1. Introduction
Aging is a fundamental biological process, yet it
remains one of the most complex and pressing challenges for modern
healthcare and society. The global aging population is increasing
at an unprecedented rate. According to the United Nations, by 2050,
1 in 6 people will be over the age of 65, compared to just 1 in 11
in 2019 (1,2). This demographic shift is accompanied
by a surge in age-related diseases, including cardiovascular
disorders, cancer, neurodegenerative diseases, and diabetes, which
collectively account for >70% of global mortality. The economic
impact is equally staggering, with healthcare costs for elderly
populations expected to markedly escalate, placing immense strain
on public health systems, social security frameworks, and family
resources (3). These statistics
underscore the urgent need for innovative strategies to address the
biological drivers of aging and its associated diseases.
Age reprogramming represents a transformative
frontier in modern medicine, offering ground-breaking solutions to
the multifaceted challenges posed by aging. As the aging process
drives a cascade of cellular and molecular changes that underlie
numerous chronic and degenerative diseases, targeting these
fundamental mechanisms has become a critical focus for advancing
healthcare (4). Age reprogramming
therapies aim to address the biological hallmarks of aging,
including genomic instability, epigenetic alterations,
mitochondrial dysfunction, cellular senescence, and telomere
attrition (5,6). By intervening in these core processes,
these therapies aspire to restore cellular health, enhance
function, and build resilience against age-related decline
(7).
Recent scientific breakthroughs have advanced the
field of age reprogramming from conceptual exploration to
actionable innovation. Techniques such as epigenetic reprogramming,
which modifies gene expression patterns without altering the
underlying DNA sequence, have shown the potential to reverse
cellular aging markers (8,9). The use of Yamanaka factors
[octamer-binding protein 4 (Oct4), SRY-box transcription factor 2
(Sox2), Kruppel-like factor 4 (Klf4), and c-Myc] to reprogram
somatic cells has been particularly ground-breaking, enabling cells
to regain a youthful phenotype while preserving their specialized
identity (6,10). This discovery paves the way for
novel therapeutic strategies to address conditions such as
neurodegenerative diseases, cardiovascular disorders, and cancer
(11,12).
Gene editing is another powerful tool in the age
reprogramming arsenal. Technologies such as CRISPR-Cas9 offer
unparalleled precision in modifying genes associated with aging,
allowing scientists to enhance DNA repair mechanisms, deactivate
genes driving cellular senescence, or correct mutations that
accelerate degeneration (13,14).
These advancements are not only transforming the
scientific understanding of aging but also challenging societal
norms and expectations. By moving beyond traditional approaches
that focus on managing symptoms of age-related diseases, age
reprogramming offers a proactive pathway to extend health span, the
period of life spent in good health (3).
Despite its transformative potential, the journey to
realizing age reprogramming therapies faces significant challenges.
Scientific hurdles include ensuring the safety and efficacy of
these interventions, particularly given the complexity of cellular
and molecular mechanisms involved in aging. Current delivery
systems, such as viral vectors and nanoparticles (15), require substantial refinement to
achieve precision targeting of aging cells without affecting
healthy tissues (16). Furthermore,
the translation of preclinical successes to human applications is
fraught with regulatory and logistical barriers. Rigorous clinical
trials, ethical frameworks, and comprehensive safety evaluations
are essential to build public trust and ensure the responsible
deployment of these therapies (17).
The societal and ethical implications of age
reprogramming are profound, particularly regarding the risk of
unequal access, which could exacerbate health disparities both
within and between nations. The high costs associated with
developing and implementing these therapies may limit their
availability to affluent populations, further widening
socio-economic divides. Additionally, extended health spans will
necessitate coordinated adjustments to workforce dynamics,
retirement policies, and social services to ensure sustainable and
equitable integration (18,19).
The present review analyzes the scientific
innovations underpinning age reprogramming and cellular
rejuvenation, exploring their potential to revolutionize medicine
and redefine human aging. It also examines the multifaceted
challenges (scientific, economic, ethical, and societal) that must
be addressed to ensure these therapies benefit humanity equitably
and sustainably.
2. Current status of cell regeneration
research
Cell regeneration research, particularly in the
context of age reprogramming, has made significant strides in
recent years. This field involves innovative strategies to reverse
or halt the biological processes that lead to aging and age-related
diseases. The current status of this research includes several key
areas of focus: Epigenetic reprogramming, gene editing, stem cell
therapy, and senolytic drugs.
Epigenetic reprogramming refers to the process of
modifying gene expression patterns without altering the underlying
DNA sequence. This ground-breaking approach leverages the inherent
plasticity of the cell to reset its biological state, effectively
reversing markers of cellular aging and restoring a youthful gene
expression profile. Unlike genetic modification, which directly
edits DNA, epigenetic reprogramming operates through mechanisms
such as DNA methylation, histone modifications, and chromatin
remodelling. These changes influence how genes are turned on or
off, offering a non-invasive way to rejuvenate cells and enhance
their functional capacity (20,21).
One of the most promising strategies in epigenetic
reprogramming involves the use of Yamanaka factors, Oct4, Sox2,
Klf4, and c-Myc (6,22). Initially discovered as a method to
induce somatic cells into pluripotent stem cells [induced
pluripotent stem cells (iPSCs)], this technique has since evolved
to allow partial reprogramming. In this process, cells regain
numerous youthful characteristics without losing their specialized
identity (23). This balance is
critical for therapeutic applications, as it ensures that
rejuvenated cells can still perform their original functions. The
ability to restore cellular health without erasing its identity
holds immense potential for treating a variety of age-related
conditions, including neurodegenerative diseases, cardiovascular
disorders, and certain types of cancer.
The work of Bruno et al (24) on the Oct4 regulatory network
highlights the role of chromatin modifiers, ten-eleven
translocation 1 and Jumonji domain-containing protein 2A, in
enhancing reprogramming efficiency and reducing variability. Their
mechanistic model emphasizes that targeted recruitment of these
epigenetic factors significantly impacts the success of cellular
reprogramming. This study not only confirms the importance of
epigenetic regulators but also provides a computational framework
for optimizing reprogramming protocols, paving the way for more
precise and predictable stem cell generation strategies.
The research by Kaemena et al (25) identifies KRAB zinc finger protein
266 (ZFP266) as a major inhibitor of iPSC generation (25). Through CRISPR/Cas9 knockout
screening, they revealed how ZFP266 impedes chromatin opening by
binding to short interspersed nuclear elements (SINEs), suppressing
reprogramming factors such as Oct4, Sox2, and Klf4. This research
underscores the significance of chromatin accessibility in
reprogramming and offers a novel avenue for enhancing iPSC
efficiency by targeting reprogramming roadblocks. Their innovative
approach of converting ZFP266 from an inhibitor to a facilitator by
modifying its co-suppressor domains highlights the potential for
reprogramming-specific protein engineering.
The study by Wang et al (26) on the nucleosome remodeling
deacetylase (NuRD) complex and spalt like transcription factor 4
(Sall4) further enriches our understanding of chromatin remodeling
during early reprogramming (26).
Their findings suggest that Sall4, in collaboration with the NuRD
complex, plays a crucial role in closing open chromatin regions
that encode genes resistant to reprogramming. This chromatin
closing is essential for successful somatic reprogramming.
Importantly, their identification of the Sall4-NuRD axis as a
critical component of reprogramming adds a layer of complexity to
our knowledge of cell fate control, demonstrating the intricate
interplay between transcription factors and chromatin-modifying
complexes.
The investigation of Xie et al (27) into the role of ring finger protein
40 (RNF40) highlights how this histone H2B ubiquitin-protein ligase
facilitates early stages of iPSC reprogramming by promoting
epigenetic modifications such as H2B monoubiquitination (27). The findings of the study which
revealed that RNF40 indirectly regulates enhancer of zeste 2
polycomb repressive complex 2 subunit, a polycomb repressive
complex component, provide a deeper understanding of how bivalent
chromatin marks are resolved during reprogramming. This research
establishes RNF40 as a central mediator of the epigenetic
transitions required for pluripotency, offering insights into
manipulating histone modifications for improved reprogramming
outcomes (27).
A study by Müller et al (28) investigated M-phase phoshoprotein 8
(MPP8), an epigenetic protein crucial for maintaining the
ground-state pluripotency of mouse embryonic stem cells. The
findings demonstrated that MPP8 operates independently of
detectable H3K9me3 levels to repress long interspesed nuclear
element-1 and protect the hypomethylated pluripotent state
(28). This highlights the
versatility and complexity of epigenetic regulators in sustaining
pluripotency and provides new insights into non-canonical pathways
of chromatin regulation in stem cell biology.
Finally, a study by Srinivasan et al
(29) on striatin interacting
protein 2 (Strip2) identified this protein as a regulator of
pluripotency and differentiation by interacting with the
NuRD/TRIM28/HDACs/SETDB1 histone methyltransferase complex
(29). Their discovery that Strip2
binds to DNA motifs akin to KRAB-ZFPs and modulates DNA methylation
adds a new dimension to our understanding of how pluripotency and
differentiation are controlled. By linking Strip2 to both
pluripotency maintenance and differentiation processes, the study
provides a potential target for fine-tuning stem cell behavior in
regenerative medicine (29).
In addition to epigenetic reprogramming using
Yamanaka factors for rejuvenation therapy, CRISPR technology
introduces a revolutionary approach to enhance both the efficiency
and safety of this process (30,31).
While Yamanaka factors have shown immense promise in resetting
cellular aging markers and restoring youthful gene expression
profiles, challenges such as incomplete reprogramming, tumorigenic
risks, and off-target effects remain significant barriers to
clinical application. CRISPR, with its precise genome-editing
capabilities, offers an innovative solution to address these
limitations by enabling highly targeted modifications at the
genetic and epigenetic levels (32).
CRISPR can be also used to fine-tune the expression
of Yamanaka factors, ensuring that their activation occurs in a
controlled and transient manner. This reduces the risk of
over-activation, which can lead to tumorigenesis or loss of
cellular identity. Additionally, CRISPR-based tools such as
CRISPR-dead Cas9 (dCas9) fused with epigenetic modifiers provide
the ability to activate or suppress specific genes without
introducing permanent changes to the DNA sequence (33,34).
This allows researchers to induce rejuvenation effects by
selectively targeting aging-related pathways while minimizing
unintended consequences.
Stem cell therapy involves using stem cells to
repair or replace damaged tissues (35,36).
This approach is being explored for its potential to rejuvenate
tissues and organs affected by aging. By leveraging the
regenerative capacity of stem cells, researchers aim to restore
tissue function and delay the onset of degenerative conditions
(37,38). Previous studies have shown promising
results with stem cell therapy in animal models (41-49).
In clinical settings, these therapies are being
tested for their safety and efficacy. Early-stage clinical trials
have shown promising results, but significant challenges remain,
including refining delivery mechanisms and overcoming regulatory
hurdles (42). For instance, viral
vectors and nanoparticles are being developed to ensure precise
targeting of aging cells without affecting healthy tissues
(43). Recent studies have
emphasized the importance of developing safe and effective delivery
systems for gene editing and stem cell therapies (44,45).
The regeneration research holds significant promise
for transforming our approach to aging and age-related diseases. By
targeting the fundamental biological processes that drive aging,
these therapies have the potential to extend health span and
improve quality of life. However, addressing the scientific,
economic, and ethical challenges associated with these innovations
will be crucial for their successful implementation in both
clinical and experimental settings.
3. Healthcare cost implications
Age reprogramming and longevity therapies could lead
to both increased and decreased healthcare costs, depending on
their efficacy, accessibility, and implementation (46,47).
If successful, longevity therapies could lead to substantial
long-term savings by reducing the financial burden of age-related
diseases. Chronic conditions such as heart disease, dementia,
diabetes, and arthritis are among the leading contributors to
healthcare expenses globally (5,48). By
targeting the underlying mechanisms that drive these conditions,
longevity therapies have the potential to mitigate or even
eliminate the need for costly interventions, surgeries, and
long-term care typically associated with aging populations.
For instance, senolytic drugs designed to
selectively eliminate senescent cells may significantly decrease
the inflammatory environment that underpins multiple age-related
diseases (5,49). By investing in preventative
measures, healthcare systems could shift from a reactive approach,
where resources are spent on treating diseases, to a proactive
strategy focused on maintaining health and wellness, ultimately
leading to a more sustainable financial model.
Conversely, the development and implementation of
age reprogramming therapies are burdened by significant upfront
costs, posing a substantial challenge to their widespread adoption
(46). Advanced technologies such
as CRISPR gene editing, sophisticated stem cell-based treatments,
mitochondrial rejuvenation techniques, and senolytic interventions
are at the cutting edge of biotechnology (50-54).
However, these innovations come with inherent complexities that
drive up costs at every stage of development and deployment.
The research and refinement of such therapies
require access to specialized laboratory facilities equipped with
state-of-the-art technologies. These include high-throughput
sequencing platforms, precision gene-editing tools, and cell
culture systems capable of handling complex biological
manipulations. Moreover, their operation demands a highly skilled
workforce, including molecular biologists, bioengineers, clinical
researchers, and regulatory specialists, whose expertise comes at a
premium (53). The recruitment,
training, and retention of such personnel further add to the
financial burden.
In addition to technological and human resource
demands, the regulatory oversight required to ensure safety and
efficacy significantly escalates costs. These therapies often
involve manipulating fundamental biological mechanisms,
necessitating rigorous preclinical testing, comprehensive clinical
trials (7), and extensive safety
evaluations to meet regulatory standards (17). Compliance with these regulations
requires long timelines, substantial financial investment, and
meticulous documentation, further straining budgets (54).
Another major contributor to high costs is the
manufacturing and delivery process. Producing personalized
therapies, such as gene-edited cells or tailored stem cell
treatments, often involves intricate and time-consuming protocols
that are challenging to scale. Technologies such as viral vectors
or nanoparticles for precise delivery of therapeutic agents also
require optimization to ensure specificity, stability, and minimal
off-target effects (53). These
production challenges lead to high per-unit costs, particularly in
the early stages when economies of scale have not been
achieved.
All these factors create financial barriers that
make these therapies initially accessible only to affluent
individuals or well-resourced healthcare systems (3). Without targeted efforts to reduce
costs such as investing in scalable manufacturing technologies,
developing universal treatment platforms, or fostering
public-private partnerships, the benefits of age reprogramming risk
being confined to a privileged minority. Addressing these economic
hurdles will be critical to ensuring that these ground-breaking
innovations achieve their full potential to improve health and
extend longevity for all.
As a result, healthcare systems may face increased
economic strain, particularly if these therapies are initially
available only to a select segment of the population or if they
require significant out-of-pocket expenses for patients.
Governments and healthcare providers will need to carefully weigh
the potential long-term savings from reduced disease burden against
the immediate financial implications of implementing these new
therapies (55). This could lead to
difficult decisions about resource allocation, possibly diverting
funds from other critical healthcare areas. A detailed picture of
the healthcare cost implications associated with age reprogramming
therapies, linking each aspect to its financial impact on
healthcare systems is provided in Table
I.
 | Table IHealthcare cost implications
associated with age reprogramming therapies. |
Table I
Healthcare cost implications
associated with age reprogramming therapies.
| Aspect | Description | Implications | Healthcare cost
implications | (Refs.) |
|---|
| Potential for cost
savings | Targeting
underlying mechanisms of age-related diseases (such as heart
disease, dementia, diabetes) to reduce reliance on interventions,
surgeries, and long-term care. | Shifts healthcare
focus from reactive treatment to preventative wellness strategies,
potentially saving billions globally. | Long-term
reductions in healthcare expenses associated with managing chronic
and age-related diseases. | (3,5) |
| Senolytic
drugs | Designed to clear
senescent cells, reducing systemic inflammation and slowing the
progression of multiple age-related conditions. | Promotes healthier
aging populations, lowering healthcare costs tied to treating
inflammation-driven diseases such as arthritis and cardiovascular
disorders. | Cost savings from
reduced treatment requirements for inflammation-related conditions
and improved patient outcomes. | (5,49) |
| High upfront
costs | Advanced
technologies (such as CRISPR, stem cell therapies, mitochondrial
rejuvenation) require specialized equipment, expert personnel, and
rigorous research processes. | Limits early
adoption to affluent individuals or nations, creating barriers to
widespread access. | Significant initial
investments for research, development, and infrastructure setup,
delaying cost-efficiency benefits. | (46) |
| Technological
demands | Reliance on
cutting-edge tools (such as gene-editing platforms, cell culture
systems) and highly skilled professionals (molecular biologists,
bioengineers, clinicians). | Increases
operational expenses for research and development, delaying
widespread availability. | High operational
costs drive up therapy prices, affecting affordability and
scalability. | (53) |
| Regulatory
compliance | Extensive clinical
trials, safety evaluations, and adherence to strict regulatory
frameworks to ensure efficacy and public trust. | Adds financial and
time burdens, requiring long-term investments to navigate complex
approval processes. | Raises the overall
cost of bringing therapies to market, impacting initial
accessibility and affordability. | (7,17) |
| Manufacturing
challenges | Personalized
therapies including gene-edited cells and stem cell treatments
involve intricate, time-consuming protocols and costly delivery
systems (for example, viral vectors). | Scaling production
while maintaining affordability is difficult, leading to high
per-unit costs and limited accessibility in early stages. | Increases per-unit
costs, particularly in early phases, until scalable and
cost-efficient production methods are developed. | (53) |
| Healthcare system
strain | High initial costs
for implementing therapies could divert resources from other
critical healthcare areas or require significant out-of-pocket
expenses for patients. | Risks overburdening
publicly funded systems and creating inequities unless offset by
future savings from reduced disease prevalence. | Short-term
budgetary strain on healthcare systems, requiring careful
allocation of resources. | (55) |
| Insurance and
funding dilemmas | Uncertainty over
whether longevity therapies should be covered by public health
insurance or considered elective treatments requiring private
funding. | Raises ethical and
economic questions about equitable access and the prioritization of
healthcare funding for innovative therapies. | Potential increases
in insurance premiums and out-of-pocket costs for patients,
affecting affordability and coverage scope. | (56) |
| Equity and
accessibility | Without
interventions such as public-private partnerships, universal
platforms, and scalable production, access may remain restricted to
affluent populations or nations. | Exacerbates health
disparities globally, creating a ‘longevity divide’ that leaves
underprivileged groups without access to transformative
therapies. | Limits the broader
economic benefits of healthier populations if access remains
exclusive to certain socio-economic groups. | (57) |
| Ethical and policy
frameworks | Policymakers must
address questions concerning access, affordability, and balancing
resources between innovative therapies and essential healthcare
needs. | Equitable
distribution frameworks are essential to ensure that these
therapies benefit all socio-economic groups and reduce societal
divides. | Investment in
inclusive policies could balance short-term costs with long-term
societal and economic benefits of healthier aging populations. | (17) |
| Long-term
impact | Potential to lower
costs by preventing age-related diseases vs. high initial
investments for research, production, and infrastructure. | A sustainable
balance is needed to offset short-term financial strain with
long-term savings, creating a healthier aging population and
reducing economic burdens. | Significant
potential for long-term healthcare savings by reducing treatment
needs for chronic diseases and improving workforce
productivity. | (19) |
The introduction of longevity therapies will also
create new pressures on insurance providers and publicly funded
healthcare systems (56). Key
questions will arise regarding the coverage of these treatments:
Should longevity therapies be classified as essential healthcare
services, warranting funding through public health insurance, or
should they be considered elective procedures, requiring
individuals to cover costs out-of-pocket?
The resolution of these questions will necessitate a
careful examination of the balance between personal and collective
responsibility in healthcare financing. As these therapies become
more widely available, the ethical implications of access and
equity in healthcare will come to the forefront. Policymakers will
need to consider the potential for exacerbating health disparities
if only wealthier individuals can afford these advanced treatments,
while also addressing the need for inclusive frameworks that ensure
equitable access to innovative healthcare solutions (57).
While age reprogramming and longevity therapies hold
the promise of reducing long-term healthcare costs by preventing
age-related diseases, their development and implementation come
with significant upfront expenses and potential challenges for
insurance coverage and equity (58). A balanced approach that considers
both immediate costs and long-term savings will be essential for
navigating this evolving landscape in healthcare.
4. Accessibility and inequality in longevity
therapies
The accessibility of longevity therapies presents a
significant challenge, with the potential to exacerbate existing
social and economic inequalities. If these treatments remain
available only to a privileged segment of the population, they
could deepen disparities in healthcare access, economic
opportunities, and overall quality of life. The high cost of
developing and administering age-reprogramming therapies is likely
to restrict their initial availability to wealthier individuals and
nations, creating a scenario where only the affluent benefit from
extended health spans and prolonged productivity. This raises
ethical concerns about the equitable distribution of healthcare
resources and the societal consequences of a growing gap between
those who can afford longevity interventions and those who
cannot.
On a global scale, disparities in access to
longevity therapies may widen the health divide between high-income
and low-income countries. Numerous developing nations already face
significant healthcare challenges, including limited resources,
inadequate infrastructure, and competing priorities such as
infectious disease management and maternal health. Integrating
advanced longevity treatments into these healthcare systems may
prove financially and logistically overwhelming, further
marginalizing populations that already struggle with basic medical
care. Without targeted efforts to address these disparities, a
‘longevity divide’ could emerge, where wealthier nations reap the
benefits of life-extending therapies while less affluent regions
remain burdened by preventable age-related diseases.
The equitable distribution of healthcare resources
is a fundamental ethical principle that could come under scrutiny
with the introduction of longevity therapies (55). The ethical debate surrounding
longevity therapies extends beyond affordability to the broader
issue of healthcare prioritization. Policymakers must navigate
difficult decisions about resource allocation, balancing
investments in extending lifespan with the pressing need to improve
primary healthcare services. In low- and middle-income countries,
where healthcare funding is already constrained, prioritizing
longevity treatments over fundamental health needs could widen
inequities in care. A thoughtful approach is required to ensure
that advancements in aging research complement, rather than
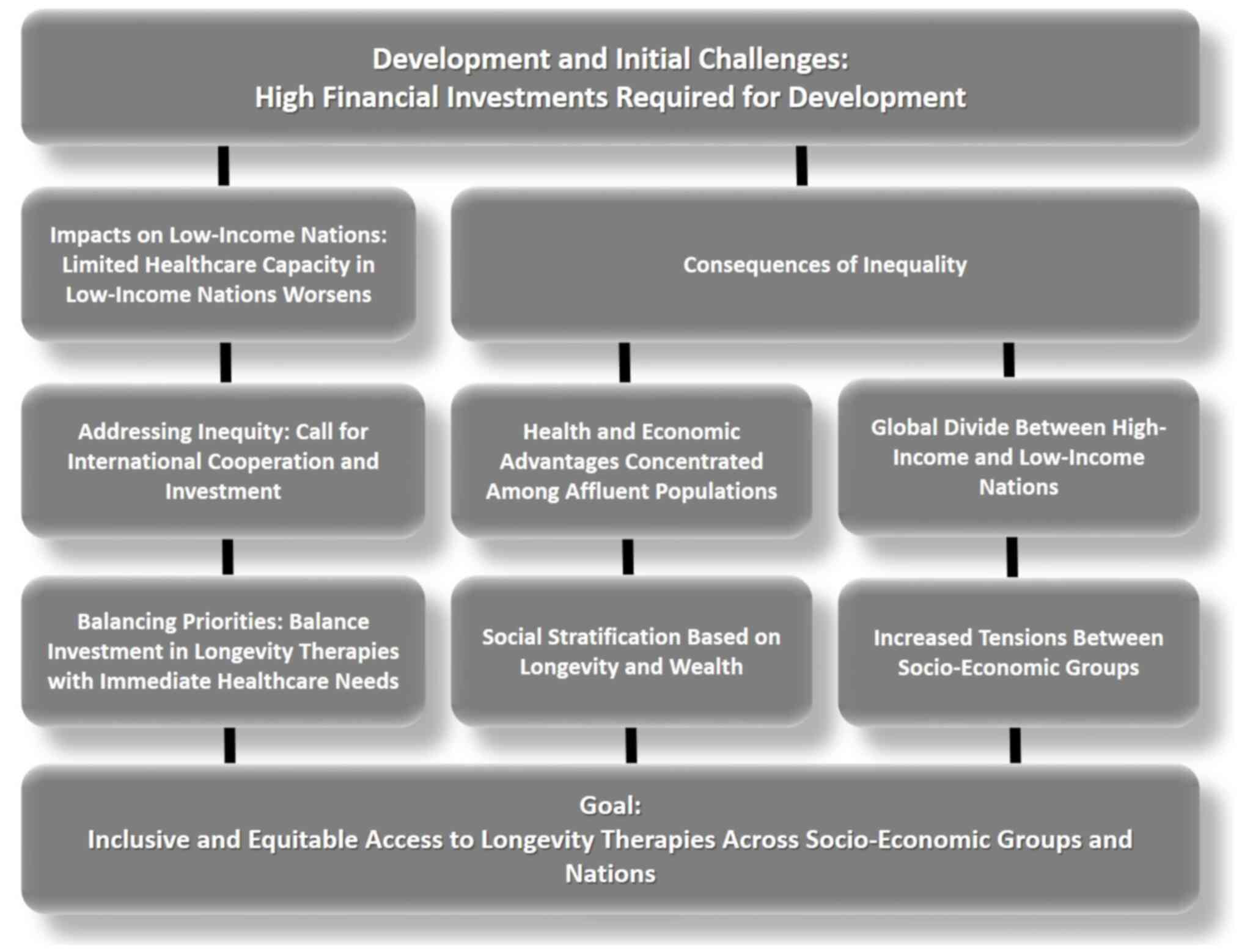
overshadow, essential healthcare initiatives (Fig. 1).
Economic disparities also shape access within
individual nations. Even in wealthier countries with strong
healthcare systems, longevity therapies may initially be restricted
to those who can afford them, creating a tiered healthcare
landscape where extended health and vitality become privileges of
the wealthy. This could reinforce social stratification, as those
with access to these therapies gain prolonged economic and social
advantages, further entrenching inequalities in wealth, employment,
and quality of life. Middle-income nations face a unique challenge,
possessing some capacity to adopt longevity therapies but often at
the cost of diverting resources from other critical healthcare
areas. The long-term consequences of such trade-offs require
careful consideration to prevent the deepening of existing health
inequities (55,59).
Moreover, the disparities also influence the global
adoption of longevity therapies in healthcare systems, economic
resources, regulatory frameworks, and cultural norms across
different countries (Table II).
Cultural and societal factors further influence the acceptance and
implementation of longevity therapies. In some societies, aging is
viewed as a natural process, and efforts to extend lifespan may be
met with skepticism or resistance. Conversely, cultures that
prioritize youthfulness and productivity may be more receptive to
these treatments, influencing policy decisions and funding
allocations. These differences highlight the need for culturally
sensitive approaches to longevity research and implementation,
ensuring that interventions align with societal values and ethical
considerations.
 | Table IIGlobal disparities in healthcare and
implications for longevity therapies. |
Table II
Global disparities in healthcare and
implications for longevity therapies.
| Metric | High-income
nations | Low-income
nations | Implications for
longevity therapies |
|---|
| Healthcare
expenditure per capita | 5,000-12,000 USD
(for example, USA, Germany, Japan) | 50-150 USD (for
example, Sub-Saharan Africa, South Asia) | High costs of
longevity therapies are prohibitive in low-income settings,
limiting adoption and innovation. |
| Percentage of GDP
spent on healthcare | 10-17% | 3-6% | Lower budget
allocation restricts funding for anti-aging research and access to
therapies. |
| Access to advanced
biotechnologies | Widely available
(including CRISPR, stem cell clinical trials) | Rare or
non-existent | Lack of access to
cutting-edge biotechnologies perpetuates inequality in health
outcomes. |
| Life
expectancy | 80-85 years | 55-65 years | Existing gaps in
longevity could widen further without equitable deployment of
anti-aging interventions. |
| Availability of
trained healthcare workers | 30-50 per 10,000
individuals | 1-5 per 10,000
individuals | Insufficient
personnel in low-income nations limits the capacity to deliver
complex treatments. |
| Healthcare
infrastructure | Advanced hospitals,
research centres, labs | Basic care
facilities with limited diagnostic tools | Complex therapies
requiring advanced infrastructure remain inaccessible in
resource-limited settings. |
| Public spending on
aging research | 1 billion USD+
annually (including NIH, EU funding programs) | <10 million USD
annually | Low investment
prevents development of localized therapies and limits
participation in global research collaborations. |
| Health equity index
(WHO) | 0.8-0.95 | 0.2-0.4 | Inequitable
healthcare systems hinder the fair distribution of longevity
benefits. |
| Availability of
anti-aging therapies | Widespread in
clinical trials and early applications | Rare or
experimental at best | Limited access
results in a ‘longevity divide’, concentrating benefits in affluent
regions. |
Addressing these problems requires a multifaceted
approach. Public-private partnerships, tiered pricing models, and
subsidized healthcare programs could help make longevity therapies
more widely accessible. Additionally, fostering research and
innovation within middle- and low-income countries could reduce
dependence on external providers and ensure that treatments are
tailored to local healthcare needs. International collaboration and
policy frameworks promoting equitable distribution will be
essential in preventing longevity therapies from becoming tools of
exclusivity.
Ultimately, ensuring fair access to longevity
therapies is not just a matter of medical innovation but of ethical
and social responsibility. Without proactive measures to bridge the
gap, these treatments could reinforce existing inequalities rather
than serving as tools for global health improvement. A
comprehensive strategy that integrates affordability,
infrastructure development, and ethical healthcare policies is
necessary to ensure that longevity advancements benefit all
segments of society, regardless of economic status or geographic
location.
5. Ethical considerations in genetic
interventions for longevity
The prospect of employing genetic interventions to
enhance longevity brings forth a complex landscape of ethical
questions that must be carefully navigated. These questions
encompass the scope of genetic modifications, their safety, and the
broader social implications that may arise from their
implementation (60). As we stand
on the brink of revolutionary advancements in genetic therapies, it
is imperative to consider the ethical ramifications that accompany
these technologies.
One of the most ethically contentious aspects of
longevity therapy is the potential for germline editing, altering
genes in such a way that these changes can be inherited by future
generations. Unlike somatic cell editing, which affects only the
individual receiving the treatment, germline editing would pass
genetic modifications to offspring, raising profound ethical
concerns regarding consent, unintended consequences, and the
long-term impact on human evolution (61).
While germline editing could theoretically shield
future generations from age-related diseases, it carries
significant risks of unforeseen genetic complications, which may
not manifest until many years later (62). The inability to obtain consent from
future generations introduces a moral dilemma: Are we justified in
making irreversible changes to the human genome without the
approval of those who will be affected? Furthermore, the potential
for germline editing to create a form of genetic determinism raises
additional ethical concerns, as the implications of modifying
traits related to health, longevity, and possibly even intelligence
or physical ability must be considered.
Longevity therapies challenge societal notions of
natural aging and what it means to grow old. Some ethicists contend
that interfering with the biological aging process represents a
fundamental shift in healthcare thus transforming it from a tool
for treating diseases into an enhancement technology aimed at
extending life beyond natural limits (62). This distinction is crucial, as it
provokes questions about the ethical implications of prioritizing
treatments that extend life in ways that deviate from biological
norms. The summary on the ethical considerations and solutions in
genetic interventions for longevity and rejuvenation therapy are
provided in Table III.
 | Table IIIEthical considerations and solutions
in genetic interventions for longevity. |
Table III
Ethical considerations and solutions
in genetic interventions for longevity.
| Aspect | Description | Ethical
concerns | Proposed
solutions |
|---|
| Scope of genetic
modifications | Genetic
interventions, including somatic and germline editing, aim to
address aging by modifying biological processes. | Risks of unintended
consequences, unforeseen genetic complications, and irreversible
impacts on human evolution. | Conduct rigorous
safety testing, establish robust ethical oversight, and involve
public discourse to ensure responsible applications. |
| Germline
editing | Alters inheritable
genes to potentially prevent age-related diseases in future
generations. | Raises concerns
about consent from future generations, unforeseen long-term
consequences, and risks of genetic determinism (modifying
non-health traits). | Limit germline
editing to essential health interventions; develop international
guidelines and enforce strict regulatory controls. |
| Redefinition of
natural aging | Longevity therapies
challenge aging as a natural process, treating it as a condition
that can be managed or reversed. | Raises questions
about disrupting biological norms, redefining aging, and
prioritizing enhancements over natural health trajectories. | Promote ethical
discussions about the societal perception of aging and ensure
alignment with cultural and moral values. |
| Social
stratification | Access to longevity
therapies may create a privileged class with significant health and
lifespan advantages. | Risk of widening
socio-economic divides, reinforcing inequities, and fostering
societal tensions between those who can and cannot afford these
therapies. | Implement equitable
pricing models, public-private partnerships, and subsidies to
ensure broader access for underserved populations. |
| Informed consent
and autonomy | Ensures individuals
can decide whether to undergo therapies with full knowledge of
risks and benefits. | Challenges in
communicating complex risks, societal pressures to conform, and
potential stigma for those who choose to opt out. | Establish
transparent consent processes, enhance public education, and
safeguard individual rights to refuse therapies without
discrimination. |
| Environmental
impacts | Extended lifespans
may increase demand for resources such as housing, food, and
energy, intensifying ecological strain. | Risk of
overburdening environmental resources, contributing to depletion,
and exacerbating global inequalities in access to essentials. | Integrate
sustainability into longevity strategies, promote eco-friendly
practices, and ensure resource-conscious planning in policy
design. |
| Societal
implications | A growing
population of healthy, long-lived individuals could disrupt
workforce dynamics and retirement systems. | Strains on
healthcare, pensions, and social services; risks of generational
inequalities and conflicts over resource allocation. | Adapt workforce
structures, introduce flexible retirement policies, and ensure fair
distribution of resources to maintain intergenerational
equity. |
| Sustainability | Balancing the
benefits of extended healthspans with societal and ecological
demands. | Ethical dilemmas of
extending life without adequately addressing resource needs and
long-term environmental impacts. | Align longevity
advancements with sustainability goals, ensuring therapies support
global ecological and social well-being. |
| Justice and
fairness | Ensures fair access
to therapies across socio-economic groups and nations. | Risk of exclusive
access for affluent populations or countries, leaving marginalized
groups without benefits of longevity therapies. | Establish global
frameworks for equitable access, incentivize affordable production,
and support low-income nations with healthcare infrastructure. |
| Ethical
governance | Development of
transparent and inclusive guidelines to regulate genetic
interventions for longevity. | Risks of misuse,
inequities, and inadequate accountability in deploying advanced
therapies. | Form
interdisciplinary coalitions to set ethical standards, ensure
global oversight, and continuously evaluate societal impacts. |
The potential for creating a biologically privileged
class raises concerns about social fragmentation. If only certain
segments of the population can afford or access longevity
therapies, a divide may emerge between those who undergo age
reprogramming and those who do not. This disparity could lead to a
society where health and longevity become markers of privilege,
further entrenching existing social inequalities. The ethical
implications of creating enhanced individuals must be critically
examined, particularly in terms of fairness and justice within
society.
A critical ethical consideration in the realm of
longevity therapies is the issue of informed consent and individual
autonomy. Individuals must retain the right to decide whether or
not to undergo these treatments, particularly given the associated
risks and unknowns, especially during the early stages of these
therapies. Upholding ethical standards requires ensuring that
patients are fully informed about potential risks, including side
effects and long-term consequences that may not yet be understood
(58).
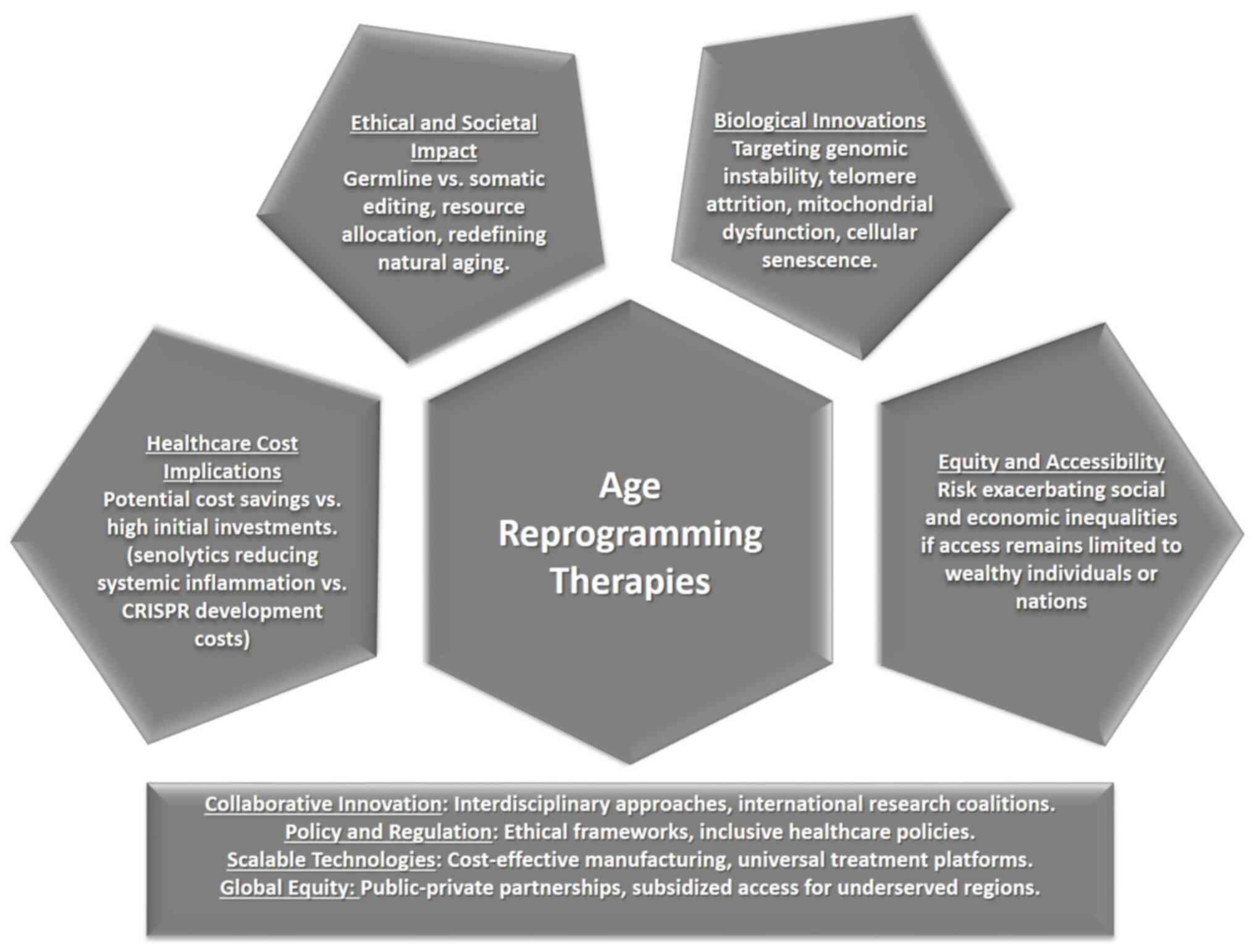
Moreover, as longevity therapies become more
commonplace, society must respect the rights of individuals to
decline these treatments without facing societal pressure, stigma,
or discrimination (Fig. 2).
Ensuring that personal autonomy is preserved in the face of
potential societal norms promoting longevity as a desirable goal is
essential for maintaining ethical integrity in healthcare. The
ethical framework surrounding informed consent must adapt to
address the nuances of these therapies, ensuring that individuals
make choices based on comprehensive information and without
coercion (62).
The extension of human health span and potential
lifespan raises crucial questions regarding the environmental and
societal impacts of a growing population of healthy, long-lived
individuals (63). While a
healthier elderly population could alleviate some economic burdens
on healthcare systems, it may simultaneously exert increased
pressures on essential resources such as housing, employment, and
social services.
Moreover, longer lifespans could have significant
environmental implications. Extended consumption patterns could
contribute to resource depletion and environmental degradation,
necessitating thoughtful planning to mitigate these effects. As we
consider the societal changes that accompany a population with an
extended health span, it is vital to ensure that longevity
therapies do not inadvertently create new challenges. Integrating
sustainability into the discussion of longevity therapies will be
essential to fostering an equitable and healthy future for all
individuals (60,64).
The genetic interventions introduce a host of
ethical considerations that must be meticulously examined (65,66).
Addressing the complexities of germline editing, the redefinition
of natural aging, informed consent, and the broader societal
impacts will be critical to navigating the ethical landscape of
these emerging technologies (58).
The complex ethical dimensions of gene intervention for aging and
cellular rejuvenation therapies, emphasizing the need for careful
regulation, informed consent, and equitable access to these
ground-breaking but potentially controversial treatments is
illustrated in Table IV.
 | Table IVEthical issues and impact of gene
intervention in rejuvenation therapy on individuals. |
Table IV
Ethical issues and impact of gene
intervention in rejuvenation therapy on individuals.
| Ethical Issues | Description | Potential impacts
on individuals | Solutions |
|---|
| Health risks | Risks related to
the safety and unintended effects of genetic interventions. | On-target risks:
Potential tumorigenesis from prolonged activation of Yamanaka
factors. Off-target risks: CRISPR may unintentionally alter genes
unrelated to aging, leading to unpredictable health issues. | Need in extensive
preclinical testing and phased clinical trials to ensure safety.
Implement robust monitoring systems for long-term follow-up. |
| Equity of
access | Unequal
availability of therapies based on socioeconomic status or
geographic location. | Wealthier
individuals may benefit disproportionately, leading to a healthcare
gap. | Develop
public-private partnerships to subsidize treatments for low-income
populations. |
| | | Limited access for
underprivileged populations could exacerbate inequalities. | Prioritize global
collaborations to ensure equitable distribution. |
| Autonomy
rights | The right of
individuals to make informed decisions about undergoing genetic
intervention. | Individuals may
feel pressured to adopt therapies due to societal or familial
expectations. | Create clear
educational campaigns to explain risks, benefits, and
alternatives. |
| | | Lack of adequate
information could hinder fully informed consent. | Ensure counselling
services are available to address societal or familial
pressures. |
| Informed
consent | The necessity of
clear and thorough communication about risks and benefits to
participants. | Complex scientific
details may confuse patients, leading to uninformed decisions. | Simplify consent
processes with layperson-friendly explanations. |
| | | Ethical concerns
arise if therapies are applied without clear, long-term safety
data. | Require third-party
oversight to ensure consent forms are properly reviewed and
understood. |
| Intergenerational
impacts | Genetic changes
that may unintentionally affect future generations if germline
cells are altered. | Children of treated
individuals might inherit unintended modifications. | Prohibit germline
editing until safety is proven and public consensus is
reached. |
| | | Raises ethical
questions about altering human biology without consent from future
generations. | Focus on somatic
cell therapies that do not affect future generations. |
| Psychological
effects | Potential mental
and social impacts of rejuvenation therapies. | Increased pressure
to maintain ‘youthful’ traits could cause psychological
stress. | Foster societal
acceptance of aging as a natural process, even with rejuvenation
options available. |
| | | Fear of missing out
on treatments might lead to societal stigmatization of untreated
individuals. | Provide
psychological support and counselling for individuals undergoing
treatments. |
| Cultural and
societal norms | Challenges to
cultural beliefs about aging and mortality. | May disrupt
traditional views on aging as a natural life process. | Engage cultural and
religious leaders in discussions to promote ethical use of
therapies. |
| | | Could exacerbate
ageism if older individuals are pressured to ‘rejuvenate’ to stay
relevant. | Need to advocate
for inclusive narratives about aging and societal value. |
| Economic
consequences | High costs
associated with developing and administering advanced genetic
therapies. | Risk of creating
‘longevity elites’ who can afford therapies. | Implementation
tiered pricing models to make treatments affordable. |
| Potential for
misuse | Risk of therapies
being used for non-therapeutic or enhancement purposes. | Could lead to the
pursuit of cosmetic or performance enhancements rather than
addressing legitimate health concerns. | Enforce regulations
restricting therapies to medically necessary applications. |
| | | | Monitor and
penalize unethical marketing or unauthorized applications. |
| Long-term
safety | Lack of data on the
long-term effects of genetic interventions. | Unknown health
consequences might emerge decades after treatment. | Requires ongoing
longitudinal studies to track the effects of therapies over
time. |
| | | Individuals may
experience unforeseen complications as they age. | Establish
transparent reporting systems for adverse effects. |
| Personal
identity | Concerns about how
altering aging trajectories could affect an individual’s sense of
self. | Reprogramming
cellular age might alter how individuals perceive themselves in
terms of their life stage and societal roles. | Counselling to help
individuals adjust to the psychological and social impacts of
therapy. |
Steps toward international ethical alignment could
include creating a globally recognized body, akin to the World
Health Organization (WHO), to oversee genetic interventions and
longevity therapies. This body could develop universal guidelines
defining permissible applications of germline editing, enforce
strict regulatory standards, and foster cooperation between
countries. Agreements such as the UNESCO Universal Declaration on
Bioethics and Human Rights could serve as a foundation for
establishing these principles (67,68).
Additionally, such a framework should emphasize equitable access to
these therapies, ensuring that advancements benefit global
populations rather than a privileged few.
6. Future directions in age reprogramming
research
Future research in age reprogramming holds the
promise of addressing critical challenges in aging and advancing
healthcare into a new era. One of the most promising areas of
investigation is targeted mitochondrial editing. Mitochondrial
dysfunction, a hallmark of aging, contributes to reduced energy
production, oxidative stress, and the progression of age-related
diseases. Precise editing of mitochondrial DNA using advanced
CRISPR-based tools, such as mitoCRISPR, could correct mutations,
restore energy metabolism, and reduce oxidative damage in aging
tissues (69). Combining
mitochondrial editing with therapies that promote mitochondrial
biogenesis and balanced fission-fusion cycles could offer a
comprehensive approach to improving cellular health and longevity
(70,71).
Another critical avenue of research is stem cell
rejuvenation for neurodegenerative conditions, which pose
significant challenges in aging populations. Neurodegenerative
diseases including Alzheimer's and Parkinson's could be addressed
by leveraging reprogrammed neural stem cells derived through
techniques such as partial reprogramming or iPSCs (72,73).
These cells have the potential to restore neural plasticity and
replenish lost or damaged neurons, offering hope for regeneration
in affected brain regions. Pairing these approaches with
extracellular vesicle-based delivery systems may enhance
therapeutic outcomes by improving the integration of stem cells and
reducing inflammation in neural tissues.
To optimize these therapies, the development of
reliable and minimally invasive biomarkers is essential. Biomarkers
that reflect biological age and therapeutic efficacy, such as
circulating DNA methylation patterns, telomere length, or
mitochondrial health indicators, could enable precise monitoring of
therapeutic progress. Artificial intelligence (AI)-driven analyses
of multi-omics datasets, encompassing genomics, proteomics, and
metabolomics, could accelerate the discovery and validation of such
biomarkers, ensuring their effective application in clinical
settings.
Epigenetic reprogramming tools also remain a
cornerstone of age-reprogramming research. Yamanaka factors have
shown great potential for reversing cellular aging, but future
efforts should focus on refining these tools to achieve controlled
and reversible reprogramming without inducing tumorigenicity or
loss of cell identity (74,75). Transient delivery of reprogramming
factors through advanced techniques, such as nanoparticles or
gene-editing technologies, could improve safety and precision,
paving the way for broader clinical applications (6).
Another promising direction involves gene therapies
targeting immune senescence. The aging immune system, which becomes
less effective at fighting infections and more prone to chronic
inflammation, could benefit from interventions aimed at
rejuvenating immune function. For example, modifying pathways
involved in T-cell regeneration or reducing pro-inflammatory
cytokines could enhance immune resilience in older adults (76). Combining such strategies with
senolytics or senomorphics could further improve outcomes by
reducing systemic inflammation (77,78).
The integration of these advances into healthcare
systems represents a paradigm shift from reactive disease
management to proactive health preservation. Age-reprogramming
therapies could form the backbone of preventive medicine, focusing
on mitigating age-related decline before clinical symptoms emerge.
Early intervention programs that screen for biological aging
markers during routine health check-ups could enable timely
therapeutic applications, such as senolytics or epigenetic
reprogramming, to delay the onset of age-related diseases (79). Personalized medicine, driven by
genetic, epigenetic, and lifestyle data, could ensure that
therapies are tailored to individual patients for maximum efficacy
(80). Additionally, longitudinal
care models, incorporating periodic interventions to maintain
cellular and tissue health over decades, could become standard
practice.
AI will play a pivotal role in this transformation
by customizing therapies, predicting patient responses, and
managing treatment schedules. AI integration could make
age-reprogramming therapies more accessible and efficient,
streamlining their adoption into mainstream healthcare. By bridging
these innovative approaches with preventive and personalized care,
age-reprogramming therapies have the potential to redefine
healthcare, shifting aging from an inevitable decline to a
manageable and reversible process. With interdisciplinary
collaboration and continued technological advancements, these
therapies could become a cornerstone of 21st-century medicine,
enhancing both lifespan and health span while fundamentally
reshaping our approach to aging.
7. Conclusions
Age reprogramming and cellular rejuvenation
therapies represent a transformative frontier in medicine, aiming
to extend health spans by addressing the biological roots of aging.
These therapies promise to reduce age-related diseases and redefine
societal views on aging, enabling longer, healthier lives. However,
realizing their potential requires overcoming key challenges.
International collaboration is essential to harmonize regulatory
frameworks, streamline clinical trials, and establish universal
safety standards to build public trust. High costs remain a
barrier, necessitating investments in scalable manufacturing,
standardized gene-editing platforms, and cost-effective delivery
systems to ensure affordability.
Equity must be prioritized to prevent widening
global health disparities. Public-private partnerships, tiered
pricing, and subsidized funding can improve accessibility,
particularly in low- and middle-income countries. At the same time,
interdisciplinary research is crucial to enhance the safety,
efficacy, and precision of these therapies, focusing on
advancements in targeted delivery, biomarkers, and personalized
treatments. By combining scientific innovation, ethical governance,
and equitable access, age reprogramming therapies can redefine
healthcare, transforming aging from an inevitable decline into a
manageable and reversible process.
Acknowledgements
Not applicable.
Funding
Funding: The study was supported by program-targeted funding
from the Ministry of Science and Higher Education of the Republic
of Kazakhstan ‘Prolonging healthy longevity: using new technologies
and machine learning to control reversal of aging in old cells’
(grant no. BR24992900).
Availability of data and materials
Not applicable.
Authors' contribution
TS conceived the study and contributed to the
writing of the original draft, as well as the methodology and
formal analysis. PBS wrote, reviewed, edited and supervised the
study, and contributed to project administration. Both authors read
and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ayalon L, Dolberg P, Mikulionienė S,
Perek-Białas J, Rapolienė G, Stypinska J, Willińska M and de la
Fuente-Núñez V: A systematic review of existing ageism scales.
Ageing Res Rev. 54(100919)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Feng Z: Global convergence: Aging and
long-term care policy challenges in the developing world. J Aging
Soc Policy. 31:291–297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mohd Tohit NF and Haque M: Gerontology in
public health: A scoping review of current perspectives and
interventions. Cureus. 16(e65896)2024.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wagner W: The link between epigenetic
clocks for aging and senescence. Front Genet.
10(303)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Alibhai FJ and Li RK: Rejuvenation of the
aging heart: Molecular determinants and applications. Can J
Cardiol. 40:1394–1411. 2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Iordache F, Petcu ACI and Alexandru DM:
Genetic and epigenetic interactions involved in senescence of stem
cells. Int J Mol Sci. 25(9708)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guarente L, Sinclair DA and Kroemer G:
Human trials exploring anti-aging medicines. Cell Metab.
36:354–376. 2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Olova NN: Epigenetic rejuvenation: A
journey backwards towards an epigenomic ground state. Epigenomics.
17:1–3. 2025.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Park K, Jeon MC, Lee D, Kim JI and Im SW:
Genetic and epigenetic alterations in aging and rejuvenation of
human. Mol Cells. 47(100137)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen Y, Li M and Wu Y: The occurrence and
development of induced pluripotent stem cells. Front Genet.
15(1389558)2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang HS, Zheng YX, Bai X, He XY and Wang
TH: Application prospects of urine-derived stem cells in
neurological and musculoskeletal diseases. World J Orthop.
15:918–931. 2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jiang B, Zhang W, Zhang X and Sun Y:
Targeting senescent cells to reshape the tumor microenvironment and
improve anticancer efficacy. Semin Cancer Biol. 101:58–73.
2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ruetz TJ, Pogson AN, Kashiwagi CM, Gagnon
SD, Morton B, Sun ED, Na J, Yeo RW, Leeman DS, Morgens DW, et al:
CRISPR-Cas9 screens reveal regulators of ageing in neural stem
cells. Nature. 634:1150–1159. 2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Falah G, Sharvit L and Atzmon G:
CRISPR-Cas9 mediated d3GHR knockout in HEK293 cells: Revealing the
longevity associated isoform stress resilience. Exp Gerontol.
196(112586)2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Somers A, Jean JC, Sommer CA, Omari A,
Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, et al:
Generation of transgene-free lung disease-specific human induced
pluripotent stem cells using a single excisable lentiviral stem
cell cassette. Stem Cells. 28:1728–1740. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Carter JL, Halmai JANM and Fink KD: The
iNs and outs of direct reprogramming to induced neurons. Front
Genome Ed. 2(7)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Esteves F, Brito D, Rajado AT, Silva N,
Apolónio J, Roberto VP, Araújo I, Nóbrega C, Castelo-Branco P and
Bragança J: ALFA Score Consortium. Reprogramming iPSCs to study
age-related diseases: Models, therapeutics, and clinical trials.
Mech Ageing Dev. 214(111854)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Towns CR: The science and ethics of
cell-based therapies for Parkinson's disease. Parkinsonism Relat
Disord. 34:1–6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Qi SD, Smith PD and Choong PF: Nuclear
reprogramming and induced pluripotent stem cells: A review for
surgeons. ANZ J Surg. 84:E1–E11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wu YL, Lin ZJ, Li CC, Lin X, Shan SK, Guo
B, Zheng MH, Li F, Yuan LQ and Li ZH: Epigenetic regulation in
metabolic diseases: Mechanisms and advances in clinical study.
Signal Transduct Target Ther. 8(98)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Dai W, Qiao X, Fang Y, Guo R, Bai P, Liu
S, Li T, Jiang Y, Wei S, Na Z, et al: Epigenetics-targeted drugs:
Current paradigms and future challenges. Signal Transduct Target
Ther. 9(332)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Generali M, Yoshihiko F, Debora K, Moe H,
Maximilian YE, Jun T, Simon PH and Hirohide S: Purification
technologies for induced pluripotent stem cell therapies. Nat Rev
Bioeng. 2:930–943. 2024.
|
|
23
|
Park J, Kim J, Shin B, Sch Ler HR, Kim J
and Kim KP: Inducing pluripotency in somatic cells: Historical
perspective and recent advances. Int J Stem Cells. 17:363–373.
2024.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bruno S, Schlaeger TM and Del Vecchio D:
Epigenetic OCT4 regulatory network: Stochastic analysis of cellular
reprogramming. NPJ Syst Biol Appl. 10(3)2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kaemena DF, Yoshihara M, Beniazza M,
Ashmore J, Zhao S, Bertenstam M, Olariu V, Katayama S, Okita K,
Tomlinson SR, et al: B1 SINE-binding ZFP266 impedes mouse iPSC
generation through suppression of chromatin opening mediated by
reprogramming factors. Nat Commun. 14(488)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang B, Li C, Ming J, Wu L, Fang S, Huang
Y, Lin L, Liu H, Kuang J, Zhao C, et al: The NuRD complex
cooperates with SALL4 to orchestrate reprogramming. Nat Commun.
14(2846)2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xie W, Miehe M, Laufer S and Johnsen SA:
The H2B ubiquitin-protein ligase RNF40 is required for somatic cell
reprogramming. Cell Death Dis. 11(287)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Müller I, Moroni AS, Shlyueva D, Sahadevan
S, Schoof EM, Radzisheuskaya A, Højfeldt JW, Tatar T, Koche RP,
Huang C and Helin K: MPP8 is essential for sustaining self-renewal
of ground-state pluripotent stem cells. Nat Commun.
12(3034)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Srinivasan SP, Nemade H, Cherianidou A,
Peng L, Cruz-Molina S, Rada-Iglesias A and Sachinidis A: Epigenetic
mechanisms of Strip2 in differentiation of pluripotent stem cells.
Cell Death Discov. 8(447)2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Saliev T and Singh PB: From bench to
bedside: Translating cellular rejuvenation therapies into clinical
applications. Cells. 13(2052)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Jing Y, Jiang X, Ji Q, Wu Z, Wang W, Liu
Z, Guillen-Garcia P, Esteban CR, Reddy P, Horvath S, et al:
Genome-wide CRISPR activation screening in senescent cells reveals
SOX5 as a driver and therapeutic target of rejuvenation. Cell Stem
Cell. 30:1452–1471.e10. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Khetpal S, Ghosh D and Roostaeian J:
Innovations in skin and soft tissue aging-A systematic literature
review and market analysis of therapeutics and associated outcomes.
Aesthetic Plast Surg. 47:1609–1622. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Maroufi F, Maali A, Abdollahpour-Alitappeh
M, Ahmadi MH and Azad M: CRISPR-mediated modification of DNA
methylation pattern in the new era of cancer therapy. Epigenomics.
12:1845–1859. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fadul SM, Arshad A and Mehmood R:
CRISPR-based epigenome editing: Mechanisms and applications.
Epigenomics. 15:1137–1155. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Oshimura M, Tabata T, Uno N, Takata S,
Hichiwa G, Kanazawa I, Endo T, Honma K, Wang Y, Kazuki K, et al:
Rejuvenation of human mesenchymal stem cells using a nonintegrative
and conditionally removable Sendai virus vector. Sci Rep.
14(23623)2024.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Salehpour A, Karimi Z, Ghasemi Zadeh M,
Afshar M, Kameli A, Mooseli F, Zare M and Afshar A: Therapeutic
potential of mesenchymal stem cell-derived exosomes and miRNAs in
neuronal regeneration and rejuvenation in neurological disorders: A
mini review. Front Cell Neurosci. 18(1427525)2024.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Saad FA: Gene therapy for skin aging. Curr
Gene Ther. 25:2–9. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wu S, Sun S, Fu W, Yang Z, Yao H and Zhang
Z: The role and prospects of mesenchymal stem cells in skin repair
and regeneration. Biomedicines. 12(743)2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ruscu M, Glavan D, Surugiu R, Doeppner TR,
Hermann DM, Gresita A, Capitanescu B and Popa-Wagner A:
Pharmacological and stem cell therapy of stroke in animal models:
Do they accurately reflect the response of humans? Exp Neurol.
376(114753)2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Li C, Qin H, Zeng L, Hu Z and Chen C:
Efficacy of stem cell therapy in animal models of intracerebral
hemorrhage: An updated meta-analysis. Stem Cell Res Ther.
13(452)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ribitsch I, Baptista PM, Lange-Consiglio
A, Melotti L, Patruno M, Jenner F, Schnabl-Feichter E, Dutton LC,
Connolly DJ, van Steenbeek FG, et al: Large animal models in
regenerative medicine and tissue engineering: To do or not to do.
Front Bioeng Biotechnol. 8(972)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zheng J, Yang B, Liu S, Xu Z, Ding Z and
Mo M: Applications of exosomal miRNAs from mesenchymal stem cells
as skin boosters. Biomolecules. 14(459)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chehelgerdi M, Chehelgerdi M, Allela OQB,
Pecho RDC, Jayasankar N, Rao DP, Thamaraikani T, Vasanthan M,
Viktor P, Lakshmaiya N, et al: Progressing nanotechnology to
improve targeted cancer treatment: Overcoming hurdles in its
clinical implementation. Mol Cancer. 22(169)2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yücel AD and Gladyshev VN: The long and
winding road of reprogramming-induced rejuvenation. Nat Commun.
15(1941)2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang S, Lee Y, Liu Y, Yu Y and Han I:
Stem cell and regenerative therapies for the treatment of
osteoporotic vertebral compression fractures. Int J Mol Sci.
25(4979)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Jothi D and Kulka LAM: Strategies for
modeling aging and age-related diseases. NPJ Aging.
10(32)2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dhanjal DS, Singh R, Sharma V, Nepovimova
E, Adam V, Kuca K and Chopra C: Advances in genetic reprogramming:
Prospects from developmental biology to regenerative medicine. Curr
Med Chem. 31:1646–1690. 2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Balducci L, Falandry C and Monfardini S:
Senotherapy, cancer, and aging. J Geriatr Oncol.
15(101671)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Abdellatif M, Schmid ST, Fuerlinger A and
Kroemer G: Anti-ageing interventions for the treatment of
cardiovascular disease. Cardiovasc Res: Aug 22, 2024 (Epub ahead of
print).
|
|
50
|
Meltzer WA, Gupta A, Lin PN, Brown RA,
Benyamien-Roufaeil DS, Khatri R, Mahurkar AA, Song Y, Taylor RJ and
Zalzman M: Reprogramming chromosome ends by functional histone
acetylation. Int J Mol Sci. 25(3898)2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kim J, Hwang Y, Kim S, Chang Y, Kim Y,
Kwon Y and Kim J: Transcriptional activation of endogenous Oct4 via
the CRISPR/dCas9 activator ameliorates Hutchinson-Gilford progeria
syndrome in mice. Aging Cell. 22(e13825)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Yang SG, Wang XW, Qian C and Zhou FQ:
Reprogramming neurons for regeneration: The fountain of youth. Prog
Neurobiol. 214(102284)2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tabibzadeh S: From genoprotection to
rejuvenation. Front Biosci (Landmark Ed). 26:97–162.
2021.PubMed/NCBI View
Article : Google Scholar
|
|
54
|
Alvarez-Kuglen M, Ninomiya K, Qin H,
Rodriguez D, Fiengo L, Farhy C, Hsu WM, Kirk B, Havas A, Feng GS,
et al: ImAge quantitates aging and rejuvenation. Nat Aging.
4:1308–1327. 2024.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pereira B, Correia FP, Alves IA, Costa M,
Gameiro M, Martins AP and Saraiva JA: Epigenetic reprogramming as a
key to reverse ageing and increase longevity. Ageing Res Rev.
95(102204)2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Suslo A, Mizia S, Horoch-Łyszczarek E and
Pochybełko E: The future of care and healthcare provision to
communitydwelling disa-bled elderly people in an ageing society.
Fam Med Prim Care Rev. 25:102–106. 2023.
|
|
57
|
Iijima K, Arai H, Akishita M, Endo T,
Ogasawara K, Kashihara N, Hayashi YK, Yumura W, Yokode M and Ouchi
Y: Toward the development of a vibrant, super-aged society: The
future of medicine and society in Japan. Geriatr Gerontol Int.
21:601–613. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ok SC: Insights into the anti-aging
prevention and diagnostic medicine and healthcare. Diagnostics
(Basel). 12(819)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kim Y, Zharkinbekov Z, Sarsenova M, Yeltay
G and Saparov A: Recent advances in gene therapy for cardiac tissue
regeneration. Int J Mol Sci. 22(9206)2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Goya RG, Lehmann M, Chiavellini P,
Canatelli-Mallat M, Hereñú CB and Brown OA: Rejuvenation by cell
reprogramming: A new horizon in gerontology. Stem Cell Res Ther.
9(349)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Santa Cruz-Pavlovich FJ, Bolaños-Chang AJ,
Del Rio-Murillo XI, Aranda-Preciado GA, Razura-Ruiz EM, Santos A
and Navarro-Partida J: Beyond vision: An overview of regenerative
medicine and its current applications in ophthalmological care.
Cells. 13(179)2024.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Gacic JM, Rascanin SR, Jovanovic MR,
Nikolovski SS, Jovanovic N, Petkovic J, Zdravkovic N, Djokic O and
Rancic NK: Comparison of knowledge about induced pluripotent stem
cells in relation to gender among healthcare professionals and in
the general population. Cureus. 16(e66821)2024.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Smajdor A: An alternative to sexual
reproduction: Artificial gametes and their implications for
society. Br Med Bull. 129:5–11. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mkrtchyan GV, Abdelmohsen K, Andreux P,
Bagdonaite I, Barzilai N, Brunak S, Cabreiro F, de Cabo R, Campisi
J, Cuervo AM, et al: ARDD 2020: From aging mechanisms to
interventions. Aging (Albany NY). 12:24484–24503. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kabata F and Thaldar D: The human genome
as the common heritage of humanity. Front Genet.
14(1282515)2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Hoffmann WA and Nortjé N: Ethics review
framework and guidelines for social science research. Soc Sci Res
Ethics Afr. 7:229–248. 2019.
|
|
67
|
Madathil LP, Palatty PL, Sacheendran D,
Jayachander M, George T, Gur A, Krishna A, D'souza RF and Baliga
MS: Bioethical and human right considerations during COVID-19
pandemic period: Reflections of integrated oncology clinical
services from India. Ind J Med Paediatr Oncol. 45:481–487.
2024.
|
|
68
|
Pimenta FJB and Gómez AG: Contemplating
the principles of the UNESCO declaration on bioethics and human
rights: A bioaesthetic experience. Int J Ethics Educ. 8:249–274.
2023.
|
|
69
|
Nikitchina N, Ulashchik E, Shmanai V,
Heckel AM, Tarassov I, Mazunin I and Entelis N: Targeting of
CRISPR-Cas12a crRNAs into human mitochondria. Biochimie. 217:74–85.
2024.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lim K: Mitochondrial genome editing:
Strategies, challenges, and applications. BMB Rep. 57:19–29.
2024.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Gao Y, Guo L, Wang F, Wang Y, Li P and
Zhang D: Development of mitochondrial gene-editing strategies and
their potential applications in mitochondrial hereditary diseases:
A review. Cytotherapy. 26:11–24. 2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Tao R, Yue C, Guo Z, Guo W, Yao Y, Yang X,
Shao Z, Gao C, Ding J, Shen L, et al: Subtype-specific neurons from
patient iPSCs display distinct neuropathological features of
Alzheimer's disease. Cell Regen. 13(21)2024.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Summers RA, Fagiani F, Rowitch DH, Absinta
M and Reich DS: Novel human iPSC models of neuroinflammation in
neurodegenerative disease and regenerative medicine. Trends
Immunol. 45:799–813. 2024.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Antón-Fernández A, Roldán-Lázaro M,
Vallés-Saiz L, Ávila J and Hernández F: In vivo cyclic
overexpression of Yamanaka factors restricted to neurons reverses
age-associated phenotypes and enhances memory performance. Commun
Biol. 7(631)2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Macip CC, Hasan R, Hoznek V, Kim J, Lu YR,
Metzger LE IV, Sethna S and Davidsohn N: Gene therapy-mediated
partial reprogramming extends lifespan and reverses age-related
changes in aged mice. Cell Reprogram. 26:24–32. 2024.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Li X, Li C, Zhang W, Wang Y, Qian P and
Huang H: Inflammation and aging: Signaling pathways and
intervention therapies. Signal Transduct Target Ther.
8(239)2023.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Calabrò A, Accardi G, Aiello A, Caruso C,
Galimberti D and Candore G: Senotherapeutics to counteract
senescent cells are prominent topics in the context of anti-ageing
strategies. Int J Mol Sci. 25(1792)2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kaur G, Sohanur Rahman M, Shaikh S, Panda
K, Chinnapaiyan S, Santiago Estevez M, Xia L, Unwalla H and Rahman
I: Emerging roles of senolytics/senomorphics in HIV-related
co-morbidities. Biochem Pharmacol. 228(116179)2024.PubMed/NCBI View Article : Google Scholar
|
|
79
|
McHugh D, Durán I and Gil J: Senescence as
a therapeutic target in cancer and age-related diseases. Nat Rev
Drug Discov. 24:57–71. 2025.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Martel J, Ojcius DM and Young JD:
Lifestyle interventions to delay senescence. Biomed J.
47(100676)2024.PubMed/NCBI View Article : Google Scholar
|