1. Background
Parkinson's disease (PD) has a great negative impact
on life quality and countless more comorbidities, significantly
affecting both physical and psychological health. Overall, the most
important unfavorable outcome of PD encompasses lack of dexterity
and ambulation, along with disturbed cognition (1). Epidemiological studies have reported
that, with the increasing life span, the prevalence of PD is facing
a significant increase in most countries of the world, thus
representing a healthcare burden not to be neglected (2). Advanced aging is also related with the
increasing prevalence of cardiovascular diseases and, therefore,
investigating possible associations between the two and PD is quite
justified.
Furthermore, aging, diabetes mellitus and the male
sex have been shown to be notable risk factors for both
cardiovascular illnesses and PD (3). Studies have shown that various
metabolic dysregulations which are found in cardiovascular
diseases, are present in PD, as well. Increased glycemia values and
insulin-resistance have been revealed to be associated with PD,
while inflammatory markers, such as reactive oxygen species, lipid
degradation products or C-reactive protein (CRP), have also been
revealed to be associated with this disease (2). Consequently, these patients have
significantly higher risk to develop cardiovascular disease, high
blood pressure or diabetes mellitus. Additionally, another link
between these two illnesses is represented by the molecular changes
within the structures of cells, including storage of protein
agglomerates, dysfunctional clearing process of proteins,
mitochondrial involvement, inflammation within the nervous system
and various gene mutations, all of which are responsible for the
phenotype and severity of a disease (4,5).
By and large, the cardiovascular involvement in
patients with PD is represented on the one hand by cardiovascular
dysautonomia and, on the other hand, by structural cardiovascular
illnesses. The most common cardiovascular abnormalities found in
patients with PD are related to dysautonomia, while structural
cardiac diseases are more scarcely identified in this category of
patients. Nevertheless, previous research has demonstrated a
positive link between cardiovascular illnesses and PD (6). Autonomic nervous system dysregulation
usually leads to the occurrence of orthostatic or postprandial
hypotension, either nocturnal or supine hypertension (2). Moreover, either independent or in
conjunction to dysautonomia, patients with PD have increased risk
of developing structural and functional cardiovascular illnesses,
such as left ventricular hypertrophy and diastolic dysfunction, in
addition to, as the diseases progress, heart failure, ischemic
heart disease and even ventricular tachyarrhythmias (2).
Furthermore, patients with PD are at risk of
developing heart disease secondary to PD medication. While levodopa
potentiates orthostatic hypotension, dopamine agonists are
responsible for restrictive valvular heart disease (7,8). As
for anticholinergic agents, donepezil has been reported to cause
ventricular tachyarrhythmias, which were reversible after treatment
cessation (9).
In the present state-of-the-art review, a unique and
novel approach of presenting cardiovascular involvement in patients
with PD is provided. To the best of our knowledge, to date, there
is no research that has been published which covers the entire
aspect of cardiovascular disease in patients with PD. For this
purpose, the latest available articles were reviewed and reported,
providing on the one hand a clear and comprehensive perspective on
the matter and, on the other hand, starting from current findings,
future research perspectives in terms of cardiovascular protection
and risk assessment (2), along with
specific possible future biomarkers. Another strength of the
present review is its clinical viewpoint and the fact that it is
addressed not only to experimental researchers, but also to
clinical researchers and doctors as well (7,8). Thus,
the present article is considered to be a comprehensive and useful
review, which reveals several aspects in the field of PD and
cardiovascular involvement.
2. Cardiovascular dysautonomia in patients
with PD
Autonomic nervous system dysfunction represents a
non-motor involvement in PD, which occurs early in disease
progression and growing evidence suggests that it may predict the
diagnosis long before the appearance of standard motor signs and
symptoms (10,11). With regard to the mechanisms
responsible for these manifestations, it has been considered that
α-synuclein and autonomic nerve denervation were the keystones of
cardiac dysautonomia, however recent research has shown that there
is more than meets the eye in terms of pathogenesis (12). Amongst all, autonomic nervous system
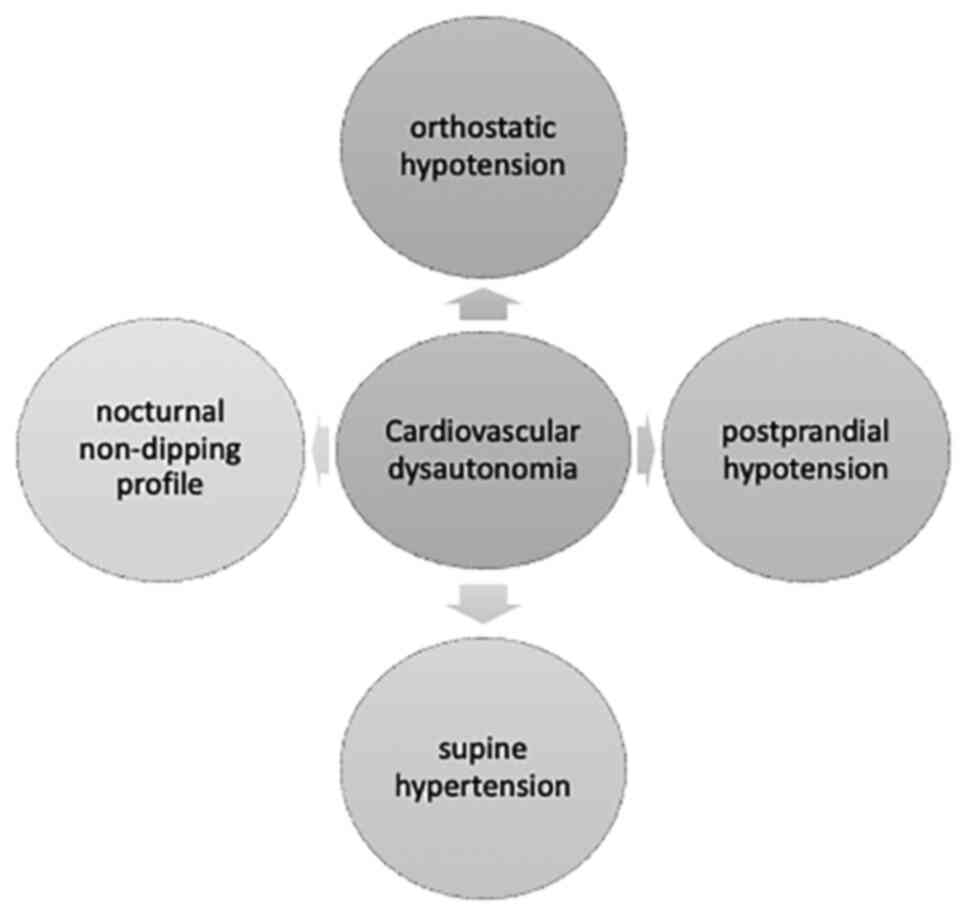
dysregulation is responsible for cardiovascular dysautonomia in
>80% of cases, including orthostatic and postprandial arterial
hypotension, supine arterial hypertension, as well as the presence
of nocturnal nondipping profile in this category of subjects
(Fig. 1) (13,14).
In a meta-analysis conducted by Velseboer et al, it was
concluded that orthostatic and postprandial hypotension usually
occur in up to one third of patients with PD (15), with postprandial hypotension being
more frequently observed in patients with orthostatic hypotension
(16). Moreover, orthostatic
hypotension has been shown to significantly impact the quality of
life of sufferers and PD progression (12).
Orthostatic hypotension is one of the major
cardiovascular illnesses, which occurs in patients with PD,
requiring watchful and careful monitoring due to the patient's risk
of falling and social isolation. Specific medications, comprising
synthetic mineralocorticoids and pressor agents, along with general
measurements, such as proper hydration, siting immediately after
feeling lightheaded upon standing, external compression, physical
counter-maneuvers that enhance venous return, are required
(16,17). According to Jain et al,
30-40% of patients with PD have orthostatic hypotension, while
others have suggested a percentage of >50% (18). In a study by Scorza et al,
the incidence of various cardiovascular diseases was low, under
3-5% for myocardial infarction, with only one case of takotsubo
cardiomyopathy reported. To date, there are no epidemiological
studies that may clarify the true proportions of various cardiac
diseases in patients with PD (6).
Senard et al revealed that 58.2% of patients with PD have
concomitant orthostatic hypotension and one third of them are
asymptomatic at the moment of diagnosis (19). In a study by Blaho et al
conducted in patients with PD, it was demonstrated that decreased
baroreflex response was closely associated with supine hypertension
and orthostatic hypotension (20).
Similarly, the adaptability of systemic peripheral resistance was
revealed to be severely dysregulated due to sympathetic nerve
degeneration, thus promoting orthostatic hypotension (21). In addition, it was reported that
parasympathetic nerve fibers are degenerated, thus contributing to
orthostatic hypotension (22).
Another interesting aspect of orthostatic
hypotension derives from the differences with Parkinson-like
syndromes, such as multiple system atrophy (MSA) in terms of
pathogenetic mechanisms. If in PD, the main mechanisms of
orthostatic hypotension are neurological lesions of the brainstem,
sympathetic denervation and postganglionic affliction, in MSA,
central nervous system damages are exclusively responsible for this
medical condition (23,24). Furthermore, in PD, the histological
lesions are represented by the accumulation of Lewy bodies, while
in MSA, α-synuclein aggregates are found in neurons and
oligodendrogial cytoplasmatic inclusions (25,26).
Moreover, in a recently published study by Cuoco et al,
patients with MSA who also suffered from orthostatic hypotension
had a significantly increased risk of cognitive degradation at
1-year follow-up (27).
As for the nocturnal nondipping profile of patients
with PD, studies have shown that almost 90% were nondippers
(12). In a study by Sommer et
al, which comprehensively characterized the presence of a
nondipping profile in a cohort of patients with PD, it was
concluded that up to 95% of patients with orthostatic hypotension
are nocturnal nondippers, however the underlying pathogenetic
mechanism is quite independent of this because up to 80% of
patients who did not present with orthostatic hypotension also had
a nondipping profile at 24 h ambulatory blood pressure monitoring
(14). This aspect represents a
paramount link between cardiovascular risk and PD because, as
demonstrated by de la Sierra et al, nondipping profiles are
strongly related to cardiovascular illnesses (28).
Supine hypertension is considerably frequent in
patients with PD, and it has been shown that this category of
cardiovascular dysautonomia is associated with general
cardiovascular risk, the occurrence of myocardial infarction and
ischemic stroke (12). Likewise,
supine hypertension has been strongly associated with left
ventricular hypertrophy and, in the long term, diastolic
dysfunction, and heart failure (29). Moreover, a recent study conducted by
Shin et al sought to investigate the clinical risk factors
of the dilated perivascular space-cognition-motor symptom axis. The
study concluded that basal ganglia-dilated perivascular space
secondary to supine hypertension is a major contributor to
cognitive failure due to cerebral white matter hyperintensities and
is responsible for more pronounced motor symptoms in patients with
PD (30).
Another keystone component in PD is α-synuclein, the
main constituent of Lewy bodies, which has significant roles in
disease development. Recent technological advances in the field of
molecular biochemistry and proteomics have led to a better
understanding of the morphological and functional hallmarks of
α-synuclein, thus providing even more enlightenment in PD (31). Furthermore, a recent study published
by Javanshiri et al revealed that up to 82% of patients with
α-synucleinopathies presented intracardiac α-synuclein at
pathological examination, as compared to those without
α-synucleinopathies for whom cardiac α-synuclein was completely
absent (32). In addition, Isonaka
et al demonstrated that in patients with neurogenic
orthostatic hypotension, the accumulation of α-synuclein within the
sympathetic ganglions, especially in the noradrenergic nerve
fibers, is considerably correlated with the loss of cardiac
noradrenaline, along with its neuronal storage. These findings may
be in favor of a possible pathogenetic link between α-synuclein
deposition, Lewy bodies and retrogression of cardiac sympathetic
nerve fibers, although further research needs to be conducted
(33). Furthermore, it has been
shown that 6-hydroxydopamine, a neurotoxin with major implications
in the pathogenesis of PD, plays significant roles in reducing
cardiac sympathetic nerve fibers (12,34).
Notwithstanding, several specific gene mutations
have been shown to be associated with autonomic nervous system
dysfunction. Tijero et al revealed that E46K mutation within
the α-synuclein gene was notably associated with significant
degeneration of cardiac sympathetic nerves in both symptomatic and
asymptomatic patients with PD (35). Moreover, another specific mutation,
namely LRRK2, led to increased myocardial uptake of
I-meta-iodobenzylguanidine (MIBG) at scintigraphy (36).
3. Appraising the risk of heart disease in
patients with PD
In addition to cardiac dysautonomia, patients with
PD commonly develop structural heart disease such as ischemic heart
disease or heart failure, representing a paramount additive factor
to their morbidity and mortality. As recently suggested by Park
et al, patients with PD are at a significantly higher risk
to develop cardiovascular disease, even though the exact
pathogenetic mechanisms are not fully understood and further
research is required in order to elucidate them (37).
In a recently published systematic review that
sought to establish pathogenetic links between PD and heart
disease, Suri et al emphasized the role of atherosclerosis
in cardiovascular and cerebrovascular disease in this category of
patients, endorsing the importance of constant evaluation for
cardiovascular diseases (38).
Notably, Driver et al conducted a prospective study on 330
patients who succumbed to PD and had similar comorbidities. They
concluded that mortality was independent of aging and smoking in
patients with PD (39).
Furthermore, Nam et al revealed that subjects
with metabolic syndrome were at an increased risk of developing PD
than healthy individuals. Moreover, the incidence of PD slightly
increased with the number of metabolic syndrome components,
especially in individuals who were ≥65 years old (40). In addition, a slight increase in
arterial pressure in high-normal blood pressure individuals was
revealed to significantly increase the risk of PD (41). Moreover, Liang et al
conducted a case-control study on 3,211 patients with PD and a
similar number of controls who underwent a 3-year follow-up. In the
diseased group, a significantly increased risk of developing acute
myocardial infarction, either fatal or not, was observed (42). Interestingly, a recently published
study revealed that statins were significantly associated with
increased risk of PD, while serum hypercholesterolemia slightly
prevented the occurrence of the disease (43). Conversely, Vikdahl et al
revealed that hypercholesterolemia, smoking, and obesity were
slightly associated with increased risk of acquiring PD, while
physical activity was a protective factor for this disease
(44).
Similar to cardiovascular diseases, in PD there is a
high grade of inflammation, insulin resistance, dyslipidemia and
oxidative stress. Physical exertion and coffee consumption are
inversely associated with the risk of both PD and cardiovascular
diseases, along with optimal diabetes and hypertension therapy
(43). Recently, Scorza et
al revealed that in addition to ischemic heart disease,
patients with PD may also develop cardiomyopathies, arrhythmias, or
sudden cardiac death, although their real incidence is a topic of
debate (6). Interestingly, Chohan
et al performed a meta-analysis in which they sought to
establish the link between diabetes mellitus and PD. The study
revealed a strong relationship between the presence of diabetes
mellitus and the development of PD, showing a 21% increased risk to
develop PD in diabetic patients (45). These findings may be elucidated by
similar pathogenetic mechanisms, including mitochondrial
malfunction, increased oxidative stress, insulin resistance and
hyperglycemia. Moreover, reported data revealed a notable
association even with the progression of PD and, conversely, the
positive effects of anti-diabetic medication on halting PD were
identified (46). In addition to
diabetes, insulin resistance itself has been shown to be associated
with PD. Insulin passes into the central nervous system and
regulates neuronal development and apoptosis, dopaminergic
transmission, and synapses functionality, while it may also promote
α-synuclein synthesis (47).
Furthermore, previously reported data support the
role of vitamin D in the progression of PD, specifically through
its own receptor, and also through various molecules which are
involved in different pathogenetic pathways. Therefore, several
genes which are associated with vitamin D, including type II major
histocompatibility complex, cytochrome P4502D6, chromosome 22, the
renin-angiotensin system, heme oxygenase-1, poly-(ADP-ribose)
polymerase-1 gene, neurotrophic factor, and Sp1 transcription
factor, have been demonstrated to be strongly associated with PD.
In addition, various inflammatory markers, such as L-type
voltage-sensitive calcium channels, nerve growth factor, matrix
metalloproteinases, prostaglandins and cyclooxygenase-2, have been
revealed to be associated with PD via vitamin D (48-53).
A possible pertinent explanation for these findings may be that
vitamin D and its activating enzyme called alpha1-hydroxylase are
considerably found in the substantia nigra. In a recently published
review of the literature, Fullard et al concluded that sera
vitamin D levels are closely related to the severity of motor
signs, memory, psychological status, orthostatic hypotension, and
loss of smell (54). It has
recently been shown that decreased levels of vitamin D leads to
apoptosis of dopaminergic neurons, while its systemic
administration counterbalances the process (55). In addition, growing evidence
suggests that vitamin D may become a feasible biomarker for PD, but
larger cohort studies are required (56).
Moreover, low serum levels of vitamin D have been
strongly associated with increased risk of numerous cardiovascular
illnesses. Patients who experienced stable coronary artery disease
and acute myocardial infarction were demonstrated to have decreased
levels of vitamin D (57).
Likewise, a meta-analysis which evaluated the relationship between
this molecule and the risk of atrial fibrillation concluded that
vitamin D deficiency significantly increases the risk of atrial
fibrillation (58). Conversely, in
a meta-analysis which included ~83,000 patients, exogenic
supplementation with vitamin D did not reduce the risk of
cardiovascular disease of any kind (59). Thus, based on these findings,
vitamin D may become a useful marker of cardiovascular dysfunction
in patients with PD.
As for biomarkers of inflammation, it has been
proposed that CRP may become a valuable marker of disease
progression in chronic inflammatory and neurodegenerative diseases,
such as PD (60). Qiu et al
conducted an interesting meta-analysis which compared patients with
PD and healthy individuals and revealed that increased levels of
CRP were identified in the diseased group, thus suggesting that
this biomarker may become a risk factor for PD (61). Moreover, Sawada et al
revealed that initial CRP levels were associated with mortality and
prognosis prediction of patients with PD, independent of disease
duration, severity, age or other confounders (62). Furthermore, urate is another
important serum biomarker in patients with PD, having a multitude
of anti-inflammatory effects within human cells and promoting
reactive oxygen species within neurons (63,64).
Clinical studies have shown that increased serum levels of urate
were associated with a considerable decrease in the progression of
PD and a lower unified PD rating scale score, promoting its
protective effects in this category of patients (65,66).
In addition to the potential role of urate as a biomarker of
disease progression, its therapeutic ability to halt the
development of PD has been recently assessed. In a recent clinical
trial, it was determined whether high plasmatic levels of urate
induced by inosine produced protective effects in patients with
early PD. The results revealed that compared with placebo, patients
with PD who were treated with inosine did not benefit in terms of
clinical disease progression (67).
Moreover, urate was also revealed to be associated with increased
risk of cardiovascular diseases. In a recently published review,
high levels of urate were closely associated with various
cardiovascular diseases, increasing the risk of arterial
hypertension, metabolic syndrome and intrinsic cardiac diseases,
mainly by promoting vascular endothelial dysfunction, atherogenesis
and lipid oxidation. Additionally, increased levels of urate have
also been revealed to be associated with cardiovascular outcome and
heart failure (68). These findings
suggest an indirect, but strong association between PD and
cardiovascular disease. Thus, although increased serum levels of
urate are associated with a decreased progression of PD, they
conversely, significantly increase the risk of cardiovascular
diseases.
Another important serum marker in patients with PD
is protein DJ-1, which is also a neuroprotector with significant
impact in neurodegenerative diseases (69). Initially, Waragai et al
revealed that this protein is increased in blood and cerebrospinal
fluid in patients with PD (70).
Furthermore, various mutations in genes of the DJ-1 protein have
been associated with significantly increased risk of PD (71), and in a recently published review,
its possible role as a biomarker of PD progression and even its
ability to serve as a therapeutic target were suggested, however
further studies still need to be conducted (72). Moreover, it has been shown that
protein DJ-1 is an endogenous protective molecule that halts
glycative stress, thus preventing heart failure induced by
myocardial ischemia (73). In
addition, protein DJ-1 may exert protective effects on the
cardiovascular system, especially in patients with heart failure,
pulmonary hypertension or those who have undergone coronary artery
by-pass, by promoting antioxidant gene expression (74). These findings suggest that protein
DJ-1 may become another pivotal biomarker which could mediate the
association between PD and cardiovascular illnesses.
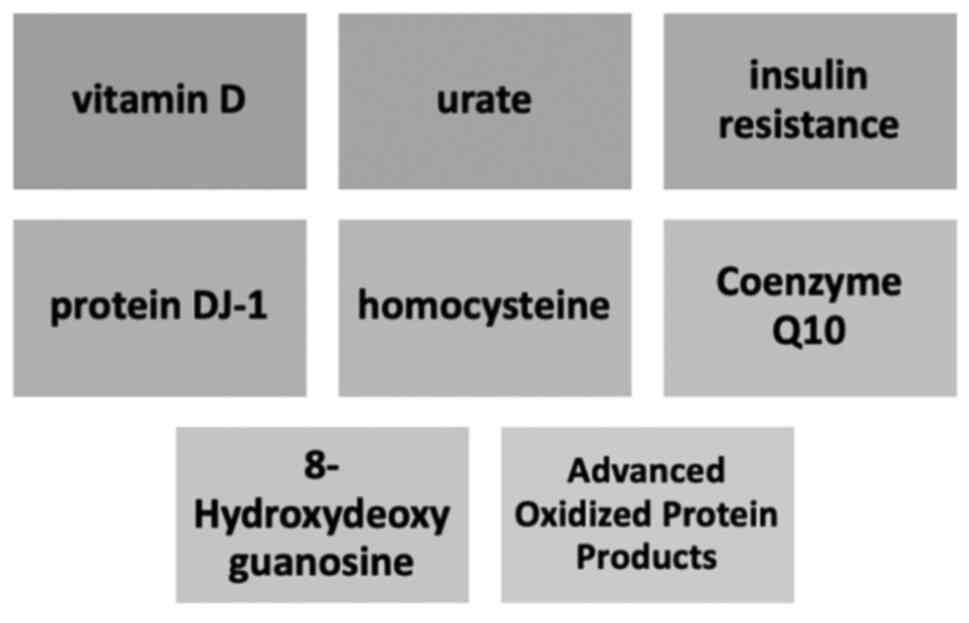
Furthermore, other serum biomarkers which are
closely related with both PD and cardiovascular disease include
coenzyme Q10, homocysteine and advanced oxidized protein products.
These molecules are involved in the pathogenesis of cardiac
diseases by promoting inflammation, myocyte disruption,
atherogenesis, and heart failure, and thus may become biomarkers of
PD progression (Fig. 2) in the near
future and are presented in Table I
(75).
 | Table IPotential future biomarkers in
PD. |
Table I
Potential future biomarkers in
PD.
| Biomarker | Neurodegenerative
effects |
|---|
| Urate | • Antioxidant
effects |
| | • May prevent
neurodegeneration of substantia nigra |
| | • Reduces
mitochondrial oxidation |
| Protein DJ-1 | • Neuroprotective
role in oxidative stress |
| | • Reduces
neurodegeneration |
| | • Modulates
chaperone proteins |
| | • Reduces
mitochondrial oxidation |
| Coenzyme Q10 | • Destabilizes the
redox equilibrium |
| | • Neuronal
toxicity |
| | • Mitochondrial
defects |
| Homocysteine | • Substantia nigra
toxicity |
| | • Neurotoxicity,
especially in dopaminergic neurons |
| | • Increases sera
levels of amyloid β |
|
8-Hydroxydeoxyguanosine | • Promotes reactive
oxygen species synthesis |
| | • Substantia nigra
toxicity |
| Advanced oxidized
protein products | • Protein
halogenation |
| | • Increases
phagocytosis |
Despite the fact that there are only a handful of
studies demonstrating significant associations between PD and
structural heart disease, there is significant evidence to endorse
further studies in this direction.
4. PD therapy related to cardiovascular
disease
The most used medications in patients with PD
include levodopa, dopamine agonists, inhibitors of monoaminoxidase
B, inhibitors of catechol-O-methyltransferase (COMT), amantadine,
and anticholinergic agents. Growing evidence has revealed the
negative impact of PD therapy on the cardiovascular system, with
research demonstrating a close association between this category of
treatment and the development and progression of heart disease
(76).
Levodopa
Considered to be the most efficient and extensively
used for the treatment of PD, levodopa has major positive effects
on survival, morbidity, quality of life, and significantly
improving symptoms of PD (77).
Previously reported data have indicated that levodopa is associated
with increased stiffness within the aorta as well as hypertension,
and that it promotes left ventricular diastolic dysfunction
(7,78). It is also responsible for promoting
orthostatic hypotension, however the pathogenetic mechanism for
this, either vasodepressor or cardioinhibitory effect, is not fully
elucidated, although certain data are in favor of a negative
inotropic mechanism (7). In
addition, levodopa is also responsible for increased sera levels of
homocysteine due to its methylation through the COMT pathway
(79). In a study by Kocer et
al, which sought to evaluate the ability of a COMT inhibitor to
prevent hyperhomocysteinemia secondary to levodopa therapy, the
study failed to identify a protective effect of entacapone on
preventing hyperhomocysteinemia (80). Furthermore, in a study by
O'Suilleabhain et al, it was demonstrated that
hyperhomocysteinemia in patients with PD was significantly
associated with depression and poorer neuropsychometric activity,
as compared to individuals with normal sera levels of homocysteine
(81).
Ergot-derived agonists
Another pharmacological class of anti-PD drugs
represented by ergot-derived dopamine agonists, such as
bromocriptine and cabergoline, are even more incriminated in
cardiovascular involvement. Initially, Van Camp et al
reported two cases of PD with restrictive valvular heart disease
and clinical signs of significant heart failure due to daily doses
of pergolide (82). Subsequently,
the same research team evaluated the incidence of restrictive
mitral stenosis in a cohort of 78 patients with PD treated with
pergolide. The results revealed that 33% of patients developed
restrictive mitral stenosis, of whom 19% had a severe form of this
illness, thus suggesting that this complication is not rare in
patients treated with this medication (8). Similarly, Horvath et al also
identified restrictive valvular heart disease and suggested a
comprehensive echocardiographic evaluation at the beginning and
during treatment with pergolide and cabergoline (83). As for non-ergot-based dopamine
agonists, pramipexole has scientific evidence for being responsible
for heart failure. In a systematic review published by Tran et
al, it was revealed that this drug was significantly associated
with the risk of heart failure (84). However, further studies are required
to elucidate the underlying mechanisms.
Anticholinergic agents
With regard to anticholinergic agents, donepezil has
been found to prolong the QT interval in long-term administration.
Additionally, a close monitoring strategy should be applied when
this drug is used in combination with tricyclic antidepressants
(85). Recently, Kho et al
demonstrated that, due to increased sera levels of acetylcholine
which blocks the potassium ion channel from the heart, donepezil
may be responsible for adverse tachyarrhythmias, namely polymorphic
ventricular tachycardia with oscillatory changes in amplitude of
the QRS complexes around the isoelectric line (9).
Dexrazoxane, a potential
cardioprotective agent in patients with PD
To date, there is no drug used in patients with PD
that does not carry any cardiovascular risk. A noteworthy
pharmacological agent which may benefit PD patients and also exert
protective cardiovascular effects is dexrazoxane. Dexrazoxane was
recently approved for the prevention of chemotherapy-induced
cardiotoxicity in both adults and children who received
anthracycline therapeutic regimens (86). A previous experimental study has
suggested that dexrazoxane may act as protective agent against
neurodegeneration, however further studies need to be conducted to
reveal its potential clinical effects in patients with PD (87).
5. Conclusion
Compelling evidence supports a cause-effect
relationship between PD and cardiovascular involvement. PD leads to
cardiac dysautonomia and promotes ischemic heart, heart failure and
arterial hypertension. Additionally, several drugs used to treat
PD, such as levodopa, dopamine agonists and anticholinergic agents,
are responsible for heart disease, as well. Nevertheless, there is
not enough evidence available to identify the underlying mechanisms
of these links. Further studies are required to provide appropriate
data regarding the pathogenetic and clinical associations between
PD and cardiovascular disease in order to deploy therapeutic
options.
Acknowledgements
This work was supported by the Internal Doctoral
Fellowship of Iuliu Hatieganu University of Medicine and Pharmacy,
Cluj-Napoca, Romania.
Funding
Funding: No funding was received.
Availability of data and material
Not applicable.
Authors's contributions
LG, AIG, and AZ researched data for the article and
wrote the manuscript. LG, DC and AIG discussed the content of the
review, and LG and LPD reviewed and edited the manuscript before
submission. Data authentication is not applicable. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pohar SL and Allyson Jones C: The burden
of Parkinson disease (PD) and concomitant comorbidities. Arch
Gerontol Geriatr. 49:317–321. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H
and Wang Z: Global trends in the incidence, prevalence, and years
lived with disability of Parkinson's disease in 204
countries/territories from 1990 to 2019. Front Public Health.
9(776847)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
James SL, Abate D, Abate KH, Abay SM,
Abbafati C, Abbasi N, Abbastabar H, Abd-Allah F, Abdela J,
Abdelalim A, et al: Global, regional, and national incidence,
prevalence, and years lived with disability for 354 diseases and
injuries for 195 countries and territories, 1990-2017: A systematic
analysis for the global burden of disease study 2017. Lancet.
392:1789–1858. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Feigin VL, Nichols E, Alam T, Bannick MS,
Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG,
et al: Global, regional, and national burden of neurological
disorders, 1990-2016: A systematic analysis for the global burden
of disease study 2016. Lancet Neurol. 18:459–480. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wanneveich M, Moisan F, Jacqmin-Gadda H,
Elbaz A and Joly P: Projections of prevalence, lifetime risk, and
life expectancy of Parkinson's disease (2010-2030) in France. Mov
Disord. 33:1449–1455. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Scorza FA, Fiorini AC, Scorza CA and
Finsterer J: Cardiac abnormalities in Parkinson's disease and
Parkinsonism. J Clin Neurosci. 53:1–5. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Noack C, Schroeder C, Heusser K and Lipp
A: Cardiovascular effects of levodopa in Parkinson's disease.
Parkinsonism Relat Disord. 20:815–818. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
van Camp G, Flamez A, Cosyns B, Weytjens
C, Muyldermans L, Van Zandijcke M, De Sutter J, Santens P, Decoodt
P, Moerman C and Schoors D: Treatment of Parkinson's disease with
pergolide and relation to restrictive valvular heart disease.
Lancet. 363:1179–1183. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kho J, Ioannou A, Mandal AKJ and Missouris
CG: Donepezil induces ventricular arrhythmias by delayed
repolarisation. Naunyn Schmiedebergs Arch Pharmacol. 394:559–560.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Palma JA and Kaufmann H: Treatment of
autonomic dysfunction in Parkinson disease and other
synucleinopathies. Mov Disord. 33:372–390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Coon EA, Cutsforth-Gregory JK and
Benarroch EE: Neuropathology of autonomic dysfunction in
synucleinopathies. Mov Disord. 33:349–358. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen Z, Li G and Liu J: Autonomic
dysfunction in Parkinson's disease: Implications for
pathophysiology, diagnosis, and treatment. Neurobiol Dis.
134(104700)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Arici Duz O and Helvaci Yilmaz N:
Nocturnal blood pressure changes in Parkinson's disease:
Correlation with autonomic dysfunction and vitamin D levels. Acta
Neurol Belg. 120:915–920. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sommer S, Aral-Becher B and Jost W:
Nondipping in Parkinson's disease. Parkinsons Dis.
2011(897586)2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Velseboer DC, de Haan RJ, Wieling W,
Goldstein DS and de Bie RMA: Prevalence of orthostatic hypotension
in Parkinson's disease: A systematic review and meta-analysis.
Parkinsonism Relat Disord. 17:724–729. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yalcin A, Atmis V, Cengiz OK, Cinar E,
Aras S, Varli M and Atli T: Evaluation of cardiac autonomic
functions in older Parkinson's disease patients: A cross-sectional
study. Aging Dis. 7:28–35. 2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palma JA and Kaufmann H: Epidemiology,
diagnosis, and management of neurogenic orthostatic hypotension.
Mov Disord Clin Pract. 4:298–308. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jain S and Goldstein DS: Cardiovascular
dysautonomia in Parkinson disease: From pathophysiology to
pathogenesis. Neurobiol Dis. 46:572–580. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Senard JM, Raï S, Lapeyre-Mestre M, Brefel
C, Rascol O, Rascol A and Montastruc JL: Prevalence of orthostatic
hypotension in Parkinson's disease. J Neurol Neurosurg Psychiatry.
63:584–589. 1997.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Blaho A, Šutovský S, Valkovič P, Šiarnik
P, Sýkora M and Turčáni P: Decreased baroreflex sensitivity in
Parkinson's disease is associated with orthostatic hypotension. J
Neurol Sci. 377:207–211. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nakamura T, Hirayama M, Hara T, Mizutani
Y, Suzuki J, Watanabe H and Sobue G: Role of cardiac sympathetic
nerves in preventing orthostatic hypotension in Parkinson's
disease. Parkinsonism Relat Disord. 20:409–414. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shibata M, Morita Y, Shimizu T, Takahashi
K and Suzuki N: Cardiac parasympathetic dysfunction concurrent with
cardiac sympathetic denervation in Parkinson's disease. J Neurol
Sci. 276:79–83. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Goldstein DS: Dysautonomia in Parkinson's
disease: Neurocardiological abnormalities. Lancet Neurol.
2:669–676. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Goldstein DS, Pechnik S, Holmes C, Eldadah
B and Sharabi Y: Association between supine hypertension and
orthostatic hypotension in autonomic failure. Hypertension.
42:136–142. 2003.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Galvin JE, Lee VMY and Trojanowski JQ:
Synucleinopathies: Clinical and pathological implications. Arch
Neurol. 58:186–190. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Iodice V, Low DA, Vichayanrat E and
Mathias CJ: Cardiovascular autonomic dysfunction in MSA and
Parkinson's disease: Similarities and differences. J Neurol Sci.
310:133–138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Cuoco S, Carotenuto I, Cappiello A,
Scannapieco S, Russillo MC, Andreozzi V, Forino L, Amboni M,
Picillo M, Erro R, et al: Relationship between orthostatic
hypotension and cognitive functions in multiple system atrophy: A
longitudinal study. Front Neurol. 12(711358)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
de la Sierra A, Gorostidi M, Banegas JR,
Segura J, de la Cruz JJ and Ruilope LM: Nocturnal hypertension or
nondipping: Which is better associated with the cardiovascular risk
profile? Am J Hypertens. 27:680–687. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Espay AJ, LeWitt PA, Hauser RA, Merola A,
Masellis M and Lang AE: Neurogenic orthostatic hypotension and
supine hypertension in Parkinson's disease and related
synucleinopathies: Prioritisation of treatment targets. Lancet
Neurol. 15:954–966. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shin NY, Park YW, Yoo SW, Yoo JY, Choi Y,
Jang J, Ahn KJ, Kim BS and Kim JS: Adverse effects of hypertension,
supine hypertension, and perivascular space on cognition and motor
function in PD. NPJ Parkinsons Dis. 7(69)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Meade RM, Fairlie DP and Mason JM:
Alpha-synuclein structure and Parkinson's disease-lessons and
emerging principles. Mol Neurodegener. 14(29)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Javanshiri K, Drakenberg T, Haglund M and
Englund E: Cardiac alpha-synuclein is Present in
alpha-synucleinopathies. J Parkinsons Dis. 12:1125–1131.
2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Isonaka R, Rosenberg AZ, Sullivan P,
Corrales A, Holmes C, Sharabi Y and Goldstein DS: Alpha-synuclein
deposition within sympathetic noradrenergic neurons is associated
with myocardial noradrenergic deficiency in neurogenic orthostatic
hypotension. Hypertension. 73:910–918. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Rodrigues LD, Oliveira LF, Shinoda L,
Scorza CA, Faber J, Ferraz HB, Britto LRG and Scorza FA:
Cardiovascular alterations in rats with Parkinsonism induced by
6-OHDA and treated with Domperidone. Sci Rep.
9(8965)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tijero B, Gómez-Esteban JC, Lezcano E,
Fernández-González C, Somme J, Llorens V, Martínez A, Ruiz-Martínez
J, Foncea N, Escalza I, et al: Cardiac sympathetic denervation in
symptomatic and asymptomatic carriers of the E46K mutation in the α
synuclein gene. Parkinsonism Relat Disord. 19:95–100.
2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tijero B, Gómez Esteban JC, Somme J,
Llorens V, Lezcano E, Martinez A, Rodríguez T, Berganzo K and
Zarranz JJ: Autonomic dysfunction in parkinsonian LRRK2 mutation
carriers. Parkinsonism Relat Disord. 19:906–909. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Park JH, Kim DH, Park YG, Kwon DY, Choi M,
Jung JH and Han K: Association of Parkinson disease with risk of
cardiovascular disease and all-cause mortality: A nationwide,
population-based cohort study. Circulation. 141:1205–1207.
2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Suri JS, Paul S, Maindarkar MA, Puvvula A,
Saxena S, Saba L, Turk M, Laird JR, Khanna NN, Viskovic K, et al:
Cardiovascular/stroke risk stratification in Parkinson's disease
patients using atherosclerosis pathway and artificial intelligence
paradigm: A systematic review. Metabolites. 12(312)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Driver JA, Kurth T, Buring JE, Gaziano JM
and Logroscino G: Parkinson disease and risk of mortality: A
prospective comorbidity-matched cohort study. Neurology.
70:1423–1430. 2008.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Nam GE, Kim SM, Han K, Kim NH, Chung HS,
Kim JW, Han B, Cho SJ, Yu JH, Park YG and Choi KM: Metabolic
syndrome and risk of Parkinson disease: A nationwide cohort study.
PLoS Med. 15(e1002640)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Qiu C, Hu G, Kivipelto M, Laatikainen T,
Antikainen R, Fratiglioni L, Jousilahti P and Tuomilehto J:
Association of blood pressure and hypertension with the risk of
Parkinson disease: The national FINRISK study. Hypertension.
57:1094–1100. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liang HW, Huang YP and Pan SL: Parkinson
disease and risk of acute myocardial infarction: A
population-based, propensity score-matched, longitudinal follow-up
study. Am Heart J. 169:508–514. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Potashkin J, Huang X, Becker C, Chen H,
Foltynie T and Marras C: Understanding the links between
cardiovascular disease and Parkinson's disease. Mov Disord.
35:55–74. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Vikdahl M, Carlsson M, Linder J, Forsgren
L and Håglin L: Weight gain and increased central obesity in the
early phase of Parkinson's disease. Clin Nutr. 33:1132–1139.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Chohan H, Senkevich K, Patel RK, Bestwick
JP, Jacobs BM, Bandres Ciga S, Gan-Or Z and Noyce AJ: Type 2
diabetes as a determinant of Parkinson's disease risk and
progression. Mov Disord. 36:1420–1429. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Hassan A, Sharma Kandel R, Mishra R,
Gautam J, Alaref A and Jahan N: Diabetes mellitus and Parkinson's
disease: Shared pathophysiological links and possible therapeutic
implications. Cureus. 12(e9853)2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Athauda D and Foltynie T: Insulin
resistance and Parkinson's disease: A new target for disease
modification? Prog Neurobiol. 145-146:98–120. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lương K and Nguyễn L: Role of vitamin D in
Parkinson's disease. ISRN Neurol. 2012(134289)2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kim JS, Kim YI, Song C, Yoon I, Park JW,
Choi YB, Kim HT and Lee KS: Association of vitamin D receptor gene
polymorphism and Parkinson's disease in Koreans. J Korean Med Sci.
20:495–498. 2005.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Pascale E, Purcaro C, Passarelli E,
Guglielmi R, Vestri AR, Passarelli F and Meco G: Genetic
polymorphism of angiotensin-converting enzyme is not associated
with the development of Parkinson's disease and of L-dopa-induced
adverse effects. J Neurol Sci. 276:18–21. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Castellani R, Smith MA, Richey GL and
Perry G: Glycoxidation and oxidative stress in Parkinson disease
and diffuse Lewy body disease. Brain Res. 737:195–200.
1996.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Soós J, Engelhardt JI, Siklós L, Havas L
and Majtényi K: The expression of PARP, NF-kappa B and parvalbumin
is increased in Parkinson disease. Neuroreport. 15:1715–1718.
2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Choi JM, Hong JH, Chae MJ, Hung NP, Kang
HS, Ma HI and Kim YJ: Analysis of mutations and the association
between polymorphisms in the cerebral dopamine neurotrophic factor
(CDNF) gene and Parkinson disease. Neurosci Lett. 493:97–101.
2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fullard ME and Duda JE: A review of the
relationship between vitamin D and Parkinson disease symptoms.
Front Neurol. 11(454)2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pignolo A, Mastrilli S, Davì C, Arnao V,
Aridon P, Dos Santos Mendes FA, Gagliardo C and D'Amelio M: Vitamin
D and Parkinson's disease. Nutrients. 14(1220)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Barichella M, Garrì F, Caronni S, Bolliri
C, Zocchi L, Macchione MC, Ferri V, Calandrella D and Pezzoli G:
Vitamin D status and Parkinson's disease. Brain Sci.
12(790)2022.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rizzoni D, Rizzoni M and Nardin M: Vitamin
D and ischaemic heart disease: A casual or A causal association?:
Commentary on: ‘Raslan E et al. Association of vitamin D
deficiency with chronic stable angina: A case-control study’. High
Blood Press Cardiovasc Prev. 26:151–155. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liu X, Wang W, Tan Z, Zhu X, Liu M, Wan R
and Hong K: The relationship between vitamin D and risk of atrial
fibrillation: A dose-response analysis of observational studies.
Nutr J. 18(73)2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Barbarawi M, Kheiri B, Zayed Y, Barbarawi
O, Dhillon H, Swaid B, Yelangi A, Sundus S, Bachuwa G, Alkotob ML
and Manson JE: Vitamin D supplementation and cardiovascular disease
risks in more than 83 000 individuals in 21 randomized clinical
trials: A Meta-analysis. JAMA Cardiol. 4:765–776. 2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Luan Y and Yao Y: The clinical
significance and potential role of C-reactive protein in chronic
inflammatory and neurodegenerative diseases. Front Immunol.
9(1302)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Qiu X, Xiao Y, Wu J, Gan L, Huang Y and
Wang J: C-reactive protein and risk of Parkinson's disease: A
systematic review and meta-analysis. Front Neurol.
10(384)2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sawada H, Oeda T, Umemura A, Tomita S,
Kohsaka M, Park K, Yamamoto K and Sugiyama H: Baseline C-reactive
protein levels and life prognosis in Parkinson disease. PLoS One.
10(e0134118)2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Hwang O: Role of oxidative stress in
Parkinson's disease. Exp Neurobiol. 22:11–17. 2013.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Perfeito R, Cunha-Oliveira T and Rego AC:
Revisiting oxidative stress and mitochondrial dysfunction in the
pathogenesis of Parkinson disease-resemblance to the effect of
amphetamine drugs of abuse. Free Radic Biol Med. 53:1791–1806.
2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ascherio A, LeWitt PA, Xu K, Eberly S,
Watts A, Matson WR, Marras C, Kieburtz K, Rudolph A, Bogdanov MB,
et al: Urate as a predictor of the rate of clinical decline in
Parkinson disease. Arch Neurol. 66:1460–1468. 2009.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Schwarzschild MA, Schwid SR, Marek K,
Watts A, Lang AE, Oakes D, Shoulson I and Ascherio A: Parkinson
Study Group PRECEPT Investigators. Hyson C, et al: Serum urate as a
predictor of clinical and radiographic progression in Parkinson
disease. Arch Neurol. 65:716–723. 2008.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Bluett B, Togasaki DM, Mihaila D, Evatt M,
Rezak M, Jain S, Schwarzschild MA, Ascherio A, Casaceli C, Curhan
GC, et al: Effect of urate-elevating inosine on early Parkinson
disease progression: The SURE-PD3 randomized clinical trial. JAMA.
326:926–939. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Borghi C and Piani F: Uric acid and risk
of cardiovascular disease: A question of start and finish.
Hypertension. 78:1219–1221. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Saito Y, Shioya A, Sano T, Sumikura H,
Murata M and Murayama S: Lewy body pathology involves the olfactory
cells in Parkinson's disease and related disorders. Mov Disord.
31:135–138. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Waragai M, Nakai M, Wei J, Fujita M,
Mizuno H, Ho G, Masliah E, Akatsu H, Yokochi F and Hashimoto M:
Plasma levels of DJ-1 as a possible marker for progression of
sporadic Parkinson's disease. Neurosci Lett. 425:18–22.
2007.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Lev N, Roncevich D, Ickowicz D, Melamed E
and Offen D: Role of DJ-1 in Parkinson's disease. J Mol Neurosci.
29:215–226. 2006.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Repici M and Giorgini F: DJ-1 in
Parkinson's disease: Clinical insights and therapeutic
perspectives. J Clin Med. 8(1377)2019.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Shimizu Y, Nicholson CK, Polavarapu R,
Pantner Y, Husain A, Naqvi N, Chin LS, Li L and Calvert JW: Role of
DJ-1 in modulating glycative stress in heart failure. J Am Heart
Assoc. 9(e014691)2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Tsoporis JN, Drosatos IA, Gupta S,
Amatullah H, Izhar S, Dos Santos CC, Salpeas V, Rigopoulos AG,
Toumpoulis IK, Triantafyllis AS, et al: Cytoprotective mechanisms
of DJ-1: Implications in cardiac pathophysiology. Molecules.
26(3795)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
He R, Yan X, Guo J, Xu Q, Tang B and Sun
Q: Recent advances in biomarkers for Parkinson's disease. Front
Aging Neurosci. 10(305)2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cuenca-Bermejo L, Almela P,
Navarro-Zaragoza J, Fernández Villalba E, González-Cuello AM,
Laorden ML and Herrero MT: Cardiac changes in Parkinson's disease:
Lessons from clinical and experimental evidence. Int J Mol Sci.
22(13488)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Dhall R and Kreitzman DL: Advances in
levodopa therapy for Parkinson disease: Review of RYTARY (carbidopa
and levodopa) clinical efficacy and safety. Neurology. 86 (14 Suppl
1):S13–S24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Günaydin ZY, Özer FF, Karagöz A, Bektaş O,
Karataş MB, Vural A, Bayramoğlu A, Çelik A and Yaman M: Evaluation
of cardiovascular risk in patients with Parkinson disease under
levodopa treatment. J Geriatr Cardiol. 13:75–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Martignoni E, Tassorelli C, Nappi G,
Zangaglia R, Pacchetti C and Blandini F: Homocysteine and
Parkinson's disease: A dangerous liaison? J Neurol Sci. 257:31–37.
2007.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Kocer B, Guven H and Comoglu SS:
Homocysteine levels in Parkinson's disease: Is entacapone
effective? Biomed Res Int. 2016(7563705)2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
O'Suilleabhain PE, Sung V, Hernandez C,
Lacritz L, Dewey RB Jr, Bottiglieri T and Diaz-Arrastia R: Elevated
plasma homocysteine level in patients with Parkinson disease:
Motor, affective, and cognitive associations. Arch Neurol.
61:865–868. 2004.PubMed/NCBI View Article : Google Scholar
|
|
82
|
van Camp G, Flamez A, Cosyns B, Goldstein
J, Perdaens C and Schoors D: Heart valvular disease in patients
with Parkinson's disease treated with high-dose pergolide.
Neurology. 61:859–861. 2003.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Horvath J, Fross RD, Kleiner-Fisman G,
Lerch R, Stalder H, Liaudat S, Raskoff WJ, Flachsbart KD, Rakowski
H, Pache JC, et al: Severe multivalvular heart disease: A new
complication of the ergot derivative dopamine agonists. Mov Disord.
19:656–662. 2004.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Tran T, Brophy JM, Suissa S and Renoux C:
Risks of cardiac valve regurgitation and heart failure associated
with ergot- and non-ergot-derived dopamine agonist use in patients
with Parkinson's disease: A systematic review of observational
studies. CNS Drugs. 29:985–998. 2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kho J, Ioannou A, Mandal AKJ, Cox A, Nasim
A, Metaxa S and Missouris CG: Long term use of donepezil and QTc
prolongation. Clin Toxicol (Phila). 59:208–214. 2021.PubMed/NCBI View Article : Google Scholar
|
|
86
|
de Baat EC, Mulder RL, Armenian S, Feijen
EA, Grotenhuis H, Hudson MM, Mavinkurve-Groothuis AM, Kremer LC and
van Dalen EC: Dexrazoxane for preventing or reducing cardiotoxicity
in adults and children with cancer receiving anthracyclines.
Cochrane Database Syst Rev. 9(CD014638)2022.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Mei M, Zhou Y, Liu M, Zhao F, Wang C, Ding
J, Lu M and Hu G: Antioxidant and anti-inflammatory effects of
dexrazoxane on dopaminergic neuron degeneration in rodent models of
Parkinson's disease. Neuropharmacology. 160(107758)2019.PubMed/NCBI View Article : Google Scholar
|