Introduction
Patients overexpressing human epidermal growth
factor receptor 2 (HER2) account for 15-20% of all breast cancer
(BC) cases and have a poor prognosis (1,2).
Pyrotinib is an irreversible small molecule tyrosine kinase
inhibitor (TKI) drug that targets HER1, HER2 and HER4 and has
robust antitumor effects in patients with HER2-positive BC
(3). It has been shown that,
compared with lapatinib plus capecitabine, pyrotinib plus
capecitabine significantly improves the progression-free survival
(PFS) of patients with HER2-positive metastatic BC after
trastuzumab and chemotherapy (4).
Dying cancer cells exert both immuno-activating and
immunosuppressive effects, depending on the intracellular signals,
extracellular context and systemic crosstalk (5). Immune cells communicate with the
microenvironment via cytokines, which are mainly produced by
macrophages, T cells, B cells and cancer cells. These cytokines can
promote or inhibit malignant tumor progression through the complex
cytokine network between tumor cells and the tumor microenvironment
(TME) (6,7). Cytokines from the TME can be released
into the circulation and detected, making them potentially useful
as biomarkers to predict disease outcomes and guide therapeutic
decisions (8). In BC, cytokines
serve a crucial role in regulating immunity (9). The levels of cytokines such as TNF,
colony stimulating factor 1, IL-6 and endogenous IFN in the
circulation of patients with BC are significant markers of tumor
progression (10). These cytokines
also form an autocrine, pro-inflammatory feed-forward loop that
facilitates the accumulation of drug-resistant BC cells (11).
The aim of the present study was to explore the
relationship between plasma cytokines and the efficacy of
pyrotinib. For this purpose, plasma cytokines such as IL-6, IL-8,
IL-10, IL-17 and IFN-γ were measured before and 4 weeks after
pyrotinib treatment.
Materials and methods
Study design
A total of 58 patients with HER2-positive advanced
BC (ABC) treated with pyrotinib at Tangshan People's Hospital
(Tangshan, China) were enrolled in the present single-center
prospective study from January 2020 to December 2022. Since only
patients who were already scheduled to receive pyrotinib-containing
treatments were included in the present study, patient
randomisation was not applicable. The study protocol was approved
by the Ethics Committee of Tangshan People's Hospital (approval no.
RMYY-LLKS-2019-058; Tangshan, China). The key inclusion criteria
were as follows: i) Histopathological confirmation of invasive
ductal carcinoma with clear immunohistochemistry; ii) HER2
3+ or 2+ detected by fluorescence in situ
hybridization (FISH); iii) Eastern Cooperative Oncology Group
(ECOG) score of 1-3; and iv) patients expected to survive ≥3
months. According to the Response Evaluation Criteria in Solid
Tumors (RECIST 1.1) for efficacy evaluation, at least one
measurable target lesion was detected, and previous
trastuzumab-based targeted therapy had failed. The key exclusion
criteria were as follows: i) Had other malignant tumors; ii) were
under the age of 18; and iii) were unwilling to provide informed
consent. Written informed consent was obtained from all
participants prior to study enrollment.
Treatment and follow-up
All patients received pyrotinib (400 mg, orally,
once daily) combined with capecitabine (1,000 mg/m2,
orally, twice daily on days 1-14) or vinorelbine soft capsules (40
mg, orally, 3 times weekly), which was for patients who had
previously taken capecitabine in a 3-week cycle. Upon the
development of grade ≥3 adverse events, symptomatic treatment was
administered and pyrotinib was suspended or reduced to 320 mg
daily. In the present study, treatment was continued until disease
progression (PD), unacceptable toxicity or withdrawal of consent
Treatment responses were determined according to the RECIST 1.1
guidelines. Adverse events were assessed according to the Common
Terminology Criteria for Adverse Events Version 4.0 and graded as
0-4. Follow-up was performed upon clinic visit, hospital admission,
and telephone contact until December 2023.
Sample and data collection
In total, 2 ml peripheral blood (PB) samples were
collected from patients before and 4 weeks after the initial
pyrotinib treatment using EDTA anticoagulant tubes. The plasma
samples were centrifuged at 1,000 x g for 10 min for cytokine
detection. The plasma cytokine levels, including IL-6, IL-8, IL-10,
IL-17 and IFN-γ, were measured using a multi-microsphere
immunofluorescence assay according to the manufacturer's
instructions (plasma cytokine test kit; cat. no. 20202400205;
Qingdao Ruisikaier Biotechnology Co., Ltd.) and a Navios flow
cytometer analyser with Kaluza 1.2 software (Beckman Coulter,
Inc.).
Statistical analysis
Statistical analyses were performed using SPSS 26.0
software (IBM Corp.). For descriptive analysis, qualitative
variables are expressed as absolute values and percentages, and
quantitative variables are expressed as the mean and range.
Kaplan-Meier curves were used for survival analysis. Based on the
results of the univariate logistic regression analysis (P<0.10),
candidate variables were selected for inclusion in the multivariate
Cox regression analysis, which was performed to assess the
prognostic variables. The relationship between non-normally
distributed variables was analysed by Spearman's rank correlation
analysis. The predictive accuracy of the nomograms was evaluated
using receiver operating characteristic (ROC) curves. P<0.05 was
considered to indicate a statistically significant defined
difference.
Results
Clinical data
A total of 58 patients were included in the present
study. The median follow-up time was 33.25 months (range, 14.0-49.0
months) and the follow-up rate was 98.28%. The median age of the
included patients was 53 years. The detailed clinical data of the
58 patients are shown in Table I.
The findings on the IL-6, IL-8, IL-10 and IL-17 plasma levels
before and 4 weeks after pyrotinib treatment are shown in Table II.
 | Table IBaseline characteristics of human
epidermal growth factor receptor 2-positive patients with advanced
breast cancer treated with pyrotinib. |
Table I
Baseline characteristics of human
epidermal growth factor receptor 2-positive patients with advanced
breast cancer treated with pyrotinib.
| Clinical
characteristics (n=58) | n (%) |
|---|
| Eastern Cooperative
Oncology Group performance status | |
|
0 | 7 (12.10) |
|
1 | 46 (79.3) |
|
2 | 5 (8.60) |
| Menopause | |
|
No | 38 (65.50) |
|
Yes | 20 (34.50) |
|
Oestrogen+ | |
|
≥20% | 19 (32.76) |
|
<20% | 39 (67.24) |
| Location of
metastasis | |
|
Lung | 39 (67.24) |
|
Liver | 14 (24.14) |
|
Brain | 7 (12.07) |
|
Bone | 18 (31.03) |
|
Serous
effusion | 3 (5.17) |
|
Lymph
node | 29 (50.0) |
| Multiple organs
metastasis | |
|
Yes | 46 (79.31) |
|
No | 12 (20.69) |
| Chemotherapy
cycles | |
|
2 lines | 21 (36.21) |
|
≥3
lines | 37 (63.79) |
| Combined | |
|
Capecitabine | 35 (60.30) |
|
Vinorelbine | 23 (39.7) |
 | Table IIPlasma cytokine levels in patients
with advanced breast cancer treated with pyrotinib. |
Table II
Plasma cytokine levels in patients
with advanced breast cancer treated with pyrotinib.
| Cytokines | Pretreatment, median
(range) | Post-treatment,
median (range) |
|---|
| IL-6, pg/ml | 7.95
(1.23-56.83) | 8.81
(1.33-71.72) |
| IL-8, pg/ml | 7.41
(0.37-32.06) | 8.03 (0.1-65.05) |
| IL-10, pg/ml | 1.98 (0.71-4.62) | 1.68 (0.18-3.63) |
| IL-17, pg/ml | 4.64
(0.53-19.23) | 2.87 (0.1-32.46) |
| Interferon-γ | 6.28
(0.29-27.09) | 6.65 (0.5-25.99) |
Short-term efficacy
The short-term efficacy evaluation according to the
RECIST 1.1 guidelines revealed that 7 patients had PD (12.1%), 28
had stable disease (48.3%), 22 had partial remission (37.9%), and 1
had complete remission (1.7%).
Log-rank univariate analysis
On December 2023 data cut-off date, the median PFS
(mPFS) time of the included patients was 11.0 months, the 2-year
survival rate was 66%, the 3-year survival rate was 52% and the
median overall survival (OS) was not reached. The results revealed
that patients with a HER2 3+ status had an improved PFS
time compared with those with a HER2 2+ status
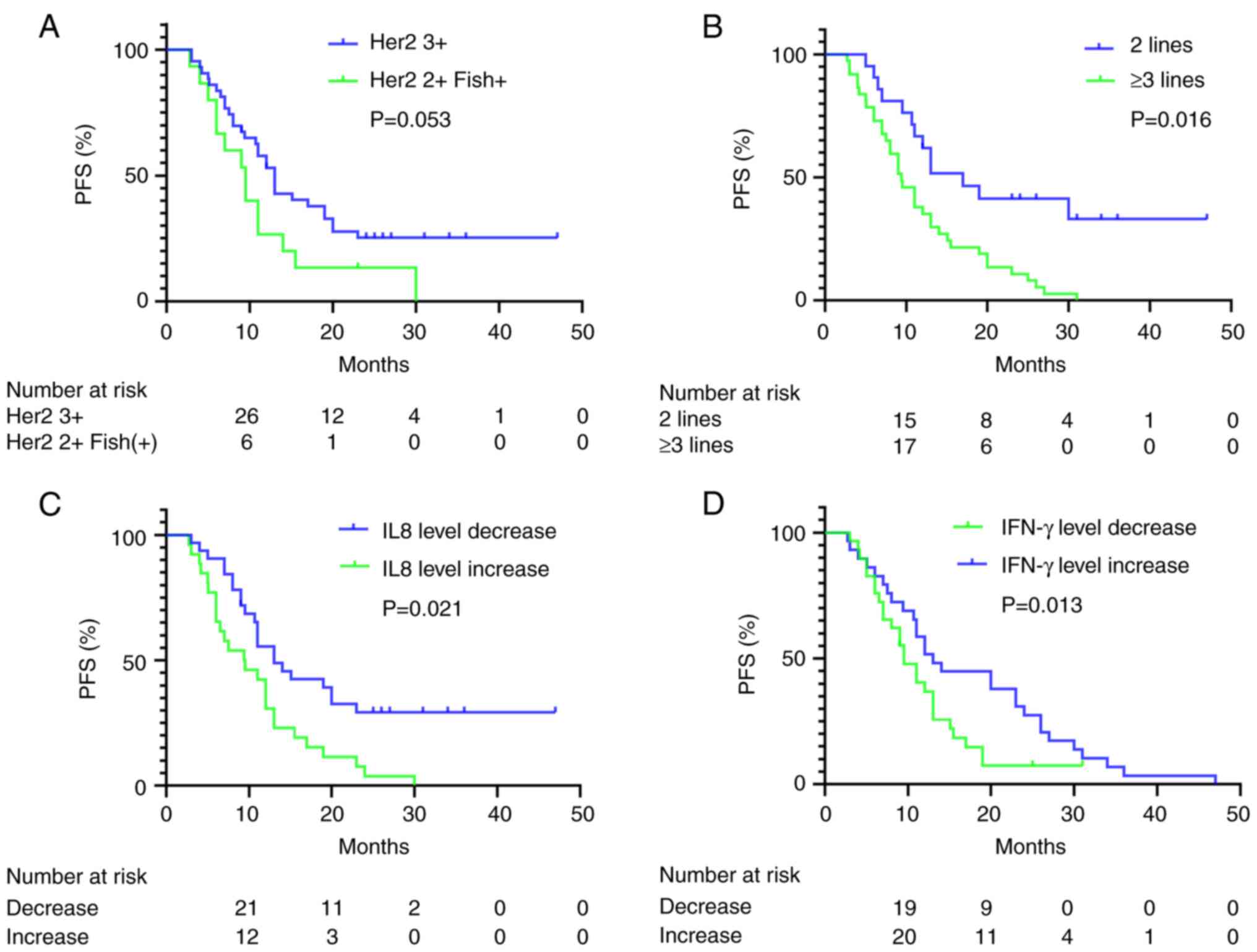
(FISH-positive) (mPFS, 13.0 vs. 9.5 months; P=0.053; Fig. 1A). Patients whose HER2 expression
status changed after recurrence exhibited a trend towards a poorer
PFS time compared with those with stabilized HER2 expression (mPFS,
9.5 vs. 13.0 months; P=0.072). Patients who received pyrotinib as
the second-line therapy had a longer PFS time than patients who
received pyrotinib as the third- or later-line therapy (mPFS, 17.0
vs. 9.5 months; P=0.016; Fig. 1B).
Patients who had primary resistance to trastuzumab also had a
poorer PFS time than those who had secondary resistance (mPFS, 9.5
vs. 13.0; P=0.057). Patients whose plasma IL-8 levels increased
after 4 weeks of pyrotinib treatment had a poorer PFS time than
those whose plasma IL-8 levels decreased (mPFS, 9.4 vs. 14.0
months; P=0.021; Fig. 1C). Patients
with increased plasma IFN-γ levels had an improved PFS time
compared with patients with decreased plasma IFN-γ levels (mPFS,
13.0 vs. 11.0 months; P=0.013; Fig.
1D). The results of the univariate analysis of PFS with various
clinical parameters in patients with pyrotinib-treated ABC are
included in Table III.
 | Table IIIUnivariate analysis of the prognosis
of patients with advanced breast cancer treated with pyrotinib. |
Table III
Univariate analysis of the prognosis
of patients with advanced breast cancer treated with pyrotinib.
| Clinical
parameters | Total (n=58) | Median
progression-free survival (months) | χ2 | P-value |
|---|
| Age, years | | | | |
|
≤53 | 32 | 13.0 | 1.801 | 0.180 |
|
>53 | 26 | 9.0 | | |
| ECOG | | | | |
|
1 | 7 | 7.0 | 0.882 | 0.643 |
|
2 | 46 | 12.0 | | |
|
3 | 5 | 6.5 | | |
| Menopause | | | | |
|
Yes | 38 | 11.0 | 1.023 | 0.880 |
|
No | 20 | 11.0 | | |
| Oestrogen
status | | | | |
|
<20% | 48 | 11.0 | 0.041 | 0.839 |
|
≥20% | 10 | 8.0 | | |
| Brain
metastasis | | | | |
|
Yes | 7 | 13.0 | 0.316 | 0.574 |
|
No | 51 | 11.0 | | |
| HER2 status when
relapse | | | | |
|
3+ | 43 | 13.0 | 3.744 | 0.053 |
|
2+
(FISH+) | 15 | 9.5 | | |
| HER2 status changed
after recurrence | | | | |
|
Yes | 13 | 9.5 | 3.231 | 0.072 |
|
No | 45 | 13.0 | | |
| Therapy cycles | | | | |
|
2 lines | 21 | 17.0 | 5.766 | 0.016 |
|
≥3
lines | 37 | 9.5 | | |
| Resistance to
trastuzumab | | | | |
|
Primary | 20 | 9.5 | 3.637 | 0.057 |
|
Secondary | 36 | 13.0 | | |
| Combined drug | | | | |
|
Capecitabine | 35 | 13.0 | 1.176 | 0.278 |
|
Vinorelbine | 23 | 9.0 | | |
| Lapatinib
application | | | | |
|
Yes | 9 | 9.0 | 1.457 | 0.227 |
|
No | 49 | 12 | | |
| IL-6 level | | | | |
|
Increase | 28 | 11.0 | 3.296 | 0.069 |
|
Decrease | 30 | 17.0 | | |
| IL-8 level | | | | |
|
Increase | 29 | 9.4 | 5.332 | 0.021 |
|
Decrease | 29 | 13.0 | | |
| IL-10 level | | | | |
|
Increase | 30 | 13.0 | 1.346 | 0.246 |
|
Decrease | 28 | 10.7 | | |
| IL-17 level | | | | |
|
Increase | 29 | 11.0 | 0.031 | 0.861 |
|
Decrease | 29 | 13.0 | | |
| Interferon-γ
level | | | | |
|
Increase | 29 | 14.0 | 6.701 | 0.013 |
|
Decrease | 29 | 9.50 | | |
Multivariate Cox regression
analysis
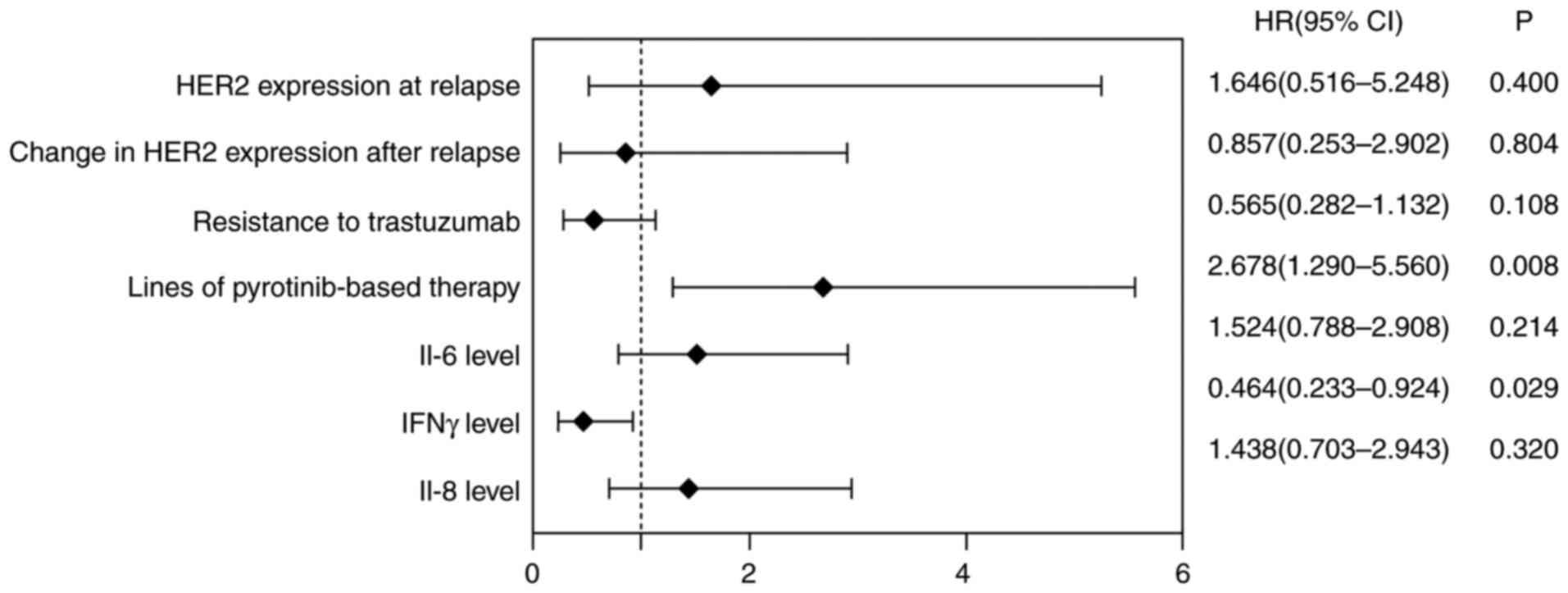
Multivariate Cox regression analysis revealed that
the number of cycles of pyrotinib treatment after recurrence and
elevated plasma IFN-γ levels after treatment were independent
predictive factors for PFS (Table
IV and Fig. 2).
 | Table IVMultivariable analysis of the
prognosis of patients with advanced breast cancer treated with
pyrotinib. |
Table IV
Multivariable analysis of the
prognosis of patients with advanced breast cancer treated with
pyrotinib.
| Clinical
parameters | B | SE | Wald | df | P-value | Exp(B) |
|---|
| HER2 expression at
relapse | 0.498 | 0.592 | 0.710 | 1 | 0.400 | 1.646
(0.516-5.248) |
| Change in HER2
expression after relapse | -0.155 | 0.623 | 0.062 | 1 | 0.804 | 0.857
(0.253-2.902) |
| Resistance to
trastuzumab | -0.571 | 0.355 | 2.590 | 1 | 0.108 | 0.565
(0.282-1.132) |
| Number of cycles of
pyrotinib-based therapy | 0.985 | 0.373 | 6.989 | 1 | 0.008 | 2.678
(1.290-5.560) |
| IL-6 level
increase | 0.414 | 0.333 | 1.547 | 1 | 0.214 | 1.514
(0.788-2.908) |
| IL-8 level
increase | 0.363 | 0.365 | 0.989 | 1 | 0.320 | 1.438
(0.703-2.943) |
| Interferon-γ level
increase | -0.768 | 0.352 | 4.769 | 1 | 0.029 | 0.464
(0.233-0.924) |
Spearman's rank correlation
analysis
Spearman's rank correlation analysis demonstrated
that there was no correlation between the levels of plasma IFN-γ
and other cytokines (P>0.05). There was a correlation between
IL-17 and IL-8 after treatment, with a correlation coefficient of
0.431 (P<0.001). There was a correlation between IL-17 and
IL-10, with a correlation coefficient of 0.666 (P<0.001) but
there was no correlation between IL-8 and IL-10, with a correlation
coefficient of 0.212 (P=0.110) (Table
V).
 | Table VCorrelations between IFN-γ and IL-6,
IL-8, IL-10, IL-17 in advanced breast cancer patients after
pyrotinib therapy. |
Table V
Correlations between IFN-γ and IL-6,
IL-8, IL-10, IL-17 in advanced breast cancer patients after
pyrotinib therapy.
| Cytokines | | IL-6 | IL-8 | IL-10 | IL-17 | IFN-γ |
|---|
| IL-6 | Coefficient | 1.000 | 0.242 | 0.194 | 0.158 | -0.113 |
| | P-value | - | 0.068 | 0.144 | 0.235 | 0.399 |
| IL-8 | Coefficient | 0.242 | 1.000 | 0.212 | 0.431 | 0.068 |
| | P-value | 0.068 | - | 0.110 | 0.001 | 0.610 |
| IL-10 | Coefficient | 0.194 | 0.212 | 1.000 | 0.666 | 0.104 |
| | P-value | 0.144 | 0.110 | - | 0.000 | 0.435 |
| IL-17 | Coefficient | 0.158 | 0.431 | 0.666 | 1.000 | -0.124 |
| | P-value | 0.235 | 0.001 | 0.000 | - | 0.355 |
| IFN-γ | Coefficient | -0.113 | 0.068 | 0.104 | -0.124 | 1.000 |
| | P-value | 0.399 | 0.610 | 0.435 | 0.355 | - |
ROC curve analysis
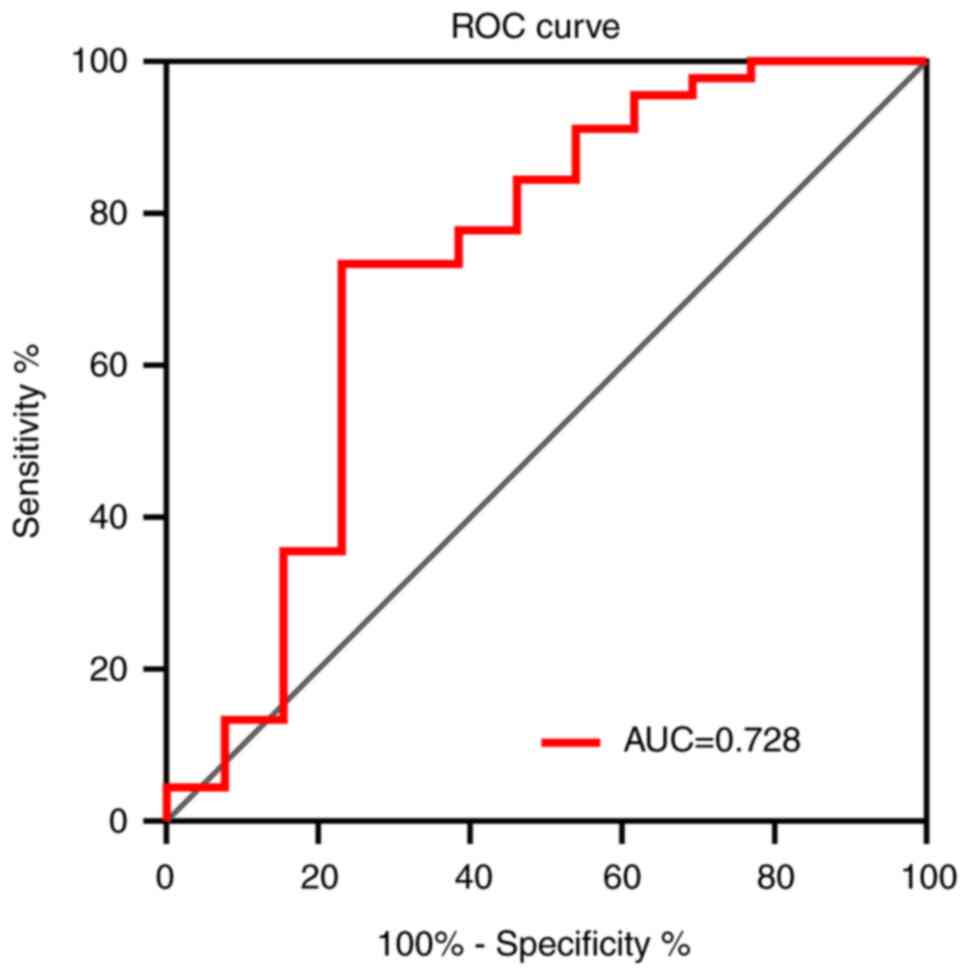
ROC curve analysis demonstrated that the change in
plasma IFN-γ level had an area under the curve of 0.728 (95%
confidence interval, 0.544-0.912) for predicting the PFS of
patients with HER-2 positive ABC treated with pyrotinib (Fig. 3).
Adverse reactions
There were no deaths associated with adverse events
in any of the included patients. The most commonly adverse events
were diarrhea (91.38%), bone marrow suppression (34.48%), hand-foot
syndrome (32.76%), nausea (21.69%), fatigue (5.17%) and oral
mucositis (6.9%) (Table VI). Most
adverse events were well tolerated in all patients and were
alleviated after diet modification, symptomatic treatment or by
adjusting the dose of pyrotinib.
 | Table VIIncidence of the main adverse effects
related to pyrotinib. |
Table VI
Incidence of the main adverse effects
related to pyrotinib.
| Adverse
effects | All patients
(%) | Ⅲ-Ⅳ patients
(%) |
|---|
| Hematology | | |
|
Neutropenia | 20 (34.48%) | 2 (3.45%) |
|
Thrombocytopenia | 6 (10.34%) | 0 (0%) |
|
Non-haematology | | |
|
Diarrhea | 53 (91.38%) | 11 (18.96%) |
|
Hand-foot
syndrome | 19 (32.76%) | 2 (3.45%) |
|
Nausea | 12 (21.69%) | 0 (0%) |
|
Fatigue | 3 (5.17%) | 0 (0%) |
|
Oral
mucositis | 4 (6.90%) | 0 (0%) |
Discussion
BC is currently the most common malignant tumor and
HER2-positive BC accounts for 15-20% of all BC cases (1,2).
Trastuzumab is the most commonly used anti-HER2 monoclonal
antibody, but ~50% of patients show resistance (12,13).
Numerous patients with HER2-positive BC do not respond to initial
therapy with trastuzumab, and a vast majority of these patients
develop resistance to this monoclonal antibody within 1 year
(14). A randomized phase II trial
demonstrated that, pyrotinib (an irreversible anti-HER2 TKI) plus
capecitabine demonstrated a significantly improved objective
response rate (78.5 vs. 57.1%) and PFS time (18.1 vs. 7.0 months)
than lapatinib plus capecitabine in patients with HER2-positive
metastatic BC (15). The phase III
PHOEBE clinical study also identified that the mPFS time was
significantly longer in patients treated with pyrotinib plus
capecitabine (12.5 months) compared with those treated with
lapatinib plus capecitabine (6.8 months) who had been previously
treated with trastuzumab and taxanes (4). The present study enrolled 58 patients,
including 7 patients with brain metastasis. The mPFS time of these
patients was 11.0 months, the 2-year survival rate was 66%, the
3-year survival rate was 52% and the median OS had not yet been
reached. Notably, in the present study, all patients exhibited
primary or secondary resistance to trastuzumab and some patients
had previously received heavily treatment of them even had been
heavily treated. A previous study demonstrated that the mPFS time
of patients with HER2-positive ABC treated with pyrotinib was 14.1
months and that there were no significant differences in PFS or OS
among patients receiving pyrotinib as first-, second-, and third or
later-line therapy (16). In the
present study, patients who received pyrotinib beyond the
third-line a had poorer PFS time than those who received pyrotinib
at the second-line (mPFS, 9.5 vs. 17.0 months; P=0.016).
Multivariate analysis also revealed that the application of
pyrotinib beyond third-line therapy was an independent risk factor
for PFS. A previous study reported that, for patients with
HER2-positive metastatic BC receiving first-, second- and third or
later-line therapy with pyrotinib, the mPFS time was 16.5, 12.4 and
9.3 months, respectively (17).
Another study suggested that patients who received second-line
pyrotinib had a significantly longer PFS time than those who
received third-line pyrotinib (18). Therefore, early application of
pyrotinib results in a longer mPFS time, however, whether early
application of pyrotinib can improve OS requires further follow-up
observation.
In total, ~15.7% of patients with BC experience a
change in HER2 status during tumor progression (17). In the present study, 13 patients
experienced HER2 status alterations during tumor progression and
had a worse PFS time than patients without HER2 alterations (mPFS,
9.5 vs. 13.0 months; P=0.072). Additionally, patients with a HER2
3+ status had a longer PFS time than patients with a
HER2 2+ status (mPFS; 13.0 vs. 9.5 months; P=0.053).
Patients who had primary resistance to trastuzumab also had a
poorer PFS time than those who had secondary resistance, when
treated with pyrotinib (mPFS; 9.5 vs. 13.0 months; P=0.057).
However, according to the results of the multivariate Cox
regression analysis in the present study, there was no
statistically significant difference. These results require
verification in further studies with larger sample sizes and
extended the follow-up periods to evaluate the long-term efficacy
of pyrotinib and the survival benefits for patients.
Pyrotinib resistance is inevitable in patients with
advanced HER2+ ABC, thus identifying a biomarker that
predicts treatment efficacy is crucial. The multicentre phase II
Panphila study evaluated pyrotinib plus trastuzumab, docetaxel and
carboplatin as neoadjuvant therapies for early BC and found a
significant association between the pathological complete response
and a greater baseline infiltration of stromal
(s)-CD20+, s-CD8+ and s-CD4+ cells
(19). Cytokines produced by
T-helper cells play a crucial role in regulating cellular responses
between tumors and immune system (20). Cytokines are also derived from
stromal, parenchymal and immune cells that reside in the tumor
tissue, under the activation of cancer-related inflammation or by
treatments for cancer (21).
Certain cytokines may not be beneficial for cancer treatment and
can cause immunosuppression, promote tumor progression and induce
drug resistance (22).
HER2-positive BC is accompanied by a significant
increase in PB IL-6 and IL-8 levels (23), particularly in the presence of
trastuzumab-resistant HER2-positive BC cells (24). The synthesis and secretion of the
IL-8 chemokine family are enhanced, which are involved in
HER2-positive BC metastasis and endocrine resistance. The
circulating levels of the IL-8 and GRO cytokines may represent new
biomarkers for monitoring the responses of patients with BC to
endocrine therapy and HER2-targeted therapy (25). In the present study, elevated IL-6
and IL-8 levels indicated a poor prognosis, but the differences
were not statistically significant. IFN-γ can cause significant
surface loss of HER2, diminished growth and induced tumor
senescence (26). IFN-γ-secreting T
cells have been observed to enhance tumor infiltration after
treatment with doxorubicin and/or lapatinib in a HER2-positive BC
animal model (27). The mechanism
of pyrotinib, an irreversible small molecule TKI of HER1, HER2 and
HER4, is similar to that of lapatinib. At present, few studies
investigating biomarkers associated with the efficacy of pyrotinib
have been conducted. In the present study, the plasma levels of the
IL-6, IL-8, IL-10, IL-17 and IFN-γ cytokines were measured before
and 4 weeks after pyrotinib treatment. Univariate analysis revealed
that increased expression levels of IL-6 and IL-8 in the PB after
treatment were risk factors for PFS. However, the prolonged mPFS
time was associated with increased IFN-γ expression. Multivariate
analysis revealed that increased PB IFN-γ levels are an independent
influencing factor for mPFS time in patients with HER2-positive ABC
tumors treated with pyrotinib. ROC curve analysis also demonstrated
that serum IFN-γ levels have value in predicting the efficacy of
pyrotinib in the treatment of HER-2 positive ABC. However, the
underlying biological mechanisms were not thoroughly investigated
in the present study. The next step is to investigate the
mechanisms of IFN-γ in pyrotinib treatment using in vitro
experiments or animal models.
The main adverse effect observed in the present
study was diarrhea, but this symptom can be managed through
targeted symptomatic treatment and diet adjustment. Overall, the
side effects included diarrhea (91.38%), bone marrow suppression
(34.48%), hand-foot syndrome (32.76%), nausea (21.69%), fatigue
(5.17%) and oral mucositis (6.9%), which are similar to a previous
study (16) and demonstrate that
the treatment is tolerable.
While the present study provides valuable insights,
its findings must be interpreted considering its sample size
limitations. The relatively small cohort (n=58) may restrict the
statistical power to detect subtle effects. Reduced power increases
the risk of Type II errors, wherein true associations may remain
undetected due to insufficient sensitivity. Despite these
limitations, the study's rigorous methodology and novel findings
offer a meaningful foundation for future research. The small size
of the present study means that a larger randomised study will be
required to define the predictive role of PB IFN-γ levels in
HER2-positive ABC treated with pyrotinib.
In summary, pyrotinib is well-tolerated by patients
with HER2-positive ABC and is more efficacious with early
application. Further follow-up and larger patient cohorts are
required to determine whether these promising findings translate
into improvements in the OS of patients. Furthermore, PB IFN-γ
levels can predict the effect of pyrotinib in patients with
HER2-positive ABC.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Health Planning
Projects in Hebei (grant no. 20191614).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JJ contributed to the design of the study, performed
the data curation and analyses, performed the literature search and
wrote the first draft of the manuscript. JW contributed to the
design of the study, performed the literature search, and edited
the manuscript. XY and JL contributed to study design, data
curation, data analysis, and manuscript editing. JJ and JW confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in line with the
principles of the Declaration of Helsinki, and in accordance with
the Australian Code for the Responsible Conduct of Research and the
National Statement on Ethical Conduct in Human Research. Approval
was granted by the Ethics Committee of Tangshan People's Hospital
(Tangshan, China; approval no. RMYY-LLKS-2019-058). Written
informed consent was obtained from all participants prior to study
enrollment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Harbeck N: Advances in targeting
HER2-positive breast cancer. Curr Opin Obstet Gynecol. 30:55–59.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li X, Yang C, Wan H, Zhang G, Feng J and
Zhang L, Chen X, Zhong D, Lou L, Tao W and Zhang L: Discovery and
development of pyrotinib: A novel irreversible EGFR/HER2 dual
tyrosine kinase inhibitor with favorable safety profiles for the
treatment of breast cancer. Eur J Pharm Sci. 110:51–61.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xu B, Yan M, Ma F, Hu X, Feng J, Ouyang Q,
Tong Z, Li H, Zhang Q, Sun T, et al: Pyrotinib plus capecitabine
versus lapatinib plus capecitabine for the treatment of
HER2-positive metastatic breast cancer (PHOEBE): A multicentre,
open-label, randomised, controlled, phase 3 trial. Lancet Oncol.
22:351–360. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hou J, Greten TF and Xia Q:
Immunosuppressive cell death in cancer. Nat Rev Immunol.
17(401)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Edwardson DW, Parissenti AM and Kovala AT:
Chemotherapy and inflammatory cytokine signalling in cancer cells
and the tumour microenvironment. Adv Exp Med Biol. 1152:173–215.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kartikasari AER, Huertas CS, Mitchell A
and Plebanski M: Tumor-induced inflammatory cytokines and the
emerging diagnostic devices for cancer detection and prognosis.
Front Oncol. 11(692142)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Habanjar O, Bingula R, Decombat C,
Diab-Assaf M, Caldefie-Chezet F and Delort L: Crosstalk of
inflammatory cytokines within the breast tumor microenvironment.
Int J Mol Sci. 24(4002)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Semesiuk NI, Zhylchuk A, Bezdenezhnykh N,
Lykhova A, Vorontsova AL, Zhylchuk VE and Kudryavets YI:
Disseminated tumor cells and enhanced level of some cytokines in
bone marrow and peripheral blood of breast cancer patients as
predictive factors of tumor progression. Exp Oncol. 35:295–302.
2013.PubMed/NCBI
|
|
11
|
Jia D, Li L, Andrew S, Allan D, Li X, Lee
J, Ji G, Yao Z, Gadde S, Figeys D and Wang L: An autocrine
inflammatory forward-feedback loop after chemotherapy withdrawal
facilitates the repopulation of drug-resistant breast cancer cells.
Cell Death Dis. 8(e2932)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Slamon DJ, Leyland-Jones B, Shak S, Fuchs
H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M,
et al: Use of chemotherapy plus a monoclonal antibody against HER2
for metastatic breast cancer that overexpresses HER2. N Engl J Med.
344:783–792. 2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vogel CL, Cobleigh MA, Tripathy D, Gutheil
JC, Harris LN, Fehrenbacher L, Slamon DJ, Murphy M, Novotny WF,
Burchmore M, et al: Efficacy and safety of trastuzumab as a single
agent in first-line treatment of HER2-overexpressing metastatic
breast cancer. J Clin Oncol. 20:719–726. 2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hubalek M, Brunner C, Matthä K and Marth
C: Resistance to HER2-targeted therapy: mechanisms of trastuzumab
resistance and possible strategies to overcome unresponsiveness to
treatment. Wien Med Wochenschr. 160:506–512. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ma F, Ouyang Q, Li W, Jiang Z, Tong Z, Liu
Y, Li H, Yu S, Feng J, Wang S, et al: Pyrotinib or lapatinib
combined with capecitabine in HER2-positive metastatic breast
cancer with prior taxanes, anthracyclines, and/or trastuzumab: A
randomized, phase II study. J Clin Oncol. 37:2610–2619.
2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhang Q, He P, Tian T, Yan X, Huang J,
Zhang Z, Zheng H, Zhong X and Luo T: Real-world efficacy and safety
of pyrotinib in patients with HER2-positive metastatic breast
cancer: A prospective real-world study. Front Pharmacol.
14(1100556)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lindström LS, Karlsson E, Wilking UM,
Johansson U, Hartman J, Lidbrink EK, Hatschek T, Skoog L and Bergh
J: Clinically used breast cancer markers such as estrogen receptor,
progesterone receptor, and human epidermal growth factor receptor 2
are unstable throughout tumor progression. J Clin Oncol.
30:2601–2608. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang X, Li Z, Han L, Lv Z, Teng Y, Cui X,
Zhou C, Wu H, Fang W, Xu L, et al: Efficacy and safety of pyrotinib
in human epidermal growth factor receptor 2-positive advanced
breast cancer: A multicenter, retrospective, real-world study. Onco
Targets Ther. 15:1067–1078. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu Z, Wang C, Chen X, Zhu J, Sun X, Xia
Q, Lu Z, Qiao J, Zhou Y, Wang H, et al: Pathological response and
predictive role of tumour-infiltrating lymphocytes in HER2-positive
early breast cancer treated with neoadjuvant pyrotinib plus
trastuzumab and chemotherapy (Panphila): A multicentre phase 2
trial. Eur J Cancer. 165:157–168. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jurisic V: Multiomic analysis of cytokines
in immuno-oncology. Expert Rev Proteomics. 17:663–674.
2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Quinn KM, Kartikasari AER, Cooke RE,
Koldej RM, Ritchie DS and Plebanski M: Impact of age-, cancer-, and
treatment-driven inflammation on T cell function and immunotherapy.
J Leukoc Biol. 108:953–965. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang Y, Li S, Wang Y, Zhao Y and Li Q:
Protein tyrosine kinase inhibitor resistance in malignant tumors:
Molecular mechanisms and future perspective. Signal Transduct
Target Ther. 7(329)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Autenshlyus A, Davletova K, Varaksin N,
Marinkin I and Lyakhovich V: Cytokines in various molecular
subtypes of breast cancer. Int J Immunopathol Pharmacol.
35(20587384211034089)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tian D, Tian M, Ma ZM, Zhang LL, Cui YF
and Li JL: Anesthetic propofol epigenetically regulates breast
cancer trastuzumab resistance through IL-6/miR-149-5p axis. Sci
Rep. 10(8858)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Vazquez-Martin A, Colomer R and Menendez
JA: Her-2/neu-induced ‘cytokine signature’ in breast cancer. Adv
Exp Med Biol. 617:311–319. 2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jia Y, Kodumudi KN, Ramamoorthi G, Basu A,
Snyder C, Wiener D, Pilon-Thomas S, Grover P, Zhang H, Greene MI,
et al: Th1 cytokine interferon gamma improves response in HER2
breast cancer by modulating the ubiquitin proteasomal pathway. Mol
Ther. 29:1541–1556. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hannesdóttir L, Tymoszuk P, Parajuli N,
Wasmer MH, Philipp S, Daschil N, Datta S, Koller JB, Tripp CH,
Stoitzner P, et al: Lapatinib and doxorubicin enhance the
Stat1-dependent antitumor immune response. Eur J Immunol.
43:2718–2729. 2013.PubMed/NCBI View Article : Google Scholar
|