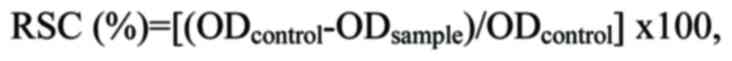Introduction
Research on the consumption of functional foods over
the last decades has established their importance for the promotion
of optimal human health. Importantly, dietary antioxidants hold a
significant role in the prevention of reactive oxygen species
formation or even in scavenging free radicals through radical chain
reaction interruption, molecules that are responsible for
triggering numerous pathological conditions and diseases (1). Previous studies have elaborated on the
chemo-preventive properties of natural food products (2,3), a
large number of which have examined the nutritional importance of
honey (4-6).
Honey is a natural product derived from the insect Apis
mellifera, which holds a significant role in the field of
agriculture (7). These insects
serve as plant and crop species pollinators to enhance the
biodiversity of agricultural and also non-agricultural landscapes
(1). The use of honey has been
recorded from ancient civilizations, not only as a food but also as
a medicine for relieving gastrointestinal disorders (8) and wound healing by reducing the
oedema, inflammation, and exudation that frequently develop in
numerous types of wounds (8,9).
Nectar selection from different plant species,
represents the main factor determining the different types of
honey, resulting in a wide variation in its colour, taste, texture
and, composition (10-12).
Honey has a complex composition, containing ~200 compounds such as
sugars, proteins, vitamins, water, free amino acids, enzymes,
minerals, and numerous phytochemicals (13). Due to its composition, enriched with
many bioactive ingredients, several studies have established its
antimicrobial (14), antiviral
(15), anticancer (16), antidiabetic (17) and antioxidant (18) properties. This multilayer activity
of honey has been suggested for the protection against pathologies
interrelated with the cardiovascular (19), nervous (20), respiratory, and gastrointestinal
system (21).
Oxidative stress has been revealed as the main
contributing factor in several pathologies, initiating structure
modifications and function modulations in nucleic acids, lipids,
and proteins (22). Specifically,
it has been associated with several pathological conditions such as
neurodegenerative disorders (Alzheimer, Parkinson, etc.) (23), cancer (16), diabetes obesity, and cardiovascular
diseases (24,25). Previous animal studies have
demonstrated the relationship between honey and oxidative
stress-induced conditions. Specifically, Abdulmajeed et al
concluded that honey consumption was able to increase glutathione
(GSH) and glutathione S-transferase (GST) levels in brain tissue of
rats after they were exposed to lead for 28 days (26). Moreover, honey appears to have the
ability to enhance the activity of major antioxidant enzymes such
as superoxide dismutase (SOD), glutathione-disulfide reductase
(GR), catalase (CAT) and glutathione peroxidase (GPx) in the liver
of rats after they consumed acetaminophen for 10 days (27). Despite the strong evidence
suggesting the chemo-preventive role of honey, it is still under
debate which specific ingredients of honey and in which
composition, are liable for its activity (28). It is common knowledge that honey
composition is mainly affected by botanical origin, however,
geographical factors can also influence its synthesis and
consequently its quality (29).
Constituents such as glucose oxidase, catalase, organic acids,
amino acids, proteins, phenolic acids, and flavonoids have been
suggested to play a significant role in the antioxidant capacity of
honey (30). Apart from the
components aforementioned, polyphenols contained in honey have been
proposed to affect its antioxidant capacity (31). Even though the antioxidant capacity
of honey is well documented, the exact antioxidant mechanism is
still unknown (32). Some of the
most studied interrelated mechanisms include, but are not limited
to, hydrogen donation, metallic ion chelation and scavenging of
free radicals by increasing the endogenous levels of important
antioxidant molecules and enzymes such as β-carotene vitamin C,
glutathione reductase and uric acid (32). In vitro studies regarding
honey are predominant in the literature (33-37),
but in vivo studies, especially concerning the metabolic
changes caused in humans are scarce. Schramm et al,
recruited 40 healthy human volunteers to test the impact of honey
consumption on the antioxidant and reducing capacity in plasma. The
research indicated that consumption of 1.5 g honey/kg of body
weight had a positive effect on their antioxidant and reducing
capacity, supporting the concept that honey consumption may have a
positive impact on the antioxidant defense system of healthy human
subjects (38). Moreover, another
human clinical trial examined the effects of a honey, rich in
probiotics, on 60 patients suffering from diabetic nephropathy
(39). In this study, patients
consumed 25 g of honey rich in probiotics per day for 12 weeks and
malondialdehyde (MDA) levels were found to be significantly lower
following the consumption of the honey rich in probiotics (39). The effects of specific types of
honey on physiological parameters have shown that consumption of 20
g/day of Tualang honey, for 12 months resulted in significant
decreases in diastolic blood pressure (BP) and fasting blood
glucose in postmenopausal women (40).
To this end, honey has been proposed as the ‘gold
treasure’ with health-promoting effects against oxidative
stress-related dysfunctions. However, the effects of consumption of
a novel natural honey-based gel on redox biomarkers, blood
chemistry and physiological characteristics have not been
investigated in healthy individuals. Therefore, the present pilot
study aimed: i) To estimate the antioxidant potential of a
honey-based gel and ii) to monitor the physiological redox and
adjustments obtained after its consumption in healthy participants,
and iii) to assess whether the responses of the assessed variables
would be different between men and women.
Materials and methods
Study design
A total number of 20 participants took part in the
present study, and were separated into two equal subgroups of 10
individuals, as a preliminary power analysis (a probability error
of 0.05, and a statistical power of 80%) showed that this sample
size per group was the appropriate, in order to detect
statistically meaningful changes between groups. The division in
two groups was performed according to sex, therefore, 10 men and 10
women with a mean age of 39.0±11.5 years (range, 22-52 years) were
recruited for this clinical study. Τhe criteria defined for the
participants included: i) Absence of musculoskeletal injury and
cardiovascular/metabolic disease; ii) not consuming nutritional
supplements and medication prior to (≥6 months) and during the
study; and iii) non-smoking. Following recruitment, volunteers
completed a health history questionnaire and provided their written
consent for participation after they were informed about the
purpose, methodology and possible risks associated with the study.
Participants received 70 g of a honey-based gel, commercially
available under the trade name of ‘Bear Strength honey gel’ (Nomad
Premium Greek Honey), per day for 14 consecutive days. The
honey-based gel is a natural product containing 87% fir honey, and
pollen. Prior to its consumption and 14 days post-consumption,
participants provided a blood sample and underwent an assessment of
their physiological profile. Along with the honey-based gel
consumption, no other restrictions or limitations were set
regarding food intake. Dietary intake was recorded for 3 days prior
to first blood sample collection and participants were instructed
to follow the same diet prior to second blood collection.
Furthermore, participants were also instructed to abstain from any
strenuous physical activity, prior to pre- and post-blood sampling
and physiological profile assessment, to avoid possible
exercise-related alterations of their redox profile (41). The first blood samples collected
from the participants prior to the honey-based gel consumption was
defined as the control group. The Institutional Review Board of the
Department of Physical Education and Sport Science of the
University of Thessaly (protocol ID: 1722/9-12-2020) approved the
methods, procedures, and ethics of this study. Procedures agreed
with the 1975 Declaration of Helsinki as revised in 2013.
Total phenolic content (TPC) of the
honey-based gel
The TPC value of the honey-based gel sample was
estimated using Folin-Ciocalteu (FC) reagent. In brief, 20 µl of
the sample, 1 ml of dH2O and 100 µl of FC reagent were
agitated and incubated for 3 min, in the dark at room temperature
(RT). Next, 25%w/v Na2CO3 (280 µl) and
dH2O (600 µl) were vortexed and incubated for 60 min,
RT, in the dark. Finally, the absorbance was measured at 765 nm
(Hitachi, U 1900 UV/VIS; Hitachi High Technologies Corporation).
The absorbance value of a blank was deducted from the absorbance
value of the tested sample, where the blank contained the FC
reagent and dH2O. Increasing concentrations of gallic
acid (0, 50, 150, 250 and 500 µg/ml) were used to create a standard
curve for TPC value estimation, which was expressed as mg of gallic
acid equivalents (GAEs) per g of honey (mg GAE/g honey) (42).
ABTS•+ radical scavenging
(RSC) assay
The ABTS•+ scavenging capacity of the
honey-based gel was determined according to Miller et al
(43) with some modifications
(44). Briefly, 400 µl of
dH2O, 1 mM of ABTS•+ solution (500 µl), 30 µM
hydrogen peroxide (H2O2) (50 µl) and 6 µM
horseradish peroxidase (HRP) (50 µl) were mixed and then incubated
for 45 min in the absence of light at RT. Subsequently, 50 µl of
each dilution (25-0.78 mg/ml) were added and the absorbance was
measured at 730 nm using a spectrophotometer (Hitachi, U 1900
UV/VIS). As a blank, an ABTS•+ radical solution without
the addition of the enzyme (HRP) was used, while the mixture in the
absence of the tested sample was used as a control. The results
were interpreted as follows:
where ODcontrol and ODsample
are the absorbance values of the control and the sample,
respectively. The capacity of the honey-based gel to scavenge the
free radicals was estimated through a half maximal inhibitory
concentration value (IC50), which was determined from
the graph-plotted percentage against the sample concentration.
Physiological characteristics
A stadiometer (Beam Balance Stadiometer 208; Seca
United Kingdom) was used in order to measure the body mass and the
height (to the nearest 0.05 kg and 0.1 cm) of the participants.
During measurements, participants were barefoot and lightly dressed
(45). Waist and hip circumferences
were obtained with a measuring tape. Waist-to-hip ratio (WHR) was
calculated by the use of the equation: WHR=waist circumference/hip
circumference. Body fat percentage was measured by dual-energy
X-ray absorptiometry (Lunar DPX NT; GE Healthcare) as previously
described (46). Resting heart rate
(RHR) was measured using a heart rate monitor (Polar Electro) with
participants in the supine position and following a 5-min rest as
previously described (47).
Systolic and diastolic BP were assessed by a physician using an arm
sphygmomanometer (Precisa N R-1362; Rudolf Riester GmbH) according
to a standardized procedure established by the American Heart
Association (48). Briefly,
participants had to be seated and rested for 5 min prior to the
exam. Systolic and diastolic BP was measured according to the first
and fifth Korotkoff sounds and each reading was performed in
duplicate (with a 1-min break between readings) (49). Participants were asked whether they
had any difficulty in consuming the product or there were any side
effects from the honey-based gel intake.
Blood collection
In EDTA tubes, 10 ml of blood were collected, and a
small portion was analyzed for the complete blood count (CBC)
parameters using an automatic hematology analyzer (Mythic 18
Orphee; Orphee-Medical; Cormay Diagnostics). The remaining blood
was centrifuged at 1,370 x g for 10 min at 4˚C and the upper layer
(plasma) was isolated. The total amount of erythrocytes was diluted
in water (1% v/v), followed by centrifugation at 4,000 x g for 15
min at 4˚C and the supernatant was collected. Both red blood cell
lysate (RBCL) and plasma samples were stored at -20˚C until use
(50).
Determination of hemoglobin
concentration
A commercial kit (Hemoglobin kit; article no. 60230;
Dutch Diagnostics BV) was used in order to assess the hemoglobin
levels, according to manufacturer's instructions. Briefly, 5 µl of
RBCL were mixed with 1 ml of hemoglobin reagent (Reagent R1, pH
7.3). After a 10-min incubation in the absence of light and at RT,
the optical density was measured at 540 nm (Hitachi, UV-1900). As a
blank, 1 ml of the reagent (R1) was used.
Determination of reduced GSH
levels
A total of 400 µl of RBCL was precipitated with 400
µl 5% (w/v) TCA, followed by centrifugation at 15,000 x g for 5 min
at 4˚C. This step was repeated and 90 µl of 5% (w/v) TCA were added
to 300 µl of the supernatant, followed by centrifugation as
aforementioned. GSH levels were quantified according to a study by
Reddy et al (51), with some
alterations (52). Briefly, 20 µl
of clarified RBCL was dissolved in 67 mmol/l (660 µl) potassium
sodium phosphate (pH 8.0) and 1 mmol/l (330 µl)
5,5'-dithiobis-2-nitrobenzoic acid followed by incubation at RT for
10 min, in the absence of light. The optical density was measured
at 412 nm (Hitachi, UV-1900) in order to interpret the results with
a calibration curve using the millimolar extinction coefficient of
2-nitro-5-thiobenzoate (13.6 l/mmol/cm) as previously reported
(52).
Catalase activity
Catalase activity was estimated according to a
method described in a study by Aebi (53), which was slightly revised (54). A 1:10 dilution of RBCL (4 µl) was
added to 2,996 ml of 67 mmol/l sodium potassium phosphate (pH 7.4)
and incubated for 10 min at 37˚C. Subsequently, 5 µl of 30%
H2O2 were added in the cuvette that was
placed in the spectrophotometer (Hitachi, UV-1900) and the optical
density was measured twice, at time point 0 and 120 sec, at 240 nm.
The calculation of catalase activity was based on the molar
extinction coefficient of H2O2 (43.6
M-1 cm-1) as previously reported (54).
Determination of total antioxidant
capacity (TAC)
TAC was performed as described in a previous study
(55). To 10 mmol/l sodium
potassium phosphate pH 7.4 (480 µl) and 0.1 mmol
2,2-diphenyl-1-picrylhydrazyl (DPPH)• (500 µl), 20 µl of
plasma were added and incubated at RT for 1 h, in the absence of
light. At the end of the incubation period, centrifugation was
performed at RT for 3 min at 15,000 x g, and the optical density
was measured at 520 nm (Hitachi, UV-1900). The results were
estimated by the reduction of DPPH to
2,2-diphenyl-1-picrylhydrazine (DPPH:H) caused by the plasma
antioxidants, and were presented as mmol DPPH/l plasma.
Thiobarbituric acid reactive
substances (TBARS)
TBARS levels were identified according to a
previously described protocol (56)
slightly modified (52).
Particularly, in a reaction tube containing 100 µl of plasma, 500
µl of 35% (w/v) TCA and 200 mM Tris-HCl buffer, pH 7.4 (500 µl)
were added and incubated for 10 min at RT. Subsequently, 1 ml of a
2-M Na2SO4-55 mM TBA solution was added,
followed by incubation in a 95˚C water bath for 45 min, and an
additional incubation in ice for 5 min. Finally, 1 ml of 70% (w/v)
TCA was combined with the samples, vortexed and 1 ml of each
testing sample was transferred to a new centrifuge tube. A
centrifugation at 11,200 x g, for 3 min at RT was performed and the
optical density was determined at 530 nm (Hitachi, UV-1900). The
results were based on the molar extinction coefficient of
malondialdehyde (156x103 M-1 cm-1)
as previously reported (56).
Protein carbonyls (PCARBS)
The determination of protein carbonyls was performed
according to a study by Skaperda et al (54). In centrifuge tubes, 50 µl of plasma
and 50 µl of 20% (w/v) TCA were added, incubated for 15 min at RT
and centrifugated at 15,000 x g for 5 min at 4˚C. Subsequently, the
supernatant was removed, and the pellet was resuspended in 10 mM
2,4-dinitrophenylhydrazine (DNPH) (500 µl; dissolved in 2.5 N HCl).
A blank was used for each sample, in which the pellet was
resuspended in 2.5 N HCl (500 µl) instead of DNPH. Samples and
their respective blanks were both incubated at RT for 60 min in the
absence of light, stirring every 15 min. After 1 h, the samples and
blanks were centrifuged (15,000 x g, for 5 min, at 4˚C), the
supernatant was removed, the pellets were resuspended in 1 ml of
10% (w/v) TCA and centrifuged as aforementioned. Subsequently, the
pellets were washed in 50% v/v ethanol-ethyl acetate solution (1
ml) and centrifuged at 15,000 x g, for 5 min, at 4˚C. The last step
was repeated twice. Next, the pellets were resuspended in 5 M, urea
pH 2.3 (1 ml) followed by a 15-min incubation at 37˚C. Following
centrifugation of the samples (15,000 x g, for 5 min, at 4˚C), the
optical density was monitored at 375 nm (Hitachi, UV-1900). The
calculation of the protein carbonyl levels was based on the molar
extinction coefficient of DNPH (22x103 M-1
cm-1) as previously reported (54).
Chemicals
Chemicals used for all the aforementioned assays
were supplied by Sigma-Aldrich; Merck KGaA.
Statistical analysis
Data normality was verified using the Shapiro-Wilk
test. A 2x2 repeated measures ANOVA [group (men and women) by time
(pre and post)] was used to identify possible sex-specific changes.
If a significant interaction was detected, pairwise comparisons
were performed through simple contrasts and simple main effects
analysis using the LSD test method. Pre- and post-measurements
within conditions were compared using paired t-tests. The
statistical analysis of the results was performed using GraphPad
Prism version 8.0.1 (for Windows; GraphPad Software, Inc.) and
expressed as the mean ± standard error of the mean (SEM). Each
assay was performed in triplicate. P<0.05 was considered to
indicate a statistically significant difference.
Results
Participants did not report any side effects from
the consumption of the honey-based gel.
The TPC of the honey-based gel was 129 mg GAE/100 g
and, the IC50 value of the ABTS•+ RSC assay
was 0.88 mg/ml (data not shown).
Physiological and CBC data are presented in Tables I and II, respectively. The consumption of the
honey-based gel resulted in significant decreases in diastolic and
mean arterial BP when the data was combined (Table I). Comparison of the responses
between the two sexes revealed changes after the consumption of the
honey-based gel, resulting in significant decreases in women but
not in men (Table I). There were no
significant changes due to honey gel consumption in the other
physiological and CBC measures (Table
II).
 | Table IAssessment of physiological
characteristics following consumption of a honey-based gel. |
Table I
Assessment of physiological
characteristics following consumption of a honey-based gel.
| | Total | Women | Men |
|---|
| Group
Parameter | Pre | Post | Pre | Post | Pre | Post |
|---|
| Body Weight
(kg) | 76.82±4.02 | 77.1±4.15 | 61.65±2.15 | 61.5±2.14 | 91.99±3.46 | 92.7±3.49 |
| Height (m) | 1.73±0.02 | 1.73±0.02 | 1.66±0.02 | 1.66±0.02 | 1.80±0.02 | 1.80±0.02 |
| BMI
(kg/m2) | 25.34±0.98 | 25.42±1.00 | 22.39±0.88 | 22.33±0.87 | 28.29±1.07 | 28.51±1.07 |
| Body fat (%) | 27.28±1.24 | 27.65±1.22 | 28.95±1.61 | 29.1±1.69 | 25.61±1.64 | 26.21±1.55 |
| Waist Circumference
(cm) | 84.82±3.02 | 84.60±3.14 | 73.50±1.67 | 72.90±1.79 | 96.15±2.59 | 96.30±2.64 |
| Hip Circumference
(cm) | 104.20±1.72 | 104.30±1.74 | 99.20±1.50 | 99.20±1.56 | 109.30±1.99 | 109.50±1.96 |
| WHR (ratio) | 0.81±0.02 | 0.81±0.02 | 0.74±0.01 | 0.73±0.01 | 0.88±0.01 | 0.88±0.01 |
| Systolic BP (mm
Hg) | 115.70±2.25 | 111.20±2.52 | 112.70±4.03 | 105.00±3.24 | 118.80±1.11 | 117.40±2.47 |
| Diastolic BP (mm
Hg) | 79.10±2.05 |
74.75±2.52a | 75.70±2.87 |
68.70±2.92a | 82.50±2.34 | 80.80±2.88 |
| Resting HR
(b/min) | 63.85±1.49 | 62.20±1.7 | 64.70±1.69 | 64.70±1.92 | 63.00±2.33 | 59.70±2.48 |
| Mean arterial BP
(mm Hg) | 91.30±2.12 |
86.88±2.52a | 88.02±3 |
80.79±2.91a | 94.58±2.83 | 92.99±2.44 |
 | Table IIComplete blood count responses
following consumption of a honey-based gel. |
Table II
Complete blood count responses
following consumption of a honey-based gel.
| | Total | Women | Men |
|---|
| Group
Parameter | Pre | Post | Pre | Post | Pre | Post |
|---|
| WBC
(103/µl) | 5.61±0.32 | 5.67±0.34 | 5.49±0.31 | 5.54±0.34 | 5.74±0.32 | 5.81±0.32 |
| LYM (%) | 36.19±1.65 | 35.96±1.55 | 37.42±1.79 | 36.7±1.96 | 34.96±1.29 | 35.23±1.73 |
| MON (%) | 4.56±0.49 | 4.05±0.46 | 4.71±0.58 | 3.38±0.30 | 4.42±0.34 | 4.73±0.48 |
| GRA (%) | 59.24±1.92 | 59.98±1.69 | 57.87±2.17 | 59.92±1.22 | 60.62±1.39 | 60.04±1.98 |
| RBC
(106/µl) | 4.66±0.18 | 4.65±0.18 | 4.38±0.08 | 4.38±0.09 | 4.94±0.2 | 4.92±0.19 |
| HGB (g/dl) | 14.17±0.36 | 14.14±0.36 | 13.54±0.32 | 13.53±0.36 | 14.81±0.27 | 14.76±0.22 |
| HCT (%) | 40.82±0.89 | 40.44±0.89 | 39.46±0.86 | 39.03±0.95 | 42.19±0.63 | 41.85±0.46 |
| MCV
(µm3) | 88.28±2.39 | 87.64±2.39 | 90.09±1.34 | 89.14±1.53 | 86.47±2.89 | 86.14±2.84 |
| MCH (pg) | 30.68±0.97 | 30.65±0.92 | 30.96±0.69 | 30.91±0.61 | 30.41±1.14 | 30.40±1.10 |
| MCHC (g/dl) | 34.71±0.39 | 34.96±0.27 | 34.33±0.41 | 34.66±0.17 | 35.09±0.28 | 35.26±0.30 |
| RDW (%) | 13.46±0.44 | 13.60±0.37 | 13.17±0.29 | 13.35±0.30 | 13.76±0.52 | 13.85±0.39 |
| PLT
(103/µl) | 268.70±17.00 | 272.08±19.65 | 276.20±14.11 | 263.30±17.22 | 261.30±18.4 | 282.30±20.45 |
| MPV
(µm3) | 7.92±0.21 | 8.12±0.21 | 7.82±0.14 | 8.19±0.27 | 8.02±0.25 | 8.05±0.24 |
| PCT (%) | 0.21±0.01 | 0.22±0.01 | 0.21±0.01 | 0.21±0.01 | 0.21±0.01 | 0.22±0.01 |
| PDW (%) | 14.89±0.47 | 15.39±0.59 | 14.4±0.36 | 15.36±0.48 | 15.39±0.49 | 15.43±0.65 |
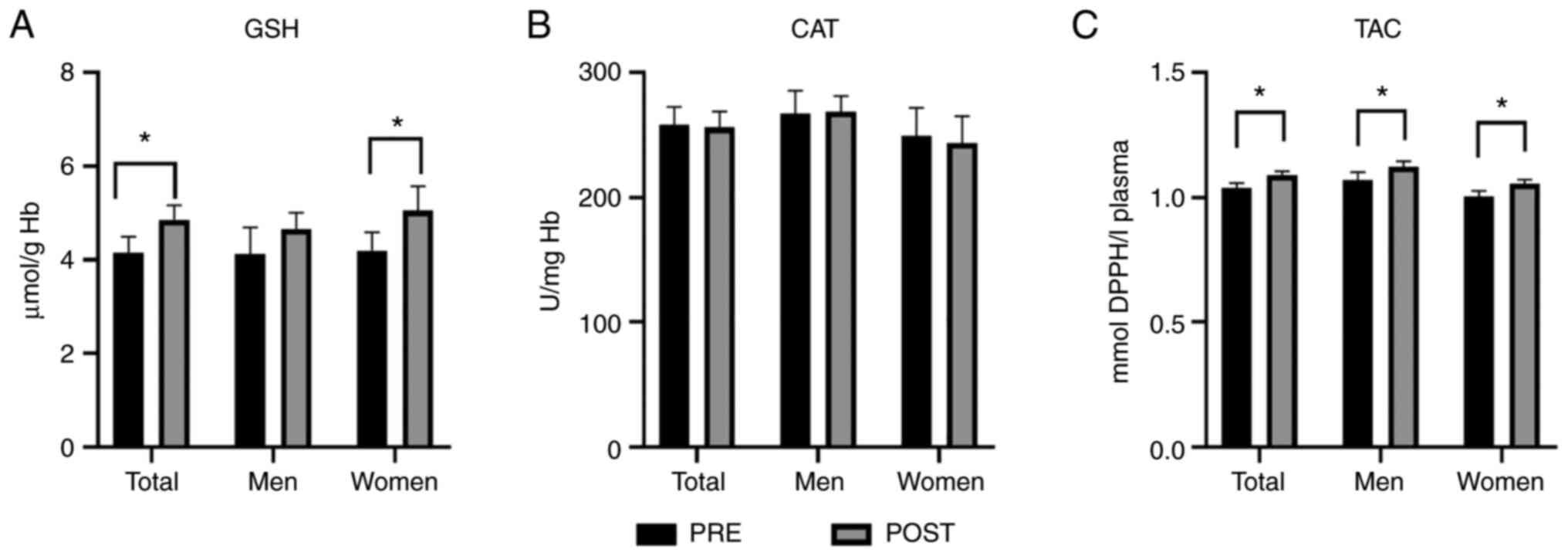
The consumption of the honey-based gel resulted in
significant increases in GSH and TAC when the data was combined
(Fig. 1A and C, respectively). Comparison of the
responses between the two sexes revealed changes after the
consumption of the honey-based gel in GSH levels, resulting in
significant increases in women but not in men (Fig. 1A). There were no significant changes
due to honey gel consumption in CAT (Fig. 1B).
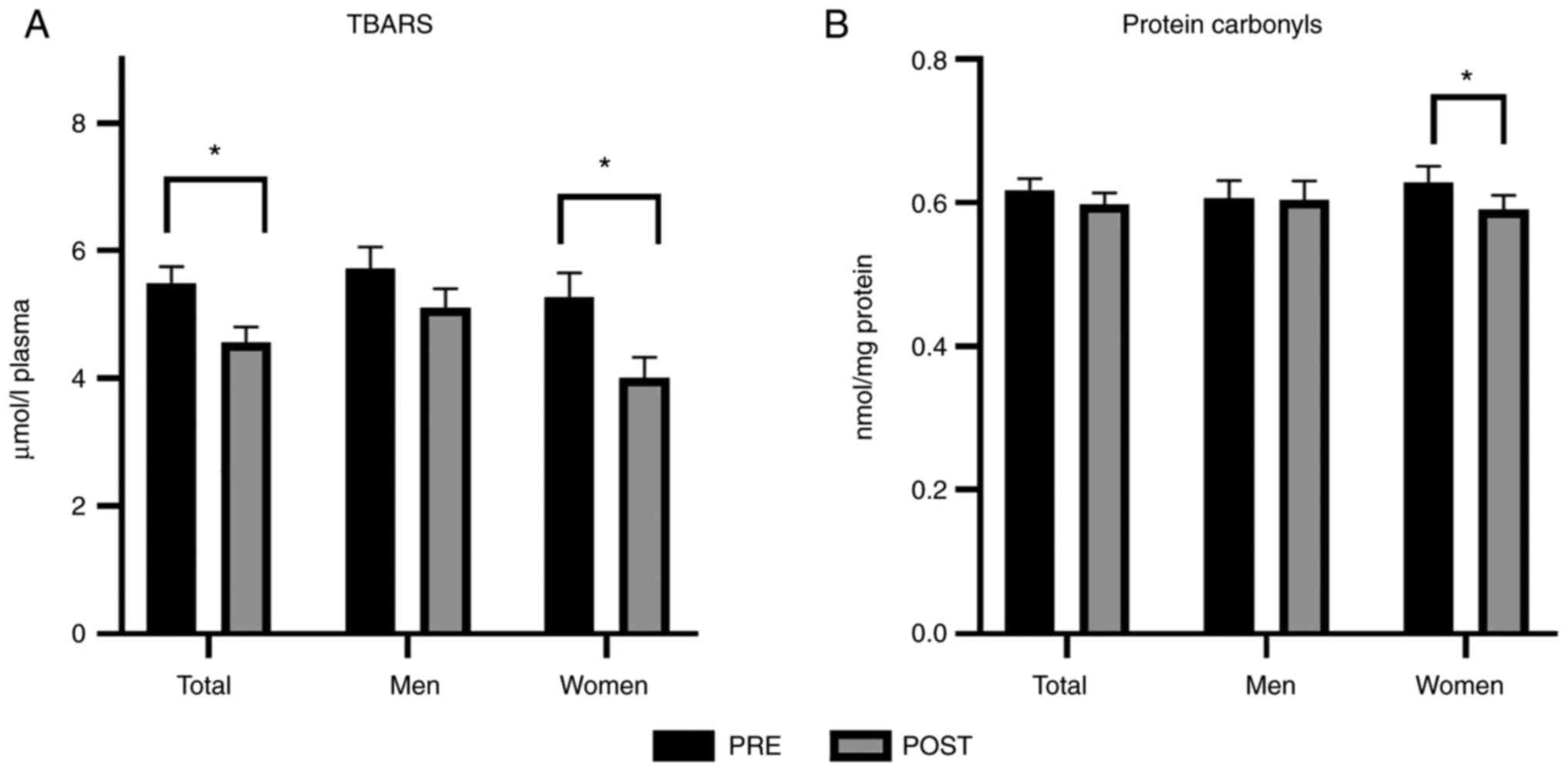
The consumption of the honey-based gel resulted in
significant decreases in TBARS when the data was combined (Fig. 2A). Comparison of the responses
between the two sexes revealed changes after the consumption of the
honey-based gel, resulting in a significant decrease in women but
not in men (Fig. 2A). Furthermore,
comparison of the responses between the two sexes with regard to
PCARBS revealed changes after the consumption of the honey gel,
resulting in a significant decrease in PCARBS in women but not in
men (Fig. 2B).
Discussion
To the best of our knowledge, the present study is
the first to determine the sex-specific metabolic changes after the
consumption of a honey-based gel in healthy humans via the
evaluation of various redox biomarkers and physiological
parameters. The results revealed a significant improvement of the
antioxidant defense system of the participants, as indicated by the
increase in TAC and GSH levels and the decrease of TBARS and PCARBS
levels in combination with a decrease of the diastolic and mean BP
of the participants, without affecting their hematological
profile.
Honey was considered from antiquity as a natural
food product with high nutritional importance, used in numerous
medical treatments worldwide due to its healing, anti-inflammatory,
and antibacterial properties (57).
Additionally, evidence in the literature has also widely debated
the chemo-preventive, immunoregulatory, antioxidant and
antiatherogenic properties of honey (58-61).
Nevertheless, information regarding the properties of Greek honey
in literature and notably from in vivo studies are scarce
even though Greece is part of the eastern Mediterranean, creating a
rich and diversified flora habitat (62,63).
This rich flora biodiversity combined with the overflowing
sunshine, creates an ideal environment resulting in a variety of
honey types originating from pine, thyme, fir and other conifers
(64,65).
According to our in vitro results with regard
to the honey-based gel, a high antioxidant capacity was observed
(TPC, 129 mg GAE/100 g honey; and ABTS assay, IC50=0.88
mg/ml), compared with other published studies, including raw honey
samples. For example, a previous study conducted at the laboratory
of the authors investigated the antioxidant and antibacterial
capacity of 21 honey types produced on the highest mountain of the
country, Mount Olympus, compared with Manuka honey (66). Regarding the results, the
honey-based gel used in the present study, exhibited the lowest
IC50 value, determined using an ABTS assay, compared
with both the 21 different Greek honey types and Manuka (66). Bazaid et al investigated the
scavenging activity of Manuka honey using ABTS RSC assay among
other assays (67). Concerning the
results of the present study, the honey-based gel exhibited a lower
IC50 value (0.88 mg/ml) than the assessed Manuka honey
(4.49 mg/ml) (67). The present
study is part of a larger project investigating Greek honey
bioactivity. Specifically, the antioxidant capacity of various
types of honey is being investigated both in vitro and in
vivo, as well as their effect on the redox state and
physiological profile of the human body. In the present
investigation, in vitro measurements concerning the
honey-based gel were performed in order to establish its
polyphenolic content as well as its ability to inhibit the ABTS
radical formation. Interestingly, the results of the present study
differ from previous data on the bioactivity of honey. For
instance, Chau et al, after examining various types of
honey, such as forest honey, indicated a TPC value ranging from
19.7 to 85 mg GAE/100 g honey (68), which is lower compared to the
honey-based gel (129 mg GAE/100 g honey) used in the present study.
Concerning a study that assessed 105 different types of raw honey,
produced by 3 countries, the honey-based gel used in the present
study exhibited higher polyphenolic content from all honey samples
except for the buckwheat honey (69). It is plausible that the high level
of TPC, determined in the product used in the present study, may be
due to substances such as caffeic acid, quercetin and kaempferol
which are also found in honey and contribute to its antioxidant
activity (70,71). In fact, the honey-based gel used is
a mix of 87% fir honey and pollen. Bee pollen is produced by the
pollen of flowering plants after mixing with nectar and bee
secretions (72). Pollen, in
addition to lipids, sugars, proteins, vitamins, carbohydrates, and
amino acids, contains polyphenols such as flavonoids (73) that are related to its antioxidant
properties (74). Based on this, it
is hypothesized that part of the honey-based gel antioxidant
capacity and polyphenolic content is due to the presence of
pollen.
In the present study, no significant changes in the
hematological profile of the participants were detected, however,
in physiological measurements, a significant decrease was observed
in diastolic BP and mean arterial BP. Hypertension is known to be a
major risk factor for renal and cardiovascular diseases (75). Furthermore, oxidative stress is also
involved in the pathogenesis of hypertension, although evidence in
the literature indicates the possibility of hypertension leading to
oxidative stress development (76,77).
Altogether, the beneficial effects of antioxidants in diminishing
oxidative damage and attenuating or decreasing high BP, endorse the
negative role of oxidative stress in the management of hypertension
(78).
A previous study investigated the effects of honey
on redox biomarkers evaluated on kidney samples of rats with both
diabetes mellitus and hypertension (79). Results from the aforementioned study
revealed an increase in left kidney intracellular GSH and
reduced/oxidized GSH (GSH/GSSG) ratio after honey supplementation
for 3 weeks in rats (79). It is
evident that GSH plays a key role in reactive species scavenging
and xenobiotic detoxification (80), processes that are closely related to
the antioxidant properties of honey (81). The present study revealed a
significant increase in GSH levels after the consumption of the
honey-based gel. The results from a previous study support the role
of honey in the regulation of GSH levels, since diabetic rats that
consumed honey for 4 weeks exhibited a significant increase in GSH
levels in the kidney tissue (82).
In the same study, a significant increase was also found in total
antioxidant status levels (82).
The aforementioned results are in agreement with the results of the
present study, since an increase in the levels of GSH and TAC was
observed.
Perturbations in the redox status were also evident
by changes in the TBARS and PCARB levels and the findings in the
present study are similar with other studies (83-85)
where honey consumption resulted in significant decreases of MDA
levels, a commonly used marker of oxidative stress that utilizes
TBA reagent, for detecting oxidation products of unsaturated fatty
acids (84), of female athletes
(83) and PCARB following a 0.2
g/kg body weight/day of Tualang honey supplementation for 18 days
(85).
One of the aims of the present study was to assess
potential differences between men and women on blood redox
biomarkers and physiological profile responses following the
consumption of a honey-based gel. The results revealed that women
had a greater response since there were significant decreases in
diastolic BP and mean arterial BP and several blood redox
biomarkers. This could be explained by the greater presence of
estrogen in the female body (86).
Indeed, estrogen levels have a positive correlation with the
antioxidant capacity of the plasma and the antioxidant enzymes
expressed throughout the menstrual cycle (87) and can prevent lipid peroxidation
(88). The antioxidant potential of
estrogens, may also have a direct effect on free RSC activity
(89), a hypothesis that has been
verified through in vitro and ex vivo animal studies
(90). However, a direct
correlation between the antioxidant enzyme system and estrogens has
yet to be determined in humans (91).
Limitations of the present study constitute the
small sample size and the lack of further biomarkers evaluated in
human blood in order to come to safe conclusions regarding the
effects on metabolic health. Finally, the evaluation of
time-dependent alterations in the levels of the proposed biomarkers
during a more extended supplementation period (>14 days) would
ensure comparability and reproducibility among future studies that
will determine redox and metabolic changes after the consumption of
various honey types in different populations. The findings of the
present study indicate that the consumption of the natural
honey-based gel results in significant changes in BP and indices of
the redox status, that are more evident in women. Further studies
should assess different dosages and length of time of
supplementation.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by a project of the
laboratory of Professor D. Kouretas, at the Department of
Biochemistry-Biotechnology, School of Health Sciences, University
of Thessaly, Larissa, Greece (Project Code: 2017ΕΠ53000002),
through the Region of Epirus.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
DK and AZJ contributed to the conceptualization,
supervision, project administration, data analysis and curation of
the study, as well as the correction and editing of the manuscript.
AP contributed to the methodology, investigation, data analysis and
curation, as well as the writing and preparation of the original
manuscript. PS contributed to the methodology, investigation, data
analysis and curation. MK contributed to the data analysis, writing
and preparation of the original manuscript. KP oversaw the
methodology, data analysis, writing and preparation of the original
manuscript. ZS conducted the formal analysis, wrote the original
manuscript, as well as corrected and edited the manuscript. DK and
AZJ confirm the authenticity of all the raw data. All authors have
read and agreed to the published version of the manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of the Department of
Physical Education and Sport Science of the University of Thessaly
(protocol ID: 1722/9-12-2020) approved the methods, procedures, and
ethics of this study. Procedures agreed with the 1975 Declaration
of Helsinki as revised in 2013. All participants provided their
written consent for participation after they were informed about
the purpose, methodology and possible risks associated with the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Andre CM, Larondelle Y and Evers D:
Dietary Antioxidants and Oxidative Stress from a Human and Plant
Perspective: A Review. Curr Nutr Food Sci. 6:2–12. 2010.
|
|
2
|
Ma L, Zhang M, Zhao R, Wang D, Ma Y and Ai
L: Plant natural products: Promising resources for cancer
chemoprevention. Molecules. 26(933)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Briguglio G, Costa C, Pollicino M, Giambò
F, Catania S and Fenga C: Polyphenols in cancer prevention: New
insights (Review). Int J Funct Nutr. 1(9)2020.
|
|
4
|
Zalibera M, Staško A, Šlebodová A,
Jančovičová V, Čermáková T and Brezová V: Antioxidant and
radical-scavenging activities of Slovak honeys-An electron
paramagnetic resonance study. Food Chem. 110:512–521.
2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alamri A: A review of the anticancer
properties of bee products and their molecular mechanisms: An
overview on lung cancer. Trop J Pharm Res. 20:1765–1774. 2022.
|
|
6
|
Mohd Kamal DA, Ibrahim SF, Kamal H, Kashim
MIAM and Mokhtar MH: Physicochemical and medicinal properties of
tualang, gelam and kelulut honeys: A comprehensive review.
Nutrients. 13(197)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Papa G, Maier R, Durazzo A, Lucarini M,
Karabagias IK, Plutino M, Bianchetto E, Aromolo R, Pignatti G,
Ambrogio A, et al: The honey bee apis mellifera: An insect at the
interface between human and ecosystem health. Biology (Basel).
11(233)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pyrzynska K and Biesaga M: Analysis of
phenolic acids and flavonoids in honey. TrAC Trends Anal Chem.
28:893–902. 2009.
|
|
9
|
Ahmed S and Othman NH: Review of the
medicinal effects of tualang honey and a comparison with manuka
honey. Malays J Med Sci. 20:6–13. 2013.PubMed/NCBI
|
|
10
|
Da Silva PM, Gauche C, Gonzaga LV, Costa
AC and Fett R: Honey: Chemical composition, stability and
authenticity. Food Chem. 196:309–323. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Piotraszewska-Pająk A and
Gliszczyńska-Świgło A: Directions of colour changes of nectar
honeys depending on honey type and storage conditions. J Apic Sci.
59:51–61. 2015.
|
|
12
|
Tornuk F, Karaman S, Ozturk I, Tokerac OS,
Tastemur B, Sagdic O, Dogan M and Kayacier A: Quality
characterization of artisanal and retail Turkish blossom honeys:
Determination of physicochemical, microbiological, bioactive
properties and aroma profile. Ind Crops Prod. 46:124–131. 2013.
|
|
13
|
Escuredo O, Míguez M, Fernández-González M
and Carmen Seijo M: Nutritional value and antioxidant activity of
honeys produced in a European Atlantic area. Food Chem.
138:851–856. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Israili ZH: Antimicrobial properties of
honey. Am J Ther. 21:304–323. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Saranraj P, Sivasakthi S and Feliciano GD:
Pharmacology of Honey: A Review. Adv Biol Res. 10:271–289.
2016.
|
|
16
|
Waheed M, Hussain MB, Javed A, Mushtaq Z,
Hassan S, Shariati MA, Khan MU, Majeed M, Nigam M, Mishra AP and
Heydari M: Honey and cancer: A mechanistic review. Clin Nutr.
38:2499–2503. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Erejuwa OO, Sulaiman SA and Wahab MS:
Honey-A novel antidiabetic agent. Int J Biol Sci. 8:913–934.
2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Alvarez-Suarez JM, Tulipani S, Romandini
S, Bertoli E and Battino M: Contribution of honey in nutrition and
human health: A review. Mediterr J Nutr Metab. 3:15–23. 2010.
|
|
19
|
Bt Hj Idrus R, Sainik NQAV, Nordin A, Saim
AB and Sulaiman N: Cardioprotective effects of honey and its
constituent: An evidence-based review of laboratory studies and
clinical trials. Int J Environ Res Public Health.
17(3613)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mijanur Rahman M, Gan SH and Khalil MI:
Neurological effects of honey: Current and future prospects. Evid
Based Complement Alternat Med. 2014(958721)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Samarghandian S, Farkhondeh T and Samini
F: Honey and health: A review of recent clinical research.
Pharmacognosy Res. 9:121–127. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pizzino G, Irrera N, Cucinotta M, Pallio
G, Mannino F, Arcoraci V, Squadrito F, Altavilla D and Bitto A:
Oxidative stress: Harms and benefits for human health. Oxid Med
Cell Longev. 2017(8416763)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gubandru M, Margina D, Tsitsimpikou C,
Goutzourelas N, Tsarouhas K, Ilie M, Tsatsakis AM and Kouretas D:
Alzheimer's disease treated patients showed different patterns for
oxidative stress and inflammation markers. Food Chem Toxicol.
61:209–214. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ungurianu A, Şeremet O, Gagniuc E, Olaru
OT, Guţu C, Grǎdinaru D, Ionescu-Tȋrgovişte C, Marginǎ D and
Dǎnciulescu-Miulescu R: Preclinical and clinical results regarding
the effects of a plant-based antidiabetic formulation versus well
established antidiabetic molecules. Pharmacol Res.
150(104522)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gradinaru D, Khaddour H, Margina D,
Ungurianu A, Borsa C, Ionescu C, Prada GI, Usher J and Elshimali Y:
Insulin-Leptin Axis, Cardiometabolic risk and oxidative stress in
elderly with metabolic syndrome. Exp Clin Endocrinol Diabetes.
126:445–452. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Abdulmajeed WI, Sulieman HB, Zubayr MO,
Imam A, Amin A, Biliaminu SA, Oyewole LA and Owoyele BV: Honey
prevents neurobehavioural deficit and oxidative stress induced by
lead acetate exposure in male wistar rats-a preliminary study.
Metab Brain Dis. 31:37–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mahesh A, Shaheetha J, Thangadurai D and
Rao DM: Protective effect of Indian honey on acetaminophen induced
oxidative stress and liver toxicity in rat. Biologia. 64:1225–1231.
2009.
|
|
28
|
Ahmed S and Othman NH: Honey as a
potential natural anticancer agent: A review of its mechanisms.
Evid Based Complement Alternat Med. 2013(829070)2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tomczyk M, Tarapatskyy M and Dżugan M: The
influence of geographical origin on honey composition studied by
Polish and Slovak honeys. Czech J Food Sci. 37:232–238. 2019.
|
|
30
|
Mushtaq S, Imtiyaz Z, Wali AF, Khan A,
Rasid SM, Amin I, Ali A, Rehman MU and Arafah A: Honey: A Powerful
Natural Antioxidant and Its Possible Mechanism of Action. Ther Appl
Honey its Phytochem. 1:11–29. 2020.
|
|
31
|
Alvarez-Suarez JM, Giampieri F and Battino
M: Honey as a source of dietary antioxidants: Structures,
bioavailability and evidence of protective effects against human
chronic diseases. Curr Med Chem. 20:621–638. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ahmed S, Sulaiman SA, Baig AA, Ibrahim M,
Liaqat S, Fatima S, Jabeen S, Shamim N and Othman NH: Honey as a
potential natural antioxidant medicine: An insight into its
molecular mechanisms of action. Oxid Med Cell Longev.
2018(8367846)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Attanzio A, Tesoriere L, Allegra M and
Livrea MA: Monofloral honeys by Sicilian black honeybee (Apis
mellifera ssp. sicula) have high reducing power and antioxidant
capacity. Heliyon. 2(e00193)2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Combarros-Fuertes P, Estevinho LM, Dias
LG, Castro JM, Tomás-Barberán FA, Tornadijo ME and Fresno-Baro JM:
Bioactive components and antioxidant and antibacterial activities
of different varieties of honey: A screening prior to clinical
application. J Agric Food Chem. 67:688–698. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Afonso AM, Gonçalves J, Luís Â, Gallardo E
and Duarte AP: Evaluation of the in vitro wound-healing activity
and phytochemical characterization of propolis and honey. Appl Sci.
10(1845)2020.
|
|
36
|
Mărgăoan R, Topal E, Balkanska R, Yücel B,
Oravecz T, Cornea-Cipcigan M and Vodnar DC: Monofloral honeys as a
potential source of natural antioxidants, minerals and medicine.
Antioxidants (Basel). 10(1023)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Do Nascimento KS, Gasparotto Sattler JA,
Lauer Macedo LF, Serna González CV, Pereira de Melo IL, Da Silva
Araújo E, Granato D, Sattler A and Almeida-Muradian L: Phenolic
compounds, antioxidant capacity and physicochemical properties of
Brazilian Apis mellifera honeys. LWT. 91:85–94. 2018.
|
|
38
|
Schramm DD, Karim M, Schrader HR, Holt RR,
Cardetti M and Keen CL: Honey with high levels of antioxidants can
provide protection to healthy human subjects. J Agric Food Chem.
51:1732–1735. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Mazruei Arani N, Emam-Djomeh Z,
Tavakolipour H, Sharafati-Chaleshtori R, Soleimani A and Asemi Z:
The effects of probiotic honey consumption on metabolic status in
patients with diabetic nephropathy: A randomized, double-blind,
controlled trial. Probiotics Antimicrob Proteins. 11:1195–1201.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ab Wahab SZ, Nik Hussain NH, Zakaria R,
Abdul Kadir A, Mohamed N, Tohit NM, Norhayati MN and Hassan II:
Long-term effects of honey on cardiovascular parameters and
anthropometric measurements of postmenopausal women. Complement
Ther Med. 41:154–160. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jamurtas AZ, Fatouros IG, Deli CK,
Georgakouli K, Poulios A, Draganidis D, Papanikolaou K, Tsimeas P,
Chatzinikolaou A, Avloniti A, et al: Effects of acute low-volume
HIIT and aerobic exercise on leukocyte count and redox status. J
Sports Sci Med. 17:501–508. 2018.PubMed/NCBI
|
|
42
|
Singleton VL, Orthofer R and
Lamuela-Raventós RM: [14] Analysis of total phenols and other
oxidation substrates and antioxidants by means of folin-ciocalteu
reagent. Methods in Enzymology. 299:152–178. 1999.
|
|
43
|
Miller NJ, Rice-Evans C, Davies MJ,
Gopinathan V and Milner A: A novel method for measuring antioxidant
capacity and its application to monitoring the antioxidant status
in premature neonates. Clin Sci (Lond). 84:407–412. 1993.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kyriazis ID, Skaperda Z, Tekos F, Makri S,
Vardakas P, Vassi E, Patouna A, Terizi K, Angelakis C and Kouretas
D: Methodology for the biofunctional assessment of honey (Review).
Int J Funct Nutr. 2(5)2021.
|
|
45
|
Tofas T, Fatouros IG, Draganidis D, Deli
CK, Chatzinikolaou A, Tziortzis C, Panayiotou G, Koutedakis Y and
Jamurtas AZ: Effects of cardiovascular, resistance and combined
exercise training on cardiovascular, performance and blood redox
parameters in coronary artery disease patients: An 8-Month
training-detraining randomized intervention. Antioxidants (Basel).
10(409)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Draganidis D, Jamurtas AZ, Stampoulis T,
Laschou VC, Deli CK, Georgakouli K, Papanikolaou K, Chatzinikolaou
A, Michalopoulou M, Papadopoulos C, et al: Disparate habitual
physical activity and dietary intake profiles of elderly men with
low and elevated systemic inflammation. Nutrients.
10(566)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Batrakoulis A, Jamurtas AZ, Draganidis D,
Georgakouli K, Tsimeas P, Poulios A, Syrou N, Deli CK, Papanikolaou
K, Tournis S and Fatouros IG: Hybrid neuromuscular training
improves cardiometabolic health and alters redox status in inactive
overweight and obese women: A randomized controlled trial.
Antioxidants (Basel). 10(1601)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pickering TG, Hall JE, Appel LJ, Falkner
BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG and Roccella EJ:
Recommendations for blood pressure measurement in humans and
experimental animals part 1: Blood pressure measurement in humans A
statement for professionals from the subcommittee of professional
and public education of the American heart association Council on
High Blood Pressure Research. Circulation. 111:697–716.
2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Georgakouli K, Fatouros IG, Fragkos A,
Tzatzakis T, Deli CK, Papanikolaou K, Koutedakis Y and Jamurtas AZ:
Exercise and redox status responses following alpha-lipoic acid
supplementation in G6PD deficient individuals. Antioxidants
(Basel). 7(162)2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Makri S, Kafantaris I, Savva S, Ntanou P,
Stagos D, Argyroulis I, Kotsampasi B, Christodoulou V, Gerasopoulos
K, Petrotos K, et al: Novel feed including olive oil mill
wastewater bioactive compounds enhanced the redox status of lambs.
In Vivo. 32:291–302. 2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Reddy YN, Murthy SV, Krishna DR and
Prabhakar MC: Role of free radicals and antioxidants in
tuberculosis patients. Indian J Tuberc. 51:213–218. 2004.
|
|
52
|
Vardakas P, Veskoukis AS, Rossiou D,
Gournikis C, Kapetanopoulou T, Karzi V, Docea AO, Tsatsakis A and
Kouretas D: A mixture of endocrine disruptors and the pesticide
roundup® induce oxidative stress in rabbit liver when administered
under the long-term low-dose regimen: Reinforcing the notion of
real-life risk simulation. Toxics. 10(190)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Skaperda Z, Kyriazis ID, Tekos F, Alvanou
MV, Nechalioti PM, Makri S, Argyriadou A, Vouraki S, Kallitsis T,
Kourti M, et al: Determination of redox status in different tissues
of lambs and kids and their in-between relationship. Antioxidants
(Basel). 11(2065)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Janaszewska A and Bartosz G: Assay of
total antioxidant capacity: Comparison of four methods as applied
to human blood plasma. Scand J Clin Lab Invest. 62:231–236.
2002.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Keles MS, Taysi S, Sen N, Aksoy H and
Akçay F: Effect of corticosteroid therapy on serum and CSF
malondialdehyde and antioxidant proteins in multiple sclerosis. Can
J Neurol Sci. 28:141–143. 2001.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Ranneh Y, Akim AM, Hamid HA, Khazaai H,
Fadel A, Zakaria ZA, Albujja M and Bakar MFA: Honey and its
nutritional and anti-inflammatory value. BMC Complement Med Ther.
21(30)2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Spilioti E, Jaakkola M, Tolonen T,
Lipponen M, Virtanen V, Chinou I, Kassi E, Karabournioti S and
Moutsatsou P: Phenolic acid composition, antiatherogenic and
anticancer potential of honeys derived from various regions in
greece. PLoS One. 9(e94860)2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Tsavea E, Vardaka FP, Savvidaki E, Kellil
A, Kanelis D, Bucekova M, Grigorakis S, Godocikova J, Gotsiou P,
Dimou M, et al: Physicochemical characterization and biological
properties of pine honey produced across greece. Foods.
11(943)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Masad RJ, Haneefa SM, Mohamed YA, Al-Sbiei
A, Bashir G, Fernandez-Cabezudo MJ and Al-Ramadi BK: The
immunomodulatory effects of honey and associated flavonoids in
cancer. Nutrients. 13(1269)2021.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Hemmati M, Karamian M and Malekaneh M:
Anti-atherogenic potential of natural honey: Anti-diabetic and
antioxidant approaches. J Pharm Pharmacol. 3:278–284. 2015.
|
|
62
|
Skaperda Z, Kyriazis ID, Vardakas P, Tekos
F, Antoniou K, Giannakeas N and Kouretas D: In vitro antioxidant
properties of herb decoction extracts derived from Epirus, Greece.
Int J Funct Nutr. 2(11)2021.
|
|
63
|
Kougioumoutzis K, Kokkoris IP, Panitsa M,
Kallimanis A, Strid A and Dimopoulos P: Plant endemism centres and
biodiversity hotspots in greece. Biology (Basel).
10(72)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Dimou M, Tananaki C, Liolios V and
Thrasyvoulou A: Pollen foraging by honey bees (Apis Mellifera L.)
in Greece: Botanical and geographical origin. J Apic Sci. 58:11–23.
2014.
|
|
65
|
Tsiapara AV, Jaakkola M, Chinou I, Graikou
K, Tolonen T, Virtanen V and Moutsatsou P: Bioactivity of Greek
honey extracts on breast cancer (MCF-7), prostate cancer (PC-3) and
endometrial cancer (Ishikawa) cells: Profile analysis of extracts.
Food Chem. 116:702–708. 2009.
|
|
66
|
Stagos D, Soulitsiotis N, Tsadila C,
Papaeconomou S, Arvanitis C, Ntontos A, Karkanta F,
Adamou-Androulaki S, Petrotos K, Spandidos DA, et al: Antibacterial
and antioxidant activity of different types of honey derived from
Mount Olympus in Greece. Int J Mol Med. 42:726–734. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Bazaid AS, Alamri A, Almashjary MN, Qanash
H, Almishaal AA, Amin J, Binsaleh NK, Kraiem J, Aldarhami A and
Alafnan A: Antioxidant, anticancer, antibacterial, antibiofilm
properties and gas chromatography and mass spectrometry analysis of
manuka honey: A nature's bioactive honey. Appl Sci.
12(9928)2022.
|
|
68
|
Chau TS, Owusu-Apenten RK and Nigam P:
Total phenols, antioxidant capacity and antibacterial activity of
manuka honey extract. J Adv Biol Biotechnol. 15:1–6. 2017.
|
|
69
|
Puścion-Jakubik A, Bielecka J, Grabia M,
Markiewicz-żukowska R, Soroczyńska J, Teper D and Socha K:
Comparative analysis of antioxidant properties of honey from
Poland, Italy, and Spain based on the declarations of producers and
their results of melissopalinological analysis. Nutrients.
14(2694)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Khalil MI and Sulaiman SA: The potential
role of honey and its polyphenols in preventing heart diseases: A
review. Afr J Tradit Complement Altern Med. 7:315–321.
2010.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Laaroussi H, Bakour M, Ousaaid D,
Ferreira-Santos P, Genisheva Z, El Ghouizi A, Aboulghazi A,
Teixeira JA and Lyoussi B: Protective effect of honey and propolis
against gentamicin-induced oxidative stress and hepatorenal
damages. Oxid Med Cell Longev. 2021(9719906)2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Graikou K, Kapeta S, Aligiannis N,
Sotiroudis G, Chondrogianni N, Gonos E and Chinou I: Chemical
analysis of Greek pollen-Antioxidant, antimicrobial and proteasome
activation properties. Chem Cent J. 5(33)2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Human H and Nicolson SW: Nutritional
content of fresh, bee-collected and stored pollen of Aloe
greatheadii var davyana (Asphodelaceae). Phytochemistry.
67:1486–1492. 2006.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Mohdaly AAA, Mahmoud AA, Roby MHH,
Smetanska I and Ramadan MF: Phenolic extract from propolis and bee
pollen: Composition, antioxidant and antibacterial activities. J
Food Biochem. 39:538–547. 2015.
|
|
75
|
Al-Waili N, Salom K, Al-Ghamdi A, Ansari
MJ, Al-Waili A and Al-Waili T: Honey and cardiovascular risk
factors, in normal individuals and in patients with diabetes
mellitus or dyslipidemia. J Med Food. 16:1063–1078. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Lopes de Faria JB, Silva KC and Lopes de
Faria JM: The contribution of hypertension to diabetic nephropathy
and retinopathy: The role of inflammation and oxidative stress.
Hypertens Res. 34:413–422. 2011.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Rodrigo R, González J and Paoletto F: The
role of oxidative stress in the pathophysiology of hypertension.
Hypertens Res. 34:431–440. 2011.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Erejuwa OO, Sulaiman SA and Ab Wahab MS:
Honey: A novel antioxidant. Molecules. 17:4400–4423.
2012.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Erejuwa OO, Sulaiman SA, Wahab MS,
Sirajudeen KN, Salleh SM and Gurtu S: Differential responses to
blood pressure and oxidative stress in streptozotocin-induced
diabetic wistar-kyoto rats and spontaneously hypertensive rats:
Effects of antioxidant (Honey) treatment. Int J Mol Sci.
12:1888–1907. 2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Forman HJ, Zhang H and Rinna A:
Glutathione: Overview of its protective roles, measurement, and
biosynthesis. Mol Aspects Med. 30:1–12. 2009.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Khalil MI, Sulaiman SA and Boukraa L:
Antioxidant properties of honey and its role in preventing health
disorder. Open Nutraceuticals J. 3:6–16. 2010.
|
|
82
|
Erejuwa OO, Sulaiman SA, Wahab MS, Salam
SK, Salleh MS and Gurtu S: Comparison of antioxidant effects of
honey, glibenclamide, metformin, and their combinations in the
kidneys of streptozotocin-induced diabetic rats. Int J Mol Sci.
12:829–843. 2011.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ahmad NS, Abdul Aziz A, Kong KW, Hamid
MSA, Cheong JPG and Hamzah SH: Dose-Response effect of tualang
honey on postprandial antioxidant activity and oxidative stress in
female athletes: A pilot study. J Altern Complement Med.
23:989–995. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Tsikas D: Assessment of lipid peroxidation
by measuring malondialdehyde (MDA) and relatives in biological
samples: Analytical and biological challenges. Anal Biochem.
524:13–30. 2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Al-Rahbi B, Zakaria R, Othman Z, Hassan A
and Ahmad AH: Protective effects of tualang honey against oxidative
stress and anxiety-like behaviour in stressed ovariectomized rats.
Int Sch Res Notices. 2014(521065)2014.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Kander MC, Cui Y and Liu Z: Gender
difference in oxidative stress: A new look at the mechanisms for
cardiovascular diseases. J Cell Mol Med. 21:1024–1032.
2017.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Michos C, Kiortsis DN, Evangelou A and
Karkabounas S: Antioxidant protection during the menstrual cycle:
The effects of estradiol on ascorbic-dehydroascorbic acid plasma
levels and total antioxidant plasma status in eumenorrhoic women
during the menstrual cycle. Acta Obstet Gynecol Scand. 85:960–965.
2006.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Serviddio G, Loverro G, Vicino M,
Prigigallo F, Grattagliano I, Altomare E and Vendemiale G:
Modulation of endometrial redox balance during the menstrual cycle:
Relation with sex hormones. J Clin Endocrinol Metab. 87:2843–2848.
2002.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Prokai-Tatrai K, Perjesi P,
Rivera-Portalatin NM, Simpkins JW and Prokai L: Mechanistic
investigations on the antioxidant action of a neuroprotective
estrogen derivative. Steroids. 73:280–288. 2008.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Dantas AP, Tostes RC, Fortes ZB, Costa SG,
Nigro D and Carvalho MH: In vivo evidence for antioxidant potential
of estrogen in microvessels of female spontaneously hypertensive
rats. Hypertension. 39 (2 Pt 2):405–411. 2002.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Bellanti F, Matteo M, Rollo T, De Rosario
F, Greco P, Vendemiale G and Serviddio G: Sex hormones modulate
circulating antioxidant enzymes: Impact of estrogen therapy. Redox
Biol. 1:340–346. 2013.PubMed/NCBI View Article : Google Scholar
|