Introduction
The placenta is the connection point between the
mother's and fetal blood flow, effectively transferring essential
nutrients and oxygen from the mother to the fetus for normal fetal
development (1). Abnormal placental
growth is the root cause of various pregnancy issues, such as fetal
growth restriction (FGR), also known as intrauterine growth
restriction (IUGR) (1). Placentae
with FGR are typically ~50% smaller than normal placentae, and
exhibit structural abnormalities in the villous tree and placental
vascular networks (2). Currently,
there is no universally accepted method for the diagnosis of FGR. A
statistical difference in fetal size typically identifies it
compared with the reference population using percentile thresholds
of 10, 5, or 3(3). The current
obstacle lies in distinguishing pregnancies affected by genuine FGR
from those where the infant is small for gestational age due to
natural reasons and those where the baby's weight exceeds
percentile limits but suffers from compromised growth and health
due to underlying pathology. The past decade has witnessed
significant progress in our comprehension of placental disorders
linked to FGR. In high-income countries, placental insufficiency is
the main cause of FGR, while in low-income countries, FGR is
primarily attributed to inadequate maternal nutrition (4,5). FGR
significantly contributes to adverse outcomes during the perinatal
period and is associated with a higher likelihood of long-term
neurological and neurodevelopmental issues (6,7).
Furthermore, infants affected by FGR have an elevated risk of
developing cardiovascular disease later in life (8). Therefore, the timely diagnosis and
management of FGR during pregnancy holds significant implications
for the healthy development of the entire society. Additionally,
immune cells are essential for the development and operation of the
placenta, and insufficient control of the maternal immune system is
related to placental issues and complications during pregnancy
(9). However, the immunological
background of FGR has received limited attention.
Notch proteins were first identified in
Drosophila as a family of transmembrane receptors that
exhibit both functional and structural conservation across various
species (10). Notch signaling
constitutes a crucial developmental pathway that plays a vital role
in maintaining tissue homeostasis and facilitating stem cell
differentiation (11). The
activation of Notch signaling relies on the direct interaction
between cells mediated by membrane-bound ligands and receptors
(12). The cell that sends the
signal expresses Serrate-like ligands Jagged 1/2 or Delta-like
ligands, which engage with the extracellular domain of Notch
receptors (Notch1-4) through their epidermal growth factor repeats.
This group of receptors is currently understood to regulate the
fate decisions of developing cells across various tissues during
placental development, embryogenesis and postnatal stages. For
example, Afshar et al (13)
posited that Notch1 may mediate a survival signal in the uterine
endometrium, enabling a rapid response to chorionic gonadotropin
and ultimately preventing menstrual sloughing. In the progression
of preeclampsia, fetal growth may be impaired, and the expression
of Notch1 and its ligand Jagged1 is found to be absent in patients
with pre-eclampsia. Furthermore, inhibition of Notch signaling
results in a reduction in the invasion of placental trophoblasts
(14). More importantly, Sahin
et al (15) indicated that
the expression of Notch1 protein is diminished in placental tissue
from pregnancies affected by IUGR. However, the precise mechanism
underlying this action remains unspecified.
In the present study, the expression and
distribution of Notch1 and its ligand Jagged was investigated.
Furthermore, the levels of inflammatory cytokines and
immune-related factors in both serum and placental tissues from
pregnancies with FGR were measured. These findings may elucidate
the pathogenesis of FGR and offer a technically efficient, safe and
economically viable diagnostic modality for FGR pregnancies.
Materials and methods
Patients
A total of 50 patients (age: 30-39 years) diagnosed
with FGR were enrolled at Jinhua Maternal and Child Health Hospital
(Jinhua, China) from January 2021 to December 2021. FGR pregnancies
were identified through ultrasonographic monitoring during the
second/third trimester, which revealed biometry measurements below
the 10th percentile and birth weights also below the 10th
percentile. The inclusion criteria were: i) singleton pregnancy;
ii) term delivery (37-41 weeks gestation). Patients presenting with
any complications associated with FGR such as multiple pregnancies,
fetal malformations, preeclampsia, chronic maternal diseases,
intrauterine infections, hypertension, or diabetes were excluded.
Concurrently, 50 normal pregnancies matched the gestational weeks,
and birth weights between 10 and 90th percentiles were recruited as
the controls. All participants had been informed the study details
and given their written consent in advance. Ethical approval was
obtained from the Ethics Committee of Jinhua Maternal and Child
Health Hospital (approval no. 2021-KY-009; Jinhua, China) and this
research adheres to the principles outlined in the Declaration of
Helsinki.
Sample collection
Within 4 h of venous blood collection, peripheral
blood mononuclear cells (PBMCs) were isolated from a 10 ml
heparinized sample using a human peripheral blood monocyte
isolation solution kit (Beijing Solarbio Science & Technology
Co., Ltd.). Concurrently, 5 ml of venous blood samples were
obtained via vein puncture and subsequently subjected to
centrifugation at 1,000 x g for 10 min to collect serum samples.
Placentae were collected from women post-delivery. Immediately, 1
cm3 of tissue block was dissected from the lobule of the
maternal surface of the placenta and divided into two parts,
followed by washing with PBS. Subsequently, one part of the samples
was stored in cryotubes at liquid nitrogen and used for reverse
transcription-quantitative PCR (RT-qPCR), western blotting and
ELISA, while the other part was subjected to fixation using 10%
formaldehyde and used for the subsequent hematoxylin & eosin
(H&E) staining, immunofluorescence (IF) staining,
immunohistochemistry (IHC) and TUNEL assays.
H&E staining
Placenta histopathology was examined using a H&E
staining Kit (Abcam). The fixed placentae were subjected to
dehydration followed by embedding in paraffin. After cutting into
4-µm thickness slices, dewaxing and rehydration, the sections were
executed for H&E staining. Images were captured under a BX53
microscope from Olympus Corporation (Scale bar, 100 µm).
TUNEL assay
In accordance with the experimental protocol in the
manufacturer's specifications of the Kit (Beijing Solarbio Science
& Technology Co., Ltd.), apoptosis was evaluated in placental
tissues. Briefly, the placental tissues were fixed using 4%
paraformaldehyde at 4˚C for 48 h, and then embedded in paraffin and
cut into 5-µm sections. The tissue sections, after
deparaffinization, were treated with 3% hydrogen peroxide in
methanol for 10 min at 25˚C under dark conditions. They were
subsequently rinsed three times using PBS and then exposed to a
solution of 0.1% Triton X-100 in freshly prepared 0.01% sodium
citrate for 8 min at the same temperature. Following this, the
sections underwent incubation with a proteinase K working solution
for 25 min at 37˚C, followed by three washes with PBS, each lasting
5 min. Each sample received 50 µl of TUNEL reagent and was
incubated at 37˚C for 1 h. Afterward, the sections were washed
three more times with PBS, and the cell nuclei were stained with a
2 µg/ml DAPI solution for 10 min in the dark at room temperature.
Finally, the samples were mounted using 50 µl of an anti-fade
mounting medium. TUNEL-positive cells were observed in five
randomly-selected fields using a fluorescence microscope (Olympus
Corporation; Scale bar, 100 µm).
Total RNA isolation and RT-qPCR
The total RNA was isolated from PBMCs or placental
tissues using a Total RNA Extraction Kit (Promega Corporation).
Subsequently, cDNA synthesis was performed utilizing a First Strand
cDNA Synthesis Kit (Vazyme Biotech Co., Ltd.) according to the
manufacturer's instructions. Following the manufacturer's
instructions, RT-qPCR analysis was conducted employing the
Hieff® qPCR SYBR Green Master Mix (Shanghai Yeasen
Biotechnology Co., Ltd.). The thermocycling conditions were as
follows: 95˚C for 5 min, 40 cycles of denaturation at 95˚C for 10
sec, annealing at 50˚C for 1 min and extension at 72˚C for 30 sec.
To assess gene expression levels, the 2-ΔΔCq method was
applied (16). Normalization of
data was performed using GAPDH as a reference gene. The primers
used are included in Table I.
 | Table IPrimer sequences used for reverse
transcription-quantitative PCR. |
Table I
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5'-3') |
|---|
| Notch1 | F:
CACGTGGTGGACCGCAGA |
| | R:
CACAGTTCTGGCCGGTGAA |
| Jagged1 | F:
AGCTATTTGCCGACAAGGCT |
| | R:
CACTGCCAGGGCTCATTACA |
| GAPDH | F:
AAGCCTGCCGGTGACTAAC |
| | R:
GCATCACCCGGAGGAGAAAT |
Western blotting
The placentae were subjected to lysis using RIPA
buffer (Boster Biological Technology), followed by the
determination of protein concentrations utilizing a BCA Protein Kit
(Beyotime Institute of Biotechnology). Subsequently, the protein
samples (30 µg per lane) were separated through 10% SDS
polyacrylamide gel electrophoresis and transferred onto PVDF
membranes. To block the membranes, a solution containing 5% non-fat
milk was employed for 2 h at 25˚C. Following this step, primary
antibodies including Notch1 (1:750; cat. no. 20687-1-AP), Jagged1
(1:20,000; cat. no. 66890-1-Ig), β-actin (1:20,000; cat. no.
66009-1-Ig), TNF-α (1:2,000; cat. no. 60291-1-Ig), vascular
endothelial growth factor (VEGF; (1:8,000; cat. no. 19003-1-AP),
GAPDH (1:50,000; cat. no. 60004-1-Ig; all from Proteintech Group,
Inc.), IL-6 (1:1,000; cat. no. bs-0782R), placental growth factor
(PLGF; 1:1,000; cat. no. bsm-54066R; both from BIOSS), C-X-C motif
chemokine ligand 1 (CXCL1; 1:100; cat. no. ab206411), soluble
fms-like tyrosine kinase-1 (sFlt-1; 1:1,000; cat. no. ab32152) and
placental protein 13 (PP13; 1:1,000; cat. no. ab218411; all from
Abcam) were introduced to the membranes for overnight incubation at
4˚C. The HRP-conjugated secondary antibodies (1:1,000; cat. nos.
A21010 & A21020; Abbkine Scientific Co., Ltd.) were then added
and allowed to incubate for 1 h at room temperature. Visualization
of immunoblotting was achieved using an ECL detection Kit (Thermo
Fisher Scientific, Inc.) and brand intensity was measured by
densitometry using Quantity One software version 4.6 (Bio-Rad
Laboratories, Inc.).
IF staining
Placenta slices were deparaffinized in xylene and
rehydrated through a graded series of ethanol, followed by antigen
retrieval in 10 mM Citrate buffer at 95˚C for 20 min. Subsequently,
the sections (5 µm) were permeabilized with 0.5% TritonX-100 in PBS
at room temperature for 1 h after being rinsed three times in PBS.
Following blocking with 5% bovine serum albumin (Beijing Solarbio
Science & Technology Co., Ltd.) at room temperature for 1 h,
the sections were incubated overnight at 4˚C with primary
antibodies Notch1 (1:200; cat. no. ab52627) and Jagged1 (1:200;
cat. no. ab300561; both from Abcam). This was followed by
incubation with corresponding secondary antibodies conjugated to
fluorescent dyes (1:200; cat. no. ab150115; Abcam) at 37˚C for 30
min. After staining with DAPI (Beyotime Institute of Biotechnology;
1 µg/ml) at 37˚C for 15 min, images were captured using a
fluorescence microscope (Olympus Corporation; Scale bar, 100
µm).
IHC
The fixed placenta samples were subsequently
embedded in paraffin, followed by deparaffinization in xylene and
rehydration through a graded series of ethanol. After antigen
retrieval, the samples were incubated overnight at 4˚C with primary
antibodies against Notch1 (1:200), Jagged1 (1:200), CD3 (1:200;
cat. no. 17617-1-AP), CD86 (1:200; cat. no. 26903-1-AP), CD206
(1:200; cat. no. 18704-1-AP; last 3 obtained from Proteintech
Group, Inc.) and Forkhead Box protein 3 (Foxp3; 1:200; cat. no.
ab36607; Abcam). Subsequently, secondary antibodies conjugated with
HRP (1:3,000; cat. no. ab205719; Abcam) were applied for 1 h at
37˚C. DAB staining (Beijing Solarbio Science & Technology Co.,
Ltd.) was then performed on each slice, followed by image capture
using a light microscope (Scale bar, 100 µm).
ELISA
According to the manufacturer's protocols provided
for the human IL-10 (cat. no. KTE6019), IL-17 (cat. no. KTE6022),
TNF-α (cat. no. KTE6032), IL-6 (cat. no. KTE6017), CXCL1 (cat. no.
KTE3051), sFlt-1 (cat. no. KTE3117), VEGF (cat. no. KTE6033) and
PLGF (cat. no. KTE3057) ELISA Kits (Abbkine Scientific Co., Ltd.),
as well as the IL-35 ELISA Kit (cat. no. ED-10371; LunChangShuo
Biotech) and PP13 (cat. no. CSB-E12733h; Cusabio Technology, LLC),
the levels of these cytokines in serum and/or placental samples
were assessed.
Determination of T helper 17 cell
(Th17) and regulatory T cells (Treg) cell frequencies
To determine the frequencies of Th17 cells, PBMCs
were incubated with APC-conjugated anti-CD4 antibodies (1:200; cat.
no. 980812; BioLegend, Inc.) for 15 min at 4˚C. Following this
incubation, the cells were stained with PE-conjugated anti-IL-17A
antibodies (1:200; cat. no. 512305; BioLegend, Inc.). For the
detection of Treg, cultured PBMCs were treated with PE-labeled
anti-human CD25 (1:200; cat. no. 302605), PE-conjugated anti-human
Foxp3 (1:200; cat. no. 320107) and FITC-labeled anti-human CD4
(1:200; cat. no. 344604; all from BioLegend, Inc.) at 4˚C for 15
min. After staining, the cells were washed and subsequently
resuspended in a solution containing 1% formaldehyde. The analysis
of all stained cells was conducted using a BD FACSCalibur flow
cytometer (BD Biosciences), operated with BD CellQuest software
version 3.5.1.
Statistical analysis
All experiments were conducted in triplicate in at
least three independent experiments. The differences among the data
were evaluated using Student's t-tests (unpaired). Data analysis
was conducted using SPSS software version 22.0 (IBM Corp.), and the
results were presented as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Placenta histopathology and
trophoblast cell apoptosis in patients with FGR
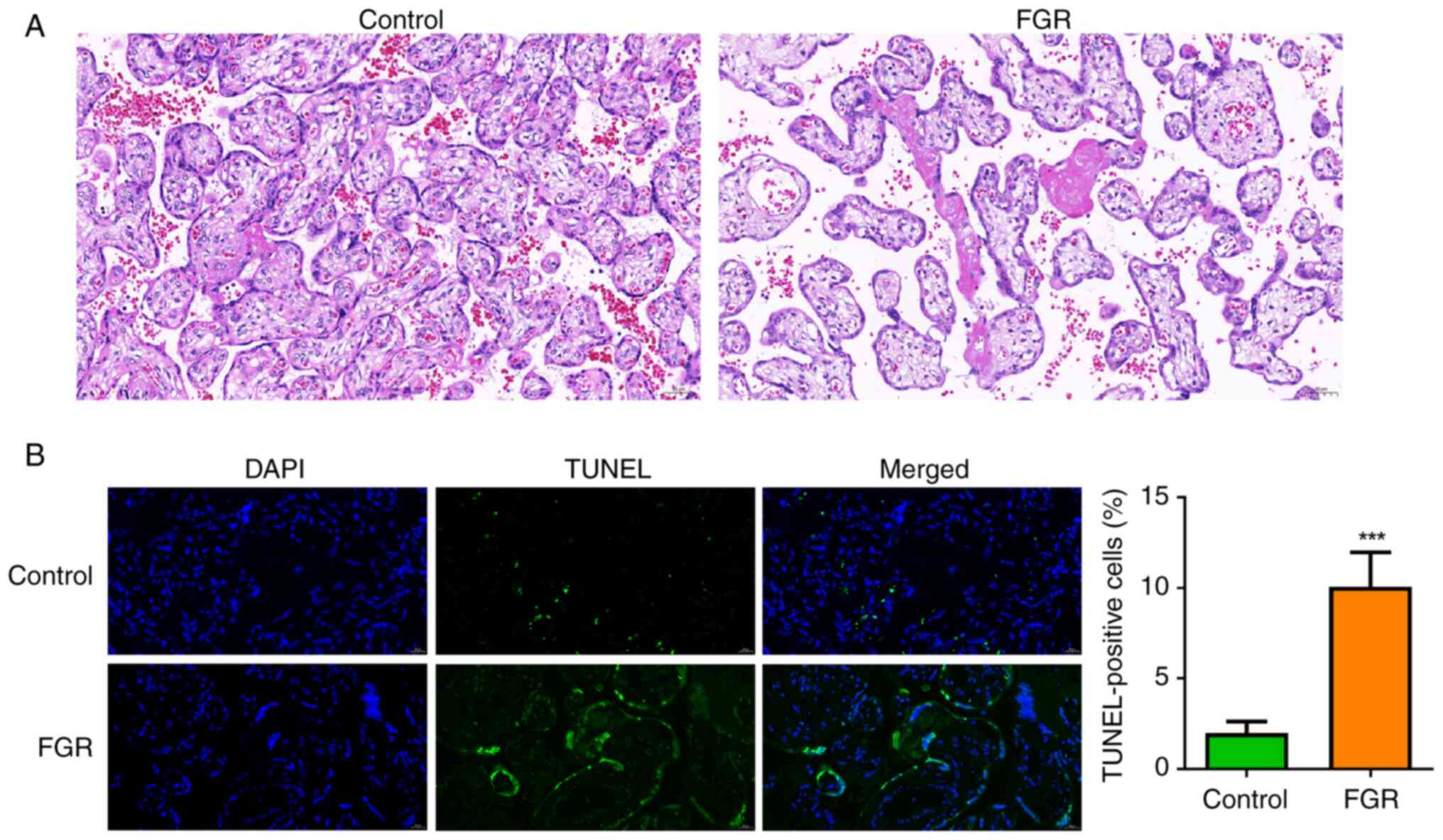
The histopathological changes in the placental
tissues of patients with FGR were initially examined. As
illustrated in Fig. 1A, the control
group exhibited a normal placental tissue structure and well-formed
blood vessels, with scattered calcifications and fibrin deposits
observed between the villi. By contrast, the FGR group displayed
extensive infarction and a reduction in villous vascularization.
Additionally, the apoptosis of trophoblast cells was also assessed.
It was demonstrated that the percentage of TUNEL-positive
trophoblast cells was significantly elevated in the FGR group when
compared with the control group (Fig.
1B, P<0.001), suggesting pronounced apoptosis of trophoblast
cells within the placental tissues of individuals affected by
FGR.
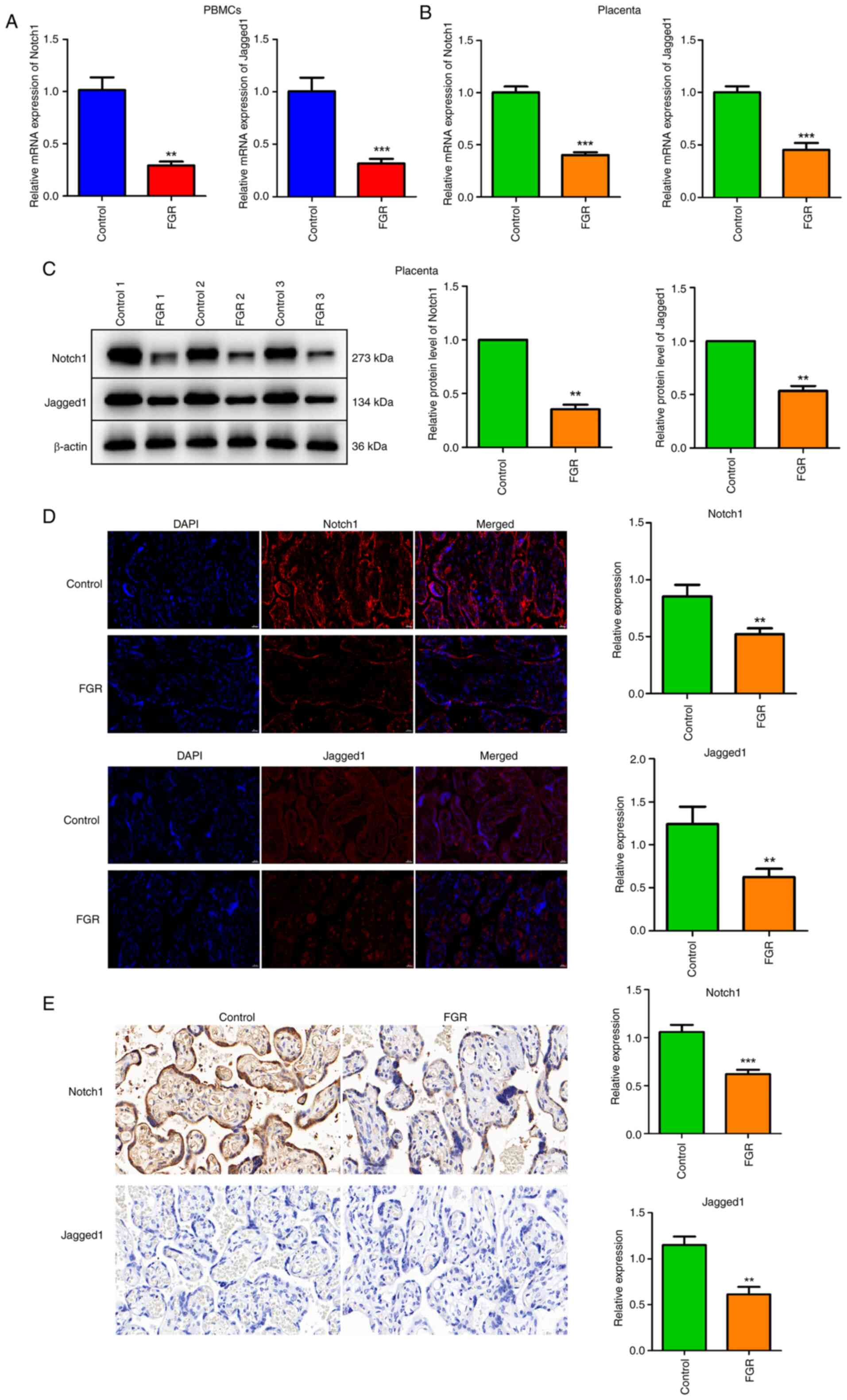
The expression and distribution of
Notch1 and Jagged1 is decreased in PBMCs and placenta
PBMCs were isolated from both healthy pregnancies
and FGR pregnancies. The mRNA expression levels of Notch1 and
Jagged1 in PBMCs were preliminarily assessed. As shown in Fig. 2A, both Notch1 and Jagged1 mRNA
expression were significantly reduced in PBMCs from FGR pregnancies
compared with those from control pregnancies (P<0.01). In
placental tissues, the mRNA expression of Notch1 and Jagged1 was
also evaluated. Low expression levels of Notch1 and Jagged1 were
observed in the FGR group by contrast to the control group
(Fig. 2B, P<0.001). Furthermore,
3 healthy pregnancies and 3 FGR pregnancies were randomly selected
for protein level analysis of Notch1 and Jagged1 in placental
tissues. Western blotting demonstrated a significant decrease in
the protein levels of Notch1 and Jagged1 within placental tissues
from FGR pregnancies in contrast to those control pregnancies
(Fig. 2C, P<0.01). IF assays
were performed to assess the distribution and expression patterns
of Notch1 and Jagged1 within placental tissues. A significantly
reduced distribution and expression of Notch1 and Jagged1 was
observed in placental tissues from FGR pregnancies compared with
those from control pregnancies (Fig.
2D, P<0.01). Additionally, IHC further validated that the
relative expression of Notch1 and Jagged1 was significantly
inhibited in the FGR group relative to the control group (Fig. 2E, P<0.01).
Inflammation- and angiogenesis-factors
in serum and placental tissue of FGR pregnancies
The occurrence of FGR is associated with numerous
critical physiological mechanisms, such as inflammation and
angiogenesis (17,18). Consequently, the expression of
relevant factors was investigated. In contrast to the control
group, the concentrations of inflammatory cytokines (TNF-α, CXCL1
and IL-6) and antiangiogenic protein sFlt-1 were significantly
increased in the FGR group (Fig.
3A, P<0.01). By contrast, the levels of pro-angiogenic
proteins including PLGF, VEGF and PP13 were significantly decreased
(P<0.05). Furthermore, similar results were observed in
placental tissues from 3 randomly selected patients with FGR
(Fig. 3B, P<0.05).
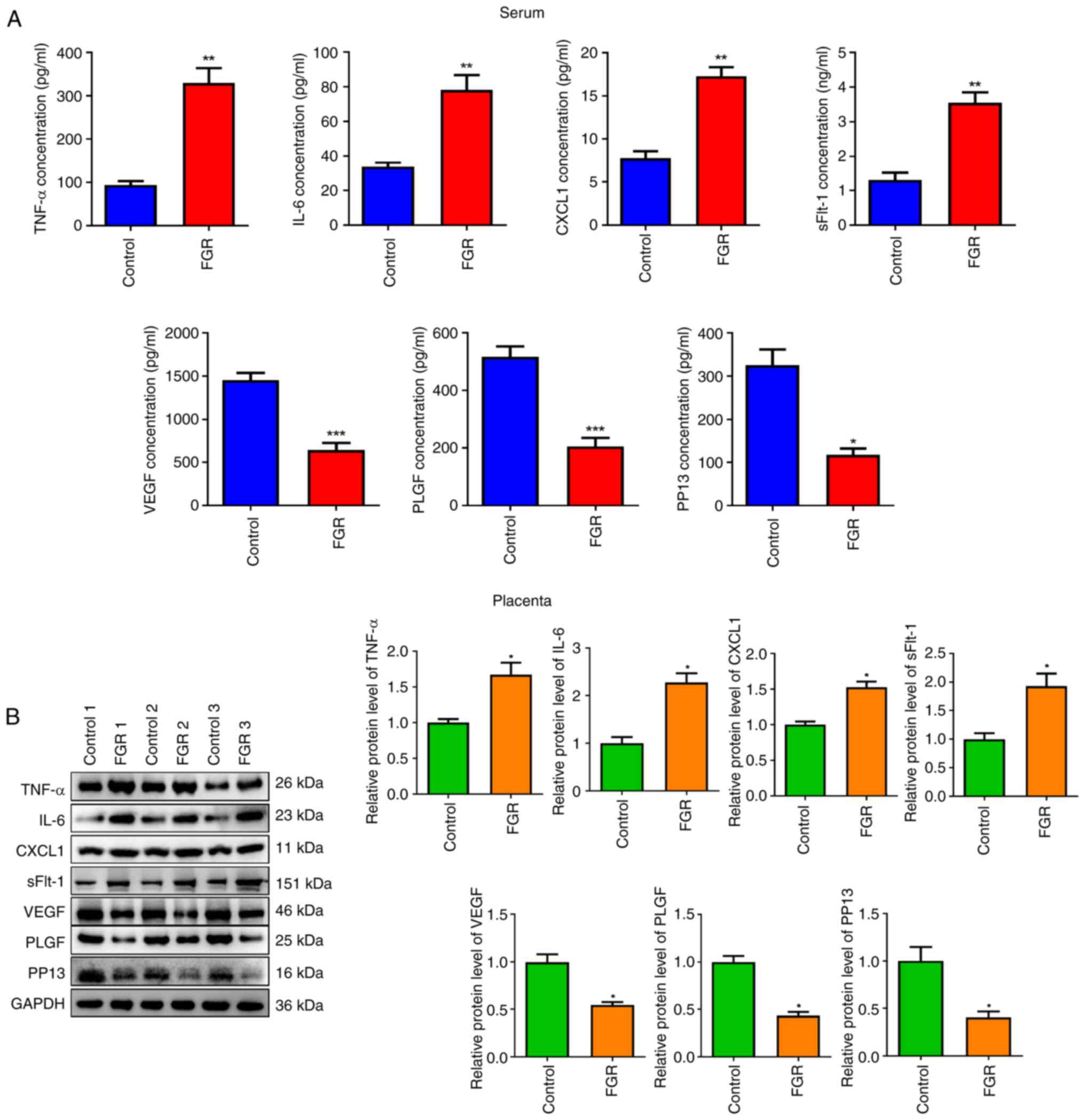 | Figure 3Inflammation- and angiogenesis-factors
in serum and placental tissue of FGR pregnancies. (A and B) The
levels of TNF-α, IL-6, CXCL1, sFlt-1, VEGF, PLGF and PP13 in serum
and placenta of FGR pregnancies were determined via (A) ELISA and
(B) western blotting. *P<0.05, **P<0.01
and ***P<0.001 vs. control. FGR, fetal growth
restriction; CXCL1, C-X-C motif chemokine ligand 1; sFlt-1, soluble
fms-like tyrosine kinase-1; VEGF, vascular endothelial growth
factor; PLGF, placental growth factor; PP13, placental protein
13. |
Immune dysfunction in patients with
FGR
It was observed that the frequency of Th17 cells was
significantly increased (Fig. 4A,
P<0.05), while the frequencies of Treg cells were significantly
reduced (P<0.05). Consequently, a significant increase in the
Th17/Treg ratio was determined (P<0.001). IHC was employed to
assess the involvement of immune cells such as macrophages, Treg
and Th17, by employing their corresponding markers. As manifested
in Fig. 4B, compared with the
control group, the relative expression CD86 (M1 macrophage marker)
and CD3 (Th17 marker) was significantly elevated (P<0.001),
whereas both CD206 (M1 macrophage marker) and Foxp3 (Treg marker)
expression levels were found to be significantly reduced
(P<0.001). IL-10, IL-17, and IL-35 are cytokines associated with
immune responses. The levels of IL-10, IL-17 and IL-35 were also
measured in both serum and placental tissue from FGR pregnancies.
The current findings indicated that compared with control
pregnancies, serum levels of IL-10 and IL-35 were significantly
decreased in FGR pregnancies (Fig.
4C, P<0.01), while serum levels of IL-17 showed a
significant increase (P<0.001). Similar patterns were also
observed in placental tissues from FGR pregnancies (Fig. 4D, P<0.001).
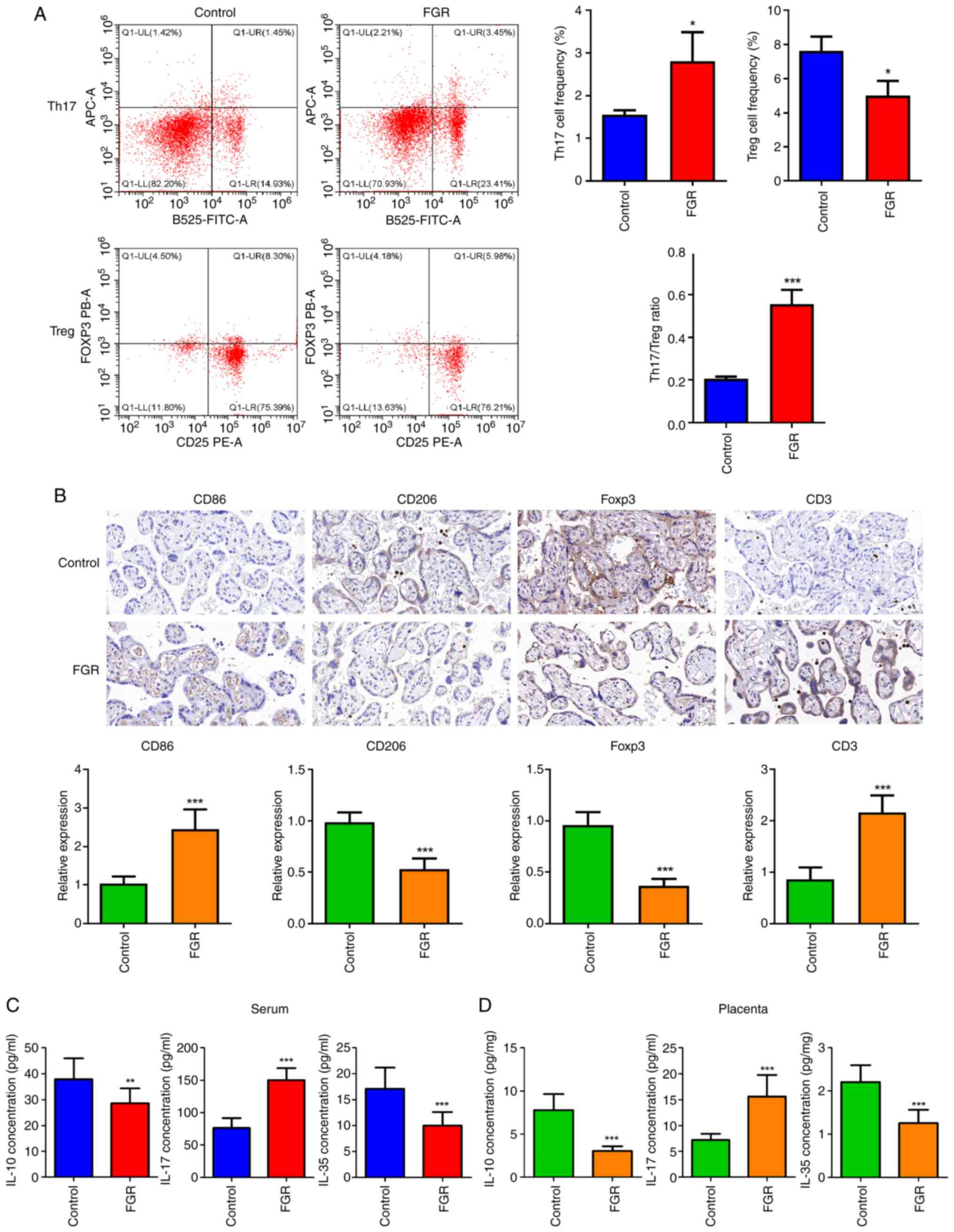 | Figure 4Immune dysfunction in patients with
FGR. (A) Th17 and Treg frequencies were assessed through flow
cytometry. (B) The expression of CD86 (M1 macrophage marker), CD206
(M1 macrophage marker), Foxp3 (Treg marker) and CD3 (Th17 marker)
was evaluated using immunohistochemistry. Scale bar, 100 µm. (C)
Serum and (D) placental levels of IL-10, IL-17, and IL-35 were
measured by ELISA. *P<0.05, **P<0.01
and ***P<0.001 vs. control. FGR, fetal growth
restriction; Th17, T helper 17 cells; Treg, regulatory T cells;
Foxp3, Forkhead Box protein 3. |
Discussion
In the present study, a preliminary mechanism
underlying the pathogenesis of FGR was uncovered, indicating that
the Notch signaling pathway mediates immune dysfunction to regulate
FGR development. The Notch signaling is a classical pathway for
inducing human trophoblast development and differentiation
(19). Research examining the
relationship between placental development and trophoblast cells
indicates that dysregulation of subcellular levels in trophoblasts,
along with increased apoptosis of these cells, are critical factors
contributing to impaired placental function in cases of FGR
(20). Consequently, it was
hypothesized that the Notch signaling pathway plays a pivotal role
in the pathogenesis of FGR. Additionally, several studies have
shown that vascular defects are observed in mice with a knockout of
Jagged1 (21,22). In the human placenta, Notch1 and its
ligand Jagged1 are prominently expressed, indicating their
involvement in the process of placental angiogenesis (23). In the current study, the expression
levels of Notch1 and its ligand Jagged1 was assessed in PBMCs and
placenta. As expected, it was found that the expression levels of
Notch1 and Jagged1 were downregulated in both PBMCs and placenta,
consistent with findings reported by Sahin et al (15). Furthermore, it was validated that
the distribution of Notch1 and Jagged1 within the placenta of FGR
pregnancy was also significantly reduced. These findings suggested
that the downregulation of Notch1 and Jagged1 may play a crucial
role in the pathogenesis of FGR.
The etiology of FGR is highly complex, with
inflammation reported to be existed throughout the FGR process
(17,24). A previous study demonstrated a
significant increase in pro-inflammatory cytokines in cases of FGR
compared with normal pregnancies (25). Similarly, the present study revealed
a significant elevation of IL-6, CXCL1 and TNF-α in both serum and
placental tissues from patients with FGR, indicating a more
pronounced pro-inflammatory response in pregnancies affected by
this condition. Additionally, angiogenic imbalance represents
another critical factor contributing to the development of FGR
(26). For example, PP13 is a
sophisticated protein that exhibits a strong affinity for glycans,
predominantly located within syncytiotrophoblasts (27). It is subsequently released into the
maternal bloodstream via microvesicles (27). This protein plays a pivotal role in
orchestrating implantation and facilitating the complex development
of placental blood vessels (28).
Both PLGF and VEGF are essential for promoting angiogenesis within
the placental tissues, as well as in transforming spiral arteries
into vessels characterized by low resistance and capacitance
(29). By contrast, sFlt-1 serves
an important function in inhibiting angiogenesis by effectively
counteracting the influences of PLGF and VEGF (30). The findings of the present study
corroborated previous research indicating that elevated levels of
sFlt-1 were present in both serum and placental tissues of
pregnancies affected by FGR. By stark contrast, the concentrations
of PLGF, VEGF and PP13 were found to be significantly diminished.
These results suggest a profound dysregulation of the angiogenic
process throughout the progression of FGR.
Additionally, the importance of immune mechanisms in
the onset and progression of pregnancy-related diseases is
increasingly acknowledged (31,32).
The phenomenon whereby the fetus is not rejected by the maternal
immune system represents a unique form of immune tolerance. Treg
cells possess immunosuppressive functions and are capable of
expressing various surface molecules, including the key
transcription factor Foxp3, which regulates both the development
and function of Treg cells while secreting cytokines such as IL-10
and IL-35(33). Numerous studies
have highlighted the crucial role of Treg cells in initiating and
sustaining maternal fetal tolerance. Deficiencies or dysfunctions
in Treg cells are closely associated with recurrent miscarriage,
infertility and preeclampsia (34).
Th17 is an identified subset of CD4+T cells in 2006,
capable of producing the cytokine IL-17. Elevated levels of Th17
and IL-17 have been closely associated with pathological conditions
such as recurrent miscarriage and infertility (35). Therefore, preserving the delicate
balance between Th17 and Treg cells is an essential prerequisite
for ensuring a normal pregnancy. In the present study, a
significant increase was confirmed in the frequency of Th17 and a
remarkable decrease in Treg cell frequency, indicating an imbalance
in the Th17/Treg ratio during the progression of FGR. Additionally,
the levels of IL-10, IL-17 and IL-35, which serve as indirect
indicators of homeostasis between Th17 and Treg cells, were
quantified. The concentrations of IL-10 and IL-35 were found to be
significantly reduced in both serum and placental tissues from FGR
pregnancies, while the levels of IL-17 were significantly
increased. This further underscored the imbalance between Th17 and
Treg cells observed in FGR pregnancies. Macrophages display
considerable diversity and function as principal antigen-presenting
cells at the maternal-fetal interface (36). Aberrant polarization of macrophages
has been associated with various pregnancy complications, including
preeclampsia, FGR and recurrent pregnancy loss (37,38).
In the current study, a shift towards the M1 phenotype was observed
in maternal macrophages within pregnancies complicated by FGR,
which was consistent with the research undertaken by Berezhna et
al (39). Collectively, these
findings elucidate the immune dysregulation that occurs throughout
the progression of FGR.
The existence of limitations in this research should
not be overlooked. First, additional in vitro investigations
are necessary to explore the direct interactions among Notch1,
Jagged1 and immune cells. Second, confirming these findings through
FGR animal models would significantly enhance the robustness of the
present study.
In conclusion, the present study provides
preliminary evidence for a novel regulatory mechanism in the
progression of FGR, pointing out that Notch signaling may mediate
immune balance to influence FGR development. These findings offer
valuable insights into potential clinical diagnostics and
interventions for the management of FGR.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL made substantial contributions to the conception
and design of the study. LY, XZ and YY made substantial
contributions to the acquisition, analysis and interpretation of
the data. LY drafted the manuscript. All authors critically revised
the manuscript for intellectual content, and confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
All the individuals had been informed the study
details and given their written consent in advance. Ethical
approval (approval no. 2021-KY-009) was obtained from the Ethics
Committee of Jinhua Maternal and Child Health Hospital (Jinhua,
China) and this research adheres to the principles outlined in the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Damhuis SE, Ganzevoort W and Gordijn SJ:
Abnormal fetal growth: small for gestational age, fetal growth
restriction, large for gestational age: Definitions and
epidemiology. Obstet Gynecol Clin North Am. 48:267–279.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gupta N and Khajuria A: Histomorphological
features of placenta in pregnancy complicated with intrauterine
growth retardation. JK Sci. 18:21–25. 2016.
|
|
3
|
Nardozza LMM, Caetano ACR, Zamarian ACP,
Mazzola JB, Silva CP, Marçal VMG, Lobo TF, Peixoto AB and Araujo
Júnior E: Fetal growth restriction: Current knowledge. Arch Gynecol
Obstet. 295:1061–1077. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Turner S, Posthumus AG, Steegers EAP,
AlMakoshi A, Sallout B, Rifas-Shiman SL, Oken E, Kumwenda B,
Alostad F, Wright-Corker C, et al: Household income, fetal size and
birth weight: An analysis of eight populations. J Epidemiol
Community Health. 76:629–636. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
González-Fernández D, Muralidharan O,
Neves PA and Bhutta ZA: Associations of maternal nutritional status
and supplementation with fetal, newborn, and infant outcomes in
low-income and middle-income settings: An overview of reviews.
Nutrients. 16(3725)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dudink I, Hüppi PS, Sizonenko SV,
Castillo-Melendez M, Sutherland AE, Allison BJ and Miller SL:
Altered trajectory of neurodevelopment associated with fetal growth
restriction. Exp Neurol. 347(113885)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Della Gatta AN, Aceti A, Spinedi SF,
Martini S, Corvaglia L, Sansavini A, Zuccarini M, Lenzi J,
Seidenari A, Dionisi C, et al: Neurodevelopmental outcomes of very
preterm infants born following early foetal growth restriction with
absent end-diastolic umbilical flow. Eur J Pediatr. 182:4467–4476.
2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Rock CR, White TA, Piscopo BR, Sutherland
AE, Miller SL, Camm EJ and Allison BJ: Cardiovascular and
cerebrovascular implications of growth restriction: Mechanisms and
potential treatments. Int J Mol Sci. 22(7555)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Bezemer RE, Schoots MH, Timmer A, Scherjon
SA, Erwich JJHM, van Goor H, Gordijn SJ and Prins JR: Altered
levels of decidual immune cell subsets in fetal growth restriction,
stillbirth, and placental pathology. Front Immunol.
11(1898)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Muskavitch MA: Delta-notch signaling and
Drosophila cell fate choice. Dev Biol. 166:415–430. 1994.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Siebel C and Lendahl U: Notch signaling in
development, tissue homeostasis, and disease. Physiol Rev.
97:1235–1294. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: Unfolding the activation mechanism. Cell.
137:216–233. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Afshar Y, Miele L and Fazleabas AT: Notch1
is regulated by chorionic gonadotropin and progesterone in
endometrial stromal cells and modulates decidualization in
primates. Endocrinology. 153:2884–2896. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hunkapiller NM, Gasperowicz M, Kapidzic M,
Plaks V, Maltepe E, Kitajewski J, Cross JC and Fisher SJ: A role
for Notch signaling in trophoblast endovascular invasion and in the
pathogenesis of pre-eclampsia. Development. 138:2987–2998.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sahin Z, Acar N, Ozbey O, Ustunel I and
Demir R: Distribution of Notch family proteins in intrauterine
growth restriction and hypertension complicated human term
placentas. Acta Histochem. 113:270–276. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Chen YH, Liu ZB, Ma L, Zhang ZC, Fu L, Yu
Z, Chen W, Song YP, Wang P, Wang H and Xu X: Gestational vitamin D
deficiency causes placental insufficiency and fetal intrauterine
growth restriction partially through inducing placental
inflammation. J Steroid Biochem Mol Biol.
203(105733)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hong J and Kumar S: Circulating biomarkers
associated with placental dysfunction and their utility for
predicting fetal growth restriction. Clin Sci (Lond). 137:579–595.
2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dietrich B, Haider S, Meinhardt G,
Pollheimer J and Knöfler M: WNT and NOTCH signaling in human
trophoblast development and differentiation. Cell Mol Life Sci.
79(292)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Scifres CM and Nelson DM: Intrauterine
growth restriction, human placental development and trophoblast
cell death. J Physiol. 587:3453–3458. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gale NW, Dominguez MG, Noguera I, Pan L,
Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, et
al: Haploinsufficiency of delta-like 4 ligand results in embryonic
lethality due to major defects in arterial and vascular
development. Proc Natl Acad Sci USA. 101:15949–15954.
2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Thurston G and Gale NW: Vascular
endothelial growth factor and other signaling pathways in
developmental and pathologic angiogenesis. Int J Hematol. 80:7–20.
2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Herr F, Schreiner I, Baal N, Pfarrer C and
Zygmunt M: Expression patterns of Notch receptors and their ligands
Jagged and Delta in human placenta. Placenta. 32:554–563.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kirici P, Çağıran FT, Kali Z, Tanriverdi
ES, Mavral N and Ecin SM: Determination of maternal serum
pro-inflammatory cytokine changes in intrauterine growth
restriction. Eur Rev Med Pharmacol Sci. 27:1996–2001.
2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Al-Azemi M, Raghupathy R and Azizieh F:
Pro-inflammatory and anti-inflammatory cytokine profiles in fetal
growth restriction. Clin Exp Obstet Gynecol. 44:98–103.
2017.PubMed/NCBI
|
|
26
|
Kluivers ACM, Biesbroek A, Visser W, Saleh
L, Russcher H, Danser AHJ and Neuman RI: Angiogenic imbalance in
pre-eclampsia and fetal growth restriction: enhanced soluble
fms-like tyrosine kinase-1 binding or diminished production of
placental growth factor? Ultrasound Obstet Gynecol. 61:466–473.
2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gadde R, Cd D and Sheela SR: Placental
protein 13: An important biological protein in preeclampsia. J Circ
Biomark. 7(1849454418786159)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yu N, Cui H, Chen X and Chang Y: First
trimester maternal serum analytes and second trimester uterine
artery Doppler in the prediction of preeclampsia and fetal growth
restriction. Taiwan J Obstet Gynecol. 56:358–361. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rana S, Burke SD and Karumanchi SA:
Imbalances in circulating angiogenic factors in the pathophysiology
of preeclampsia and related disorders. Am J Obstet Gynecol. 226
(2S):S1019–S1034. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Garcia-Manau P, Mendoza M, Bonacina E,
Garrido-Gimenez C, Fernandez-Oliva A, Zanini J, Catalan M, Tur H,
Serrano B and Carreras E: Soluble fms-like tyrosine kinase to
placental growth factor ratio in different stages of early-onset
fetal growth restriction and small for gestational age. Acta Obstet
Gynecol Scand. 100:119–128. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Z, Liu H, Shi Y, Xu N, Wang Y, Li A
and Song W: Increased circulating Th22 cells correlated with Th17
cells in patients with severe preeclampsia. Hypertens Pregnancy.
36:100–107. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Liu ZZ, Sun GQ, Hu XH, Kwak-Kim J and Liao
AH: The transdifferentiation of regulatory T and Th17 cells in
autoimmune/inflammatory diseases and its potential implications in
pregnancy complications. Am J Reprod Immunol. 78:2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Niedźwiecki M, Budziło O, Zieliński M,
Adamkiewicz-Drożyńska E, Maciejka-Kembłowska L, Szczepański T and
Trzonkowski P:
CD4+CD25highCD127low/-FoxP3+
regulatory T cell subpopulations in the bone marrow and peripheral
blood of children with ALL: Brief report. J Immunol Res.
2018(1292404)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Matsuda M, Terada T, Kitatani K, Kawata R
and Nabe T: Analyses of Foxp3+ Treg cells and Tr1 cells
in subcutaneous immunotherapy-treated allergic individuals in
humans and mice. Nihon Yakurigaku Zasshi. 154:17–22.
2019.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
35
|
Laresgoiti-Servitje E: A leading role for
the immune system in the pathophysiology of preeclampsia. J Leukoc
Biol. 94:247–257. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang LL, Li ZH, Wang H, Kwak-Kim J and
Liao AH: Cutting edge: The regulatory mechanisms of macrophage
polarization and function during pregnancy. J Reprod Immunol.
151(103627)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yao Y, Xu XH and Jin L: Macrophage
polarization in physiological and pathological pregnancy. Front
Immunol. 10(792)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bezemer RE, Faas MM, van Goor H, Gordijn
SJ and Prins JR: Decidual macrophages and Hofbauer cells in fetal
growth restriction. Front Immunol. 15(1379537)2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Berezhna VA, Mamontova TV and Gromova AM:
Cd68+ M1 macrophages is associated with placental insufficiency
under fetal growth restriction. Wiad Lek. 74:213–219.
2021.PubMed/NCBI
|