Introduction
Immunodeficiency leads to the onset of cancer and
the development of infectious diseases, and increased mortality
from these diseases has become a medical and social problem. It is
considered that there is a positive correlation between aging and
the incidence of cancer, which may be in part due to prolonged
exposure periods to cancerous substances, genetic changes with
aging, and the decrease in immunity of the aging population.
Various immune cells, such as T cells, B cells, and natural killer
(NK) cells, are involved in immunity. These immune cells originate
from hematopoietic stem cells (HSCs), which sparsely exist in the
bone marrow. HSCs with pluripotency and self-renewal ability
differentiate into all types of immune cells, thereby supplying
immune cells continuously for a lifetime (1). However, both proliferative and
differentiating abilities of HSCs decrease with age, contributing
to the decline of immunity with age (2-4).
Ganoderma, one of the most well-known
medicinal mushrooms, has various physiological functions such as
immunomodulatory, antitumor, hypolipidemic, antidiabetic, and
antiarteriosclerotic effects (5-9).
Ganoderma is generally extracted with hot water, and the
efficacy of Ganoderma is mostly confirmed with hot water
extracts. Furthermore, the use of subcritical water with a higher
temperature and pressure than hot water has recently attracted
attention as an extraction technique for natural products.
Subcritical water with a high temperature (100-374˚C) and high
pressure allows efficient elution of components from natural
products and extraction of relatively low-polarity components and
low-molecular-weight peptides generated by hydrolysis (10-12).
The immunomodulatory effects of the hot water
extract of Ganoderma have already been reported (13,14).
However, the effects of subcritical water extracts on the immune
system have not been investigated in detail. Particularly, few
studies have been published on the effects of subcritical water
extracts on HSCs. Thus, the present study investigated the effects
of subcritical water extract of Ganoderma (SWEG) on
immunity.
Materials and methods
Antibodies
Antibodies against CD34-FITC (cat. no. 11-0341-82),
Sca1-PE (cat. no. 12-5981-82), CD117-APC (cat. no. 17-1171-82),
CD11b-biotin (cat. no. 13-0112-82), Gr1-biotin (cat. no.
13-5931-82), B220-biotin (cat. no. 13-0452-82), TER119-biotin (cat.
no. 13-5921-82), and streptavidin-PE-Cy7 (cat. no. 25-4317-82) were
purchased from Thermo Fisher Scientific, Inc. Biotinylated
antibodies were detected using streptavidin-PE-Cy7. Antibodies
against CD19-FITC (cat. no. 115505), NKp46-PE (cat. no. 137603),
and CD3e-AF488 (cat. no. 300319) were obtained from BioLegend,
Inc.
Preparation and molecular weight
analysis of SWEG
Ganoderma [mixture of fruiting bodies of
Ganoderma lucidum (GL) and Ganoderma sinense (GS);
Nikkei Co., Ltd.] was subjected to extraction in a container for
subcritical water treatment at 140-180˚C. Following filtration of
the extract, the filtrate was concentrated and lyophilized to
prepare SWEG. Ganoderma was then extracted with hot water at
95-100˚C, and filtered, concentrated, and lyophilized to prepare a
hot water extract of Ganoderma (HWEG) as a reference sample
for comparison of chemical properties with SWEG. As reference
samples for comparison of the efficacy tests using cultured cells,
hot water extracts of GL and GS were also prepared as
aforementioned.
The molecular weights of SWEG and HWEG were measured
using gel filtration HPLC. Develosil 100-Diol-5 (particle diameter
5 µm, 8.0 mmφ x 500 mm, Nomura Chemical Co., Ltd.) was used as a
column, and Shimadzu RID-10A as a detector (Shimadzu Corporation).
The system conditions were as follows: The mobile phase consisted
of 0.1 M phosphate buffer solution (pH 6.8); the required pH of the
solution was prepared by mixing 0.1 M Na2HPO4
and 0.1 M NaH2PO4 solutions; the injected
volume was 100 µl at a flow rate of 1.0 ml/min; and the column
temperature was set at 25˚C. Pullulan (Molecular Weight Markers for
Gel Filtration Chromatography; cat. no. 53168; Sigma-Aldrich; Merck
KGaA) was employed as a standard sample for the molecular
weight.
Measurement of β-glucan contents
α-Amylase (cat. no. 635-53982; FUJIFILM Wako Pure
Chemical Corporation), protease (cat. no. P5380; Sigma-Aldrich;
Merck KGaA), and amyloglucosidase (cat. no. A9913; Sigma-Aldrich;
Merck KGaA) were added to SWEG and HWEG, dissolved in 50 mM
phosphate buffer, for the enzymatic decomposition of macromolecules
other than β-glucan. Subsequently, ethanol was added at 4-fold the
amount of the reaction solution to precipitate β-glucan. To this
precipitate, sulfuric acid was added for acid-hydrolysis of
β-glucan. Glucose contained in this solution was quantified by
Glucose Assay Kit-WST (cat. no. 346-09411; Dojindo Laboratories,
Inc.) to calculate the β-glucan contents (%) in SWEG and HWEG
(15).
Cells
A-6 cells (ES derived; cell no. RCB1517) were used
as a model of HSCs (16,17). YAC-1 cells (cell no. RCB1165) were
used as a target for the measurement of NK cell activity. A-6 cells
and YAC-1 cells were provided by the RIKEN BRC through the National
BioResource Project of the MEXT/AMED, Japan.
Cell viability test
A-6 cells were suspended in DMEM/F12 (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 1% fetal bovine serum
(FBS) (Sigma-Aldrich; Merck KGaA), 1% antibiotic-antimycotic
solution (Gibco; Thermo Fisher Scientific, Inc.), 10 µg/ml human
transferrin (Sigma-Aldrich; Merck KGaA), 10 µg/ml human insulin
(Sigma-Aldrich; Merck KGaA), 100 µM 2-mercaptoethanol (Gibco;
Thermo Fisher Scientific, Inc.), and 5 ng/ml fibroblast growth
factor 2 (PeproTech, Inc.). The suspension (5x104 cells)
was seeded into a 96-well plate and incubated at 37˚C. At 24 h
after seeding, SWEG, GL, or GS were added at concentrations of 40,
80, 160, and 325 µg/ml, followed by culturing for an additional 24
h at 37˚C. The cell proliferation-promoting effects of each of the
extracts was examined by cell viability assay with Cell Counting
Kit-8 (CCK-8; cat. no. 343-07623; Dojindo Laboratories, Inc.).
Following the addition of 10 µl CCK-8 solution into each well, the
cells were incubated for 2 h at 37˚C, and then the absorbance of
each well was detected at a wavelength of 450 nm.
Cell differentiation induction
test
A-6 cells were suspended in each differentiation
induction media for T cells, B cells, and NK cells (18-20).
T cell differentiation medium consisted of DMEM/F12 medium
supplemented with 20% FBS, 1% antibiotic-antimycotic solution, 10
µg/ml human transferrin, 10 µg/ml human insulin, 100 µM
2-mercaptoethanol, 30 ng/ml FMS-related tyrosine kinase 3 ligand
(Flt3L) (GenScript), 30 ng/ml stem cell factor (SCF) (NKMAX Co.,
Ltd.), and 30 ng/ml interleukin-7 (IL-7) (GenScript). B cell
differentiation medium consisted of DMEM/F12 medium supplemented
with 20% FBS, 1% antibiotic-antimycotic solution, 10 µg/ml human
transferrin, 10 µg/ml human insulin, 100 µM 2-mercaptoethanol, 50
ng/ml SCF, 50 ng/ml Flt3L, 10 ng/ml IL-3 (GenScript), and 20 ng/ml
IL-7. NK cell differentiation medium consisted of DMEM/F12 medium
supplemented with 20% FBS, 1% antibiotic-antimycotic solution, 10
µg/ml human transferrin, 10 µg/ml human insulin, 100 µM
2-mercaptoethanol, 10 ng/ml Flt3L, 20 ng/ml SCF, 10 ng/ml IL-15
(GenScript), 5 ng/ml IL-3, and 20 ng/ml IL-7. Each suspension
(7.5x105 cells) was seeded into a 12 well plate and
incubated for 7 days at 37˚C. During the 7-day differentiation
induction, SWEG, GL, or GS were added at 325 µg/ml. Each of the
media was once changed on the 3rd day. Subsequently, 7 days later,
total RNA was extracted from the cells using RNAiso Plus (cat. no.
9109; TaKaRa Bio, Inc.) solution for the expression analysis of
marker genes, Cd3e, Ptprc, and Id3,
characteristic of T cells, B cells, and NK cells, respectively, by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) to examine the promoting effects of differentiation
induction of the each of the extracts. As positive controls,
high-dose cytokines for each differentiation media were added,
i.e., 50 ng/ml IL-7 for T cell differentiation medium, 20 ng/ml
IL-3 and 50 ng/ml IL-7 for B cell differentiation medium, and 20
ng/ml IL-15 and 50 ng/ml IL-7 for NK cell differentiation medium,
respectively.
Animal breeding
A total of 17 female, 12-week-old mice weighing
35-45 g were purchased from Japan SLC, Inc. A controlled housing
environment, at 23±1˚C with humidity at 55±15%, was used to
maintain the animals, with alternate 12-h light/dark cycles. A
commercial pellet diet and tap water were provided ad
libitum. The animal study protocols were approved (approval no.
HS201708) by the Animal Experiment Committee of Nippon Menard
Cosmetic Co., Ltd.
Administration of SWEG and tissue
removal
After 1 week of preliminary breeding, the mice were
divided into control (n=9) and SWEG (n=8) groups. The SWEG group
was fed ad libitum with MF feed (Oriental Yeast Co., Ltd.)
supplemented with 2% SWEG, and the control group was fed ad
libitum with MF feed supplemented with 2% cornstarch (FUJIFILM
Wako Pure Chemical Corporation) instead of SWEG for 30 days.
Regarding the breeding period, in previous studies using mice, GL
was confirmed to promote NK cell activity at oral administration
for 30 days (21), and Ganoderma
formosanum was also confirmed to promote the gene expression
related to immune function of the spleen at oral administration for
32 days (22). With reference to
these studies, the breeding period was set to 30 days in this
study. Following breeding with SWEG-supplemented feed,
pentobarbital sodium (200 mg/kg body weight, ip) was used for
euthanasia. When the animal ceased breathing and no heartbeat was
detected, the femurs, thymus, and spleen were removed. Bone marrow
cells collected from the femurs and the thymus cells were used to
conduct the population analysis of immune cells. Spleen cells
collected from the spleen were used to examine the immune
functions. Furthermore, the spleen was partially cut into small
pieces and homogenized in RNAiso Plus (TaKaRa Bio, Inc.) solution
for RNA extraction to analyze gene expression relevant to NK cell
activity by RT-qPCR.
Population analysis of immune cells by
flow cytometer (FCM)
The femurs removed from the mice were resected at
both ends to collect bone marrow cells. To calculate the number of
immune cells in the bone marrow, the surface antigens of the
collected bone marrow cells were analyzed using FACSAria flow
cytometer and FlowJo software (version 10.5.3) (Becton, Dickinson
and Company) to identify the cell types. Bone marrow cells
(0.5-1.0x107 cells) were stained with aforementioned
antibodies in 0.1% BSA containing PBS buffer at 4˚C for 30 min.
Each analysis was assessed with 1.0x106 cells on the
FCM. Cells identified as CD34-, Sca1+,
CD117+, lineage- (Lin-) were
analyzed as HSCs, those identified as Sca1+,
CD117+, Lin- as hematopoietic precursor cells
(HPCs), those identified as B220+, CD19- as
immature B cells, and those identified as NKp46+ as NK
cells. The lineage marker was defined as the combination of the
following antibodies: CD3e (Clone: 145-2C11), CD11b (Clone: M1/70),
Gr1 (Clone: RB6-8C5), B220 (Clone: RA3-6B2), and TER119 (Clone:
TER-119).
Furthermore, the thymus removed from the mice was
ground on a metal mesh and suspended in 0.1% BSA containing PBS
buffer. The thymus cells (0.5-1.0x107 cells) were
stained with CD3e antibody at 4˚C for 30 min to calculate the
number of T cells by FCM as aforementioned. Cells identified as
CD3e+ were analyzed as T cells.
NK cell activity and cytokine
expression
To prepare the spleen cells, the spleen was ground
on a metal mesh and suspended in RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% FBS. The spleen
cells (1x106 cells) were seeded into a U-shaped 96-well
plate, to which 2.5x104 target cells (YAC-1) were added,
followed by culturing at 37˚C for 20 h. Subsequently, the 96-well
plate was centrifuged at 250 x g for 10 min at 25˚C, and the
supernatant was subjected to assessment of lactate dehydrogenase
activity with an LDH Cytotoxicity Detection Kit (cat. no. MK401;
TaKaRa Bio, Inc.), to determine NK cell activity, according to the
manufacturer's instructions. Furthermore, granzyme B and
interferon-gamma (IFN-γ) in the culture supernatant of spleen cells
were quantified using an enzyme-linked immunosorbent assay (ELISA)
kit (for granzyme B, cat. no. ELM-GranzymeB-1; for IFN-γ, cat. no.
ELM-IFNg-1; RayBiotech Life, Inc.). The expression levels of mRNAs
relevant to NK cell activity were determined using RT-qPCR to
examine the effects of SWEG on transcription. In addition,
correlation analysis between granzyme B and IFN-γ expression and NK
cell activity in the spleen for all mice, including both control
and SWEG groups, were carried out. Measured values of granzyme B
and IFN-γ expression and NK cell activity were plotted, calculating
Pearson's product-moment correlation coefficients to reveal which
cytokine was more closely related to NK cell activity.
RT-qPCR
First strand cDNA synthesis was performed with the
RNA as the template (500 ng) using High Capacity RNA-to-cDNA Kit
(cat. no. 4374967; Thermo Fisher Scientific, Inc.). Reverse
transcription was performed at 37˚C for 60 min and then at 95˚C.
qPCR amplification was performed using SYBR Select Master Mix (cat.
no. 4472919; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were: Denaturation at 95˚C for 15 sec, annealing and
extension at 60˚C for 60 sec for 40 cycles. qPCR was performed
using Step One Plus Real-Time PCR System (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the
internal control. The results were calculated using the
2-ΔΔCq method (23), normalized to GAPDH mRNA levels and
reported as the relative fold change. Primers synthesized by Nippon
Gene Co., Ltd. were used for mRNA amplification. The sequences of
the primers are listed in Table
SI.
Statistical analysis
All experimental results are expressed as the means
± standard error (SE). Statistical analyses were conducted in R
(Version 3.6.0). Differences between two groups were assessed using
unpaired Student's t-test. Differences between multiple
groups were compared using one-way ANOVA with post-hoc Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference. The analysis of correlations between
granzyme B and IFN-γ expression and NK cell activity was based on
Pearson's product-moment correlation coefficient. Correlation
coefficients were graded as follows: Low (r<0.5), moderate
(0.5≤r<0.7), and high (r≥0.7) (24,25).
P<0.05 indicated a significant correlation.
Results
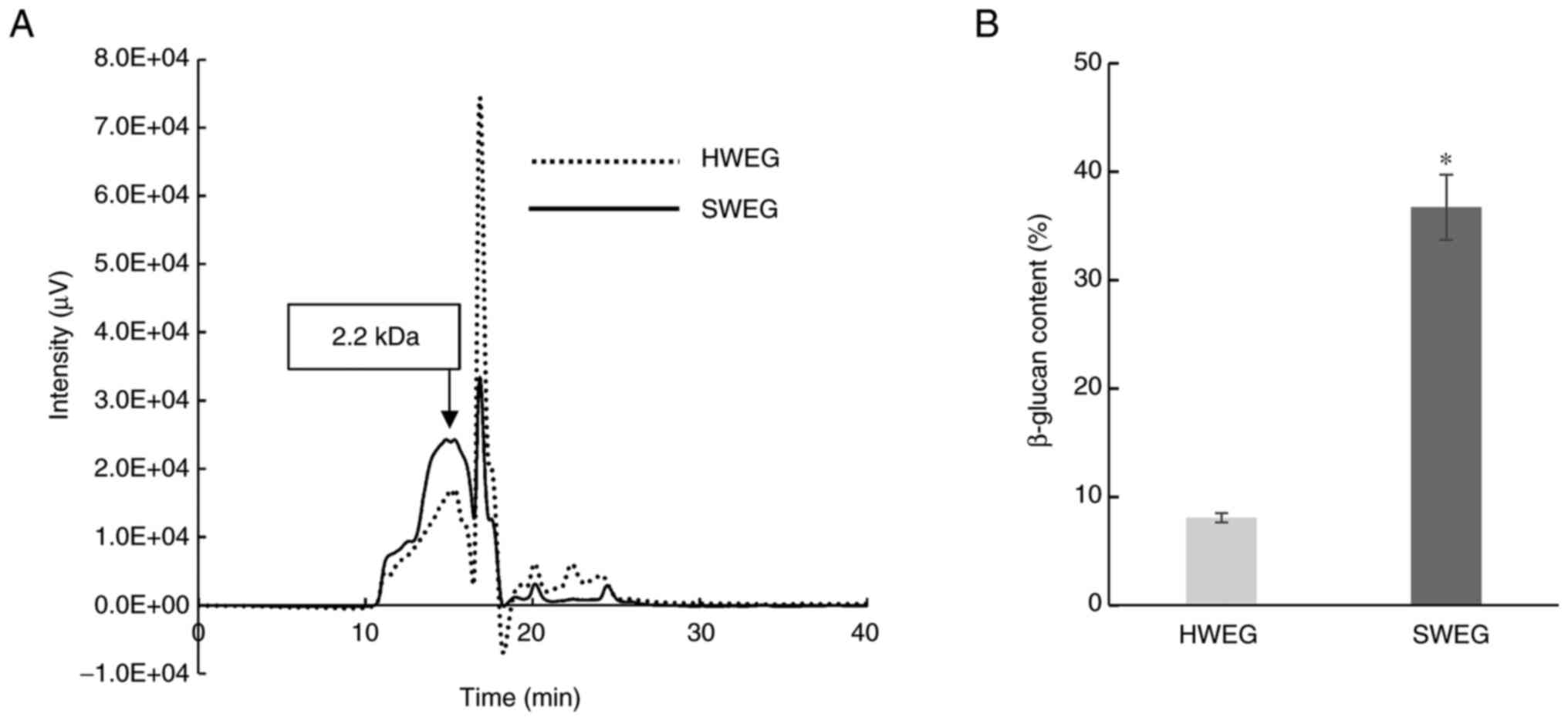
Comparison of chemical properties
between SWEG and HWEG
The distributions of the molecular weights of SWEG
and HWEG were examined using pullulan as the standard sample of
molecular weight, demonstrating an increase in the peak of ~2.2 kDa
for SWEG (Fig. 1A). The β-glucan
contents in SWEG and HWEG were 36.7 and 8.1%, respectively
(Fig. 1B). The partial degradation
and conformation changes of β-glucan have been reported after heat
treatment (26), hence these
differences in SWEG and HWEG appear to have occurred due to the
thermal energy of the extraction temperature.
The ~18-min HWEG signal below zero in Fig. 1A (dotted line) may be caused by
bubbles dissolved in the mobile phase solution or sample solution
used for HPLC analysis. This may be caused due to insufficient
deaeration in the mobile phase solution or sample solution.
Effects on the self-renewal and
differentiation abilities of HSCs
A-6 cells cultured for 1 day after the addition of
SWEG exhibited enhanced cell viability in a concentration-dependent
manner, as compared with the control group (Fig. 2A). Since the addition of 325 µg/ml
SWEG exhibited a clear effect on the cell viability, this
concentration (325 µg/ml) was applied to the subsequent cell
differentiation test. As a result, the addition of SWEG to A-6
cells during differentiation induction promoted the expression of
marker genes, Cd3e and Id3, characteristic of T cells
and NK cells, respectively (Fig.
2B). Thus, SWEG promoted both self-renewal and differentiation
into immune cells in the A-6 cells, whereas GL and GS had little
effect. Regarding the gene expression analysis conducted on
differentiation induction test in the A-6 cells, high-dose
cytokines as positive controls also promoted the differentiation
into immune cells (Fig. S1).
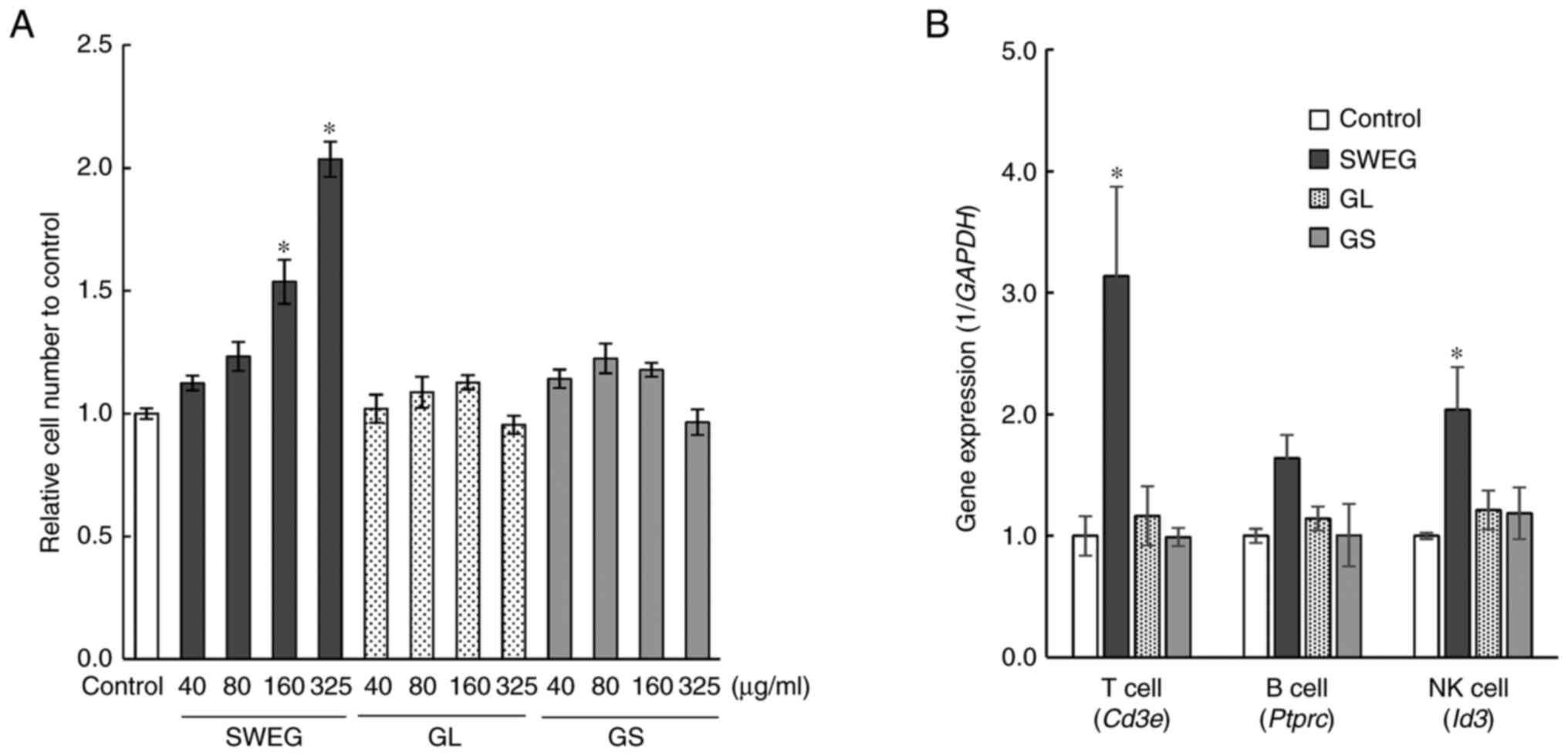 | Figure 2Cell viability and differentiation
tests with A-6 cells. (A) SWEG, GL, or GS were added to the cells
at concentrations of 40, 80, 160 and 325 µg/ml, followed by
culturing for 24 h. The promoting effects on the cell proliferation
of these extracts were examined by cell viability assay. Data are
presented as the mean ± SE (n=6). *P<0.05 compared
with the control. (B) A-6 cells were cultured to differentiate into
T cells, B cells, and NK cells under respective
differentiation-inducing conditions. During the differentiation
induction, SWEG, GL, or GS were added at 325 µg/ml, followed by the
analysis of the expression of marker genes, Cd3e,
Ptprc, and Id3, characteristic of T cells, B cells,
and NK cells, respectively. Data are presented as the mean ± SE
(n=3). *P<0.05 compared with the control. SWEG,
subcritical water extract of Ganoderma; GL, hot water
extract of Ganoderma lucidum; GS, hot water extract of
Ganoderma sinense; NK, natural killer. |
Increases in immune cells in vivo
The bone marrow cells removed from femurs and the
thymus cells were examined for the population analysis of immune
cells by FCM. Since the cell surface antigens CD3e, CD11b, Gr1,
B220, and TER119 are expressed in immune cells such as NK cells, T
cells, monocytes, macrophages, and dendritic cells, and also, these
antigens are considered not to be expressed in immature cells, the
immature cell population in bone marrow was first separated as
Lin- (CD3e-, CD11b-,
Gr1-, B220-, and TER119-) cells
(27,28). To detect HSCs and HPCs, CD117, Sca1,
and CD34 antibodies on the subpopulation of Lin- cells
were used and the Lin- Sca1+
CD117+ cells (HPCs) and Lin- Sca1+
CD117+ CD34- cells (HSCs) (Fig. 3A and B) were quantified. As a result, FCM
analysis demonstrated significant increases in HPCs, immature B
cells, and NK cells from femurs, and T cells from the thymus after
the administration of SWEG, as compared with the control group
(Fig. 3C).
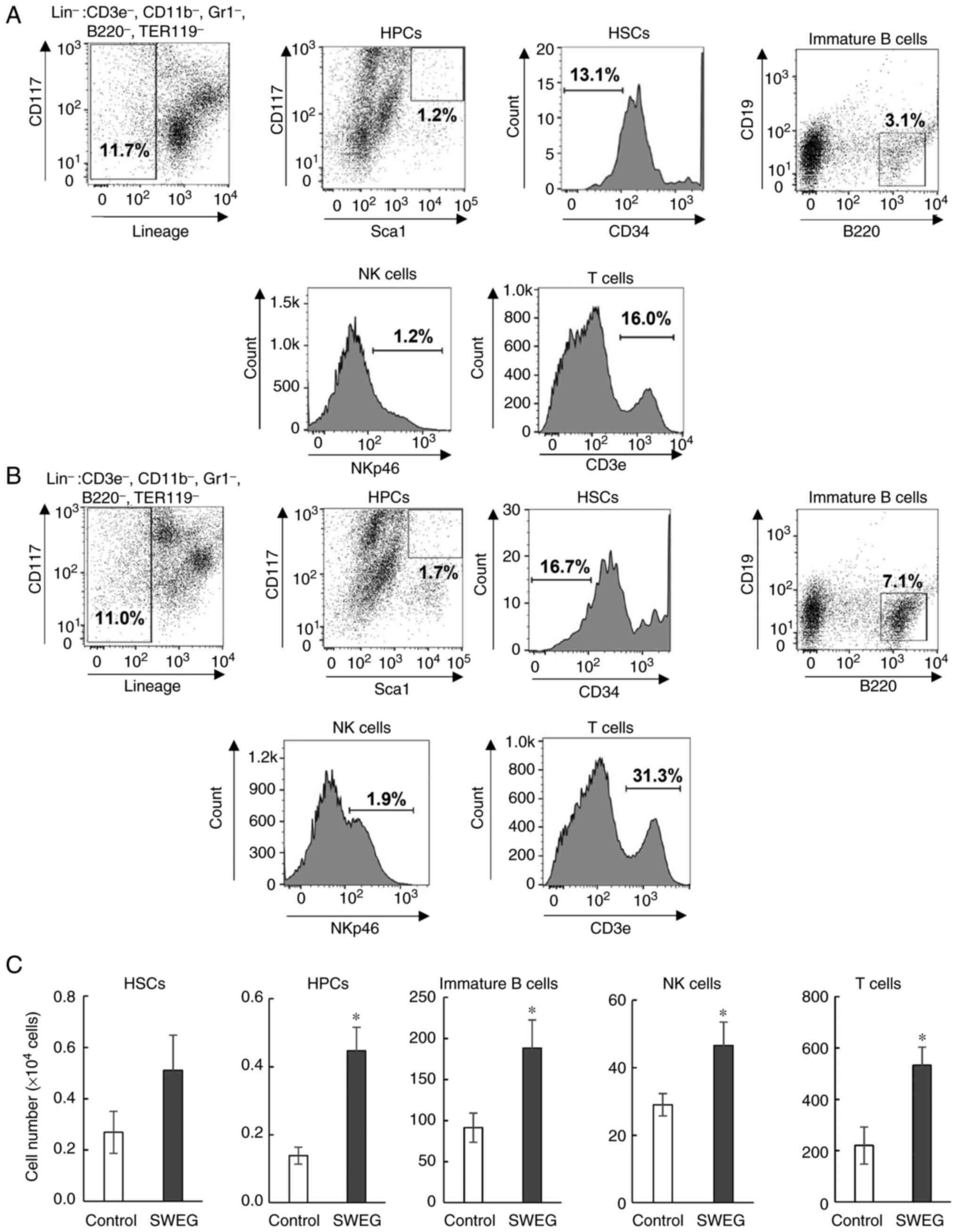 | Figure 3Representative flow cytometric dot
plots and histograms of immune cells in (A) the control group and
(B) the SWEG group. The far left dot plot represents
Lin- (CD3e-, CD11-,
Gr1-, B220-, and TER119-) bone
marrow cells. (C) Comparison of the number of HSCs, HPCs, immature
B cells, NK cells, and T cells in the control group and SWEG group.
Data are presented as the mean ± SE (n=9, control group; n=8, SWEG
group). *P<0.05 compared with the control. SWEG,
subcritical water extract of Ganoderma; HSCs, hematopoietic
stem cells; HPCs, hematopoietic precursor cells; NK, natural
killer. |
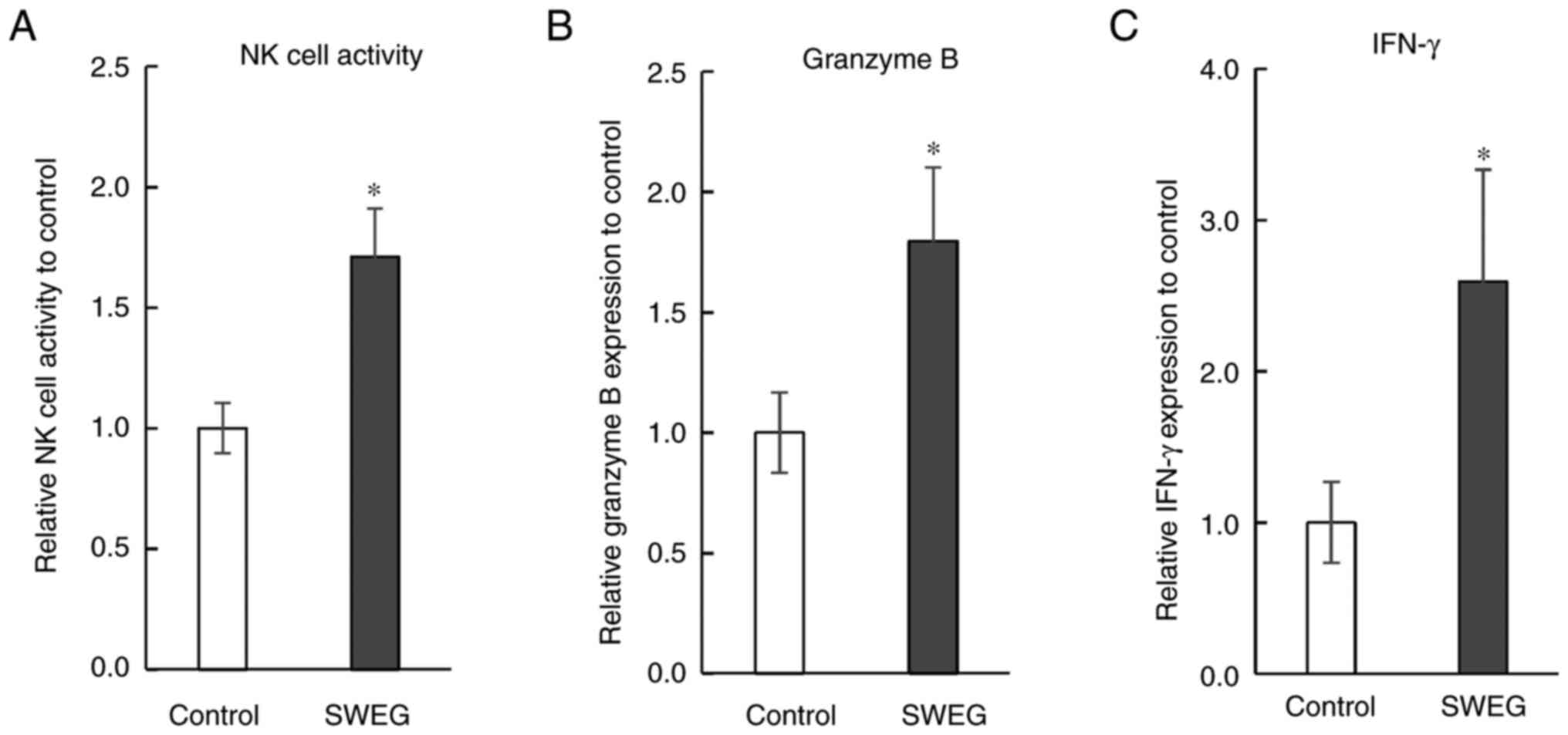
Enhancing effects on immune
functions
Immune functions were examined with the spleen cells
prepared from the spleens of the reared mice. The NK cell activity
in the SWEG group was significantly higher than that in the control
group (Fig. 4A). The expression of
granzyme B and IFN-γ in the spleen cells of the SWEG group were
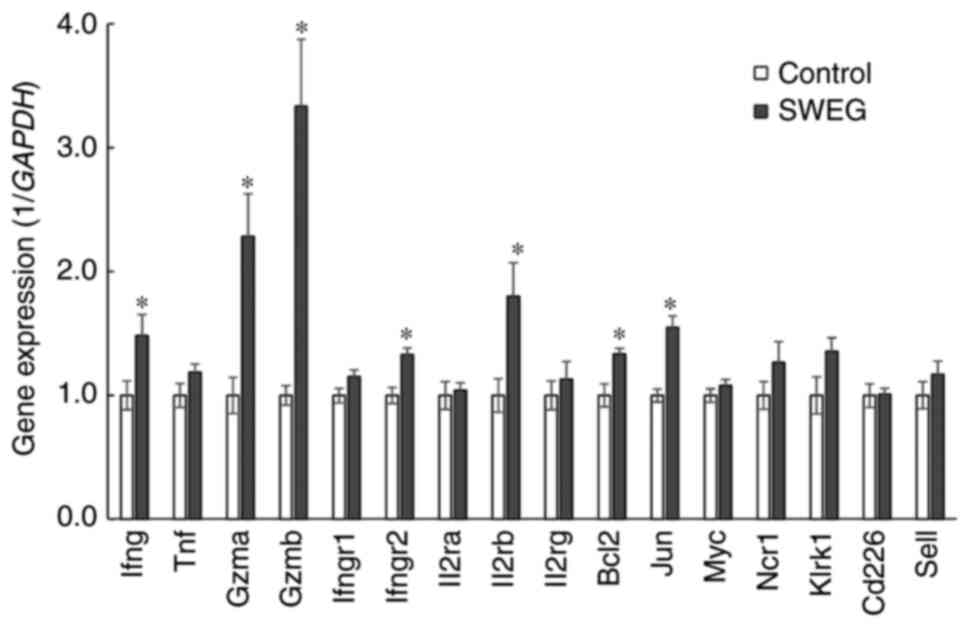
significantly higher than those of the control group (Fig. 4B and C). To reveal the relevant mechanisms, the
gene expression levels relevant to NK cell activity were examined
in the removed spleens, demonstrating the enhanced gene expression
levels of multiple factors critical for NK cell activity,
especially Gzmb (granzyme B) (Fig. 5).
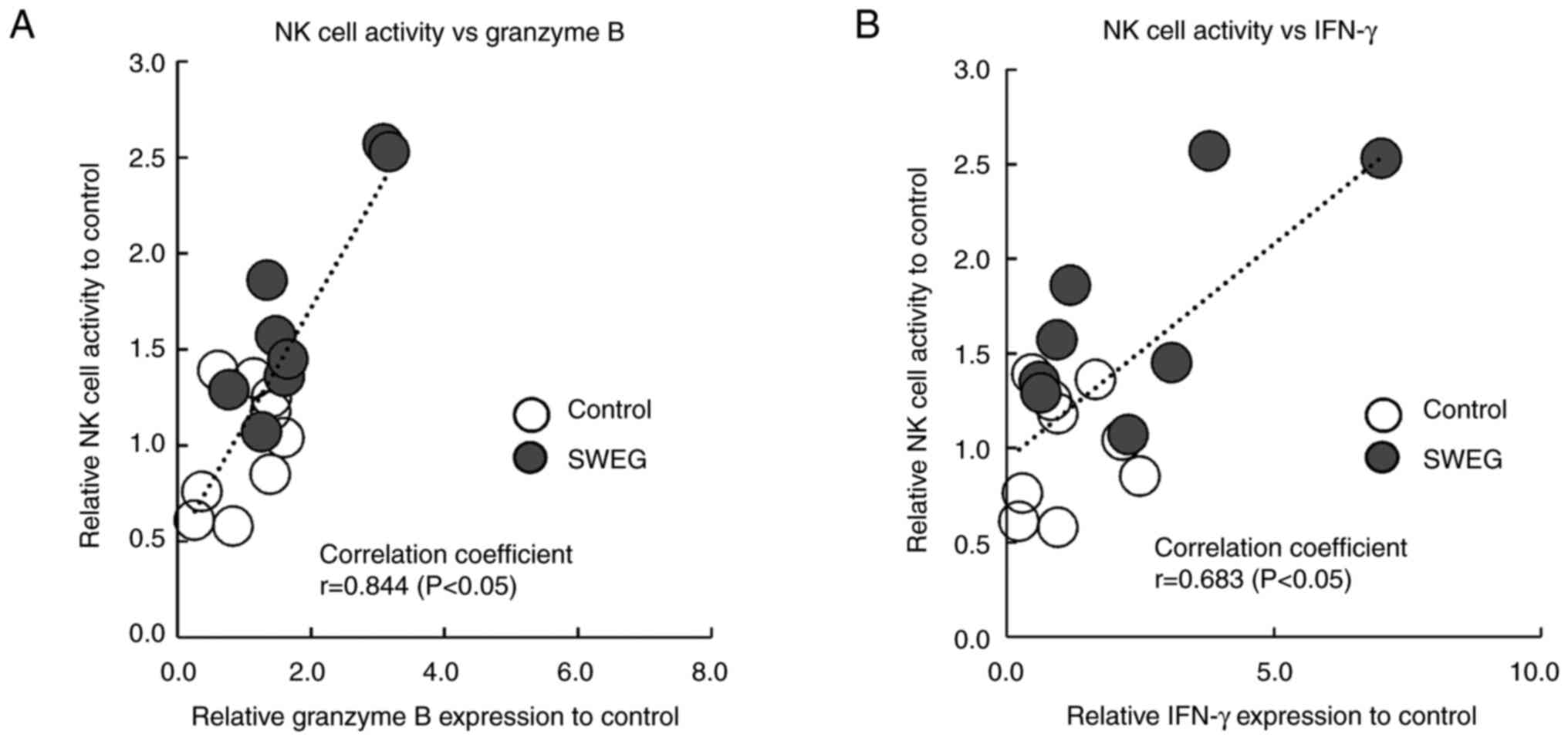
Correlation between granzyme B and
IFN-γ expression and NK cell activity
There were significant correlations both between
granzyme B expression and NK cell activity (P<0.05), and IFN-γ
expression and NK cell activity (P<0.05). The correlation
coefficients were 0.844 (graded as high) for granzyme B and 0.683
(graded as moderate) for IFN-γ, respectively (Fig. 6A and B).
Discussion
Subcritical water may enhance the potentials of
natural products because it allows extraction of components that
cannot be easily obtained by routine hot water extraction (29,30).
In the present study, Ganoderma, a type of medicinal
mushroom, was subjected to subcritical water extraction to examine
its effects on immunity. Ganoderma with immunomodulatory
effects contains polysaccharides, and various polysaccharides have
been separated depending on the molecular weight, constituent
monosaccharides, and branched structures of polysaccharides
(31-33).
A purified polysaccharide with a molecular weight of >3,000 kDa
has also been reported (34). SWEG
and HWEG, used in the present study, contained large amounts of
relatively low-molecular-weight components. However, SWEG differed
from HWEG, i.e., SWEG contained larger amounts of components with a
molecular weight of ~2.2 kDa than HWEG. This may be explained by
the fact that larger amounts of components of ~2.2 kDa were
extracted due to the hydrolysis of high-molecular-weight
polysaccharides in Ganoderma by high-temperature and
high-pressure subcritical water treatment. Furthermore, SWEG
contained β-glucan at >4-fold compared with HWEG. β-glucan forms
a strong triple helix structure in water, but it has been reported
that the structure collapses when dissolved at temperatures
>135˚C, and then adopts less organized conformations to form
random coils (35,36). Since over 140˚C of extraction
temperature was employed to obtain SWEG from Ganoderma in
the present study, β-glucan extraction from the structure with
reduced solidity was presumed to be more efficient. Although the
binding energy of β-glucan is considered to be closely related to
the ease of extraction of β-glucan, experimental information on the
association between the thermal energy of the subcritical water
treatment and the binding energy of β-glucan is not available at
this stage. The cleavage of β-glucan during the heating process can
be detected as the formation of the oxidized functional groups,
i.e., carbonyl groups along the chain (37). In addition, the structural changes
in β-glucan due to heat treatment can be investigated by analysis
methods such as X-ray fiber diffraction (38), carbon-13 nuclear magnetic resonance
(13C-NMR) spectroscopy (39), fluorescence resonance energy
transfer (FRET) spectroscopy (40),
and molecular dynamic simulation (41). By using such analytical techniques,
it may be possible to investigate the effect of subcritical water
treatment on Ganoderma in more detail. At present, the
details of the extract from Ganoderma by subcritical water
treatment are not clear, but at least, SWEG was confirmed to differ
from HWEG in the molecular weight distributions and β-glucan
contents. Therefore, the efficacy study focused on the effects of
SWEG on immunity, especially on HSCs.
The effects of SWEG on the self-renewal and
differentiation abilities of A-6 cells with the same properties as
HSCs were examined. GL and GS, which are routine hot water
extracts, were also subjected to the experiments as reference
samples. As a result, among these extracts, only SWEG promoted both
self-renewal and differentiation into immune cells. Recent studies
have indicated that several factors may be responsible for efficacy
of Ganoderma. With regard to extraction temperature, a
previous study found that extracts of Ganoderma with water
below 100˚C exhibit high antioxidant capacity and cytoprotective
effects against oxidative damage (42). In terms of molecular weight of
Ganoderma polysaccharides, the association between molecular
weights and biological activities of the polysaccharides have been
demonstrated in several studies. For instance, high-molecular
weight polysaccharides exhibited better mitigation effects on
ethanol-induced acute gastric injury than low-molecular weight
polysaccharides in rats (43). By
contrast, another study revealed that low-molecular weight
polysaccharides exhibited stronger antioxidant activities than
high-molecular weight polysaccharides in several in vitro
assays (44). Thus, the extraction
temperature for Ganoderma and the molecular weight of the
resulting extract are closely related to bioactivity. In the
present study, SWEG was confirmed to contain components with a
molecular weight of ~2.2 kDa, and to have unique effects on A-6
cells. In previous studies focusing on immunomodulatory effects, GL
polysaccharides with relatively high molecular weights have been
reported, e.g., inhibition of the growth of Sarcoma 180 tumor in
mice (45), antitumor activity to
Lewis lung cancer model (46), and
the effect on stimulation of humoral immune responses in
immunosuppressed mice (47). The
average molecular weight of these polysaccharides was >20 kDa.
SWEG, containing larger amounts of components of ~2.2 kDa, also
exhibited an immunomodulatory effect, although the molecular size
was small compared with the Ganoderma polysaccharides
reported in the aforementioned studies. Thus, polysaccharides with
various molecular weights derived from Ganoderma appear to
have multiple mechanisms of action against the immune system.
The polysaccharides of fungi represented by β-glucan
have been demonstrated to be recognized by receptors on the cell
surface, and the signal is transmitted into the cell (48,49).
Dectin-1, regarded as a key β-glucan receptor, has been reported to
bind to polysaccharides with lengths longer than a decasaccharide
(50). The details with regard to
the components of ~2.2 kDa of SWEG are yet to be elucidated, but
there is a possibility that a certain component in SWEG may bind to
some type of receptor on the cell surface and has the cell
proliferation- and differentiation-promoting effects on A-6 cells.
In addition, it is important to examine what type of
three-dimensional structure in polysaccharides is necessary for
stimulation of the receptors of immune cells. For further
investigation of the components with a molecular weight of ~2.2 kDa
in SWEG, isolation of the polysaccharides and detailed structure
studies, such as monosaccharide composition analysis and glycosidic
linkage pattern analysis, are required.
Since the promoting effect of SWEG on cell viability
was observed only for one day, the long-term effect has not been
verified. In addition, the cell differentiation test was conducted
based on the expression marker genes characteristic of T cells, B
cells, and NK cells. Therefore, the effects of SWEG were verified
using animals. As a result, the oral administration of SWEG in mice
demonstrated that SWEG increased immune cells in the bone marrow
and thymus. This effect of SWEG appears to be consistent with the
in vitro results in A-6 cells, however it is not yet clear
what type of mechanism is involved in the increase in HPCs and
lymphocytes in vivo. For this determination, further
research is required. In a previous study, mitogen-activated
protein kinase (MAPK) signals were indicated to be markedly
involved in the proliferation and differentiation of HSCs, which
were maintained by signaling pathways such as extracellular
signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and
p38 MAPK (51). By examining
whether SWEG is involved in the activation of these signaling
pathways, the effect of SWEG on the immune system will be
clarified.
In addition to the increase of immune cells
demonstrated in the animal study, SWEG significantly promoted NK
cell activity and expression of cytokines in the spleen, compared
with the control group. Therefore, gene expression analysis was
conducted to investigate its mechanism, and the results revealed
that SWEG promoted the gene expression of multiple factors involved
in immune function, such as IFN-γ, granzyme A, granzyme B, and
interleukin-2 receptor beta. Notably, SWEG markedly promoted the
gene expression of granzyme B, an apoptosis-inducing factor
(52,53), among the factors relevant to NK cell
activity. This indicates that SWEG may have a potent effect on the
transcription of the granzyme B gene.
To clarify the importance of granzyme B for NK cell
activity, correlation analysis between granzyme B and IFN-γ
expression and NK cell activity in the spleen were carried out. As
was revealed, the correlation coefficient between granzyme B
expression and NK cell activity was higher than in the case of
IFN-γ, which is also known as an enhancer of NK cell activity
(54). The correlation coefficients
were compared, demonstrating that granzyme B was more strongly
correlated with NK cell activity than IFN-γ. Thus, granzyme B was
once again confirmed to be critical for NK cell activity.
Zhu et al reported the effect of
polysaccharides with a molecular weight of >500 kDa, isolated
from GL polysaccharides, in the promotion of granzyme B expression
in cytokine-induced killer (CIK) cells (55). In that study, the promotion of
granzyme B expression by GL polysaccharides, at the protein and
mRNA level in vitro was demonstrated, but the in vivo
effect of the polysaccharide was not fully elucidated. By contrast,
the present study demonstrated that SWEG had an effect on A-6
cells, which are model cells of HSCs, and that SWEG promoted
granzyme B expression in the spleen when taken orally in
vivo. A previous study using the human colon cancer-derived
cell line Caco-2 revealed that polysaccharides extracted from
Lycium barbarum (>10 kDa) could be absorbed by
endocytosis from the small intestine (56). For this reason, it is quite possible
that components in SWEG with a molecular weight of ~2.2 kDa, which
is a relatively small size among polysaccharides derived from
Ganoderma, were absorbed from the small intestine and
interacted with the immune system. Therefore, SWEG may have
beneficial effects on health as an immunomodulatory food that can
be orally ingested, due to its potent effect of promotion of
granzyme B expression. In addition, the systemic immune functions
enhanced through intestinal immunity by SWEG may have been extended
to the spleen. To clarify the absorption mechanisms and immune
responses of SWEG in vivo, further research is required.
In A-6 cells, SWEG was demonstrated to promote the
cell differentiation into immune cells, but its effects on immune
function, such as NK cell activity and expression of granzyme B and
IFN-γ, have yet to be examined. In the future, analysis of immune
function at the protein level, even in differentiated A-6 cells, is
warranted. In the present study, both the differentiation-promoting
effect of SWEG on A-6 cells and the immunostimulatory effect on
mice were evaluated at only one dose. In order to further confirm
the effectiveness of SWEG, experiments with various doses are
necessary. By conducting experiments under various conditions and
considering the results of both in vitro and in vivo
studies combined, the understanding of the effect of SWEG on
immunity would be further advanced.
In conclusion, SWEG, prepared by treating
Ganoderma at a high temperature and high pressure, differed
in the molecular weight distribution and β-glucan content from
HWEG, a common hot-water extract. SWEG influenced immunity, i.e.,
acted on HSCs and induced highly functional immune cells. The
results of the present study indicated that SWEG is a beneficial
food material for immunoregulation, including enhancement of
granzyme B expression and NK cell activity.
Supplementary Material
Cell differentiation assessment of A-6
cells to ensure the validity of the evaluation. High-dose cytokines
for each of the differentiation media were added as positive
controls during the differentiation induction, followed by the
analysis of the expression of marker genes, Cd3e,
Ptprc, and Id3, characteristic of T cells, B cells,
and NK cells, respectively. Data are presented as the mean ± SE
(n=3). *P<0.05 compared with the control. NK, natural
killer.
Primer sets used for RT-qPCR.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KH, YY and SH conceived the research. KH, HTakagi,
YO, TY, TS and HTanaka designed the research. KH, YO, HH and KF
performed the experiments, prepared all the figures, and wrote the
first draft of the manuscript. YY, SH and HTanaka supervised the
research. KH, HTakagi, TY, TS, YY and SH wrote, reviewed and edited
the final manuscript. HTanaka provided instructions for performing
the experiments and assisted in the preparation of the manuscript.
KH, HTakagi, TY and TS confirm the authenticity of all the raw
data. All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The animal study protocols were approved (approval
no. HS201708) by the Animal Experiment Committee of Nippon Menard
Cosmetic Co., Ltd.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eaves CJ: Hematopoietic stem cells:
Concepts, definitions, and the new reality. Blood. 125:2605–2613.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sudo K, Ema H, Morita Y and Nakauchi H:
Age-associated characteristics of murine hematopoietic stem cells.
J Exp Med. 117:1273–1280. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Geiger H, Haan G and Florian CM: The
ageing haematopoietic stem cell compartment. Nat Rev Immunol.
13:376–389. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Akunuru S and Geiger H: Aging, clonality,
and rejuvenation of hematopoietic stem cells. Trends Mol Med.
22:701–712. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shiao MS: Natural products of the
medicinal fungus Ganoderma lucidum: Occurrence, biological
activities, and pharmacological functions. Chem Rec. 3:172–180.
2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sato N, Zhang Q, Ma CM and Hattori M:
Anti-human immunodeficiency virus-1 protease activity of new
lanostane-type triterpenoids from Ganoderma sinense. Chem
Pharm Bull (Tokyo). 57:1076–1080. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sanodiya BS, Thakur GS, Baghel RK, Prasad
GB and Bisen PS: Ganoderma lucidum: A potent pharmacological
macrofungus. Curr Pharm Biotechnol. 10:717–742. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Andersen CJ, Murphy KE and Fernandez ML:
Impact of obesity and metabolic syndrome on immunity. Adv Nutr.
7:66–75. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cao Y, Xu X, Liu S, Huang L and Gu J:
Ganoderma: A cancer immunotherapy review. Front Pharmacol.
9:1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang L, Qu H, Mao G, Zhao T, Li F, Zhu B,
Zhang B and Wu X: Optimization of subcritical water extraction of
polysaccharides from Grifola frondosa using response surface
methodology. Pharmacogn Mag. 9:120–129. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park Y, Han BK, Choi HS, Hong YH, Jung EY
and Suh HJ: Effect of porcine placenta extract from subcritical
water extraction on photodamage in human keratinocytes. Korean J
Food Sci Anim Resour. 35:164–170. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu J, Li Y, Liu W, Qi Q, Hu X, Li S, Lei
J and Rong L: Extraction of polysaccharide from Dendrobium
nobile Lindl. by subcritical water extraction. ACS Omega.
4:20586–20594. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chang YW and Lu TJ: Molecular
characterization of polysaccharides in hot-water extracts of
Ganoderma lucidum fruiting bodies. J Food Drug Anal.
12:59–67. 2004.
|
|
14
|
Wang C, Shi S, Chen Q, Lin S, Wang R, Wang
S and Chen C: Antitumor and immunomodulatory activities of
Ganoderma lucidum polysaccharides in glioma-bearing rats.
Integr Cancer Ther. 17:674–683. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mccleary BV and Draga A: Measurement of
β-glucan in mushrooms and mycelial products. J AOAC Int.
99:364–373. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Anzai H, Nagayoshi M, Obata M, Ikawa Y and
Atsumi T: Self-renewal and differentiation of a basic fibroblast
growth factor-dependent multipotent hematopoietic cell line derived
from embryonic stem cells. Dev Growth Differ. 41:51–58.
1999.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Anzai H, Ikawa Y and Atsumi T: Stem cell
factor and interleukin-3 induce stepwise generation of erythroid
precursor cells from a basic fibroblast growth factor-dependent
hematopoietic stem cell line, A-6. Biochem Biophys Res Commun.
282:940–946. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vodyanik MA, Bork JA, Thomson JA and
Slukvin II: Human embryonic stem cell-derived CD34+
cells: Efficient production in the coculture with OP9 stromal cells
and analysis of lymphohematopoietic potential. Blood. 105:617–626.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Woll PS, Martin CH, Miller JS and Kaufman
DS: Human embryonic stem cell-derived NK cells acquire functional
receptors and cytolytic activity. J Immunol. 175:5095–5103.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Awong G, Herer E, Surh CD, Dick JE, La
Motte-Mohs RN and Zúñiga-Pflücker JC: Characterization in vitro and
engraftment potential in vivo of human progenitor T cells generated
from hematopoietic stem cells. Blood. 114:972–982. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin Y, Fu W, Fu M, He G and Traore L: The
immune effects of edible fungus polysaccharides compounds in mice.
Asia Pac J Clin Nutr. 16:258–260. 2007.PubMed/NCBI
|
|
22
|
Kuo HC, Liu YW, Lum CC, Hsu KD, Lin SP,
Hsieh CW, Lin HW, Lu TY and Cheng KC: Ganoderma formosanum
exopolysaccharides inhibit tumor growth via immunomodulation. Int J
Mol Sci. 22(11251)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Stoner L, Meyer ML, Kucharska-Newton A,
Stone K, Zieff G, Dave G, Fryer S, Credeur D, Faulkner J,
Matsushita K, et al: Associations between carotid-femoral and
heart-femoral pulse wave velocity in older adults: The
atherosclerosis risk in communities (ARIC) study. J Hypertens.
38:1786–1793. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Krebs S, O'Donoghue JA, Biegel E, Beattie
BJ, Reidy D, Lyashchenko SK, Lewis JS, Bodei L, Weber WA and
Pandit-Taskar N: Comparison of 68Ga-DOTA-JR11 PET/CT
with dosimetric 177Lu-satoreotide tetraxetan
(177Lu-DOTA-JR11) SPECT/CT in patients with metastatic
neuroendocrine tumors undergoing peptide receptor radionuclide
therapy. Eur J Nucl Med Mol Imaging. 47:3047–3057. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kiss A, Grünvald P, Ladányi M, Papp V,
Papp I, Némedi E and Mirmazloum I: Heat treatment of Reishi medical
mushroom (Ganoderma lingzhi) basidiocarp enhanced its
β-glucan solubility, antioxidant capacity and lactogenic
properties. Foods. 10(10092015)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fan Z, Enjoji K, Tigges JC, Toxavidis V,
Tchipashivili V, Gong W, Storm TB and Koulmanda M: Bone marrow
derived hematopoietic stem and progenitor cells infiltrate
allogeneic and syngeneic transplants. Am J Transplant.
14:2869–2873. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Boulais PE, Mizoguchi T, Zimmerman S,
Nakahara F, Vivié J, Mar JC, Oudenaarden A and Frenette PS: The
majority of CD45- CD31- Ter119-
bone marrow cell fraction is of hematopoietic origin and contains
erythroid and lymphoid progenitors. Immunity. 49:627–639.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee KA, Kim KT, Chang PS and Paik HD: In
vitro cytotoxic activity of ginseng leaf/stem extracts obtained by
subcritical water extraction. J Ginseng Res. 38:289–292.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kim DS and Lim SB: Semi-continuous
subcritical water extraction of flavonoids from Citrus
unshiu peel: Their antioxidant and enzyme inhibitory
activities. Antioxidants. 9(360)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Han XQ, Yue GL, Yue RQ, Dong CX, Chan CL,
Ko CH, Cheung WS, Luo KW, Dai H, Wong CK, et al: Structure
elucidation and immunomodulatory activity of a beta glucan from the
fruiting bodies of Ganoderma sinense. PLoS One.
9(e100380)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu Y, Tang Q, Zhang J, Xia Y, Yang Y, Wu
D, Fan H and Cui WS: Triple helix conformation of β-d-glucan from
Ganoderma lucidum and effect of molecular weight on its
immunostimulatory activity. Int J Biol Macromol. 114:1064–1070.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li LF, Liu HB, Zhang QW, Li ZP, Wong TL,
Fung HY, Zhang JX, Bai SP, Lu AP and Han QB: Comprehensive
comparison of polysaccharides from Ganoderma lucidum and G.
sinense: Chemical, antitumor, immunomodulating and gut-microbiota
modulatory properties. Sci Rep. 8(6172)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu Y, Zhang J, Tang Q, Yang Y, Guo Q,
Wang Q, Wu D and Cui SW: Physicochemical characterization of a high
molecular weight bioactive β-D-glucan from the fruiting bodies of
Ganoderma lucidum. Carbohydr Polym. 101:968–974.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Legentil L, Paris F, Ballet C, Trouvelot
S, Daire X, Vetvicka V and Ferrières V: Molecular Interactions of
β-(1→3)-glucans with their receptors. Molecules. 20:9745–9766.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yanaki T, Tabata K and Kojima T: Melting
behaviour of triple helical polysaccharide schizophyllan in aqueous
solution. Carbohydr Polym. 5:275–283. 1985.
|
|
37
|
Kivelä R, Henniges U, Sontag-Strohm T and
Potthast A: Oxidation of oat β-glucan in aqueous solutions during
processing. Carbohydr Polym. 87:589–597. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chuah CT, Sarko A, Deslandes Y and
Marchessault RH: Triple-helical crystalline structure of curdlan
and paramylon hydrates. Macromolecules. 16:1375–1382. 1983.
|
|
39
|
Yoshioka Y, Uehara N and Saito H:
Conformation-dependent change in antitumor activity of linear and
branched (1→3)-β-D-glucans on the basis of conformational
elucidation by carbon-13 nuclear magnetic resonance spectroscopy.
Chem Pharm Bull (Tokyo). 40:1221–1226. 1992.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Young SH, Dong WJ and Jacobs RR:
Observation of a partially opened triple-helix conformation in
1->3-beta-glucan by fluorescence resonance energy transfer
spectroscopy. J Biol Chem. 275:11874–11879. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Okubira T, Miyoshi K, Uezu K, Sakurai K
and Shinkai S: Molecular dynamics studies of side chain effect on
the beta-1,3-D-glucan triple helix in aqueous solution.
Biomacromolecules. 9:783–788. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tulsawani R, Sharma P, Manimaran M,
Koganti P, Singh M, Meena DK, Negi PS and Misra K: Effects of
extraction temperature on efficacy of Lingzhi or Reishi medical
mushroom, Ganoderma lucidum (Agaricomycetes), aqueous
extract against oxidative stress. Int J Med Mushrooms. 22:547–558.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tian B, Zhao Q, Xing H, Xu J, Li Z, Zhu H,
Yang K, Peilong S and Cai M: Gastroprotective effects of
Ganoderma lucidum polysaccharides with different molecular
weights on ethanol-induced acute gastric injury in rats. Nutrients.
14(1476)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gao X, Qi J, Ho CT, Li B, Xie Y, Chen S,
Hu H, Chen Z and Wu Q: Purification, Physicochemical properties,
and antioxidant activities of two low-molecular-weight
polysaccharides from Ganoderma leucocontextum fruiting
bodies. Antioxidants. 10(1145)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li P and Zhang K: Isolation, purification
and bioactivities of exopolysaccharides from fermented broth of
Ganoderma lucidum. Wei Sheng Wu Xue Bao. 40:217–220.
2000.PubMed/NCBI(In Chinese).
|
|
46
|
Wang Y, Fan X and Wu X: Ganoderma
lucidum polysaccharide (GLP) enhances antitumor immune response
by regulating differentiation and inhibition of MDSCs via a
CARD9-NF-κB-IDO pathway. Biosci Rep. 40(BSR20201170)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu Y, Wang Y, Zhou S, Yan M, Tang Q and
Zhang J: Structure and chain conformation of bioactive β-D-glucan
purified from water extracts of Ganoderma lucidum unbroken
spores. Int J Biol Macromol. 180:484–493. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Goodridge HS, Wolf AJ and Underhill DM:
β-glucan recognition by the innate immune system. Immunol Rev.
230:38–50. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Seong SK and Kim HW: Potentiation of
innate immunity by β-glucans. Mycobiology. 38:144–148.
2010.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Palma AS, Feizi T, Zhang Y, Stoll MS,
Lawson AM, Díaz-Rodríguez E, Campanero-Rhodes MA, Costa J, Gordon
S, Brown GD and Chai W: Ligands for the beta-glucan receptor,
Dectin-1, assigned using ‘designer’ microarrays of oligosaccharide
probes (neoglycolipids) generated from glucan polysaccharides. J
Biol Chem. 281:5771–5779. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Geest CR and Coffer PJ: MAPK signaling
pathway in the regulation of hematopoiesis. J Leukoc Biol.
86:237–250. 2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Shresta S, MacIvor DM, Heusel JW, Russell
JH and Ley TJ: Natural killer and lymphokine-activated killer cells
require granzyme B for the rapid induction of apoptosis in
susceptible target cells. Proc Natl Acad Sci USA. 92:5679–5683.
1995.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Safta TB, Ziani L, Favre L, Lamendour L,
Gros G, Mami-Chouaib F, Martinvalet D, Chouaib S and Thiery J:
Granzyme B-activated p53 interacts with Bcl-2 to promote cytotoxic
lymphocyte-mediated apoptosis. J Immunol. 194:418–428.
2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Aquino-López A, Senyukov VV, Vlasic Z,
Kleinerman ES and Lee DA: Interferon gamma induces changes in
natural killer (NK) cell ligand expression and alters NK
cell-mediated lysis of pediatric cancer cell lines. Front Immunol.
8(391)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhu X and Lin Z: Modulation of cytokines
production, granzyme B and perforin in murine CIK cells by
Ganoderma lucidum polysaccharides. Carbohydr Polym.
63:188–197. 2006.
|
|
56
|
Feng L, Xiao X, Liu J, Wang J, Zhang N,
Bing T, Liu X, Zhang Z and Shangguan D: Immunomodulatory effects of
Lycium barbarum polysaccharide extract and its uptake
behaviors at the cellular level. Molecules. 25(1351)2020.PubMed/NCBI View Article : Google Scholar
|