Introduction
Immune thrombocytopenic purpura (ITP) is an
acquired, immune and hemorrhagic disease characterized by isolated
platelet count reduction without clear triggers (1). The main pathogenesis is immune
intolerance, which mediates platelet immune destruction and
insufficient platelet generation. The diagnostic criterion for ITP
is a platelet count of <100x109/l, with no
significant abnormalities in blood cell morphology (2). Steroids are powerful
immunosuppressants that reduce the production of platelet
autoantibodies and the phagocytosis of platelets by macrophages
(3). They have been used as
first-line treatments for ITP for many years, and initial reactions
have been observed in ~70% of patients (4). However, the side effects of long-term
steroid usage have forced numerous patients with persistent or
chronic ITP to choose other drug types.
Thrombopoietin receptor agonists (TPO-RAs) are
second-line drugs used to treat ITP. They can bind to and activate
thrombopoietin receptors on the membranes of megakaryocytes in the
bone marrow, thus promoting megakaryocyte maturation and increasing
platelet production (4,5). Currently, TPO-RAs that have been
approved by China, the US and the EU for the clinical treatment of
ITP include romiplostim, eltrombopag, avatrombopag and hetrombopag
(5,6). Over the past decade, TPO-RAs have
become increasingly common for treating ITP. An increasing number
of adverse events, including thrombosis, have been observed in
clinical practice. TPO-RAs can potentially increase the risk of
thrombosis by increasing platelet count and stimulating the
production of younger and more hemostatic platelets (7). Several multicenter clinical trials
have reported a thrombotic event incidence of 2-6% during the
treatment of ITP with TPO-RAs, while others have not reported such
events (8-10).
It thus remains unclear whether TPO-RAs increase the incidence of
thrombosis. Therefore, the present study aimed to evaluate the
incidence of thrombotic events in the treatment of ITP with TPO-RAs
and provide a theoretical basis for monitoring adverse drug
reactions in clinical practice by a systematic review of the
literature and a meta-analysis.
Materials and methods
Literature search
This systematic review and meta-analysis was
performed following the Preferred Reporting Items for Systematic
review and Meta-Analysis guidelines (11). Two researchers (JY and RW)
independently searched five databases-PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase
(https://www.embase.com), Web of Science
(https://www.webofscience.com), China
National Knowledge Infrastructure (CNKI; https://www.cnki.net) and Wanfang Data (https://www.wanfangdata.com.cn)-from the
establishment of the database until April 1, 2023. Literature
retrieval was performed by combining theme words with free words,
while also tracing the citations included in the literature to
expand the search scope. The search terms included the following:
‘immune thrombocytopenia’, ‘immune thrombocytopenic purpura’,
‘ITP’, ‘purpura, thrombocytopenic, idiopathic’, ‘thrombocytopenic
purpura’, ‘idiopathic thrombocytopenic purpura’, ‘romiplostim’,
‘eltrombopag’, ‘avatrombopag’, ‘hetrombopag’, ‘thrombopoietin
receptor agonists’, ‘TPO-RA’ and ‘TPO-RAs’. The languages used for
retrieval were Chinese and English. No filters were used to reduce
the possibility of accidental exclusion of relevant articles.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) The type
of study needed to be a randomized controlled trial (RCT), with the
intervention group being treated with romiplostim, eltrombopag,
avatrombopag or hetrombopag; ii) all participants were adults (≥18
years old) with ITP, regardless of whether they had newly diagnosed
ITP, persistent ITP or chronic ITP; and iii) presence of a detailed
record of the number of thrombotic events in the study. The
exclusion criteria were as follows: i) Duplicate publications of
the same data; ii) not written in English or Chinese; and iii)
inability to obtain the full text.
Quality analysis
Two researchers (JY and RW) used the revised
Cochrane Risk of Bias Assessment Tool (ROB 2.0; The Cochrane
Collaboration) to evaluate the quality of each RCT. Based on the
evaluation results, studies were divided into low-, high- and
unclear risk groups. Differences between the ratings from the two
researchers were resolved through discussion. If they could not be
resolved, adjudication by a third researcher (HY) was sought.
Data extraction
The retrieved literature was imported into the
EndNote X9 software (Clarivate PLC) and checked for duplicates,
which were deleted. Two researchers (JY and RW) referred to the
inclusion and exclusion criteria and initially excluded articles
that did not meet the requirements by reading the titles and
abstracts. Clinical data, including the name of the main author,
year of publication, study design, sample size, intervention
measures, treatment duration and number of thrombotic events, were
independently extracted. After data extraction, comprehensive
recording and crosschecking were performed. Differences between the
two researchers were resolved through discussion.
Statistical methods
Statistical analyses were conducted using the RevMan
5.4.1 software (https://revman.cochrane.org). Dichotomous data were
represented as the odds ratios (OR) and 95% confidence interval
(CI). Statistical heterogeneity analysis was conducted using the
Q-test and the size of heterogeneity was estimated using the
I2 test. Regardless of the P-value and I²-value, the
random-effects model was chosen for meta-analysis. Statistical
significance was set at P<0.05.
Results
Study selection
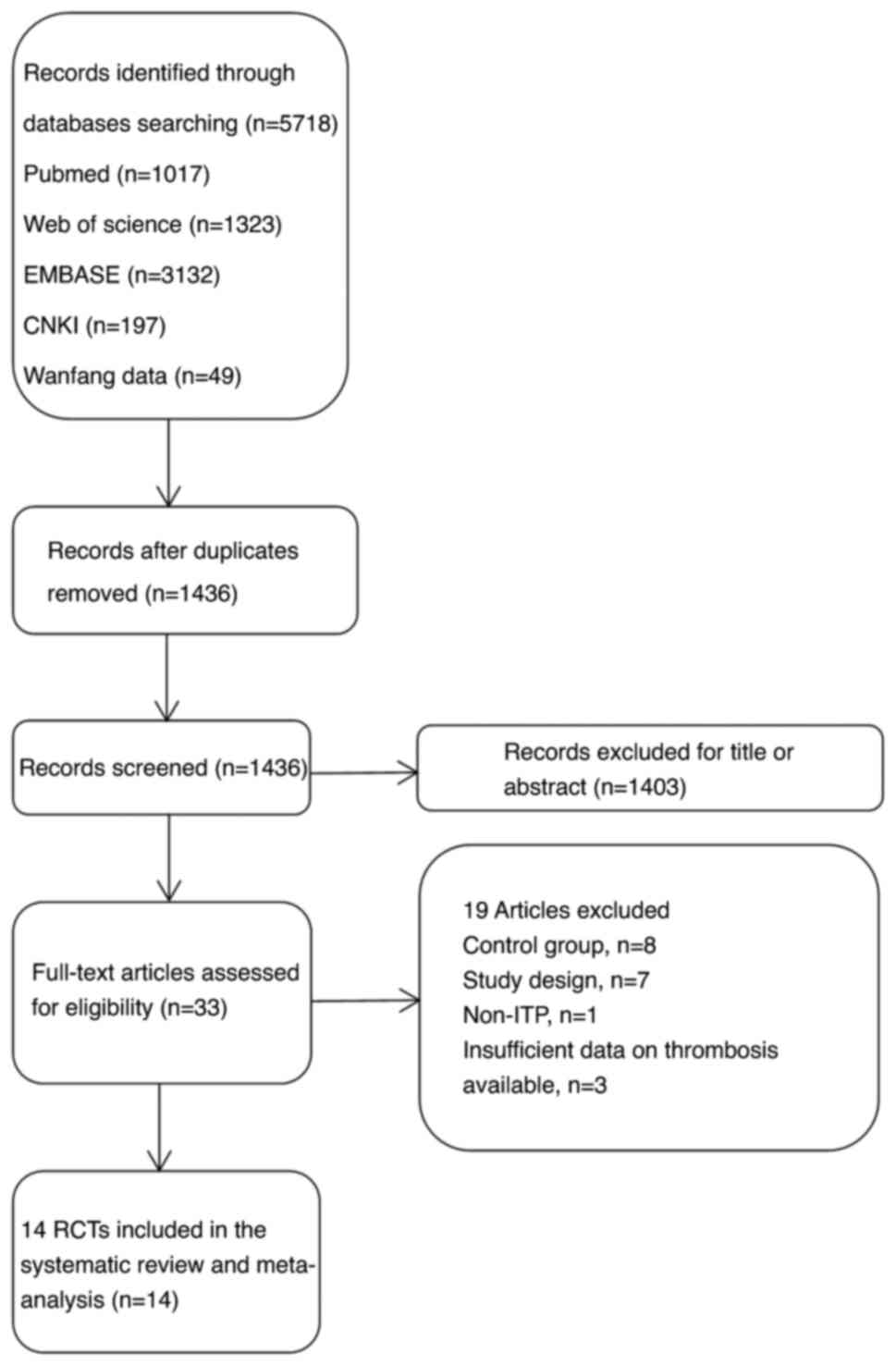
Based on the search strategy, 5,718 articles were
retrieved after a preliminary search, including 246 Chinese
articles. After eliminating duplicate records using EndNote X9
software, 1,436 publications remained. After reading the titles and
abstracts, 1,403 articles that did not meet the inclusion criteria
were removed and 33 articles were preliminarily screened. After
reading the full texts, 14 RCTs were included in this
meta-analysis. The screening process is depicted in Fig. 1.
Study characteristics
The main characteristics of the included studies are
summarized in Table I. All of the
included studies were multicenter clinical trials, including eight
Phase III clinical trials and one Phase II clinical trial. The
studies were published between 2006 and 2021. A total of 1,698
adult patients with ITP were enrolled, including 1,171 and 527
patients in the intervention and control groups, respectively. The
duration of the intervention ranged from 2 to 52 weeks and the
sample size ranged from 21 to 424 participants. Participants in 12
studies had a history of ITP for >3 months. The control group in
11 studies was treated with a placebo, while the remaining three
controls received standard-of-care, intravenous immunoglobulin and
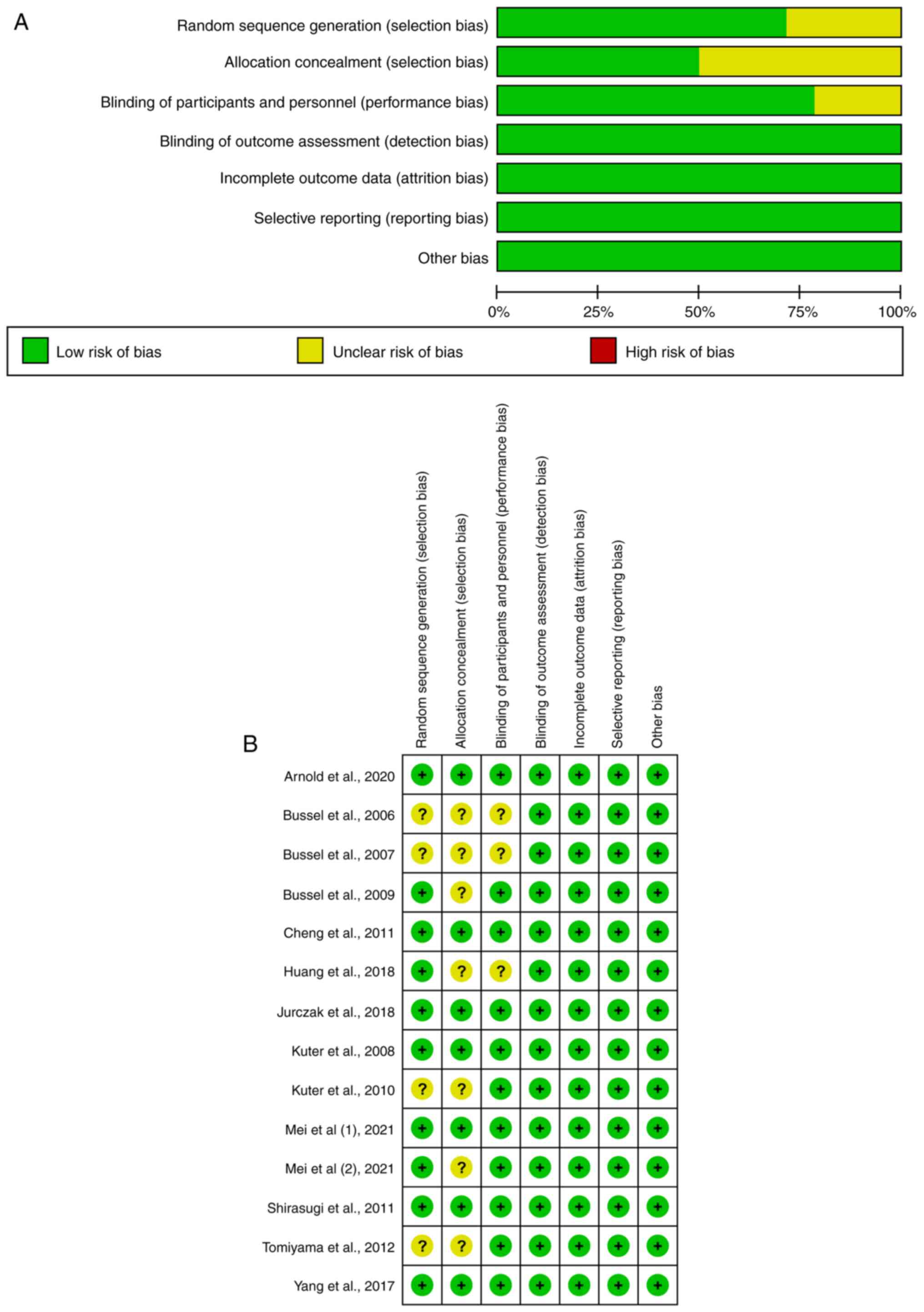
thrombopoietin. Eight articles were deemed low-risk and six
articles were deemed to have unclear risk based on bias assessment
(Fig. 2). High risk was not
observed in the quality evaluation of the included articles.
 | Table IStudy characteristics and summary of
findings. |
Table I
Study characteristics and summary of
findings.
| | Intervention | Control | Thrombotic
events | |
|---|
| Author, year | Study design | Study duration,
weeks | Population | Drug (dose) | No. of patients | Method | No. of patients | Intervention
group | Control group | (Refs.) |
|---|
| Bussel, 2006 | Multicenter,
randomized, double-blind, placebo controlled, Phase II | 6 | pITP and cITP
(n=21) | Romiplostim (1, 3 or
6 µg/kg) | 17 | Placebo | 4 | 0 | 1 | (12) |
| Bussel, 2007 | Multicenter,
randomized, double-blind, placebo controlled | 6 | pITP and cITP
(n=117) | Eltrombopag (30, 50
or 75 mg) | 88 | Placebo | 29 | 1 | 0 | (13) |
| Kuter, 2008 | Multicenter,
randomized, double-blind, placebo controlled, Phase III | 24 | pITP and cITP
(n=125) | Romiplostim (1 or 2
µg/kg) | 83 | Placebo | 42 | 2 | 1 | (14) |
| Bussel, 2009 | Multicenter,
randomized, double-blind, placebo controlled, Phase III | 6 | pITP and cITP
(n=114) | Eltrombopag (50 or
75 mg) | 76 | Placebo | 38 | 0 | 0 | (15) |
| Kuter, 2010 | Multicenter,
randomized, open-label, controlled | 52 | ITP (n=234) | Romiplostim (3-10
µg/kg) | 157 | Standard of
care | 77 | 11 | 2 | (16) |
| Shirasugi,
2011 | Multicenter,
randomized, double-blind, placebo controlled, Phase III | 12 | pITP and cITP
(n=34) | Romiplostim (3-10
µg/kg) | 22 | Placebo | 12 | 0 | 0 | (17) |
| Cheng, 2011 | Multicenter,
randomized, double-blind, placebo controlled, Phase III | 24 | pITP and cITP
(n=197) | Eltrombopag (25-75
mg) | 135 | Placebo | 62 | 3 | 0 | (8) |
| Tomiyama, 2012 | Multicenter,
randomized, double-blind, placebo controlled | 6 | pITP and cITP
(n=23) | Eltrombopag
(12.5-25 mg) | 15 | Placebo | 8 | 1 | 0 | (18) |
| Yang, 2017 | Multicenter,
randomized, double-blind, placebo controlled, Phase III | 8 | cITP (n=155) | Eltrombopag (25-75
mg) | 104 | Placebo | 51 | 2 | 0 | (19) |
| Jurczak, 2018 | Multicenter,
randomized, double-blind, placebo controlled, Phase III | 26 | cITP (n=49) | Avatrombopag (5-40
mg) | 32 | Placebo | 17 | 3 | 0 | (20) |
| Huang, 2018 | Multicenter,
randomized, double-blind, placebo controlled, Phase III | 6 | cITP (n=35) | Eltrombopag (25-75
mg) | 17 | Placebo | 18 | 1 | 0 | (21) |
| Arnold, 2020 | Multicenter,
randomized, open-label, controlled | 3 | ITP (n=74) | Eltrombopag (50
mg) | 38 | Intravenous
immunoglobulin | 36 | 1 | 0 | (22) |
| Mei (i), 2021 | Multicenter,
randomized, double-blind, placebo controlled, Phase III | 10 | pITP or cITP
(n=424) | Hetrombopag (2.5 or
5 mg) | 339 | Placebo | 85 | 1 | 0 | (23) |
| Mei (ii), 2021 | Multicenter,
randomized, double-blind, controlled | 2 | pITP or cITP
(n=96) | Eltrombopag (25
mg) | 48 | Thrombopoietin | 48 | 0 | 0 | (10) |
Thrombosis events on total
TPO-RAs
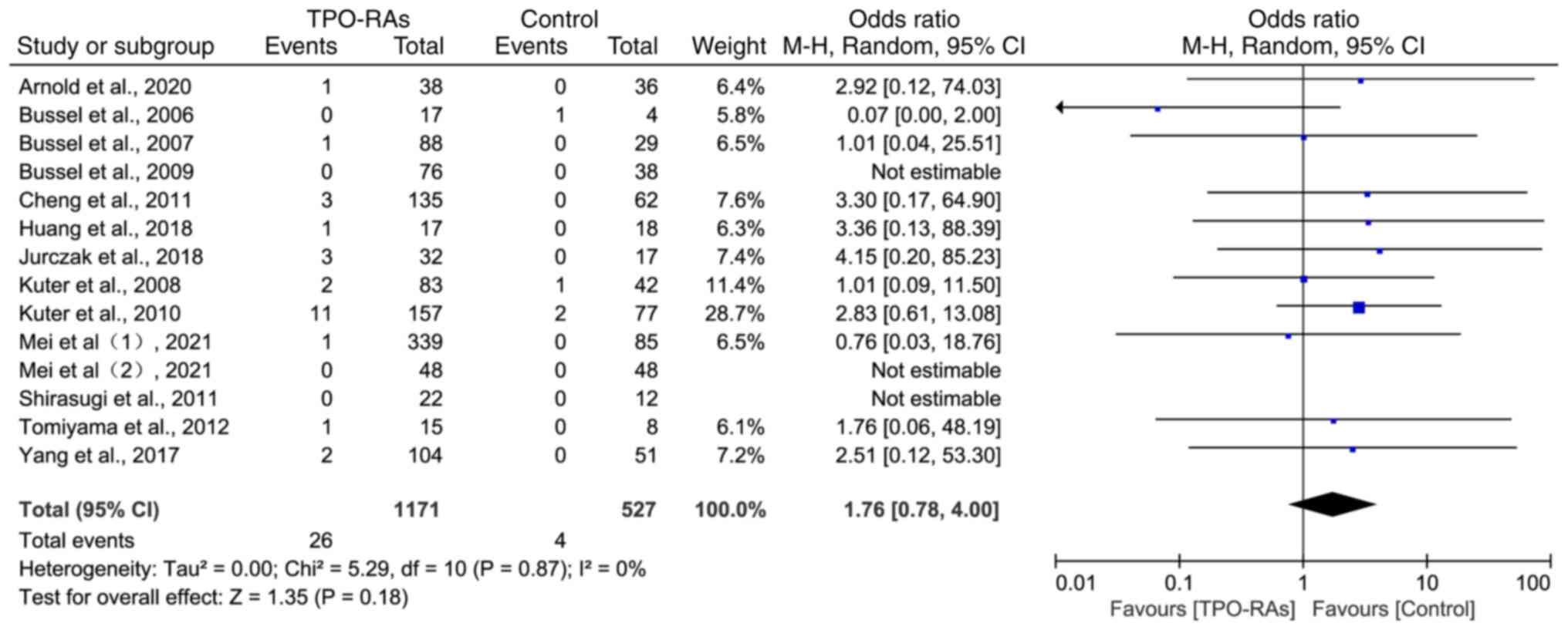
The heterogeneity test analysis results showed that
there was a high degree of homogeneity among the 14 included
studies (χ2=5.29, P=0.87; I2=0%) and a
random-effects model was selected for meta-analysis. The results
showed that, compared with the control group, the incidence of
thrombotic events in the TPO-RAs group did not significantly
increase (OR=1.76, 95% CI: 0.78-4.00, P=0.18; Fig. 3).
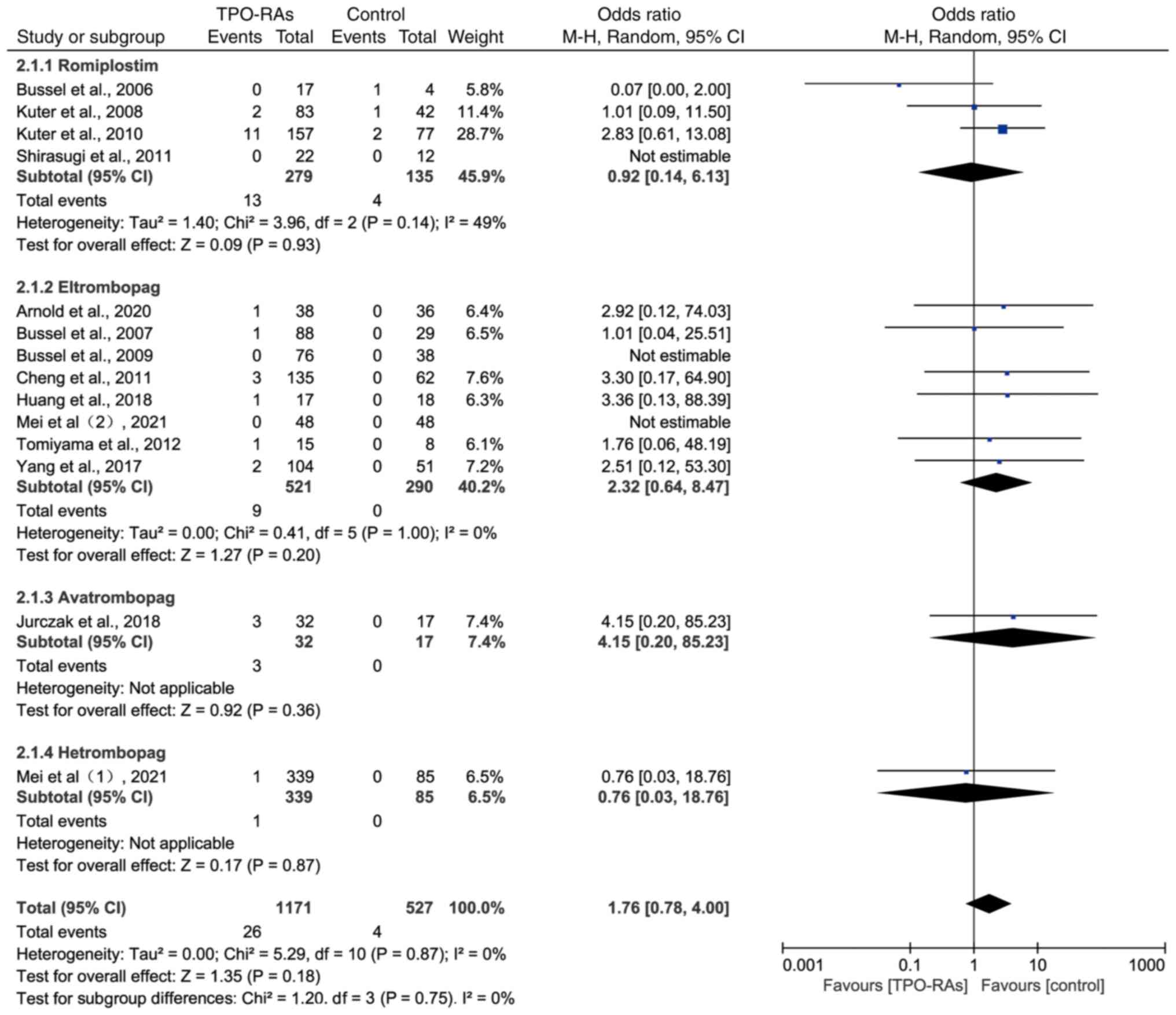
Subgroup analysis of thrombosis events
on TPO-RAs
The analysis was divided into four subgroups based
on the names of the drugs. The results showed that there was no
statistically significant difference in the probability of
thrombotic events occurring during the treatment of ITP with
romiplostim (OR=0.92, 95% CI: 0.14-6.13, P=0.93), eltrombopag
(OR=2.32, 95% CI: 0.64-8.47, P=0.20), avatrombopag (OR=4.15, 95%
CI: 0.20-85.23, P=0.36) or hetrombopag (OR=0.76, 95% CI:
0.03-18.76, P=0.87) compared to the control group (Fig. 4).
Thrombosis events on TPO-RAs treatment
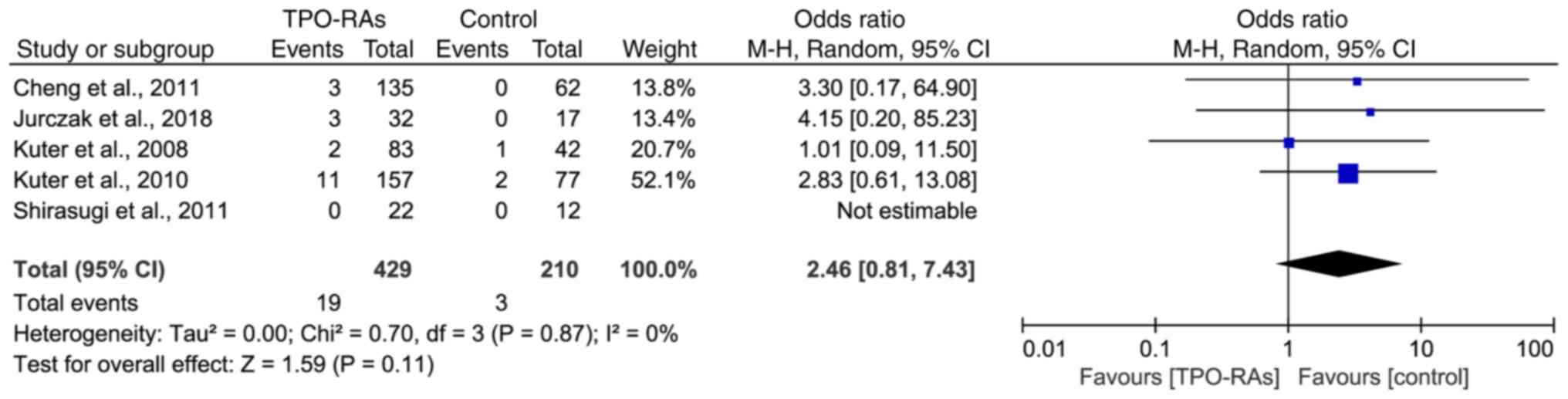
time >12 weeks
There were 5 studies with medication durations
exceeding 12 weeks, including 3 with romiplostim (12, 24 and 52
weeks), 1 with eltrombopag (24 weeks) and 1 with avatrombopag (26
weeks). The results showed that there was no statistically
significant difference in the incidence of thrombosis between the
TPO-RAs treatment groups and the control groups (OR=2.46, 95% CI:
0.81-7.43, P=0.11), as shown in Fig.
5.
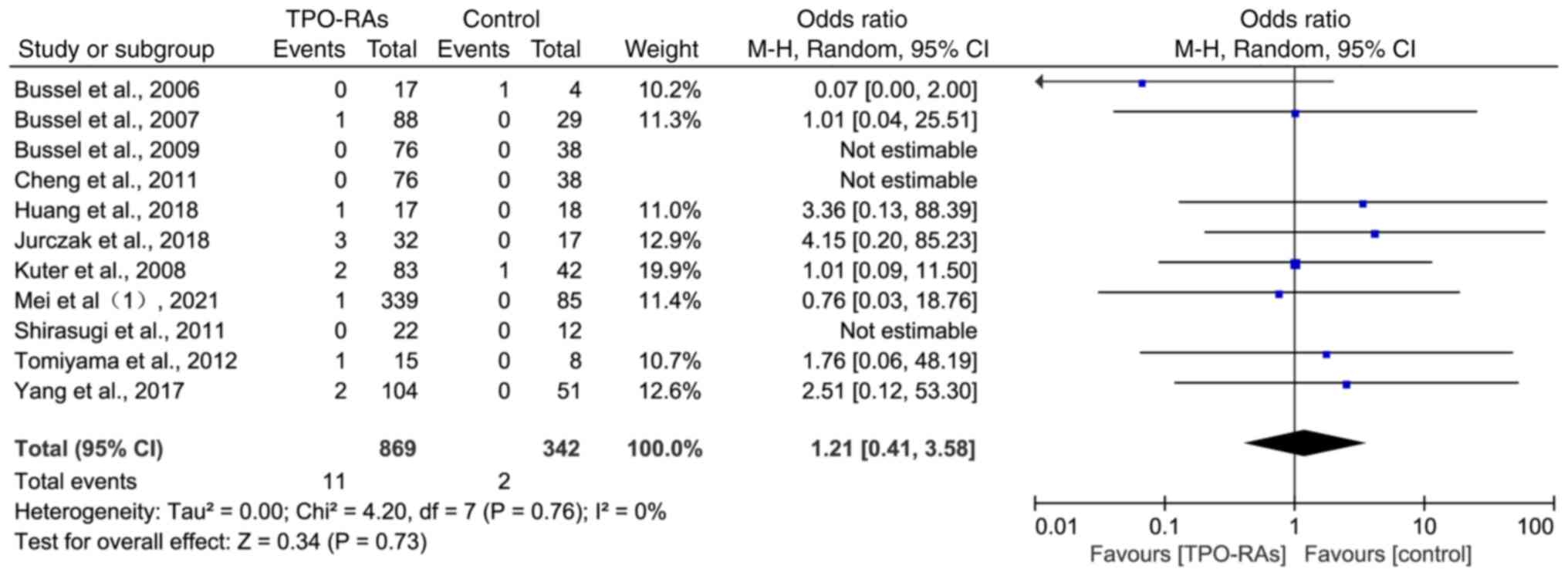
Thrombosis events on TPO-RAs in
double-blinded, placebo-controlled RCTs
A total of 11 studies were included in the analysis
with a total of 1,121 participants, including 869 in the
intervention group and 342 in the placebo control group. Compared
with placebo, there was no statistically significant increase in
the incidence of thrombotic events in the treatment of ITP with
TPO-RAs (OR=1.21, 95% CI: 0.41-3.58, P=0.73; Fig. 6).
Discussion
This systematic review and meta-analysis aimed to
determine the effect of TPO-RAs on thrombotic events in ITP. All
relevant RCTs (n=14) were included and the results showed that
TPO-RAs did not significantly increase the incidence of thrombotic
events, even when the treatment time was ≥12 weeks. Subgroup
analysis showed that administering romiplostim, eltrombopag,
avatrombopag and hetrombopag did not increase the incidence of
thrombotic events. These findings suggest no statistically
significant association between thrombosis and TPO-RAs, signifying
that patients with ITP treated with TPO-RAs do not face elevated
thromboembolism risks compared to those treated without
TPO-RAs.
A thrombus is a blood clot that clogs the vessels,
composed of insoluble fibrin, aggregated platelets, white blood
cells and red blood cells. The function and quantity of platelets
have important roles in thrombosis (24). TPO-RAs, which are second-line drugs
for immune thrombocytopenia, have been widely used for the
treatment of ITP. They can stimulate the bone marrow to produce
younger and more active platelets. However, theoretically, they
also increase the risk of thrombosis (7). Both single-arm and controlled trials
have reported cases of thrombosis treated with TPO-RAs with an
incidence rate of 2-6% (8,10,25). A
previous systematic review compared the risks of thrombotic events
between TPO-RA and non-TPO-RA treatments (26). The results showed that the incidence
rates of thrombotic events were 3.37% (25/740) and 1.13% (4/352),
respectively, with a relative risk of 1.82 (95% CI: 0.78-4.24,
P=0.16). In the present study, the incidence of thrombotic events
during TPO-RAs treatment was 2.22% (26/1171), which was higher than
the incidence of 0.76% (4/527) in the control group (OR=1.76, 95%
CI: 0.78-4.00, P=0.18). To the best of our knowledge, although no
systematic review has reported a significant increase in the
incidence of thrombotic events during the treatment of ITP with
TPO-RAs, the incidence in the treatment group was more than twice
that in the control group. Therefore, it may be necessary to
monitor thrombotic events during the use of TPO-RAs for ITP
treatment in clinical practice. There are two other systematic
reviews comparing the risk of thrombotic events between TPO-RA and
non-TPO-RA treatments (27,28) and their results are similar to those
of the present study. Unlike the previous two systematic reviews,
the present study was the only one that covers all TPO-RA drugs
currently approved for the treatment of ITP (romiplostim,
eltrombopag, avatrombopag and hetrombopag), and the types of
studies included herein were RCTs in adult ITP populations.
Subgroup analyses by the four different drugs, the duration of
medication and randomized double-blinded controlled trials were
also conducted to validate the results.
As a large RCT on the safety and efficacy of
eltrombopag, the RAISE study reported a 2% incidence of thrombosis
when the TPO-RA was used, compared with 0% in the control group
(8). In the EXTEND study, an
open-label extension of the RAISE study, the incidence of
thromboembolic events in the TPO-RA group was reported to be 6% in
total (10). This may indicate that
the duration of medication use is related to the incidence of
thrombosis. Therefore, in the present study, a separate statistical
analysis of studies with treatment durations of >12 weeks (12-52
weeks) was performed. The results also showed no significant
difference in the incidence of thrombotic events between the TPO-RA
and control groups during treatment. However, considering TPO-RA
use often persists in patients with ITP, long-term safety
necessitates further study (29).
Recent studies have shown that plasminogen activator
inhibitor-1 levels increase in patients with ITP receiving TPO-RA
treatment, leading to the formation of more fibrinolytic-resistant
clots (30). Furthermore, their
platelets exhibit increased apoptosis, which leads to increased
exposure to phosphatidylserine, resulting in a larger surface area
for the binding of thrombin precursor complexes (30). These two points indirectly support
the possibility that TPO-RAs increase the incidence of thrombosis.
However, patients with ITP have a potential risk of developing
thrombosis and the probability is much higher than that in the
general population, which may be related to the underlying
mechanisms of the disease (31-33).
In addition, patient age, individual factors (such as hypertension,
diabetes and smoking history) and early treatment of ITP
(glucocorticoids and splenectomy) may also be risk factors for
thrombosis (34,35). These factors were not included in
the exclusion criteria for the 14 RCTs included in the present
study. Therefore, the occurrence of thrombotic events in patients
with ITP should also consider individual factors.
The present study had certain limitations. First,
all study participants were adults. Due to the differences in the
pathogenesis and thrombosis incidence of ITP between adults and
children, the results of this analysis are not applicable to
children. Furthermore, TPO-RAs are second-line agents for ITP,
meaning that most participants had been receiving hormonal therapy
for some time or splenectomy; thus, the number of TPO-RA-related
thrombotic events that occur during treatment may be amplified. In
addition, owing to the lack of peripheral blood platelet count at
the time of thrombotic events in most of the included studies, no
association analysis between platelet counts and thrombotic events
was performed. Finally, publication bias was not evaluated.
In conclusion, there was no significant association
between TPO-RA treatment and thrombotic events. However, caution
should be exercised when prescribing TPO-RAs for patients with a
history of thromboembolic events, given the lack of studies that
include this population in their analysis. Furthermore, the risk of
thrombosis in patients with ITP and elevated platelet counts
remains unknown. Future studies should address these aspects.
Acknowledgements
The authors thank Dr Jing Zhang and Dr Youqing Shen
(Department of Pediatrics, Suqian Hospital Affiliated to Xuzhou
Medical University, Suqian, China) for their helpful guidance
during the writing period.
Funding
Funding: This work was supported by Jiangsu Province Maternal
and Child Health Research Project (grant no. F202153) and the
Suqian Science and Technology Plan Project (grant no. K202118).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NS performed the statistical analysis and wrote the
manuscript. JQ and YJ extracted the data, interpreted data and
wrote the manuscript. JY and RW performed the searches, evaluated
study quality and performed the statistical analysis. HY evaluated
study quality and interpreted the data. SZ and JL designed the
study and reviewed the manuscript. JQ and SZ confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cooper N and Ghanima W: Immune
thrombocytopenia. N Engl J Med. 381:945–955. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Audia S, Mahévas M, Samson M, Godeau B and
Bonnotte B: Pathogenesis of immune thrombocytopenia. Autoimmun Rev.
16:620–632. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bolton-Maggs PHB and George JN: Immune
thrombocytopenia treatment. N Engl J Med. 385:948–950.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sandal R, Mishra K, Jandial A, Sahu KK and
Siddiqui AD: Update on diagnosis and treatment of immune
thrombocytopenia. Expert Rev Clin Pharmacol. 14:553–568.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim DS: Recent advances in treatments of
adult immune thrombocytopenia. Blood Res. 57 (S1):S112–S119.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Syed YY: Hetrombopag: First approval.
Drugs. 81:1581–1585. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rodeghiero F: Is ITP a thrombophilic
disorder? Am J Hematol. 91:39–45. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cheng G, Saleh MN, Marcher C, Vasey S,
Mayer B, Aivado M, Arning M, Stone NL and Bussel JB: Eltrombopag
for management of chronic immune thrombocytopenia (RAISE): A
6-month, randomised, phase 3 study. Lancet. 377:393–402.
2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wong RSM, Saleh MN, Khelif A, Salama A,
Portella MSO, Burgess P and Bussel JB: Safety and efficacy of
long-term treatment of chronic/persistent ITP with eltrombopag:
Final results of the EXTEND study. Blood. 130:2527–2536.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mei H, Xu M, Yuan G, Zhu F, Guo J, Huang
R, Qin J, Lv T, Qin F, Cai H, et al: A multicentre double-blind,
double-dummy, randomised study of recombinant human thrombopoietin
versus eltrombopag in the treatment of immune thrombocytopenia in
Chinese adult patients. Br J Haematol. 195:781–789. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Page MJ, Moher D, Bossuyt PM, Boutron I,
Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: PRISMA 2020 explanation and elaboration: Updated
guidance and exemplars for reporting systematic reviews. BMJ.
372(n160)2021.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Bussel JB, Kuter DJ, George JN, McMillan
R, Aledort LM, Conklin GT, Lichtin AE, Lyons RM, Nieva J, Wasser
JS, et al: AMG 531, a thrombopoiesis-stimulating protein, for
chronic ITP. N Engl J Med. 355:1672–1681. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bussel JB, Cheng G, Saleh MN, Psaila B,
Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, et
al: Eltrombopag for the treatment of chronic idiopathic
thrombocytopenic purpura. N Engl J Med. 357:2237–2247.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kuter DJ, Bussel JB, Lyons RM, Pullarkat
V, Gernsheimer TB, Senecal FM, Aledort LM, George JN, Kessler CM,
Sanz MA, et al: Efficacy of romiplostim in patients with chronic
immune thrombocytopenic purpura: A double-blind randomised
controlled trial. Lancet. 371:395–403. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bussel JB, Provan D, Shamsi T, Cheng G,
Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer
B, et al: Effect of eltrombopag on platelet counts and bleeding
during treatment of chronic idiopathic thrombocytopenic purpura: A
randomised, double-blind, placebo-controlled trial. Lancet.
373:641–648. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kuter DJ, Rummel M, Boccia R, Macik BG,
Pabinger I, Selleslag D, Rodeghiero F, Chong BH, Wang X and Berger
DP: Romiplostim or standard of care in patients with immune
thrombocytopenia. N Engl J Med. 363:1889–1899. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shirasugi Y, Ando K, Miyazaki K, Tomiyama
Y, Okamoto S, Kurokawa M, Kirito K, Yonemura Y, Mori S, Usuki K, et
al: Romiplostim for the treatment of chronic immune
thrombocytopenia in adult Japanese patients: A double-blind,
randomized phase III clinical trial. Int J Hematol. 94:71–80.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tomiyama Y, Miyakawa Y, Okamoto S,
Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S,
Ozaki K, et al: A lower starting dose of eltrombopag is efficacious
in Japanese patients with previously treated chronic immune
thrombocytopenia. J Thromb Haemost. 10:799–806. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang R, Li J, Jin J, Huang M, Yu Z, Xu X,
Zhang X and Hou M: Multicentre, randomised phase III study of the
efficacy and safety of eltrombopag in Chinese patients with chronic
immune thrombocytopenia. Br J Haematol. 176:101–110.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jurczak W, Chojnowski K, Mayer J, Krawczyk
K, Jamieson BD, Tian W and Allen LF: Phase 3 randomised study of
avatrombopag, a novel thrombopoietin receptor agonist for the
treatment of chronic immune thrombocytopenia. Br J Haematol.
183:479–490. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang YT, Liu XF, Chen YF, Fu RF, Liu W,
Zhang L and Yang RC: The efficacy and safety of eltrombopag in
Chinese patients with chronic immune thrombocytopenia. Zhonghua Xue
Ye Xue Za Zhi. 39:32–36. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
22
|
Arnold DM, Heddle NM, Cook RJ, Hsia C,
Blostein M, Jamula E, Sholzberg M, Lin Y, Kassis J, Larratt L, et
al: Perioperative oral eltrombopag versus intravenous
immunoglobulin in patients with immune thrombocytopenia: A
non-inferiority, multicentre, randomised trial. Lancet Haematol.
7:e640–e648. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mei H, Liu X, Li Y, Zhou H, Feng Y, Gao G,
Cheng P, Huang R, Yang L, Hu J, et al: A multicenter, randomized
phase III trial of hetrombopag: A novel thrombopoietin receptor
agonist for the treatment of immune thrombocytopenia. J Hematol
Oncol. 14(37)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koupenova M, Kehrel BE, Corkrey HA and
Freedman JE: Thrombosis and platelets: An update. Eur Heart J.
38:785–791. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mei H, Chen X, Zhou J, Luo J, Shi Q, Liu
J, Wu D, Chen G, Tai Y, Xiong J, et al: Safety and efficacy of
hetrombopag in patients with chronic immune thrombocytopenia: A
single-arm, open-label, multi-center phase 1 study. Ann Transl Med.
10(30)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tjepkema M, Amini S and Schipperus M: Risk
of thrombosis with thrombopoietin receptor agonists for ITP
patients: A systematic review and meta-analysis. Crit Rev Oncol
Hematol. 171(103581)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Catalá-López F, Corrales I, de la
Fuente-Honrubia C, González-Bermejo D, Martín-Serrano G, Montero D
and Saint-Gerons DM: Risk of thromboembolism with thrombopoietin
receptor agonists in adult patients with thrombocytopenia:
Systematic review and meta-analysis of randomized controlled
trials. Med Clin (Barc). 145:511–519. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Birocchi S, Podda GM, Manzoni M, Casazza G
and Cattaneo M: Thrombopoietin receptor agonists for the treatment
of primary immune thrombocytopenia: A meta-analysis and systematic
review. Platelets. 2:216–226. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bussel J, Cooper N, Boccia R, Zaja F and
Newland A: Immune thrombocytopenia. Expert Rev Hematol.
14:1013–1025. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Justo Sanz R, Monzón Manzano E, Fernández
Bello I, Teresa Álvarez Román M, Martín Salces M, Rivas Pollmar MI,
Jiménez Yuste V and Butta NV: Platelet apoptosis and PAI-1 are
involved in the pro-coagulant state of immune thrombocytopaenia
patients treated with thrombopoietin receptor agonists. Thromb
Haemost. 119:645–659. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Machin N, Ragni MV, Comer DM and Yabes JG:
Prevalence and correlates of thrombosis in adults with immune
thrombocytopenia: An NIS study. Thromb Res. 172:80–85.
2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Swan D, Newland A, Rodeghiero F and
Thachil J: Thrombosis in immune thrombocytopenia-current status and
future perspectives. Br J Haematol. 194:822–834. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jain A, Saluja S, Chaudhry S and Gupta DK:
Recurrent arterial and venous thrombosis in chronic immune
thrombocytopenia: Clinical paradox and therapeutic challenges.
Indian J Hematol Blood Transfus. 35:590–592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lakshmanan S and Cuker A: Contemporary
management of primary immune thrombocytopenia in adults. J Thromb
Haemost. 10:1988–1998. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Boyle S, White RH, Brunson A and Wun T:
Splenectomy and the incidence of venous thromboembolism and sepsis
in patients with immune thrombocytopenia. Blood. 121:4782–4790.
2013.PubMed/NCBI View Article : Google Scholar
|