Introduction
Chronic stress affects brain function and induces
long-term alterations in various neural systems related to anxiety,
depression, cognition and insomnia (1-3).
Prolonged exposure to stress has been found to play a crucial
factor in the progression of dementia and in the development of
neurological disorders, including Alzheimer's disease (AD) and
major depressive disorder (4-6).
Animal models of chronic restraint stress (CRS) are commonly used
to induce learning and memory deficits, and neuronal damage to the
hippocampus (7,8). The hippocampus is one of the brain
regions which is most vulnerable to stress-induced damage, due to
the high expression of mineralocorticoid receptors and
glucocorticoid receptors (GR) (9,10).
Previous studies have demonstrated that exposure to stress induces
alterations in hippocampal neurogenesis, synaptic plasticity and
neuronal cell survival (11-13).
Additionally, chronic stress also provokes dendritic atrophy and
causes spatial memory impairment (14,15).
Furthermore, exposure to chronic stress can interrupt the balance
of oxidants and antioxidants, leading to the overproduction of free
radicals which can in turn induce brain damage (16). Oxidative stress is considered a
crucial factor involved in the pathogenesis of neurodegenerative
diseases, including AD, Parkinson's disease (PD), depression and
memory impairment (17,18). High levels of oxidative stress can
induce memory impairment mediated by changes in hippocampal
synaptic plasticity (19). In
addition, there is also evidence to indicate that antioxidants can
ameliorate oxidative stress-induced neuronal damage (20). Therefore, the use of antioxidant
agents may represent a successful strategy for the prevention and
treatment of cognitive dysfunction.
Herbal medicinal substances exerting neuroprotective
effects have been considered to be pharmacological agents for the
treatment of several neurodegenerative diseases. Pinostrobin, a
natural bioflavonoid compound isolated from the rhizomes of
Boesenbergia rotunda (L.) or Krachai in Thai, has been
revealed to exhibit numerous biological properties, including
antioxidant, anti-inflammatory and neuroprotective activities
(21-23).
Pinostrobin has been shown to ameliorate β-amyloid-induced
neurotoxicity in PC12 cells by suppressing reactive oxygen species
(ROS) and calcium overload (23).
In addition, pinostrobin has been reported to reduce the loss of
dopaminergic neurons by suppressing oxidative stress in a model of
PD (24). However, to date, at
least to the best of our knowledge, there are no available data on
the protective effects of pinostrobin in an animal model of
CRS-induced cognitive deficits. Therefore, the present study aimed
to examine the neuroprotective potential and possible mechanisms of
action of pinostrobin in chronic stress-induced brain damage and
cognitive dysfunction in rats.
Materials and methods
Animals
A total of 28 adult male Wistar rats (weighing
200-220 g, at the commencement of the experiment) were obtained
from the Nomura Siam International (Bangkok, Thailand). The animals
were allowed a week for acclimatization prior to the commencement
of the experiments. All experimental procedures were conducted
during the dark phase of the light cycle with standard chow and
water available ad libitum in a room with a constant
temperature (21±1˚C) and humidity (35-60%). All experiments were
approved by the Ethics Committee of the Laboratory Animal Research
Center, University of Phayao, Thailand (approval no.
640104006).
Preparation of pinostrobin
Fresh rhizomes of B. rotunda were collected
from Phayao, Thailand. The voucher specimen (no. S. Sedlak 19-1)
was authenticated by the Walai Rukhavej Botanical Research
Institute, Mahasarakham University, Maha Sarakham, Thailand. The
ethanolic crude extract (251 g) was resuspended in methanol to
yield a pale yellow methanol-insoluble solid (50 g). The obtained
solid was further subjected to an open column chromatography using
silica gel (Arch. No. 7734, pore size 60 Å, particle size 70-230
mesh, Merck) as an adsorbent. The column was eluted with the
mixture of 40-80% dichloromethane-hexane under gradient separation.
The similar pattern fractions were combined based on thin-layer
chromatography analysis and recrystallized with methanol to yield
pure pinostrobin, while the purity was found to be >98% based on
HPLC analysis. Structural elucidation was performed using 13C- and
1H-NMR spectroscopy (Bruker Avance DRX500 Spectrometer) and the NMR
spectra were compared with those in the published literature
(25) as follows: 1H NMR (500 MHz,
acetone-d6): 2.80 (dd, J=17.2, 3.0 Hz; 1H, H-3a), 3.06 (dd, J=17.2,
13.0 Hz; 1H, H-3b), 3.79 (s, 3H; -OCH3), 5.39 (dd, J=13.0, 3.0 Hz;
1H, H-2), 6.06 (m, 2H, H-6, H-8), 7.41 (m, 5H; H-2', H-3', H-3',
H-5', H-6'). 13C NMR (150 MHz, acetone-d6): 43.1 (C-3), 55.7
(C-7-OCH3), 79.2 (C-2), 94.3 (C-8), 95.1 (C-6), 103.1 (C-10), 126.1
(C-2', C-3', C-5', C-6'), 128.9 (C-4'), 138.4 (C-1'), 162.8 (C-5),
164.1 (C-9), 167.9 (C-7), 195.8 (C-4). (NMR data were recorded on
January 13, 2021, Central Science Laboratory, Faculty of Science,
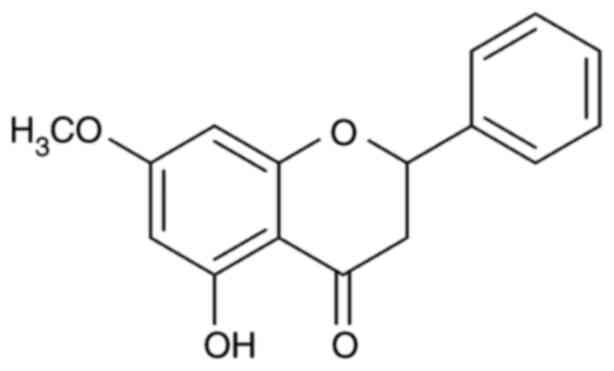
Chiang Mai University, Thailand). The chemical structure of
pinostrobin is illustrated in Fig.
1.
Experimental procedure
All animals were randomly divided into four
experimental groups (7 rats per group) as follows: i) The control
group, no stress; ii) the vehicle + CRS group; iii) the pinostrobin
20 mg/kg + CRS goup; and iv) the pinostrobin 40 mg/kg + CRS group.
The rats were exposed to restraint stress using a wire mesh
restraint secured with butterfly clips that closely fit the body of
the rats, as described in a previous study (26). The restraint stress procedure was
repeated once daily for 6 h per day (10.00 am to 16.00 pm) for 21
consecutive days. During the restraint sessions, the unstressed
control animals were handled for 2 min. The animals were
administered 1% carboxymethylcellulose (CMC) used as the vehicle or
pinostrobin (at doses of 20 or 40 mg/kg) via oral gavage daily at
30 min prior to stress exposure for 21 days. The learning and
memory performance of all animals were assessed by using a Y-maze
and novel object recognition (NOR) tests (as described below) on
day 0 (prior to beginning the experiment) for baseline data and on
the last day of the experiment (day 21) for the therapeutic effects
of pinostrobin. At 24 h after the last behavioral test, all animals
were sacrificed by transcardial perfusion with 0.1 M PBS for the
evaluation of biochemical and immunohistochemical parameters. The
doses of pinstrobin were selected based on a previous study
(27).
Tissue processing and
immunohistochemistry
All animals were deeply anesthetized via an
intraperitoneal injection of thiopental sodium (70 mg/kg body
weight) and perfused with ice-cold 0.1 M PBS. The brains (n=28)
were immediately removed and cut at the midline, dividing the brain
into two hemispheres. The hippocampus from the left hemisphere was
separated and stored at -80˚C for biochemical determination. The
right hemisphere was fixed with ice-cold 4% paraformaldehyde and
cryopreserved in 12.5% sucrose for immunohistochemistry. The brains
were sectioned into 30-µm-thick sections using a cryostat microtome
(AST500, Amos Scientific Pty Ltd.) and stored in an anti-freeze
solution (4˚C). The coronal sections were rinsed and incubated in
3% H2O2 and followed by 3% normal horse serum
(cat. no. A9647, Sigma-Aldrich; Merck KGaA) for non-specific
blocking. Subsequently, sections were incubated with the primary
antibodies, mouse anti-glial fibrillary acidic protein (GFAP;
1:500, cat. no. MAB5628, MilliporeSigma), or rabbit anti-excitatory
amino acid transporter 2 (EAAT2; (1:200, cat. no. ab41621, Abcam)
at 4˚C overnight. The sections were washed in 0.1 M PBS for 30 min
and incubated for 2 h at room temperature with biotinylated donkey
anti-mouse secondary antibody (1:500, cat. no. 715-065-150, Jackson
ImmunoResearch Europe, Ltd.) or anti-rabbit (1:500, cat. no.
711-065-152, Jackson ImmunoResearch Europe, Ltd.). The sections
were rinsed in 0.1 M PBS followed by 1 h of incubation in 0.1%
extravidin peroxidase (1:1,000, cat. no. E2886, Sigma-Aldrich;
Merck KGaA) at room temperature, and then rinsed again.
Immunolabeling was developed using a nickel-enhanced
3,3'-diaminobenzidine (DAB) reaction (cat. no. D12384,
Sigma-Aldrich; Merck KGaA). Finally, the sections were washed in
0.1 M PBS and, then mounted on positive charged slides, dehydrated
using graded alcohols, cleared in xylene, and cover-slipped using
mounting medium (cat. no. 107961, Sigma-Aldrich; Merck KGaA). For
Nissl staining, the sections were stained with 0.1% cresyl violet
(cat. no. 1.05235.0025, Sigma-Aldrich; Merck KGaA) for 8 min at
60˚C, dehydrated with ethanol, cleared in xylene, and coverslipped
using mounting medium.
Determination of the malondialdehyde
(MDA) level
The level of MDA was evaluated as an indicator of
lipid peroxidation. A total of seven hippocampi from each group
were homogenized in 0.1 M PBS (pH 7.4), centrifuged at 9,279 x g at
4˚C for 15 min, and the supernatant was subjected to the
thiobarbituric acid reaction according to the protocol previously
described by Nakmareong et al (28) with minor modifications. Briefly, the
mixture consisted of 75 µl sample or standard
(1,1,3,3-tetraethoxypropane) (cat. no. 108383, Sigma-Aldrich; Merck
KGaA), 10% TCA (cat. no. 100807, Merck KGaA), 5 mM EDTA (cat. no.
AR1240, RCI Labscan Ltd.), 8% SDS (cat. no. S/5200/53, Thermo
Fisher Scientific, Inc.) and 0.5 µg/ml BHT (cat. no. 02381, LOBA
Chemie Pvt. Ltd.) was incubated at room temperature for 10 min. The
mixture was then supplemented with 250 µl 0.6% TBA (cat. no.
108180, Merck KGaA), and boiled in a water bath for 30 min. After
cooling, the mixture was centrifuged at 10,000 x g for 5 min at
4˚C. The absorbance was measured at 532 nm by microplate reader
(Synergy H1, BioTek Instruments, Inc.). The results are as
expressed as µmol/mg protein.
Determination of superoxide dismutase
(SOD) activity
SOD activity was determined using a colorimetric
assay kit (cat. no. S19160, Sigma-Aldrich; Merck KGaA) according to
the manufacturer's instructions. The results were presented as the
inhibition rate (%).
Determination of catalase (CAT)
activity
Catalase activity was measured based on the enzyme
degradation of H2O2 according to previously
published study with some modifications (29). Briefly, 20 µl of sample were mixed
with 100 µl of 6 mM H2O2 for initiating the
enzymatic reactions followed by incubation at 37˚C for 1 min. The
reaction was then terminated with 100 µl of 32.4 mM ammonium
molybdate and the absorbance at 405 nm was measured using a
microplate reader (Synergy H1, BioTek Instruments, Inc.). The
results are presented as U/mg protein.
Cell count analysis and
thresholding
To determine the density of neurons, hippocampal
images were captured at x40 magnification using a bright-field
microscope (Nikon Corporation). Images of subregions of the
hippocampus (at x40 magnification), including CA1, CA2 and CA3 were
subjected to exhaustive manual counts using NIS Elements imaging
software version 5 (Nikon Corporation). For thresholding function,
the immunoreactivity of the astrocytes within the hippocampus was
determined using the thresholding function of Image J software
(Version 1.53, National Institutes of Health). Image thresholding
is the frequently used technique to quantitatively determine the
alterations in immunolabelled material as previously described
(30). The data are presented as
the percentage of threshold material.
Y-maze test
The Y-maze test was used to measure working memory
in animal by recording a spontaneous alternation (31). The Y-maze consisted of three arms
(40 cm long x 33 cm high x 15 cm wide, separated by an angle of
120˚, Laboratory Animal Research Center, University of Phayao,
Thailand). All animals were individually placed at one of three
enclosed arms for free exploration for total of 8 min. A
spontaneous alternation was defined as any combination of three
sequential entries in which the animal entered all three arms
(e.g., ABC, CAB, or BCA, but not ABB). The percentage of
spontaneous alternation was used as an index of working memory and
calculated according to the following equation: The spontaneous
alternation (%)=[(number of alternations)/(total arm entries-2)]
x100(32). After each session, the
maze was cleaned with 70% ethanol to avoid odors.
Novel object recognition (NOR)
test
The NOR test was performed in a black open field box
(45x65x45 cm, Laboratory Animal Research Center, University of
Phayao, Thailand) to determine the recognition memory. The test
composed of three sessions (habituation phase, training phase and
test phase) using a previously described method with minor
modifications (33). At the end of
the treatment period, the animals were allowed to explore the empty
open field for 5 min during the habituation phase. During the
training phase, two identical objects (A1 and A2) were placed in
two corners of the open field. The rats were placed in the middle
of the open field and allowed to freely explore these two identical
objects for 5 min and then the animals were returned to home cage.
After 4 h of post-training phase, a new object (B) was placed and
the animals were left to investigate the two objects for 5 min. The
exploration time identifying as pointing the nose to the object at
a distance ≤2 cm was manually recorded using a stopwatch. A
recognition index (RI) was calculated using the following formula:
[TB/(TA + TB) x100], where TA and TB are the time spent exploring
familiar object A and novel object B, respectively (34).
Statistical analysis
All data were analyzed using GraphPad Prism 9
(GraphPad Software, Inc.). Data are expressed as the mean ± SEM.
Statistical analysis was performed using one-way ANOVA, followed by
Tukey's post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effects of pinostrobin against
oxidative stress in the hippocampus
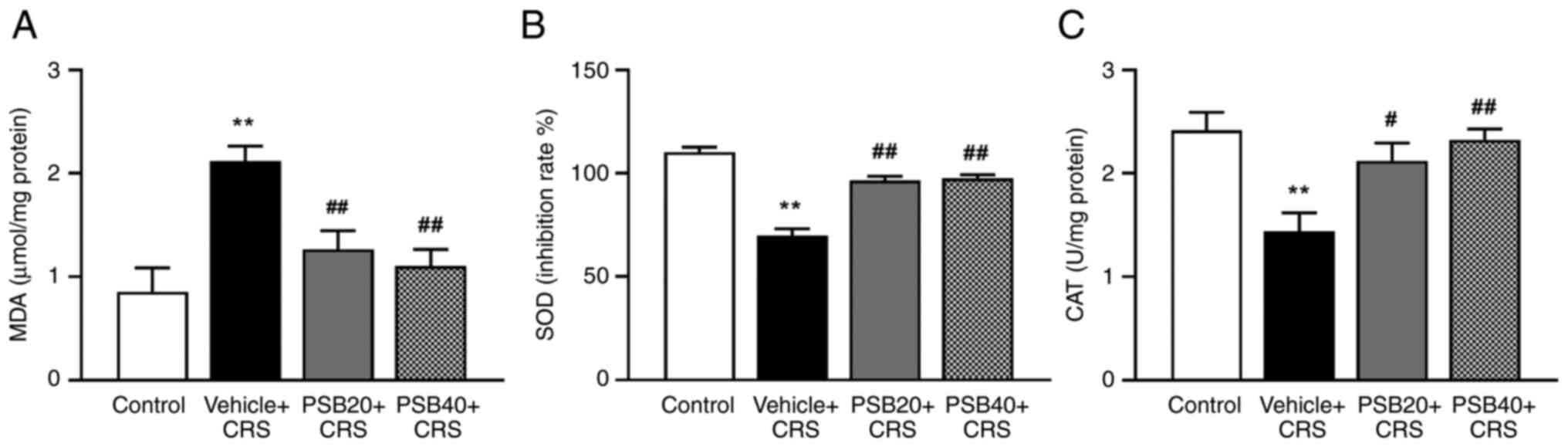
The antioxidant activities of pinostrobin were
determined by measuring the levels of MDA, which is an indicator of
lipid peroxidation, and the activities of some of the main
scavenging enzymes, such as SOD and CAT in the hippocampus
(Fig. 2). The results demonstrated
that the amount of hippocampal MDA was markedly enhanced, whereas
the activities of SOD and CAT were significantly decreased in the
rats subjected to CRS (P<0.01, Fig.
2). Notably, treatment with pinostrobin (20 and 40 mg/kg)
significantly decreased the level of MDA in the hippocampus
compared to the vehicle-chronic stress group (P<0.01, Fig. 2A). In addition, treatment with
pinostrobin at a low dose (20 mg/kg) markedly attenuated the
chronic stress-induced reduction in the levels of antioxidant
enzymes in the hippocampus compared with vehicle chronic
stress-treated group (SOD, P<0.01, Fig. 2B; CAT, P<0.05, Fig. 2C). Furthermore, treatment with
pinostrobin at a dose of 40 mg/kg also significantly increased the
activities of SOD and CAT (SOD, P<0.01, Fig. 2B; CAT, P<0.01, Fig. 2C).
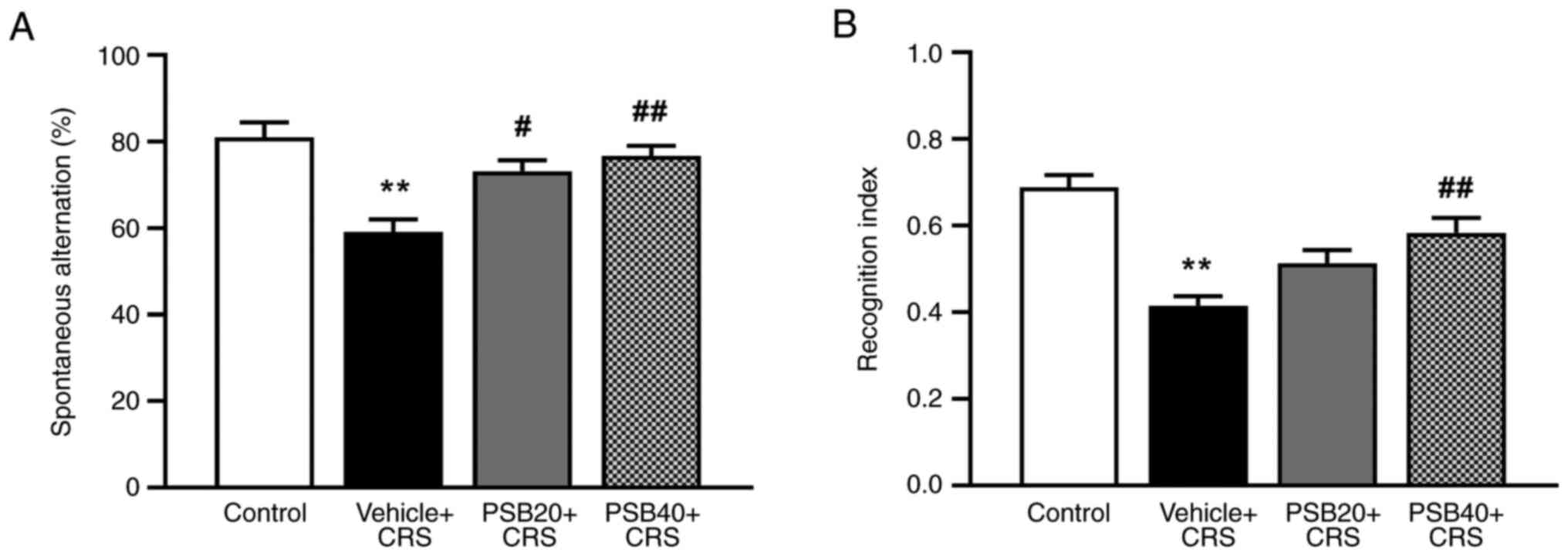
Effects of pinostrobin on CRS-induced
memory impairment of rats in the Y-maze and NOR tests
The Y-maze and NOR tests were used to determine
whether pinostrobin could reverse CRS-induced cognitive impairment
(Fig. 3). Prior to the commencement
of the experiment (day 0), the cognitive function of the rats was
determined using Y-maze and NOR tests for baseline data. It was
found that there were no significant differences between the groups
(data not shown). Additionally, behavioral assessment revealed that
21 consecutive days of exposure to restraint stress affected the
working memory of the animals by decreasing the percentage of
spontaneous alternation in the Y-maze compared to the control
animals (P<0.01). However, this impairment was considerably
restored following treatment with pinostrobin (20 mg/kg, P<0.05;
40 mg/kg, P<0.01; Fig. 3A).
After the Y-maze test, the animals were immediately examined for
recognition memory using the NOR test. In rats exposed to CRS, the
administration of pinostrobin at a dose of 40 mg/kg significantly
improved cognitive function by enhancing the recognition index
compared to vehicle-treated group (P<0.01, Fig. 3B).
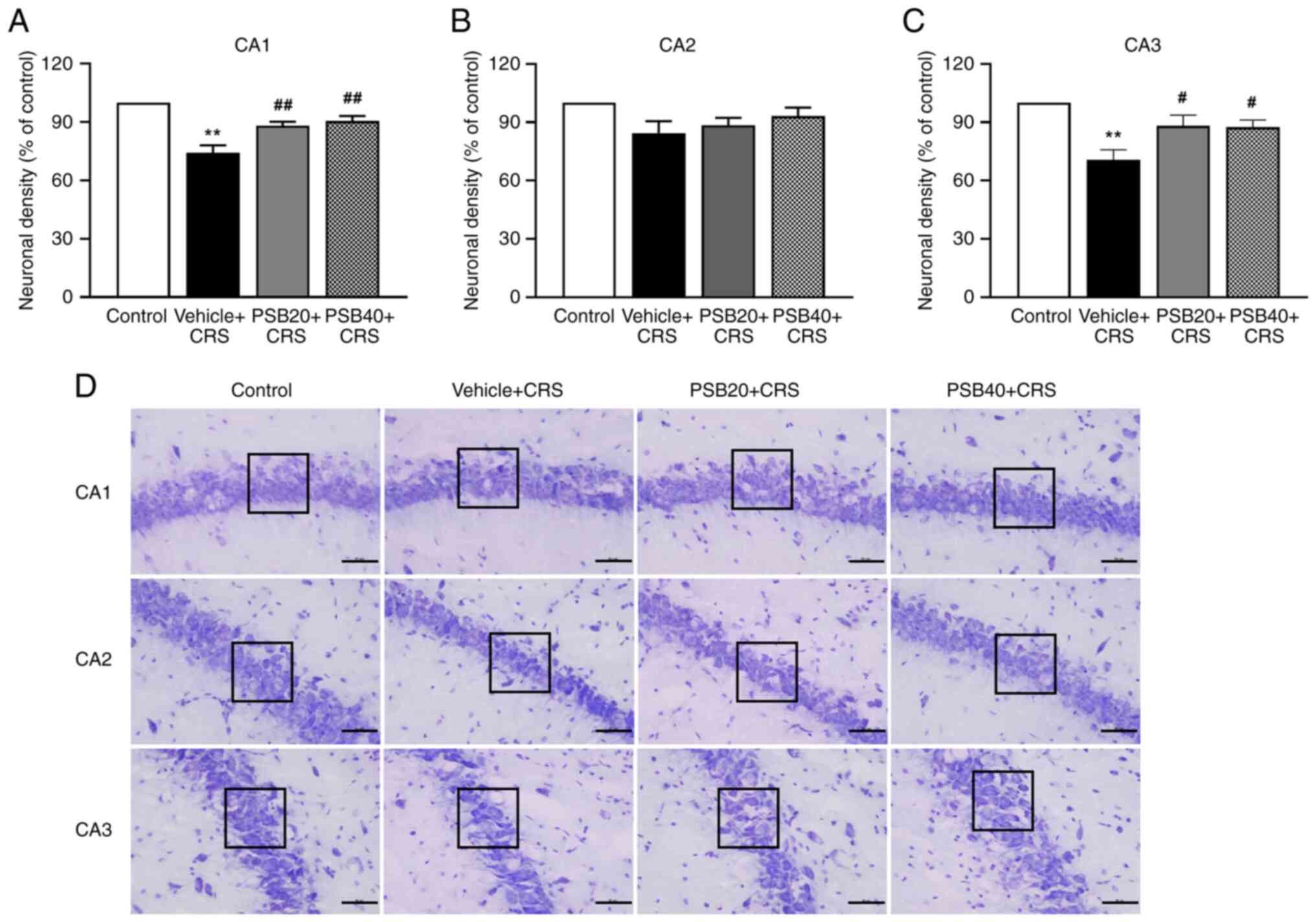
Effects of pinostrobin on neuronal
damage in the hippocampus
Chronic stress is considered an important risk
factor that can induce neuronal damage (7). Therefore, in the present study, the
neuronal density in the hippocampus was assessed for the possible
involvement of neuroprotective mechanism. The effects of
pinostrobin on neuronal damage were investigated by detecting Nissl
staining in the hippocampus (Fig.
4). The animals exposed to CRS exhibited a significant
reduction in neuronal density in the hippocampal CA1 and CA3
regions. As shown in Fig. 4D, the
neurons in the hippocampal CA1 and CA3 regions of the CRS group
were evidently damaged, and missing. However, the administration
with pinostrobin at doses of 20 and 40 mg/kg markedly improved the
density of surviving cells in the CA1 and CA3 regions of the
hippocampus (CA1, P<0.01 at 20 and 40 mg/kg, Fig. 4A; CA3, P<0.05 at 20 and 40 mg/kg,
Fig. 4C). However, no significant
difference was found in the neuronal density in the hippocampal CA2
region.
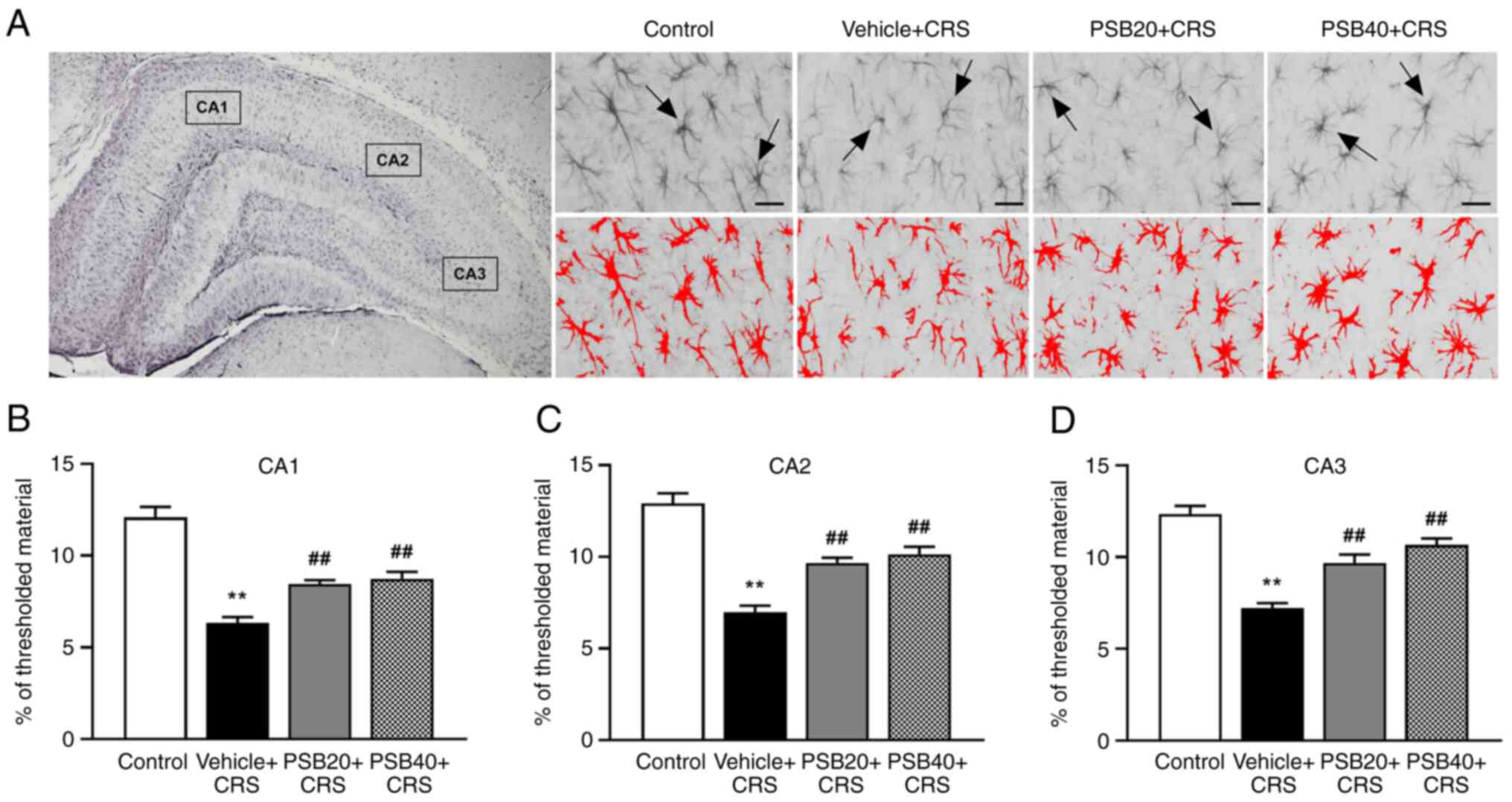
Effects of pinostrobin on the
alteration of GFAP immunoreactivity in the hippocampus
To examine whether CRS affects astrocytes, the
alteration of GFAP-immunoreactive astrocytes in the hippocampus was
further evaluated using immunohistochemistry and threshold
analysis. As shown by the results illustrated in Fig. 5, the rats exposed to CRS exhibited a
significant reduction in GFAP immunostaining in all regions of the
hippocampus compared to the controls (P<0.01). Notably, it was
found that the astrocytes of the rats exposed to CRS exhibited
small cellular bodies with less and thinner processes,
characterized as atrophy (Fig. 5A).
However, treatment of these rats with pinostrobin at 20 and 40
mg/kg markedly ameliorated the CRS-induced downregulation of GFAP
in the hippocampus compared with the vehicle-treated group
(P<0.01, Fig. 5B-D).
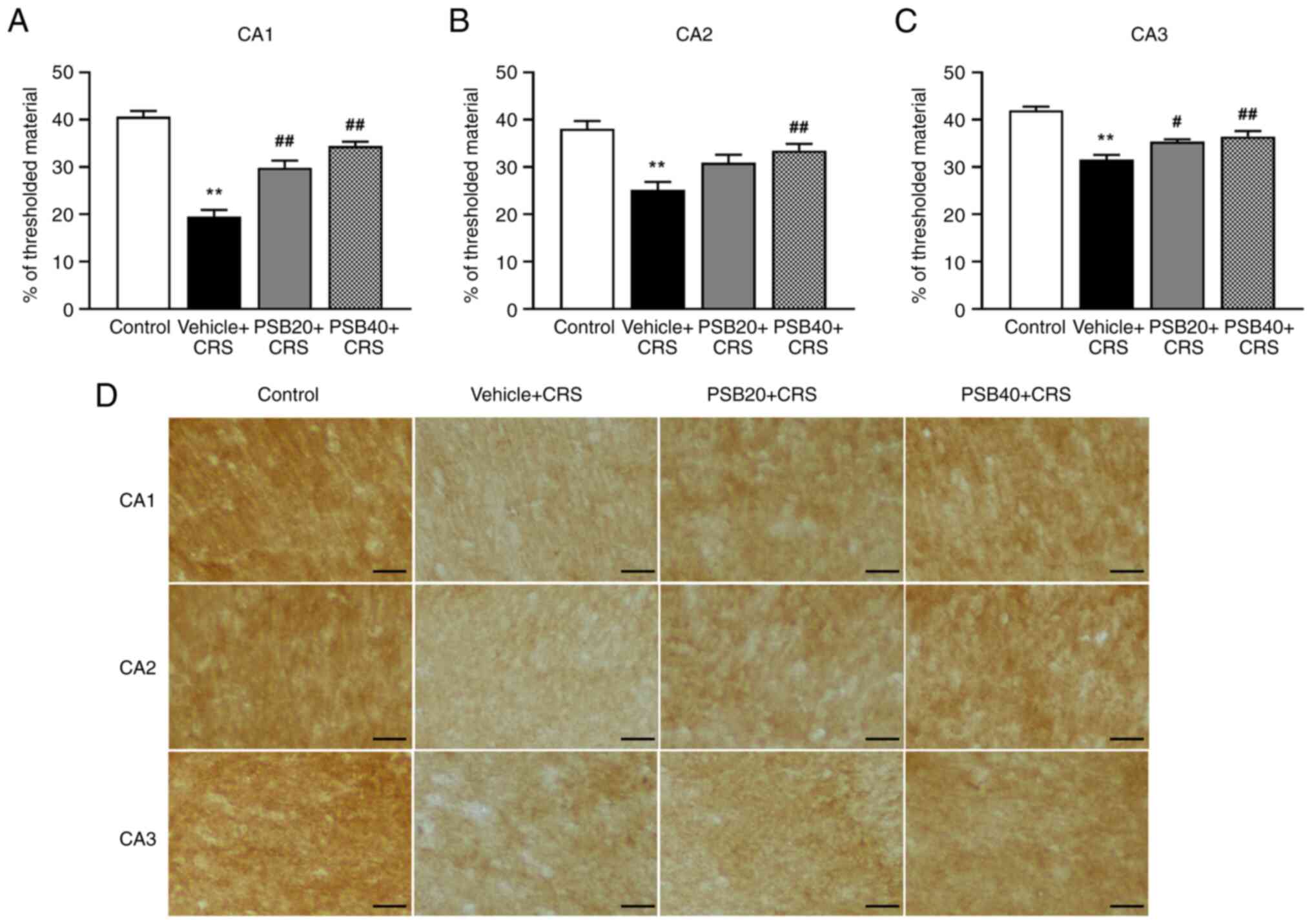
Effects of pinostrobin on the
alteration of EAAT2 immunolabeling in the hippocampus
To determine the mechanisms through which
pinostrobin altered the level of glutamate transporter expressed in
astrocytes, the immunoreactivity of EAAT2 was further investigated
using immunohistochemical analysis. As illustrated in Fig. 6, EAAT2 immunoreactivity was markedly
decreased in the rats exposed to CRS, whereas treatment with
pinostrobin at a dose of 20 mg/kg upregulated the expression of
EAAT2 in the CA1 and CA3 regions of the hippocampus (CA1,
P<0.01; CA3, P<0.05; Fig. 6A
and C). Notably, a high dose of
treatment (40 mg/kg) enhanced the expression of EAAT2 in all
subregions of the hippocampus (P<0.01).
Discussion
The present study investigated the neuroprotective
effects of pinostrobin on cognitive performance in rats exposed to
CRS. The results suggested that the administration of pinostrobin
ameliorated chronic stress-induced cognitive impairment by exerting
antioxidant effects, reducing neuronal cell damage, and enhancing
the expression of astrocytic GFAP and EAAT2 in the hippocampus.
Previous studies have revealed that chronic stress is an important
regulatory factor for the development of cognitive dysfunction
(35-37).
As the main structure of the brain associated with cognition and
mood, the hippocampus is extremely susceptible to exposure to
chronic stress. The hippocampus contains high quantities of GRs.
High glucocorticoid levels trigger ROS production, inducing
oxidative damage in the hippocampus, resulting in cognitive
impairment. Additionally, chronic stress promotes the structural
and functional alterations of the hippocampus (38,39),
leading to disruptions in cognitive function. The enhanced
production of ROS and reduced levels of antioxidants have been
implicated in the pathogenesis of neurodegenerative diseases, such
as depression and cognitive disorders (40). Exposure to chronic stress can alter
the balance of oxidants and antioxidants, leading to a large
generation of free radicals, thus inhibiting the antioxidant
efficacy. The elevated amounts of lipid peroxidation and diminished
antioxidant levels lead to the incidence of oxidative damage in the
hippocampus of rats subjected to chronic stress. The findings of
the present study demonstrated that chronic stress significantly
enhanced MDA levels, accompanied by decreased levels of the major
antioxidant enzymes, SOD and CAT, in the hippocampus; these
findings are consistent with those of previous studies (41,42).
According to previous findings, the supplementation
of natural compounds can ameliorate learning and memory deficits
involved in the CRS-induced production of oxidative stress
(37,43). Pinostrobin exhibits a several of
pharmacological activities, such as antioxidant, anti-inflammatory
and neuroprotective properties (21-23).
Pinostrobin has been previously reported to exert antioxidant
effects on ethanol-induced ulcers in rats by reducing the MDA and
nitric oxide levels (27). In
addition, pinostrobin has been found to inhibit the production of
inflammatory cytokines, such as TNF-α and IL-1β in both in
vitro and in vivo experiments, indicating
anti-inflammatory activity (22).
Pre-treatment with pinostrobin has been reported to exert
neuroprotective effects against β-amyloid-induced neurotoxicity by
suppressing oxidative damage and neuronal apoptosis (23). Moreover, pinostrobin has been shown
to reduce the loss of dopaminergic neurons in a model of PD by
exerting antioxidant and neuroprotective effects by decreasing
lipid peroxidation and enhancing the levels of antioxidant enzymes
(24). Therefore, in the present
study, the antioxidant and neuroprotective potential of pinostrobin
was determined. The results demonstrated that chronic treatment
with pinostrobin at doses of 20 and 40 mg/kg significantly reduced
the level of hippocampal lipid peroxidation induced by CRS. In
addition, treatment with pinostrobin significantly reversed the
CRS-induced decrease in hippocampal SOD and CAT activities. This
may be one of the possible underlying mechanisms for the
neuroprotective potential of pinostrobin in the cognitive
impairment of rats with CRS. However, the present study determined
oxidative stress markers only in the hippocampal homogenate, and
not by using histological analysis. This is a limitation of the
present study. Thus, further study are required to examine the
alterations in oxidative stress using histological methods.
Moreover, the ameliorative effects of pinostrobin on
cognitive deficits were determined using the Y-maze test. Working
memory is one of the short-term memories that can be decreased in
Alzheimer's disease. The Y-maze test was extensively used to
evaluate the function of hippocampal spatial working memory by
counting the number of arm entries and calculating the percentage
of spontaneous alternation (31).
The animals typically prefer to explore a new arm of a maze instead
of going to the previously visited arm, which is related to the
spatial working memory. Similar to a previous study, the present
study demonstrated that repeated stress for 21 days impaired
working memory by decreasing the percentage of spontaneous
alternations in the Y-maze compared to the control group not
exposed to stress (44). However,
treatment with pinostrobin significantly enhanced the percentage of
spontaneous alternations, indicating its ability in ameliorating
cognitive impairment. Moreover, the effects of pinstrobin on the
recognition memory were examined by conducting the novel object
recognition test. The results demonstrated that the administration
of pinostrobin at a dose of 40 mg/kg expressively attenuated the
impairment of recognition memory by increasing the recognition
index following prolonged exposure to stress. These findings
demonstrated that pinostrobin improved cognitive performance in
both the Y-maze and NOR tests. There are numerous behavioral tests
to determine cognitive function in rodent models, such as the
Morris water maze (MWM), NOR test, Y-maze, T-maze and passive
avoidance task. The previous study by Xu et al (45) used the MWM as a test of spatial
memory in a model of chronic stress. Additionally, some studies
have used two behavioral tests, including the NOR and Y-maze tests
(46), or MWM and NOR test
(47). Therefore, the use of the
NOR and Y-maze tests was considered adequate to evaluate cognitive
deficits in the present study. However, further research is
required using several cognitive tests to provide more
comprehensive data associated with hippocampal functions.
There is accumulating scientific evidence to
demonstrate that chronic stress can provoke hippocampal
neurodegeneration, including neuronal apoptosis, decreased synaptic
plasticity and the reduction of dendritic spine density, which
results in learning and memory impairment (7,48).
Therefore, the present study examined the protective effects of
pinostrobin on neuronal damage by detecting Nissl staining in the
hippocampus. The animals exposed to chronic stress exhibited
noticeable neuronal damage in the CA1 and CA3 regions of the
hippocampus, while CA2 did not exhibit a significant reduction in
neuronal density. Chronic stress has been shown to induce
morphological alterations in the hippocampus. Chronic stress causes
the reduction of dendrites in the hippocampal CA3 and dentate gyrus
(DG) neurons. The synaptic connection of the hippocampus involves
the input from the entorhinal cortex to the CA3 and DG, with the
feed-forward and feedback connections between these two subregions
enhancing memory formation (49).
In addition, alterations in CA3 associational axons can stimulate
their neighbors, thereby magnifying the output of CA3.
Consequently, the disruptions of the CA3 associational system
causes the reduced activation of CA1(50). As demonstrated in previous studies,
exposure to chronic stress for 21 days promotes a substantial
reduction of dendritic spine density in the hippocampal CA1 and CA3
regions (51,52). Additional studies have revealed that
CRS leads to dendritic retraction in the CA3, which is an extremely
vulnerable region related to the alterations in
N-methyl-d-aspartic acid receptors, and leads to
neurotoxicity and neuronal death in the hippocampus (52,53).
On the other hand, the hippocampal CA2 region has been reported to
exhibit several features that distinguish it from the CA1 and CA3
regions, including a distinctive gene expression profile, inability
to display long-term potentiation (LPT) and relative resistance to
cell death (54). This may be the
possible explanation as to why, in the present study, no
significant difference was found in neuronal cell loss in the
hippocampal CA2 region. Moreover, the structural and functional
alterations in different hippocampal subregions may be due to the
type, duration and intensity of stress stimuli (53). However, the results demonstrated
that treatment with pinostrobin at doses of 20 and 40 mg/kg
markedly enhanced the density of surviving cells in the hippocampal
CA1 and CA3 regions, suggesting the occurrence of a neuroprotective
effect in the chronically stressed rats.
To further elucidate the possible neuroprotective
mechanisms of pinostrobin, the disruptions of astrocytes involved
in CRS-induced memory deficits were determined. Previous studies
have suggested that CRS-induced astrocyte dysfunction may be
associated with learning and memory deficits (55,56).
GFAP is a critical marker of intermediate filament protein in
astrocytes. Astrocytes are the most common population of glial
cells in the central nervous system, as they play a key role in
neuronal homeostasis, supporting neurons and regulating synaptic
transmission. In addition, astrocytes can remove excess glutamate
out of the synaptic cleft through EAATs in neuronal protection
against excitotoxicity. Previous studies have demonstrated that
exposure to CRS markedly decreases the expression of GFAP in the
prefrontal cortex and the hippocampus (26,57,58).
Similarly, the present study revealed that exposure to chronic
stress led to a significant reduction in the immunostaining of GFAP
in the hippocampus compared to the controls. On the other hand, it
was found that treatment with pinostrobin markedly and
dose-dependently enhanced GFAP immunoreactivity in the hippocampal
CA1, CA2 and CA3 regions following exposure to CRS. Apart from
astrocytes, excitatory glutamate receptors, particularly EAAT2,
which is the principal glutamate transporter expressed in
astrocytes, plays a crucial role in the capacity of glutamate
uptake and in preventing neuronal damage from glutamate
neurotoxicity. A previous study suggested that the disruption of
GFAP provokes a disruption in the clearance of glutamate over the
synaptic cleft. Thus, it is possible that the reduction in GFAP
expression induced by CRS may produce a decrease of EAAT2 in the
hippocampus (59). Consistently,
the findings of the present study revealed a reduction in EAAT2
immunoreactivity in the hippocampus of animals exposed to CRS. Of
note, these changes were effectively restored by pinostrobin
treatment. However, the present study did not measure the amount of
glutamate and the synaptic plasticity in the hippocampus for the
possible underlying mechanisms in neurotoxicity. Thus, further
investigations are warranted in this regard.
The results demonstrated herein revealed the
dose-dependent effects of pinostrobin in treating CRS-induced
cognitive deficits. In a previous study on the toxicity of
pinostrobin, it was reported that pinostrobin was non-toxic and was
not mutagenic to male rats within the 1-100 mg/kg dose range
(60). Therefore, the doses of
pinostrobin (20 and 40 mg/kg) used in the present study are within
the non-toxic dose range and as indicated by the results, these
doses of pinostrobin significantly and dose-dependently improved
cognitive impairments in rats exposed to CRS. Consistent with the
findings of the present study, the study by Abdelwahab et al
(27) demonstrated that pinostrobin
at doses of 20 and 40 mg/kg significantly protected against
ethanol-induced peptic ulcers in rats. In addition, another study
demonstrated that pinostrobin at a dose of 40 mg/kg significantly
inhibited the renal expression of cystic fibrosis transmembrane
conductance regulator in rats with polycystic kidney disease
(61). It is possible that
treatment with higher concentrations of pinostrobin may exert
therapeutic effects without toxicity. Thus, further studies use
various doses (20, 40, and 80 mg/kg) of pinostrobin are required to
confirm the dose-dependent effects of pinostrobin.
In conclusion, the present study indicated that
treatment with pinostrobin significantly attenuated chronic
stress-induced cognitive impairment by decreasing the levels of
oxidative stress, reducing neuronal damage, and by enhancing the
function of astrocytes and EAAT2 in the hippocampus of rats.
Therefore, these findings suggest that pinostrobin may have
potential medicinal value as a neuroprotective agent for the
prevention and treatment of chronic stress-induced cognitive
deficits and other cognitive disorders.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported by the Unit
of Excellence in Translational Neurosciences Initiative, University
of Phayao, Phayao, Thailand (grant no. FF64-UoE021).
Availability of data and materials
The database used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
RK designed the study, performed all the experiments
and wrote the manuscript. ST was involved in the study methodology
and wrote the manuscript. SS was involved in the preparation of the
extract. TP and JJ were involved in the data analysis and in
editing the manuscript. All authors have read and approved the
final manuscript. RK and ST confirmed the authenticity of all the
raw data.
Ethics approval and consent to
participate
The experimental protocol was approved by the Ethics
Committee of the Laboratory Animal Research Center, University of
Phayao, Phayao, Thailand (approval no. 640104006).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Samarghandian S, Azimi-Nezhad M,
Farkhondeh T and Samini F: Anti-oxidative effects of curcumin on
immobilization-induced oxidative stress in rat brain, liver and
kidney. Biomed Pharmacother. 87:223–229. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Radley JJ, Kabbaj M, Jacobson L,
Heydendael W, Yehuda R and Herman JP: Stress risk factors and
stress-related pathology: Neuroplasticity, epigenetics and
endophenotypes. Stress. 14:481–497. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Kloet ER, Joëls M and Holsboer F:
Stress and the brain: From adaptation to disease. Nat Rev Neurosci.
6:463–475. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Briones A, Gagno S, Martisova E, Dobarro
M, Aisa B, Solas M, Tordera R and Ramírez M: Stress-induced
anhedonia is associated with an increase in Alzheimer's
disease-related markers. Br J Pharmacol. 165:897–907.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Jeong YH, Park CH, Yoo J, Shin KY, Ahn SM,
Kim HS, Lee SH, Emson PC and Suh YH: Chronic stress accelerates
learning and memory impairments and increases amyloid deposition in
APPV717I-CT100 transgenic mice, an Alzheimer's disease model. FASEB
J. 20:729–731. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Najjar S, Pearlman DM, Devinsky O, Najjar
A and Zagzag D: Neurovascular unit dysfunction with blood-brain
barrier hyperpermeability contributes to major depressive disorder:
A review of clinical and experimental evidence. J
Neuroinflammation. 10(142)2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Huang RR, Hu W, Yin YY, Wang YC, Li WP and
Li WZ: Chronic restraint stress promotes learning and memory
impairment due to enhanced neuronal endoplasmic reticulum stress in
the frontal cortex and hippocampus in male mice. Int J Mol Med.
35:553–559. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Y, Kan H, Yin Y, Wu W, Hu W, Wang M
and Li W and Li W: Protective effects of ginsenoside Rg1 on chronic
restraint stress induced learning and memory impairments in male
mice. Pharmacol Biochem Behav. 120:73–81. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Morimoto M, Morita N, Ozawa H, Yokoyama K
and Kawata M: Distribution of glucocorticoid receptor
immunoreactivity and mRNA in the rat brain: An immunohistochemical
and in situ hybridization study. Neurosci Res. 26:235–269.
1996.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Smith MA: Hippocampal vulnerability to
stress and aging: Possible role of neurotrophic factors. Behav
Brain Res. 78:25–36. 1996.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luo C, Xu H and Li XM: Quetiapine reverses
the suppression of hippocampal neurogenesis caused by repeated
restraint stress. Brain Res. 1063:32–39. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim JJ and Diamond DM: The stressed
hippocampus, synaptic plasticity and lost memories. Nat Rev
Neurosci. 3:453–462. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Levone BR, Cryan JF and O'Leary OF: Role
of adult hippocampal neurogenesis in stress resilience. Neurobiol
Stress. 1:147–155. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McEwen BS and Magarinos AM: Stress and
hippocampal plasticity: Implications for the pathophysiology of
affective disorders. Hum Psychopharmacol. 16 (Suppl 1):S7–S19.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
McLaughlin KJ, Gomez JL, Baran SE and
Conrad CD: The effects of chronic stress on hippocampal morphology
and function: An evaluation of chronic restraint paradigms. Brain
Res. 1161:56–64. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sahin E and Gümüşlü S: Immobilization
stress in rat tissues: Alterations in protein oxidation, lipid
peroxidation and antioxidant defense system. Comp Biochem Physiol C
Toxicol Pharmacol. 144:342–347. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim GH, Kim JE, Rhie SJ and Yoon S: The
role of oxidative stress in neurodegenerative diseases. Exp
Neurobiol. 24:325–340. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yang HJ, Kim KY, Kang P, Lee HS and Seol
GH: Effects of Salvia sclarea on chronic immobilization stress
induced endothelial dysfunction in rats. BMC Complement Altern Med.
14(396)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Serrano F and Klann E: Reactive oxygen
species and synaptic plasticity in the aging hippocampus. Ageing
Res Rev. 3:431–443. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kelsey NA, Wilkins HM and Linseman DA:
Nutraceutical antioxidants as novel neuroprotective agents.
Molecules. 15:7792–7814. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kong Y, Fu YJ, Zu YG, Liu W, Wang W, Hua X
and Yang M: Ethanol modified supercritical fluid extraction and
antioxidant activity of cajaninstilbene acid and pinostrobin from
pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Food Chem.
117:152–159. 2009.
|
|
22
|
Patel NK and Bhutani KK: Pinostrobin and
Cajanus lactone isolated from Cajanus cajan (L.) leaves
inhibits TNF-α and IL-1β production: In vitro and in vivo
experimentation. Phytomedicine. 21:946–953. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Xian YF, Ip SP, Lin ZX, Mao QQ, Su ZR and
Lai XP: Protective effects of pinostrobin on β-amyloid-induced
neurotoxicity in PC12 cells. Cell Mol Neurobiol. 32:1223–1230.
2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li C, Tang B, Feng Y, Tang F, Pui-Man Hoi
M, Su Z and Ming-Yuen Lee S: Pinostrobin exerts neuroprotective
actions in neurotoxin-induced Parkinson's disease models through
Nrf2 induction. J Agric Food Chem. 66:8307–8318. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ching AYL, Wah TS, Sukari MA, Cheng Lian
GE, Rahmani M and Khalid K: Characterization of flavonoid
derivatives from Boesenbergia rotunda (L). Malaysian J Anal
Sci. 11:154–159. 2007.
|
|
26
|
Tynan RJ, Beynon SB, Hinwood M, Johnson
SJ, Nilsson M, Woods JJ and Walker FR: Chronic stress-induced
disruption of the astrocyte network is driven by structural atrophy
and not loss of astrocytes. Acta Neuropathol. 126:75–91.
2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abdelwahab SI, Mohan S, Abdulla MA, Sukari
MA, Abdul AB, Taha MM, Syam S, Ahmad S and Lee KH: The methanolic
extract of Boesenbergia rotunda (L.) Mansf. and its major
compound pinostrobin induces anti-ulcerogenic property in vivo:
Possible involvement of indirect antioxidant action. J
Ethnopharmacol. 137:963–970. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nakmareong S, Kukongviriyapan U,
Pakdeechote P, Donpunha W, Kukongviriyapan V, Kongyingyoes B,
Sompamit K and Phisalaphong C: Antioxidant and vascular protective
effects of curcumin and tetrahydrocurcumin in rats with
L-NAME-induced hypertension. Naunyn Schmiedebergs Arch Pharmacol.
383:519–529. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Góth L: A simple method for determination
of serum catalase activity and revision of reference range. Clin
Chim Acta. 196:143–151. 1991.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Johnson SJ and Walker FR: Strategies to
improve quantitative assessment of immunohistochemical and
immunofluorescent labelling. Sci Rep. 5(10607)2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Galeano P, Martino Adami PV, Do Carmo S,
Blanco E, Rotondaro C, Capani F, Castaño EM, Cuello AC and Morelli
L: Longitudinal analysis of the behavioral phenotype in a novel
transgenic rat model of early stages of Alzheimer's disease. Front
Behav Neurosci. 8(321)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mooshekhian A, Sandini T, Wei Z, Van
Bruggen R, Li H, Li XM and Zhang Y: Low-field magnetic stimulation
improved cuprizone-induced depression-like symptoms and
demyelination in female mice. Exp Ther Med. 23(210)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jamali-Raeufy N, Kardgar S,
Baluchnejadmojarad T, Roghani M and Goudarzi M: Troxerutin exerts
neuroprotection against lipopolysaccharide (LPS) induced oxidative
stress and neuroinflammation through targeting SIRT1/SIRT3
signaling pathway. Metab Brain Dis. 34:1505–1513. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Batool Z, Sadir S, Liaquat L, Tabassum S,
Madiha S, Rafiq S, Tariq S, Batool TS, Saleem S, Naqvi F, et al:
Repeated administration of almonds increases brain acetylcholine
levels and enhances memory function in healthy rats while
attenuates memory deficits in animal model of amnesia. Brain Res
Bull. 120:63–74. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lupien SJ, McEwen BS, Gunnar MR and Heim
C: Effects of stress throughout the lifespan on the brain,
behaviour and cognition. Nat Rev Neurosci. 10:434–445.
2009.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sandi C and Pinelo-Nava MT: Stress and
memory: Behavioral effects and neurobiological mechanisms. Neural
Plast. 2007(78970)2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pourheydar B, Abar M, Farjah G, Pourheydar
M and Derafshpour L: Curcumin alleviates restraint stress-induced
learning and memory deficit and activity via modulation of
biochemical, morphology changes, and apoptosis in the prefrontal
cortex and hippocampus. Behav Neurosci. 136:149–158.
2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
McEwen BS: The neurobiology of stress:
From serendipity to clinical relevance. Brain Res. 886:172–189.
2000.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Vyas A, Mitra R, Shankaranarayana Rao BS
and Chattarji S: Chronic stress induces contrasting patterns of
dendritic remodeling in hippocampal and amygdaloid neurons. J
Neurosci. 22:6810–6818. 2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Samarghandian S, Azimi-Nezhad M and Samini
F: Preventive effect of safranal against oxidative damage in aged
male rat brain. Exp Anim. 64:65–71. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liang S, Wang T, Hu X, Luo J, Li W, Wu X,
Duan Y and Jin F: Administration of lactobacillus helveticus NS8
improves behavioral, cognitive, and biochemical aberrations caused
by chronic restraint stress. Neuroscience. 310:561–577.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Samarghandian S, Azimi-Nezhad M, Borji A,
Samini M and Farkhondeh T: Protective effects of carnosol against
oxidative stress induced brain damage by chronic stress in rats.
BMC Complement Altern Med. 17(249)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Jiang N, Wang K, Zhang Y, Huang H, Lv JW,
Wang Q, Wang HX, Xia TJ and Liu XM: Protective effect of
ginsenoside Rb1 against chronic restraint stress (CRS)-induced
memory impairments in rats. Behav Brain Res.
405(113146)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Conrad CD, Galea LA, Kuroda Y and McEwen
BS: Chronic stress impairs rat spatial memory on the Y maze, and
this effect is blocked by tianeptine pretreatment. Behav Neurosci.
110:1321–1334. 1996.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu Y, Lin D, Li S, Li G, Shyamala SG,
Barish PA, Vernon MM, Pan J and Ogle WO: Curcumin reverses impaired
cognition and neuronal plasticity induced by chronic stress.
Neuropharmacology. 57:463–471. 2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sithisarn P, Rojsanga P, Jarikasem S,
Tanaka K and Matsumoto K: Ameliorative effects of acanthopanax
trifoliatus on cognitive and emotional deficits in olfactory
bulbectomized mice: An animal model of depression and cognitive
deficits. Evid Based Complement Alternat Med.
2013(701956)2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Phachonpai W and Tongun T: Cognition
enhancing effects of Clausena lansium (Lour.) peel extract
attenuate chronic restraint stress-induced memory deficit in rats.
Heliyon. 7(e07003)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
McEwen BS and Magarinos AM: Stress effects
on morphology and function of the hippocampus. Ann N Y Acad Sci.
821:271–284. 1997.PubMed/NCBI View Article : Google Scholar
|
|
49
|
McEwen BS: Stress and hippocampal
plasticity. Annu Rev Neurosci. 22:105–122. 1999.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Brunson KL, Kramar E, Lin B, Chen Y,
Colgin LL, Yanagihara TK, Lynch G and Baram TZ: Mechanisms of
late-onset cognitive decline after early-life stress. J Neurosci.
25:9328–9338. 2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Huang P, Li C, Fu T, Zhao D, Yi Z, Lu Q,
Guo L and Xu X: Flupirtine attenuates chronic restraint
stress-induced cognitive deficits and hippocampal apoptosis in male
mice. Behav Brain Res. 288:1–10. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Christian KM, Miracle AD, Wellman CL and
Nakazawa K: Chronic stress-induced hippocampal dendritic retraction
requires CA3 NMDA receptors. Neuroscience. 174:26–36.
2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sun DS, Zhong G, Cao HX, Hu Y, Hong XY, Li
T, Li X, Liu Q, Wang Q, Ke D, et al: Repeated restraint stress led
to cognitive dysfunction by NMDA receptor-mediated hippocampal CA3
dendritic spine impairments in juvenile sprague-dawley rats. Front
Mol Neurosci. 13(552787)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dudek SM, Alexander GM and Farris S:
Rediscovering area CA2: Unique properties and functions. Nat Rev
Neurosci. 17:89–102. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hei M, Chen P, Wang S, Li X, Xu M, Zhu X,
Wang Y, Duan J, Huang Y and Zhao S: Effects of chronic mild stress
induced depression on synaptic plasticity in mouse hippocampus.
Behav Brain Res. 365:26–35. 2019.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Shilpa BM, Bhagya V, Harish G, Srinivas
Bharath MM and Shankaranarayana Rao BS: Environmental enrichment
ameliorates chronic immobilisation stress-induced spatial learning
deficits and restores the expression of BDNF, VEGF, GFAP and
glucocorticoid receptors. Prog Neuropsychopharmacol Biol
Psychiatry. 76:88–100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Araya-Callis C, Hiemke C, Abumaria N and
Flugge G: Chronic psychosocial stress and citalopram modulate the
expression of the glial proteins GFAP and NDRG2 in the hippocampus.
Psychopharmacology (Berl). 224:209–222. 2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Czéh B, Simon M, Schmelting B, Hiemke C
and Fuchs E: Astroglial plasticity in the hippocampus is affected
by chronic psychosocial stress and concomitant fluoxetine
treatment. Neuropsychopharmacology. 31:1616–1626. 2006.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Hughes EG, Maguire JL, McMinn MT, Scholz
RE and Sutherland ML: Loss of glial fibrillary acidic protein
results in decreased glutamate transport and inhibition of
PKA-induced EAAT2 cell surface trafficking. Brain Res Mol Brain
Res. 124:114–123. 2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Charoensin S, Punvittayagul C, Pompimon W,
Mevatee U and Wongpoomchai R: Toxicological and clastogenic
evaluation of pinocembrin and pinostrobin isolated from
Boesenbergia pandurata in Wistar rats. Thai J Toxicol. 25:29–40.
2010.
|
|
61
|
Tonum K, Chabang N, Fongsupa S,
Chantawarin S, Jiarpinitnun C, Tuchinda P and Soodvilai S:
Pinostrobin inhibits renal CFTR-mediated Cl- secretion
and retards cyst growth in cell-derived cyst and polycystic kidney
disease rats. J Pharmacol Sci. 148:369–376. 2022.PubMed/NCBI View Article : Google Scholar
|