Introduction
Urolithiasis, or kidney stone formation, is one of
the most common urological conditions and is characterized by the
deposition of crystals or stones within the kidneys (1). Calcium oxalate (CaOX) and/or calcium
phosphate are the predominant types of renal stones and their
formation is closely associated with increased production of
reactive oxygen species (ROS) (2).
The key processes underlying stone formation are influenced by
multiple factors, including elevated urinary calcium and phosphate
levels, as well as the relationship between urinary volume and CaOX
crystal formation (3). Renal
calculi in urolithiasis may also result from increased glycolic
acid oxidase activity induced by sodium oxalate (NaOX), which
generates glycolate and oxalate (2). When oxalate crystals deposit and
adhere to renal tubular epithelial cells, these cells become
injured and impaired (4). In
response to renal injury, epithelial cells initiate proliferation
and migration to promote regeneration and tissue repair. Collective
cell migration is a tightly regulated process essential for normal
physiological activities, including tissue repair and wound healing
(5). Among the key molecules
involved in cell migration and polarization are E-cadherin and
vimentin, which play critical roles in regulating these processes.
E-cadherin is a key integral protein that mediates lateral
cell-to-cell adhesion via adherens junctions, playing a crucial
role in maintaining tissue integrity and inhibiting relative cell
mobility. By contrast, vimentin functions as a critical regulator
of the wound repair process, serving as an intermediate filament
that facilitates cell motility and migration (6). Epithelial tight junction proteins,
such as zonula occludens (ZOs), occludin, claudins and junctional
adhesion molecules, are also essential for regulating paracellular
transport in renal tubular epithelial cells (7). These proteins can be altered following
renal stone-induced damage. For example, treatment of Madin-Darby
canine kidney (MDCK) cells with calcium oxalate monohydrate (COM)
crystals has been shown to impair tight junction function by
reducing the expression of occludin and ZO-1(8). Similarly, treatment of rat renal
tubular epithelial cells (NRK-52E) with COM crystals induces
changes in adhesion molecules, including hyaluronic acid,
osteopontin and CD44(9). Thus,
alterations in cellular signaling and adhesion molecules in renal
tubular epithelial cells are critical determinants of oxalate
crystal-induced injury and tissue repair (10). Understanding these changes may
provide insights into potential therapeutic approaches for diseases
related to oxalate crystal deposition.
Currently, there is no specific drug that
effectively treats or prevents urolithiasis, despite numerous
biological and physical studies aimed at preventing its development
(2). However, a number of
researchers have turned to natural medicinal agents to evaluate
their anti-urolithiasis potential (11,12).
The increasing interest in medicinal herbs can be attributed to
their wide-spread availability, low cost, long history of
traditional use and minimal side effects.
Gracilaria fisheri is a common red seaweed
widely found along the coastal areas of Thailand. It is rich in
sulfated galactan (SG), which primarily consists of repeating units
of D-galactose and 3,6-anhydrogalactose with sulfate residues
(13). SG is known for its various
biological activities, including immunostimulant (13), antioxidant (14) and wound healing properties (15). Our previous study demonstrated that
modifying SG to increase its sulfation, resulting in sulfated
galactan from G. fisheri (SGS), inhibited oxalate crystal
formation and provided enhanced protection against NaOX-induced
human kidney (HK-2) cell death (16). It is considered that certain
moieties of the polysulfated chain of SGS can serve as mimetics of
natural ligand-protein receptor-glycosaminoglycans, which interact
with various proteins, leading to post-translational modifications
and modulation of signaling molecules inside the cells (17). These modifications determine cell
behavior and responses (18). As
previously reported, SG from G. fisheri has been
demonstrated to interact with the epidermal growth factor receptor
(EGFR), resulting in the regulation of EGFR signaling activity,
including p-EGFR and p-Erk levels (19). In addition, studies have also
revealed that the expression of adhesion molecules, such as EpCAM,
is regulated by EGFR signaling (20,21).
Accumulating evidence suggests that SGS may modulate cell signaling
pathways and upregulate the expression of cell adhesion molecules,
potentially facilitating cell migration and tissue repair. It was
hypothesized that SGS promotes cell migration, regulates the
expression of adhesion molecules and downstream signaling pathways
in HK-2 cells and provides a protective effect against oxalate
crystal-induced injury. In addition, the effects of SGS on wound
healing and the expression of adhesion molecules in NaOX-induced
HK-2 cell injury have not yet been investigated. The present study
aimed to evaluate the effects of SGS on cell migration and adhesion
molecule expression in NaOX-induced HK-2 cell injury. HK-2 cell
migration was assessed using scratch migration and Transwell
assays. The mRNA and protein expression levels of adhesion
molecules were analyzed using reverse transcription-quantitative
(RT-q) PCR, western blotting and immunofluorescence confocal
microscopy under NaOX-induced conditions. Additionally, the
morphology of HK-2 cells treated with SGS and NaOX was examined
using scanning electron microscopy. The present study, for the
first time to the best of the authors' knowledge, explored the
effects of SGS on cell migration, the expression of cell adhesion
molecules and cell signaling pathways under NaOX-induced
conditions. These findings are expected to provide evidence
supporting the potential use of SGS in the prevention and treatment
of urolithiasis and related renal injuries.
Materials and methods
Materials, chemicals and reagents
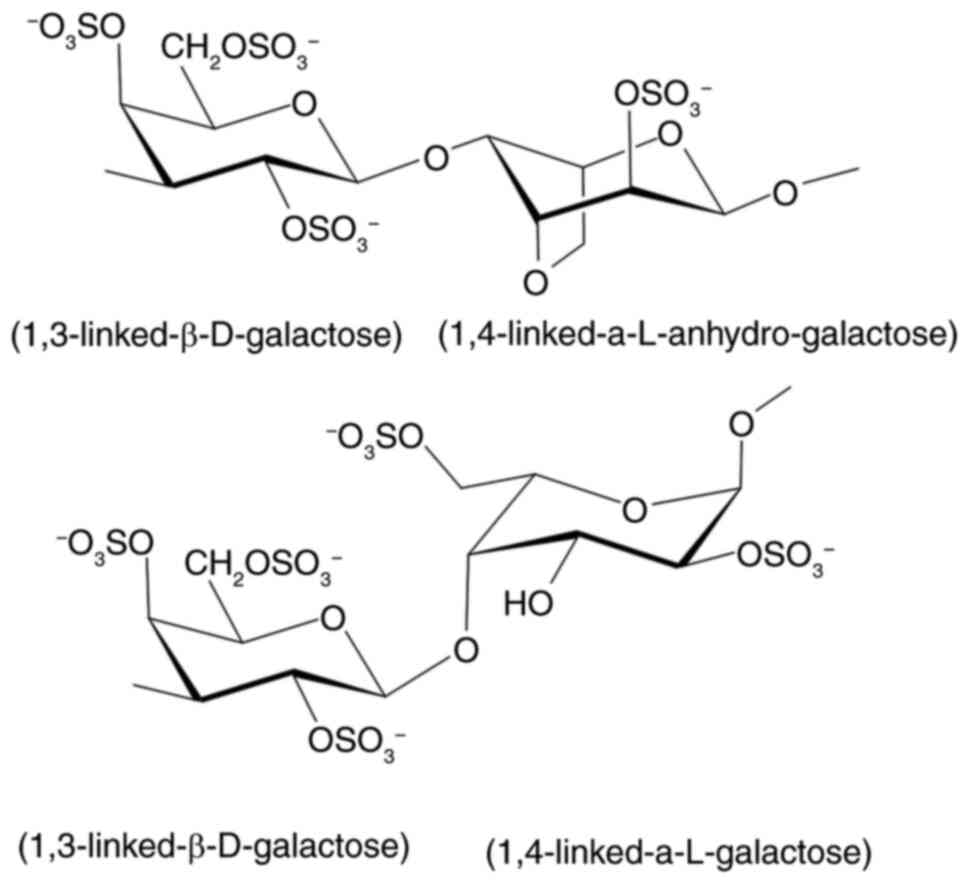
SGS was prepared as described by Rudtanatip et
al (15). SGS, with a molecular
weight of 97.07 kDa and a sulfation level of 26.53±1.09%, consists
of a complex structure of alternating 3-linked β-D-galactopyranose
and 4-linked 3,6-anhydro-α-L-galactopyranose or
α-L-galactopyranose, containing sulfate groups at C-2, C-4 and C-6
of D-galactopyranose, C-2 of L-anhydro-galactopyranose and C-2 and
C-6 of L-galactopyranose. The backbone structure of SGS is shown in
Fig. 1. The human kidney cell line
(HK-2) was purchased from ATCC. Dulbecco's Modified Eagle's Medium
(DMEM), fetal bovine serum (FBS) and Anti-Anti (100X)
antibiotic-antimycotic were obtained from Thermo Fisher Scientific,
Inc. Cystone was procured from the Himalaya Wellness Company.
Transwell permeable supports (6.5 mm inserts, 24-well plates) were
purchased from Corning Life Sciences. TRIzol® reagent
and the RevertAid First Strand cDNA Synthesis Kit were obtained
from Thermo Fisher Scientific, Inc. PowerUp SYBR™ Green Master Mix
was purchased from Applied Biosystems (Thermo Fisher Scientific,
Inc.). The Protease Inhibitor Cocktail (100X) was procured from
MedChemExpress. Nitrocellulose membrane was purchased from Global
Life Science Operations. Clarity Western ECL substrate was obtained
from Bio-Rad Laboratories, Inc. CellMask Deep Red Plasma Membrane
Stain was purchased from Thermo Fisher Scientific, Inc. All other
chemicals were purchased from Merck KGaA.
HK-2 cell culture and experimental
design
HK-2 cells were cultured in DMEM supplemented with
2.2 g/l sodium bicarbonate, 10% FBS and 1% antibiotic-antimycotic
in a humidified incubator at 37˚C with 5% CO2. To
evaluate the effects of SGS on NaOX-induced injury in HK-2 cells,
1.25 mmol/l of NaOX was used for induction, as described in our
previous study (16). The
cytotoxicity of NaOX on HK-2 cells was observed at the
concentrations ranging from 0.156-5.0 mmol/l, with NaOX showing a
dose-dependent reduction in cell proliferation. This concentration
(1.25 mmol/l) decreased cell viability by <50% (16). The cells were divided into 5 groups:
i) Control-no treatment; ii) NaOX control-treated with 1.25 mmol/l
of NaOX; iii) 100-SGS + NaOX-treated with 100 µg/ml of SGS combined
with 1.25 mmol/l of NaOX; iv) 1000-SGS + NaOX-treated with 1,000
µg/ml of SGS combined with 1.25 mmol/l of NaOX; v) Cystone +
NaOX-treated with 100 µg/ml of Cystone, a positive control drug,
combined with 1.25 mmol/l of NaOX. Cystone was used as a positive
control because it is commonly used to relieve urological problems,
including nephrolithiasis (22). It
has also been employed as a positive control in numerous
experimental studies evaluating its anti-urolithiasis activity,
both in vitro and in vivo (23,24).
Prior to treatment with NaOX and SGS, the cells were incubated
overnight in a serum-free culture medium to achieve
synchronization.
Scratch wound healing assay
The rate of HK-2 cell migration was assessed using a
scratch wound healing assay. Cells were seeded in 24-well plates at
a density of 4.5x104 cells/well in DMEM supplemented
with 10% FBS. Once the cells reached 80-100% confluence, they were
incubated overnight in a serum-free culture medium and a scratch
was created on the bottom of the well using a 200-µl sterile
pipette tip. Detached cells were removed by washing twice with 1X
PBS. The remaining cells were treated with either the SGS mixed
NaOX solution or the Cystone mixed NaOX solution. Cell migration
was monitored and imaged at 0, 6, 12 and 24 h post-treatment using
a Nikon Eclipse TS100 inverted microscope (Nikon Corporation). The
average distance between the edges of the scratch was calculated to
determine the wound width.
Transwell cell migration assay
To evaluate cell migration, HK-2 cells were seeded
into the upper chamber of a 24-well HTS Transwell Permeable
Supports plate at a density of 4.5x104 cells/well in
serum-free DMEM. The cells were treated with either the SGS mixed
NaOX solution or the Cystone mixed NaOX solution, while DMEM
supplemented with 10% FBS was added to the lower chamber as a
chemoattractant. After 24 h, the migrated cells in the lower
chamber were fixed with 10% neutral buffered formalin at room
temperature for 20 min, stained with toluidine blue O at room
temperature for 10 min and visualized under an inverted microscope
(Leica Biosystems).
Analysis of adhesion mRNA
transcription by quantitative polymerase chain reaction (qPCR)
assay
HK-2 cells were seeded in a 6-well plate at a
density of 1.6x105 cells/well in DMEM supplemented with
10% FBS and cultured at 37˚C for 24 h. The cells were then treated
with either the SGS mixed NaOX solution or the Cystone mixed NaOX
solution and incubated for an additional 24 h. Following treatment,
the cells were harvested for RNA extraction. Total RNA was
extracted using the TRIzol® reagent (Thermo Fisher
Scientific, Inc.) and cDNA was synthesized using the RevertAid
First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Inc.),
following the manufacturer's instructions. mRNA expression of CD44,
EpCAM, E-cadherin, vimentin, occludin, ZO-1 and GAPDH was analyzed
by qPCR using PowerUp SYBR Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.), as per the manufacturer's
protocol. The specific primer sequences used in the experiment are
listed in Table I. Each 20-µl PCR
reaction mixture contained 10 µl of PowerUp SYBR Green Master Mix,
0.8 µl of 500 nM forward and reverse primers, 7.2 µl of
nuclease-free water and 2 µl of cDNA. The cycling conditions were
as follows: 50˚C for 2 min, 95˚C for 10 min, followed by 40 cycles
of 95˚C for 15 sec, 60˚C for 30 sec and 72˚C for 30 sec.
Amplification and analysis were performed using the QuantStudio 6
Flex Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). GAPDH was used as the housekeeping gene for
normalization and relative gene expression was calculated using the
2-∆∆Cq method (25). The
experiments were performed in triplicate.
 | Table IPrimer sequences used for qPCR
analysis. |
Table I
Primer sequences used for qPCR
analysis.
| Gene | Sequence |
|---|
| CD44-Forward |
5'-TGCCGCTTTGCAGGTGTAT-3' |
| CD44-Reverse |
5'-GGCCTCCGTCCGAGAGA-3' |
| EpCAM-Forward |
5'-ATAACCTGCTCTGAGCGAGTG-3' |
| EpCAM-Reverse |
5'-TGAAGTGCAGTCCGCAAACT-3' |
|
E-cadherin-Forward |
5'-GAACAGCACGTACACAGCCCT-3' |
|
E-caherin-Reverse |
5'-GCAGAAGTGTCCCTGTTCCAG-3' |
|
Vimentin-Forward |
5'-AAAACACCCTGCAATCTTTCAGA-3' |
|
Vimentin-Reverse |
5'-CACTTTGCGTTCAAGGTCAAGAC-3' |
|
Occludin-Forward |
5'-GTCCAATATTTTGTGGGACAAGG-3' |
|
Occludin-Reverse |
5'-GGCACGTCCTGTGTGCCT-3' |
| ZO-1-Forward |
5'-AGAAGGATGTTTATCGTCGCATT-3' |
| ZO-1-Reverse |
5'-CCAAGAGCCCAGTTTTCCAT-3' |
| GAPDH-Forward |
5'-GGTGAAGGTCGGTGTGAACG-3' |
| GAPDH-Reverse |
5'-CTCGCTCCTGGAAGATGGTG-3' |
Determination of adhesion molecule and
signaling protein expression by western blot assay
HK-2 cells were harvested after treatment and used
for protein extraction in a protein lysis buffer containing 20 mM
Tris-HCl, 100 mM NaCl, 50 mM PMSF and 1X protease inhibitor
cocktail. Total protein extracts were quantified using a NanoDrop
2000 spectrophotometer (Thermo Fisher Scientific, Inc.) to
determine protein concentration. The protein samples (50 µg/lane)
were then separated on a 12.5% SDS-PAGE gel and transferred onto a
nitrocellulose membrane. The membrane was blocked with 4% bovine
serum albumin (BSA; Merck KGaA) in 1X Tris-buffered saline at room
temperature for 2 h and then incubated overnight at 4˚C with
primary antibodies specific to CD44, EpCAM, E-cadherin, vimentin,
occludin, ZO-1, PI3K, Akt, Erk1/2 and p38 (1:1,000 dilution).
Subsequently, the membrane was incubated at room temperature for 1
h with a secondary antibody: HRP-conjugated goat anti-rabbit IgG
(1:2,000 dilution) for CD44, EpCAM, occludin, ZO-1, PI3K, Akt,
Erk1/2 and p38, or HRP-conjugated goat anti-mouse IgG (1:2,000
dilution) for E-cadherin and vimentin. The antibodies used in the
experiment are listed in Table II.
The protein signals were developed using a Clarity Western ECL
substrate (Bio-Rad Laboratories, Inc.) and band intensities were
analyzed relative to the internal control (β-actin) using ImageJ
version 1.32j (National Institutes of Health).
 | Table IIList of antibodies used for western
blotting and fluorescence analyses. |
Table II
List of antibodies used for western
blotting and fluorescence analyses.
| Name | Host | Cat. no. | Supplier |
|---|
| CD44 | Rabbit | Ab157107 | Abcam |
| EpCAM | Rabbit | Ab71916 | Abcam |
| E-cadherin | Mouse | M3612 | Dako (Agilent
Technologies, Inc.) |
| Vimentin | Mouse | M0725 | Dako (Agilent
Technologies, Inc.) |
| Occludin | Rabbit | 71-1500 | Thermo Fisher
Scientific, Inc. |
| ZO-1 | Rabbit | 40-2200 | Thermo Fisher
Scientific, Inc. |
| PI3K | Rabbit | 4255 | Cell Signaling
Technology, Inc. |
| Akt | Rabbit | 4058 | Cell Signaling
Technology, Inc. |
| Erk1/2 | Rabbit | 4695 | Cell Signaling
Technology, Inc. |
| p38 | Rabbit | MA5-15177 | Thermo Fisher
Scientific, Inc. |
| β-actin | Rabbit | AF7018 | Affinity
biosciences |
| HRP-conjugated goat
anti-rabbit | | 31466 | Thermo Fisher
Scientific, Inc. |
| HRP-conjugated goat
anti-mouse | | 31430 | Thermo Fisher
Scientific, Inc. |
| FITC-conjugated
goat anti-rabbit | | 12-507 | Merck KGaA |
| FITC-conjugated
goat anti-mouse | | 12-506 | Merck KGaA |
Immunofluorescence confocal
microscopy
HK-2 cells were seeded onto round glass coverslips
in a 24-well plate at a density of 4.5x104 cells/well in
DMEM supplemented with 10% FBS and cultured at 37˚C for 24 h. The
cells were then treated with either the SGS mixed NaOX solution or
the Cystone mixed NaOX solution and allowed to grow for an
additional 24 h before being fixed with 10% formalin at room
temperature for 20 min. The fixed cells were washed three times
with 1X PBS and incubated overnight with primary antibodies
specific to CD44, E-cadherin, vimentin, EpCAM, occludin and ZO-1
(1:250 dilution), as shown in Table
II. They were then incubated at room temperature for 1 h with
FITC-conjugated goat anti-rabbit IgG (1:500 dilution) for CD44,
EpCAM, occludin and ZO-1, or FITC-conjugated goat anti-mouse IgG
(1:500 dilution) for E-cadherin and vimentin. Finally, the cells
were stained at room temperature for 20 min with DAPI for nuclear
visualization and CellMask Deep Red Plasma Membrane Stain to label
the cytoplasm and plasma membranes, following the manufacturer's
protocols. Images were captured using a Zeiss LSM800 inverted
confocal laser scanning microscope (Carl Zeiss AG) equipped with a
63x Plan-Apochromat 1.4 NA oil immersion objective lens
(magnification, x630). Laser wavelengths and pinhole size were set
as follows: 488 nm with a 43 µm pinhole for FITC staining, 405 nm
with a 42 µm pinhole for DAPI staining and 561 nm with a 53 µm
pinhole for CellMask Deep Red Plasma Membrane staining.
Observation of cell morphology by
scanning electron microscopy
HK-2 cells were seeded onto round glass coverslips
in a 24-well plate at a density of 4.5x104 cells/well in
DMEM supplemented with 10% FBS and cultured at 37˚C for 24 h. The
cells were then treated with either the SGS mixed NaOX solution or
the Cystone mixed NaOX solution and allowed to grow for an
additional 24 h. Following incubation, the cells were fixed with
2.5% glutaraldehyde at room temperature for 30 min. The coverslips
were then washed with 1X PBS, dehydrated and air-dried overnight.
The dried coverslips were mounted onto aluminum stubs and coated
with gold particles. Cell morphology was subsequently examined
using a JSM-IT200 InTouchScope scanning electron microscope (JEOL,
Ltd.).
Statistical analysis
All data were expressed as mean ± SEM from three
independent experiments. Statistical analysis was performed using
one-way ANOVA, followed by Tukey's multiple comparison test using
GraphPad Prism version 9 (Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
Gracilaria fisheri sulfated galactan
with increased sulfation (SGS) enhances HK-2 cell migration,
counteracting NaOX-induced inhibition of cell migration
The present study investigated the effect of SGS on
cell migration using a scratch wound assay. The initial wound edges
were marked to measure cell migration by tracking the decrease in
wound width over time. The scratch distances between wound edges
were compared across time points (0, 6, 12 and 24 h). The results
showed no significant differences in wound gape among the groups at
0 and 6 h after scratching. However, at 12 and 24 h after
scratching, the wound gape in the NaOX group was markedly larger
compared with the normal control group. Notably, a significant
reduction in wound gape was observed at 12 h in NaOX-induced HK-2
cells treated with SGS (1,000 µg/ml) compared with the NaOX group.
By 24 h, NaOX-induced HK-2 cells treated with SGS (100 and 1,000
µg/ml) and Cystone showed a significant decrease in wound gape
compared with the NaOX group (Fig.
2A and B). To further confirm
the cell migration promoting ability of SGS, a Transwell plate
assay was performed. As shown in Fig.
3, NaOX treatment reduced the number of migratory HK-2 cells
compared with the control. However, treatment with SGS (100 and
1,000 µg/ml) and Cystone markedly increased the number of migratory
cells in NaOX-induced HK-2 cells. These results suggest that SGS
markedly enhances HK-2 cell migration at the wound edge and
promotes cell migration in NaOX-induced HK-2 cells.
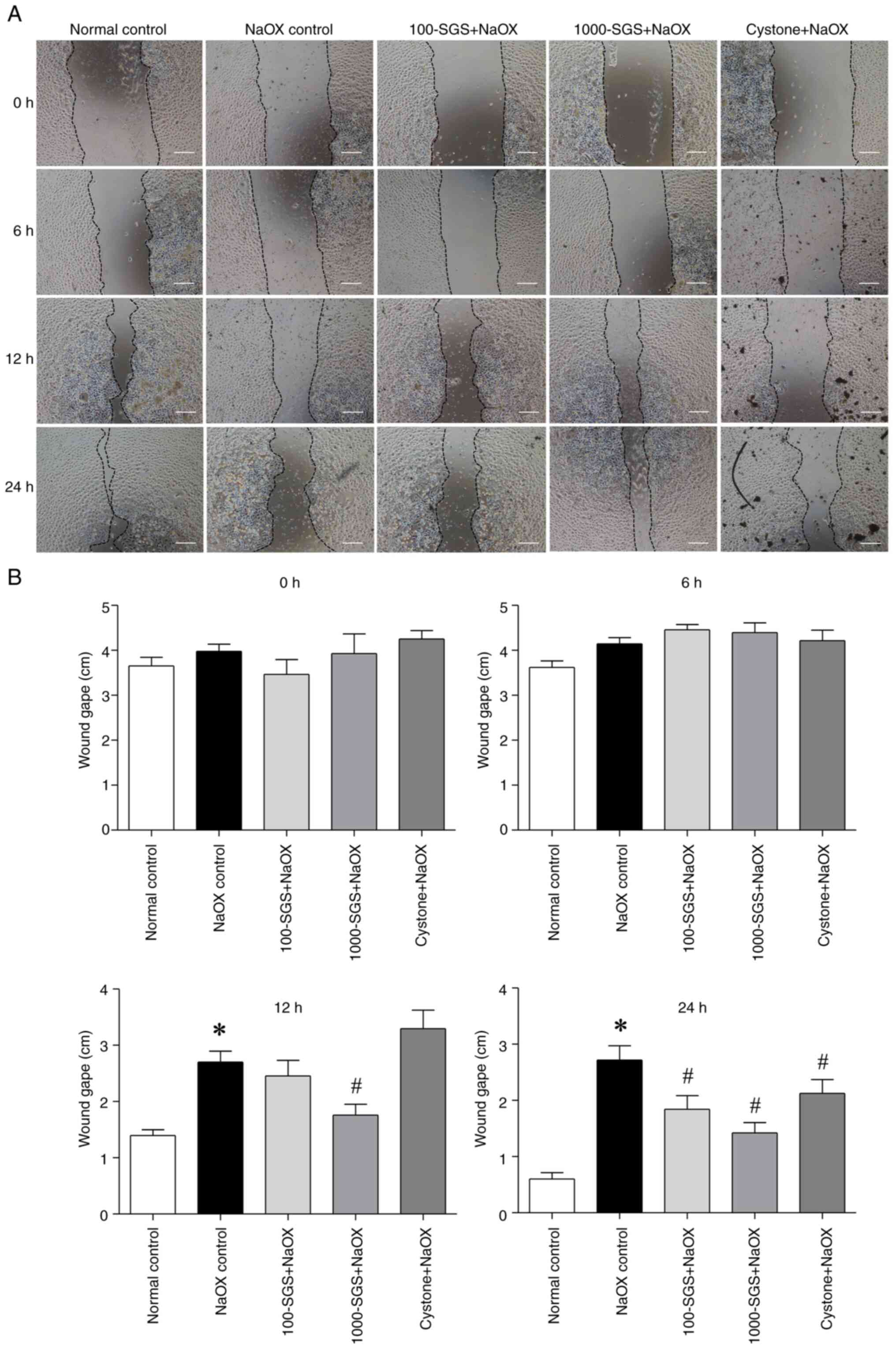 | Figure 2The effect of SGS on HK-2 cell
migration, evaluated using a scratch wound healing assay. (A) Phase
contrast images show the wound distance in HK-2 cells treated with
NaOX, SGS + NaOX and Cystone + NaOX at 0, 6, 12 and 24 h after
scratching. (B) Bar graphs represent the wound gape at 0, 6, 12 and
24 h after scratching. Data are presented as mean ± SEM (n=3) from
three independent experiments. *P<0.05 vs. normal
control; #P<0.05 vs. NaOX control. Scale bars, 100
µm. SGS, sulfated galactan with increased sulfation; NaOX, sodium
oxalate; Control, no treatment; NaOX control, treated with 1.25
mmol/l of NaOX; 100-SGS + NaOX, treated with 100 µg/ml of SGS
combined with 1.25 mmol/l of NaOX; 1000-SGS + NaOX, treated with
1,000 µg/ml of SGS combined with 1.25 mmol/l of NaOX; Cystone +
NaOX, treated with 100 µg/ml of Cystone combined with 1.25 mmol/l
of NaOX. |
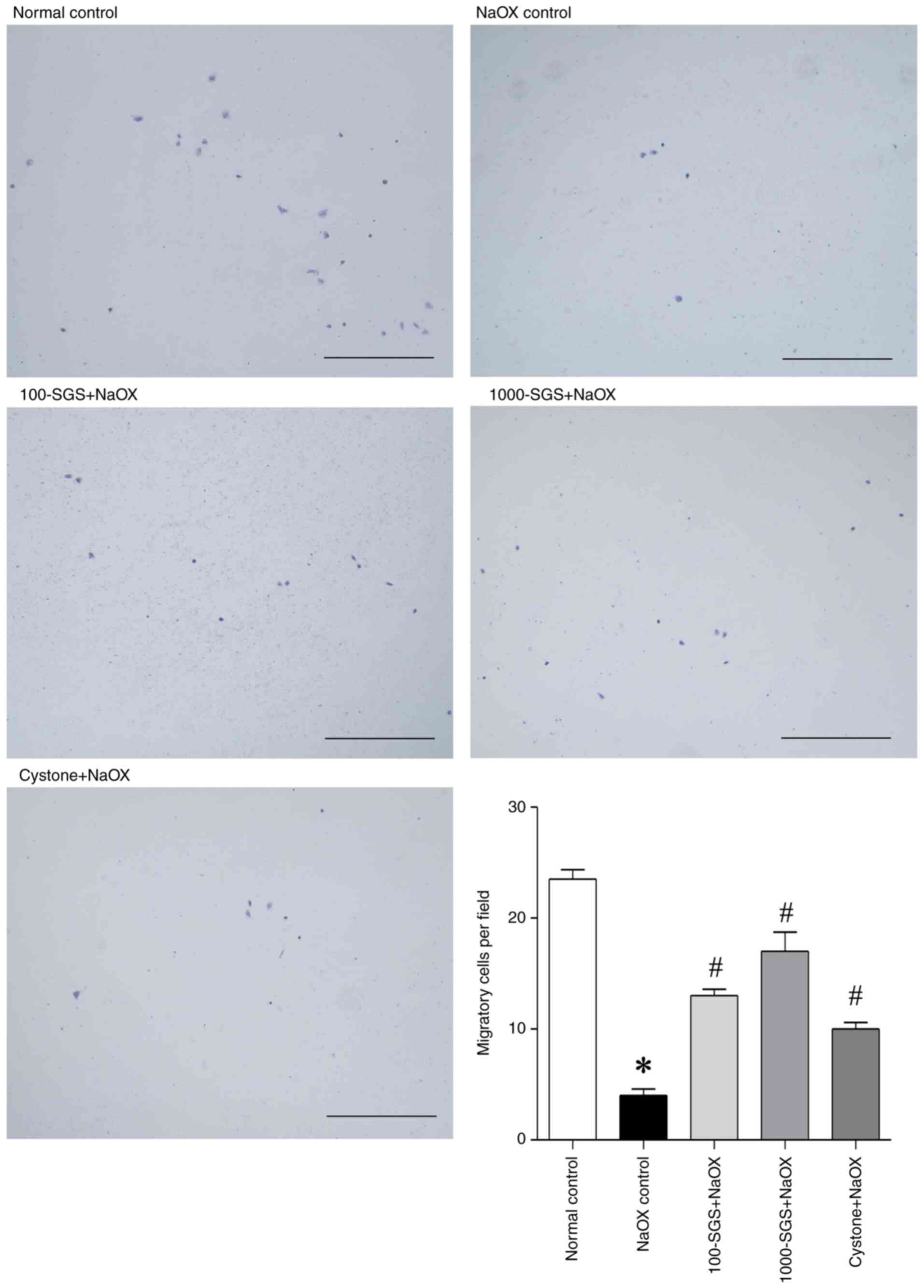 | Figure 3Photomicrographs show the effect of
SGS on the Transwell migration of HK-2 cells, stained with
toluidine blue O at 24 h, with accompanying graphs quantifying the
number of migrated cells. Data are presented as mean ± SEM (n=3)
from three independent experiments. *P<0.05 vs.
normal control; #P<0.05 vs. NaOX control. Scale bar,
500 µm. SGS, sulfated galactan with increased sulfation; NaOX,
sodium oxalate; Control, no treatment; NaOX control, treated with
1.25 mmol/l of NaOX; 100-SGS + NaOX, treated with 100 µg/ml of SGS
combined with 1.25 mmol/l of NaOX; 1000-SGS + NaOX, treated with
1,000 µg/ml of SGS combined with 1.25 mmol/l of NaOX; Cystone +
NaOX, treated with 100 µg/ml of Cystone combined with 1.25 mmol/l
of NaOX. |
SGS positively regulates the
expression of adhesion molecules in HK-2 cells induced by NaOX
Cell adhesion molecules play a critical role in
maintaining cell polarity, integrity and function (7). To evaluate the effects of SGS on the
expression of adhesion molecules in NaOX-induced HK-2 cell damage,
qPCR and western blot analyses were conducted to detect the
expression levels of CD44, EpCAM, E-cadherin, vimentin, occludin
and ZO-1. The qPCR results showed significant alterations in mRNA
expression in HK-2 cells treated with NaOX, SGS + NaOX and Cystone
+ NaOX. Specifically, the expression levels of EpCAM, E-cadherin,
occludin and ZO-1 were markedly downregulated following NaOX
exposure, while CD44 and vimentin were markedly upregulated.
Treatment with SGS (1,000 µg/ml) markedly upregulated the
expression levels of CD44, EpCAM, E-cadherin and occludin and
markedly downregulated the expression of vimentin, compared with
the NaOX control. However, no significant changes in ZO-1
expression were observed. Similarly, the expression levels of
adhesion molecule mRNA in NaOX-induced HK-2 cells treated with
Cystone (positive control) were consistent with those in
SGS-treated cells (Fig. 4). Western
blotting results (Fig. 5) further
corroborated the qPCR findings. In NaOX-induced HK-2 cell damage,
the protein expression levels of adhesion molecules followed a
similar trend. Following treatment with SGS, the protein expression
levels of CD44 and vimentin were markedly downregulated, while the
expression levels of EpCAM, E-cadherin, occludin and ZO-1 were
markedly upregulated, compared with the NaOX control. These results
suggested that SGS can modulate the expression of adhesion
molecules at both the mRNA and protein levels, providing protective
effects against NaOX-induced damage in HK-2 cells.
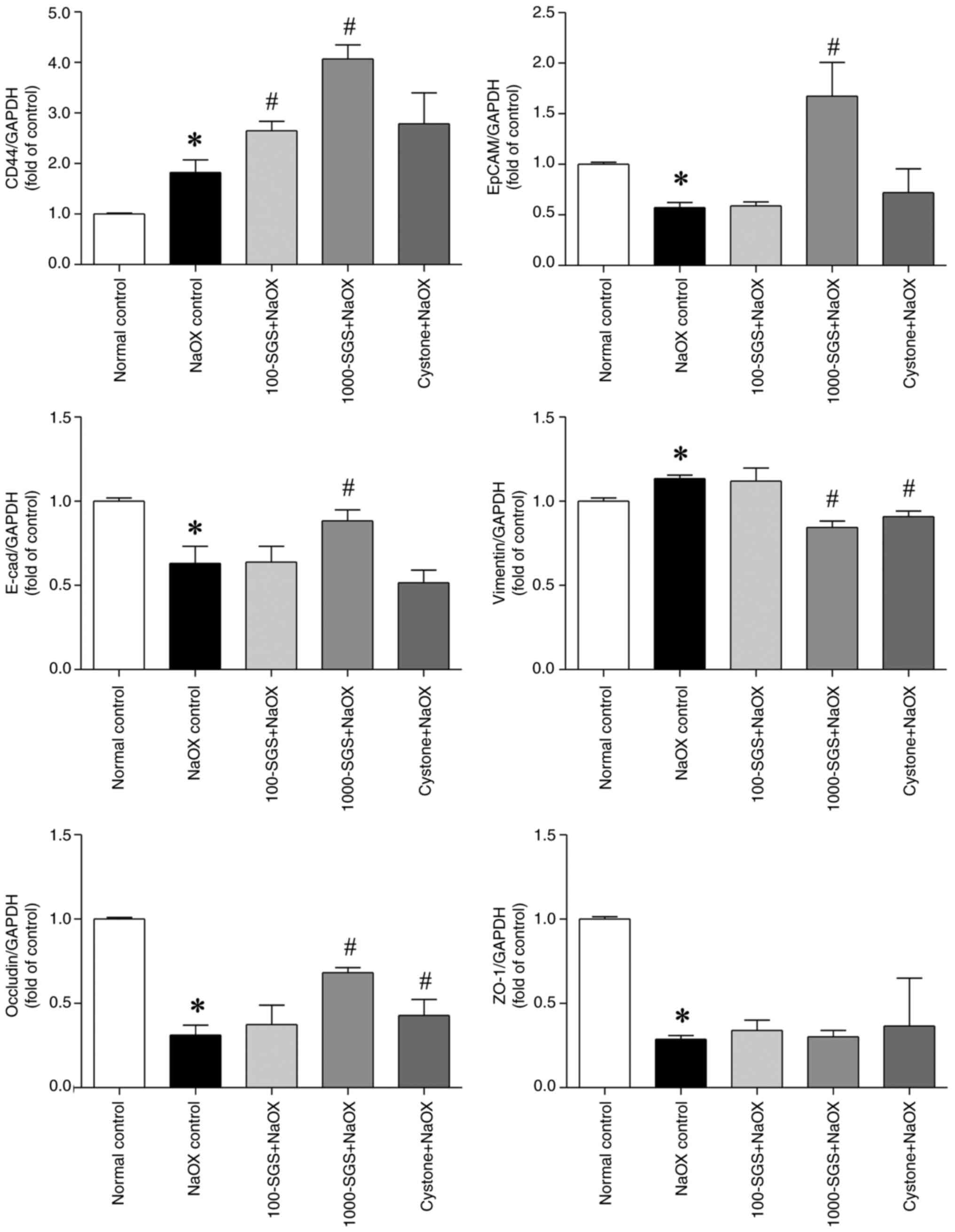 | Figure 4mRNA expression levels of adhesion
molecules in HK-2 cells treated with NaOX, SGS + NaOX and Cystone +
NaOX, assessed by qPCR analysis. The transcription levels of CD44,
EpCAM, E-cadherin, vimentin, occludin and ZO-1 are shown. Data are
presented as mean ± SEM (n=3) from three independent experiments.
*P<0.05 vs. normal control; #P<0.05 vs.
NaOX control. NaOX, sodium oxalate; SGS, sulfated galactan with
increased sulfation; Control, no treatment; NaOX control, treated
with 1.25 mmol/l of NaOX; 100-SGS + NaOX, treated with 100 µg/ml of
SGS combined with 1.25 mmol/l of NaOX; 1000-SGS + NaOX, treated
with 1,000 µg/ml of SGS combined with 1.25 mmol/l of NaOX; Cystone
+ NaOX, treated with 100 µg/ml of Cystone combined with 1.25 mmol/l
of NaOX. |
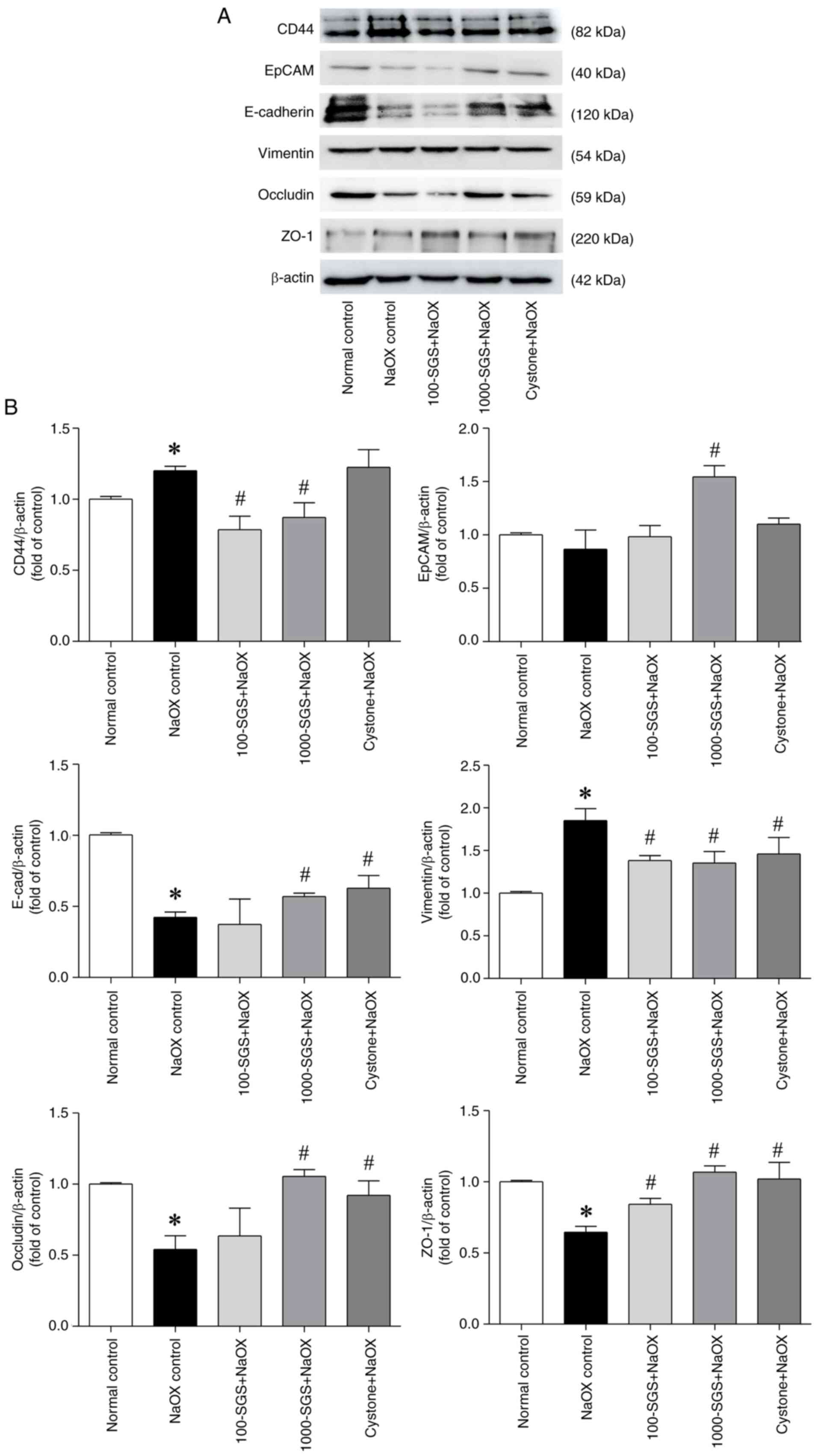 | Figure 5Expression levels of adhesion
molecules in NaOX-induced HK-2 cells following SGS treatment. (A)
Protein expression levels of adhesion molecules in HK-2 cells
treated with NaOX, SGS + NaOX and Cystone + NaOX, analyzed by
western blotting. (B) Quantitative data of CD44, EpCAM, E-cadherin,
vimentin, occludin and ZO-1 normalized to β-actin, with fold
changes relative to the control group. Data are presented as mean ±
SEM (n=3) from three independent experiments. *P<0.05
vs. normal control; #P<0.05 vs. NaOX control. NaOX,
sodium oxalate; SGS, sulfated galactan with increased sulfation;
Control, no treatment; NaOX control, treated with 1.25 mmol/l of
NaOX; 100-SGS + NaOX, treated with 100 µg/ml of SGS combined with
1.25 mmol/l of NaOX; 1000-SGS + NaOX, treated with 1,000 µg/ml of
SGS combined with 1.25 mmol/l of NaOX; Cystone + NaOX, treated with
100 µg/ml of Cystone combined with 1.25 mmol/l of NaOX. |
Additionally, confocal immunofluorescence staining
was performed to confirm the distribution of adhesion molecules in
HK-2 cells. The results showed that NaOX-induced HK-2 cells
exhibited increased expression of CD44 and vimentin, while the
expression of EpCAM, E-cadherin, occludin and ZO-1 was decreased
(Fig. 6). By contrast, HK-2 cells
treated with SGS + NaOX showed decreased expression of CD44 and
vimentin, alongside increased expression of EpCAM, E-cadherin,
occludin and ZO-1. These findings indicate that NaOX alters the
expression patterns of adhesion molecules, while SGS mitigates
NaOX-induced toxicity and restores adhesion molecule expression to
a more normal state.
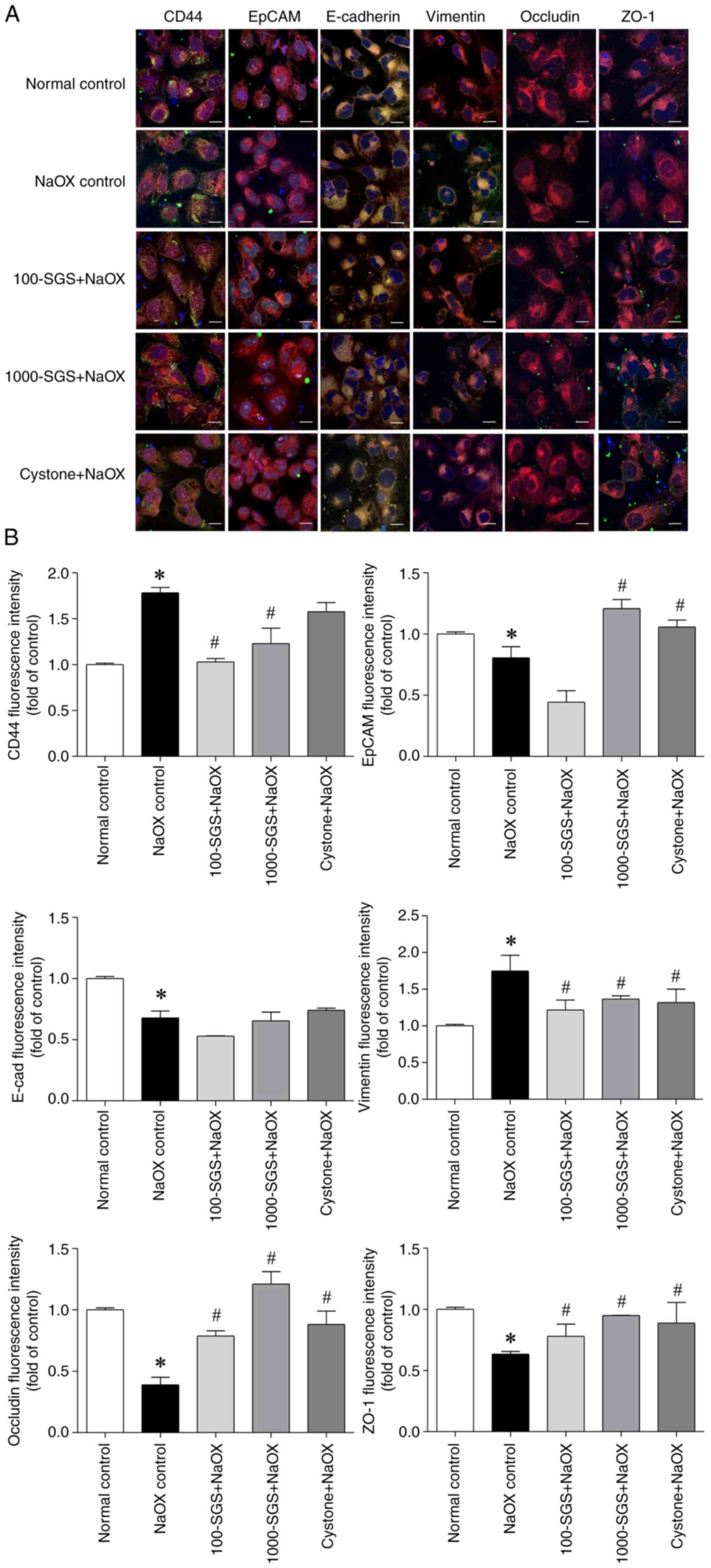 | Figure 6Distribution of adhesion molecules in
NaOX-induced HK-2 cells following SGS treatment. (A) Confocal
fluorescence micrographs show the expression of adhesion molecules
(CD44, EpCAM, E-cadherin, vimentin, occludin and ZO-1) in HK-2
cells treated with NaOX, SGS + NaOX and Cystone + NaOX. Scale bars,
10 µm. (B) Quantitative analysis of fluorescence intensities for
each adhesion molecule. Data are presented as mean ± SEM (n=3) from
three independent experiments. *P<0.05 vs. normal
control; #P<0.05 vs. NaOX control. NaOX, sodium
oxalate; SGS, sulfated galactan with increased sulfation; Control,
no treatment; NaOX control, treated with 1.25 mmol/l of NaOX;
100-SGS + NaOX, treated with 100 µg/ml of SGS combined with 1.25
mmol/l of NaOX; 1000-SGS + NaOX, treated with 1,000 µg/ml of SGS
combined with 1.25 mmol/l of NaOX; Cystone + NaOX, treated with 100
µg/ml of Cystone combined with 1.25 mmol/l of NaOX. |
NaOX-induces morphological changes of
HK-2 cells were reversed by SGS
The effect of SGS on the morphological changes of
NaOX-induced HK-2 cells was examined using scanning electron
microscopy, as shown in Fig. 7.
Normal HK-2 cells (control) exhibited thick, long, slender and
regular cytoplasmic processes that were tightly interconnected with
the cytoplasmic processes of neighboring cells. By contrast,
NaOX-induced HK-2 cell damage resulted in significant morphological
alterations, including cell volume retraction and the presence of
very thin, long, slender and irregular cytoplasmic processes.
However, NaOX-induced HK-2 cells treated with SGS displayed thin,
long, slender and regular cytoplasmic processes, resembling those
observed in the control and Cystone-treated cells. These findings
suggested that SGS exerts a cell recovery effect, mitigating the
morphological damage caused by NaOX in HK-2 cells.
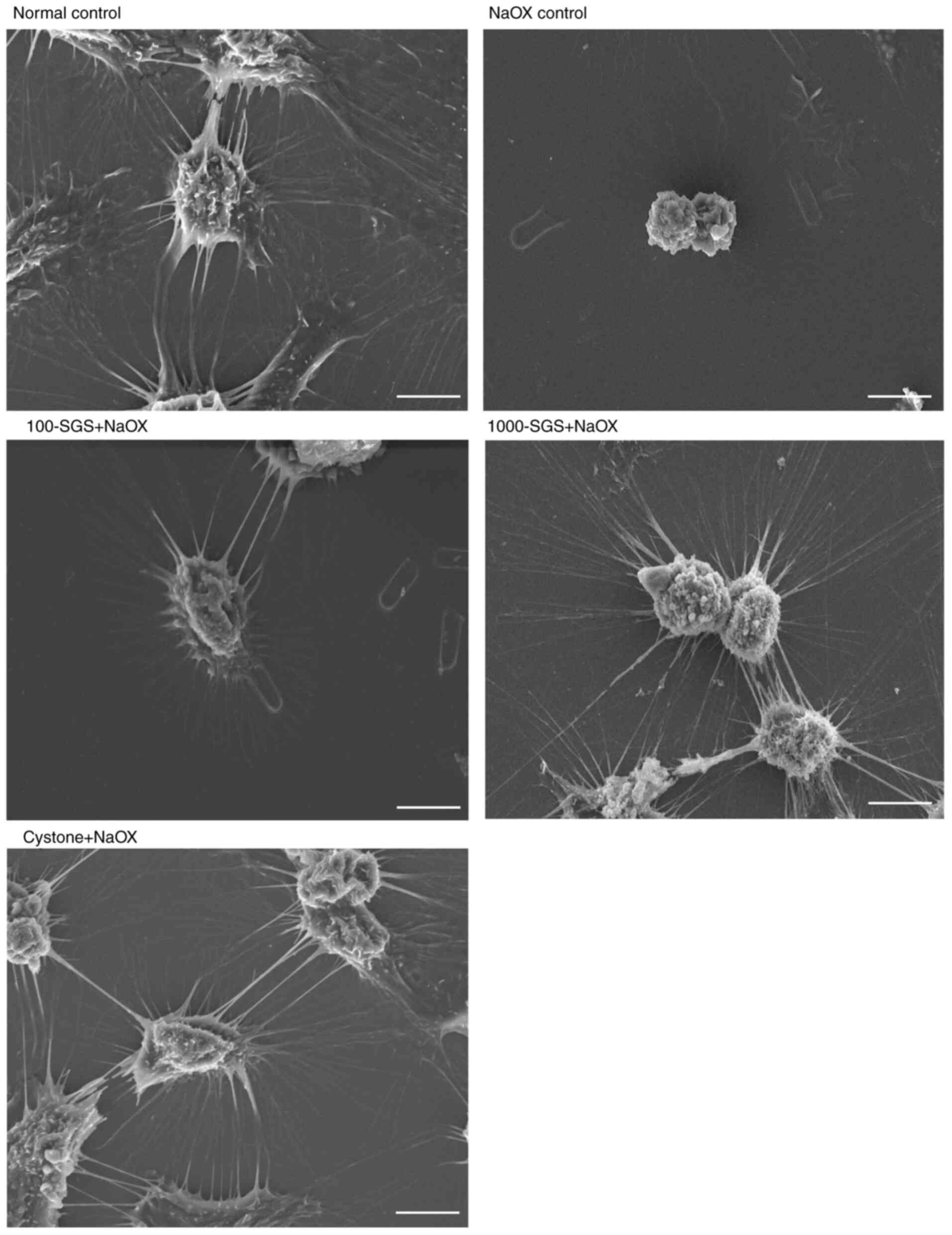 | Figure 7Scanning electron microscopy images
show morphological changes in HK-2 cells from various treatment
groups, including normal control, NaOX control, 100-SGS + NaOX,
1000-SGS + NaOX and Cystone + NaOX. Scale bar, 10 µm. NaOX, sodium
oxalate; SGS, sulfated galactan with increased sulfation; Control,
no treatment; NaOX control, treated with 1.25 mmol/l of NaOX;
100-SGS + NaOX, treated with 100 µg/ml of SGS combined with 1.25
mmol/l of NaOX; 1000-SGS + NaOX, treated with 1,000 µg/ml of SGS
combined with 1.25 mmol/l of NaOX; Cystone + NaOX, treated with 100
µg/ml of Cystone combined with 1.25 mmol/l of NaOX. |
SGS alters the expression of PI3K,
Akt, Erk1/2 and p38 signaling proteins in NaOX-induced HK-2 cell
damage
The PI3K/Akt and MAPK signaling pathways play a
critical role in regulating various cellular processes, including
the cell cycle, survival and death. These pathways are closely
associated with the pathological mechanisms of kidney diseases
(26). To evaluate the effects of
SGS on signaling pathways, we investigated the expression of key
signaling proteins, including PI3K, Akt, Erk1/2 and p38, in
NaOX-induced HK-2 cell damage. As shown in Fig. 8, NaOX exposure markedly upregulated
the expression levels of Akt and p38, while downregulating the
expression levels of PI3K and Erk1/2. However, treatment of
NaOX-induced HK-2 cells with SGS markedly reversed these changes.
Specifically, SGS treatment (1,000 µg/ml) markedly upregulated the
expression levels of PI3K and Erk1/2 while downregulating the
expression levels of Akt and p38, compared with the NaOX control.
These effects were consistent with those observed in
Cystone-treated NaOX-induced HK-2 cells.
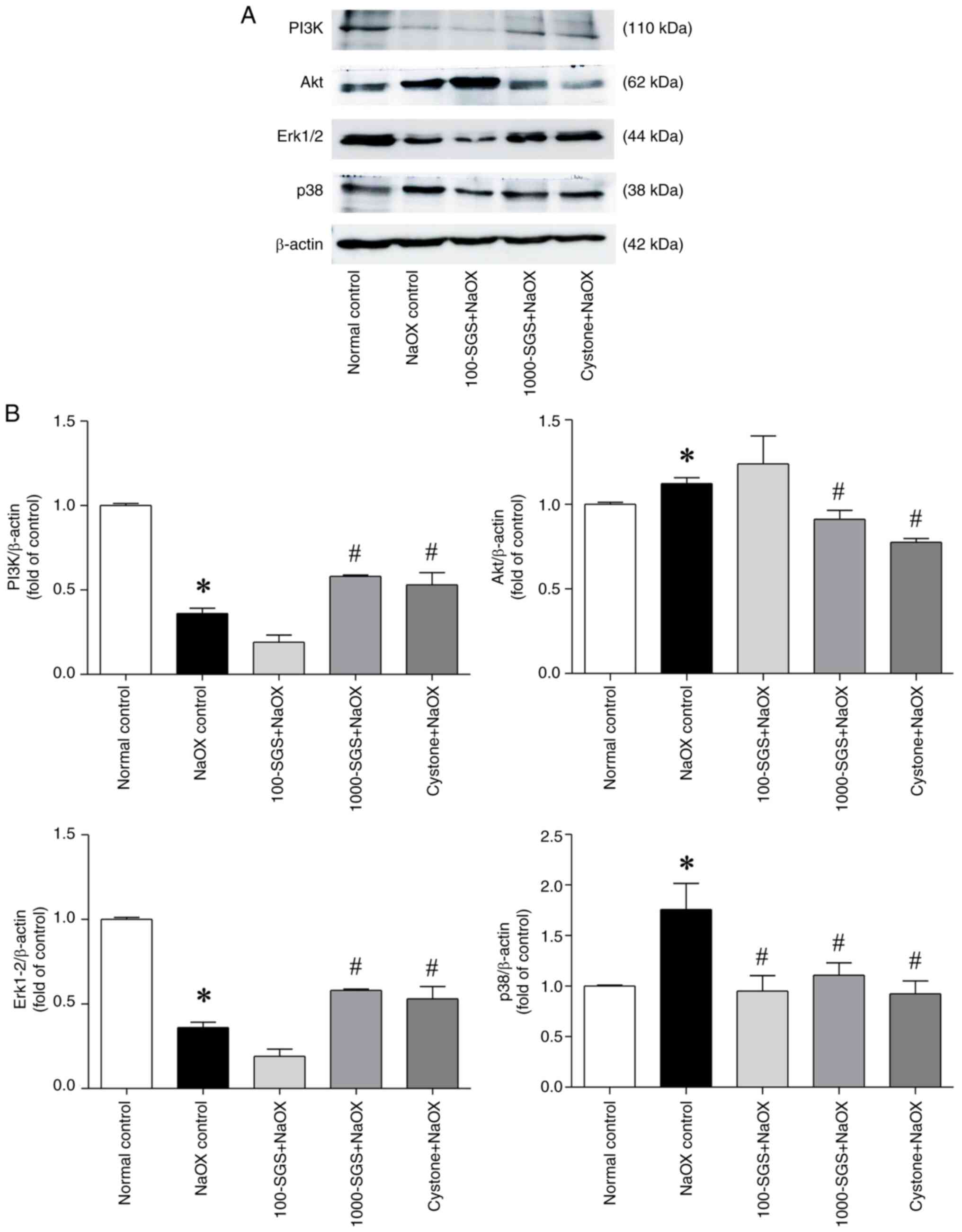 | Figure 8Expression levels of PI3K/Akt and
MAPK signaling proteins in NaOX-induced HK-2 cells following SGS
treatment. (A) Protein expression levels of PI3K, Akt, Erk1/2 and
p38 in HK-2 cells treated with NaOX, SGS + NaOX and Cystone
+ NaOX, assessed by western blotting. (B) Quantitative data
of PI3K, Akt, Erk1/2 and p38 normalized to β-actin, with fold
changes relative to the control group. Data are presented as mean ±
SEM (n=3) from three independent experiments. *P<0.05
vs. normal control; #P<0.05 vs. NaOX control. NaOX,
sodium oxalate; SGS, sulfated galactan with increased sulfation;
Control, no treatment; NaOX control, treated with 1.25 mmol/l of
NaOX; 100-SGS + NaOX, treated with 100 µg/ml of SGS combined with
1.25 mmol/l of NaOX; 1000-SGS + NaOX, treated with 1,000 µg/ml of
SGS combined with 1.25 mmol/l of NaOX; Cystone + NaOX, treated with
100 µg/ml of Cystone combined with 1.25 mmol/l of NaOX. |
Discussion
Biological activities of sulfated polysaccharides
are strongly influenced by their molecular structures, including
molecular weight and degree of sulfation (27). The present study demonstrated that
SGS enhanced cell migration ability and wound healing activity in
NaOX-induced HK-2 cells. Kidney stone (oxalate crystal) formation
has been linked to increased glycolic acid oxidase activity induced
by NaOX, leading to injury and impairment of renal tubular
epithelial cells (2,28). In response to injury, renal tubular
epithelial cells compensate through proliferation and migration to
promote tissue repair (5). However,
NaOX inhibits renal epithelial cell migration, potentially
exacerbating kidney disease progression, as observed in MDCK cells
exposed to NaOX (29). In the
present study, SGS markedly enhanced wound healing activity in HK-2
cells, which aligns with previous findings showing that SGS
promoted wound closure in an L929 fibroblast scratch model
(15). This suggests that increased
sulfation of polysaccharides is closely associated with enhanced
wound healing properties (15).
Cell adhesion molecules play a crucial role in the
processes of tissue injury and repair (30). Dysregulation of these molecules is
also critical in triggering signaling cascades that can lead to
apoptotic or necrotic cell death. The detachment of injured cells
is linked to changes in adhesion molecules such as cadherins,
integrins and occludin junction proteins, which are essential for
maintaining cell-cell and cell-extracellular matrix adhesions
(31). Oxalate crystals interact
with renal epithelial cells, altering tight junctions by decreasing
the expression levels of key proteins such as ZO-1, occludin and
claudin (32). Additionally,
oxalate crystals promote renal epithelial cell damage by
upregulating adhesion proteins, including hyaluronic acid,
osteopontin, CD44 and annexin A2, on the cell surface (9). Conversely, oxalate crystal deposition
in the kidneys of hyperoxaluric rats is reduced when osteopontin
expression is inhibited, highlighting its role in crystal formation
and retention (33). Additionally,
E-cadherin and vimentin are key proteins involved in regulating
cell migration and polarization. E-cadherin, an integral membrane
protein, mediates lateral cell-to-cell adhesion through adherens
junctions, playing a critical role in maintaining tissue integrity
and inhibiting cell mobility. By contrast, vimentin serves as an
intermediate filament that regulates the wound repair process by
promoting cell motility and migration (6). The inhibition of MDCK cell migration
by oxalate crystals, as reported by Ji et al (29), may be linked to the dysregulation of
E-cadherin and vimentin. In the present study, NaOX exposure
altered the expression of CD44, EpCAM, E-cadherin, vimentin,
occludin and ZO-1 in HK-2 cells, suggesting damage to barrier
function, loss of cell adhesion and impaired cell migration.
Additionally, increased expression of CD44 has been shown to
upregulate cytoskeleton function through ankyrin, activating the
actomyosin contractile complex to mediate cell migration (34) and may directly affect wound healing
(35). The increased expression of
CD44 induced by NaOX in the present study may reflect a
compensatory cellular response by enhancing contraction, migration
and wound healing. Another possible reason for the increased CD44
expression under NaOX-induced conditions is an imbalance between
cellular oxidants and antioxidants, leading to the expression of
inflammatory molecules (36).
However, SGS treatment alleviated the effects of
NaOX by restoring the expression levels of these molecules to
near-normal levels. Several studies further support the protective
effects of compounds on the expression of proteins and RNA involved
in cell adhesion and migration, which mitigate kidney injury. For
example, downregulation of osteopontin by caffeic acid inhibited
kidney stone formation (37). In a
glyoxylate-induced nephrolithiasis mouse model, curcumin
administration reduced calcium oxalate crystal deposition and renal
tissue damage by decreasing the expression of osteopontin and
CD44(38). Similarly, treatment of
MDCK cells with the plant alkaloid trigonelline attenuated
oxalate-induced epithelial-to-mesenchymal transition by increasing
the expression of epithelial markers E-cadherin and ZO-1(39).
Oxalate crystals not only alter the transcription
and translation of adhesion molecules but also markedly alter cell
morphology. Renal cells exposed to oxalate crystals exhibit
distinct morphological changes, including structural
disorganization, cell edema, chromatin condensation, translocation
of phosphatidylserine to the cell surface and displacement of
membrane proteins (40,23). These morphological changes are
closely linked to and functionally integrated with the actin-based
cytoskeleton (41). In the present
study, NaOX exposure induced marked morphological changes in HK-2
cells, as observed under scanning electron microscopy. However,
treatment with SGS restored the morphology of oxalate-injured
cells, which gradually returned to a normal appearance. These
findings align with a previous study demonstrating the
cytoprotective effects of D. pedicellata ethanolic extract
and Cystone in oxalate crystal-induced cell damage, where
restoration of cell morphology was also observed (23).
The mechanisms underlying kidney injury caused by
oxalate crystals are multifactorial and complex, with a number of
aspects that remain to be elucidated. The PI3K/Akt and MAPK
signaling pathways are closely linked to the pathological processes
of kidney diseases and play crucial roles in cellular functions,
including the cell cycle, survival and tissue repair (26,42).
The findings of the present study revealed that NaOX exposure
increased the expression of Akt and p38 while decreasing the
expression of PI3K and Erk1/2. Conversely, SGS treatment reversed
these effects, increasing the expression of PI3K and Erk1/2 and
reducing the expression of Akt and p38, highlighting the possible
protective effects of SGS against NaOX-induced damage. The
potential mechanisms by which SGS modulates Akt, p38, PI3K and
Erk1/2 in NaOX-induced cell death may involve its high sulfate
ester content, which imparts a strong negative charge, enabling
binding to cell surface receptors and regulation of the
PI3K/Akt/MAPK pathways (43,44).
Additionally, SGS interferes with crystal formation, reducing
crystal-cell binding and subsequently inhibiting reactive oxygen
species (ROS) production, which contributes to tight junction
alteration via regulation of the ROS/Akt/p38 MAPK signaling pathway
(8). The antioxidant properties of
SGS may also neutralize NaOX-induced free radicals, thereby
suppressing oxidative stress and modulating downstream signaling
molecules (16). However, the
protective effect of SGS against NaOX-induced damage in HK-2 cells
requires further confirmation using PI3K/Akt and MAPK signaling
inhibitors.
Oxalate crystal induction is associated with
increased cell death and dysregulation of the PI3K/Akt and MAPK
signaling pathways. Consistent with this, treating MDCK cells with
oxalate crystals has been shown to reduce the expression of
junctional proteins, such as ZO-1 and occludin, while increasing
the expression of Akt/ASK1/p38 MAPK signaling proteins (8). Transcriptomic analysis of renal
tubular epithelial cells and mouse kidneys exposed to oxalate
crystals has revealed that kidney stone formation is associated
with activation of the PI3K/Akt signaling pathway (45). Additionally, p38 MAPK has been
implicated in oxalate crystal-induced tight junction alteration in
rat renal tubular epithelial (NRK-52E) cells. The mRNA and protein
expression levels of p38 MAPK-related molecules [such as
phosphorylated (p-)p38] and adhesion molecules (such as
osteopontin, hyaluronic acid and CD44) were markedly increased in
NRK-52E cells treated with oxalate crystals (9,32).
These findings are consistent with the present study, which showed
that NaOX increased the expression of Akt and p38 while decreasing
the expression of PI3K and Erk1/2. This response may reflect
cellular compensation and repair mechanisms in response to oxalate
crystal-induced ROS generation and cell injury (46,47).
In addition, the decreased expression of Erk1/2 by NaOX may be
attributed to increased ROS generation through the inhibition of
the AMPK/mTOR/ERK signaling axis (48).
SGS treatment demonstrated opposing effects on the
expression levels of PI3K, Akt, p38 and Erk1/2, effectively
alleviating the effect of NaOX by restoring these signaling
molecules to near-normal levels. Inhibition of p38 MAPK has been
shown to reduce oxalate crystal-induced cell adhesion and injury.
For example, treatment with SB239063, a p38 MAPK inhibitor,
decreases p38 expression and inhibits oxalate crystal adhesion and
cell damage in NRK-52E cells (9).
Similarly, quercetin, a molecule with potent antioxidant and
anti-inflammatory effects, suppresses p38 MAPK activation,
mitigating oxidative stress and reducing oxalate crystal-induced
injury in HK-2 cells (49). These
findings are consistent with the results of the present study, in
which SGS decreased the expression of p38 in NaOX-induced HK-2 cell
injury. By contrast, Puerarin, an isoflavone derived from the root
of Pueraria lobata, has been reported to activate the
SIRT1/Akt/p38 signaling pathways, thereby reducing oxalate
crystal-induced oxidative stress and autophagy, suggesting its
potential to directly inhibit autophagy activation (50). Similarly, it can be implied that SGS
suppressed Akt and p38 activation, mitigating ROS generation and
reducing HK-2 cell death induced by NaOX. The observed increase in
PI3K and Erk1/2 expression following SGS treatment may be related
to the migration of non-injured epithelial cells adjacent to the
site of injury, promoting wound healing activity (46). PI3K/Akt activation mediates
VEGF-driven cell survival (47),
while Erk1/2 signaling regulates cell migration and proliferation
during wound healing. Specifically, inhibition of Erk1/2 delays
wound healing, whereas its activation stimulates cell migration and
proliferation (51). Two effects of
SGS on NaOX-induced cell death can be proposed: i) SGS inhibits
NaOX-induced cell death by suppressing ROS production mediated by
Akt and p38 and ii) SGS promotes cell migration by activating PI3K
and Erk1/2, thereby enhancing wound healing. These findings
suggested that SGS may contribute to the prevention and treatment
of urolithiasis by modulating the expression of molecules involved
in cell migration, adhesion and related signaling pathways in
response to oxalate crystal-induced injury. However, a limitation
of the present study is that the modulation of the PI3K/Akt and
MAPK (p38 and Erk1/2) signaling pathways, through the examination
of phospho-protein levels, was not assessed. Future studies should
incorporate western blot or ELISA to assess phosphorylated proteins
(p-Akt, p-p38, p-ERK1/2) to provide direct evidence of signaling
pathway regulation. This would strengthen the mechanistic
understanding of how SGS modulates the expression of molecules in
response to oxalate crystal-induced injury.
In conclusions, the present study demonstrated that
NaOX exposure altered the expression of cell adhesion molecules and
impaired the migration ability of HK-2 cells. Notably, SGS
treatment alleviated these effects by restoring cell adhesion
molecule expression to near-normal levels and enhancing cell
migration. These protective effects are likely mediated through the
regulation of the PI3K/Akt and MAPK (p38 and Erk1/2) signaling
pathways. Furthermore, the findings highlighted the potential of
SGS in the prevention and treatment of urolithiasis, providing
valuable new insights into therapeutic strategies for this
disease.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Faculty of
Medicine, Khon Kaen University, Thailand (grant no. IN63318),
Research and Graduate Studies, Khon Kaen University (grant no.
RP65-2-002) and Postgraduate Study Support Grant of the Faculty of
Medicine, Khon Kaen University (grant no. 647070001-4).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TR, JP and WS conceived and designed the
experiments. TR, JP, SS and WS performed the experiments. TR, JP,
SS, JEA and WS were responsible for the analysis of data. TR and JP
participated in the drafting of the manuscript. KW, JK and TR
edited and finalized the final version of the manuscript. KW and JK
supervised the study. WS provided funding and project
administration. TR, JP and WS confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yasui T, Okada A, Hamamoto S, Ando R,
Taguchi K, Tozawa K and Kohri K: Pathophysiology-based treatment of
urolithiasis. Int J Urol. 24:32–38. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pandhare RB, Shende RR, Avhad MS, Deshmukh
VK, Mohite PB, Sangameswaran B and Daude RB: Anti-urolithiatic
activity of Bryophyllum pinnatum Lam. hydroalcoholic extract
in sodium oxalate-induced urolithiasis in rats. J Tradit Complement
Med. 11:545–551. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Patel VB and Acharya N: Effect of
Macrotyloma uniflorum in ethylene glycol induced
urolithiasis in rats. Heliyon. 6(e04253)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Geraghty R, Wood K and Sayer JA: Calcium
oxalate crystal deposition in the kidney: Identification, causes
and consequences. Urolithiasis. 48:377–384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Costa P, Blowes LM, Laly AC and Connelly
JT: Regulation of collective cell polarity and migration using
dynamically adhesive micropatterned substrates. Acta Biomater.
126:291–300. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yao W, Wang Z, Ma H, Lin Y, Liu X, Li P
and He X: Epithelial-mesenchymal plasticity (EMP) in wound healing:
Exploring EMT mechanisms, regulatory network, and therapeutic
opportunities. Heliyon. 10(e34269)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jayne D, Herbert C, Anquetil V and
Teixeira G: Exploring the critical role of tight junction proteins
in kidney disease pathogenesis. Nephron. 149:240–250.
2025.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yu L, Gan X, Liu X and An R: Calcium
oxalate crystals induces tight junction disruption in distal renal
tubular epithelial cells by activating ROS/Akt/p38 MAPK signaling
pathway. Ren Fail. 39:440–451. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qi S, Wang Q, Xie B, Chen Y, Zhang Z and
Xu Y: P38 MAPK signaling pathway mediates COM crystal-induced
crystal adhesion change in rat renal tubular epithelial cells.
Urolithiasis. 48:9–18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liu RN, Zou DM, Tian MY, Li K, Du JL, Liu
MJ and Ma YZ: Effect of magnesium ammonium phosphate on the
expression of adhesion molecules in sheep renal tubular epithelial
cells. Res Vet Sci. 138:167–177. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sayed AA, Soliman A.M, Fahmy SR and Hosny
R: Antiurolithiatic effect of a polyherbal formulation against
sodium oxalate-induced urolithiasis in rats. J Basic Appl Zool.
84(15)2023.
|
|
12
|
Gopika S, Nisha MK, Gaayathiri Devi E,
Raja Rajeswari A and Vasandhlakshmi R: Evaluation of
antiurolithiatic potential of methanolic stem extract of
Spermacocce articularis L.f.: An in vitro and in vivo
approach. Pharmacogn J. 16:770–778. 2024.
|
|
13
|
Wongprasert K, Rudtanatip T and Praiboon
J: Immunostimulatory activity of sulfated galactans isolated from
the red seaweed Gracilaria fisheri and development of resistance
against white spot syndrome virus (WSSV) in shrimp. Fish Shellfish
Immunol. 36:52–60. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rudtanatip T, Pariwatthanakun C, Somintara
S, Sakaew W and Wongprasert K: Structural characterization,
antioxidant activity, and protective effect against hydrogen
peroxide-induced oxidative stress of chemically degraded Gracilaria
fisheri sulfated galactans. Int J Biol Macromol. 206:51–63.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rudtanatip T, Somintara S, Sakaew W,
El-Abid J, Cano ME, Jongsomchai K, Wongprasert K and Kovensky J:
Sulfated galactans from Gracilaria fisheri with supplementation of
octanoyl promote wound healing activity in vitro and in vivo.
Macromol Biosci. 22(e2200172)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sakaew W, Phanphak J, Somintara S, Hipkaeo
W, Wongprasert K, Kovensky J, Pariwatthanakun C and Rudtanatip T:
Increased sulfation in Gracilaria fisheri sulfated galactans
enhances antioxidant and antiurolithiatic activities and protects
HK-2 cell death induced by sodium oxalate. Mar Drugs.
20(382)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Sheng GJ, Oh YI, Chang SK and Hsieh-Wilson
LC: Tunable heparan sulfate mimetics for modulating chemokine
activity. J Am Chem Soc. 135:10898–10901. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zaporozhets T and Besednova N: Prospects
for the therapeutic application of sulfated polysaccharides of
brown algae in diseases of the cardiovascular system: Review. Pharm
Biol. 54:3126–3135. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sae-Lao T, Luplertlop N, Janvilisri T,
Tohtong R, Bates DO and Wongprasert K: Sulfated galactans from the
red seaweed Gracilaria fisheri exerts anti-migration effect on
cholangiocarcinoma cells. Phytomedicine. 36:59–67. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liang KH, Tso HC, Hung SH, Kuan II, Lai
JK, Ke FY, Chuang YT, Liu IJ, Wang YP, Chen RH and Wu HC:
Extracellular domain of EpCAM enhances tumor progression through
EGFR signaling in colon cancer cells. Cancer Lett. 433:165–175.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Brown TC, Sankpal NV and Gillanders WE:
Functional implications of the dynamic regulation of EpCAM during
epithelial-to-mesenchymal transition. Biomolecules.
11(956)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Erickson SB, Vrtiska TJ and Lieske JC:
Effect of Cystone® on urinary composition and stone
formation over a one year period. Phytomedicine. 18:863–867.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Singh A, Tandon S, Kaur T and Tandon C: In
vitro studies on calcium oxalate induced apoptosis attenuated by
Didymocarpus pedicellata. Biointerface Res Appl Chem.
12:7342–7355. 2022.
|
|
24
|
González Mosquera DM, Ortega YH, Quero PC,
Martínez RS and Luc Pieters L: Antiurolithiatic activity of
Boldoa purpurascens aqueous extract: An in vitro and in vivo
study. J Ethnopharmacol. 253(112691)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang H, Gao L, Zhao C, Fang F, Liu J, Wang
Z, Zhong Y and Wang X: The role of PI3K/Akt signaling pathway in
chronic kidney disease. Int Urol Nephrol. 56:2623–2633.
2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kang J, Jia X, Wang N, Xiao M, Song S, Wu
S, Li Z, Wang S, Cui SW and Guo Q: Insights into the
structure-bioactivity relationships of marine sulfated
polysaccharides: A review. Food Hydrocoll. 123(107049)2022.
|
|
28
|
De Araújo L, Costa-Pessoa JM, de Ponte MC
and Oliveira-Souza M: Sodium oxalate-induced acute kidney injury
associated with glomerular and tubulointerstitial damage in rats.
Front Physiol. 11(1076)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ji Y, Fang S, Yang Y and Wu Z:
Inactivation of the Wnt/β-catenin signaling contributes to the
epithelial barrier dysfunction induced by sodium oxalate in canine
renal epithelial cells. J Anim Sci. 99:1–10. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Uwaezuoke SN: The role of adhesion
molecules in nephropathies: The diagnostic applications. Integr Mol
Med. 6:1–5. 2019.
|
|
31
|
Hu Q, Saleem K, Pandey J, Charania AN,
Zhou Y and He C: Cell adhesion molecules in fibrotic diseases.
Biomedicines. 11(1995)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Peerapen P and Thongboonkerda V: p38 MAPK
mediates calcium oxalate crystal-induced tight junction disruption
in distal renal tubular epithelial cells. Sci Rep.
3(1041)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tsuji H, Shimizu N, Nozawa M, Umekawa T,
Yoshimura K, De Velasco MA, Uemura H and Khan SR: Osteopontin
knockdown in the kidneys of hyperoxaluric rats leads to reduction
in renal calcium oxalate crystal deposition. Urolithiasis.
42:195–202. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Senbanjo LT and Chellaiah MA: CD44: A
multifunctional cell surface adhesion receptor is a regulator of
progression and metastasis of cancer cells. Front Cell Dev Biol.
5(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jordan AR, Racine RR, Hennig MJP and
Lokeshwar VB: The role of CD44 in disease pathophysiology and
targeted treatment. Front Immunol. 6(182)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hong SY and Qin BL: The protective role of
dietary polyphenols in urolithiasis: Insights into antioxidant
effects and mechanisms of action. Nutrients.
15(3753)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yasir F, Wahab AT and Choudhary MI:
Protective effect of dietary polyphenol caffeic acid on ethylene
glycol-induced kidney stones in rats. Urolithiasis. 46:157–166.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li Y, Zhanga J, Liu H, Yuan J, Yin Y, Wang
T, Cheng B, Sun S and Guo Z: Curcumin ameliorates
glyoxylate-induced calcium oxalate deposition and renal injuries in
mice. Phytomedicine. 61(152861)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Peerapen P and Thongboonkerd V: Protective
roles of trigonelline against oxalate-induced
epithelial-to-mesenchymal transition in renal tubular epithelial
cells: An in vitro study. Food Chem Toxicol.
135(110915)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang Z, Li MX, Xu CZ, Zhang Y, Deng Q, Sun
R, Hu QY, Zhang SP, Zhang JW and Liang H: Comprehensive study of
altered proteomic landscape in proximal renal tubular epithelial
cells in response to calcium oxalate monohydrate crystals. BMC
Urol. 20(136)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Sun XY, Xu M and Ouyang JM: Effect of
crystal shape and aggregation of calcium oxalate monohydrate on
cellular toxicity in renal epithelial cells. ACS Omega.
2:6039–6052. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bonnici L, Suleiman S, Schembri-Wismayer P
and Cassar A: Targeting signalling pathways in chronic wound
healing. Int J Mol Sci. 25(50)2024.
|
|
43
|
Sun C, Liu M, Sun P, Yang M, Yates EA, Guo
Z and Fernig DG: Sulfated polysaccharides interact with fibroblast
growth factors and protect from denaturation. FEBS Open Bio.
9:1477–1487. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huang L, Shen M, Morris GA and Xie J:
Sulfated polysaccharides: Immunomodulation and signaling
mechanisms. Trends Food Sci Technol. 92:1–11. 2019.
|
|
45
|
Song Q, Song C, Chen X, Xiong Y, He Z, Su
X, Zhou J, Ke H, Dong C, Liao W and Yang S: Oxalate regulates
crystal-cell adhesion and macrophage metabolism via JPT2/PI3K/AKT
signaling to promote the progression of kidney stones. J Pharm
Anal. 14(100956)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lee S, Kim MS, Jung SJ, Kim D, Park HJ and
Cho D: ERK activating peptide, AES16-2M promotes wound healing
through accelerating migration of keratinocytes. Sci Rep.
8(14398)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kasowanjete P, Kumar SSD and Houreld NN: A
review of photobiomodulation on PI3K/AKT/mTOR in wound healing. J
Photochem Photobiol. 19(100215)2024.
|
|
48
|
Ashraf R and Kumar S: Mfn2-mediated
mitochondrial fusion promotes autophagy and suppresses ovarian
cancer progression by reducing ROS through AMPK/mTOR/ ERK
signaling. Cell Mol Life Sci. 79(573)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Guzel A, Yunusoglu S, Calapoglu M, Candan
IA, Onaran I, Oncu M, Ergun O and Oksay T: Protective effects of
quercetin on oxidative stress-induced tubular epithelial damage in
the experimental rat hyperoxaluria model. Medicina.
57(566)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Jing GH, Liu YD, Liu JN, Jin YS, Yu SL and
An RH: Puerarin prevents calcium oxalate crystal-induced renal
epithelial cell autophagy by activating the SIRT1-mediated
signaling pathway. Urolithiasis. 50:545–556. 2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Wang Y, Zheng J, Han Y, Zhang Y, Su L, Hu
D and Fu X: JAM-A knockdown accelerates the proliferation and
migration of human keratinocytes, and improves wound healing in
rats via FAK/Erk signaling. Cell Death Dis. 9(848)2018.PubMed/NCBI View Article : Google Scholar
|