Introduction
Lactic acid bacteria are probiotics that adhere to
the living body and interact with the host. The bacterial cell
components also have an immunoregulatory mechanism via the
intestinal mucosa to prevent infection. Previously, it has been
shown that oral administration of live lactic acid bacteria and
their metabolites, as well as dead bacteria, affects the immune
system. Oral administration of heat-killed Enterococcus
faecalis (HkEf) increases immunoglobulin A concentration
(1) and exhibits antiviral activity
(2).
The authors have previously reported on increased
survival rates after oral administration of HkEf in systemic
bacterial infection animal models using antimicrobial-resistant
bacteria (3,4). Moreover, an increase has been also
demonstrated in spleen immune cells in mice that were orally
administered with HkEf (5).
However, this immunostimulatory effect, which occurred via systemic
infection, was difficult to explain only in terms of gut immunity;
hence, it was hypothesized that gut cells may have interacted with
the remote immune system. The immunostimulatory effects of
exosome-mediated γ-aminobutyric acid and carnosine were
investigated, and it was clarified that mice orally administered
with HkEf released exosomes that functioned as mediators in
transmitting immune response signals after phagocytosis via
macrophages (6-8).
This eventually affected remote immune cells (5).
The present study aimed to elucidate the effect of
HkEf phagocytosed by intestinal dendritic cells (DCs) to secrete
exosomes, on immune cells and their underlying mechanisms using
mouse bone marrow-derived DCs [BMDCs; mainly plasmacytoid (pDCs) or
conventional (cDCs)] as intestinal DCs, to examine the effects of
exosomes purified and isolated from HkEf-stimulated BMDCs on a
subset of immune cells derived from mouse spleen, and analyzing
microRNAs (miRNAs) in exosomes derived from BMDCs and macrophages
(BMDMs).
Materials and methods
Experiment 1
Effects of mouse BMDC-derived HkEf-stimulated
exosomes on mouse spleen cells. HkEf (cat. no. EF-2001; Nihon
Berumu Co. Ltd.) was supplied as a heat-killed, dried powder.
EF-2001 was also used in Experiments 2 and 3. HkEf (300 mg) was
suspended in 3 ml of Penicillin-Streptomycin-Amphotericin B
Suspension (x100) (cat. no. 161-23181; FUJIFILM Wako Pure Chemical
Corporation) for aseptic processing, and a 100-mg/ml solution was
prepared from this suspension.
Preparation of cells used in the experiment.
Dendritic progenitor cells (mouse bone marrow-derived) were
obtained from BALB/c mouse bone marrow derivatives [Bone
Marrow-Derived Dendritic Precursor (BALB/c); Mouse; cat. no.
BMDC01C; Cosmo Bio Co., Ltd.].
Spleen cells were aseptically extracted under
anesthesia from five female BALB/c mice aged 4-5 weeks and weighing
14-19 g at the start of the study. The mice purchased from Japan
SLC, Inc. were used immediately in the experiments upon arrival.
Mice were euthanized via 5% isoflurane and cervical dislocation was
used as a secondary euthanasia measure, followed by rapid
harvesting of the spleen.
These cells were added into 10 ml of 0.02%
EDTA-2Na/PBS (cat. no. 14367-74; Nacalai Tesque, Inc., for
dispersion. After centrifugation (190 x g for 7 min at room
temperature (~22-25˚C), the supernatant liquid was discarded, and
the cell cluster was added to RPMI-1640 culture medium (cat. no.
189-02025; FUJIFILM Wako Pure Chemical Corporation). After
pipetting, it was centrifuged (190 x g for 7 min at room
temperature; ~22-25˚C), and the supernatant liquid was discarded.
This cell cluster was dismantled and stirred for 10 min with RBC
Lysis Buffer (cat. no. 420302; BioLegend Inc.) for hemolysis
treatment. Following this, the mixture was centrifuged (190 x g for
7 min at room temperature; ~22-25˚C), the supernatant was
discarded, cells were resuspended in RPMI-1640 culture medium, to
which 5 ml of 2% exosome-depleted fetal bovine serum (FBS; cat. no.
EXO-FBSHI-50A-1; System Biosciences) was added, following which the
cells were counted.
Induction of BMDCs (pDC/cDC). DCs were
induced to differentiate from bone marrow cells according to the
standard method (9). Dendritic
progenitor cells (mouse bone marrow-derived) were inducted for
differentiation using the Flt3 ligand (Flt3-L; cat. no. 060-04803;
FUJIFILM Wako Pure Chemical Corporation) to obtain pDCs and cDCs.
For their differentiation, Flt3, a cytokine, was essential
(10,11). The pDCs and normal DC fraction of
cDCs can reportedly be inducted via culturing bone marrow cells in
Flt3-L-containing culture medium (11).
Commercially available dendritic progenitor cells
were dispersed using 10% exosome-depleted FBS and Dulbecco's
modified Eagle's medium (DMEM, High Glucose) with L-Glutamine,
phenol red, and sodium pyruvate (cat. no. 043-30085; FUJIFILM Wako
Pure Chemical Corporation). Aliquots of the differentiating media
for pDCs and cDCs (1 ml/well) were dispensed in six wells of a
12-well plate and cultured for three days under 5% carbon dioxide
(CO2) at 37˚C and 100% humidity. After washing in DMEM
at 7˚, the differentiating medium was added and cultured in a
CO2 incubator. Thereafter, the culture medium was
changed every other day. Cells cultured on day 11 were used for the
study.
Purification and quantification of BMDC
(pDC/cDC)-derived exosomes. After the differentiation culture
medium was removed, DMEM (producing medium) including 2%
exosome-depleted FBS was added. For HkEf (+), 30 µl of aseptic HkEf
was added; for HkEf (-), only the
Penicillin-Streptomycin-Amphotericin B Suspension was added and
cultured for three days, and then centrifuged at 1,000 x g for 15
min.
The supernatant was processed to isolate exosomes
using the EXO-Prep kit (cat. no. HBM-EXP-C25; HansaBioMed Life
Sciences; https://hansabiomed.eu/). Exosome
quantity was determined by the ExoELISA-ULTRA Complete Kit (CD81
Detection) (cat. no. EXEL-ULTRA-CD81-1; System Biosciences;
https://www.systembio.com/), following
the manufacturer's instructions.
Mouse spleen cell stimulation test with BMDC
(pDC/cDC)-derived exosomes. The spleen cell suspension prepared
as aforementioned was seeded on a 6-well plate such that the
survival cell count was 2x106 cells. After measuring the
exosome quantity using ELISA, the following cultures were
performed: 1. HkEf (-) group: 40 µl of non-stimulated exosome
purified fraction was added (n=5); 2. HkEf (+) group: 40 µl of
stimulated pDC/cDC exosome purified fraction was added (n=5). These
were cultured for five days in a CO2 incubator at 37˚C.
After culture, the cells were collected, and flow cytometry was
performed.
These experiments were approved (approval no. 003)
of the Institutional Animal Care and Use Committee of the OBM
Research Center (Osaka, Japan).
Analyzing the spleen cell subset using flow
cytometry. The spleen cell subset was measured to identify each
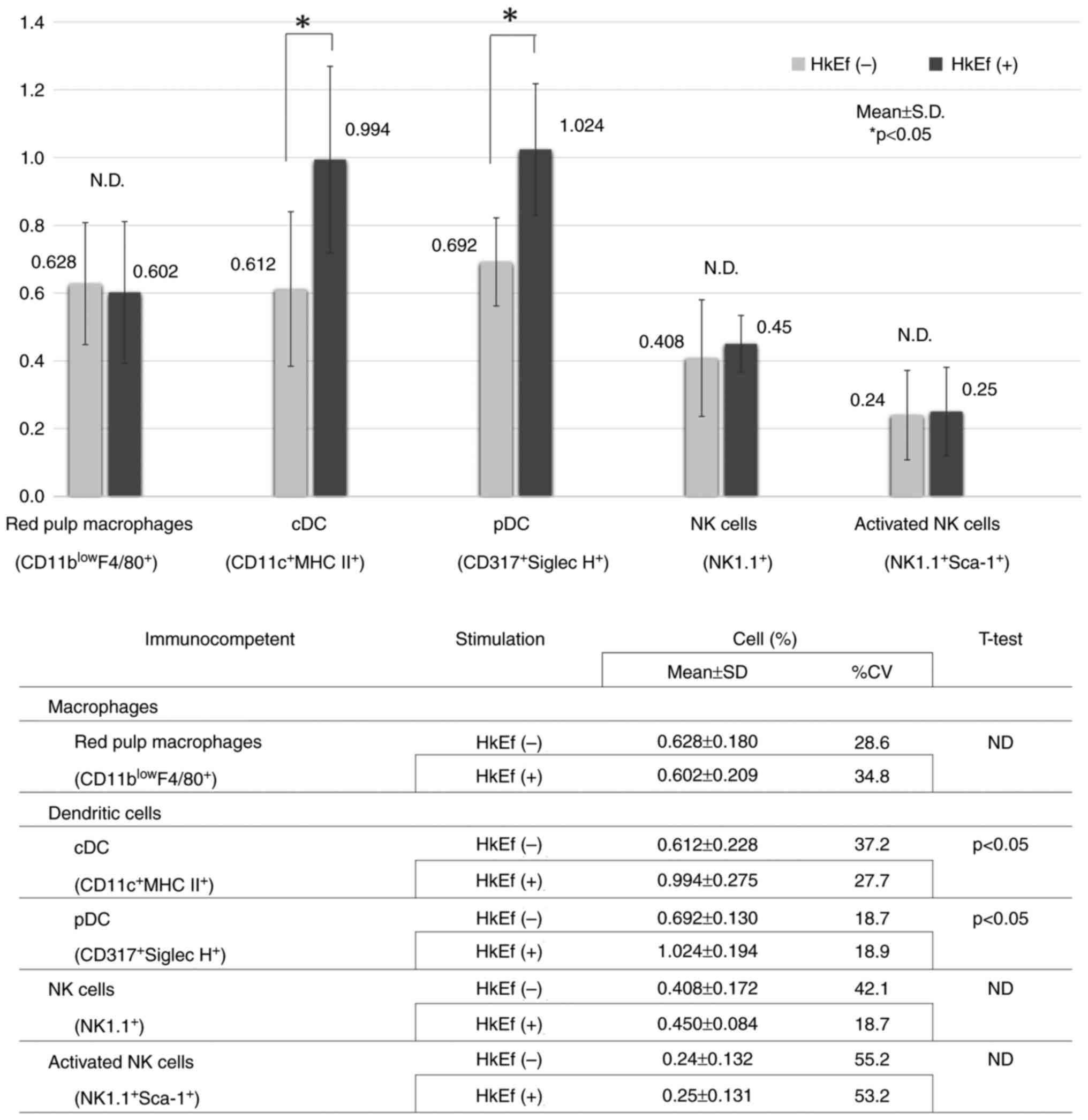
immune cell via double staining. As shown in Table I, immunocompetent cell-specific
markers were red pulp macrophages
(CD11blowF4/80+) in macrophages, and cDCs
(CD11c+MHCII+) and pDCs (CD317+
SiglecH+) in DCs. The assay was performed using a
Guava® easyCyte HT System flow cytometer (Merck
Millipore) and analyzed using guavaSoft™ software (version 4.0;
Merck Millipore). Flow cytometry settings were as follows: Count,
50000; Threshold, 9262 (FSC); Gain FSC, 19.9; SSC, 9.93; GTN-B,
9.93; YEL-B, 9.93; RED-B, 1.04.
 | Table IImmunocompetent cell-specific
markers. |
Table I
Immunocompetent cell-specific
markers.
| Immunocompetent
cell | Specific marker |
|---|
| Macrophages | | |
|
Red pulp
macrophages (CD11blowF4/80+) | CD11b | F4/80 |
| Dendritic
cells | | |
|
Conventional
dendritic cells (CD11c+MHCII+) | CD11c | MHCII |
|
Plasmacytoid
dendritic cells (CD317+ SiglecH+) | CD317 | SiglecH |
| NK cells
(NK1.1+) | NK1.1 | - |
| Activated NK cells
(NK1.1+Sca-1+) | NK1.1 | Sca-1 |
Experiment 2
MiRNA assay of mouse BMDC-derived HkEf-stimulated
exosome fractions. HkEf (-) and HkEf (+) exosome fractions
obtained from BMDCs in Experiment 1 were assayed. The following
four samples were used as controls: i) Negative control:
DNase/RNase-free water (without dissolution step, AC); ii) Negative
control: DNase/RNase-free water (with dissolution step, DC); iii)
Positive control: RNA control; and iv) Positive control: healthy
human pooled serum.
Quantification of miRNA. miRNA quantification
was performed using the FirePlex® miRNA Assay-Core
Reagent Kit V3 (Abcam). Briefly, purified exosomes were hybridized
with FirePlex® Particles and labeled with target miRNAs
by PCR (FirePlex® miRNA Panel-Immunology V2; cat. no.
ab218369; Abcam). Subsequently, the labeled targets were analyzed
using a flow cytometer (Guava® easyCyte™ 5HT, Cytek
Biosciences). The sequences of primers have not been provided by
the manufacturer. As the FirePlex® miRNA Panel uses
homologous human and mouse regions for detection, allowing for the
detection of both human and mouse miRNAs.
Experiment 3
MiRNA assay of mouse BMDM-derived HkEf-stimulated
exosome fractions. Mouse bone marrow cells were stimulated
using GM-CSF (077-04674; FUJIFILM Wako Pure Chemical Corporation).
HkEf (-) and (+) exosome fractions were obtained from BMDMs as
previously described by Matsuo et al (5).
miRNA assay using flow cytometry. According
to the Fireplex miRNA Assay V3-Assay Protocol, particles equivalent
to each miRNA were assayed via flow cytometry using the
FirePlex® miRNA Panel.
Statistical analysis
For comparison between HkEf (-) and (+) groups
related to the effect on immune subsets of mouse spleen cells in
BMDC-derived exosomes, an unpaired t-test was performed using a
commercially available statistical program (Numbers; Apple, Inc.),
with the significance level set to P<0.05. For miRNA, the
adjusted P-value was calculated using the Benjamini-Hochberg
multiple comparison test, which allows adjustment of family-wise
error rates in multiple significance testing. Statistical analysis
was performed using a commercially available statistical program
(Analysis Workbench FirePlex®; Abcam PLC) with the
significance level set to P<0.05.
Results
HkEf-stimulated BMDC-derived exosome
purification
BMDC-derived exosome production in Experiment 1 was
compared with or without HkEf stimulation. The results revealed
that HkEf (-) exosomes and HkEf (+) exosomes had almost the same
concentration of exosomes, ~1.21x1011 particles per 50
µl. Based on this result, 40 µl (~9.6x1010 particles) of
exosomes were added to mouse spleen cells.
Effect of mouse BMDC-derived
HkEf-stimulated exosomes on mouse spleen cells
The number of cDCs (CD11c+, MHC
II+) in the HkEf (+) group (0.994 ± 0.275) increased
more significantly than that in the HkEf (-) group (0.612±0.228)
(P=0.044; Fig. 1). The number of
pDC (CD317+, Siglec H+) in the HkEf (+) group
(1.024±0.194) increased more significantly than that in the HkEf
(-) group (0.692±0.130) (P=0.013; Fig.
1).
However, no significant differences were observed in
red pulp macrophages (CD11blowF4/80+),
natural killer (NK) cells (NK1.1+), and activated NK
cells (NK1.1+, Sca-1+) between both groups
(P=0.84, 0.64, and 0.94, respectively; Fig. 1).
miRNA assay of mouse BMDC-derived
HkEf-stimulated exosome fractions
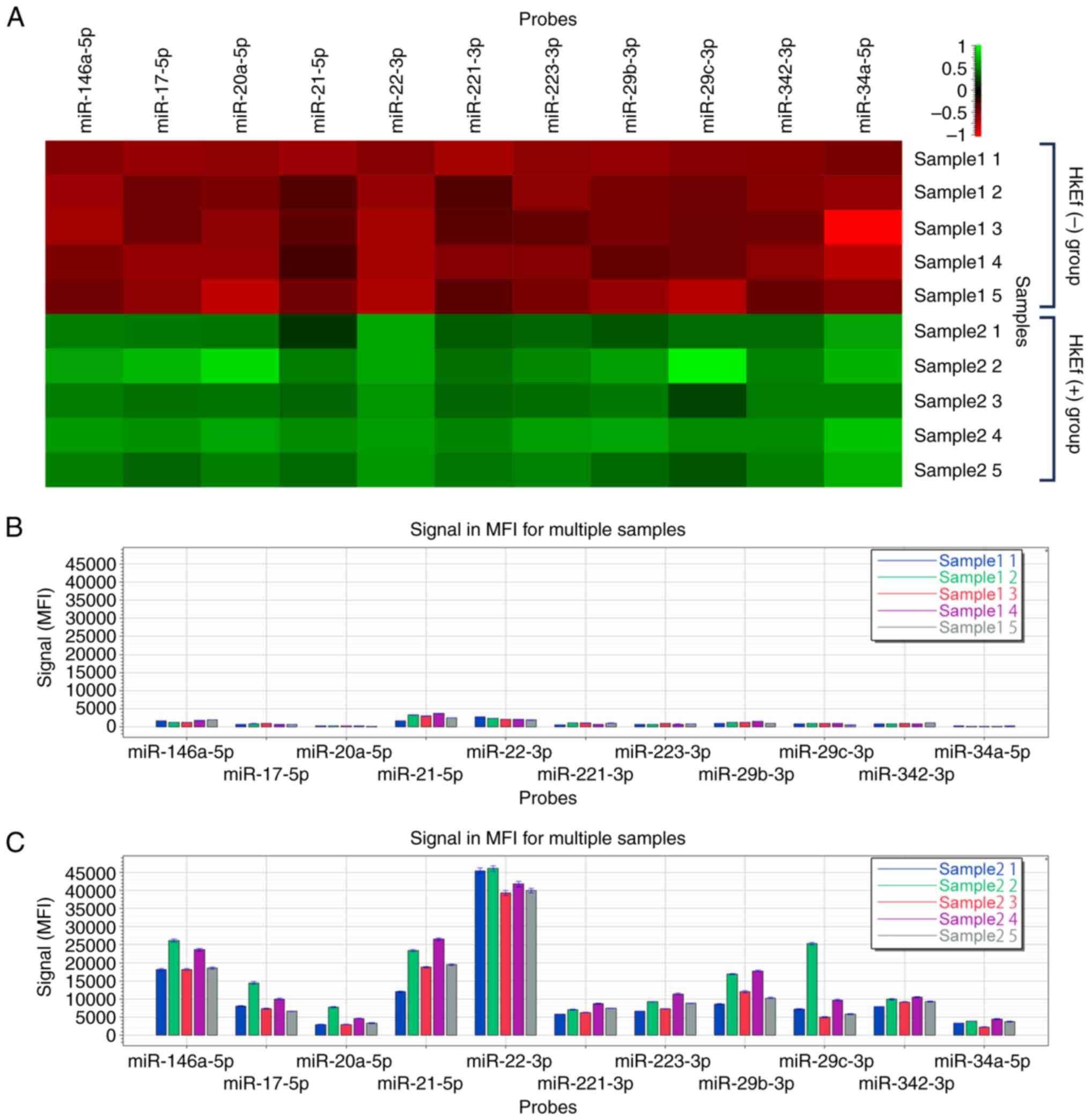
The heatmap of FirePlex®miRNA multiplex
assay of BMDC-derived exosome fractions in Experiment 2 is shown in
Fig. 2.
In the HkEf (+) group, the exosome fractions
significantly increased compared with that in the HkEf (-) group at
a fluorescence intensity of ≥2,000 (P<0.05, multiple comparison
test, Benjamini-Hochberg procedure). The miRNAs detected were
miR-146a-5p (13.54-fold), miR-17-5p (12.17-fold), miR-20a-5p
(16.32-fold), miR-21-5p (7.12-fold), miR-22-3p (19.37-fold),
miR-221-3p (8.25-fold), miR-223-3p (11.01-fold), miR-29b-3p
(11.01-fold), miR-29c-3p (11.15-fold), miR-342-3p (10.66-fold) and
miR-34a-5p (23.62-fold) (Table
II).
 | Table IIMultiple comparison of immune
microRNA of mouse BMDC-derived HkEf-stimulated exosome
fractions. |
Table II
Multiple comparison of immune
microRNA of mouse BMDC-derived HkEf-stimulated exosome
fractions.
| | HkEf (-) group | HkEf (+) group | | |
|---|
| Probe | Mean (MFI) | CV% | Mean (MFI) | CV% | HkEf (+) group/HkEf
(-) group | Multiple
comparisona |
|---|
| miR-146a-5p | 1527.05 | 20.12 | 20671.12 | 17.33 | 13.54 | 0.00000011 |
| miR-17-5p | 731.01 | 18.59 | 8899.87 | 31.83 | 12.17 | 0.0000013 |
| miR-20a-5p | 244.62 | 23.60 | 3993.22 | 43.21 | 16.32 | 0.0000038 |
| miR-21-5p | 2721.55 | 32.14 | 19376.33 | 30.91 | 7.12 | 0.000020 |
| miR-22-3p | 2190.91 | 15.36 | 42439.20 | 7.29 | 19.37 | 0.0000000043 |
| miR-221-3p | 845.66 | 33.73 | 6976.36 | 15.92 | 8.25 | 0.0000039 |
| miR-223-3p | 771.19 | 15.53 | 8487.27 | 21.71 | 11.01 | 0.0000002 |
| miR-29b-3p | 1143.64 | 20.70 | 12596.98 | 32.18 | 11.01 | 0.0000023 |
| miR-29c-3p | 784.83 | 29.34 | 8746.94 | 72.00 | 11.15 | 0.00013 |
| miR-342-3p | 873.58 | 14.15 | 9310.07 | 11.26 | 10.66 | 0.000000021 |
| miR-34a-5p | 144.50 | 54.89 | 3413.23 | 25.77 | 23.62 | 0.0000053 |
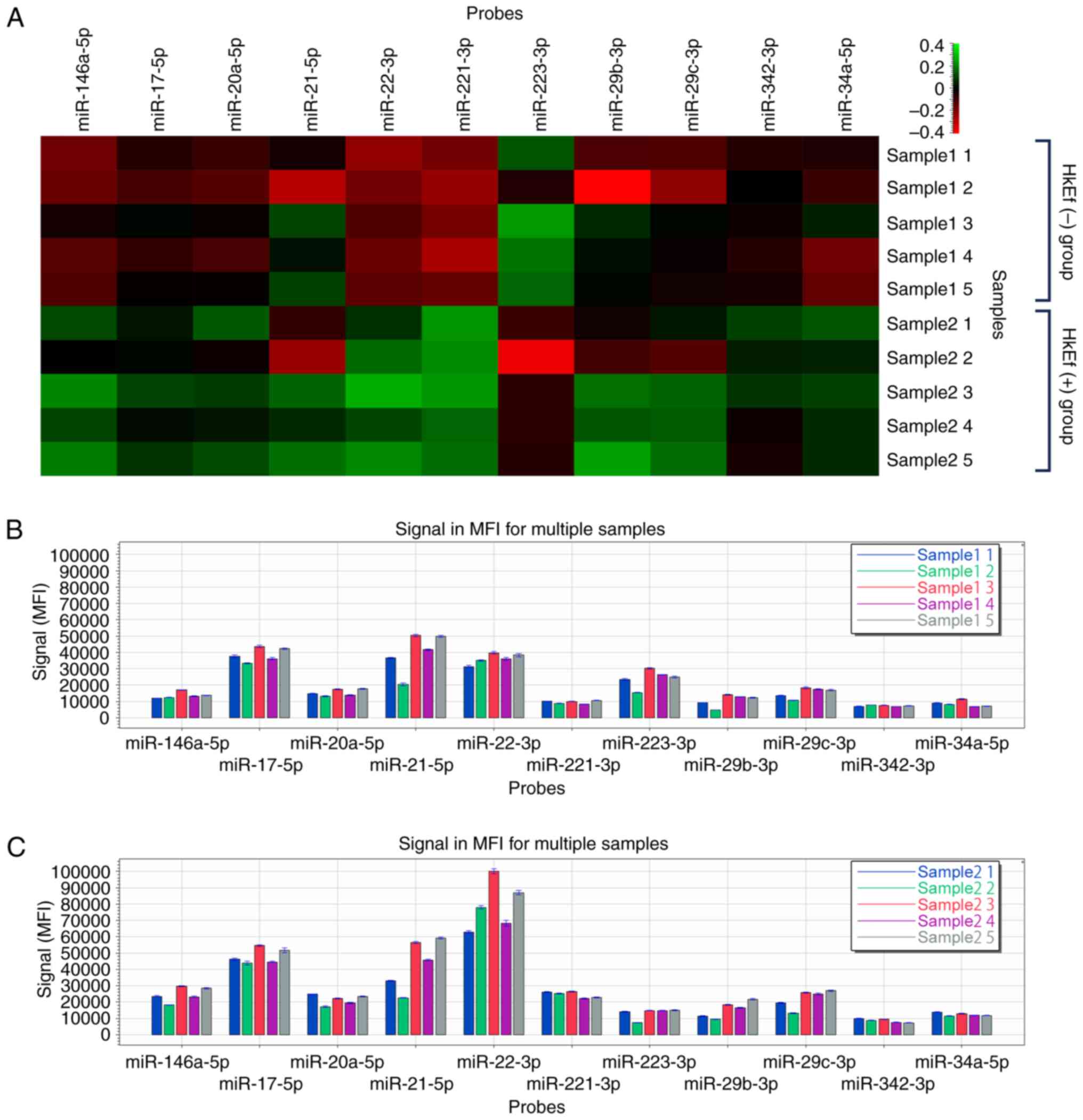
miRNA assay of mouse BMDM-derived
HkEf-stimulated exosome fractions
The heatmap of the FirePlex®miRNA
multiplex assay of BMDM-derived exosome fractions in Experiment 3
is revealed in Fig. 3.
In the HkEf (+) group, the exosome fractions
significantly increased compared with those in the HkEf (-) group
at a fluorescence intensity ≥2,000 (P<0.05, multiple comparison
test, Benjamini-Hochberg procedure). The miRNAs detected were:
miR-146a-5p (1.79-fold), miR-17-5p (1.25-fold), miR-20a-5p
(1.39-fold), miR-22-3p (2.17-fold), miR-221-3p (2.57-fold) and
miR-34a-5p (1.48-fold). A significant decrease in miR-223-3p
(0.54-fold) was confirmed (Table
III).
 | Table IIIMultiple comparison of immune
microRNA of mouse BMDM-derived HkEf-stimulated exosome
fractions. |
Table III
Multiple comparison of immune
microRNA of mouse BMDM-derived HkEf-stimulated exosome
fractions.
| | HkEf (-) group | HkEf (+) group | | |
|---|
| Probe | Mean (MFI) | CV% | Mean (MFI) | CV% | HkEf (+) group/HkEf
(-) group | Multiple
comparisona |
|---|
| miR-146a-5p | 13569.78 | 13.60 | 24267.01 | 19.54 | 1.79 | 0.0042 |
| miR-17-5p | 38445.69 | 11.11 | 48028.35 | 9.60 | 1.25 | 0.034 |
| miR-20a-5p | 15252.93 | 13.46 | 21277.14 | 14.98 | 1.39 | 0.020 |
| miR-22-3p | 35997.20 | 9.34 | 78147.10 | 18.71 | 2.17 | 0.00052 |
| miR-221-3p | 9514.26 | 10.11 | 24486.83 | 8.28 | 2.57 | 0.0000069 |
| miR-223-3p | 23559.91 | 25.82 | 12793.83 | 31.86 | 0.54 | 0.030 |
| miR-34a-5p | 8358.58 | 21.03 | 12338.22 | 7.77 | 1.48 | 0.015 |
Discussion
In a previous study by the authors (5), BMDMs were used as test cells. Orally
administered HkEf was suggested to have been phagocyted by gut
macrophages after which they released exosomes as signal
transmitters for immune regulation. In Experiment 1 of the present
study, a stimulation test on mouse spleen cells was also conducted
using BMDCs (pDC/cDC) obtained via differentiation with Flt3-L.
Quantitative changes were predicted in exosomes upon HkEf
stimulation; however, no marked quantitative differences were
observed between the HkEf (-) and (+) groups in terms of exosome
release. While cDC and pDC significantly increased in the HkEf (+)
group compared with that in the HkEf (-) group based on the effect
on the immune spleen cell subset, no such influence was observed in
NK cells, activated NK cells, and red pulp macrophages. This
suggests that the qualitative change of exosomes is accompanied by
quantitative changes in cDC and pDC. Furthermore, exosomes related
to immune response signals were released from gut immune cells due
to HkEf intake and were transported to remote tissues; this finding
is supported by a previous study (5) on systemic immunostimulatory
regulation.
In Experiment 2, the miRNAs of BMDC-derived exosome
fractions with or without HkEf stimulation were further examined.
In human monocyte-derived cells, miR-146, which was significantly
increased due to HkEf stimulation, is reportedly increased upon
inflammatory stimulation, such as by inflammatory cytokines (tumor
necrosis factor-α, interleukin-1β), lipopolysaccharide and
peptidoglycan (12). Natural
immunity is significantly affected by miR-146. When miR-146
forcibly expressed in mouse hematopoietic stem and progenitor cells
and is transplanted in mouse bone marrow after eliminating the
cells via radiation, their differentiation to myelocytes in the
blood is promoted, whereas their differentiation to lymphocytes is
impaired (13). This is because of
a filling mechanism; when natural immunity cells combat infectious
pathogens, the number of cells is often reduced (14,15).
miR-146 is suggested to play a part in this mechanism and
preferentially induces the myeloid differentiation pathway rather
than the lymphocyte differentiation pathway.
As other miRNAs increased significantly upon HkEf
stimulation, miRNAs are related to DC differentiation and maturity.
miR-29 has been suggested to play an important role in early
hematopoietic stem cell self-renewal and is involved in the onset
of acute myeloid leukemia (16).
When the expression of miR-146, miR-29b and miR-29c is increased,
the survival rate of pDC is lowered (17,18).
Conversely, miR-21 expression has been shown to
inhibit apoptosis (19). In the
present study, the miRNAs that acted against survival upon HkEf
stimulation (specifically, miR-146, miR-29b, miR-29c and miR-21)
were greatly increased. An increase in pDC was confirmed in
exosome-added mouse spleen cells; hence, it was hypothesized that
apoptosis can, overall, be inhibited. In miR-34a-overexpressing
bone marrow chimera and transgenic mice, increased DCs were
confirmed (20). miR-21 and miR-34a
promote monocyte to DC differentiation by inhibiting the expression
of JAG1 and WNT1, proteins that are necessary for hematopoietic
differentiation and generation (21). miR-22 acts as a negative control
factor of IRF8, which is a transcription factor that determines the
cell fate of neutrophils, monocytes and DCs. Inducing miR-22
expression in DC generation promotes cDC generation with
compensation of pDC; miR-22 inhibition shows the opposite effect
(22). It influences the
differentiation of the DC subset. Upon miR-221 expression, pDC
generation is inhibited (23).
During DC maturation, miR-221 expression is inversely related to
the expression of the cell-cycle regulator p27kip1.
Therefore, the apoptosis of DCs due to the accumulation of
p27kip1 appears to be inhibited (24). In the differentiation from monocytes
to immature and mature DCs, the expression of miR-29c, miR-21 and
miR-342 is increased (24). miR-223
is highly expressed in hematopoietic cells and is essential for
natural immune reactions to control bone marrow differentiation and
granulocyte function (25,26). In hematopoietic cells, miR-223
overexpression promotes granulocyte differentiation, whereas the
differentiation of erythrocytes and macrophages is inhibited
(27). Although miR-223 expression
increased in all processes upon the induction of differentiation of
human embryonic stem cells to DCs, the expression of the
differentiation marker is decreased by the miR-223 inhibitor
(28).
In Experiment 3, miRNAs of exosome fractions derived
from BMDMs were examined.
The significantly increased miRNAs were almost
common among the BMDC- and BMDM-derived exosome fractions, despite
small differences in the quantity. Unlike the measured value of
BMDC-derived exosome fractions, the rate of increase in miR-17 and
miR-20a, which inhibited differentiation to macrophages targeting
RUNX1(29), were smaller in
BMDM-derived exosome fractions (Experiment 3: miR-17, 1.25-fold,
miR-20a, 1.39-fold; Experiment 2: miR-17, 12.17-fold, miR-20a,
9.59-fold). In a previous study of BMDM-derived mouse spleen cells
(5), the number of red pulp
macrophages increased. This may be because the rate of increase in
BMDM-derived miR-17 and miR-20a was smaller than that of BMDCs,
because of which differentiation to macrophages may be induced.
Although in Experiment 3 miR-223 decreased more in the HkEf (+)
than in the HkEf (-) groups, in Experiment 2, miR-223 conversely
increased significantly (Experiment 3: 0.54-fold; Experiment 2:
11.01-fold). As previously mentioned, the inhibition of miR-223
induces an increase in erythrocytes and macrophages, while
differentiation to DCs is inhibited.
Thus, HkEf induces the release of miRNA-rich
exosomes, which act as immune response signaling mediators from
intestinal DCs and macrophages. These exosomes preferentially
induce the myeloid differentiation pathway through the effect of
miRNAs, primarily miR-146, in spleen immune cells. Moreover,
induction of differentiation to DCs and macrophages appears to be
promoted by altered expression of miRNAs, such as miR-223.
The aforementioned discussion is a hypothesis from
existing literature. On the other hand, phenotypic analysis
revealed that there were no differences in the frequency and cell
number of DC and macrophage populations in the spleen of
miR-223-deficient mice (25). This
may not be due to the effect on spleen immune cells of miRNA-rich
exosomes from intestinal DCs and macrophages, but on bone
marrow-derived stem cells in the bone marrow.
This result could be explained as an effect on
extramedullary hematopoiesis (30)
in the spleen, but this was not clear from the experimental design
of the present study. The final targets of these changed miRNAs
should be clarified, and the biological significance of miRNAs
should be confirmed in further studies.
The present study has certain limitations. First,
these investigations were animal studies, and a similar effect on
humans cannot be guaranteed. The effect of exosomes on remote cells
using mouse spleen cells was studied in vitro.
The surface of exosomes contains proteins and
lipids. Inside the exosomes, mRNA, DNA and various proteins exist;
hence, these may play a role in signal transmission as well,
requiring further study for more definitive conclusions.
In conclusion, BMDCs induced from mouse bone marrow
progenitor cells were stimulated with HkEf, and the released
exosomes were purified and isolated. As a result of examining the
effect of this exosome on immune cell subsets derived from mouse
spleen cells, it can be suggested that this exosome has an
immunostimulatory effect mediated by DCs, mainly that of pDC (in
vitro).
Moreover, as a result of measuring miRNAs in
HkEf-stimulated exosome fractions derived from BMDCs (pDC/cDCs) and
BMDMs (macrophages), effects mediated by miRNA-rich exosomes such
as miR-146, which play a part in the complement mechanism of cells
of the innate immune system are hypothesized. Orally administered
HkEf releases exosomes involved in immune response signals by
intestinal immune cells (macrophages and DCs), and regulates immune
activation as an effect on distant immune tissues via blood
circulation. Thus, it can be hypothesized that this is one of the
mechanisms to prevent systemic infection.
Acknowledgements
The authors would like to thank the OBM Research
Center and its members who performed the animal experiments.
Funding
Funding: The present study was supported by Nutri Co., Ltd.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KN conceptualized and designed the study, conducted
formal analysis, interpreted the data, and wrote the original
draft. TM and IS conceptualized and investigated the study,
reviewed and edited the manuscript. KH conceptualized the study,
interpreted the data, reviewed the manuscript and supervised the
study. KN and KH confirm the authenticity of all the raw data. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Animal experiments were conducted under the approval
(approval no. 003) of the Institutional Animal Care and Use
Committee of the OBM Research Center (Osaka, Japan).
Patient consent for publication
Not applicable.
Competing interests
All authors are employees of NUTRI Co., Ltd.
References
|
1
|
Inatomi T and Otomaru K: Effects of
heat-killed Enterococcus faecalis T-110 supplementation on
gut immunity, gut flora, and intestinal infection in naturally aged
hamsters. PLoS One. 15(e0240773)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Watanabe T, Hayashi K, Kan T, Ohwaki M and
Kawahara T: Anti-influenza virus effects of Enterococcus
faecalis KH2 and Lactobacillus plantarum SNK12 RNA.
Biosci Microbiota Food Health. 40:43–49. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hara K, Nakao K and Kawaguchi S: Efficacy
of heat-killed lactic acid bacteria Enterococcus faecalis on
methicillin-resistant Staphylococcus aureus (MRSA) infected mice. J
New Rem Clin. 67:35–44. 2018.(In Japanese).
|
|
4
|
Nakao K, Hara K, Matsuo T and Kawauchi S:
Effects of heat-killed lactic acid bacteria Enterococcus
faecalis on life-threatening antibiotic-resistant bacteria in
animal models. J New Rem Clin. 69:276–287. 2020.(In Japanese).
|
|
5
|
Matsuo T, Nakao K, Hara K and Kawaguchi S:
Blood-derived exosomes released after the oral administration of
heat-killed Enterococcus faecalis activate immunity. Biosci
Biotechnol Biochem. 86:1699–1704. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Inotsuka R, Udono M, Yamatsu A, Kim M and
Katakura Y: Exosome-mediated activation of neuronal cells triggered
by γ-aminobutyric acid (GABA). Biosci Microb Food Health. 40:43–49.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Inotsuka R, Uchimura K, Yamatsu A, Kim M
and Katakura Y: γ-Aminobutyric acid (GABA) activates neuronal cells
by inducing the secretion of exosomes from intestinal cells. Food
Funct. 11:9285–9290. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sugihara Y, Onoue S, Tashiro K, Sato M,
Hasegawa T and Katakura Y: Carnosine induces intestinal cells to
secrete exosomes that activate neuronal cells. PLoS One.
14(e0217394)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hemmi H, Kaisho T, Takeda K and Akira S:
The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein
kinase catalytic subunit in the effects of two distinct CpG DNAs on
dendritic cell subsets. J Immunol. 170:3059–3064. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Steinman RM: Decisions about dendritic
cells: Past, present, and future. Annu Rev Immunol. 30:1–22.
2012.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gilliet M, Boonstra A, Paturel C,
Antonenko S, Xu XL, Trinchieri G, O'Garra A and Liu YJ: The
development of murine plasmacytoid dendritic cell precursors is
differentially regulated by FLT3-ligand and granulocyte/macrophage
colony-stimulating factor. J Exp Med. 195:953–958. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Starczynowski DT, Kuchenbauer F, Wegrzyn
J, Rouhi A, Petriv O, Hansen CL, Humphries RK and Karsan A:
MicroRNA-146a disrupts hematopoietic differentiation and survival.
Exp Hematol. 39:167–178.e4. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ueda Y, Kondo M and Kelsoe G: Inflammation
and the reciprocal production of granulocytes and lymphocytes in
bone marrow. J Exp Med. 201:1771–1780. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Nagai Y, Garrett KP, Ohta S, Bahrun U,
Kouro T, Akira S, Takatsu K and Kincade PW: Toll-like receptors on
hematopoietic progenitor cells stimulate innate immune system
replenishment. Immunity. 24:801–812. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Han YC, Park CY, Bhagat G, Zhang J, Wang
Y, Fan JB, Liu M, Zou Y, Weissman IL and Gu H: microRNA-29a induces
aberrant self-renewal capacity in hematopoietic progenitors, biased
myeloid development, and acute myeloid leukemia. J Exp Med.
207:475–489. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Karrich JJ, Jachimowski LC, Libouban M,
Iyer A, Brandwijk K, Taanman-Kueter EW, Nagasawa M, de Jong EC,
Uittenbogaart CH and Blom B: MicroRNA-146a regulates survival and
maturation of human plasmacytoid dendritic cells. Blood.
122:3001–3009. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hong Y, Wu J, Zhao J, Wang H, Liu Y, Chen
T, Kan X, Tao Q, Shen X, Yan K and Zhai Z: miR-29b and miR-29c are
involved in Toll-like receptor control of glucocorticoid-induced
apoptosis in human plasmacytoid dendritic cells. PLoS One.
8(e69926)2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Buscaglia LEB and Li Y: Apoptosis and the
target genes of microRNA-21. Chin J Cancer. 30:371–380.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang A, Yang Y, Chen S, Xia F, Sun D,
Fang D, Xiong S, Jin L and Zhang J: MiR-34a promotes DCs
development and inhibits their function on T cell activation by
targeting WNT1. Oncotarget. 8:17191–17201. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hashimi ST, Fulcher JA, Chang MH, Gov L,
Wang S and Lee B: MicroRNA profiling identifies miR-34a and miR-21
and their target genes JAG1 and WNT1 in the coordinate regulation
of dendritic cell differentiation. Blood. 114:404–414.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li HS, Greeley N, Sugimoto N, Liu YJ and
Watowich SS: miR-22 controls Irf8 mRNA abundance and murine
dendritic cell development. PLoS One. 7(e52341)2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kuipers H, Schnorfeil FM and Brocker T:
Differentially expressed microRNAs regulate plasmacytoid vs
conventional dendritic cell development. Mol Immunol. 48:333–340.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lu C, Huang X, Zhang X, Roensch K, Cao Q,
Nakayama KI, Blazar BR, Zeng Y and Zhou X: miR-221 and miR-155
regulate human dendritic cell development, apoptosis, and IL-12
production through targeting of p27kip1, KPC1, and SOCS-1. Blood.
117:4293–4303. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhou H, Xiao J, Wu N, Liu C, Xu J, Liu F
and Wu L: MicroRNA-223 regulates the differentiation and function
of intestinal dendritic cells and macrophages by targeting C/EBPβ.
Cell Rep. 13:1149–1160. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bros M, Youns M, Kollek V, Buchmüller D,
Bollmann F, Seo EJ, Schupp J, Montermann E, Usanova S, Kleinert H,
et al: Differentially tolerized mouse antigen presenting cells
share a common miRNA signature including enhanced mmu-miR-223-3p
expression which is sufficient to imprint a Protolerogenic State.
Front Pharmacol. 9(915)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yuan X, Berg N, Lee JW, Le TT, Neudecker
V, Jing N and Eltzschig H: MicroRNA miR-223 as regulator of innate
immunity. J Leukoc Biol. 104:515–524. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu MX, Wan WL, Hu K, Wang YF, Wang J, Zhu
XW, Yan XX, Jing HM and Ke XY: MicroRNA-223 regulates the
differentiation of human embryonic stem cells to dendritic cells.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 25:1275–1282. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
29
|
Fontana L, Pelosi E, Greco P, Racanicchi
S, Testa U, Liuzzi F, Croce CM, Brunetti E, Grignani F and Peschle
C: MicroRNAs 17-5p-20a-106a control monocytopoiesis through AML1
targeting and M-CSF receptor upregulation. Nat Cell Biol.
9:775–787. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Kim CH: Homeostatic and pathogenic
extramedullary hematopoiesis. J Blood Med. 1:13–19. 2010.PubMed/NCBI View Article : Google Scholar
|