|
1
|
Cooper N and Ghanima W: Immune
thrombocytopenia. N Engl J Med. 381:945–955. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Audia S, Mahévas M, Samson M, Godeau B and
Bonnotte B: Pathogenesis of immune thrombocytopenia. Autoimmun Rev.
16:620–632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bolton-Maggs PHB and George JN: Immune
thrombocytopenia treatment. N Engl J Med. 385:948–950.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sandal R, Mishra K, Jandial A, Sahu KK and
Siddiqui AD: Update on diagnosis and treatment of immune
thrombocytopenia. Expert Rev Clin Pharmacol. 14:553–568.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim DS: Recent advances in treatments of
adult immune thrombocytopenia. Blood Res. 57 (S1):S112–S119.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Syed YY: Hetrombopag: First approval.
Drugs. 81:1581–1585. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rodeghiero F: Is ITP a thrombophilic
disorder? Am J Hematol. 91:39–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
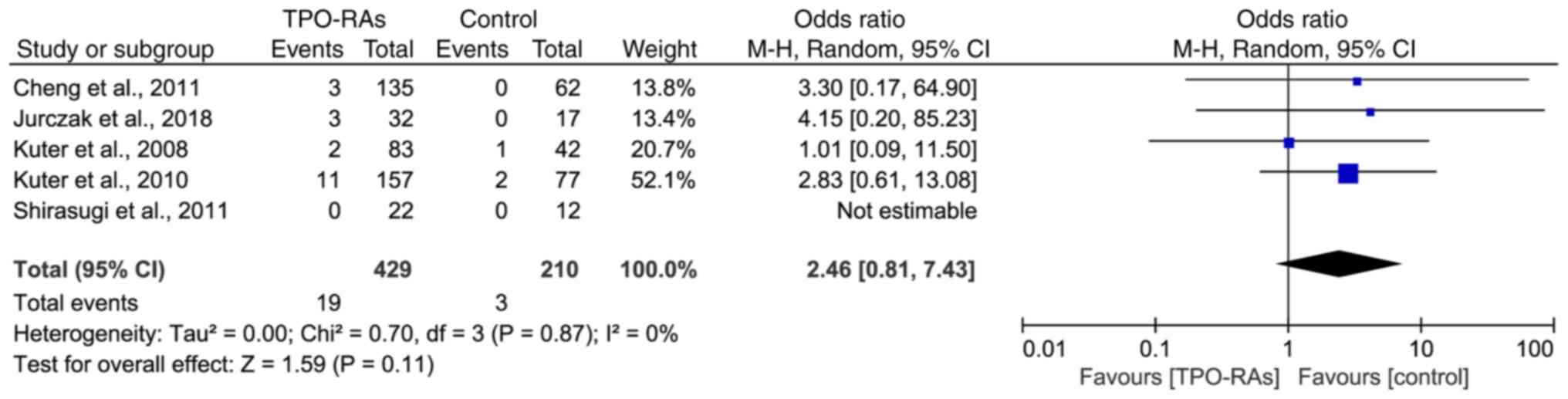
Cheng G, Saleh MN, Marcher C, Vasey S,
Mayer B, Aivado M, Arning M, Stone NL and Bussel JB: Eltrombopag
for management of chronic immune thrombocytopenia (RAISE): A
6-month, randomised, phase 3 study. Lancet. 377:393–402.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wong RSM, Saleh MN, Khelif A, Salama A,
Portella MSO, Burgess P and Bussel JB: Safety and efficacy of
long-term treatment of chronic/persistent ITP with eltrombopag:
Final results of the EXTEND study. Blood. 130:2527–2536.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mei H, Xu M, Yuan G, Zhu F, Guo J, Huang
R, Qin J, Lv T, Qin F, Cai H, et al: A multicentre double-blind,
double-dummy, randomised study of recombinant human thrombopoietin
versus eltrombopag in the treatment of immune thrombocytopenia in
Chinese adult patients. Br J Haematol. 195:781–789. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Page MJ, Moher D, Bossuyt PM, Boutron I,
Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: PRISMA 2020 explanation and elaboration: Updated
guidance and exemplars for reporting systematic reviews. BMJ.
372(n160)2021.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
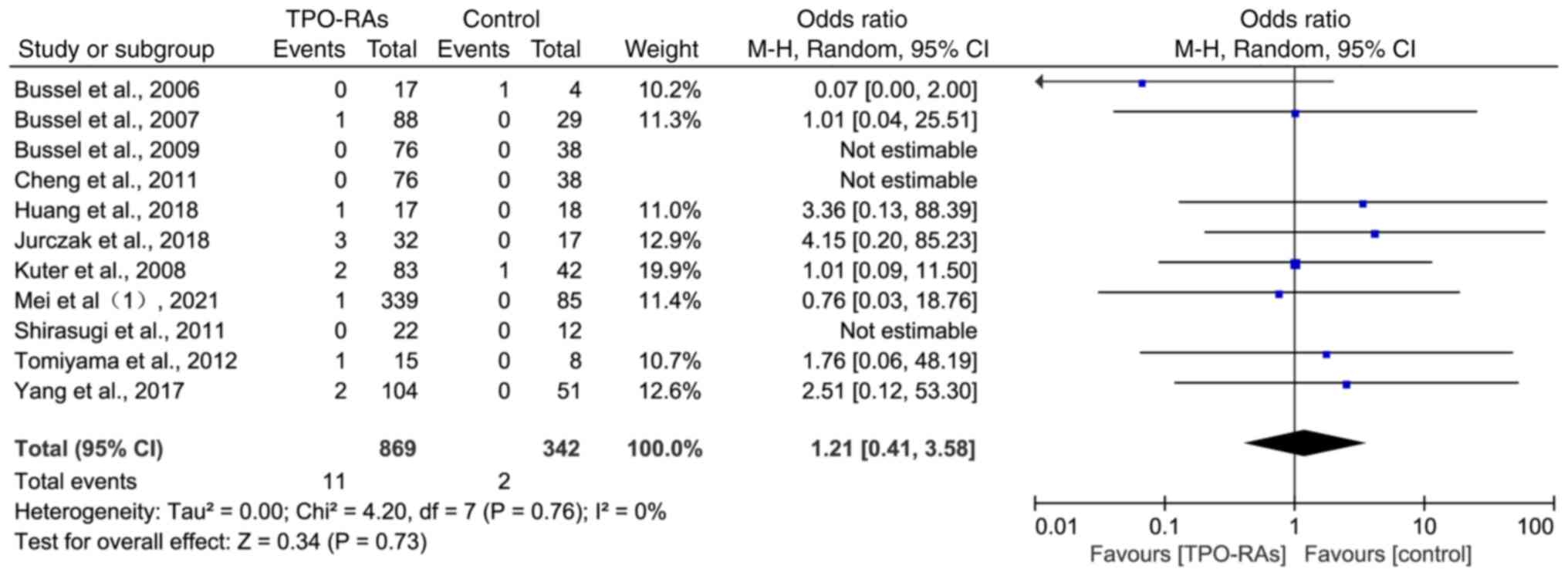
Bussel JB, Kuter DJ, George JN, McMillan
R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser
JS, et al: AMG 531, a thrombopoiesis-stimulating protein, for
chronic ITP. N Engl J Med. 355:1672–1681. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bussel JB, Cheng G, Saleh MN, Psaila B,
Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, et
al: Eltrombopag for the treatment of chronic idiopathic
thrombocytopenic purpura. N Engl J Med. 357:2237–2247.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kuter DJ, Bussel JB, Lyons RM, Pullarkat
V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM,
Sanz MA, et al: Efficacy of romiplostim in patients with chronic
immune thrombocytopenic purpura: A double-blind randomised
controlled trial. Lancet. 371:395–403. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bussel JB, Provan D, Shamsi T, Cheng G,
Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer
B, et al: Effect of eltrombopag on platelet counts and bleeding
during treatment of chronic idiopathic thrombocytopenic purpura: A
randomised, double-blind, placebo-controlled trial. Lancet.
373:641–648. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kuter DJ, Rummel M, Boccia R, Macik BG,
Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X and Berger
DP: Romiplostim or standard of care in patients with immune
thrombocytopenia. N Engl J Med. 363:1889–1899. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shirasugi Y, Ando K, Miyazaki K, Tomiyama
Y, Okamoto S, Kurokawa M, Kirito K, Yonemura Y, Mori S, Usuki K, et
al: Romiplostim for the treatment of chronic immune
thrombocytopenia in adult Japanese patients: A double-blind,
randomized phase III clinical trial. Int J Hematol. 94:71–80.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tomiyama Y, Miyakawa Y, Okamoto S,
Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S,
Ozaki K, et al: A lower starting dose of eltrombopag is efficacious
in Japanese patients with previously treated chronic immune
thrombocytopenia. J Thromb Haemost. 10:799–806. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang R, Li J, Jin J, Huang M, Yu Z, Xu X,
Zhang X and Hou M: Multicentre, randomised phase III study of the
efficacy and safety of eltrombopag in Chinese patients with chronic
immune thrombocytopenia. Br J Haematol. 176:101–110.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jurczak W, Chojnowski K, Mayer J, Krawczyk
K, Jamieson BD, Tian W and Allen LF: Phase 3 randomised study of
avatrombopag, a novel thrombopoietin receptor agonist for the
treatment of chronic immune thrombocytopenia. Br J Haematol.
183:479–490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang YT, Liu XF, Chen YF, Fu RF, Liu W,
Zhang L and Yang RC: The efficacy and safety of eltrombopag in
Chinese patients with chronic immune thrombocytopenia. Zhonghua Xue
Ye Xue Za Zhi. 39:32–36. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Arnold DM, Heddle NM, Cook RJ, Hsia C,
Blostein M, Jamula E, Sholzberg M, Lin Y, Kassis J, Larratt L, et
al: Perioperative oral eltrombopag versus intravenous
immunoglobulin in patients with immune thrombocytopenia: A
non-inferiority, multicentre, randomised trial. Lancet Haematol.
7:e640–e648. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mei H, Liu X, Li Y, Zhou H, Feng Y, Gao G,
Cheng P, Huang R, Yang L, Hu J, et al: A multicenter, randomized
phase III trial of hetrombopag: A novel thrombopoietin receptor
agonist for the treatment of immune thrombocytopenia. J Hematol
Oncol. 14(37)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koupenova M, Kehrel BE, Corkrey HA and
Freedman JE: Thrombosis and platelets: An update. Eur Heart J.
38:785–791. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mei H, Chen X, Zhou J, Luo J, Shi Q, Liu
J, Wu D, Chen G, Tai Y, Xiong J, et al: Safety and efficacy of
hetrombopag in patients with chronic immune thrombocytopenia: A
single-arm, open-label, multi-center phase 1 study. Ann Transl Med.
10(30)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tjepkema M, Amini S and Schipperus M: Risk
of thrombosis with thrombopoietin receptor agonists for ITP
patients: A systematic review and meta-analysis. Crit Rev Oncol
Hematol. 171(103581)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Catalá-López F, Corrales I, de la
Fuente-Honrubia C, González-Bermejo D, Martín-Serrano G, Montero D
and Saint-Gerons DM: Risk of thromboembolism with thrombopoietin
receptor agonists in adult patients with thrombocytopenia:
Systematic review and meta-analysis of randomized controlled
trials. Med Clin (Barc). 145:511–519. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Birocchi S, Podda GM, Manzoni M, Casazza G
and Cattaneo M: Thrombopoietin receptor agonists for the treatment
of primary immune thrombocytopenia: A meta-analysis and systematic
review. Platelets. 2:216–226. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bussel J, Cooper N, Boccia R, Zaja F and
Newland A: Immune thrombocytopenia. Expert Rev Hematol.
14:1013–1025. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Justo Sanz R, Monzón Manzano E, Fernández
Bello I, Teresa Álvarez Román M, Martín Salces M, Rivas Pollmar MI,
Jiménez Yuste V and Butta NV: Platelet apoptosis and PAI-1 are
involved in the pro-coagulant state of immune thrombocytopaenia
patients treated with thrombopoietin receptor agonists. Thromb
Haemost. 119:645–659. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Machin N, Ragni MV, Comer DM and Yabes JG:
Prevalence and correlates of thrombosis in adults with immune
thrombocytopenia: An NIS study. Thromb Res. 172:80–85.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Swan D, Newland A, Rodeghiero F and
Thachil J: Thrombosis in immune thrombocytopenia-current status and
future perspectives. Br J Haematol. 194:822–834. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jain A, Saluja S, Chaudhry S and Gupta DK:
Recurrent arterial and venous thrombosis in chronic immune
thrombocytopenia: Clinical paradox and therapeutic challenges.
Indian J Hematol Blood Transfus. 35:590–592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lakshmanan S and Cuker A: Contemporary
management of primary immune thrombocytopenia in adults. J Thromb
Haemost. 10:1988–1998. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Boyle S, White RH, Brunson A and Wun T:
Splenectomy and the incidence of venous thromboembolism and sepsis
in patients with immune thrombocytopenia. Blood. 121:4782–4790.
2013.PubMed/NCBI View Article : Google Scholar
|