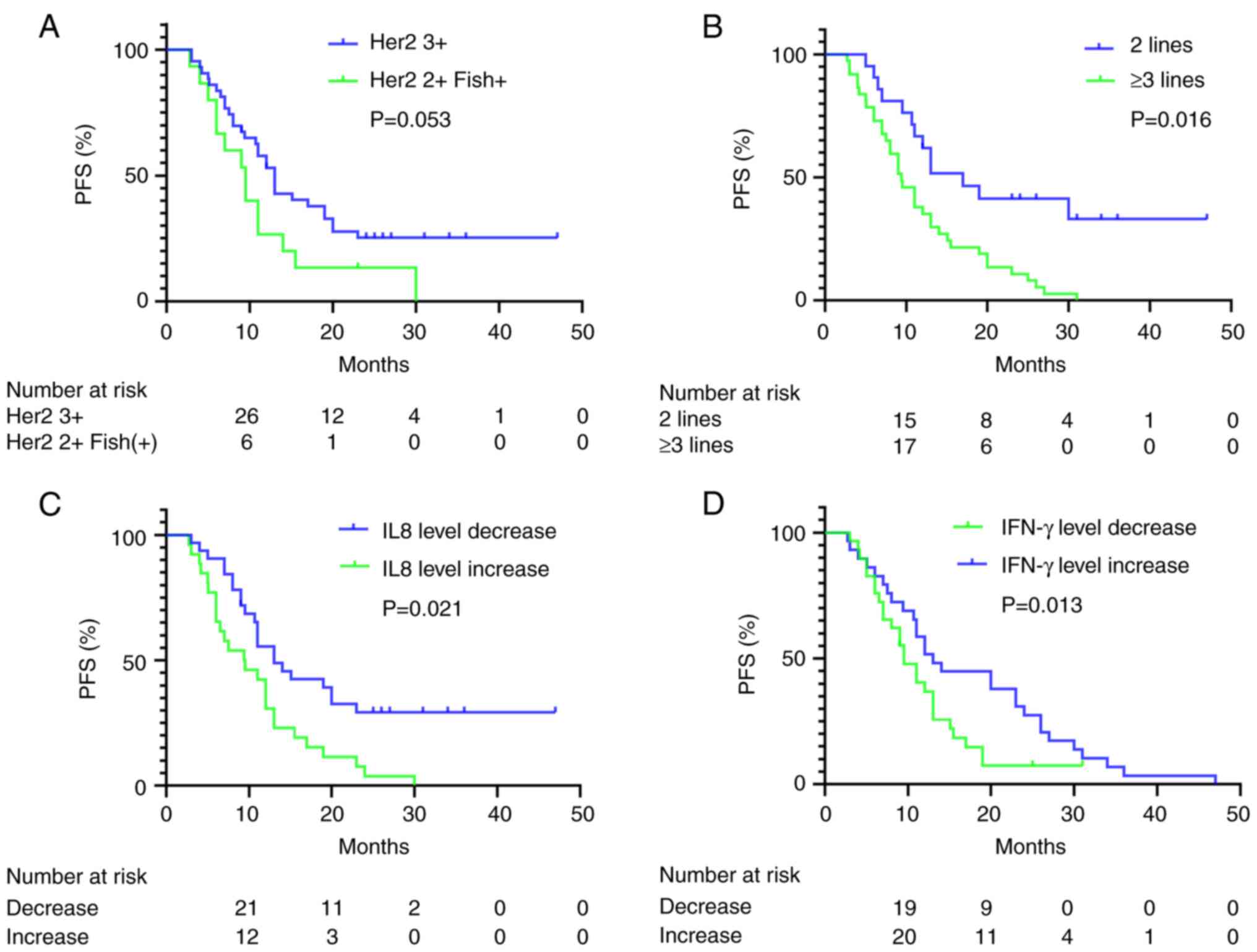
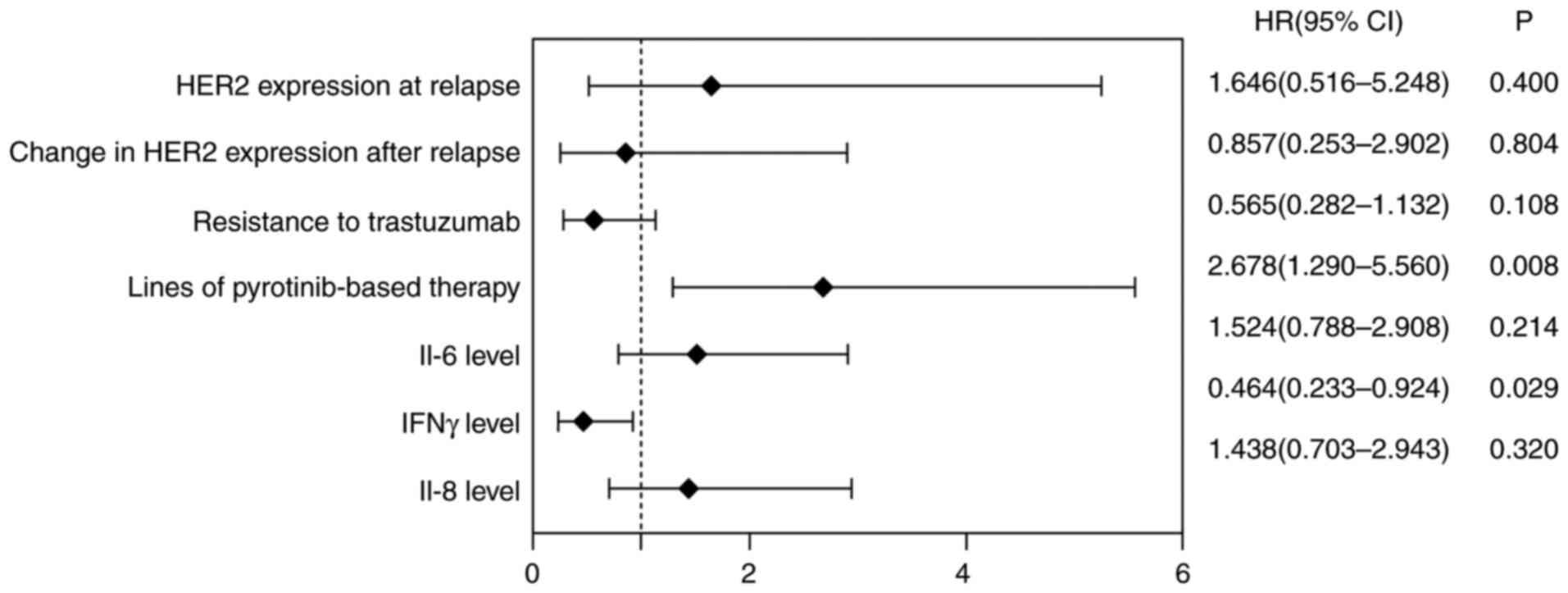
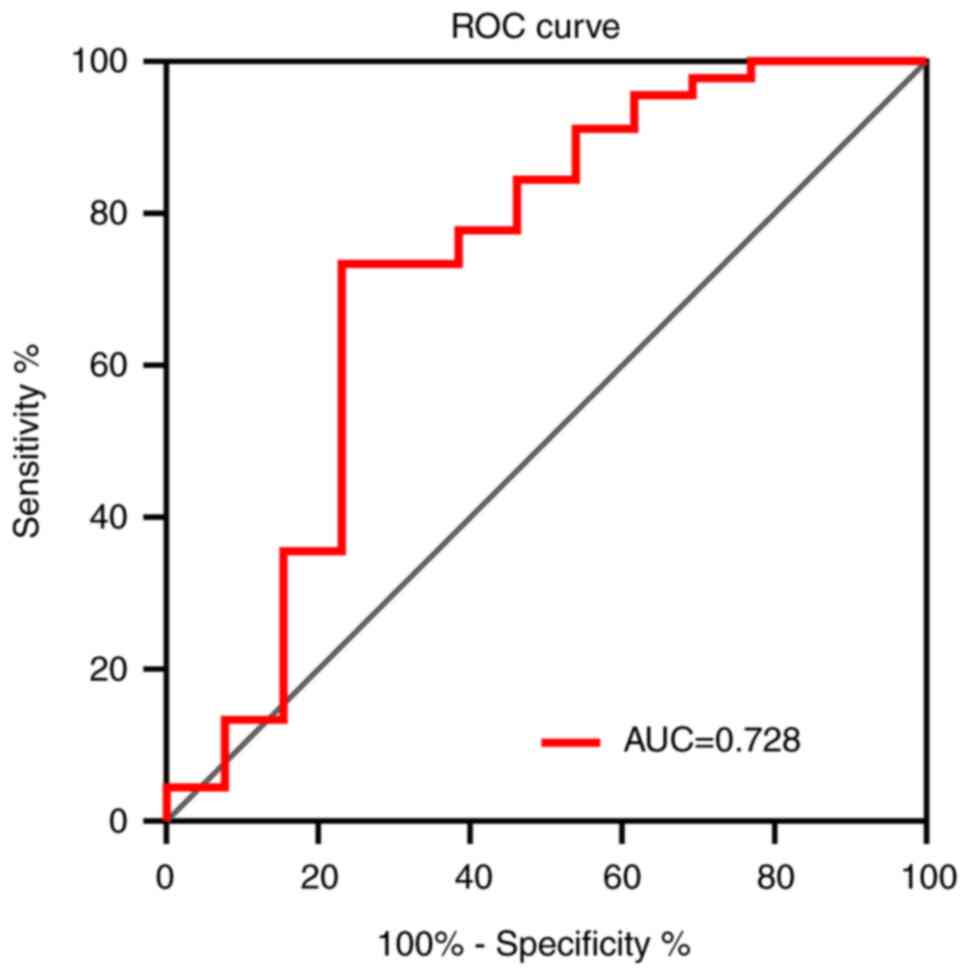
|
1
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harbeck N: Advances in targeting
HER2-positive breast cancer. Curr Opin Obstet Gynecol. 30:55–59.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li X, Yang C, Wan H, Zhang G, Feng J and
Zhang L, Chen X, Zhong D, Lou L, Tao W and Zhang L: Discovery and
development of pyrotinib: A novel irreversible EGFR/HER2 dual
tyrosine kinase inhibitor with favorable safety profiles for the
treatment of breast cancer. Eur J Pharm Sci. 110:51–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q,
Tong Z, Li H, Zhang Q, Sun T, et al: Pyrotinib plus capecitabine
versus lapatinib plus capecitabine for the treatment of
HER2-positive metastatic breast cancer (PHOEBE): A multicentre,
open-label, randomised, controlled, phase 3 trial. Lancet Oncol.
22:351–360. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hou J, Greten TF and Xia Q:
Immunosuppressive cell death in cancer. Nat Rev Immunol.
17(401)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Edwardson DW, Parissenti AM and Kovala AT:
Chemotherapy and inflammatory cytokine signalling in cancer cells
and the tumour microenvironment. Adv Exp Med Biol. 1152:173–215.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kartikasari AER, Huertas CS, Mitchell A
and Plebanski M: Tumor-induced inflammatory cytokines and the
emerging diagnostic devices for cancer detection and prognosis.
Front Oncol. 11(692142)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Habanjar O, Bingula R, Decombat C,
Diab-Assaf M, Caldefie-Chezet F and Delort L: Crosstalk of
inflammatory cytokines within the breast tumor microenvironment.
Int J Mol Sci. 24(4002)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Semesiuk NI, Zhylchuk A, Bezdenezhnykh N,
Lykhova A, Vorontsova AL, Zhylchuk VE and Kudryavets YI:
Disseminated tumor cells and enhanced level of some cytokines in
bone marrow and peripheral blood of breast cancer patients as
predictive factors of tumor progression. Exp Oncol. 35:295–302.
2013.PubMed/NCBI
|
|
11
|
Jia D, Li L, Andrew S, Allan D, Li X, Lee
J, Ji G, Yao Z, Gadde S, Figeys D and Wang L: An autocrine
inflammatory forward-feedback loop after chemotherapy withdrawal
facilitates the repopulation of drug-resistant breast cancer cells.
Cell Death Dis. 8(e2932)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, et al: Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol. 20:719–726. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hubalek M, Brunner C, Matthä K and Marth
C: Resistance to HER2-targeted therapy: mechanisms of trastuzumab
resistance and possible strategies to overcome unresponsiveness to
treatment. Wien Med Wochenschr. 160:506–512. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu
Y, Li H, Yu S, Feng J, Wang S, et al: Pyrotinib or lapatinib
combined with capecitabine in HER2-positive metastatic breast
cancer with prior taxanes, anthracyclines, and/or trastuzumab: A
randomized, phase II study. J Clin Oncol. 37:2610–2619.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Q, He P, Tian T, Yan X, Huang J,
Zhang Z, Zheng H, Zhong X and Luo T: Real-world efficacy and safety
of pyrotinib in patients with HER2-positive metastatic breast
cancer: A prospective real-world study. Front Pharmacol.
14(1100556)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lindström LS, Karlsson E, Wilking UM,
Johansson U, Hartman J, Lidbrink EK, Hatschek T, Skoog L and Bergh
J: Clinically used breast cancer markers such as estrogen receptor,
progesterone receptor, and human epidermal growth factor receptor 2
are unstable throughout tumor progression. J Clin Oncol.
30:2601–2608. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang X, Li Z, Han L, Lv Z, Teng Y, Cui X,
Zhou C, Wu H, Fang W, Xu L, et al: Efficacy and safety of pyrotinib
in human epidermal growth factor receptor 2-positive advanced
breast cancer: A multicenter, retrospective, real-world study. Onco
Targets Ther. 15:1067–1078. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Z, Wang C, Chen X, Zhu J, Sun X, Xia
Q, Lu Z, Qiao J, Zhou Y, Wang H, et al: Pathological response and
predictive role of tumour-infiltrating lymphocytes in HER2-positive
early breast cancer treated with neoadjuvant pyrotinib plus
trastuzumab and chemotherapy (Panphila): A multicentre phase 2
trial. Eur J Cancer. 165:157–168. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Quinn KM, Kartikasari AER, Cooke RE,
Koldej RM, Ritchie DS and Plebanski M: Impact of age-, cancer-, and
treatment-driven inflammation on T cell function and immunotherapy.
J Leukoc Biol. 108:953–965. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang Y, Li S, Wang Y, Zhao Y and Li Q:
Protein tyrosine kinase inhibitor resistance in malignant tumors:
Molecular mechanisms and future perspective. Signal Transduct
Target Ther. 7(329)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Autenshlyus A, Davletova K, Varaksin N,
Marinkin I and Lyakhovich V: Cytokines in various molecular
subtypes of breast cancer. Int J Immunopathol Pharmacol.
35(20587384211034089)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tian D, Tian M, Ma ZM, Zhang LL, Cui YF
and Li JL: Anesthetic propofol epigenetically regulates breast
cancer trastuzumab resistance through IL-6/miR-149-5p axis. Sci
Rep. 10(8858)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vazquez-Martin A, Colomer R and Menendez
JA: Her-2/neu-induced ‘cytokine signature’ in breast cancer. Adv
Exp Med Biol. 617:311–319. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jia Y, Kodumudi KN, Ramamoorthi G, Basu A,
Snyder C, Wiener D, Pilon-Thomas S, Grover P, Zhang H, Greene MI,
et al: Th1 cytokine interferon gamma improves response in HER2
breast cancer by modulating the ubiquitin proteasomal pathway. Mol
Ther. 29:1541–1556. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hannesdóttir L, Tymoszuk P, Parajuli N,
Wasmer MH, Philipp S, Daschil N, Datta S, Koller JB, Tripp CH,
Stoitzner P, et al: Lapatinib and doxorubicin enhance the
Stat1-dependent antitumor immune response. Eur J Immunol.
43:2718–2729. 2013.PubMed/NCBI View Article : Google Scholar
|