Introduction
Portal vein aneurysm (PVA), first documented by
Barzilai and Kleckner in 1956(1),
represents an uncommon vascular anomaly with <200 reported cases
worldwide (2), the preponderance of
which are isolated case reports or limited surgical series. The
diagnostic frequency of this condition has shown a progressive
increase, paralleling the expanded utilization of cross-sectional
abdominal imaging modalities in clinical practice (2). Despite this advancement, the
pathophysiology remains incompletely characterized, with debates
persisting regarding congenital vs. acquired origins (3), and no consensus guidelines exist for
surgical intervention criteria. Current diagnostic thresholds
define PVA as portal vein dilatation >19 mm in cirrhotic
patients or >15 mm in individuals with normal hepatic
architecture. Anatomical classification distinguishes intrahepatic
(affecting segmental portal branches) and extrahepatic (involving
main portal trunk) subtypes, with the intrahepatic type being less
common (3). The present study
details the case of a patient with PVA, highlighting the diagnostic
significance of abdominal ultrasonography (US) and
contrast-enhanced computed tomography (CT) in delineating both
morphological characteristics and hemodynamic patterns of this
vascular anomaly, serving as critical imaging biomarkers for
clinical decision-making.
Case report
A 74-year-old man presented to The Second Affiliated
Hospital of Xuzhou Medical University (Xuzhou, China) in September
2022, with a 1-month history of abdominal discomfort that had
progressively worsened over the preceding week. The patient had a
well-documented history of hypertension spanning over four decades,
which was effectively managed with regular antihypertensive
medication, achieving optimal blood pressure control. Aside from
hypertension, the patient had no significant medical history,
including prior surgical interventions, hepatitis, cirrhosis,
portal hypertension or malignancy. Additionally, there was no
relevant family history of these conditions.
Physical examination revealed a blood pressure of
148/83 mmHg, with no other notable findings, and laboratory
investigations were unremarkable as follows: Alanine
aminotransferase (ALT), 17.1 U/l (reference range, 0.0-40.0 U/l);
aspartate aminotransferase (AST), 12.0 U/l (reference range,
0.0-40.0 U/l); γ-glutamyl transpeptidase (γ-GT), 14.5 U/l
(reference range, 7.0-32.0 U/l); albumin, 41.2 g/l (reference
range, 38.0-55.0 g/l); globulin, 32.7 g/l (reference range,
20.0-40.0 g/l); prothrombin time (PT), 15.3 sec (reference range,
11.0-17.0 sec); D-dimer, 268 ng/ml (reference range, 0-500 ng/ml);
platelet count, 162x109/l (reference range,
100-300x109/l); hepatitis B virus surface antigen
(HBsAg)(-), anti-hepatitis C virus (HCV) antibodies(-),
anti-nuclear antibodies (ANA)(-), anti-smooth muscle antibodies
(ASMA)(-) and liver-kidney microsomal (LKM) antibodies(-).
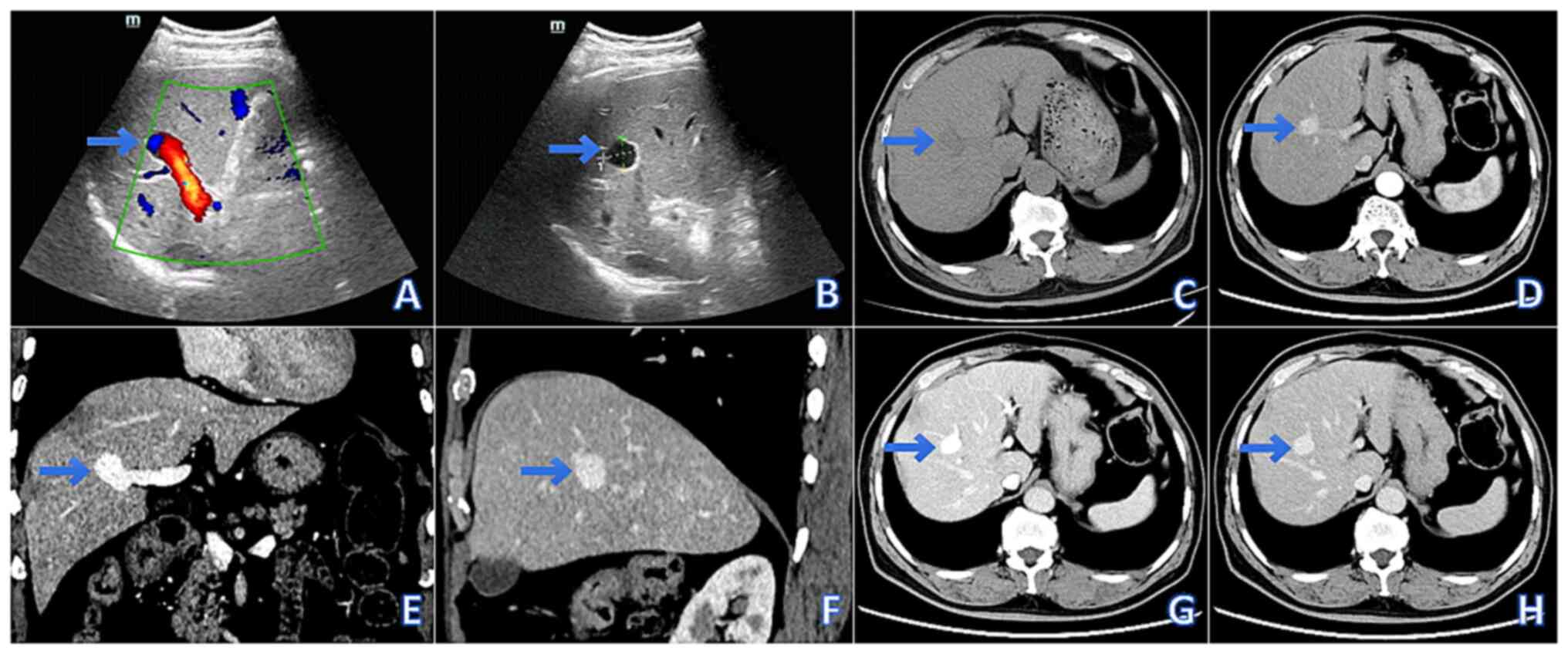
Abdominal US revealed a localized dilatation
(20.2x17.1 mm) of the right anterior branch of the portal vein,
characterized by thin walls, well-defined margins and the absence
of intraluminal abnormalities. The main portal vein measured 9 mm
in diameter. Color Doppler flow imaging demonstrated alternating
red-blue flow signals within the dilated lumen, and pulsed-wave
Doppler confirmed a typical portal venous waveform (Fig. 1A and B).
Non-contrast abdominal CT identified a round
hypodense lesion (20x17x15 mm) in the right anterior liver lobe,
exhibiting homogeneous density and well-defined margins.
Contrast-enhanced CT revealed synchronous enhancement of the lesion
with the portal vein, and multi-planar reconstruction confirmed
saccular dilation of the right anterior portal vein branch. The
main portal vein measured 9 mm in diameter, with no abnormalities
observed in the splenic vein, superior mesenteric vein or other
intrahepatic portal branches (Fig.
1C-H).
Based on the US and CT findings, the patient was
diagnosed with a right anterior PVA. Given the small size of the
lesion and the absence of notable symptoms, no immediate
intervention was deemed necessary. The patient was advised to
undergo regular follow-up imaging and to report any new or
worsening symptoms promptly.
To investigate the etiology of the abdominal
discomfort, an upper gastrointestinal endoscopy was performed,
which identified a polypoid lesion measuring ~0.6x0.6 cm in the
gastric fundus. The lesion was successfully resected using an
endoscopic mucosal resection technique. Postoperative management
included proton pump inhibitor administration for acid suppression
(40 mg pantoprazole, intravenous/oral daily, 4 weeks
postoperatively) and gastric mucosal protection (1 g sucralfate
orally, 4 times daily, 4 weeks postoperatively), along with
intravenous fluid replacement therapy (lactated Ringer's, 0.9%
normal saline, 1-2 ml/kg/h, 24 h). The patient tolerated the
procedure well and remained asymptomatic during the postoperative
period.
During the follow-up visit in September 2024, the
patient reported no abdominal discomfort or related symptoms.
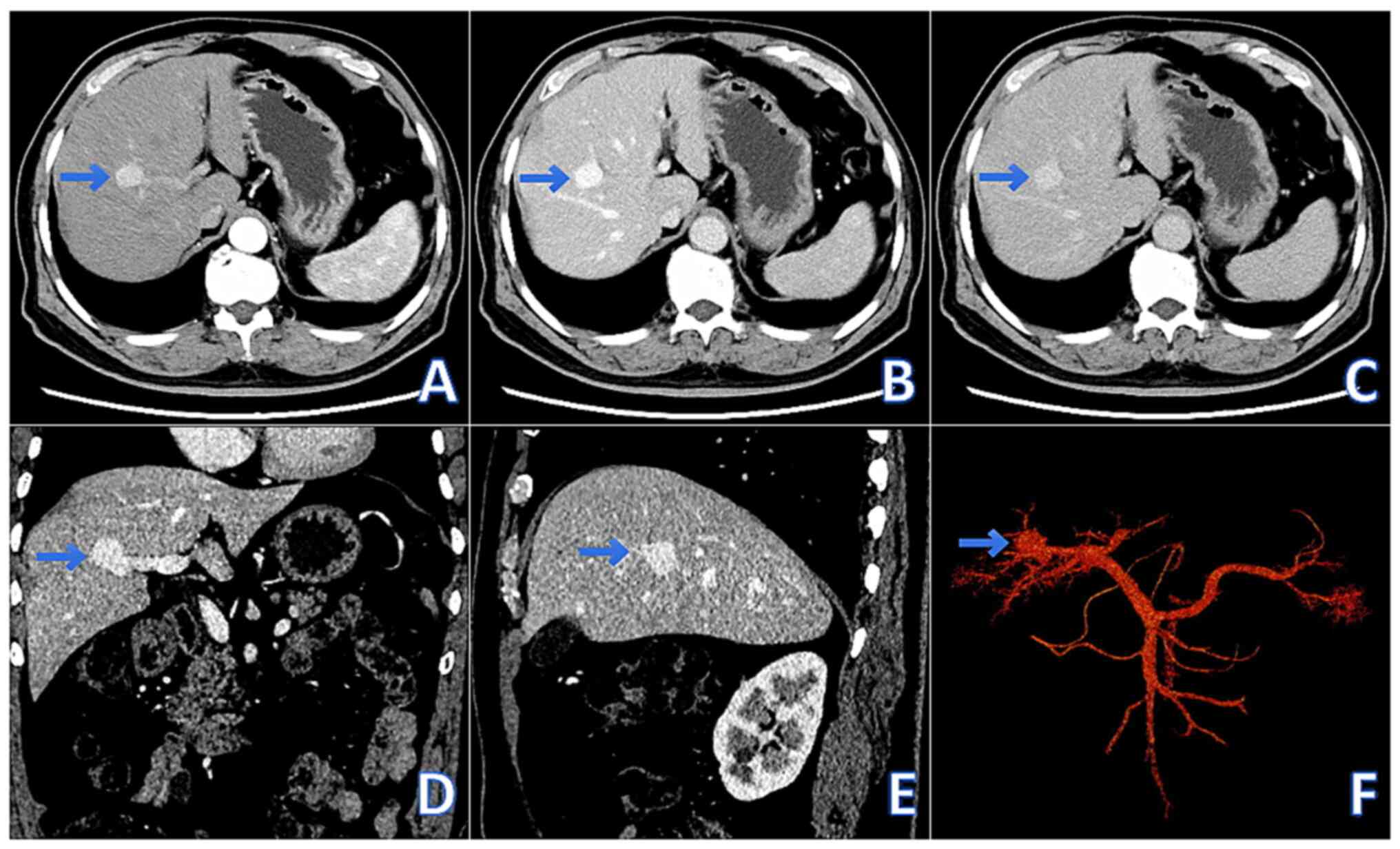
Comparative analysis of contrast-enhanced abdominal CT scans
revealed stable imaging findings, with no significant progression
or morphological changes in the PVA (Fig. 2A-F). Considering that the diameter
of the PVA in the patient was <3 cm and there were no
complications, a standardized follow-up plan was recommended for a
period of 6 months (4). The
monitoring included tumor size and the presence of complications.
Thus far, the patient has not experienced a rapid increase in tumor
size or any complications. The rupture risk of PVA is generally
low, but significantly increases in cases of extrahepatic PVA,
large aneurysms or those with complications (4). Current evidence predominantly stems
from case reports, highlighting the need for future multicenter
studies to establish robust risk stratification. In clinical
practice, risk assessment should integrate imaging characteristics
with dynamic monitoring to enable individualized clinical
management.
Discussion
PVA represents an uncommon vascular anomaly
characterized by localized dilatation of the portal venous system.
Histopathological examination typically reveals thinning of the
venous wall with marked reduction of both intimal and medial layer
components (2,4,5).
Anatomically, PVA can be classified into two distinct types based
on its location: Intrahepatic and extrahepatic. The intrahepatic
type involves the portal vein segments within the hepatic
parenchyma, while the extrahepatic type, which is more prevalent,
affects the main portal vein trunk extending from the confluence of
the splenic and superior mesenteric veins (6-8).
Current diagnostic criteria, as established by clinical studies,
define PVA as portal vein dilatation >15 mm in diameter for
intrahepatic segments and >20 mm for extrahepatic segments
(9).
Currently, there is no universally established
threshold for defining the rapid growth of a PVA. However, in
clinical practice, the following parameters are commonly considered
for assessment (10): i) Diameter
progression rate: First, a growth rate exceeding 20-30% in diameter
over a short-term period (typically 3-6 months) may indicate rapid
progression; second, when the diameter of a PVA increases by ≥0.5
cm within 1 year or ≥0.3 cm within 6 months, it may be considered
as rapid progression; and third, when the diameter of a PVA is ≥3
cm (especially when it reaches or exceeds this value in the short
term), it may raise clinical concerns as it is associated with the
risk of rupture or thrombosis. ii) Volumetric changes: The
evaluation of rapid progression of PVA is still mainly based on
diameter changes, but volume changes can be an important
supplement, especially when the tumor morphology is complex.
Quantitative volumetric analysis through advanced imaging
techniques demonstrating a >50% increase in aneurysm volume
within a defined period (e.g., 6 months) may suggest rapid
expansion (10). Some researchers
have proposed, based on insights from studies on abdominal aortic
aneurysms, that a PVA with an annual volume growth rate of ≥20% or
a ≥10% increase within 6 months may suggest rapid growth, although
this threshold requires further validation through additional
studies (11). iii) Clinical
manifestations and complications: Even in the absence of
significant dimensional changes, the development of complications,
such as intraluminal thrombosis, progressive portal hypertension
secondary to mass effect on adjacent structures or gastrointestinal
hemorrhage, may indicate clinically significant progression
requiring immediate intervention. In addition, persistent or
progressively worsening abdominal pain, especially in the upper
right or upper abdomen, may indicate tumor expansion or compression
of surrounding tissues. If patients experience non-specific
symptoms such as fever, fatigue and weight loss, it may be related
to tumor infection or thrombosis. In clinical practice, early
identification of high-risk patients and intervention measures are
key to preventing serious complications (12).
The precise etiology and pathogenesis of PVA remain
incompletely understood. Current hypotheses, supported by clinical
evidence, primarily focus on three potential mechanisms (13): i) Congenital developmental anomaly:
This theory proposes that a PVA may result from abnormal regression
of the primitive vitelline venous system during embryogenesis, with
subsequent dilatation of persistent venous diverticula leading to
aneurysm formation. ii) Acquired vascular wall weakness: Structural
compromise of the portal vein wall may occur secondary to various
pathological processes, including traumatic injury, chronic
pancreatitis or neoplastic infiltration. iii) Portal
hypertension-related vascular remodeling: Chronic elevation of
portal venous pressure may induce progressive vascular wall changes
and subsequent aneurysmal dilatation (13).
Based on comprehensive evaluation of the medical
history, clinical presentation and imaging characteristics of the
present patient, the congenital origin appears to be the most
plausible etiology in this case. Congenital factors contributing to
PVA formation can be categorized into three main etiological
pathways: First, developmental anomalies during portal vein
embryogenesis may result in structural defects (14). Second, there appears to be an
association with hereditary connective tissue disorders, with
clinical evidence documenting PVA occurrence in patients with
Ehlers-Danlos syndrome (15) and
Marfan syndrome (16). Third,
portal vein cavernous transformation may predispose to aneurysm
development (14).
In the current case, the patient's medical history
revealed no significant congenital abnormalities or family history
of connective tissue disorders. Radiological evaluation, including
comprehensive imaging studies, demonstrated no evidence of portal
vein branch cystic dilatation or other vascular malformations.
The acquired etiologies of PVA, although rare, can
be categorized into three primary mechanisms (17): First, conditions associated with
increased splanchnic blood flow, such as occult splenic
arteriovenous fistula or congenital hepatic artery-portal vein
fistula, may create abnormal venous pressure gradients. Second,
idiopathic vascular wall degeneration, potentially resulting from
aberrant extracellular matrix remodeling or chronic low-grade
inflammatory processes, could predispose to aneurysm formation.
Third, the development of microthrombi and subsequent vascular
remodeling may contribute to PVA pathogenesis. Subclinical portal
vein microthrombosis can induce localized flow turbulence, leading
to compensatory vascular dilation (17).
In the present case, Doppler ultrasonography
demonstrated normal portal venous flow velocity and hemodynamic
parameters. Furthermore, the patient's coagulation profile was
within normal limits. For comprehensive evaluation, additional
investigations are recommended during the next follow-up, including
immunological markers (serum IgG4 and antinuclear antibody
profile), as well as thrombophilia screening (anticardiolipin
antibodies and JAK2 V617F mutation analysis).
Contemporary imaging modalities, including US, CT,
magnetic resonance imaging (MRI), and digital subtraction
angiography (DSA), constitute the diagnostic cornerstone for PVA
evaluation (18). US provides
real-time visualization of the aneurysmal segment, demonstrating
characteristic findings such as turbulent flow patterns on Doppler
analysis and intraluminal thrombus formation. Cross-sectional
imaging techniques (CT and MRI) enable comprehensive assessment
through three-dimensional vascular reconstruction, precisely
delineating the lesion's spatial relationship with adjacent
anatomical structures, while concurrently evaluating potential
parenchymal abnormalities and associated vascular malformations.
Contrast-enhanced MRI further enhances diagnostic accuracy through
multiparametric tissue characterization and quantitative
hemodynamic parameters (19). DSA,
while less frequently employed, remains the diagnostic gold
standard for preoperative planning by providing dynamic blood flow
visualization and detailed angioarchitectural mapping essential for
endovascular intervention or pre-surgical roadmap creation
(20).
The clinical presentation of PVA typically ranges
from an asymptomatic status to non-specific manifestations,
including epigastric discomfort, postprandial pain or occult
gastrointestinal hemorrhage, with incidental detection through
routine abdominal imaging constituting the predominant diagnostic
pathway. Current evidence-based management protocols stratify
patients into two principal categories: Surveillance cohorts and
interventional candidates (21).
Conservative management with serial imaging surveillance (6- to
12-month intervals) is recommended for asymptomatic patients with
aneurysmal diameters <25 mm and the absence of portal
hypertension sequelae. Surgical intervention becomes imperative
when demonstrating progression of ≥5 mm/year in diameter,
thrombosis risk stratification scores ≥3 (incorporating flow stasis
parameters and hypercoagulable status) or impending rupture signs
on contrast-enhanced studies (22).
For patients with existing portal hypertension without clinical
symptoms, some scholars advocate preventive surgical treatment
(23).
Therapeutic strategies for PVA require meticulous
hemodynamic evaluation and anatomical consideration. For
intrahepatic variants, management algorithms should integrate
three-dimensional flow analysis to assess hepatic perfusion
compromise and coexisting pathologies, such as cirrhotic
transformation or portal hypertensive manifestations (24). The treatment methods may include
tumor resection, venous reconstruction or artificial blood vessel
reconstruction; however, the specific plan depends on the patient's
condition (25). Extrahepatic PVA
management typically involves portal axis reconstruction through
splenomesenteric confluence remodeling, often combined with
splenectomy when demonstrating hypersplenism or risk of left-sided
portal hypertension (26). Some
scholars believe that preventive surgery can effectively and safely
alleviate symptoms, and avoid the occurrence of complications for
extrahepatic PVA (27).
Given that the diameter of the PVA in the present
patient was <3 cm and no complications were present, a
standardized 6-month follow-up protocol was recommended. This
protocol should incorporate multimodal imaging evaluation alongside
systematic laboratory monitoring as follows: i) Imaging evaluation:
Utilize CT three-dimensional volume measurement technology with a
recommended slice thickness of ≤1 mm, including arterial and portal
dual-phase enhanced scans, to dynamically monitor the rate of tumor
volume changes.
Perform ultrasound Doppler to assess hemodynamic
parameters, specifically monitoring peak portal vein velocity,
intra-tumoral eddy current index, and resistance index (28). ii) Laboratory monitoring: Measure
ALT, AST, γ-GT, albumin levels and prothrombin time (PT) as live
function tests. Test for HBsAg and anti-HCV antibodies to assess
hepatitis status. Evaluate autoimmune activity through ANA, ASMA
and LKM tests. Conduct a complete blood cell count, with particular
attention to dynamic changes in platelet counts and indicators of
anemia. Monitor fibrinogen and D-dimer levels, and perform
thromboelastography to assess coagulation status (29,30).
This comprehensive follow-up plan ensures thorough
monitoring of the patient's condition, facilitating timely
detection and management of any potential changes or
complications.
The prognosis of patients with PVA is influenced by
multiple factors. Early diagnosis, tailored treatment strategies
based on individual conditions and proactive management of
complications are critical for improving patient outcomes. Patients
with smaller, regularly shaped tumors generally have a lower risk
of rupture and a more favorable prognosis. Conversely, those with
larger tumors (diameter >5 cm) or irregular, lobulated shapes
face an increased risk of rupture and may experience poorer
prognoses. Additionally, the development of complications, such as
intratumoral thrombosis, tumor rupture and hemorrhage, or portal
hypertension, can significantly worsen the prognosis (31,32).
Given the rarity of PVA, current clinical decisions
are based on limited evidence. There is an urgent need to establish
an international, multicenter registry study to collect long-term
follow-up data, which will facilitate the development of
evidence-based intervention guidelines.
The primary objective of the present study was to
characterize the clinical and imaging profiles of PVA through
representative case analysis, aiming to establish a practical
reference framework for clinical diagnosis and management. While we
acknowledge that the limited sample size inherent to rare disease
studies constrains the generalizability of conclusions, this study
systematically synthesizes multimodal imaging criteria and clinical
manifestations of PVA, thereby proposing a standardized
methodological framework for future investigations. Importantly,
the findings underscore the necessity of multi-center
collaborations to address current knowledge gaps. To advance this
field, we are initiating a cross-institutional consortium to
aggregate heterogeneous PVA cases, which will serve as a critical
foundation for elucidating pathogenic mechanisms and optimizing
evidence-based interventions.
In summary, the etiology and pathogenesis of PVA
remain poorly understood, and standardized management guidelines
have yet to be established. For asymptomatic patients with small
volume tumors and no evidence of portal hypertension, long-term
clinical observation and regular follow-up are recommended.
However, surgical intervention should be considered in cases of
rapid tumor expansion, intratumoral thrombosis or tumor rupture.
Radiological examinations, including US and CT, play a critical
role in both the diagnosis and ongoing monitoring of PVA.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Key R and D Project of
Xuzhou Science and Technology Bureau (grant no. KC23208),
Development Fund Project of Xuzhou Medical University Affiliated
Hospital (grant no. XYFY202460), Medical Research Project of
Jiangsu Provincial Health Commission (grant no. Z2024021) and
Xuzhou City Clinical Technology Key Personnel Advanced Training
Program.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW, AC and PD were responsible for study conception
and design. Collection and assembly of data (medical images) was
performed by YW and PD. All authors wrote and revised the
manuscript. All authors have read and approved the manuscript. YW
and PD confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The study was reviewed and approved by the Ethics
Committee of The Second Affiliated Hospital of Xuzhou Medical
University (Xuzhou, China; approval no. KY-20242717). The patient
provided written informed consent to participate in the study.
Patient consent for publication
The patient provided written informed consent for
the publication of data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Barzilai R and Kleckner MS Jr:
Hemocholecyst following ruptured aneurysm of portal vein; report of
a case. AMA Arch Surg. 72:725–727. 1956.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Laurenzi A, Ettorre GM, Lionetti R,
Meniconi RL, Colasanti M and Vennarecci G: Portal vein aneurysm:
What to know. Dig Liver Dis. 47:918–923. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
López-Sánchez J, Santabrígida Oreja G,
Quiñones Sampedro JE and Muñoz-Bellvís L: Portal vein aneurysm
associated with extensive portal-mesenteric thrombosis. Cir Esp
(Engl Ed). 101(863)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vine HS, Sequeira JC, Widrich WC and Sacks
BA: Portal vein aneurysm. AJR Am J Roentgenol. 132:557–560.
1979.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chaubard S, Lacroix P, Kennel C and
Jaccard A: Aneurysm of the portal venous system: A rare and unknown
pathology. Rev Med Interne. 39:946–949. 2018.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
6
|
Kurtcehajic A, Vele E and Hujdurovic A:
Portal vein aneurysm and portal biliopathy. J Hepatobiliary
Pancreat Sci. 23(658)2016.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Levi Sandri GB, Sulpice L, Rayar M,
Bosquet E, Boudjema K and Meunier B: Extrahepatic portal vein
aneurysm. Ann Vasc Surg. 28:1319.e5–7. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
De Vloo C, Matton T, Meersseman W, Maleux
G, Houthoofd S, Op de Beeck K, Laleman W, Van Malenstein H, Nevens
F, Verbeke L, et al: Thrombosis of a portal vein aneurysm: A case
report with literature review. Acta Clin Belg. 74:115–120.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ohnami Y, Ishida H, Konno K, Naganuma H,
Hamashima Y, Zeniya A and Masamune O: Portal vein aneurysm: report
of six cases and review of the literature. Abdom Imaging.
22:281–286. 1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Binko MA, Andraska EA, Reitz KM, Handzel
RM, Singh MJ, Sridharan ND, Chaer RA and Hager ES: The natural
history of portal venous system aneurysms. J Vasc Surg Venous
Lymphat Disord. 13(102163)2025.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Andraus W, Amico EC, Machado MA, Bacchella
T and Machado MC: Portal vein aneurysm. Clinics (Sao Paulo).
62:203–205. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tanaka H, Muromachi K, Tamai T, Hashiguchi
M, Enokizono R, Nakajyo Y, Iryo Y, Hori T, Tsubouchi H and Ido A:
Extrahepatic portal vein aneurysm in which the acute thrombogenic
process triggered by trauma confirmed by abdominal ultrasonography:
A case report. Clin J Gastroenterol. 16:702–708. 2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moreno JA, Fleming MD, Farnell MB and
Gloviczki P: Extrahepatic portal vein aneurysm. J Vasc Surg.
54:225–226. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yedlicka GM, Maatman TK, Mangus RS and
Nakeeb A: Operative treatment of portal vein aneurysm. Surg Open
Sci. 10:165–167. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Amato ACM, da Silva AEC, Bernal IM, de
Oliveira JC, Di Paschoal Almeida Ribeiro M, Schinzari PS and Dos
Santos RV: Combined nutcracker and ehlers-danlos syndromes: A case
report. EJVES Vasc Forum. 47:12–17. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moretti G, Staeffen J, Broustet A and Le
Bras M: Congenital malformations associated with stenosis of the
portal vein. Sem Hop. 44:893–897. 1968.PubMed/NCBI(In French).
|
|
17
|
Passi N, Wadhwa AC and Naik S:
Radiological evaluation of extrahepatic and intrahepatic portal
vein aneurysms: A report of two cases. Radiol Case Rep.
17:4784–4789. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hirji SA, Robertson FC, Casillas S, McPhee
JT, Gupta N, Martin MC and Raffetto JD: Asymptomatic portal vein
aneurysms: To treat, or not to treat? Phlebology. 33:513–516.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Koc Z, Oguzkurt L and Ulusan S: Portal
venous system aneurysms: Imaging, clinical findings, and a possible
new etiologic factor. AJR Am J Roentgenol. 189:1023–1030.
2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Murty T and Negrete L: Portal venous
aneurysm. Radiology. 307(e221311)2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tan RLW and Ng ZQ: Portal venous aneurysm.
BMJ Case Rep. 14(e244704)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nangou P, Bertrand P, Samann I, Filali A,
el Hassani R, Benabdellah C, Elhadj R, Boulakia C and Icard P:
Portal vein aneurysm. Ann Chir. 125:476–478. 2000.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
23
|
Qi X, Yin Z, He C, Guo W, Han G and Fan D:
Extrahepatic portal vein aneurysm. Clin Res Hepatol Gastroenterol.
37:1–2. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Haddad A, Fraiman M and Mackey R: Portal
vein aneurysm. Am Surg. 77:503–505. 2011.PubMed/NCBI
|
|
25
|
Cho SW, Marsh JW, Fontes PA, Daily MF,
Nalesnik M, Tublin M, De Vera ME, Geller DA and Gamblin TC:
Extrahepatic portal vein aneurysm-report of six patients and review
of the literature. J Gastrointest Surg. 12:145–152. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kurtcehajic A, Zerem E, Alibegovic E,
Kunosic S, Hujdurovic A and Fejzic JA: Portal vein
aneurysm-etiology, multimodal imaging and current management. World
J Clin Cases. 11:725–737. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ma R, Balakrishnan A, See TC, Liau SS,
Praseedom R and Jah A: Extra-hepatic portal vein aneurysm: A case
report, overview of the literature and suggested management
algorithm. Int J Surg Case Rep. 3:555–558. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liu Y, Arief J, Xiu W, Hao X, Wang F, Xia
N and Dong Q: Case Report: Management of a congenital intrahepatic
portosystemic shunt with portal vein aneurysm in a child using 3D
computer-assisted partial right hepatectomy. Front Pediatr.
12(1429537)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim HU, Mateja HL, Neris R, Kimyaghalam A
and DeVito PM: Asymptomatic portal vein aneurysm uncovered during
the evaluation of a gastrointestinal hemorrhage: A rare clinical
case. Cureus. 16(e68388)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lenaerts YF, Labarque V and Limantoro I:
Thrombosed massive portal vein aneurysm in an adolescent boy.
Pediatr Radiol. 54:1928–1932. 2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Higashi S, Nakabori T, Mukai K, Seiki Y,
Watsuji K, Hirao T, Kawamoto Y, Urabe M, Kai Y, Takada R, et al:
Portal vein aneurysm in a patient with cirrhosis type C controlled
by direct-acting antiviral treatment. Case Rep Gastroenterol.
18:74–80. 2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Monville JF, Meurisse N and Dondelinger
RF: Anticoagulant Treatment of a Thrombosed Giant Portal Vein
Aneurysm. J Belg Soc Radiol. 108(8)2024.PubMed/NCBI View Article : Google Scholar
|