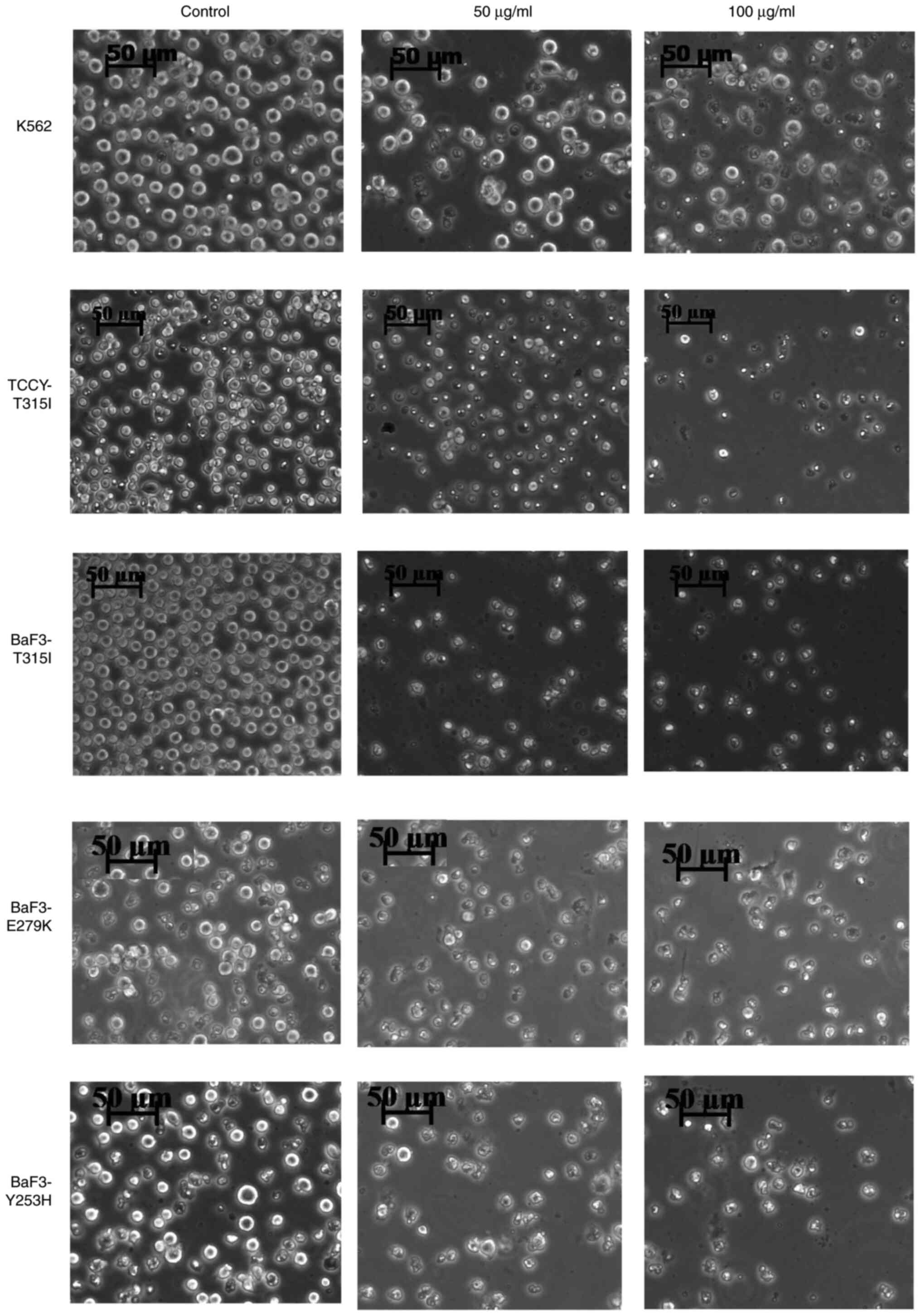
The morphological changes of these cells were
observed using a phase contrast inverted microscope following
treatment with AVM at concentrations of 50 as well as 100 µg/ml for
24 h. Cells appeared to have shrunken in size (Fig. 5). Interestingly, this finding
corresponds to the results presented in Figs. 2 and 4.
Effect of AVM on the viability of
TCCY-T315I cells in a time-dependent manner
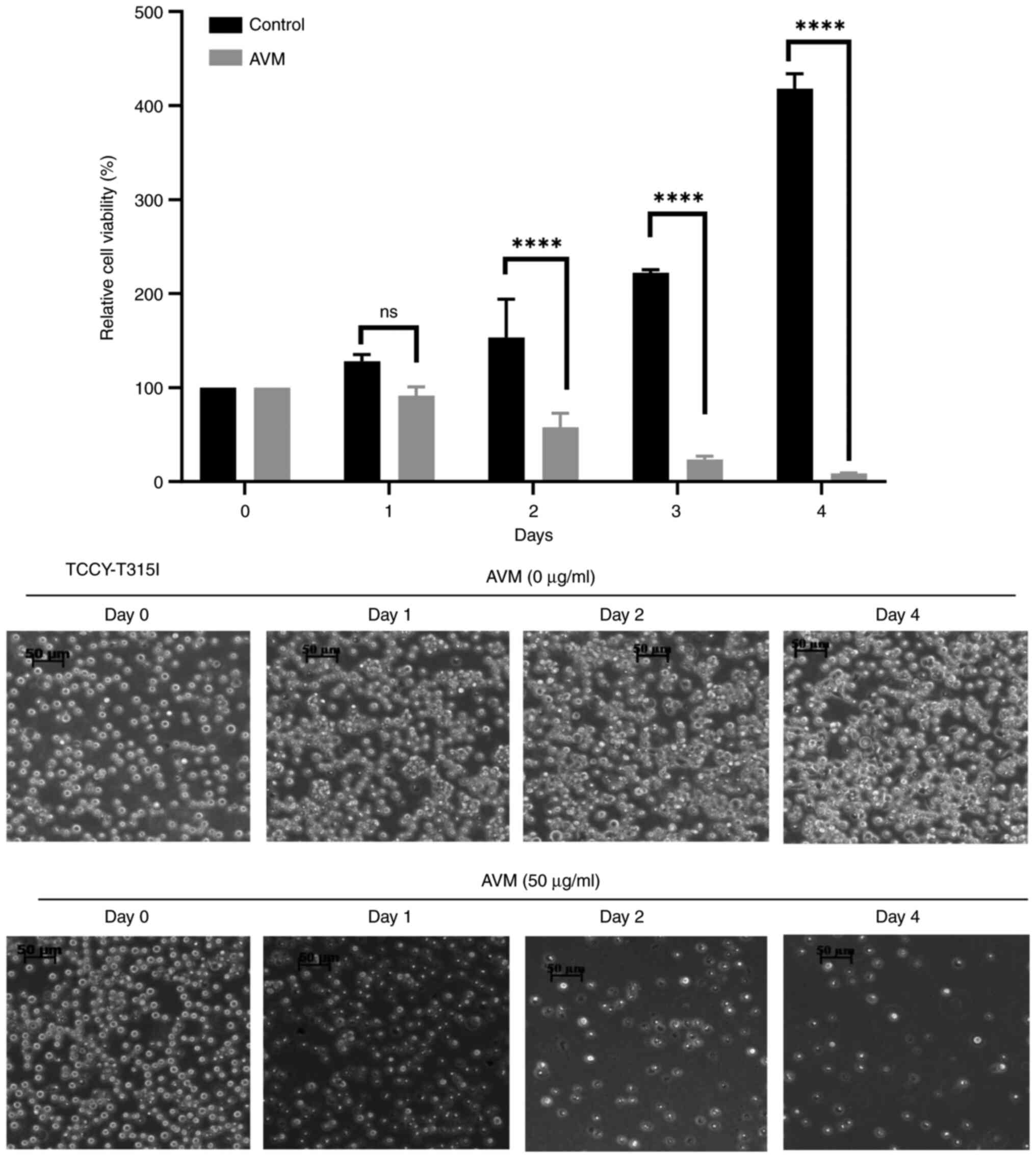
To determine whether AVM has a time-dependent effect
on cells, a time-dependent experiment with TCCY-T315I cells was
performed. The results indicated that cells treated with control
(methanol alone) continued to develop well up to the 4th day.
However, cells treated with 50 µg/ml AVM extract did not grow and
were almost completely attenuated by day 4 of culture (Fig. 6). This study demonstrated, once
again, that AVM may decrease cell viability in a dose- and
time-dependent manner, as previously demonstrated.
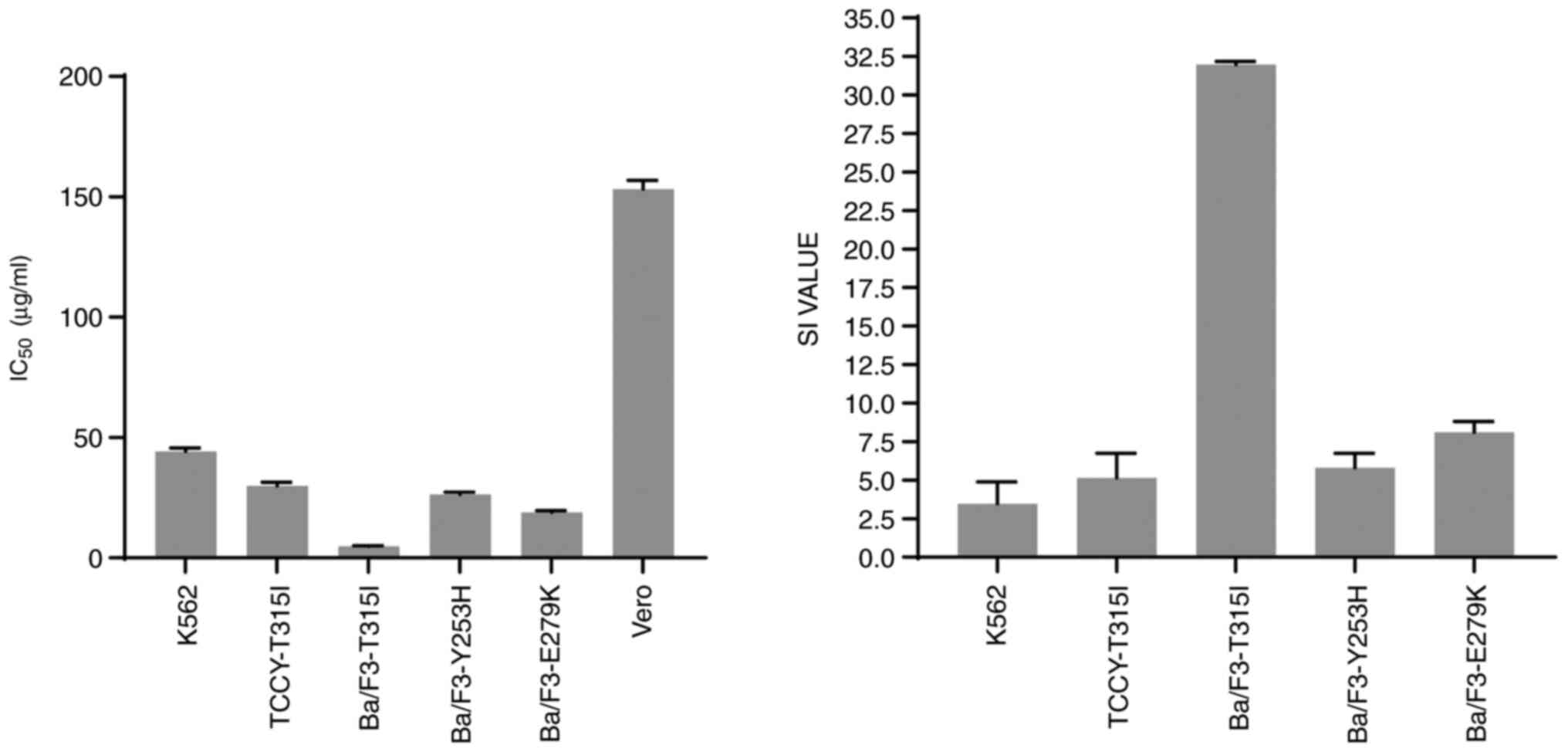
Because the SI value reflects the differential
behavior of the extract, the higher the SI value, the more
selective the extract. An SI number >3 units reflects the
general toxicity of the pure chemical (14). The SI value in this study was
calculated as the difference between the IC50 values of
the Vero cells and cancer cell lines. It was clear from the results
obtained that the Vero cells were the least sensitive to the action
of AVM extract compared with the other cell-lines (left panel,
Fig. 7). The AVM extract had SI
values >3 (Table I and Fig. 7, right panel), indicating that it
had a significant cytotoxic action and strong selectivity against
BCR-ABL leukemia cells.
 | Table IEffect of AVM on the cell
proliferation of leukemia cell lines, determined using the
IC50 value and SI. |
Table I
Effect of AVM on the cell
proliferation of leukemia cell lines, determined using the
IC50 value and SI.
| Leukemia cell
lines | IC50
(µg/ml) | SI |
|---|
| K562 | 44.27±1.42 | 3.46 |
| TCCY-T315I | 29.81±1.61 | 5.14 |
| Ba/F3-T315I | 4.78±0.19 | 31.98 |
| Ba/F3-Y253H | 26.34±0.94 | 5.81 |
| Ba/F3-E279K | 18.89±0.70 | 8.11 |
Discussion
Cancer is a leading cause of death worldwide, owing
to factors such as late diagnosis, poor prognosis, and resistance
to chemotherapy and radiation. A significant increase in
cancer-related mortality is projected in the following several
decades (19). Although treatment
with imatinib, for CML with BCR-ABL, or erlotinib, for non-small
cell lung cancer have been found to be effective, treatment
resistance continues to be a challenge that must be resolved in
order to provide improved healthcare (20,21).
Reagents for evaluating possible candidates for therapeutic use can
be found in natural chemicals generated from plants. In fact, a
significant number of cancer-fighting drugs are natural compounds
or are derived from natural substances (22). Artemisia santolina, Artemisia
kulbadica, Artemisia diffusa, Artemisia turanica and Artemisia
sieberi extracts demonstrated cytotoxic activity against a
variety of cancer cell lines (23).
Recently, it was demonstrated that AVM significantly decreases the
viability of a hepatocellular cancer cell line (24) Additionally, A. vulgaris
essential oil has been demonstrated to cause apoptosis in leukemic
cell line, HL-60(25). In the
present study, it was determined that AVM inhibited the development
of leukemia cells and induced death in CML cells. The present study
is consistent with a previous study (10). In a previous study, it was
discovered that AVM contains a significant amount of courmarin and
flavonoid (10). As a result, it is
reasonable to assert that these secondary metabolite groups
contributed to the anti-leukemic action of AVM.
Previous research has demonstrated that the
IC50 values of AVM vary greatly among cancer cell lines,
such as 50 ng/ml for human HCT-15 colon cancer (24), 100 mg/ml for hepatocellular
carcinoma (HepG2) (24), 190 µg/ml
for MCF7, 778 µg/ml for A549, 284 µg/ml for HeLa, 317 µg/ml for
A7R5, and 317 µg/ml for 293T cells (26). Compared with the preceding findings,
leukemia cells appear to be significantly more sensitive. Further
study in an in vivo model is required to further elucidate
the toxicity of AVM.
As was revealed, AVM may also alter the levels of
BCR-ABL, which in turn affects the stimulation of MAPK and AKT
(Fig. 1). The oncoprotein BCR-ABL
was revealed to play a role in the development of leukemia. BCR-ABL
inhibitors have been developed and successfully used in clinical
treatment based on earlier findings (27). Additional therapeutic strategies,
however, have aimed to decrease BCR-ABL expression in order to
inhibit BCR-ABL signals. In the present study it was demonstrated
that AVM treatment inhibits BCR-ABL. Subsequently, the downstream
signals of the BCR-ABL cascade may be impacted as well. This
finding may shed light on the mechanism by which AVM affects
leukemia cells.
Several important post-translational modifications,
such as ubiquitination, SUMOylation, phosphorylation, neddylation,
and acetylation, control how active and stable the BCR-ABL protein
is (28). It has been shown that
the ubiquitin-proteasome (UPP) pathway can be used to break down
the BCR-ABL protein (29). Protein
ubiquitination is a process that uses a bioactive enzyme called the
ubiquitin-conjugate enzyme and a protein called the ubiquitin
ligase (30). It has been reported
that the ubiquitin ligases CHIP11, c-CBL, and SH2-U-box12 cause
BCR-ABL to multiply and then break down. However, adding ubiquitin
to a protein is a dynamic process, and a certain enzyme called
deubiquitinase can remove ubiquitin molecules that have already
been attached (Dub) (31-33).
Several Dubs, such as USP25(31),
HAUSP (USP7) (32), and USP9x
(33), have been found to be linked
to BCR-ABL1. Recent research has revealed that Dub USP7 keeps
BCR-ABL and USP7/BCR-ABL from becoming unstable (32). USP7 interacts with the Y-kinase
domain of BCR-ABL. Due to this, USP7 and BCR-ABL can form a
positive feedback loop in which USP7 stabilizes BCR-ABL and BCR-ABL
phosphorylates and activates USP7, which further promotes the
pathophysiology of CML (32). Thus,
drugs that inhibit the function of USP7 could also be used to treat
CML.
Artesunate is a semisynthetic version of the active
ingredient in the Chinese herb Artemisia annua, which is
called artemisinin (34). It has
been shown that artesunate inhibited the interaction between USP7
and BCR-ABL. This led to more polyubiquitination of BCR-ABL, which
accelerated its breakdown. Notably, artesunate functioned well with
imatinib, which is the main drug used to treat CML and is a
specific inhibitor of BCR-ABL kinase, to cause CML cells to commit
suicide (apoptosis) (32).
Artemisinin is the primary bioactive compound in A.
vulgaris. (8). Thus, it is
considered that it is possible that AVM slows the viability of CML
cells through a mechanism that affects how USP7 and BCR-ABL work
together. However, this hypothesis needs to be assessed in more
detail to be confirmed.
It is important to note that AVM has a negligible
effect on Vero cells when compared to leukemia cells in the
laboratory (Fig. 7). In addition,
in the present study it was determined that AVM had a high
selective effect on aberrant leukemia cells or Ba/F3 cells that had
been transfected with the oncogene BCR-ABL (Fig. 7 and Table I). This finding suggests that AVM
may have potential in treatment for leukemia. However, additional
research is necessary before AVM can be applied in practice.
Acknowledgements
We would like to thank Professor Yuko Sato
(University of Tokyo, Japan) for providing the cell lines used in
the present study.
Funding
Funding: The present research was funded by Thu Dau Mot
University (Thu Dau Mot, Vietnam).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HTC designed the study and performed the
experiments. BTKL analyzed the data and wrote the manuscript. BTKL
and HTC confirm the authenticity of all the raw data. Both authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chereda B and Melo JV: Natural course and
biology of CML. Ann Hematol. 94 (Suppl 2):S107–S121.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hochhaus A, Larson RA, Guilhot F, Radich
JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F,
Fujihara S, et al: Long-term outcomes of imatinib treatment for
chronic myeloid leukemia. N Engl J Med. 376:917–927.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Waller CF: Imatinib mesylate. Recent
Results Cancer Res. 212:1–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chandran RK, Geetha N, Sakthivel KM,
Aswathy CG, Gopinath P, Raj TVA, Priya G, Nair JKKM and Sreedharan
H: Genomic amplification of BCR-ABL1 fusion gene and its impact on
the disease progression mechanism in patients with chronic
myelogenous leukemia. Gene. 686:85–91. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yurttas NO and Eskazan AE: Novel
therapeutic approaches in chronic myeloid leukemia. Leuk Res.
91(106337)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yan Z, Lai Z and Lin J: Anticancer
properties of traditional Chinese medicine. Comb Chem High
Throughput Screen. 20:423–429. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moreira D, Teixeira SS, Monteiro MHD,
De-Oliveira AC and Paumgartten FJR: Traditional use and safety of
herbal medicines1. Rev Bras Farmacogn. 24:248–257. 2014.
|
|
8
|
van Agtmael MA, Eggelte TA and van Boxtel
CJ: Artemisinin drugs in the treatment of malaria: From medicinal
herb to registered medication. Trends Pharmacol Sci. 20:199–205.
1999.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Abiri R, Silva ALM, de Mesquita LSS, de
Mesquita JWC, Atabaki N, de Almeida EB Jr, Shaharuddin NA and Malik
S: Towards a better understanding of Artemisia vulgaris:
Botany, phytochemistry, pharmacological and biotechnological
potential. Food Res Int. 109:403–415. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ly BTK, Ly DM, Linh PH, Son HK, Ha NL and
Chi HT: Screening of medicinal herbs for cytotoxic activity to
leukemia cells. J BUON. 25:1989–1996. 2020.PubMed/NCBI
|
|
11
|
Li S, Ilaria RL Jr, Million RP, Daley GQ
and Van Etten RA: The P190, P210, and P230 forms of the BCR/ABL
oncogene induce a similar chronic myeloid leukemia-like syndrome in
mice but have different lymphoid leukemogenic activity. J Exp Med.
189:1399–1412. 1999.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ly BTK and Chi HT: The potential effects
of green tea (-)-epigallocatechin-3-gallate on overcoming
imatinib-resistance in chronic myeloid leukemia bearing BCR-ABL. Ho
Chi Minh City University of Education: J Sci. 14:134–142. 2017.
|
|
13
|
Ly BT, Chi HT, Yamagishi M, Kano Y, Hara
Y, Nakano K, Sato Y and Watanabe T: Inhibition of FLT3 expression
by green tea catechins in FLT3 mutated-AML cells. PLoS One.
8(e66378)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mahavorasirikul W, Viyanant V,
Chaijaroenkul W, Itharat A and Na-Bangchang K: Cytotoxic activity
of Thai medicinal plants against human cholangiocarcinoma,
laryngeal and hepatocarcinoma cells in vitro. BMC Complement Altern
Med. 10(55)2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Arana-Trejo RM, Sánchez ER, Ignacio-Ibarra
G, de la Fuente EB, Garces O, Morales EG, Granados MC, Martínez RO,
Rubio-Borja ME, Anaya LS, et al: BCR/ABL p210, p190 and p230 fusion
genes in 250 Mexican patients with chronic myeloid leukaemia (CML).
Clin Lab Haematol. 24:145–150. 2002.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hehlmann R, Lauseker M, Saußele S,
Pfirrmann M, Krause S, Kolb HJ, Neubauer A, Hossfeld DK, Nerl C,
Gratwohl A, et al: Assessment of imatinib as first-line treatment
of chronic myeloid leukemia: 10-year survival results of the
randomized CML study IV and impact of non-CML determinants.
Leukemia. 31:1476–5551. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Branford S, Melo JV and Hughes TP:
Selecting optimal second-line tyrosine kinase inhibitor therapy for
chronic myeloid leukemia patients after imatinib failure: Does the
BCR-ABL mutation status really matter? Blood. 114:5426–5435.
2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Apperley JF: Part I: Mechanisms of
resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol.
8:1018–1029. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bray F, Laversanne M, Weiderpass E and
Soerjomataram I: The ever-increasing importance of cancer as a
leading cause of premature death worldwide. Cancer. 127:3029–3030.
2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mauro MJ and Druker BJ: STI571: Targeting
BCR-ABL as therapy for CML. Oncologist. 6:233–238. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Herbst RS: Erlotinib (Tarceva): An update
on the clinical trial program. Semin Oncol. 30:34–46.
2003.PubMed/NCBI
|
|
22
|
Cragg GM and Pezzuto JM: Natural products
as a vital source for the discovery of cancer chemotherapeutic and
chemopreventive agents. Med Princ Pract. 25 (Suppl 2):S41–S59.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Abad MJ, Bedoya LM, Apaza L and Bermejo P:
The artemisia L. Genus: A review of bioactive essential oils.
Molecules. 17:2542–2566. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sharmila K and Padma PR: Anticancer
activity of Artemisia vulgaris on hepatocellular carcinoma
(HepG2) cells. Int J Pharmacy Pharm Sci. 5:479–483. 2013.
|
|
25
|
Saleh AM, Aljada A, Rizvi SA, Nasr A,
Alaskar AS and Williams JD: In vitro cytotoxicity of Artemisia
vulgaris L. essential oil is mediated by a
mitochondria-dependent apoptosis in HL-60 leukemic cell line. BMC
Complement Altern Med. 14(226)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Erel SB, Şenol SK, Köse FA and Ballar P:
In vitro cytotoxic properties of six Artemisia L. species. Turk J
Pharm Sci. 8:247–252. 2011.
|
|
27
|
Deguchi Y, Kimura S, Ashihara E, Niwa T,
Hodohara K, Fujiyama Y and Maekawa T: Comparison of imatinib,
dasatinib, nilotinib and INNO-406 in imatinib-resistant cell lines.
Leuk Res. 32:980–983. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu C, Nie D, Li J, Du X, Lu Y, Li Y, Zhou
J, Jin Y and Pan J: Antitumor effects of blocking protein
neddylation in T315I-BCR-ABL leukemia cells and leukemia stem
cells. Cancer Res. 78:1522–1536. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Burslem GM, Schultz AR, Bondeson DP, Eide
CA, Stevens SL, Druker BJ and Crews CM: Targeting BCR-ABL1 in
chronic myeloid leukemia by PROTAC-mediated targeted protein
degradationdual inhibition and degradation of BCR-ABL1. Cancer Res.
79:4744–4753. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Huang H, Weng H, Dong B, Zhao P, Zhou H
and Qu L: Oridonin triggers chaperon-mediated proteasomal
degradation of BCR-ABL in leukemia. Sci Rep. 7:1–12.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shibata N, Ohoka N, Tsuji G, Demizu Y,
Miyawaza K, Ui-Tei K, Akiyama T and Naito M: Deubiquitylase USP25
prevents degradation of BCR-ABL protein and ensures proliferation
of Ph-positive leukemia cells. Oncogene. 39:3867–3878.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jiang S, Wang X, He Y, Huang H, Cao B,
Zhang Z, Liu J, Wang Q, Huang Z and Mao X: Suppression of USP7
induces BCR-ABL degradation and chronic myelogenous leukemia cell
apoptosis. Cell Death Dis. 12(456)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Carrà G, Panuzzo C, Crivellaro S, Morena
D, Taulli R, Guerrasio A, Saglio G and Morotti A: The targetable
role of herpes virus-associated ubiquitin-specific protease (HAUSP)
in p190 BCR-ABL leukemia. Oncol Lett. 12:3123–3126. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI View Article : Google Scholar
|