Introduction
The incidence of type 2 diabetes mellitus (T2DM) is
rapidly increasing worldwide, which is linked to higher medical
costs (1,2). Some progress has been made in
treatments for diabetes but their effect is not always sustained
and their use may be associated with undesirable side effects, such
as hypoglycemia. Insufficient blood glucose control leads to
complications and early mortality. The complications of diabetes,
such as cardiovascular disease, nephropathy, neuropathy and liver
damage, occur shortly after the onset of T2DM (3). Clinical experiments have confirmed the
‘legacy effect’ or ‘metabolic memory’ of prior glycemic control.
The long-term cardiovascular benefits of good glycemic control
early in the course of diabetes is receiving increasing attention
(4,5). Increasing evidence suggests that
numerous patients with T2DM can follow a non-albuminuric pathway to
renal function loss, even after accounting for the use of
renoprotective agents (6,7). It is important to clarify the
association between high glucose intake and the formation of
diabetes. Hyperglycemia is critical in the genesis of diabetic
complications. Poor glycemic control is an independent predictor of
the development and progression of diabetic complications. The
tight control of glucose concentration is determined by glucose
absorption from the intestine, glucose production by the liver and
glucose uptake from the plasma (8).
Patients with T2DM are prone to developing nonalcoholic fatty liver
disease (NAFLD), and NAFLD itself is associated with a risk of
T2DM. It has been revealed that a number of patients with chronic
liver disease have an abnormal glucose metabolism, which ultimately
leads to impaired glucose tolerance and the development of
diabetes. The pathogenesis of impaired glucose metabolism during
chronic liver disease has yet to be fully elucidated. The potential
of targeting the liver to normalize blood glucose levels has not
been fully exploited (9-11).
The molecular mechanisms controlling hepatic gluconeogenesis and
glycogen storage are not very clear, deeming further clinical and
experimental studies necessary. The time it takes for high glucose
to induce liver lesions needs to be determined.
The gut microbiota is a large and complex microbial
ecosystem that maintains the homeostasis of the body and
environment. The microbiota consumes carbohydrates that are not
easily digested by the body. The human gut microbiome is a
promising target for managing T2DM. Short-chain fatty acids (SCFAs)
are produced through the fermentation of dietary fiber by the gut
microbiota and are beneficial to the health of the body.
Insufficient production of SCFAs is associated with T2DM (12,13).
This indicates that intestinal mucosa and gut microbiota play an
important role in glycemic control. However, the changes that occur
in the intestinal mucosa and gut microbiota following short-term
high glucose intake are not clear.
Sugar consumption is regarded as a major risk for
the development of obesity. Diets enriched in sugars including the
intake of sugar-sweetened beverages have been consistently linked
to the increased risk of obesity, T2DM, and cardiovascular disease.
Dietary glucose has been revealed to increase serum glucose and
insulin concentrations in the postprandial state. Studies in
experimental animals and in humans have demonstrated that chronic
elevation in the plasma glucose concentration impairs insulin
action. Chronic hyperglycemia causes insulin resistance, but the
short-term glucotoxicity and the underlying mechanisms are unclear
(14,15).
High glucose plays a critical role in the formation
and progression of diabetes. However, the mechanism through which
it induces diabetes and the organ that triggers diabetes remain
unclear. The scope of most studies is restricted to studying the
effects of oral high glucose (OHG) or high glucose infusion (IHG)
on only one or two organs. Therefore, the aim of the present study
was to evaluate the different effects of short-term OHG or IHG on
different organs concurrently. Rats were fed or infused with liquid
high glucose, and the effects on the liver, pancreas, kidneys and
intestine, as well as the development of gut microbial dysbiosis,
were analyzed.
Materials and methods
Experimental animals
The present study was performed using 30 male
specific pathogen-free Sprague-Dawley rats aged 12 weeks and
weighing ~200±20 g. The rats were purchased from the Experimental
Animal Center of Guangdong Province [approval no. SCXK
(Yue)-2013-0002]. All rats were raised under light-controlled
conditions (12-h light/dark cycle) in a temperature- (23±2˚C) and
humidity (40%)-controlled room with food and water freely
available.
All animal experiments were conducted in accordance
with the guidelines of the Ethics Committee of the Guangdong
Provincial People's Hospital (Guangzhou, China) and approved
(approval no. KY-D-2019-082-01) by the Institutional Animal Care
and Use Committee of Guangdong Provincial People's Hospital.
Experimental design
Following adaptive feeding, all rats were randomly
divided into three groups: The normal diet (ND; n=10), the OHG
(n=10) and the IHG (n=10) groups. OHG group rats were fed with 50%
high glucose at a dose of 2.5 g/kg/day for 2 weeks. The IHG group
rats were treated with 50% high glucose via tail vein injection at
a dose of 2 g/kg/day for 2 weeks. The ND group received an
equivalent amount of saline orally for the same period. During the
experiment, all rats received the same standard chow. Fasting blood
sugar (FBS) levels were measured in all animals weekly using a
glucometer (Abbott Diabetes Care). Finally, all rats were
sacrificed by cervical dislocation following anesthesia with an
intraperitoneal injection of sodium pentobarbital (45 mg/kg;
MilliporeSigma) to reduce pain. The following samples were
collected from the rats: Feces were collected individually for at
least 3 days for each animal. Blood samples were collected via the
abdominal aorta (4 ml) for biochemical assays after anesthesia with
an intraperitoneal injection of sodium pentobarbital. Intestine and
liver samples were also collected.
Weight of rats
The weight of the rats was measured at different
time-points (days 1, 8, 16, 24 and 32), to determine the effect of
high glucose on the ND, OHG and IHG groups.
Changes in blood glucose levels
In order to compare the effects of IHG and OHG on
blood glucose levels, FBS levels were measured on days 0 (prior to
glucose intake), 18, 24 and 32.
Detection of cytokines and
chemokines
ELISA kits were used to measure the serum levels of
pro-inflammatory cytokines interleukin (IL)-6 (cat. no. ERA31RB),
tumor necrosis factor (TNF)-α (cat. no. BMS622), blood insulin (BI)
(cat. no. ERINS; all from Invitrogen; Thermo Fisher Scientific,
Inc.) and 24-h microalbuminuria (cat. no. BJ003256; Shanghai
Bangjing Industrial Co., Ltd.) according to the manufacturer's
instructions. The blood glutamic pyruvic transaminase (ALT; cat.
no. BC1555) and glutamic oxalic transaminase (AST; cat. no. BC1565;
both from Beijing Solarbio Science & Technology Co., Ltd.)
levels were determined using standard enzymatic kits.
Histopathological examinations
The jejunum was removed, divided longitudinally, and
washed. The intestine, liver, kidney and pancreatic sections (4 mm)
were fixed in 10% formalin for 2 h at 25˚C, embedded in paraffin
and stained with hematoxylin and eosin for 3 min at 25˚C. Oil Red O
staining for 5 min at 25˚C was performed on frozen sections (2 mm)
fixed in 4% paraformaldehyde for 6 h at 25˚C and sucrose-protected.
For the comparison of histological differences, three sections were
blindly selected per sample from each rat and quantified using
ImageJ software (version 1.6.0; National Insitutes of Health).
Kidney sections (2 mm) were fixed in 2.5% neutral glutaraldehyde
(cat. no. A17876; Alfa Aesar; Thermo Fisher Scientific, Inc.) for 3
h at 4˚C, stained with 2% uranyl acetate (cat. no. 22400; Electron
Microscopy Sciences) for 30 min at 25˚C, for assessment using
transmission electron microscopy (JEM-1400 PLUS; Japan Electron
Optics Laboratory Co., Ltd.).
Western blotting
In order to detect changes in renal cells, the
levels of gasdermin D (GSMD), NLR family pyrin domain containing 3
(NLRP3) and caspase-1 protein were assessed to determine whether
apoptosis occurred in renal cells. Western blotting was performed
on total protein extracts from rat kidneys (n=3 per group). Lysis
buffer (cat. no. ab270054, Abcam) was used for protein extraction.
BCA protein assay was used for protein determination. A total of 50
µg protein/lane was separated on 13.5% SDS-PAGE gel and transferred
onto a PVDF membrane for 1 h at 100V. Subsequently, 10X Blocking
Buffer (cat. no. ab126587; Abcam) was used to block the membrane to
prevent non-specific background binding of the primary and
secondary antibodies to the membrane at 25˚C for 1 h. The blots
were immunoprobed with anti-GSMD (dilution, 1:500; cat. no.
219800), anti-caspase-1 (dilution, 1:500; cat. no. ab138483), and
mouse anti-NLRP3 (dilution, 1:1,000; cat. no. ab263899; all from
Abcam) and rabbit anti-GAPDH (dilution, 1:1,000; cat. no. 25778;
Santa Cruz Biotechnology, Inc.) antibodies, incubated at 25˚C for 1
h. Pierce ECL Western Blotting Substrate (cat. no. 32106; Thermo
Fisher Scientific, Inc.) was used for the detection of the
anti-rabbitt HRP-conjugated secondary antibody (dilution, 1:500;
cat. no. AC2114; Azure Biosystems) at 25˚C for 2 h. Films were
scanned using the RICOH scanner (600 dpi and grey scale) and
analyzed with ImageJ software (version 1.6.0; National Institutes
of Health).
Gut microbiota analysis. DNA
extraction and PCR amplification
Microbial DNA was extracted from the feces of rats
using the HiPure Soil DNA Kits (cat. no. D3143-03; Shanghai Maige
Biotechnology Co., Ltd.), according to the manufacturer's
instructions. As the most popular method used for 16S assessment,
the 16S rDNA V4 region of the ribosomal RNA gene was amplified
using PCR (95˚C for 2 min, followed by 27 cycles at 98˚C for 10
sec, 62˚C for 30 sec and 68˚C for 30 sec, followed by a final
extension at 68˚C for 10 min). The following primers were used:
341F CCTACGGGAGGCAGCAG, 806R GGACTACHVGGGTWTCTAAT, 515F
GTGYCAGCMGCCGCGGTAA, 806R GGACTACHVGGGTWTCTAAT. Arch519F,
CAGCTGCCGCGGTAA and Arch915R, GTGCTCCCCCGCCAATTCCT were used as a
supplement, where the barcode is an 8-base sequence unique to each
sample. PCR reactions were performed in triplicate in 50 µl mixture
containing 5 µl of 10X KOD Buffer, 5 µl of 2.5 mM dNTPs, 1.5 µl of
each primer (5 µM), 1 µl KOD Polymerase, and 100 ng template DNA.
Amplicons were extracted from 2% agarose gels and purified using
the AxyPrep DNA Gel Extraction Kit (Corning, Inc.), according to
the manufacturer's instructions, and quantified using ABI
StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, Inc.).
Purified amplicons were pooled in equimolar and paired-end
sequenced (2x250) on an Illumina platform according to the
manufacturer's instructions. The raw reads were deposited into the
NCBI Sequence Read Archive database (accession no. PRJNA884142;
ID:884142).
Bioinformatics analysis
Raw data were further filtered using FASTP
(https://github.com/OpenGene/fastp) as
follows: i) Removing reads containing >10% of unknown
nucleotides (N); ii) Removing reads containing <80% of bases
with quality (Q-value) >20. Paired end clean reads were merged
as raw tags using FLASH (version 1.2.11; http://ccb.jhu.edu/software/FLASH/index.shtml)
with a minimum overlap of 10 bp and mismatch error rates of 2%.
Noisy sequences of raw tags were filtered using QIIME (version
1.9.1; http://www.wernerlab.org/software/macqiime) under
specific filtering conditions to obtain the high-quality clean
tags. Clean tags were searched against the reference database
(http://drive5.com/uchime/uchime_download.html) to
perform reference-based chimera checking using UCHIME algorithm
(http://www.drive5.com/usearch/manual/uchime_algo.html).
All chimeric tags were removed and the effective tags that were
finally obtained were used for further analysis. The effective tags
were clustered into operational taxonomic units (OTUs) of ≥97%
similarity using UPARSE (https://ext.dcloud.net.cn/). The tag sequence with
highest abundance was selected as a representative sequence within
each cluster. Venn analysis between groups was performed in the R
project (version 3.4.1; http://ruanfujia.com/vendor/3191173) to identify
unique and common OTUs. The representative sequences were
classified into organisms using RDP classifier (version 2.2), a
naive Bayesian model, on the SILVA database (https://www.arb-silva.de/), with the confidence
threshold values ranging from 0.8 to 1. The abundance statistics of
each taxonomy were visualized using Krona (version 2.6; https://github.com/marbl/Krona/wiki). Biomarker
features in each group were screened by Metastats (version
20090414; http://www.mothur.org/wiki/Metastats) and LEfSe
software (version 1.0; http://cloud.magigene.com. Chao1, Simpson and all
other alpha diversity indices were calculated in QIIME. OTU
rarefaction curve and rank abundance curves were plotted in QIIME.
Alpha index comparison between groups was calculated via Welch's
t-test and Wilcoxon rank test in the R project. Alpha index
comparison among groups was computed by Tukey's HSD test and
Kruskal-Wallis H test in the R project. Weighted and unweighted
unifrac distance matrices were generated by QIIME. Multivariate
statistical techniques, including principal component analysis,
principal coordinates analysis and non-metric multi-dimensional
scaling of unweighted nifrac distances were calculated and plotted
in the R project. Statistical analysis of Welch's t-test, Wilcoxon
rank test, Tukey's HSD test, Kruskal-Wallis H test, Adonis (also
called Permanova) and Anosim test was calculated using the R
project. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
analysis of the OTUs was conducted using Tax4Fun (version 1.0;
http://tax4fun.gobics.de/).
Statistical analysis
All the experiments were independently performed
more than three times. The data are expressed as the mean ±
standard deviation. Statistical analysis was performed using SPSS
21.0 (IBM, Corp.). Statistical differences were determined using a
one-way ANOVA with Tukey's post hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
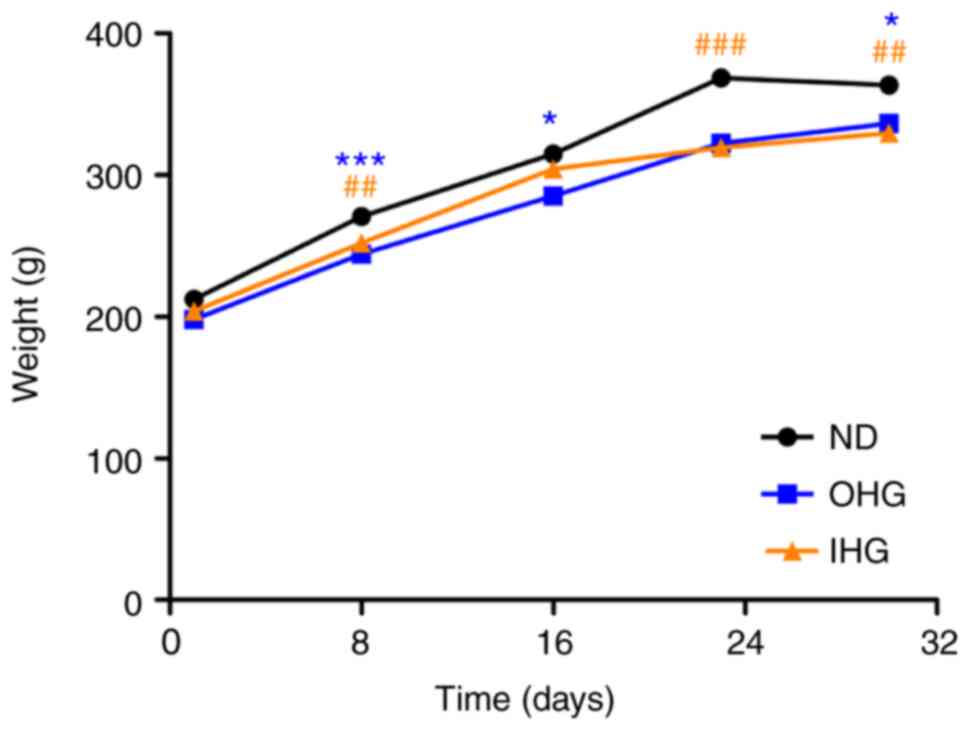
Weight of rats
To compare the effect of a IHG and OHG on the weight
of rats, their weight was measured on days 1, 8, 16, 24 and 32. The
weight of the rats in the OHG and IHG groups was lower than that in
the ND group (Fig. 1). Loss of
appetite caused by high blood glucose and damage of intestinal
mucosa may be the reasons for the recorded weight loss. However,
the exact reasons warrant further study.
Biochemical observations
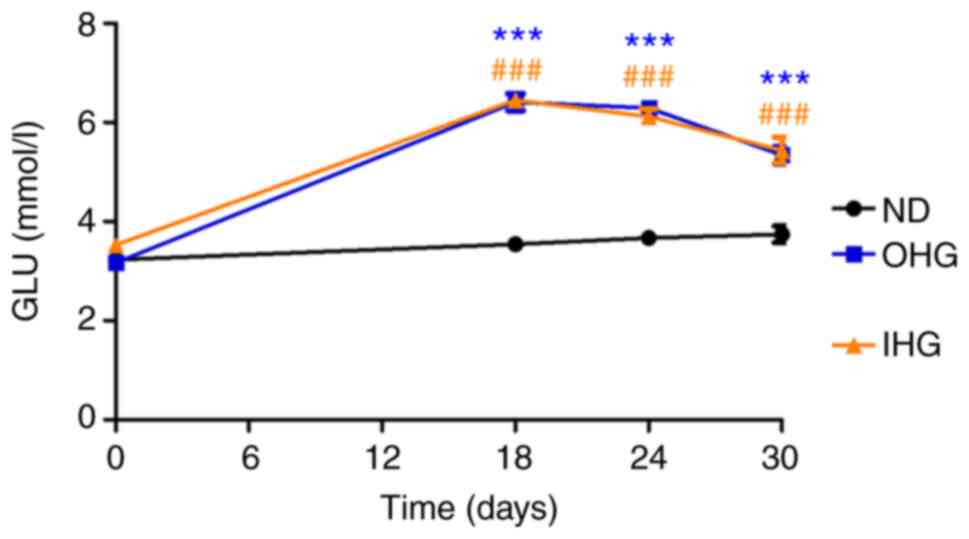
In order to compare the influence of IHG and OHG on
blood glucose levels, FBS levels were measured at days 0 (prior to
glucose intake), 18 24 and 30. Both OHG and IHG significantly
increased blood glucose levels. Blood glucose was slowly decreased
7 days after ceasing glucose intake in both groups. The blood
glucose levels in both groups of rats were higher than that in rats
with a ND until 14 days after ceasing high glucose intake. There
was no difference between the OHG and IHG groups (Fig. 2).
At the end of high glucose intake, the serum levels
of inflammatory cytokines IL-6 and TNF-α were measured to evaluate
the level of inflammation in rats. The serum levels of IL-6 and
TNF-α were markedly increased in both the OHG and IHG groups.
However, the increase was slightly more in the IHG group than in
the OHG group (Table I).
 | Table IExpression levels of cytokines, IL-6
and TNF-α, in the blood. |
Table I
Expression levels of cytokines, IL-6
and TNF-α, in the blood.
| Groups (n=10) | IL-6 (pg/ml) | TNF-α (pg/ml) |
|---|
| OHG |
122±11.16a |
295.28±36.95a |
| IHG |
127.8±16.42a,b |
300.28±30.1a,b |
| ND | 89.49±18.24 | 245.85±34.46 |
The blood insulin, blood ALT and AST levels, as well
as urinary protein content, were assessed to detect the function of
the pancreas, liver and kidneys. No obvious differences were
identified among the three groups (Table II). In addition, no urinary protein
was detected (data not shown).
 | Table IIAssessment of BI, ALT and AST. |
Table II
Assessment of BI, ALT and AST.
| Groups (n=10) | BI (U/l) | ALT (U/l) | AST (U/l) |
|---|
| OHG |
22.3±5.16a |
45.28±3.95a |
123.45±5.42a |
| IHG |
22.8±4.42a,b |
46.28±4.1a,b |
124.33±5.26a,b |
| ND | 21.9±4.24 | 45.85±4.46 | 123.98±6.12 |
Histopathological observations
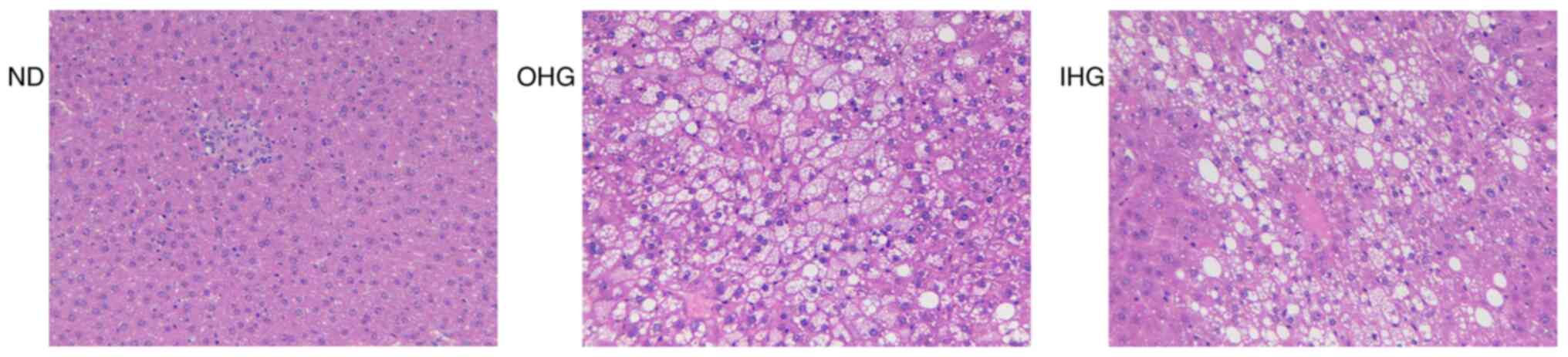
Hepatic fat accumulation was investigated 14 days
after ceasing high glucose intake. Both OHG and IHG induced obvious
steatosis in the livers of rats. A large number of hepatocytes were
injured. A large amount of white fat could be observed in the
sections of the OHG and the IHG groups, which was due to liver cell
lipolysis. No differences between the OHG and IHG groups were
identified (Fig. 3).
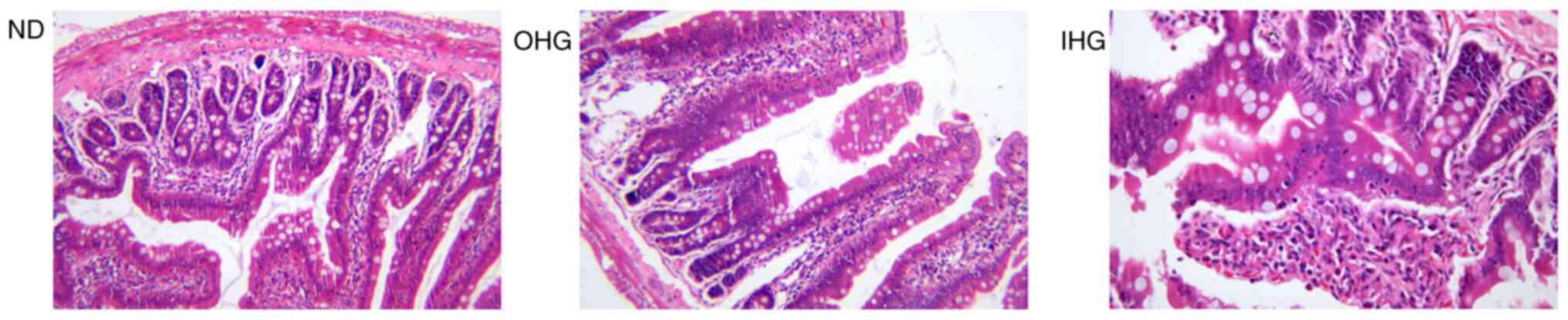
Jejunum mucosae were collected from rats for
pathological assessment. Both OHG and IHG induced jejunum mucosa
injury. In the jejunum tissue, there was no obvious lesion in the
intestinal mucosa of the ND group. In the OHG group, a small amount
of intestinal mucosal villi had atrophied, villi epithelial cells
were damaged, individual villi had swelled and died, and epidermal
epithelial cells had disappeared. In the IHG group, intestinal
mucosal villi had atrophied. In addition, a large number of cells
in the upper intestinal mucosa were necrotic, and the normal
structure of the villi had disappeared, but the lesions were
limited to the mucosal layer. The results revealed that high
glucose or IHG for 2 weeks causes jejunum lesions (Fig. 4).
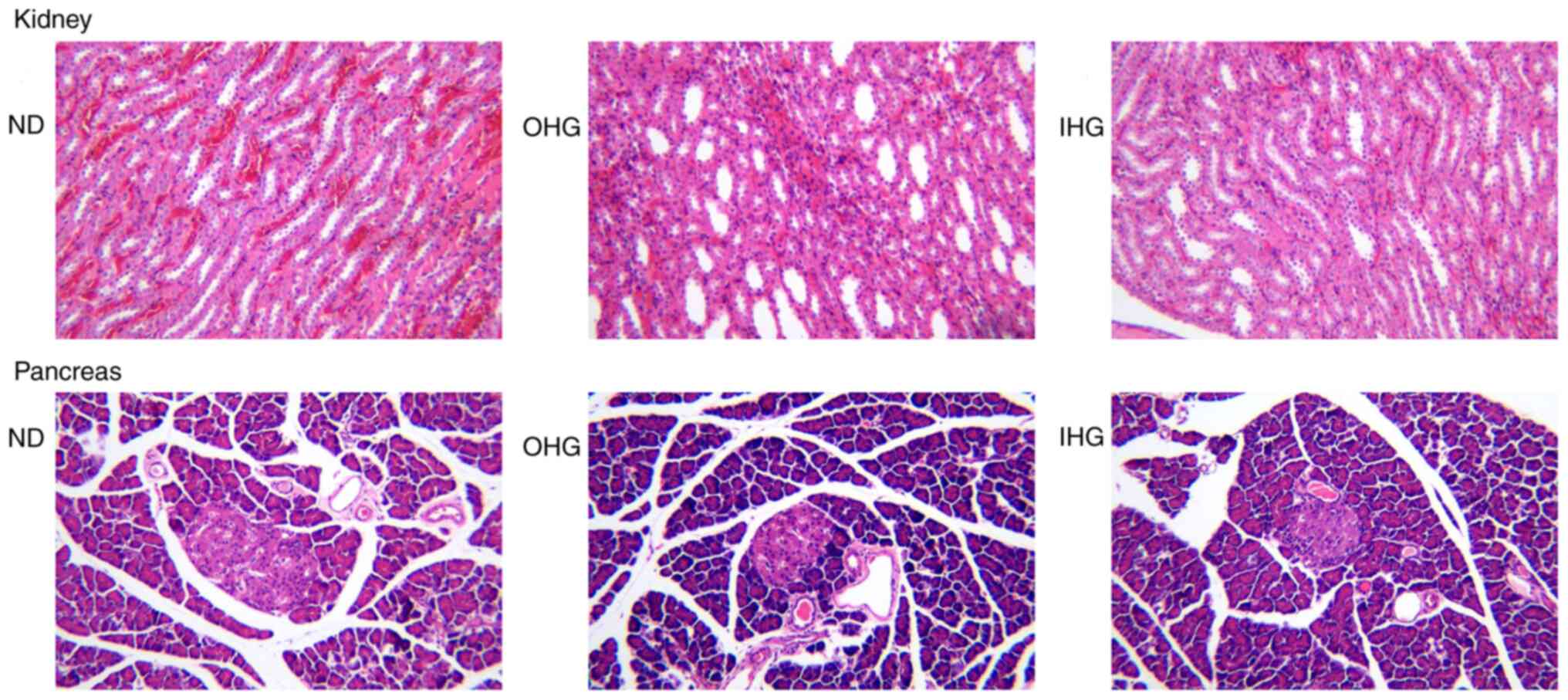
Following observation under an optical microscope,
no obvious pathological lesions were identified in the kidney and
pancreatic tissues of the rats (Fig.
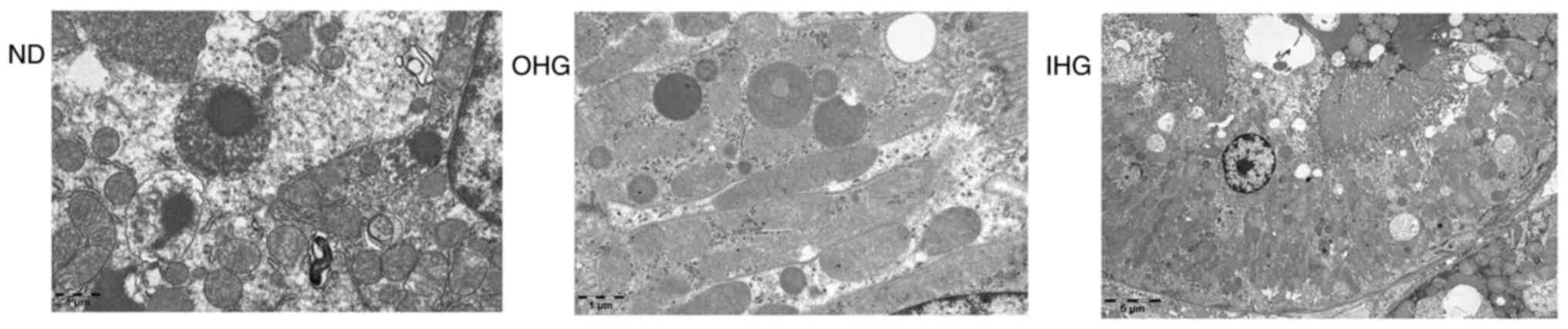
5). Following transmission electron microscopy, however, a
degree of glomerular cell swelling was identified, the internal
structure of mitochondria was found to be empty and mitochondria
were disintegrated (Fig. 6).
Glomerular cell apoptosis
Since the kidney is one of the targets of
glycotoxins, the kidney was examined for pathological lesions; no
obvious lesions were identified (Fig.
5). One of the objectives of the present study was to clarify
whether high glucose induces renal cell damage. Apoptosis is a
common type of cell death (16).
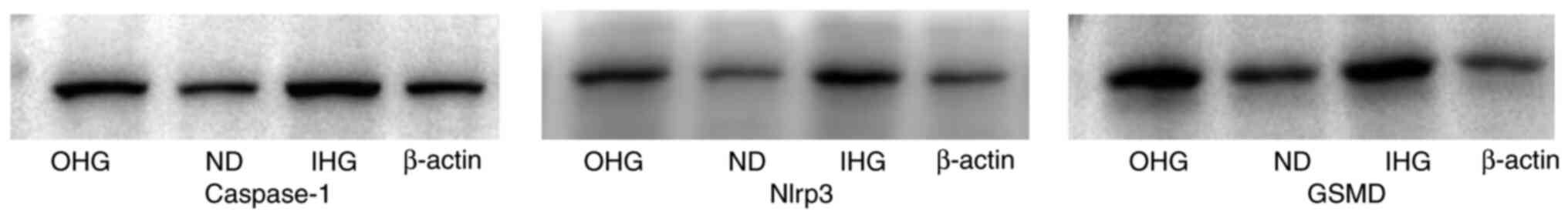
GSMD, caspase-1 and NLRP3 were assessed to confirm the apoptosis of
renal cells. The expression levels of GSMD, caspase-1 and NLRP3
were increased in the kidney (Fig.
7). This indicated that glomerular cells were damaged to a
certain extent.
Changes in the gut microbiota
To elucidate the mechanism of the effect of high
glucose, the impact of high glucose on the gut microbiota was
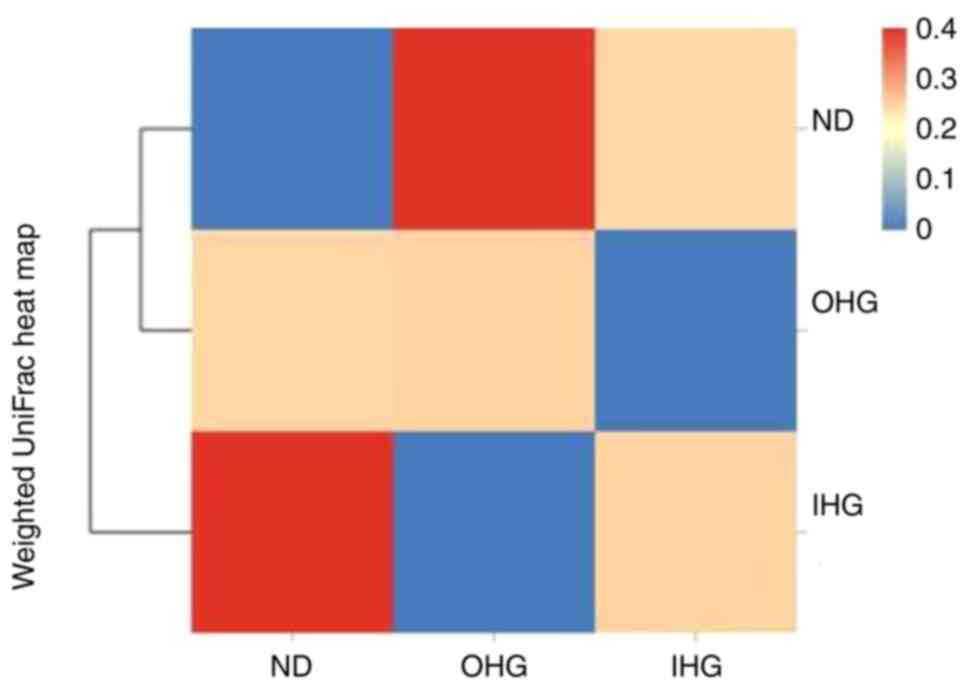
investigated in SD rats. 16S rDNA sequencing was used to assess
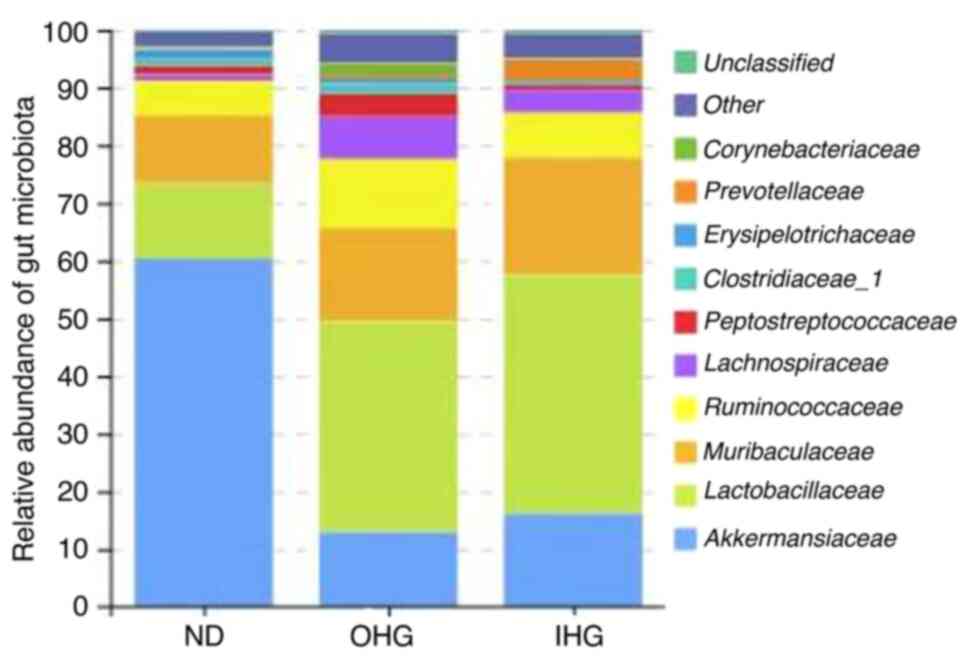
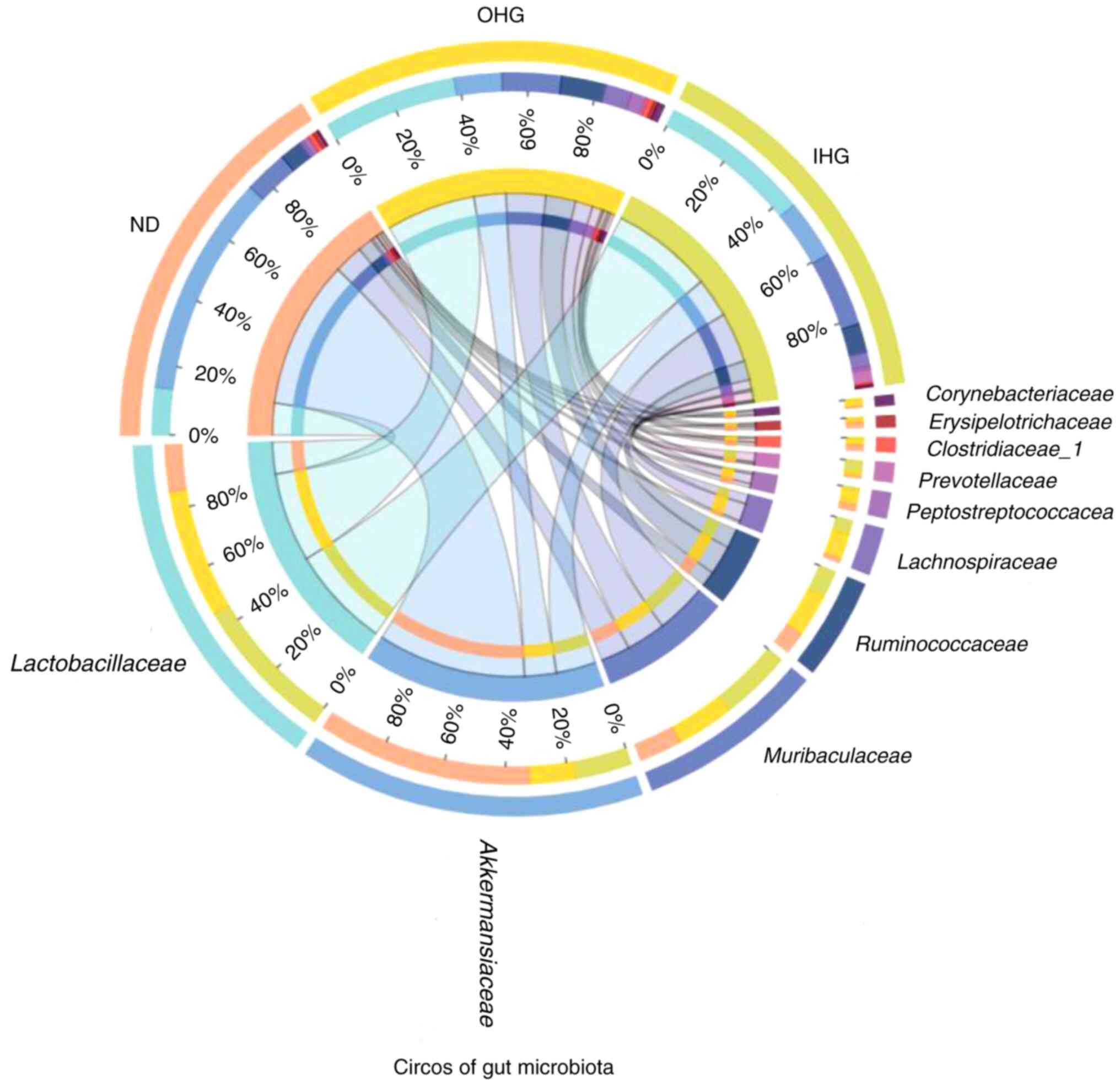
changes in the fecal microbiota of OHG and IHG rats. The dominant
floras in the rat intestines were Akkermansiaceae,
Lactobacillaceae, Muribaculaceae,
Ruminococcaceae, Peptostreptococcacea,
Clostridiaceae_1; these accounted for 60% of the gut
microbiota in SD rats. Rats in the two groups lost gut microbial
diversity. It was characterized by a lower proportion of
Akkermansiaceae and a markedly increased proportion of
Lactobacillaceae, Muribaculaceae,
Ruminococcaceae, Peptostreptococcacea.
Compared with the ND group, the abundance of
Akkermansiaceae was decreased in the OHG group, while that
of Lactobacillaceae, Muribaculaceae,
Ruminococcaceae and Clostridiaceae_1 was increased in
the OHG group. The abundance of Akkermansiaceae and
Clostridiaceae_1 was decreased in the IHG group. The
abundance of Lactobacillaceae and Muribaculaceae was
increased in the IHG group (Fig. 8,
Fig. 9 and Fig. 10).
Discussion
Sugar consumption is regarded as a major risk for
the development of obesity and diabetes. Prediabetes or
intermediate hyperglycemia is a high-risk state for developing T2DM
(17,18). The effects of OHG and IHG on blood
glucose were compared, and it was revealed that both markedly
increased blood glucose. In addition, both also led to persistent
hyperglycemia that lasted two weeks. Notably, it was demonstrated
that both OHG and IHG caused weight loss in rats. These results
confirmed that sugar is a critical cause of diabetes.
Inflammation is divided into two complementary
subsystems: The innate immune system and the highly adaptive immune
system. An increasing number of studies has indicated that diabetes
is a type of inflammation (19,20).
It is now accepted that obesity-associated chronic low-grade
systemic inflammation is a major underlying factor for the
development of several metabolic diseases (21). The present results indicated that
only 2 weeks of OHG or IHG induced inflammation in rats.
Clinical and pathophysiological studies have shown
that T2DM is a condition mainly caused by excess fat accumulation
in the liver and pancreas. Excess fat worsens hepatic
responsiveness to insulin, leading to increased glucose production.
The removal of excess fat from the liver through substantial weight
loss can normalize hepatic insulin responsiveness (22,23).
Negative energy balance in T2DM causes a marked decrease in liver
fat content, which results in the normalization of hepatic insulin
sensitivity within 7 days. As the period of negative energy balance
extends, liver fat levels decrease to the normal range and the rate
of export of triacylglycerols from the liver also decreases
(24).
The primary care-based Diabetes Remission Clinical
Trial revealed that 46% of patients with T2DM could achieve
remission, which was mediated by weight loss, at 12 months, and 36%
at 24 months (25,26). Glucotoxicity and lipotoxicity are
key factors of T2DM, but their molecular nature during the early
stages of the disease remains to be elucidated (27). The present study indicated that both
OHG and IHG induced obvious steatosis in the livers of rats in just
2 weeks. However, there were no obvious lesions in the kidney and
pancreas. The liver may be the first organ to be damaged by high
glucose. Further study is required to determine whether the liver
is the trigger of diabetes.
The intestinal epithelium is characterized by a
remarkable self-renewal ability. The crypt base columnar cells
marked by Lgr5 represent the actively proliferating stem cells that
mediate the daily renewal of the intestinal epithelium. The
constant renewal cycle takes place in a hostile environment
characterized by the presence of bacterial toxins and metabolites,
dietary antigens and mutagens, as well as immunological cytokines
and oxidative stress. The intestine has been implicated as a key
organ that critically contributes to the development of
obesity-associated chronic inflammation and systemic insulin
resistance, as well as metabolic dysregulation (28,29).
Glucose directly stimulates intestinal epithelial cells (27). The results of the present study
indicated that both OHG and IHG led to jejunum mucosal injury. The
damage from OHG was more serious than that of IHG. It influenced
the villi and mucosa at the same time, which may have been the
result of direct contact between mucosa and high glucose.
The gastrointestinal (GI) tract is a highly complex
organ composed of the intestinal epithelium layer, intestinal
microbiota and local immune system. The gut microbiota is one of
the most diverse communities. It constantly interacts with the
cells and systems of the body. Distinct proportions of the
intestinal microbiota are contained in different sections of the GI
tract (30-32).
Gut microbiota and its metabolites play pivotal roles in host
physiology and pathology. Diet is one of the various factors
influencing the microbiota. Intestinal microbiota modulate
metabolism and are closely associated with epithelial cells in the
intestine (33,34). The intestinal microbiota converts
ingested nutrients into metabolites that target either the
intestinal microbiota population or host cells. As metabolites of
intestinal microbiota, SCFAs are a major energy source for
intestinal epithelial cells in the colon and reinforce the
intestinal barrier function through multiple mechanisms. SCFAs can
reduce inflammation and protect the kidneys (35). However, lipopolysaccharides (LPS)
are mainly derived from microbiota and induce inflammation and
injury in the kidneys, heart, cerebrovascular as well as other
organs (28). A vast number of
studies have demonstrated that the gut microbiota and their
metabolites play an important role in the pathogenesis of T2DM.
Accumulating evidence suggests that SCFAs regulate inflammation,
energy metabolism and blood pressure, which affects kidney function
through the gut-kidney axis (36,37).
The present study indicated that rats treated with high glucose
lost gut microbial diversity in 2 weeks. Gut microbiota was
characterized by a decreased proportion of Akkermansiaceae
and a markedly increased proportion of Lactobacillaceae,
Muribaculaceae, Ruminococcaceae and
Peptostreptococcacea. As compared with the ND group, the
abundance of Akkermansiaceae decreased in the OHG group, and
the abundance of Lactobacillaceae, Muribaculaceae,
Ruminococcaceae and Clostridiaceae_1 increased. The
abundance of Akkermansiaceae and Clostridiaceae_1
decreased, while the abundance of Lactobacillaceae and
Muribaculaceae increased in the IHG group. A decrease in
Akkermansiaceae was revealed to be closely associated with
obesity and diabetes (38).
Diabetic nephropathy (DN) is the leading cause of
end-stage renal disease worldwide. Chronic hyperglycemia is the
main risk factor for the development of DN (39,40).
However, the time of DN onset remains unclear and clinical studies
have yet to provide a conclusive answer. In the present study, no
obvious pathological lesions were identified in the kidney tissues
of the rats. However, glomerular cell swelling and mitochondria
disintegration were identified, using transmission electron
microscopy, after 2 weeks of high glucose intake. Apoptosis of
glomerular cells was increased to a certain degree in the present
study. This suggests that formation of glycotoxins in renal cells
occurred quickly, however, further research is required to confirm
this.
Low-grade inflammation affects the pathogenesis of
the metabolic syndrome and T2DM. Chronic inflammation is considered
to be one of the key factors of atherosclerosis development and is
present from the earliest stages of pathology initiation.
Hyperglycemia increases the magnitude and duration of systemic
inflammatory responses, which promotes the development of T2DM and
cardiovascular disease (41).
Inflammation leads to cell apoptosis and oxidative DNA damage of
the heart, liver and kidneys (42).
Inflammation in the progression of steatohepatitis is a complex
response to microbial dysbiosis, loss of barrier integrity in the
intestine, hepatocellular stress and death, as well as inter-organ
crosstalk. In response to chronic, heavy alcohol exposure,
hepatocytes express a large number of chemokines and inflammatory
mediators and can also release damage-associated molecular patterns
during injury and death (43).
Disruption of the intestinal epithelial barrier and gut vascular
barrier are early events in the development of NASH (44). The present results indicated that
short-term high glucose increased the systemic inflammatory
responses of rats. The association between organ damage and
preinflammation warrants further research.
There were some limitations in the present study.
First, the dose-dependent effect of high glucose was not evaluated.
Second, insulin sensitivity was not assessed. In addition, the
reason why high blood glucose induced weight loss warrants further
research.
In conclusion, in the present study it was revealed
that high glucose may induce lesions in the liver and intestinal
epithelium, disturb the balance of the gut microbiota and
consequently induce inflammation. Glomerular cells were revealed to
be damaged, however, no obvious pathological lesions were
identified in the kidney tissues of the rats.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
Department of Science and Technology of Guangdong Province (grant
no. 2021A1515220050) and the Guangdong Provincial Bureau of
Traditional Chinese Medicine (grant no. 20223001).
Availability of data and materials
The datasets used in the present study are available
from the corresponding author upon reasonable request. The raw
reads were deposited into the NCBI Sequence Read Archive database
(accession no. PRJNA884142; ID:884142).
Authors' contributions
CM conceived and designed the study. TF, YD and WT
collected and analyzed the data. XH and TW wrote the manuscript.
CM, TF, WT, TW, YD and XH revised the manuscript critically for
important intellectual content. CM and TF confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the committee guidelines of Guangdong Provincial People's
Hospital (Guangzhou, China) and approved (approval no.
KY-D-2019-082-01) by the Institutional Animal Care and Use
Committee of Guangdong Provincial People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dall TM, Yang W, Gillespie K, Mocarski M,
Byrne E, Cintina I, Beronja K, Semilla AP, Iacobucci W and Hogan
PF: The economic burden of elevated blood glucose levels in 2017:
Diagnosed and undiagnosed diabetes, gestational diabetes mellitus,
and prediabetes. Diabetes Care. 42:1661–1668. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cowie CC: Diabetes diagnosis and control:
Missed opportunities to improve health: The 2018 Kelly West award
lecture. Diabetes Care. 42:994–1004. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Filla LA and Edwards JL: Metabolomics in
diabetic complications. Mol Biosyst. 12:1090–1105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zou X, Zhou X, Ji L, Yang W, Lu J, Weng J,
Jia W, Shan Z, Liu J, Tian H, et al: The characteristics of newly
diagnosed adult early-onset diabetes: A population-based
cross-sectional study. Sci Rep. 7(46534)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Riddle MC and Gerstein HC: The
cardiovascular legacy of good glycemic control: Clues about
mediators from the DCCT/EDIC study. Diabetes Care. 42:1159–1161.
2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gaballa M and Farag MK: Predictors of
diabetic nephropathy. Eur J Med. 8:287–296. 2013.
|
|
7
|
MacIsaac RJ and Ekinci EI: Progression of
diabetic kidney disease in the absence of albuminuria. Diabetes
Care. 42:1842–1844. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rines AK, Sharabi K, Tavares CD and
Puigserver P: Targeting hepatic glucose metabolism in the treatment
of type 2 diabetes. Nat Rev Drug Discov. 15:786–804.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kahl S, Gancheva S, Straßburger K, Herder
C, Machann J, Katsuyama H, Kabisch S, Henkel E, Kopf S, Lagerpusch
M, et al: Empagliflozin effectively lowers liver fat content in
well-controlled type 2 diabetes: A randomized, double-blind, phase
4, placebo-controlled trial. Diabetes Care. 43:298–305.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen M, Zheng H, Xu M, Zhao L, Zhang Q,
Song J, Zhao Z, Lu S, Weng Q, Wu X, et al: Changes in hepatic
metabolic profile during the evolution of STZ-induced diabetic rats
via an 1H NMR-based metabonomic investigation. Biosci
Rep. 39(BSR20181379)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rai RC, Bagul PK and Banerjee SK: NLRP3
inflammasome drives inflammation in high fructose fed diabetic rat
liver: Effect of resveratrol and metformin. Life Sci.
253(117727)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ju M, Liu Y, Li M, Cheng M, Zhang Y, Deng
G, Kang X and Liu H: Baicalin improves intestinal microecology and
abnormal metabolism induced by high-fat diet. Eur J Pharmacol.
857(172457)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang Y, Gu Y, Ren H, Wang S, Zhong H,
Zhao X, Ma J, Gu X, Xue Y, Huang S, et al: Gut microbiome-related
effects of berberine and probiotics on type 2 diabetes (the PREMOTE
study). Nat Commun. 11(5015)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Malik VS, Willett WC and Hu FB: Global
obesity: Trends, risk factors and policy implications. Nat Rev
Endocrinol. 9:13–27. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shannon C, Merovci A, Xiong J, Tripathy D,
Lorenzo F, McClain D, Abdul-Ghani M, Norton L and DeFronzo RA:
Effect of chronic hyperglycemia on glucose metabolism in subjects
with normal glucose tolerance. Diabetes. 67:2507–2517.
2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tsuchiya K: Inflammasome-associated cell
death: Pyroptosis, apoptosis, and physiological implications.
Microbiol Immunol. 64:252–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chiu THT, Pan WH, Lin MN and Lin CL:
Vegetarian diet, change in dietary patterns, and diabetes risk: A
prospective study. Nutr Diabetes. 8(12)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Taylor R and Barnes AC: Translating
aetiological insight into sustainable management of type 2
diabetes. Diabetologia. 61:273–283. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Prasad M, Chen EW, Toh SA and Gascoigne
NRJ: Autoimmune responses and inflammation in type 2 diabetes. J
Leukoc Biol. 107:739–748. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Purnamasari D, Khumaedi AI, Soeroso Y and
Marhamah S: The influence of diabetes and or periodontitis on
inflammation and adiponectin level. Diabetes Metab Syndr.
13:2176–2182. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Biscetti F, Ferraro PM, Hiatt WR, Angelini
F, Nardella E, Cecchini AL, Santoliquido A, Pitocco D, Landolfi R
and Flex A: Inflammatory cytokines associated with failure of
lower-extremity endovascular revascularization (LER): A prospective
study of a population with diabetes. Diabetes Care. 42:1939–1945.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kheiripour N, Karimi J, Khodadadi I,
Tavilani H, Goodarzi MT and Hashemnia M: Silymarin prevents lipid
accumulation in the liver of rats with type 2 diabetes via sirtuin1
and SREBP-1c. J Basic Clin Physiol Pharmacol. 29:301–308.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Taylor R, Al-Mrabeh A and Sattar N:
Understanding the mechanisms of reversal of type 2 diabetes. Lancet
Diabetes Endocrinol. 7:726–736. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ma Z, Fang L, Ungerfeld E, Li X, Zhou C,
Tan Z, Jiang L and Han X: Supplementation of rumen-protected
glucose increased the risk of disturbance of hepatic metabolism in
early postpartum holstein cows. Antioxidants (Basel).
11(469)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bril F, Portillo Sanchez P, Lomonaco R,
Orsak B, Hecht J, Tio F and Cusi K: Liver safety of statins in
prediabetes or T2DM and nonalcoholic steatohepatitis: Post Hoc
analysis of a randomized trial. J Clin Endocrinol Metab.
102:2950–2961. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tsujita M, Hossain MA, Lu R, Tsuboi T,
Okumura-Noji K and Yokoyama S: Exposure to high glucose
concentration decreases cell surface ABCA1 and HDL biogenesis in
hepatocytes. J Atheroscler Thromb. 24:1132–1149. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Leviatan S and Segal E: Identifying gut
microbes that affect human health. Nature. 587:373–374.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Haase S, Haghikia A, Wilck N, Müller DN
and Linker RA: Impacts of microbiome metabolites on immune
regulation and autoimmunity. Immunology. 154:230–238.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Botchlett R, Li H, Guo X, Qi T, Zhao J,
Zheng J, Woo SL, Pei Y, Liu M, Hu X, et al: Glucose and palmitate
differentially regulate PFKFB3/iPFK2 and inflammatory responses in
mouse intestinal epithelial cells. Sci Rep. 6(28963)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sittipo P, Shim JW and Lee YK: Microbial
metabolites determine host health and the status of some diseases.
Int J Mol Sci. 20(5296)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Do MH, Lee E, Oh MJ, Kim Y and Park HY:
High-glucose or -fructose diet cause changes of the gut microbiota
and metabolic disorders in mice without body weight change.
Nutrients. 10(761)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou T, Heianza Y, Chen Y, Li X, Sun D,
DiDonato JA, Pei X, LeBoff MS, Bray GA, Sacks FM and Qi L:
Circulating gut microbiota metabolite trimethylamine N-oxide (TMAO)
and changes in bone density in response to weight loss diets: The
POUNDS lost trial. Diabetes Care. 42:1365–1371. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee SA, Cozzi M, Bush EL and Rabb H:
Distant organ dysfunction in acute kidney injury: A review. Am J
Kidney Dis. 72:846–856. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Li YJ, Chen X, Kwan TK, Loh YW, Singer J,
Liu Y, Ma J, Tan J, Macia L, Mackay CR, et al: Dietary fiber
protects against diabetic nephropathy through short-chain fatty
acid-mediated activation of G protein-coupled receptors GPR43 and
GPR109A. J Am Soc Nephrol. 31:1267–1281. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu X, Lu J, Liao Y, Liu S, Chen Y, He R,
Men L, Lu C, Chen Z, Li S, et al: Dihydroartemisinin attenuates
lipopolysaccharide-induced acute kidney injury by inhibiting
inflammation and oxidative stress. Biomed Pharmacother.
117(109070)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Whitt J, Woo V, Lee P, Moncivaiz J,
Haberman Y, Denson L, Tso P and Alenghat T: Disruption of
epithelial HDAC3 in intestine prevents diet-induced obesity in
mice. Gastroenterology. 155:501–513. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li L, Ma L and Fu P: Gut
microbiota-derived short-chain fatty acids and kidney diseases.
Drug Des Devel Ther. 11:3531–3542. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cao H, Li C, Lei L, Wang X, Liu S, Liu Q,
Huan Y, Sun S and Shen Z: Stachyose improves the effects of
berberine on glucose metabolism by regulating intestinal microbiota
and short-chain fatty acids in spontaneous type 2 diabetic KKAy
mice. Front Pharmacol. 11(578943)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Samsu N: Diabetic nephropathy: Challenges
in pathogenesis, diagnosis, and treatment. Biomed Res Int.
2021(1497449)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Oshima M, Shimizu M, Yamanouchi M, Toyama
T, Hara A, Furuichi K and Wada T: Trajectories of kidney function
in diabetes: A clinicopathological update. Nat Rev Nephrol.
17:740–750. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Poznyak A, Grechko AV, Poggio P,
Myasoedova VA, Alfieri V and Orekhov AN: The diabetes
mellitus-atherosclerosis connection: The role of lipid and glucose
metabolism and chronic inflammation. Int J Mol Sci.
21(1835)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Neves-Costa A and Moita LF: Modulation of
inflammation and disease tolerance by DNA damage response pathways.
FEBS J. 284:680–698. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gao B, Ahmad MF, Nagy LE and Tsukamoto H:
Inflammatory pathways in alcoholic steatohepatitis. J Hepatol.
70:249–259. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Mouries J, Brescia P, Silvestri A, Spadoni
I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini
L, et al: Microbiota-driven gut vascular barrier disruption is a
prerequisite for non-alcoholic steatohepatitis development. J
Hepatol. 71:1216–1228. 2019.PubMed/NCBI View Article : Google Scholar
|