Introduction
Coronavirus disease 2019 (COVID-19), caused by
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2),
necessitates urgent preventive measures and vaccination,
particularly in high-risk populations (1,2), such
as individuals aged >60 years and those with lung or hearth
disease or diabetes. Among the currently known mRNA vaccines
developed, BNT162b2 encodes the SARS-CoV-2 spike protein (S
protein) and was authorized in 2021 by the U.S. Food and Drug
Administration and recommended by the European Medicines Agency for
medical use following a large, placebo-controlled, randomized phase
III study, which demonstrated its efficacy in preventing COVID-19
and safety of two 30-µg doses administered 21 days apart (3,4). A
third dose of BNT162b2, tested to boost its efficacy against
SARS-CoV-2 in a placebo-controlled, randomized, phase III trial
(5), was administered at a median
of 10.8 months after the second dose (5). At a median follow-up of 2.5 months,
the third dose showed 95.5% effectiveness against COVID-19 without
any corresponding new unexpected adverse events.
COVID-19 infection typically starts with flu-like
symptoms (6) and can either remain
asymptomatic or has a mild to severe disease course (7). The infection is characterized by a
significant burden of inflammation (8). The association between inflammatory
parameters, such as neutrophil/lymphocyte ratio,
platelet/lymphocyte ratio, mean platelet volume (MPV), red cell
distribution width (RDW), monocyte to lymphocyte ratio (MLR) and
COVID-19 infection, has been previously established (9). In addition, decreased hemoglobin and
increased red cell distribution width have been associated with
recurrent hospitalizations by patients with COVID-19(10). A hemoglobin A1C (HbA1C) test, MPV
and MLR were introduced as possible predictors of frailty, a
geriatric state of reduced functional reserve and vulnerability, in
patients with diabetes during COVID-19(11). Considering the association between
COVID-19 vaccines and increased levels of inflammatory biomarkers,
such as TNF-α, IL-1β, IL-6, IL-8 and IL-10(12), the study of their effects in a
health-care setting is reasonable.
Healthcare facilities pose a high risk for infection
acquisition (13). Therefore,
personal protective equipment combined with vaccination is crucial
for COVID-19 protection. Real world data from US and Israel
(14,15) confirmed the high efficacy of
BNT162b2 in healthcare workers (HCWs), consistent with findings
from previous randomized trials (16,17).
Despite the high effectiveness of this vaccine, new COVID-19 cases
have increased worldwide over 1-12 months (18,19),
raising concerns of waning immunity. A prospective study involving
vaccinated HCWs with BNT162b2 previously reported a consistent
decline in IgG and neutralizing antibody titers over 6 months. In
addition, 6 months after the second vaccine dose, significant
declines in neutralizing antibodies were observed in male patients,
in individuals aged >65 years and in those with
immunosuppression (20). However,
another previous report (21)
showed a low rate (2.1%) of BNT162b2 failure (defined as ratio of
subjects diagnosed with COVID-19 to overall study population)
despite a rapid decline in IgG antibodies and an absence of
cellular immune response after 6 months in the majority of enrolled
HCWs. In addition, at 6 months, IgG antibodies and the levels of
T-cell activation had significantly declined in individuals with
obesity (21).
The purpose of the present study was to determine
the incidence of symptomatic COVID-19 following the third dose of
BNT162b2 and to assess its safety profile, with focus on sex and
BMI, in a real-world medical facility setting (National Cancer
Institute, Bratislava, Slovakia). Additionally, IgG levels
following vaccination were explored, which were then used to assess
their possible association with sex, BMI, number of adverse events
(AEs) and the presence of COVID-19.
Materials and methods
Study design and end points
The present study is a case-control study with the
primary objective of determining the incidence of symptomatic
COVID-19, defined as onset within ≤7 days after the third dose of
BNT162b2. Another objective of the present study was to assess
vaccine safety with focus on sex and BMI. As in a real-world
scenario, a symptomatic SARS-CoV-2 infection was confirmed using
rapid antigen tests, rapid antigen real-time reverse transcription
PCR (RT-PCR) or loop-mediated isothermal amplification tests. In
the present study, patients only reported symptomatic infection and
that they had tested positive for SARS-CoV-2. The investigators did
not provide precise data on the test used, rendering it impossible
to state the criteria used to define infection using each of these
kits. A secondary objective was to explore potential differences in
plasma IgG levels following vaccination and their association with
sex, BMI, AEs and positivity for SARS-CoV-2. The recruitment period
was between October and December 2021. All subjects were recruited
at the National Cancer Institute (NCI; Bratislava, Slovakia).
Enrollment criteria
Participants had to be current employees of the NCI,
vaccinated with three doses of BNT162b2 and aged ≥18 years old.
Exclusion criteria included the use of corticosteroids, such as
prednisone, prednisolone, methylprednisolone and hydrocortisone,
COVID-19 diagnosed between the second and third vaccine doses,
cigarette smoking and concomitant vaccines administered.
Data validation
Data were entered into electronic forms by EM, KR,
AS, which were subsequently validated by LS and JO to ensure
accuracy.
Process of vaccination
All individuals were vaccinated with the dilution of
0.3 ml BNT162b2 (batch no. FE7010 01/01/2022; supplied by Pfizer,
Inc. and BioNTech) at the NCI. The process of the vaccine
preparation, as previously described (13), was conducted in accordance with the
product license and manufacturer recommendations. Briefly, the
vials of BNT162b2 were thawed and diluted with 1.8 ml sodium
chloride 9 mg/ml (0.9%) solution for injection. After dilution, 6
doses were extracted from each vial and administered
intramuscularly.
Measurement of IgG antibodies
Blood samples were obtained from fasting
participants by 6 ml venipuncture in the morning using BD
Vacutainer® tubes with citrate (BD Biosciences). IgG
antibodies to the receptor-binding domain (RBD) of the S1 antigen
of SARS-CoV-2 were determined using the Atellica® IM
SARS-CoV-2 IgG assay (cat. no. 11207388 Siemens Healthineers).
Results were reported qualitatively as non-reactive (<1.00
index; IgG negative) or reactive (≥1.00 index; IgG positive) and
quantitatively within a measuring interval of 1.0-150.0 index
(22).
Statistical analysis
All statistical analyses were performed using NCSS
24 Statistical Software Version 24.0.3 (NCSS) (23). Data were summarized as mean ±
standard deviation (SD) and range for continuous variables, or as
frequencies for categorical variables. For some continuous
variables, data were also analyzed using the median. For normally
distributed continuous variables, defined by Shapiro-Wilk test,
P-values were determined using the paired t-test and unpaired
t-test when comparing two independent groups (IgG levels in males
vs. females or high vs. low BMI groups). When the distribution of
values was found to be non-normal, the Wilcoxon rank-sum test was
used to calculate the P-values. For categorical variables, P-values
were determined using Fisher's exact test or the χ² test.
To examine predictors of adverse events (AEs) and
immune response, multiple logistic regression analysis and multiple
linear regression analysis were performed. The models included the
following: i) Headache occurrence as a dependent variable (yes vs.
no) with sex (male vs. female), BMI (continuous, kg/m²), IgG index
(continuous), prior COVID-19 infection (yes vs. no) and the number
of AEs (continuous count) as independent variables; ii) presence of
AEs as a binary dependent variable (yes vs. no) with sex (male vs.
female), BMI (continuous, kg/m²) and IgG index (continuous) as
independent variables; and iii) IgG levels as a continuous
dependent variable, with sex (male vs. female), BMI (continuous,
kg/m²), number of AEs (continuous count) and prior COVID-19
infection (yes vs. no) as independent variables. In addition, a
comprehensive multivariate analysis was conducted to evaluate the
combined effects of multiple predictors on single outcome
variables. The logistic regression model included sex, BMI and IgG
levels as independent variables, with presence of AEs as the
dependent variable evaluated as binary outcome (yes vs. no).
Additionally, linear regression was used to assess IgG levels
(continuous variable) as the dependent variable, with sex (male vs.
female), BMI (continuous, kg/m²), number of AEs (continuous count)
and prior COVID-19 (yes vs. no) infection as independent variables.
P≤0.05 was considered to indicate a statistically significant
difference. Multicollinearity was assessed using variance inflation
factors (VIFs) (24), where the
variable BMI ≥30 kg/m2 was removed due to collinearity
issues. Briefly, VIF was measured, and the threshold was defined as
VIF ≤10 to detect multicollinearity among independent variables. If
a variable had VIF >10, it was considered highly collinear and
potentially redundant in the model. As a result, BMI ≥30 kg/m² was
removed due to excessive collinearity with other predictors,
ensuring that the regression analysis provided stable and
interpretable estimates.
Results
Subjects
In total, 273 eligible individuals who met the
inclusion criteria were enrolled into the present study. Of this
cohort, 232 were HCWs (48 doctors, 103 nurses, 8 pharmacists, 52
medical technicians, 1 psychologist, 2 physiotherapists 18 other
HCWs) and 41 were non-HCWs in administration. The mean age of the
study cohort was 47±11 years, with a median of 47 years (range,
22-80 years). The majority of the participants were female
individuals (N=233; 85.3%). The median of BMI was 24.8
kg/m2 (95% CI, 23.7-25.7; range, 16.3-44.6
kg/m2), 53 participants had a high BMI of ≥30
kg/m2. All individuals had been vaccinated with three
doses of BNT162b2. The median time between second and third vaccine
dose was 8.5 months (range, 4.9-10.9 months). Baseline
characteristics are shown in Table
I.
 | Table ICharacteristics of subjects. |
Table I
Characteristics of subjects.
| Total
population | N (%) | Median (range) |
|---|
| Overall | 273 (100.0) | |
|
Healthcare
workers | 232 (85.0) | |
|
Doctors | 48 (17.6) | |
|
Nurses | 103 (37.7) | |
|
Pharmacists | 8 (2.9) | |
|
Medical
technicians | 52 (19.0) | |
|
Psychologist | 1 (0.4) | |
|
Physiotherapists | 2 (0.7) | |
|
Other
HCWs | 18 (6.6) | |
|
Non-HCWs | 41 (15.0) | |
| Age, years | | 47 (22-80) |
| Sex | | |
|
Male | 40 (14.7) | |
|
Female | 233 (85.3) | |
| Body mass index,
kg/m2 | | 24.8
(16.3-44.6) |
|
<30 | 220 (80.6) | |
|
≥30 | 53 (19.4) | |
Incidence of symptomatic SARS-CoV-2
infection following vaccination
At the median follow-up of 4.7 months (range,
2.9-5.0 months), 38 participants contracted COVID-19 following
vaccination, resulting in an incidence rate of 13.9%. During the
spring of 2022, the predominant circulating SARS-CoV-2 strain in
Slovakia was the Omicron variant (BA.5) (25). The median time from receiving the
third dose of BNT162b2 to the diagnosis of COVID-19 was 2.8 months
(range, 0.2-4.4 months). All patients who contracted COVID-19
experienced a mild course of infection and survived.
AEs following BNT162b2 third dose
At least one AE was reported by 258 participants
(94.5%), with the median number of BNT162b2 AEs being 3 (range,
0-13). In total, 143 individuals (52.4%) experienced three or more
AEs. The most frequently reported AEs were pain at the injection
site (200, 73.3%), fatigue (120, 44.0%) and pain in extremity (90,
33.0%). A comprehensive summary of all AEs is presented in Tables II and III.
 | Table IIAdverse events of the BNT162b2 third
dose by sex (N=273). |
Table II
Adverse events of the BNT162b2 third
dose by sex (N=273).
| Study
population | Total incidence N
(%) | Female (n=233), n
(%) | Male (n=40), n
(%) | P-values |
|---|
| Any adverse
events | 256 (93.8) | 220 (94.4) | 36 (90.0) | |
| Headache | 62 (22.7) | 56 (24.0) | 6 (15.0) | 0.2086 |
| Muscle pain | 68 (24.9) | 60 (25.8) | 8 (20.0) | 0.4381 |
| Joint pain | 47 (17.2) | 45 (19.3) | 2 (5.0) | 0.0271 |
| Pain at injection
site | 200 (73.3) | 175 (75.1) | 25 (62.5) | 0.0967 |
| Fatigue | 120 (44.0) | 105 (45.1) | 15 (37.5) | 0.3741 |
| Chills | 55 (20.1) | 49 (21.0) | 6 (15.0) | 0.3806 |
| Pyrexia | 51 (18.7) | 43 (18.5) | 8 (20.0) | 0.8172 |
| Swelling at
injection site | 36 (13.2) | 33 (14.2) | 3 (7.5) | 0.2508 |
| Nausea | 25 (9.2) | 22 (9.4) | 3 (7.5) | 0.6946 |
| Redness at
injection site | 29 (10.6) | 27 (11.6) | 2 (5.0) | 0.2125 |
| Swallen lymph
nodes | 47 (17.2) | 42 (18.0) | 5 (12.5) | 0.3933 |
| Insomnia | 19 (7.0) | 15 (6.4) | 4 (10.0) | 0.4143 |
| Pain in
extremity | 90 (33.0) | 84 (36.1) | 6 (15.0) | 0.0091 |
| Itching at
injection site | 12 (4.4) | 12 (5.2) | 0 (0.0) | 0.1429 |
| Lethargy | 23 (8.4) | 32 (13.7) | 3 (7.5) | 0.2769 |
| Hypoaesthesia | 9 (3.3) | 8 (3.4) | 3 (7.5) | 0.2278 |
 | Table IIIAdverse events of the third dose of
the BNT162b2 by BMI (N=273). |
Table III
Adverse events of the third dose of
the BNT162b2 by BMI (N=273).
| Study
population | N (%) | BMI <30
kg/m2 (n=233), n (%) | BMI ≥30
kg/m2 (n=40), n (%) | P-values |
|---|
| Any adverse
event | 256 (93.8) | 220 (94.4) | 36 (90.0) | |
| Headache | 62 (22.7) | 46 (20.9) | 16 (30.2) | 0.1485 |
| Muscle pain | 68 (24.9) | 48 (21.8) | 20 (37.7) | 0.0164 |
| Joint pain | 47 (17.2) | 33 (15.0) | 14 (26.4) | 0.0486 |
| Pain at injection
site | 200 (73.3) | 160 (72.7) | 40 (75.5) | 0.6859 |
| Fatigue | 120 (44.0) | 91 (41.4) | 29 (54.7) | 0.0793 |
| Chills | 55 (20.1) | 41 (18.6) | 14 (26.4) | 0.2059 |
| Pyrexia | 51 (18.7) | 41 (18.6) | 10 (18.9) | 0.9691 |
| Swelling at
injection site | 36 (13.2) | 24 (10.9) | 12 (22.6) | 0.0237 |
| Nausea | 25 (9.2) | 20 (9.1) | 5 (9.4) | 0.9382 |
| Redness at
injection site | 29 (10.6) | 18 (8.2) | 11 (20.8) | 0.0078 |
| Swallen lymph
nodes | 47 (17.2) | 39 (17.7) | 8 (15.1) | 0.6492 |
| Insomnia | 19 (7.0) | 14 (6.4) | 5 (9.4) | 0.4313 |
| Pain in
extremity | 90 (33.0) | 70 (31.8) | 20 (37.7) | 0.4116 |
| Itching at
injection site | 12 (4.4) | 6 (2.7) | 6 (11.3) | 0.0061 |
| Lethargy | 23 (8.4) | 28 (12.7) | 7 (13.2) | 0.9254 |
| Hypoaesthesia | 9 (3.3) | 10 (4.5) | 1 (1.9) | 0.3778 |
In addition to the common AEs, 17 participants
(6.2%) reported other AEs possibly associated with the vaccination,
including herpes infection (3, 1.1%), shivering (3, 1.1%), vomitus
(2, 0.7%), vomitus and diarrhea (1, 0.4%), diarrhea (1, 0.4%),
tinnitus (1, 0.4%), dry cough (1, 0.4%), abdominal pain (1, 0.4%),
loss of appetite (1, 0.4%), lower back pain (1, 0.4%), tachycardia
(1, 0.4%), and eyelid edema (1, 0.4%). The median number of AEs in
all participants was 3 (0-13) and it was significantly higher in
female individuals compared with that in male individuals (3.0±2.8
vs. 1.5±2.6; P<0.05) and in participants with high BMI compared
to those with low BMI (4.0±3.0 vs. 2.0±2.8; P<0.05). No serious
AEs (SAEs), such as myocarditis, anaphylaxis or severe
thromboembolic events, were reported following vaccination.
In terms of sex, the incidence of joint pain and
pain in extremity was statistically significantly higher in female
patients compared with those in their male counterpart (P<0.05;
Table II). In addition,
participants with high BMI more frequently reported muscle pain,
joint pain, swelling, redness and itching at injection site
(P<0.05; Table III).
Plasma IgG following vaccination
The median time to plasma IgG measurement after the
third dose of the vaccine was 3.4 months (range, 2.1-4.8 months),
with a median plasma IgG level of 114.9 index (range, 4.7-150.0
index).
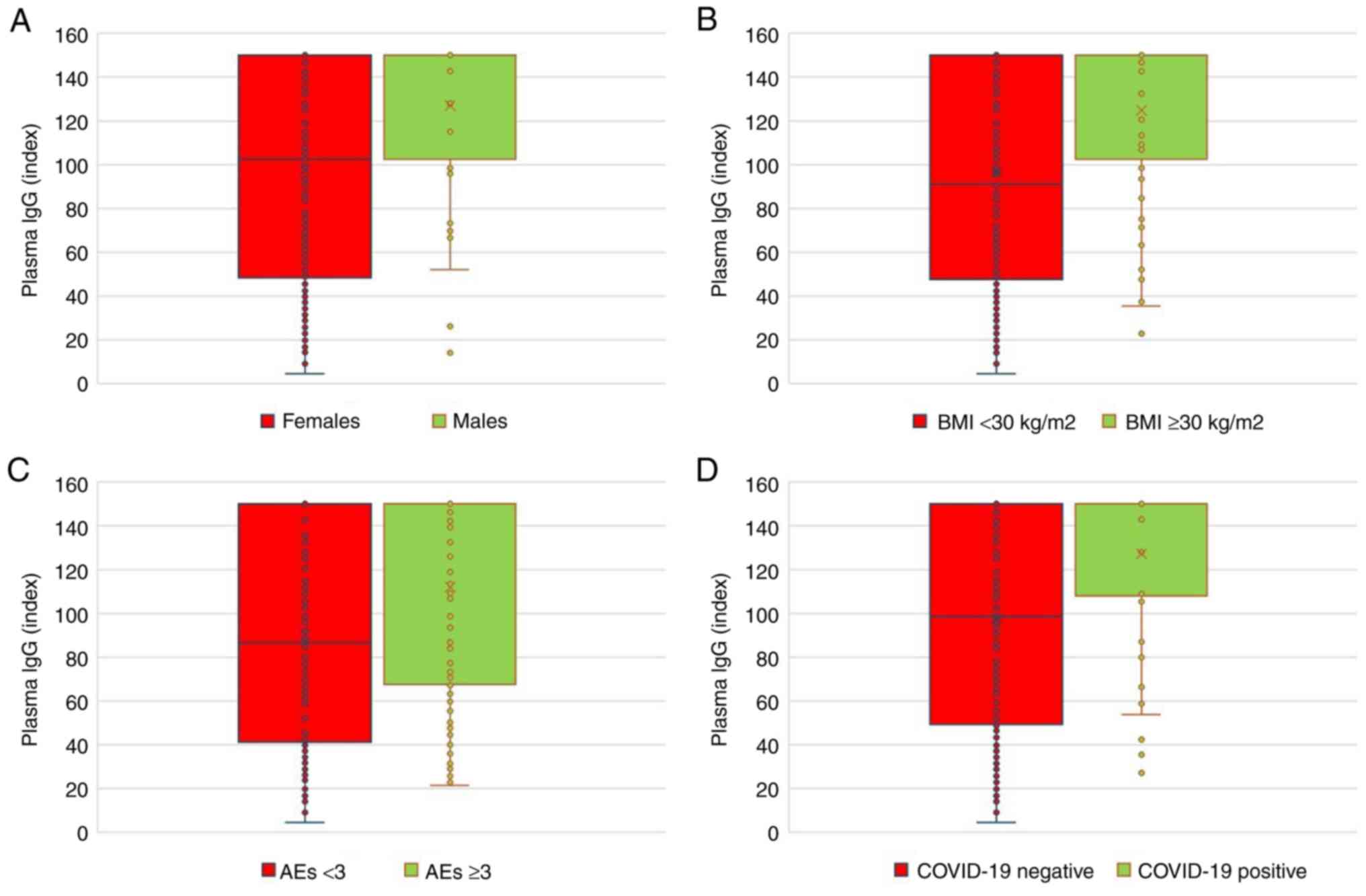
IgG antibody levels were found to be significantly
higher in male patients compared with those in female patients, in
participants with high BMI compared with those with low BMI, in
individuals experiencing a high number of AEs of ≥3 compared with
those with a low number of AEs and in participants who tested
positive for COVID-19 compared with those who did not (P<0.05;
Fig. 1 and Table IV).
 | Table IVPlasma IgG antibodies following the
BNT162b2 third dose. |
Table IV
Plasma IgG antibodies following the
BNT162b2 third dose.
| Study
population | N | Mean ± SD
(Index) | P-values |
|---|
| Sex | | | 0.0005 |
|
Female | 233 | 97.48±49.98 | |
|
Male | 40 | 126.99±39.37 | |
| Body mass
index | | | 0.0001 |
|
<30
kg/m2 | 220 | 96.25±50.47 | |
|
≥30
kg/m2 | 53 | 124.84±38.44 | |
| Number of adverse
events | | | 0.0003 |
|
<3 | 130 | 90.56±50.83 | |
|
≥3 | 143 | 112.03±46.35 | |
| Presence of
Coronavirus disease 2019 | | | 0.0006 |
|
No | 235 | 97.69±49.92 | |
|
Yes | 38 | 127.27±39.52 | |
Multivariate regression results from the model
explained 28.03% of the variance in headache occurrence (R²=0.2803;
adjusted R²=0.2612). The F-statistic (F=14.741; P<0.0001)
confirmed that at least one predictor significantly contributed.
All adverse events (not only headache) were included in the
multivariate analysis, which showed that the only AE of
significance was headache occurrence. Table V only shows the multivariate
analysis (multiple regression) for headache occurrence for
transparency. The number of AEs was the only significant predictor
of headache occurrence (β=0.0789; P<0.0001). Other factors,
including IgG index, sex, BMI and prior COVID-19 infection, were
not significant predictors (Table
V). The comparison calculations were not performed for the BMI
≤30 kg/m2 due to collinearity issues, whereas other
predictors had acceptable VIFs.
 | Table VMultiple regression analysis for
headache occurrence. |
Table V
Multiple regression analysis for
headache occurrence.
| Variable | Regression
Coefficient | Standard Error | OR (95% CI) | P-value |
|---|
| Sex (male vs.
female) | 0.006 | 0.065 | 1.006
(0.886-1.143) | 0.927 |
| Body mass index
(continuous) | -0.0049 | 0.0068 | 0.995
(0.982-1.008) | 0.473 |
| IgG
(continuous) | -0.0003 | 0.0005 | 0.9997
(0.999-1.001) | 0.554 |
| COVID-19 after 3rd
dose (yes vs. no) | 0.0805 | 0.0651 | 1.083
(0.954-1.231) | 0.217 |
| Number of AEs
(<3 AEs vs. ≥3 AEs) | 0.0789 | 0.0083 | 1.082
(1.065-1.100) | <0.0001 |
The univariate analyses were not performed as they
could provide biased data in this case. Univariate analysis does
not control the confounding variables, meaning the observed
associations may be misleading. If the number AEs is associated
with both IgG levels and prior COVID-19 infection, univariate
analysis may wrongly suggest that AEs alone are driving IgG
changes, when in fact prior COVID-19 infection is a confounding
factor. Additionally, reporting univariate results alongside
multivariate findings may create confusion by presenting unadjusted
associations that do not accurately reflect real-world
interactions. Since the primary aim of the present study was to
identify independent predictors of IgG levels, univariate analysis
would not provide meaningful additional insights. Instead, it could
introduce interpretation bias and misrepresentation of causal
relationships. Therefore, a multivariate approach was chosen to
ensure that results account for multiple influencing factors
simultaneously, providing a more accurate and reliable assessment
of predictors.
Discussion
In the present study with enrolled >270
individuals, a third 30-µg dose of BNT162b2, received at a median
of 8.5 months after the second dose, was effective in terms of
percentage of subjects tested positive to SARS-CoV-2 and safety
according to the absence of serious AEs. The incidence of COVID-19
following vaccination was found to be <14%, where ~95%
participants reported at least one AE. The most frequent AEs were
injection site pain (73.3%), fatigue (44.0%) and extremity pain
(33.0%). Statistically significant differences in the incidence of
pain (joints and extremity) favored male patients, whilst pain
(joints and muscles) with local reactions at the injection site
(swelling, itching, and redness) were more common in participants
with low BMI. These observations associated with a significantly
higher median number of AEs in female patients compared with male
patients and in participants with high compared with low BMI.
The use of the BNT162b2 third dose was authorized by
European Medicine Agency for individuals aged ≥16 years to improve
protection against COVID-19 based on the results of a phase III
study (5). At a median follow-up of
2.5 months, 7 cases of COVID-19 were identified among 5,056
subjects who received three doses of the vaccine (5). In the present study, the detection of
38 cases of infection in a population of 273 individuals can be
explained by the longer follow-up period and the circulation of a
different predominant SARS-CoV-2 strain when COVID-19 was
diagnosed. Additionally, the fact that the majority of the present
study population consisted of HCWs, who tended to be more aware of
even clinically minor symptoms and signs, may have led to the more
frequent testing for SARS-CoV-2, resulting in a higher proportion
of COVID-19 cases diagnosed. The higher overall incidence of AEs
may also be attributed to the constitution of the present study
population, mostly comprising of HCWs who are better informed about
AEs and are more likely to attribute new symptoms to the
administered vaccine.
The present findings demonstrate that the number of
AEs was the strongest predictor of headache occurrence, supporting
a dose-response relationship between systemic reactogenicity and
headache risk. Other demographic and clinical factors, including
sex, BMI, IgG levels and prior COVID-19 infection, were not
significantly associated with headaches. These results emphasize
that immune response intensity (as measured by IgG) does not
directly associate with headache occurrence. In addition, the use
of multivariate regression revealed a moderate model fit
(R²=0.2803; adjusted R²=0.2612), suggesting that additional
unmeasured factors may contribute to headache risk. These findings
provide potential insights into the reactogenicity of the third
BNT162b2 dose and suggest that headache risk is primarily
influenced by overall reactogenicity instead of specific
demographic or immunological factors. Future studies should
consider larger sample sizes, additional confounding factors as
pre-existing autoimmune diseases (such as rheumatoid arthritis and
lupus), medication use (such as corticosteroids and
immunosuppressants), lifestyle factors (including smoking and
alcohol consumption) or genetic predisposition to immune response
and extended follow-up periods to confirm these findings.
Evidence of the effective protection provided by
BNT162b2 has been previously yielded by various countries. In
Hungary, the risk of mortality associated with COVID-19 was reduced
by 82% in the general population immunized with a booster dose of
vaccines, including BNT162b2(26).
Furthermore, the booster dose was found to enhance BNT162b2 vaccine
efficacy even in immunocompromised subjects in Hungary (27). Similarly, Berec et al
(28) provided real-world evidence
on the waning protection against COVID-19 at 8 months and the
restoration of efficacy following booster dose administration in
Czechia (28). In a medical center
in Israel, known for its mass vaccination policy during the
pandemic (29), the BNT162b2
booster dose was found to significantly reduce the breakthrough
infection rate regardless of age in HCWs (29).
The higher incidence of AEs in female patients
compared with male counterparts and in those with high BMI compared
with low BMI can stem from the recognized germline-encoded
differences in innate immune responses, evident in polymorphisms or
variability in sex chromosomes and the autosomal genes encoding
immunological proteins (30). These
differences include the varying activity and number of innate
immune cells, CD4+ and CD8+ T cells, B lymphocyte subsets and the
production of cytokines and chemokines. Hormonal mediators, such as
estradiol, progesterone and androgens, serve significant roles in
innate and adaptive immunity (30).
Environmental factors, such as nutrition and microbiota, can also
impact vaccine efficacy (30).
Results from the present study are consistent with those of a
previous meta-analysis including 46 studies, which showed higher
rates of AEs in female patients compared with those in male
patients following immunization with the seasonal influenza vaccine
(31). Differences in the incidence
of AEs following the BNT162b2 mRNA vaccine by sex were also shown
in a meta-analysis conducted by Green et al (32).
The present study revealed statistically
significantly higher plasma IgG antibodies following the third
vaccine dose measured at a median time of 3.4 months in male
patients, participants with high BMI, those with a high number of
AEs and those who contracted COVID-19. Multivariate analysis showed
all these factors to be independent predictors of high plasma IgG
antibodies. Data regarding sex as a predictor of the humoral immune
response to vaccination are inconsistent. A number of studies
identified males to be low responders (33,34),
whilst others did not confirm such an association (35). In addition, Papaioannidou et
al (36) did not find any
significant relationship between BMI and IgG antibodies after two
doses of the BNT162b2 vaccine among HCWs in their hospital in
Northern Greece (36). However, in
a previous study on IgG dynamics following two doses of
BNT162b2(21), high vaccine
efficacy despite the rapid decline of plasma IgG and a negative or
borderline cellular immune response was observed in a specific
population of the NCI employees. There were significant differences
in IgG among the BMI subgroups (<18.5 kg/m2,
18.5-24.9 kg/m2, 25.0-29.9 kg/m2, 30.0-34.9
kg/m2 and ≥35.0 kg/m2). However, this must be
interpreted cautiously due to the small number of subjects in the
subgroups with BMI <18.5 kg/m2 and ≥35.0
kg/m2. Previous reports have shown associations between
SARS-CoV-2 IgG antibody titers and the incidence of AEs following
two doses of BNT162b2(37) and
greater IgG levels in individuals infected after their booster mRNA
vaccine compared with those in uninfected subjects (38-40).
It appeared that the level of IgG could also be associated with the
type of vaccine used (BNT162b2 vs. ChAdOx1) (41).
Interpreting results from various studies and making
indirect comparisons is challenging in the present study due to the
use of different methods for determining IgG following vaccination
developed by various manufacturers. In a previous report (21) and the present study, the
Atellica® IM SARS-CoV-2 IgG (sCOVG) assay from Siemens
was used with numeric results reported as an index from 1 to 150.
Wei et al (33) measured IgG
antibody levels using an ELISA method developed by the University
of Oxford, with results reported in ng/ml and positive samples
defined as ≥42 ng/ml (33).
Wolszczak-Biedrzycka et al (42) used the Elecsys anti-SARS-CoV-2 S
assay developed by Roche to determine the total IgG, IgA and IgM
antibodies against the spike RBD protein, with results reported in
U/ml and positive results defined as ≥0.80 U/ml (42).
COVID-19 has been shown to reduce mitochondrial
bioenergetics functions (43),
where vaccination against SARS-CoV-2 can prevent declines in energy
production and mitochondrial respiration in platelets (44). Hypothetical dysregulation in immune
cell mitochondrial bioenergetics may explain the differing plasma
IgG levels following vaccination in SARS-CoV-2 infected compared
with non-infected participants in the present study.
In the present study, significantly higher IgG
levels after vaccination were found in male patients, those with
high BMI and the individuals following COVID-19. Therefore, they
all could represent the populations of special interest for subject
stratifications within future clinical trials with novel vaccines.
Once these observations are confirmed and an association with lower
incidence of COVID-19 demonstrated in a prospective manner, this
may have a potential impact on public health policy due to the
reduction of costs incurred for the vaccination of individuals who
do not need to be vaccinated repeatedly or for whom a lower dose of
vaccine is sufficient. Reducing the number or doses of vaccine may
also mitigate the incidence of its late or long-lasting toxicities.
In addition, a positive impact on public healthcare and saving
resources may accordingly manifest. In the light of the present
findings, monitoring of IgG levels following vaccination against
COVID-19 seems to be reasonable.
The present study has certain limitations, including
the absence of data on IgG levels prior to vaccination due to the
time of its designing and the various methods for confirming the
diagnosis of symptomatic COVID-19. With the vast majority of the
study population being female, such a sex imbalance could render
the generalizability of the results in assessing the impact of sex
differences on the immune response difficult. The present study
also did not account for potential confounding factors, such as
comorbidities, medications or lifestyle factors. The comorbidities,
such as end stage renal disease, cancer, autoimmune disease or
hypertension, are associated with the impairment of macrophage and
lymphocyte functions, affecting immune response following
vaccination negatively (45). In
addition, cigarette smoking can attenuate the immune system by
activating MAPK and NF-κB signaling with activation of enzymes
regulating posttranslational modifications (methylation,
acetylation, phosphorylation and ubiquitination) of histones
(46). Nevertheless, taking
corticosteroids with a negative impact on humoral immune response
were among the exclusion criteria in the present study.
The absence of unvaccinated individuals or those who
received only two doses as control groups limits the ability to
draw clear conclusions about the effectiveness of the third vaccine
dose in the present study. However, the study design was adapted to
the overall number of employees who were not vaccinated with three
doses of vaccine being <1% in the NCI. In addition, the
relatively short median follow-up period limits the ability to
provide insights into the long-term effects of the vaccination. A
short follow-up period was chosen for the present study, which is
indirectly comparable to the median follow-up 2.5 months in a
previous phase III study with BNT162b2(5), predominantly in the context of
fast-changing SARS-CoV-2 variants in the population, making the
interpretation of the efficacy results difficult. By contrast, the
present results provide real-world evidence of the benefit of
vaccination in terms of the low number of COVID-19 cases and the
safety of a third dose of the BNT162b2 mRNA vaccine in a specific
medical facility providing healthcare for patients with cancer.
The current global context is determined by the
background immunity of the population and reduced severity of
SARS-CoV-2 causing clinically less serious infectious disease
(47). Even though COVID-19 is no
longer considered to be a serious threat at present (48,49),
it has nevertheless been acknowledged that there is a need for the
development of effective preventive strategies, which may influence
potential pandemics in future (49,50).
The recent developments in a field of preventive measures against
COVID-19 are comprised of the research of novel vaccines with
different mechanisms of action, including adenovirus vectors,
protein subunit, inactivated viruses and mRNAs (51-54).
However, several obstacles remain in relation to mRNA vaccines,
such as the strict temperature requirements, the delivery systems
of high effectiveness and resistance to degradation. In addition,
among the problems preventing their spread in low-income countries
are the relatively high costs for production (55).
To conclude, the present study showed the low
percentage of COVID-19 cases and the safety of a third dose of
BNT162b2 in employees of the NCI. In addition, a greater occurrence
of vaccine-related AEs was observed in female patients and
participants with high BMI. Furthermore, plasma IgG antibody levels
were found to be dependent on several factors, including sex,
frequency of vaccine adverse events, BMI and COVID-19 diagnosis
following vaccination. Understanding the importance of IgG titers
against COVID-19 infection may facilitate the setting up of
vaccination programs and adaptation of the vaccine dosage and/or
dose interval according to the characteristics of individual
subjects.
Acknowledgements
For technical support in terms of administrative
activities, gratitude went to Ms. Miroslava Augustínová from the
National Cancer Institute (Bratislava, Slovakia).
Funding
Funding: National Cancer Institute, Bratislava, SK (Atellica
assays) and OncoReSearch, Rovinka, SK (APC).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PP and MV initiated and designed the study concept,
and confirm the authenticity of all the raw data. EM, KR, AS, LS
and JO participated in data collection and manuscript writing. All
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study adhered to the principles of the
Declaration of Helsinki and the International Council for
Harmonization of Good Clinical Practice Guidelines (ICH GCPG). The
study protocol (code Covid-SK001; version 3.0, No. 11/2021) was
approved by the Ethics Committee of the NCI (Bratislava, Slovakia)
on September 30, 2021. All participants provided written informed
consent before enrollment; no minors were included.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization: COVID-19:
Vulnerable and high-risk groups.
|
|
2
|
CDC. Underlying conditions and the higher
risk for severe COVID-19.
|
|
3
|
Polack FP, Thomas SJ, Kitchin N, Absalon
J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED,
Zerbini C, et al: Safety and efficacy of the BNT162b2 mRNA Covid-19
vaccine. N Engl J Med. 383:2603–2615. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Thomas SJ, Moreira ED Jr, Kitchin N,
Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack
FP, Zerbini C, et al: Safety and efficacy of the BNT162b2 mRNA
Covid-19 vaccine through 6 months. N Engl J Med. 385:1761–1773.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moreira ED Jr, Kitchin N, Xu X, Dychter
SS, Lockhart S, Gurtman A, Perez JL, Zerbini C, Dever ME, Jennings
TW, et al: Safety and efficacy of a third dose of BNT162b2 Covid-19
vaccine. N Engl J Med. 386:1910–1921. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Aktaş G: A comprehensive review on
rational and effective treatment strategies against an invisible
enemy; SARS Cov-2 infection. Exp Biomed Res. 3:293–311. 2020.
|
|
7
|
Wu Z and McGoogan JM: Characteristics of
and important lessons from the coronavirus disease 2019 (COVID-19)
outbreak in china: summary of a report of 72 314 cases from the
Chinese center for disease control and prevention. JAMA.
323:1239–1242. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aktas G, Balci B, Yilmaz S, Bardak H,
Duman TT and Civil C: Characteristics of Covid-19 infection with
the original SARS-Cov-2 virus and other variants: A comparative
review. J Bionic Mem. 2:96–112. 2022.
|
|
9
|
Aktas G: Hematological predictors of novel
coronavirus infection. Rev Assoc Med Bras (1992). 67 (Suppl
1):S1–S2. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Atak BM, Kahveci G, Bilgin S, Kurtkulagi
O, Taslamacioglu Duman T, Demirkol ME and Aktas G: Haemoglobin and
red cell distribution width levels in internal medicine patients
indicate recurrent hospital admission during COVID-19. Fam Med Prim
Care Rev. 24:32–36. 2022.
|
|
11
|
Atak BM, Bilgin S, Kurtkulagi O, Kahveci
G, Taslamacioglu Duman T, Sagdic T and Aktas G: Frailty in diabetic
subjects during Covid-19 and its association with HbA1c, mean
platelet volume and monocyte/lymphocyte ratio. Clin Diabetol.
11:119–126. 2022.
|
|
12
|
Ostrowski SR, Søgaard OS, Tolstrup M,
Stærke NB, Lundgren J, Østergaard L and Hvas AM: Inflammation and
platelet activation after COVID-19 vaccines-possible mechanisms
behind vaccine-induced immune thrombocytopenia and thrombosis.
Front Immunol. 12(779453)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kumar G, Adams A, Hererra M, Rojas ER,
Singh V, Sakhuja A, Meersman M, Dalton D, Kethireddy S, Nanchal R
and Guddati AK: Predictors and outcomes of healthcare-associated
infections in COVID-19 patients. Int J Infect Dis. 104:287–292.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pilishvili T, Gierke R, Fleming-Dutra KE,
Farrar JL, Mohr NM, Talan DA, Krishnadasan A, Harland KK, Smithline
HA, Hou PC, et al: Effectiveness of mRNA Covid-19 vaccine among
U.S. health care personnel. N Engl J Med. 385(e90)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Benenson S, Oster Y, Cohen MJ and Nir-Paz
R: BNT162b2 mRNA Covid-19 vaccine effectiveness among health care
workers. N Engl J Med. 384:1775–1777. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Paris C, Perrin S, Hamonic S, Bourget B,
Roué C, Brassard O, Tadié E, Gicquel V, Bénézit F, Thibault V, et
al: Effectiveness of mRNA-BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19
vaccines against COVID-19 in healthcare workers: An observational
study using surveillance data. Clin Microbiol Infect.
27:1699.e5–e8. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hall VJ, Foulkes S, Saei A, Andrews N,
Oguti B, Charlett A, Wellington E, Stowe J, Gillson N, Atti A, et
al: COVID-19 vaccine coverage in health-care workers in England and
effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A
prospective, multicentre, cohort study. Lancet. 397:1725–1735.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Feikin DR, Higdon MM, Abu-Raddad LJ,
Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O'Brien KL,
Smith PG, et al: Duration of effectiveness of vaccines against
SARS-CoV-2 infection and COVID-19 disease: Results of a systematic
review and meta-regression. Lancet. 399:924–944. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bobrovitz N, Ware H, Ma X, Li Z, Hosseini
R, Cao C, Selemon A, Whelan M, Premji Z, Issa H, et al: Protective
effectiveness of previous SARS-CoV-2 infection and hybrid immunity
against the omicron variant and severe disease: A systematic review
and meta-regression. Lancet Infect Dis. 23:556–567. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Levin EG, Lustig Y, Cohen C, Fluss R,
Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al:
Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6
months. N Engl J Med. 385(e84)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Palacka P, Pol'anová M, Svobodová A,
Žigmond J, Zanchetta K, Gombárová V, Vulganová M, Slopovský J,
Obertová J, Drgoňa Ľ, et al: Effectiveness, adverse events, and
immune response following double vaccination with BNT162b2 in staff
at the national comprehensive cancer center (NCCC). Vaccines
(Basel). 10(558)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Siemens Healthineers: SARS-CoV-2 IgG
(sCOVG) assay for the detection of IgG antibodies to SARS-CoV-2.
https://www.fda.gov/media/146931/download. Accessed
August 26, 2023.
|
|
23
|
National Council for the Social Studies
(NCSS): NCSS Statistical Software. NCSS, LLC, Kaysville, UT, 2024.
https://www.ncss.com/.
|
|
24
|
Thompson CG, Kim RS, Aloe AM and Becker
BJ: Extracting the variance inflation factor and other
multicollinearity diagnostics from typical regression results.
Basic Appl Soc Psychol. 39:81–90. 2017.
|
|
25
|
Monitorovacia správa cirkulujúcich
variantov vírusu SARS-CoV-2 v Slovenskej republike zachytených v
systéme NarCoS-Národné Covid-19 Sekvenovanie.
|
|
26
|
Kiss Z, Wittmann I, Polivka L, Surján G,
Surján O, Barcza Z, Molnár GA, Nagy D, Müller V, Bogos K, et al:
Nationwide effectiveness of first and second SARS-CoV2 booster
vaccines during the delta and omicron pandemic waves in Hungary
(HUN-VE 2 study). Front Immunol. 13(905585)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Szekanecz Z, Vokó Z, Surján O, Rákóczi É,
Szamosi S, Szűcs G, Szekanecz É, Müller C and Kiss Z: Effectiveness
and waning of protection with the BNT162b2 vaccine against the
SARS-CoV-2 delta variant in immunocompromised individuals. Front
Immunol. 14(1247129)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Berec L, Šmíd M, Přibylová L, Májek O,
Pavlík T, Jarkovský J, Zajíček M, Weiner J, Barusová T and Trnka J:
Protection provided by vaccination, booster doses and previous
infection against covid-19 infection, hospitalisation or death over
time in Czechia. PLoS One. 17(e0270801)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oster Y, Benenson S, Nir-Paz R, Buda I and
Cohen MJ: The effect of a third BNT162b2 vaccine on breakthrough
infections in health care workers: A cohort analysis. Clin
Microbiol Infect. 28:735.e1–e3. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Klein SL and Flanagan KL: Sex differences
in immune responses. Nat Rev Immunol. 16:626–638. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tadount F, Doyon-Plourde P, Rafferty E,
MacDonald S, Sadarangani M and Quach C: Is there a difference in
the immune response, efficacy, effectiveness and safety of seasonal
influenza vaccine in males and females?-A systematic review.
Vaccine. 38:444–459. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Green MS, Peer V, Magid A, Hagani N, Anis
E and Nitzan D: Gender differences in adverse events following the
Pfizer-BioNTech COVID-19 vaccine. Vaccines (Basel).
10(233)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wei J, Stoesser N, Matthews PC, Ayoubkhani
D, Studley R, Bell I, Bell JI, Newton JN, Farrar J, Diamond I, et
al: Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from
the general population of the United Kingdom. Nat Microbiol.
6:1140–1149. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Amodio E, Capra G, Casuccio A, De Grazia
S, Genovese D, Pizzo S, Calamusa G, Ferraro D, Giammanco GM, Vitale
F and Bonura F: Antibodies responses to SARS-CoV-2 in a large
cohort of vaccinated subjects and seropositive patients. Vaccines
(Basel). 9(714)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Đaković Rode O, Bodulić K, Zember S,
Cetinić Balent N, Novokmet A, Čulo M, Rašić Ž, Mikulić R and
Markotić A: Decline of anti-SARS-CoV-2 IgG antibody levels 6 months
after complete BNT162b2 vaccination in healthcare workers to levels
observed following the first vaccine dose. Vaccines (Basel).
10(153)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Papaioannidou P, Skoumpa K, Bostanitis C,
Michailidou M, Stergiopoulou T, Bostanitis I and Tsalidou M: Age,
sex and BMI relations with anti-SARS-CoV-2-Spike IgG antibodies
after BNT162b2 COVID-19 vaccine in health care workers in Northern
Greece. Microorganisms. 11(1279)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Braun E, Horowitz NA, Leiba R, Weissman A,
Mekel M, Shachor-Meyouhas Y, Hussein K, Halberthal M, Azzam ZS and
Berger G: Association between IgG antibody levels and adverse
events after first and second Bnt162b2 mRNA vaccine doses. Clin
Microbiol Infect. 28:1644–1648. 2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ailsworth SM, Keshavarz B, Richards NE,
Workman LJ, Murphy DD, Nelson MR, Platts-Mills TAE and Wilson JM:
Enhanced SARS-CoV-2 IgG durability following COVID-19 mRNA booster
vaccination and comparison of BNT162b2 with mRNA-1273. Ann Allergy
Asthma Immunol. 130:67–73. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wolszczak-Biedrzycka B, Bieńkowska A and
Dorf J: Assessment of post-vaccination antibody response eight
months after the administration of BNT1622b2 vaccine to healthcare
workers with particular emphasis on the impact of previous COVID-19
infection. Vaccines (Basel). 9(1508)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wolszczak-Biedrzycka B, Bieńkowska A,
Zaborowska JE, Smolińska-Fijołek E, Biedrzycki G and Dorf J:
Anti-SARS-CoV-2S antibody levels in healthcare workers 10 months
after the administration of two BNT162b2 vaccine doses in view of
demographic characteristic and previous COVID-19 infection.
Vaccines (Basel). 10(741)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wolszczak Biedrzycka B, Bieńkowska A,
Smolińska-Fijołek E, Biedrzycki G and Dorf J: The influence of two
priming doses of different anti-COVID-19 vaccines on the production
of anti-SARS-CoV-2 antibodies after the administration of the
Pfizer/BioNTech booster. Infect Drug Resist. 15:7811–7821.
2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wolszczak-Biedrzycka B, Bieńkowska A,
Cieślikiewicz B, Smolińska-Fijołek E, Biedrzycki G and Dorf J: The
effect of the third dose of the BNT162b2 vaccine on anti-SARS-CoV-2
spike antibody levels in healthcare workers with and without
COVID-19 infection. Ann Med. 55:722–732. 2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sumbalova Z, Kucharska J, Palacka P,
Rausova Z, Langsjoen PH, Langsjoen AM and Gvozdjakova A: Platelet
mitochondrial function and endogenous coenzyme Q10 levels are
reduced in patients after COVID-19. Bratisl Lek Listy. 123:9–15.
2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gvozdjáková A, Kucharská J, Rausová Z,
Lopéz-Lluch G, Navas P, Palacka P, Bartolčičová B and Sumbalová Z:
Effect of vaccination on platelet mitochondrial bioenergy function
of patients with post-acute COVID-19. Viruses.
15(1085)2023.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Notarte KI, Ver AT, Velasco JV, Pastrana
A, Catahay JA, Salvagno GL, Yap EPH, Martinez-Sobrido L, B
Torrelles J, Lippi G and Henry BM: Effects of age, sex, serostatus,
and underlying comorbidities on humoral response post-SARS-CoV-2
Pfizer-BioNTech mRNA vaccination: A systematic review. Crit Rev
Clin Lab Sci. 59:373–390. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Francisco V, Pino J, Campos-Cabaleiro V,
Ruiz-Fernández C, Mera A, Gonzalez-Gay MA, Gómez R and Gualillo O:
Obesity, fat mass and immune system: Role for leptin. Front
Physiol. 9(640)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Petrone D, Mateo-Urdiales A, Sacco C,
Riccardo F, Bella A, Ambrosio L, Lo Presti A, Di Martino A,
Ceccarelli E, Del Manso M, et al: Reduction of the risk of severe
COVID-19 due to omicron compared to delta variant in Italy
(November 2021-February 2022). Int J Infect Dis. 129:135–141.
2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sarker R, Roknuzzaman ASM, Nazmunnahar
Hossain MJ and Islam MR: Benefits and probable ill effects of WHO's
declaration of end of COVID-19 pandemic: A way back to
pandemic-free normal life. Ann Med Surg (Lond). 85:3199–3201.
2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Roknuzzaman A, Sarker R, Nazmunnahar
Shahriar M, Mosharrafa RA and Islam MR: The WHO has declared
COVID-19 is no longer a pandemic-level threat: A perspective
evaluating potential public health impacts. Clin Pathol.
17(2632010X241228053)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Koolaparambil Mukesh R, Yinda CK, Munster
VJ and van Doremalen N: Beyond COVID-19: The promise of
next-generation coronavirus vaccines. NPJ Viruses.
2(39)2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Singh C, Verma S, Reddy P, Diamond MS,
Curiel DT, Patel C, Jain MK, Redkar SV, Bhate AS, Gundappa V, et
al: Phase III pivotal comparative clinical trial of intranasal
(iNCOVACC) and intramuscular COVID 19 vaccine
(Covaxin®). NPJ Vaccines. 8(125)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Vishwanath S, Carnell GW, Ferrari M,
Asbach B, Billmeier M, George C, Sans MS, Nadesalingam A, Huang CQ,
Paloniemi M, et al: A computationally designed antigen eliciting
broad humoral responses against SARS-CoV-2 and related
sarbecoviruses. Nat Biomed Eng. 9:153–166. 2025.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Carreño JM, Raskin A, Singh G, Tcheou J,
Kawabata H, Gleason C, Srivastava K, Vigdorovich V, Dambrauskas N,
Gupta SL, et al: An inactivated NDV-HXP-S COVID-19 vaccine elicits
a higher proportion of neutralizing antibodies in humans than mRNA
vaccination. Sci Transl Med. 15(eabo2847)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Xu K, Lei W, Kang B, Yang H, Wang Y, Lu Y,
Lv L, Sun Y, Zhang J, Wang X, et al: A novel mRNA vaccine, SYS6006,
against SARS-CoV-2. Front Immunol. 13(1051576)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Ao D, He X, Liu J and Xu L: Strategies for
the development and approval of COVID-19 vaccines and therapeutics
in the post-pandemic period. Signal Transduct Target Ther.
8(466)2023.PubMed/NCBI View Article : Google Scholar
|