Introduction
Focal ischemia occurs when an embolus or thrombus
occludes an artery, causing the rapid obstruction of cerebral blood
flow (CBF). There is increasing evidence to indicate that stroke is
a main cause of disability and mortality in developing countries
(1). Moreover, the global economic
burden of stroke on health care is enormous. At present, stroke
accounts for ~34% of total global healthcare-related costs
(2). In addition, effective
therapeutic strategies for stroke remain very limited. Novel
treatment options are thus required.
Normally, the average CBF for an adult is ~50 ml/100
g/min, which is crucial for brain function (3). In focal infarcts, the CBF decreases to
<10 ml/100 g/min, causing irreversible neuronal damage (4). Folioing the onset of complete
ischemia, the levels of adenosine triphosphate (ATP) are depleted,
disrupting ionic homeostasis. Radical species then form and these
are involved in mediating damage. Cells have enzymatic and
non-enzymatic defense mechanisms to protect themselves from this
type of damage (5). Enzymatic
mechanisms include neutralization by superoxide dismutase (SOD),
glutathione peroxidase (GSH-Px) and catalase (CAT). Previous
studies have demonstrated that increasing the levels of these
antioxidant enzymes can mitigate brain damage caused by ischemic
stroke (5-7).
Neuronal inflammation and apoptosis are also induced
by the oxidative stress described above (8). Arachidonic acid derivatives and
prostaglandins are the major inflammatory mediators. With the onset
of ischemia, the levels of intracellular calcium increase, with the
consequent activation of phospholipase. The metabolites of
arachidonic acid are the most critical contributors to the
pathophysiology of ischemic stroke. A number of studies have found
that interleukin (IL)-1 and IL-6 are also mediators of ischemic
damage (9-11).
Cyclooxygenase-2 (COX-2) is also a mediator of inflammation
(12,13), and there is accumulating evidence to
indicate that COX-2 suppression can attenuate ischemic injury
following middle cerebral artery occlusion (14). Herbal bioactive components exhibit
anti-inflammatory, antioxidant and anti-apoptotic properties that
may have therapeutic potential against the neuronal injury caused
by ischemic stroke.
The main phytochemical present in ginger
(Zingiber officinale; Zingiberaceae family) is 6-gingerol.
This compound exhibits a variety of pharmacological properties,
including anti-inflammatory, antioxidant (15,16),
anti-apoptotic (17) and anticancer
activities (18). Additionally, a
previous study demonstrated that 6-gingerol significantly reduced
the infarct volume and brain damage following ischemia/reperfusion
injury by inhibiting NLR family pyrin domain containing 3
inflammasome-induced inflammation and neuronal apoptosis (19). However, the effects of 6-gingerol
against focal ischemic stroke-induced brain damage have not yet
been evaluated, at least to the best of our knowledge. Thus, the
present study examined the effects of 6-gingerol on the brain
infarct volume, neuronal loss and on the oxidative stress
parameters, COX-2 and IL-6, in rats following focal ischemic
stroke.
Materials and methods
Test treatments
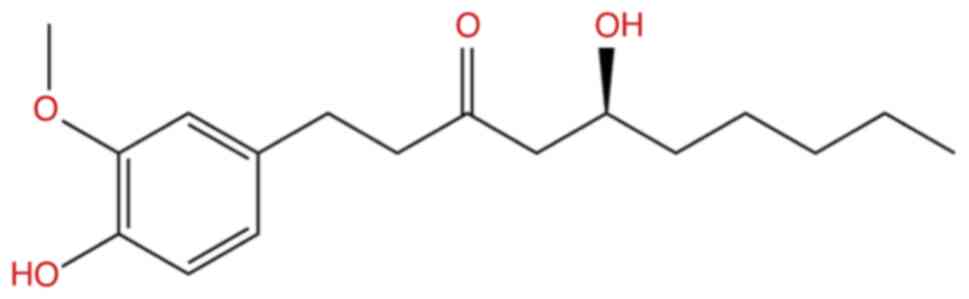
The test compound, 6-gingerol (98.7% purity;
chemical structure illustrated in Fig.
1), was purchased from Chengdu Biopurify Phytochemicals Ltd.
Piracetam, the positive control, was obtained from Glaxosmithkline
(Thailand) Ltd. and DMSO, the vehicle, was obtained from Thermo
Fisher Scientific, Inc. (product code: D/4121/PB15).
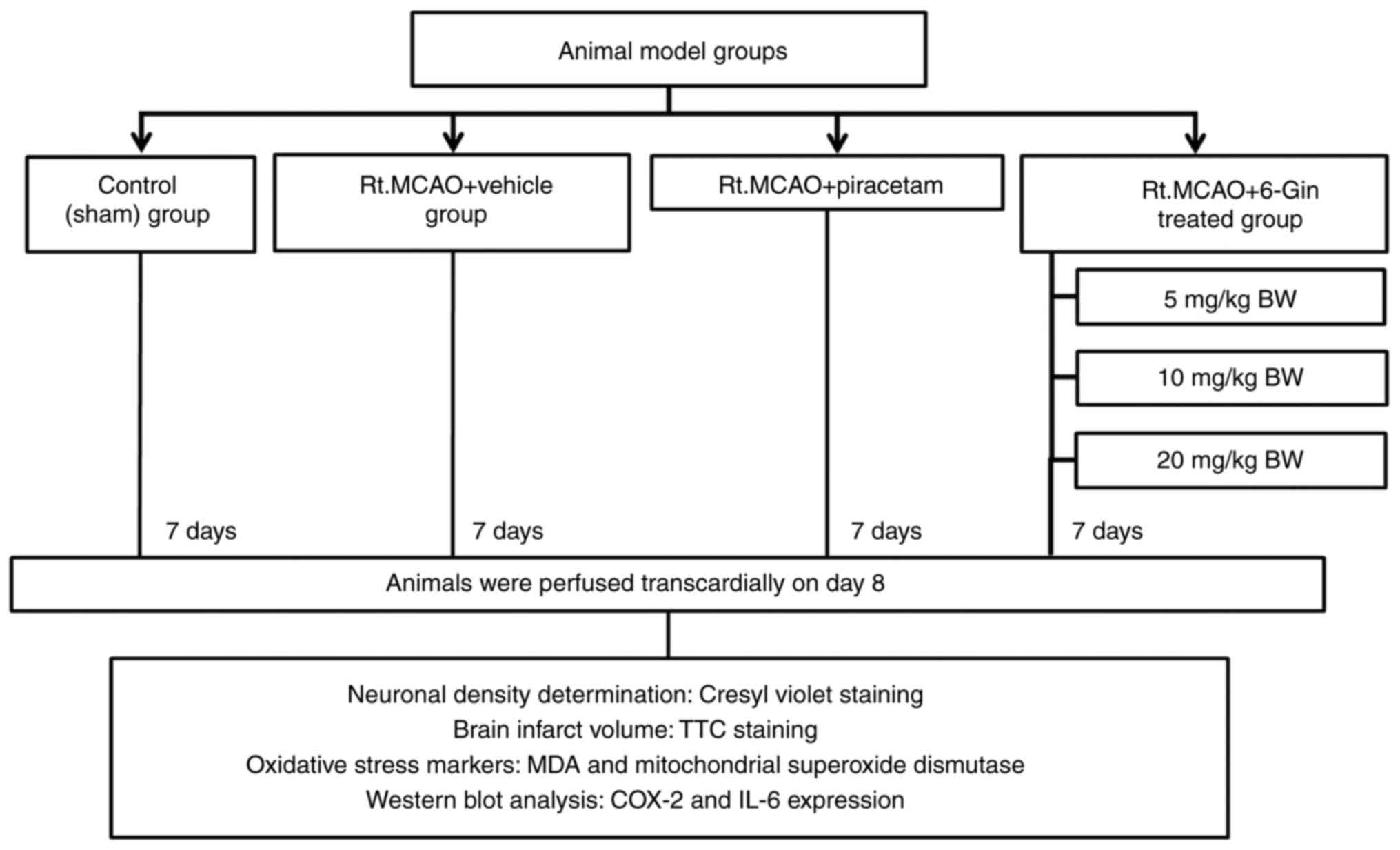
Study design
A total of 90 healthy male Wistar rats (weighing
250-300 g, 8 weeks old, from the Northeastern Laboratory Animal
Center, Khon Kaen University, Khon Kaen, Thailand) were randomly
divided into six groups (n=15 per group) as follows: i) The
control; ii) right middle cerebral artery occlusion (Rt.MCAO) +
vehicle; iii) Rt.MCAO + piracetam at 250 mg/kg body weight (BW);
iv) Rt.MCAO + 6-gingerol (6-Gin) at 5 mg/kg BW; v) Rt.MCAO + 6-Gin
at 10 mg/kg BW; and vi) the Rt.MCAO + 6-Gin at 20 mg/kg BW group.
The animals in all the groups received their treatments by
intraperitoneal (i.p.) injection once daily for 7 consecutive days
following Rt.MCAO. All rats were housed in groups of five in
standard metal cages, maintained under standard conditions with a
12-h on/off light/dark cycle, relative humidity controlled at
~30-60%, and a temperature controlled at 23±2˚C. The rats had ad
libitum access to water and commercial pellets (24 h/day). The
piracetam at 250 mg/kg BW and 6-gingerol at 5, 10 and 20 mg/kg BW
were selected based on previous research by the authors,
preliminary studies and literature reviews (6,16,20).
Moreover, as previously reported, the median lethal dose of
gingerol (i.p. administration) has been determined in mice and
rats. The dose required to kill half the members of a tested
population following a specified test duration value (LD50) has
been reported to be ~58.1 mg/kg BW (21). Thus, the doses used in the present
study were noted to be non-toxic. The infarct volume was examined
for 5 rats in each group using 2,3,5-triphenyltetrazolium chloride
(TTC) staining. Another 5 rats per group were used to measure
cerebral cortex and the hippocampus neuronal density using cresyl
violet staining. The remaining 5 animals per group were used to
examine the malondialdehyde (MDA) levels and SOD activities in the
cortex and hippocampus mitochondria using biochemical assays. IL-6
and COX-2 expression levels were also measured in the cortex and
hippocampus of the rats treated with those doses of 6-gingerol that
produced optimum changes in infarct volume, neuronal density and
oxidative stress markers (Fig. 2).
Two replicates were performed for each test.
Model of Rt.MCAO
All animals were fasted overnight, but were allowed
free access to water before their surgery. The animals were
anesthetized using isoflurane (5% for induction and 1-3% for
maintenance) delivered in 100% oxygen. The model of focal ischemia
was established by the permanent intraluminal occlusion of the
right middle cerebral artery, as previously described (22). Briefly, a 4-0 silicone-coated
monofilament (USS DGTM Division of United States
Surgical; Tyco Healthcare Group LP, Norwalk, CT, USA) was inserted
into the internal carotid artery ~17 mm or until a slight
resistance was detected. The wound was then sutured and 10%
povidone iodine solution was applied at the incision site for
antiseptic postoperative care. In the sham operation, all the
arteries were exposed as described above, but monofilament
insertion was not performed. The criteria for humane endpoints was
defined as the inability to move, wound infection following
surgery, a weight loss of >20%, dehydration, dyspnea,
progressive pain, lack of response to external stimuli and bleeding
from any orifice. However, all animals in the present study
survived to the end of the study period (8 days).
Determination of brain infarct
volume
At the end of the study period, the rats were
anesthetized with thiopental sodium (80 mg/kg BW; i.p.
administration) prior to cardiac perfusion with cold normal saline
solution. The brains were then removed from the skull and the
2-mm-thick coronal sections were stained with 2% TTC
(MilliporeSigma) in normal saline for 30 min at 37˚C. Images were
then obtained using a digital camera and the infarct volume was
determined using Image J® software (version 1.53e,
National Institutes of Health). The infarct volumes were then
calculated using the formula described in a previous study by the
authors (6).
Cresyl violet staining for neuronal
density determination
Serial coronal sections of the cortex and
hippocampus (30-µm-thick) were stained with cresyl violet acetate
solution (MilliporeSigma) for 16 min at 60˚C to determine the
neuronal density. Regions of the cortex and hippocampus (CA1, CA2
and CA3) were then examined using an Olympus light microscope
(model CX23; Olympus Corporation) at x40 magnification. Images of
the cortex and hippocampus at stereotaxic co-ordinates, selected as
described in a previous study by the authors (23), were used to measure neuronal
density. This was achieved by blinded analysis, and the data are
expressed as a percentage of the control.
Isolation of brain mitochondria for
biochemical assays
Following the completion of perfusion, brain tissues
from the cerebral cortex and hippocampal regions were isolated and
prepared for mitochondrial extraction, using a protocol described
in a previous study by the authors (24). The brain tissues were stored at
-80˚C until use.
Protein determination
The method described in the study by Lowry et
al (25) was used to determine
the mitochondrial protein concentrations in the brain areas
aforementioned, using bovine serum albumin (MilliporeSigma) as a
standard.
Determination of the MDA level
The lipid peroxidation product, MDA, was used as an
indicator of oxidative stress. Its levels were measured using the
thiobarbituric acid (MilliporeSigma) reaction in all samples,
according to the method described in the study by Ohkawa et
al (26). The results are
reported as nmol/mg protein mitochondria.
Determination of SOD activity
A SOD assay kit from MilliporeSigma (19160-1K-F) was
used to determine the SOD activity. Data are expressed as U/mg
protein mitochondria.
Western blot analysis
At the end of the study period, COX-2 and IL-6
expression levels were measured in the cortex and hippocampus of
the rats using western blot analysis as described in a previous
study by the authors (22). The
brain tissues were homogenized in lysis buffer (cat. no. 87792,
Thermo Fisher Scientific, Inc.) and the method of Lowry et
al (25) was used to determine
the total protein concentrations. Equal quantities of protein (40
µg protein) were subjected to 10% SDS-polyacrylamide gel
electrophoresis, transferred to a Hybond-P (PVDF) membrane (Cytiva)
and then incubated with rabbit monoclonal anti-COX-2 (1:1,000, cat.
no. ab179800, Abcam), mouse monoclonal anti-IL-6 (1:2,000, cat. no.
ab9324, Abcam) and rabbit monoclonal anti-β-actin (1:5,000, cat.
no. AC026, ABclonal Biotech Co., Ltd.) antibodies at 4˚C overnight.
The membranes were then incubated with anti-rabbit (1:2,000, cat.
no. AS063, ABclonal Biotech Co., Ltd.) or anti-mouse (1:2,000, cat.
no. 12-349, MilliporeSigma) secondary antibodies for 1 h at room
temperature. The immunoreactive proteins on the blots were
visualized using chemiluminescent substrate (Supersignal West Pico;
Pierce; Thermo Fisher Scientific, Inc.). The density of the COX-2
and IL-6 bands were normalized to β-actin, and the protein
expression was calculated using a ChemiDocTM MP imaging
system with Image Lab software (version 6.0.0 build 25, Bio-Rad
Laboratories Inc.).
Statistical analysis
The data are expressed as the mean ± the standard
error of the mean. Statistical analysis was assessed using one-way
analysis of variance (ANOVA), followed by a Tukey’s post hoc test
using SPSS® software (version 25, SPSS-IBM Inc.). A
P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Effects of 6-gingerol on brain injury
in rats subjected to Rt.MCAO
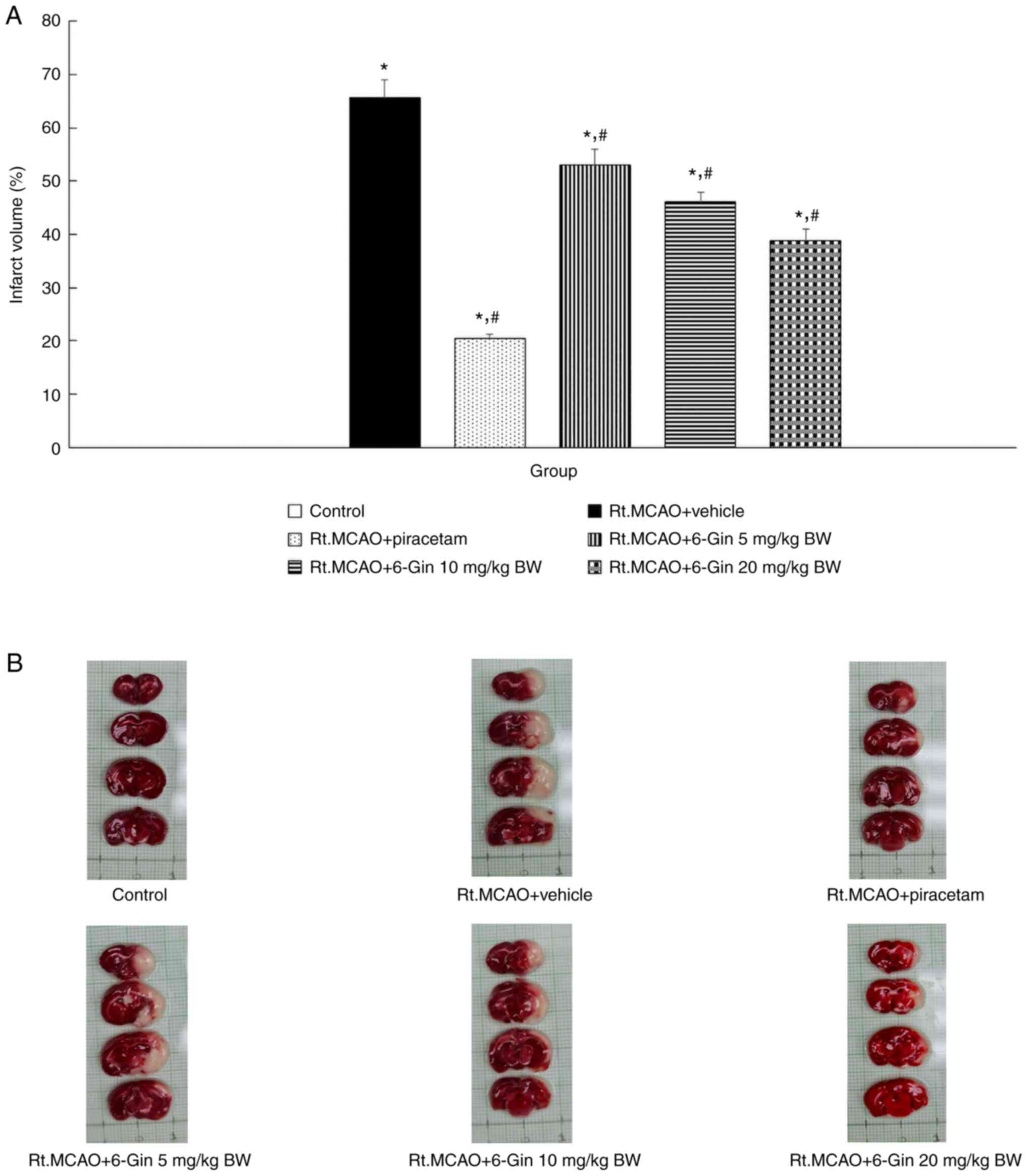
The present study measured the brain infarct volume
using TTC staining following treatment of the rats with 6-gingerol.
Rats undergoing permanent occlusion (Rt.MCAO) and who received the
vehicle exhibited a significantly increased infarct volume
(P<0.05) compared to the rats in the control group (Fig. 3). However, the rats treated with
piracetam and 6-gingerol exhibited a marked decrease in their
infarct volumes compared to the vehicle-treated ischemic stroke
group (P<0.05).
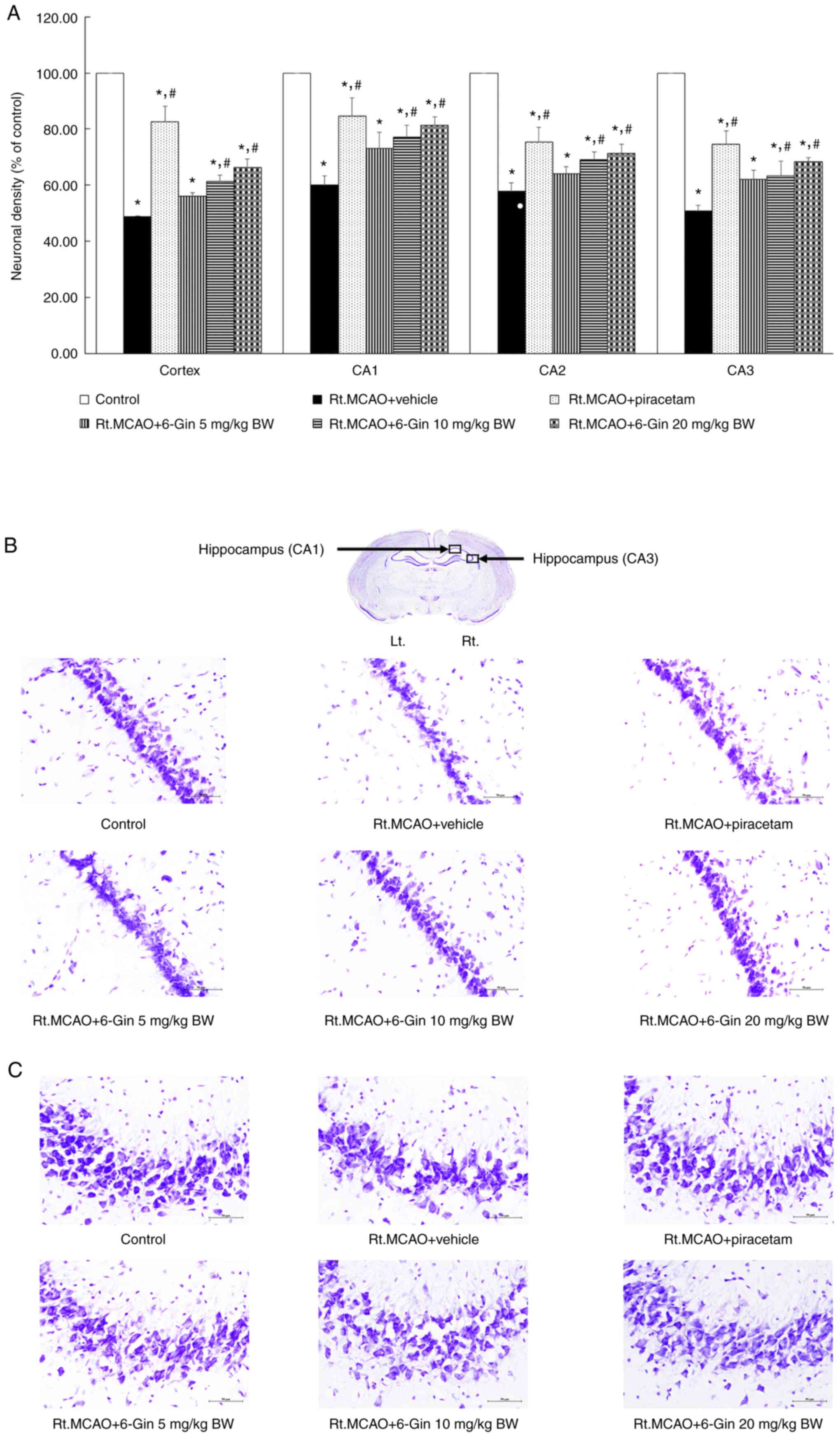
Effects of 6-gingerol on neuronal
damage in the cortex and hippocampus of rats subjected to
Rt.MCAO
Cerebral ischemia induced major neuronal damage in
the cortex and all subregions of the hippocampus (CA1, CA2 and
CA3). The Rt.MCAO + vehicle (DMSO) group exhibited a significant
decrease in neuronal survival in the cortex or CA1, CA2 or CA3
regions of the hippocampus compared to the control. By contrast,
piracetam (at a dose of 250 mg/kg BW) and 6-gingerol (at doses of
10 and 20 mg/kg BW) markedly reduced ischemic stroke-induced
neuronal loss within the cortex and all subregions of the
hippocampus (P<0.05) compared to the Rt.MCAO + vehicle group
(Fig. 4). At the lower dose 5 mg/kg
BW), 6-gingerol did not lead to any significant difference in
neuronal damage in all areas compared to the Rt.MCAO + vehicle
group.
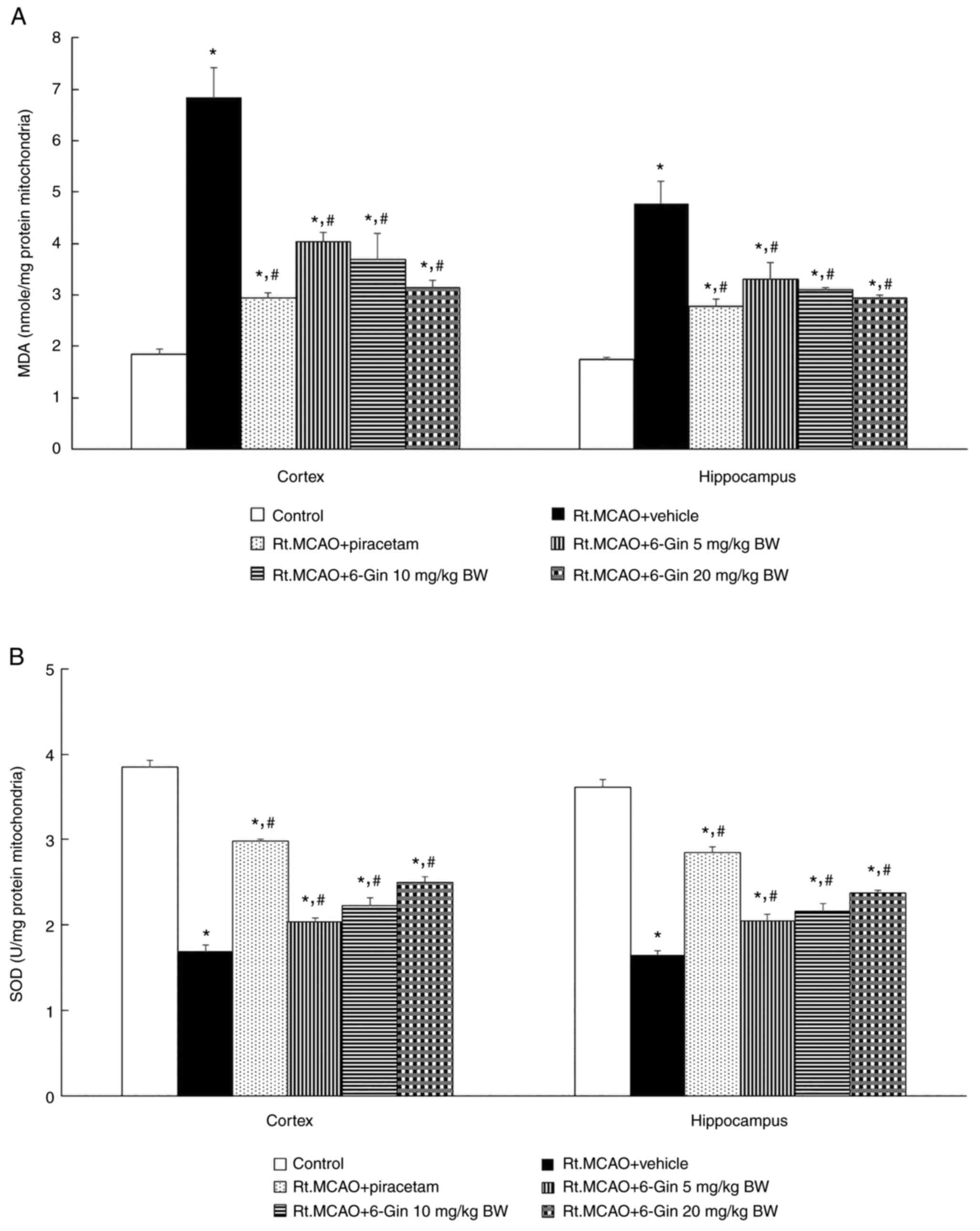
Effects of 6-gingerol on oxidative
stress in the mitochondria from the cortex and hippocampus of rats
subjected to Rt.MCAO
As oxidative stress plays a key role in the
pathogenesis of ischemic stroke, the present study also examined
the effects of 6-gingerol on oxidative stress markers, including
MDA levels and activity of the scavenging enzyme, SOD, in the
mitochondria. Rats that underwent permanent Rt.MCAO exhibited
increased mitochondrial MDA levels (Fig. 5A) and significantly reduced
mitochondrial SOD activities (Fig.
5B) compared to the control group. By contrast, the rats
treated with piracetam or 6-gingerol (5, 10 and 20 mg/kg BW)
exhibited mitochondrial SOD activities that were significantly less
diminished and mitochondrial MDA levels that were significantly
less elevated than the Rt.MCAO + vehicle group both in the cerebral
cortex and hippocampus (P<0.05; Fig.
5).
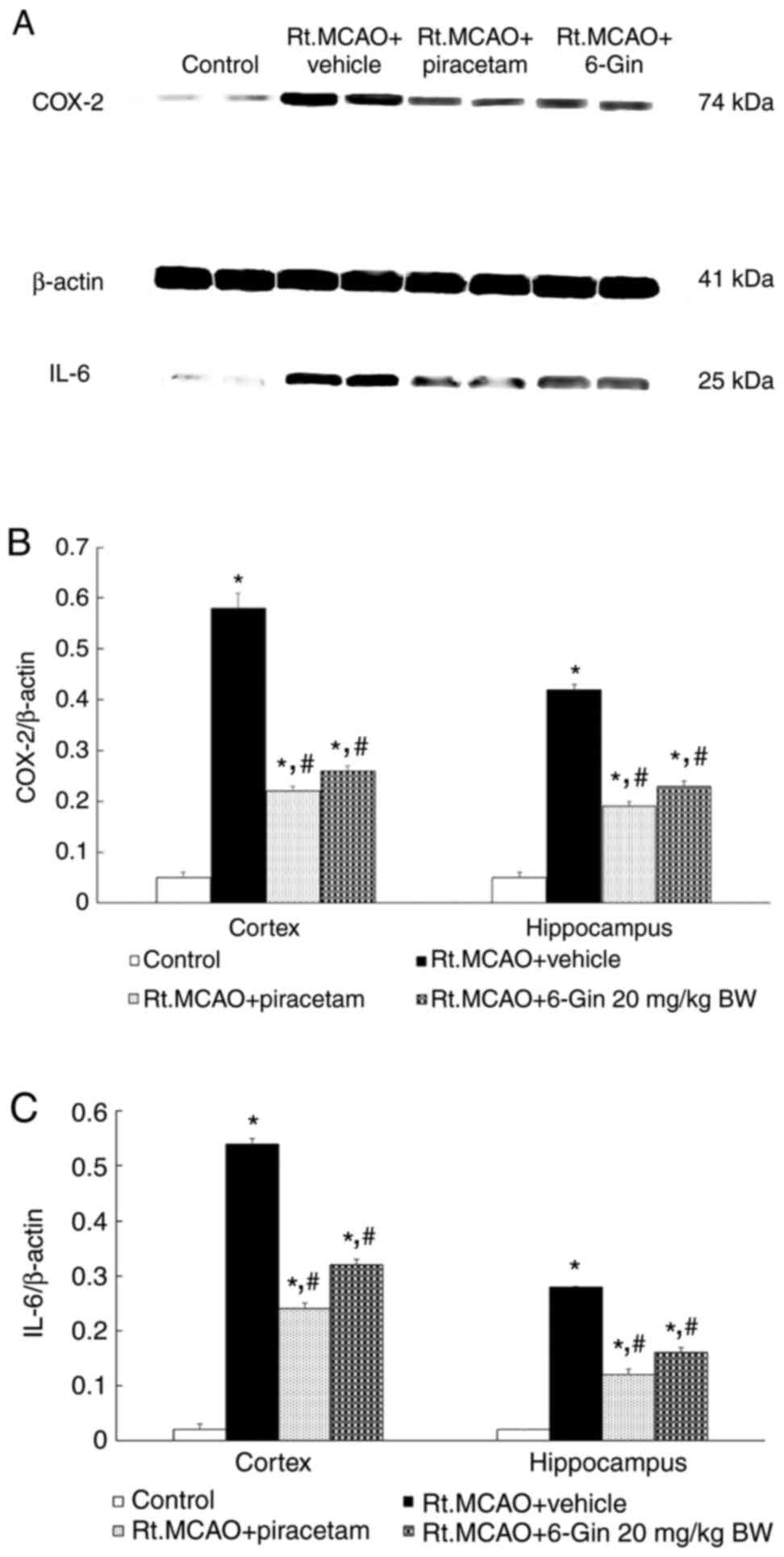
Effects of 6-gingerol on COX-2 and
IL-6 expression in the cortex and hippocampus of rats subjected to
Rt.MCAO
To assess the anti-inflammatory activity of
6-gingerol, the present study measured COX-2 and IL-6 protein
expression in the cortex and hippocampus using western blot
analysis. As 20 mg/kg BW 6-gingerol led to optimum changes in
infarct volume, neuronal density and oxidative stress markers, this
dose was selected to investigate the effects of 6-gingerol on COX-2
and IL-6 levels. Bands showing positive immunoreactivities against
COX-2, β-actin and IL-6 were detected at 74, 41 and 25 kDa,
respectively (Fig. 6A). Treatment
with 6-gingerol at a dose of 20 mg/kg BW markedly decreased the
density ratios of COX-2 and IL-6 to the β-actin band compared to
the Rt.MCAO + vehicle group (P<0.05, Fig. 6B and C).
Discussion
Animal models are widely used to gain understanding
of the pathophysiology of cerebral ischemic injury. In such
studies, MCAO is one of the most commonly employed surgical
procedures to induce ischemic stroke (27). This model, known as the permanent
MCAO model, has been developed to mimic human ischemic stroke, and
generates cerebral infarction in both cortical and subcortical
areas (28). The present study used
the MCAO model to investigate 6-gingerol as an experimental
therapy. As was expected, cortical and subcortical region infarct
volumes were large in rats treated with only DMSO, whereas these
volumes were significantly smaller in the rats treated with
6-gingerol and piracetam.
In addition to the infarcts described above,
cortical and hippocampal neuronal loss and brain damage are
reportedly induced in the MCAO model (29-31),
with apoptotic cell death occurring after 24 h (32-34).
Data from the present study are in agreement with these previous
findings. Herein, the positive control drug, piracetam, which
increases CBF, was found to enhance the density of neurons within
the cortex and hippocampus. This result is also in agreement with
those of previous studies, which reported the nootropic action of
this drug and its ability to ameliorate cerebral ischemia-induced
brain damage (35-37).
In the present study, the experimental treatment agent, 6-gingerol,
markedly alleviated ischemic stroke-induced neuronal damage within
the cortex and hippocampus regions CA1, CA2 and CA3. Although this
has not been previously demonstrated, at least to the best of our
knowledge, it does correspond well with other reported activities
of 6-gingerol. For example, 6-gingerol has previously been shown to
reduce the in vitro apoptosis of PC12 cells (38). It has also been previously
demonstrated that 6-gingerol protects rats from
lipopolysaccharide-induced brain injury by improving the expression
of brain-derived neurotrophic factor (BDNF) within the cerebral
cortex and hippocampus (20). In
another in vivo study, 6-gingerol improved the hippocampal
levels of BDNF and nerve growth factor in rats exposed to gold
nanoparticles (17). Moreover,
during pathophysiological processes in the subacute phase of
ischemic stroke (up to 7 days following complete ischemia onset),
ATP levels are depleted, disrupting ionic homeostasis. Radical
species then form and these are involved in mediating damage. Cells
have enzymatic and non-enzymatic defense mechanisms to protect
themselves from this type of damage (5). In addition, the initiation of the
inflammatory response followed by the release of mediators
exacerbates the effects of neuronal inflammation and primary damage
(39). Therefore, the present study
selected the duration of 6-gingerol treatment to be 7 days.
Reactive oxygen species (ROS) contribute to the
progression of numerous diseases (40-42),
and both ROS and lipid peroxidation (LPO) are known to mediate
tissue damage in ischemic stroke. Cells have enzymatic and
non-enzymatic defense mechanisms to protect themselves from some of
this damage (5). Enzymatic
mechanisms include neutralization by SOD, GSH-Px and CAT. Previous
studies have found that increasing these antioxidant enzymes can
mitigate brain damage from ischemic stroke (5-7).
Another study demonstrated that 6-gingerol is a potent antioxidant,
with the potential to treat and prevent chronic diseases (43). As mitochondrial dysfunction and SOD
deficiency contribute to increased neuronal death, increased
numbers of superoxide anions and increased cerebral infarction
(44,45), the present study examined the
effects of 6-gingerol on LPO (by examining the MDA levels) and SOD
activity. The results revealed that both the positive control,
piracetam, and the experimental treatment agent, 6-gingerol (5, 10
and 20 mg/kg BW), decreased the LPO product and increased SOD
activity in cortical and hippocampal mitochondria compared to the
Rt.MCAO + vehicle group. Several lines of evidence demonstrate that
6-gingerol has antioxidant, anti-inflammatory (15,16),
anti-apoptotic (17) and anticancer
properties (18). In addition, a
6-gingerol rich fraction from Z. officinale has been shown
to prevent acrylonitrile-induced cerebral cortex damage partly via
its antioxidant and anti-inflammatory activities (46).
In the development of ischemic damage, the
production of free radicals is markdly elevated, and oxidative
stress causes neuronal inflammation and apoptosis (8). Arachidonic acid derivatives and
prostaglandins are two of the major inflammatory mediators
involved. With the onset of ischemia, intracellular calcium
increases, with the consequent activation of phospholipase.
Arachidonic acid metabolites are then formed, contributing to the
pathophysiological process. A number of studies have found that
IL-1 and IL-6 are also inflammatory mediators of ischemic damage
(9-11).
COX-2 is a key inflammatory mediator in cerebral ischemia and
neurodegenerative disorders too (12,13).
Recent evidence indicates that the suppression of COX-2 attenuates
ischemic injury following MCAO (14). In the present study, significant
decreases in the density ratio of COX-2 and IL-6 to the β-actin
band were detected in rats subjected to Rt.MCAO receiving 20 mg/kg
BW 6-gingerol for 7 days. This is in accordance with the findings
of previous studies demonstrating that the inhibition of
inflammation can decrease the brain infarct volume in experimental
stroke (47) and 6-gingerol can
alleviate inflammatory damage by inhibiting the production of
pro-inflammatory cytokines (48-51).
Consequently, 6-gingerol can attenuate focal cerebral ischemic
stroke-induced neuronal injury by suppressing oxidative stress and
inflammatory mediators.
In animal studies, it has been demonstrated that the
mitochondria are the main source of free radical generation
following focal cerebral ischemia. It is possible that mutations in
nuclear genes following cerebral ischemia alter the apoptotic
process. Gene mutations in mitochondria may be an initiating event
that leads to apoptotic neuronal death. Therefore, drugs or other
interventions that enhance repair efficiency or reduce apoptosis in
the brain offer the possibility of improved outcomes following
ischemic stroke. An imbalance between mitochondrial fusion and
fission can also enhance free radical generation and has been
detected in stroke and other diseases. Mitogen-activated protein
kinase (MAPK) is associated with mitochondrial dynamics.
Bioinformatics research has demonstrated that some miRNAs are
involved in the modulation of MAPK, which is crucial for the
alleviation of inflammation and apoptosis in ischemic stroke
(52-54).
Furthermore, research using rat models of MCAO has demonstrated
that reductions in mitofusin (Mfn)1 and Mfn2 induce Ca2+
overload mitochondrial Bax translocation (55) and promote neuronal apoptosis.
Additionally, Mfn2 has been reported to decrease caspase-3 and
increase the Bcl-2/Bax ratio, these two factors reducing cellular
susceptibility to apoptosis after cerebral ischemic stroke
(54). Further studies are t
required in order better understand the effects of 6-gingerol on
these pathways and its potential as a supportive treatment for
patients with ischemic stroke.
The present study has a few limitations. Firstly, a
weakness of the MCAO model is that the filament may not always be
inserted far enough to completely occlude the middle cerebral
artery. To address this issue, some researchers use laser doppler
flowmetry (LDF) to measure the reduction in CBF and confirm
successful occlusion. The present study did not use LDF or micro
computed tomography (micro-CT), although the authors did always
check that the filament was of optimal size and that the point of
occlusion was associated with the infarct size after the rats were
euthanized. A second limitation is that the present study did not
measure LPO or SOD activity in the blood or other tissues. LPO and
SOD activity were measured in just the cerebral cortex and
hippocampus mitochondria. Lastly, the small number of replicates
used in the biochemical assays represents another study limitation.
Three or more replicates would have been preferable to two;
however, pipetting was performed by an experienced laboratorian and
the standard curves had high R2 values.
In conclusion, 6-gingerol (10 and 20 mg/kg BW for 7
days) exerted antioxidant and anti-inflammatory effects, which
effectively reduced brain damage in a rat model of focal cerebral
ischemic stroke. However, further investigations are warranted to
determine whether any additional mechanism(s) contribute to the
ameliorative effects detected in the present study.
Acknowledgements
The authors would like to thank Dr Tim Cushnie
(Faculty of Medicine, Mahasarakham University, Mahasarakham,
Thailand) for language-editing assistance. As Mahasarakham
University does not have any animal housing facility, the animal
experiments were performed at the Northeastern Laboratory Animal
Center, Khon Kaen University, Khon Kaen, Thailand, which is close
to Mahasarakham University.
Funding
Funding: The present study received funding from the
Mahasarakham University Faculty of Medicine (Med MSU 2/65).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RK was involved in the study methodology, and in the
writing, reviewing and editing of the manuscript. JJ was involved
in the conception and design of the study, in funding acquisition,
data curation, in the study methodology, as well as in the writing
of the original draft and in the writing, reviewing and editing of
the manuscript and in project administration. Both authors have
read and approved the final manuscript and confirmed the
authenticity of all the raw data.
Ethics approval and consent to
participate
All animal-related protocols were designed to
minimize animal suffering, and were performed according to the
approval of the Institutional Animal Care and Use Committee at Khon
Kaen University, Khon Kaen, Thailand (Record No.
IACUC-KKU-6/65).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gebreyohannes EA, Bhagavathula AS, Abebe
TB, Seid MA and Haile KT: In-Hospital mortality among ischemic
stroke patients in Gondar University Hospital: A retrospective
cohort study. Stroke Res Treat. 2019(7275063)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rochmah TN, Rahmawati IT, Dahlui M,
Budiarto W and Bilqis N: Economic burden of stroke disease: A
systematic review. Int J Environ Res Public Health.
18(7552)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fantini S, Sassaroli A, Tgavalekos KT and
Kornbluth J: Cerebral blood flow and autoregulation: current
measurement techniques and prospects for noninvasive optical
methods. Neurophotonics. 3(031411)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Jaffer H, Morris VB, Stewart D and
Labhasetwar V: Advances in stroke therapy. Drug Deliv Transl Res.
1:409–419. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Davis SM and Pennypacker KR: Targeting
antioxidant enzyme expression as a therapeutic strategy for
ischemic stroke. Neurochem Int. 107:23–32. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jittiwat J, Suksamrarn A, Tocharus C and
Tocharus J: Dihydrocapsaicin effectively mitigates cerebral
ischemia-induced pathological changes in vivo, partly via
antioxidant and anti-apoptotic pathways. Life Sci.
283(119842)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jittiwat J, Chonpathompikunlert P and
Sukketsiri W: Neuroprotective effects of Apium graveolens
against focal cerebral ischemia occur partly via antioxidant,
anti-inflammatory, and anti-apoptotic pathways. J Sci Food Agric.
101:2256–2263. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wu L, Xiong X, Wu X, Ye Y, Jian Z, Zhi Z
and Gu L: Targeting oxidative stress and inflammation to prevent
ischemia-reperfusion injury. Front Mol Neurosci.
13(28)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vidale S, Consoli A, Arnaboldi M and
Consoli D: Postischemic Inflammation in Acute Stroke. J Clin
Neurol. 13:1–9. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kawabori M and Yenari MA: Inflammatory
responses in brain ischemia. Curr Med Chem. 22:1258–1277.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: Role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fathali N, Ostrowski RP, Lekic T, Jadhav
V, Tong W, Tang J and Zhang JH: Cyclooxygenase-2 inhibition
provides lasting protection against neonatal hypoxic-ischemic brain
injury. Crit Care Med. 38:572–578. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Minghetti L: Cyclooxygenase-2 (COX-2) in
inflammatory and degenerative brain diseases. J Neuropathol Exp
Neurol. 63:901–910. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yan W, Ren D, Feng X, Huang J, Wang D, Li
T and Zhang D: Neuroprotective and Anti-Inflammatory Effect of
pterostilbene against cerebral ischemia/reperfusion injury via
suppression of COX-2. Front Pharmacol. 12(70329)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Alsahli MA, Almatroodi SA, Almatroudi A,
Khan AA, Anwar S, Almutary AG, Alrumaihi F and Rahmani AH:
6-Gingerol, a major ingredient of ginger attenuates
Diethylnitrosamine-Induced liver injury in rats through the
modulation of oxidative stress and anti-inflammatory activity.
Mediators Inflamm. 2021(6661937)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Almatroodi SA, Alnuqaydan AM, Babiker AY,
Almogbel MA, Khan AA and Husain Rahmani A: 6-Gingerol, a bioactive
compound of ginger attenuates renal damage in
Streptozotocin-Induced diabetic rats by regulating the oxidative
stress and inflammation. Pharmaceutics. 13(317)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Majdi Yazdi G, Vaezi G, Hojati V and
Mohammad-Zadeh M: The Effect of 6-gingerol on Growth factors and
apoptosis indices in rats exposed to gold nanoparticles. Basic Clin
Neurosci. 12:301–308. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang S, Zhang C, Yang G and Yang Y:
Biological properties of 6-gingerol: A brief review. Nat Prod
Commun. 9:1027–1030. 2014.PubMed/NCBI
|
|
19
|
Luo J, Chen J, Yang C, Tan J, Zhao J,
Jiang N and Zhao Y: 6-Gingerol protects against cerebral
ischemia/reperfusion injury by inhibiting NLRP3 inflammasome and
apoptosis via TRPV1/FAF1 complex dissociation-mediated autophagy.
Int Immunopharmacol. 100(108146)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Adetuyi BO and Farombi EO: 6-Gingerol, an
active constituent of ginger, attenuates lipopolysaccharide-induced
oxidation, inflammation, cognitive deficits, neuroplasticity, and
amyloidogenesis in rat. J Food Biochem. 45(e13660)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Suekawa M, Ishige A, Yuasa K, Sudo K,
Aburada M and Hosoya E: Pharmacological studies on ginger. I.
Pharmacological actions of pungent constitutents, (6)-gingerol and
(6)-shogaol. J Pharmacobiodyn. 7:836–848. 1984.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Jittiwat J: Baihui point laser acupuncture
ameliorates cognitive impairment, motor deficit, and neuronal loss
partly via antioxidant and anti-inflammatory effects in an animal
model of focal ischemic stroke. Evid Based Complement Alternat Med.
2019(1204709)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wattanathorn J, Jittiwat J, Tongun T,
Muchimapura S and Ingkaninan K: Zingiber officinale
Mitigates Brain Damage and Improves Memory Impairment in Focal
Cerebral Ischemic Rat. Evid Based Complement Alternat Med.
2011(429505)2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jittiwat J: Laser Acupuncture at GV20
Improves brain damage and oxidative stress in animal model of focal
ischemic stroke. J Acupunct Meridian Stud. 10:324–330.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
26
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rossmeisl JH Jr, Rohleder JJ, Pickett JP,
Duncan R and Herring IP: Presumed and confirmed striatocapsular
brain infarctions in six dogs. Vet Ophthalmol. 10:23–36.
2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wayman C, Duricki DA, Roy LA, Haenzi B,
Tsai SY, Kartje G, Beech JS, Cash D and Moon L: Performing
permanent distal middle cerebral with common carotid artery
occlusion in aged rats to study cortical ischemia with sustained
disability. J Vis Exp. (53106)2016.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Teertam SK and Prakash Babu P:
Differential role of SIRT1/MAPK pathway during cerebral ischemia in
rats and humans. Sci Rep. 11(6339)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wang C, Ma Z, Wang Z, Ming S, Ding Y, Zhou
S and Qian H: Eriodictyol attenuates MCAO-Induced brain injury and
neurological deficits via reversing the autophagy dysfunction.
Front Syst Neurosci. 15(655125)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee B, Choi EJ, Lee EJ, Han SM, Hahm DH,
Lee HJ and Shim I: The neuroprotective effect of methanol extract
of gagamjungjihwan and fructus euodiae on ischemia-induced neuronal
and cognitive impairment in the rat. Evid Based Complement Alternat
Med. 2011(685254)2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shah FA, Li T, Kury LTA, Zeb A, Khatoon S,
Liu G, Yang X, Liu F, Yao H, Khan AU, et al: Pathological
comparisons of the hippocampal changes in the transient and
permanent middle cerebral artery occlusion rat models. Front
Neurol. 10(1178)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chung JY, Yi JW, Kim SM, Lim YJ, Chung JH
and Jo DJ: Changes in gene expression in the rat hippocampus after
focal cerebral ischemia. J Korean Neurosurg Soc. 50:173–178.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Genovese T, Mazzon E, Paterniti I,
Esposito E, Bramanti P and Cuzzocrea S: Modulation of NADPH oxidase
activation in cerebral ischemia/reperfusion injury in rats. Brain
Res. 1372:92–102. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Paliwal P, Dash D and Krishnamurthy S:
Pharmacokinetic study of piracetam in focal cerebral ischemic rats.
Eur J Drug Metab Pharmacokinet. 43:205–213. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Muley MM, Thakare VN, Patil RR, Bafna PA
and Naik SR: Amelioration of cognitive, motor and endogenous
defense functions with silymarin, piracetam and protocatechuic acid
in the cerebral global ischemic rat model. Life Sci. 93:51–57.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
He Z, Liao Y, Zheng M, Zeng FD and Guo LJ:
Piracetam improves cognitive deficits caused by chronic cerebral
hypoperfusion in rats. Cell Mol Neurobiol. 28:613–627.
2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rezazadeh-Shojaee FS, Ramazani E, Kasaian
J and Tayarani-Najaran Z: Protective effects of 6-gingerol on
6-hydroxydopamine-induced apoptosis in PC12 cells through
modulation of SAPK/JNK and survivin activation. J Biochem Mol
Toxicol. 36(e22956)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao M, Yao Y, Du J, Kong L, Zhao T, Wu D,
Man L and Zhou W: 6-Gingerol Alleviates neonatal hypoxic-ischemic
cerebral and white matter injury and contributes to functional
recovery. Front Pharmacol. 12(707772)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Liu Z, Ren Z, Zhang J, Chuang CC,
Kandaswamy E, Zhou T and Zuo L: Role of ROS and Nutritional
antioxidants in human diseases. Front Physiol.
9(477)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gorjão R, Takahashi HK, Pan JA and Massao
Hirabara S: Molecular mechanisms involved in inflammation and
insulin resistance in chronic diseases and possible interventions.
J Biomed Biotechnol. 2012(841983)2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mohd Yusof YA: Gingerol and its role in
chronic diseases. Adv Exp Med Biol. 929:177–207. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
El-Senousey HK, Chen B, Wang JY, Atta AM,
Mohamed FR and Nie QH: Effects of dietary vitamin C, vitamin E, and
alpha-lipoic acid supplementation on the antioxidant defense system
and immune-related gene expression in broilers exposed to oxidative
stress by dexamethasone. Poult Sci. 97:30–38. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Watts LT, Lloyd R, Garling RJ and Duong T:
Stroke neuroprotection: Targeting mitochondria. Brain Sci.
3:540–560. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Farombi EO, Abolaji AO, Adetuyi BO,
Awosanya O and Fabusoro M: Neuroprotective role of 6-Gingerol-rich
fraction of Zingiber officinale (Ginger) against
acrylonitrile-induced neurotoxicity in male Wistar rats. J Basic
Clin Physiol Pharmacol. 30:2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Whiteley W, Jackson C, Lewis S, Lowe G,
Rumley A, Sandercock P, Wardlaw J, Dennis M and Sudlow C:
Inflammatory markers and poor outcome after stroke: A prospective
cohort study and systematic review of interleukin 6. PLoS Med.
6(e1000145)2009.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ju SA, Nguyen QT, Nguyen TT, Suh JH, An
WG, Callaway Z, Joe Y, Chung HT and Kim BS: Pretreatment with
6-Gingerol Ameliorates Sepsis-Induced Immune Dysfunction by
Regulating the Cytokine Balance and Reducing Lymphocyte Apoptosis.
Oxid Med Cell Longev. 2021(5427153)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hwang YH, Kim T, Kim R and Ha H: The
Natural Product 6-Gingerol Inhibits Inflammation-Associated
Osteoclast Differentiation via Reduction of Prostaglandin
E2 Levels. Int J Mol Sci. 19(2068)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tripathi S, Maier KG, Bruch D and Kittur
DS: Effect of 6-gingerol on pro-inflammatory cytokine production
and costimulatory molecule expression in murine peritoneal
macrophages. J Surg Res. 138:209–213. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kim SO, Chun KS, Kundu JK and Surh YJ:
Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression
and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors.
21:27–31. 2004.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gugliandolo A, Silvestro S, Sindona C,
Bramanti P and Mazzon E: MiRNA: Involvement of the MAPK Pathway in
Ischemic Stroke. A Promising Therapeutic Target. Medicina (Kaunas).
57(1053)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Safa A, Abak A, Shoorei H, Taheri M and
Ghafouri-Fard S: MicroRNAs as regulators of ERK/MAPK pathway: A
comprehensive review. Biomed Pharmacother.
132(110853)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yang M, He Y, Deng S, Xiao L, Tian M, Xin
Y, Lu C, Zhao F and Gong Y: Mitochondrial Quality Control: A
pathophysiological mechanism and therapeutic target for stroke.
Front Mol Neurosci. 14(786099)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Vongsfak J, Pratchayasakul W, Apaijai N,
Vaniyapong T, Chattipakorn N and Chattipakorn SC: The alterations
in mitochondrial dynamics following cerebral ischemia/reperfusion
injury. Antioxidants (Basel). 10(1384)2021.PubMed/NCBI View Article : Google Scholar
|