1. Introduction
Photoaging due to exposure to ultraviolet (UV)
radiation results in the appearance of rough skin, wrinkles,
xerosis, hyperpigmentation and epidermal thickening owing to the
progressive loss of function and regenerative potential of the skin
tissue (1). Collagen is the main
supporting skin structure; its excessive degradation is directly
related to wrinkle formation (2).
Matrix metalloproteinase (MMP) is a matrix-degrading enzyme that
degrades and modifies some components of the extracellular matrix
(ECM). UV radiation can increase MMP-1 production, which in turn
increases collagen degradation in human skin (3).
An increase in the ROS content caused by UV exposure
can cause oxidative stress accumulation, which activates MMP and
increases collagen damage (4).
Antioxidants are chemical substances that neutralize or interfere
with the action of free radicals (5); these agents are often used to prevent
or slow down the oxidation of macromolecules that cause oxidative
stress. One natural ingredient that has antioxidant properties by
reducing ROS production is black cumin or Nigella sativa
(N. sativa) seeds (6).
N. sativa is a small shrub, 20-90 cm in
height, which belongs to the Ranunculaceae family; it is
known for its antioxidant, antiviral, antifungal, antiparasitic and
anti-inflammatory effects (7). The
effects of these agents against free radicals are related to their
activities of free radical scavenging and inhibiting
5-lipooxygenase during the inflammatory phase (8). This ability is attributed to several
important components, such as thymoquinone (TQ), flavonoids,
essential fatty acids and various mineral components, which have
been widely studied (9). N.
sativa has a therapeutic effect by reducing cytotoxicity,
oxidative stress, inflammatory response and cell death caused by
mitochondrial dysfunction in keratinocyte cells irradiated with
ultraviolet A (UVA) light (10).
Another study found that N. sativa extract inhibits the
formation of advanced glycation end products, collagen
cross-linking, collagenase activity and elastase activity (2). An animal study with rats exposed to
UVB light showed a significant increase in collagen levels after
being given topical N. sativa extract (11).
2. Photoaging
Photoaging is a group of clinical and pathological
manifestations caused by skin exposure to UV radiation (12). UVA and UVB both play a role in
photoaging, although UVA rays have longer wavelengths because UVA-1
rays (340-400 nm) can be absorbed deeply into the human skin and
exert effects directly through the dermal fibroblast level
(13); however, UVC is fully
reflected by the ozone layer (14).
Photoaging involves all skin layers, with primary effects on the
dermis and paracrine effects on other skin layers. This condition
comprises acute and chronic cellular processes characterized by
rough wrinkling, decreased skin elasticity, pigmentation, xerosis,
keratosis and telangiectasis (15).
The clinical manifestations of normal aging and
photoaging often appear simultaneously; however, there are several
differences (12). Normal aging is
characterized by sagging skin and an excessive expression of frown
lines. Aging skin has decreased hydration and elasticity,
permeability and susceptibility to irritation owing to skin barrier
damage (16). The manifestations of
photoaging include deep wrinkles, rough skin texture,
dyspigmentation and skin elasticity disturbance (17). The distribution of photoaging is
mostly limited to the face, neck and hands and rarely to the arms
and lower legs. Of photoaging cases ~80% are caused by chronic
low-level UV exposure, although exposure to high levels of acute UV
light can also cause skin burning, darkening and skin structure
disorders (18). Microscopic
examination showed an increase in the epidermal thickness,
dystrophic elastin accumulation and irregular collagen in the
dermal layer. Skin susceptibility to UV damage depends on the
melanin content and Fitzpatrick skin types I, II and III are more
susceptible to photoaging than skin types IV, V and VI (19).
There are three main causes of photoaging: i)
Dysfunction of keratinocytes in the basal layer, which inhibits
their ability to regenerate and repair the skin; ii) loss of the
ability of fibroblasts to synthesize and produce collagen; iii)
changes in intracellular homeostasis through certain paracrine
mechanisms (20). These changes are
related to UV exposure on the skin, which causes oxidative stress
(21).
Photoaging and UV exposure
Large amounts of UVB can reach the skin due to
direct sunlight exposure during the day (22). UVA is rarely absorbed when passing
through the atmosphere (23). High
UVA radiation throughout the day causes greater UVA exposure on the
skin than UVB exposure under normal conditions (24). UVB exposure can cause erythema,
burning, skin damage and skin cancer, whereas intense UVA exposure
can cause erythema and blood vessel damage (25). To produce this effect, UVA requires
an amount 1,000 times greater than UVB. However, UVA can penetrate
deeper than UVB, causing damage to collagen and elastic fibers in
the dermal layer (24,26).
UV radiation as photons needs to be absorbed by
chromophores, followed by various photochemical events to produce
photoaging skin manifestations (19); this process involves fibroblasts,
keratinocytes and neutrophils (27). Therefore, the skin is a notable part
of the immune system and consists of numerous molecular mechanisms
that protect the skin from UV radiation (28). The epidermal layer provides the
first line of defense against hazardous external agents consisting
of keratinocytes, antigen-presenting cells, Langerhans cells, T
lymphocytes and melanocytes (29).
Keratinocytes have an immune function against UV by
producing cytokines, hormones, chemokines, neuropeptides and
antimicrobial peptides (30).
Melanin is produced by melanocytes; it works by inhibiting and
absorbing UV rays that enter the epidermis layer. As UV exposure
increases, the melanin formation rate increases, causing the skin
to appear darker and providing additional protection against
further radiation injury (22).
Furthermore, DNA damage caused by UV rays can be repaired by
nucleotide repair and base excision mechanisms, apoptosis and cell
cycle checkpoint system activation (31).
Photoaging and oxidative stress
Humans are constantly exposed to oxidants daily;
oxidative stress occurs because of a disturbance in the
oxidant-antioxidant balance (32,33).
Excessive UV radiation can induce ROS production while
downregulating peroxidase and glutathione reductase production,
which depends on the amount of UV radiation absorbed (34). UV rays can not only directly damage
the skin by destroying lipids, proteins and DNA cells but also
indirectly through the increased oxidation of various substances,
especially lipids and DNA (24).
The effects of UVB radiation in human keratinocyte
cells are characterized by enhanced ROS production, release of
nicotinamide adenine dinucleotide phosphate (NADPH) oxidase,
cyclooxygenase (COX) and nuclear factor-κB (NF-κB) (24). The aging process of fibroblasts
during photoaging owing to UVB exposure is associated with ROS
accumulation, which escalates proteasomes inhabitation and
autophagy initiation (35).
Autophagy suppression is required to switch the cell pathway from
senescence to the apoptotic process (36). Inactivation of the proteasome system
in fibroblast cells is related to the generation of singlet oxygen,
protein oxidation and transcriptional agents that regulate MMP-1
expression activation (31).
Molecular impact of ROS on
photoaging
Human bodies produce ROS consisting of superoxide
anion (O2-), hydrogen peroxide
(H2O2), hydroxyl radicals (OH), singlet
oxygen (1O2), lipid peroxide and nitrogen
oxide (37). O2
molecules gain a single unpaired electron to establish
O2- and thus become the early stage of ROS in
cells (38).
O2- molecules will form
H2O2 through antioxidant enzyme catalysis
(39). O2-
can release iron ions under stress conditions from the iron-sulfur
of proteins, participating in the Fenton reaction and converting
H2O2 to OH (40). Hydroxyl radicals are easily reacted
with other molecules with a half-life of 10-9 sec
(41).
Normal skin aging and photoaging both involve
similar signaling pathways, although they are induced differently
(42,43). In photoaging, ROS can increase MMP
synthesis by stimulating mitogen-activated protein kinase (MAPK)
and protein-1 (AP-1) heterodimers (44). MMP-1 is capable of breaking down the
integrated fibrillar collagen, whereas MMP-2, MMP-3 and MMP-9 break
down the fragments and degraded forms (44-46).
ROS and activated MAPK signaling pathways stimulate NF-κB and
induce the expression of several proinflammatory cytokines
(46,47). NF-κB organizes the expression of
hemeoxygenase-1 and increases free iron levels in cells, thereby
encouraging ROS execution through the Fenton reaction (48,49).
NF-κB also excites MMP-8 to accelerate collagen breakdown; at the
same time, AP-1 downregulates the expression of transforming growth
factor-β (TGF-β) receptor type II, which disrupts the downstream
Smad/TGF-β signal pathway, thereby reducing the transcription of
the COL1A1 and COL3A1 genes encoding type I and type III collagen
(41,50-52).
Photoaging is caused not only by increased degradation of collagen
by various MMPs, serine and other proteases but also by decreased
collagen production by fibroblasts (1).
Photoaging and MMP activities
The main function of MMPs is to degrade the ECM,
glycoproteins, cytokines, receptors and growth factors (52). Impaired regulation of MMP activity
causes various pathologies and is divided into the following: i)
Tissue damage; ii) fibrosis; and iii) matrix weakness (53). UV exposure can increase MMPs levels,
which are responsible for ECM damage, including collagen, elastin,
fibronectin and proteoglycans (54). This exaggerated degradation can
cause clinical signs of photoaging, resulting in deep wrinkles and
sagging skin through photodestruction, phototransformation and
photo-oxidation of collagen and elastin fiber processes (31).
DNA damage following UVB exposure induces MMP-1
production by increasing MMP-1 gene expression in keratinocytes
(31,54,55).
Protein expression changes have a substantial role in photoaging,
including the transformation of TGF-β, MMP-1, MMP-3 and
MMP-9(56). ROS stimulates the MAPK
signal pathway, which is responsible for AP-1 formation (57). AP-1 plays a crucial role in the
transcription regulation of MMP-1, MMP-3 and MMP-9, which
progressively damage collagen (58). In addition, AP-1 can inhibit TGF-β,
which regulates type I procollagen production and subsequently
results in decreased collagen synthesis (59). Another notable transcription factor
involved in the UV response is NF-κB (60). It can regulate the production of
MMPs, such as MMP-1 and MMP-3, by fibroblasts (31).
Photoaging and collagen levels
Collagen is a structural protein responsible for
maintaining skin elasticity (61).
Collagen produced by fibroblasts varies according to the amino acid
primer sequence of the polypeptide chain (62). Type I collagen is found in 90% of
the human body (63); it is notably
discovered in bones, tendons, ligaments, corneas and various
interstitial connective tissues (64).
Type I collagen production is regulated by two
genes, COL1A1 and COL1A2, which structurally consist of α-1 chain
and α-2 chain (62). The COL1A1
gene is located in chromosome 17q21.33(65); its synthesis and maturation are
arranged at various levels: Epigenetic and transcriptional,
posttranscriptional and posttranslational (66). In photoaged skin, COL1A1 mRNA
expression levels are reduced, whereas MMP-1 mRNA expression levels
are increased in association with collagen metabolism (67).
Collagen fibers are thicker and coarser in the inner
dermal layer than in the outer layer, with thinner and more tenuous
collagen fibers (68). Collagen
fibers in younger skin are intact, dehydrated, elastic and
damage-resistant (69). Collagen
levels in aging skin begin to decrease, fade, stretch and lose
their elasticity (70). The
destruction of collagen and damage of other structural components,
including elastic and reticular fibers, underlie the characteristic
changes in aging skin and photoaging (64). Significant reductions in the amount
and collagen structure can weaken the flexibility of the dermal
layer, causing wrinkles and xerosis (71).
UV radiation exposure indirectly initiates MMP
activation without upregulating collagen production, causing
decreased collagen levels and ECM fragmentation (72). MMP activation is influenced by
photochemical reactions that change the UV energy absorbed by
cellular macromolecules into ROS (73). Over time, ROS levels exceed
antioxidant defense, which promotes increased collagen-degrading
MMP production. ROS inhibits the activation of protein tyrosine
phosphatase, causing receptor tyrosine kinase phosphorylation and
activating the MAPK signaling pathway that forms AP-1(74). AP-1 is important for upregulating
the transcription of MMP-1, MMP-3 and MMP-9, where excessive MMP
expression can cause increased dermal ECM fragmentation (56,58).
Apart from collagen degradation owing to excessive
MMP expression, ROS also triggers a decrease in collagen production
by inhibiting the TGF-β signal pathway (75). ROS-induced AP-1 prevents the
TGF-β/Smad signal pathway in fibroblasts that control collagen
synthesis to prevent a decrease in collagen production (76). TGF-β interacts with cell surface
receptors and triggers the Smad2/3 transcription factor. The
phosphorylated Smad2/3 then associates with, and moves to, Smad4,
thereby triggering the transcription of TGF-responsive genes
(71); this leads to decreased
phosphorylation and production of the Smad 2/3 transcription factor
required for type I procollagen transcription (75).
3. N. sativa seed (black cumin)
N. sativa is a member of the
Ranunculaceae family and is known as Black Seed or Black
Cumin (77). N. sativa seeds
originate from Africa and Southwest Asia (78). High-quality seeds come from Egypt,
where a suitable environment exists for this plant (77). N. sativa is an annual herbal
plant, 20-30 cm tall, with linear leaves, 5-10 pale bluish-white
flower petals and tiny black seeds similar to cumin; therefore, it
is called black cumin (79). This
plant has green branching stems and green leaves that turn red as
they grow (80); it starts
flowering between April and August and its fruit consists of 3-6
carpels, each of which contains seeds (81). The seeds are oval and 2-3.5 mm in
size, composed of three to four fine-grained corners; the color
turns black after being cooked and exposed to air (77,79).
The nutritional value of N. sativa seeds
includes protein, fat, fiber and total carbohydrates (82). N. sativa seeds also contain
significant amounts of iron, copper and zinc (83). N. sativa has
phytoconstituents such as alkaloids, sterols, saponins and
essential oils (84). Their seeds
comprise primary fatty acids, linoleic acid, palmitic acid and
phenolics and quinones (TQ, thymol, dithymoquinone and
thymohydroquinone) (85,86). TQ
(C10H12O2) has a basic quinone
structure with methyl and isopropyl side chain groups added at 2
and 5 positions (87). TQ has COX
and 5-lipooxygenase activities, which can inhibit eicosanoid
formation during inflammatory phases and lipid membrane
peroxidation (88).
Quercetin and kaempferol are the main flavonoid
glycosides in N. sativa (86). N. sativa also contains
phenolic and triterpene compounds such as saponins (89). Saponin is a group of glycosides
comprising one or more hydrophilic groups combined with lipophilic
triterpenes or steroid derivatives. Triterpene saponins are typical
compounds found in N. sativa seeds (85).
N. sativa extract has been accepted and
recognized as safe by the Food and Drug Association in the United
States (90). N. sativa is
available in dietary supplements, oils, topical creams and powders
(91). The acceptable and effective
dose is equivalent to 1-3 g of N. sativa extract powder once
daily, 40 mg/Kgbw of N. sativa extract once daily, or 1 ml
of N. sativa extract cream three times daily, based on
clinical trial data on humans (92). However, there is considerable
variability in doses and extract preparation, including oil-based
formulations, methanol extracts and advanced nanoformulations,
which might affect bioavailability and clinical efficacy.
The in vivo TQ pharmacokinetic dose study
showed a maximum concentration (Tmax) of 3.96±0.19 h reaching
4811.33±55.52 ng/ml as the maximum blood concentration (Cmax),
while the elimination half-life (T1/2) was 4.4933±0.015 h. This
indicates the suitability of TQ for extravascular administration
through nanoparticle formulation, which has been shown to increase
bioavailability (93). The seed
extract and its constituents appear to have low levels of toxicity
(94). The lethal dose 50 (LD50) of
TQ is 2.4 g/kg bw in Swiss albino mice (95). Giving 6.4 g/kg bw of N.
sativa extract orally every day for 6 weeks to mice caused the
death of one mouse following 2 weeks of therapy, while the second
and third mice died in the third and fifth weeks after being given
21 and 60 g/kg bw of N. sativa extract, respectively
(83). Toxicity in mice after using
fixed oil content from N. sativa extract administered
intraperitoneally and intraorally showed that the LD50 was found to
be 89.7-119. 7 mg/Kgbw and 647.1-1,094.8 mg/Kgbw, respectively
(96). Additional studies are
needed to confirm the refined dosage recommendations and evaluate
N. sativa's long-term safety in other animals and also in
clinical studies.
In recent decades, a number of extraction methods,
such as cold pressing, supercritical fluid extraction, Soxhlet
extraction, hydro distillation method, microwave-assisted
extraction, ultrasonic-assisted extraction, steam distillation and
accelerated solvent extraction, have been used to extract oil from
black seeds under optimal conditions (97). The cold pressing method using hexane
as a solvent does not involve heating or chemical treatment during
oil extraction but gives low yields, whereas the Soxhlet extraction
method using methanol as a solvent has low costs; however, solvent
residues remain in the extracted oil. Thymoquinone in black cumin
oil is encapsulated to enhance its oxidative stability.
Microencapsulation was achieved through emulsification, spray
drying and nanoprecipitation. These processes are more time
consuming and involve hot gas flow; therefore, they are not
suitable for heat-sensitive bioactives. For sensitive compounds,
alternative techniques are required to overcome the drawbacks of
conventional encapsulation methods. A recently developed
encapsulation technology is electrospray; this process is
cost-effective and simple for producing a wide range of
nanoparticles. No heat is required during drying and the formation
of smaller encapsulations of 1-5 µm is increasingly important for
the encapsulation of temperature-sensitive bioactives (97). Continued investigation is required
to assess the formulation-dependent differences in bioavailability
to ensure consistent therapeutic outcomes, optimal absorption and
effective systemic distribution.
No severe side effects have been reported after
using the N. sativa extract, either orally or topically.
Three women were reported to have acute allergic contact dermatitis
after using the topical pure oil of N. sativa extract
(90). Other studies have reported
the side effects of gastrointestinal disorders, gastric irritation
and stomach cramps (98).
Gastrointestinal disturbances occurred in 9 of 49 children who
orally consumed N. sativa extract at a dose of 80 mg/kg
twice daily on an empty stomach (99). Further clinical research under
controlled conditions is necessary to comprehensively examine the
safety profile of N. sativa in dermatological
applications.
4. N. sativa seed (black cumin)
mechanism against photoaging
N. sativa mechanism is mainly because of its
active ingredients, especially TQ (91,100).
TQ has a quinone molecular structure for redox activity and the
infinite capability to cover all physiological barriers so that it
has easy access to subcellular compartments, which affects radical
scavenging (101). TQ is known to
have anti-inflammatory and antioxidant effects that can protect
cells from free radicals. In addition, TQ stimulates the
proliferation and migration of cells involved in tissue repair,
encouraging the formation of new tissue (102). TQ reduces NF-κB transcription
factor activity so that MMP-1 expression can be suppressed
(103). Decreased expression of
MMP-1 can further reduce collagen degradation. TQ also acts
directly on type I collagen synthesis by preserving the
physiological state of the interstitial system (104). Currently, there is no recommended
dose of TQ for its role in preventing photoaging. Research by
Ghorbanibirgani et al (105) using a topical extract of N.
sativa twice a day for 6 months in vitiligo cases found
significant improvement in patient scores. Unfortunately, this
study did not clearly state the manufacture of the topical cream
and its concentration. Research by Yousefi et al (106) using a topical extract of N.
sativa at a dose of 2% twice a day for 4 weeks in hand eczema
cases showed equally effective results as the use of topical
betamethasone. The two studies mentioned the anti-inflammatory and
antioxidant effects of N. sativa and TQ as their working
mechanisms.
Flavonoids, especially quercetin and kaempferol,
play a role as strong antioxidants by interrupting free radical
chain reactions and protecting against UV radiation (107). Several studies have reported that
quercetin has a photoaging defense mechanism by inhibiting MMP-1 in
a dose-dependent manner (108-111).
Quercetin shows strong antioxidant activity through in vitro
chemical tests, showing an increase in quercetin activity following
efficient formulation at the cellular level, as quercetin shows
cell-protective action on keratinocytes and in vivo on
animal skin. This antioxidant effect is also supported by the
ability of quercetin to exert anti-inflammatory actions, such as
the inhibition of NF-κB and IL-6 induction by UV irradiation. The
combination of antioxidant and anti-inflammatory actions and their
cross-linking mechanism highlight quercetin as a novel sunscreen.
Furthermore, as quercetin has antioxidant and anti-inflammatory
activities, it could be beneficial for wound healing; here,
fibroblasts are the main target in contrast to keratinocytes in
sunscreens. Furthermore, quercetin has a promising rejuvenating
effect on keratinocytes with a supportive whitening effect
(112). Kaempferol protects
against UVB-induced photoaging by preventing p38 MAPK and c-Jun
N-terminal kinase activation (113). These agents prevent the action and
amount of MMP-1 and increase collagen levels in the dermal layer.
Flavonoids can also stimulate macrophage activity, trigger the
epithelialization process and escalate ECM production, growth
factors, cytokines and angiogenesis by releasing keratinocyte
growth factor (10).
Saponin is a type of glycoside from pentacyclic
triterpenoids that has pharmaceutical, nutritional and cosmetic
potentials with anti-inflammatory and antioxidant properties. Thus,
it has the potential to protect against the detrimental effects of
photoaging (114). Saponin
modulates the inflammatory response, protects against ECM
degradation, enhances collagen production and improves wrinkle
appearance by preventing UVB-induced MAPK signaling, AP-1 and NF-κB
activation (111,115).
Linoleic acid comprises >50% of the total fatty
acids and can preserve water permeability and skin integrity
(116,117). Linoleic acid, which is known for
its percutaneous absorption, can increase drug absorption, whereas
the oil emulsion of the substance can reduce skin irritation and
improve the skin's moisturizing function (118). Topical application rich in
linoleic and oleic acids can suppress transepidermal water loss
changes, increase skin hydration, reduce skin erythema and protect
against histological changes caused by UVR exposure (119).
N. sativa seeds contain various elements,
such as Cu, Zn and Fe (100). Cu
is a common cofactor for a number of enzymes and in the skin acts
by provoking fibroblast proliferation, increasing collagen (types
I, II and V) synthesis and acting as a cofactor for superoxide
dismutase (SOD) to interfere with cellular oxidative effects
(100,120). Zn also inhibits the accumulation
of oxidative stress markers and proinflammatory cytokines. Zn ties
up to the sulfhydryl groups of oxidation-protecting biomolecules
and increases the activation of glutathione (GSH), catalase and SOD
and decreases the activity of enzymes that increase oxidant levels,
such as inducible nitric acid synthase (iNOS) and NADPH oxidase and
slows the formation of peroxidation products, lipids (121). Iron plays a role in oxygen
transport and oxidation-reduction reactions as well as in various
oxygenases, including procollagen-proline dioxygenase, which is
associated with the skin (122).
Table I lists the active
ingredients of N. sativa seeds and their mechanism against
photoaging.
 | Table INigella sativa contents and
their possible mechanism against photoaging. |
Table I
Nigella sativa contents and
their possible mechanism against photoaging.
| Ingredients | Role against
photoaging | Mechanism | (Refs.) |
|---|
| Thymoquinone | Prevents oxidative
injury and prevent membrane lipid peroxidation | • Inhibits the
activation of NF-κB | (97-99) |
| | | • Works directly on
the fibrillogenesis of type I collagen | |
| Flavonoids
(quercetin and kaempferol) | Interrupts free
radical chain reactions and protect against UV radiation | • Inhibits MMP-1
mRNA levels | (101-105) |
| | | • Inhibits ERK and
p38 MAPK activation | |
| | | • Reduction in
NF-κB | |
| Saponins | Modulates
inflammatory response, protects against ECM degradation, enhances
collagen production and improves wrinkle appearance | • Inhibits MAPK
signaling, AP-1, and NF-κB activation | (35,104) |
| Linoleic and oleic
acids | Maintains water
permeability barrier and epidermal integrity of the skin | • Suppresses
UV-induced changes in TEWL, skin hydration, and erythema | (105,108,111) |
| Minerals (Cu, Zn
and Fe) | Decreases oxidative
stress biomarkers and inflammatory cytokines | • Cofactor for many
enzymes against oxidants | (96,112,114) |
Sunlight contains UVA and UVB, which reach the skin
surface, triggering oxidative reactions that result in ROS
(13). Excessive ROS accumulation
in the skin activates the MAPK signaling pathway and the NF-κB
pathway (123). The activated MAPK
pathway triggers AP-1 production. ROS-induced AP-1 inhibits the
TGF-β/Smad signaling pathway in dermal fibroblasts, which controls
collagen synthesis to prevent a decrease in collagen production.
TGF-β interacts with cell surface receptors and triggers the
transcription factor Smad2/3. The phosphorylated Smad2/3 then
connects and moves to Smad4, thereby triggering the transcription
of genes responsive to TGF-ß. This causes a decrease in the
phosphorylation and activation of the transcription factor Smad
2/3, which is required for the transcription of type I procollagen,
a precursor of type I collagen, namely the COL3A1 and COL1A1 genes
(1,55,57).
ROS and activated MAPK signaling pathways can also
activate NF-κB; this activation can increase the expression of
proinflammatory cytokines such as IL-1β, IL-6, TNF-α and COX-2 to
regulate the inflammatory response and unbalance the MMPs/tissue
inhibitors of metalloproteinases (TIMPs) ratio by activating MMPs
and reducing TIMPs expression, thereby decompressing the ECM
(6,31,41).
NF-κB also increases the formation of the MMP1 enzyme to accelerate
collagen degradation, especially type 1 collagen (31).
N. sativa contains an active ingredient, TQ,
which is a potent antioxidant (6,9,10). The
active substance TQ has a therapeutic effect by reducing oxidative
stress and inflammatory responses, thereby preventing the
activation of the MAPK and NF-κB pathways. Kaymak et al
(124) reported that
intraperitoneal administration of TQ 10 mg/kg bw in rats for 10
days showed a significant decrease in various inflammatory mediator
levels, such as P38 MAPK and NF-kb, in the testes of rats induced
by methotrexate. Activating NF-κB is directly associated with an
increase in MMP-1(125). Chen
et al (103) investigated
the effect of TQ on MMP expression in an animal model of
osteoarthritis. Accordingly, the downregulation of MMP-1, MMP-3 and
MMP-13 expressions and the upregulation of TIMP-1 expression were
reported due to the use of TQ in chondrocytes and cartilage in the
animal model. The authors also showed that TQ could inhibit the
NF-κB p65 protein level. MMP-1 can degrade fibrillar collagen in
its three-helical domain, which causes the molecules to be
thermally unstable so that they break down to form gelatin, which
can then be degraded by other members of the MMP family (126).
Several photoaging therapies are often used with
different working mechanisms. Topical retinoids during photoaging
are mediated by their interaction with specific cellular and
nucleic acid receptors. They improve photoaging by modifying
cellular differentiation programs by increasing epidermal
proliferation and thickening, compacting the stratum corneum and
improving the deposition of glycosaminoglycans in the stratum
corneum and intercellular spaces of the epidermis (127). Vitamin C is a naturally occurring
antioxidant that has been shown to be effective for preventing and
treating sun-damaged skin; it not only has an antioxidant,
anti-inflammatory and photoprotective effect against UVA and UVB
but also enhances collagen synthesis (128). The major adverse effects of
topical retinoids and topical vitamin C are local skin irritation,
including erythema, peeling, dryness, burning and itching, which
depend on the concentration and formulation of the product
(129,130). In addition, the use of retinoid
agents is not recommended in pregnant women and topical vitamin C
preparations are occasionally unstable, limiting their clinical
use.
The summarized mechanism of N. sativa seed
extract therapy in combating photoaging is shown in Fig. 1. N. sativa plays a protective
role in inhibiting photoaging manifestations by modulating
oxidative stress and inflammatory pathways. UV exposure induces the
production of ROS, which subsequently activate the NF-κB and MAPK
signaling pathways, leading to increased transcription of
pro-inflammatory cytokines (IL-1β, TNF-α, IL-6 and COX-2) and
MMP-1, both of which contribute to inflammation and collagen
degradation. However, N. sativa inhibits ROS generation,
thereby downregulating NF-κB and AP-1 activity. These result in
reduced inflammatory cytokine expression and decreased MMP-1
levels, preserving collagen density. Additionally, N. sativa
enhances TIMPs and TGF-β receptor Smad signaling, which promotes
the expression of collagen-related genes (COL1A1 and COL3A1).
Collectively, these actions reduce inflammation, prevent collagen
breakdown and promote skin integrity, ultimately inhibiting the
visible manifestations of photoaging.
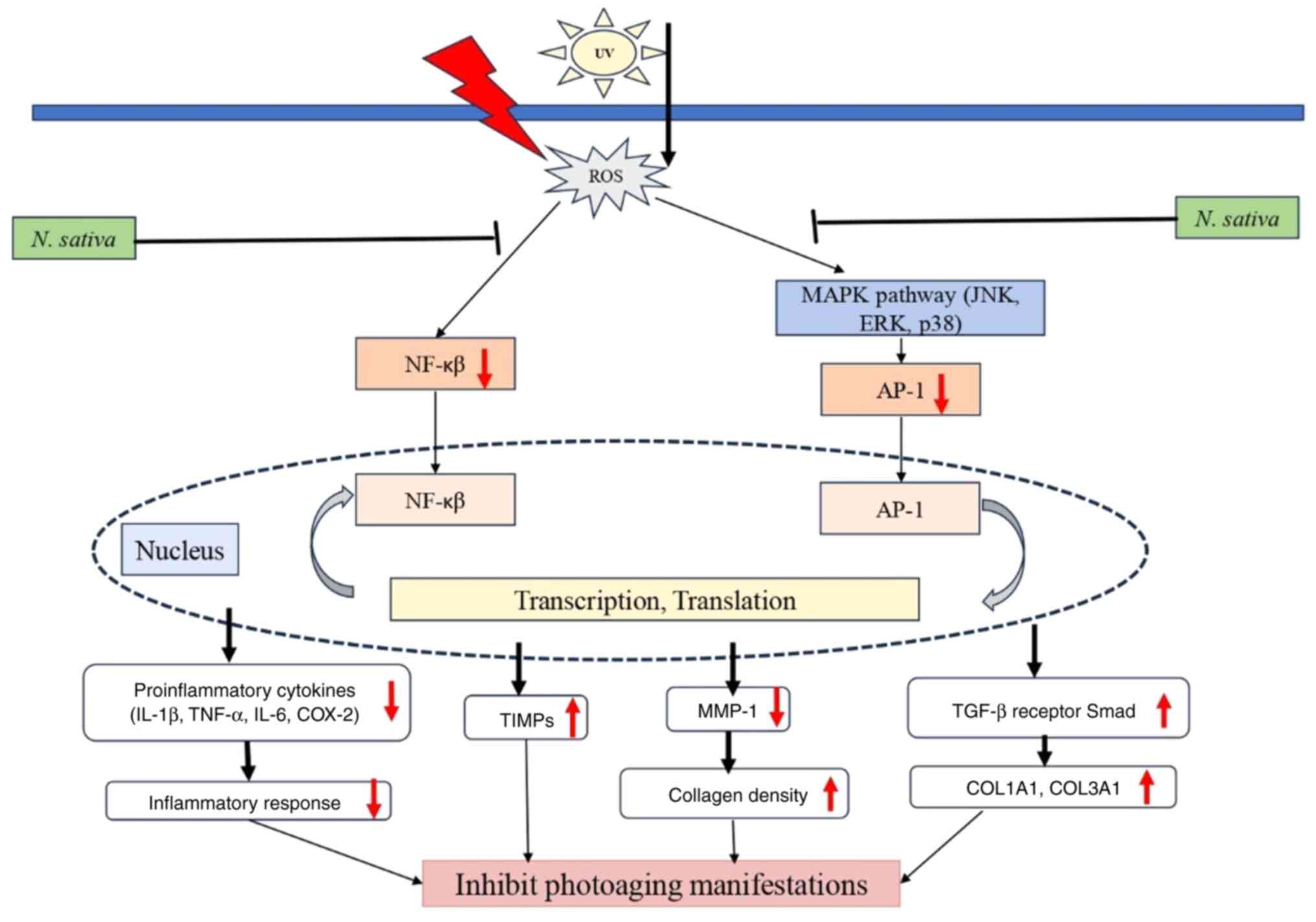 | Figure 1Possible mechanism of N.
sativa and thymoquinone against photoaging. UV, ultraviolet;
ROS, reactive oxygen species; NF-κB, nuclear factor-kappa B; MAPK,
mitogen activated protein kinase; JNK, c-Jun N-terminal kinase;
ERK, extracellular signal-regulated kinase; AP-1, activator
protein-1; TIMP, tissue inhibitor of metallopreoteinase; MMP-1,
matrix metalloproteinase-1; IL-1ß, interleukin 1ß; TNF-α, tumor
necrosis factor-α; IL-6, interleukin-6; COX-2, cyclooxygenase-2;
TGF-ß, tumor growth factor-ß. |
While multiple studies highlight the
anti-photoaging effects of N. sativa, inconsistencies exist
in its reported efficacy. Some studies emphasize strong antioxidant
and anti-inflammatory benefits, while others suggest limited or
unclear results (2,10,131-134).
The concentration of TQ in different extracts varies widely, which
may contribute to the heterogeneity in study outcomes. Furthermore,
variations in study design, including animal models compared with
human clinical trials, need further standardization to ensure
comparability.
Despite promising preliminary findings, several
research gaps remain. The optimal dosage of TQ for anti-photoaging
effects has not been established and most studies lack standardized
extraction methods. Future research should focus on
well-controlled, long-term clinical trials to assess both efficacy
and safety. Additionally, the interaction of N. sativa with
other dermatological treatments (such as retinoids, vitamin C, or
chemical peels) warrants further investigation to determine
possible synergistic or antagonistic effects. A number of studies
on N. sativa and photoaging have been conducted with small
sample sizes, often in preclinical settings, making it difficult to
generalize findings. Additionally, industry-funded research may
introduce bias in reporting only positive results and there is a
lack of large-scale, double-blind, placebo-controlled clinical
trials. This raises concerns about publication bias, which may
interfere the perceived efficacy of N. sativa in photoaging
treatment.
5. N. sativa seed (black cumin)
potential role against photoaging
The use of N. sativa, both orally and
topically, has been widely studied and its use has been shown to
have a potential role against photoaging. The potential antioxidant
and anti-inflammatory effects are possibly caused by the active
composition of N. sativa, TQ (6). There are two in vitro studies
regarding antiaging and photoaging effects of TQ. Li et al
(2) analyzed Thymocid®,
a standardized TQ extract, which can inhibit the formation of
advanced glycation end products, collagen cross-linking,
collagenase and elastase activities in vitro.
Thymocid® has potential as an antiaging property by
maintaining its protein structure against glycation (135). In addition, Thymocid®
can also protect the type I collagen structure from
glycation-induced cross-linking; there is a possibility that its
antiglycation effect may be because of the antioxidant effect of
Thymocid® (136).
Unfortunately, this study states that other phytochemical compounds
besides TQ in Thymocid® are present, such as alipathic
compounds, so there could be a bias that the effect is caused
purely by the TQ compound, not the other compounds. In addition,
further research on the effects of Thymocid® on elastase
in human dermal fibroblast cells is needed to confirm its
antiwrinkle effects.
Liang et al (10) then conducted research on TQ for its
photoprotective action on human skin keratinocytes irradiated with
UVA, finding that the use of pure TQ extract at doses of 6 and 12
µM could improve inflammation, oxidative stress, cytotoxicity and
mitochondrial dysregulation caused by UVA radiation. From this
research, it was concluded that TQ mechanism for skin damage caused
by UVA radiation inhibits COX-2 expression by activating the
NrF2/ARE pathway (10). The
NrF2/ARE pathway is an important signaling pathway that reduces
skin damage caused by UVB radiation (137). In addition, COX-2 is the main
trigger of oxidative and inflammatory responses that are often
detected in photoaging and other stress/inflammatory disorders
(138). Nevertheless, this study
suggested that high doses of TQ (>16 µM) may exhibit
cytotoxicity to keratinocytes and the most effective protective
concentration of TQ should be explored in further studies.
Pandey et al (131) investigated the methanol extract of
N. sativa given orally at a dose of 200 mg/100 g bw/day in
mice to determine its protective effect against radiation exposure.
Oxidative stress caused by free radicals following ionizing
radiation can damage normal cells; therefore, protecting normal
tissue against radiation injury is important (139). This study showed that N.
sativa can prevent radiation-induced increases in lipid
peroxide levels in intestinal tissue homogenates. In addition,
histological examination showed the prevention of villous loss,
shortening of villous height and collagen deposition owing to
radiation exposure (131). In this
study, it was found that the use of N. sativa extract
markedly increased the antioxidant properties of the irradiated
rats compared with the control group. This study also stated that
repeated oral administration of N. sativa extract for 14
days resulted in a survival rate of 100% up to 200 mg/100 g bw, so
that this dose was considered the optimum dose. The antiaging
effect of the oral administration of N. sativa extract was
also supported by Khalaf and Mostafa (140), who administered N. sativa
at a dose of 10 ml/kg bw/day to adult male rats exposed to
cigarette smoke. Photoaging and tobacco smoke-induced aging are
similar and include the adverse effects of oxidative stress
(141). This study also found that
N. sativa oil can prevent smoking-induced skin changes
because of its antioxidant effects (140). In both studies, the antioxidant
effect of N. sativa on organs other than the skin was due to
free radicals originating from ionizing radiation and smoking.
Furthermore, oral administration is not uniform, so further
research is needed to assess the optimal dose of N. sativa
extract.
Several studies have investigated the topical use
of N. sativa extract and TQ as antiaging agents. Çanakci
et al (132) used
cold-pressed N. sativa oil extract at 100% concentration
topically on the nasal mucosa of mice following exposure to
radiotherapy radiation and found that its use could lower
inflammatory cell infiltration, superficial erosion, vascular
dilatation and exudates compared with the control. This study
demonstrated the antioxidant and anti-inflammatory effects of N.
sativa extract, even with topical use. In fact, in this study,
histological images showed the protective role of N. sativa
through its antioxidant, cytoprotective, anti-inflammatory and
antineoplastic activities. Although this study did not review
further the immunohistochemical and biochemical features, it is
sufficient to confirm the role of N. sativa in preventing
the detrimental effects of radiation.
Increased collagen density and accelerated wound
repair in mice following topical application of N. sativa
extract was studied by Turhan et al (133) who compared the use of N.
sativa extract, nano-silver solution gold as the standard agent
and a combination of the two agents applied twice daily for 15 days
on 20 albino rats. The use of N. sativa extract alone
resulted in improved collagen density and wound repair than that of
the control group, although combined use had the best effect
(133). In conclusion, N.
sativa can play a synergistic role with other topical agents
for wound healing. This research also suggests that N.
sativa extract can be used as an agent that protects against
sunburn and wounds caused by exposure to sunlight. In addition, the
small sample size is a limitation of this study.
This wound healing enhancer effect was also
supported by Han et al (142) regarding the topical use of 50%
N. sativa oil cream twice daily for 14 days on the skin of
mice with skin defects. The use of N. sativa extract reduced
oxidant parameters, such as malondialdehyde (MDA), glutathione
(GSH) and skin catalase (CAT) and increased antioxidant-supporting
enzyme parameters, such as glutathione peroxidase (GPx) and SOD
levels, markedly compared with controls. It can be concluded that
N. sativa extract exerts wound healing effects through its
antioxidant properties. Topical application of N. sativa in
the form of oil cream at a concentration of 50% enhances the
healing of open wounds in a reliable manner.
Algahtani et al (134) proved the effect of
nanoemulsion-based hydrogel of TQ 0.5% (w/w) on collagen fibers in
mice with skin defects. Nanoemulsion-based hydrogel systems provide
more efficient drug delivery to increase the biopharmaceutical
attributes of drugs that are difficult to dissolve. TQ, which is
difficult to dissolve in water, can be encapsulated into the
lipophilic environment of nano-dimensional oil droplets and
stabilized to be more optimal (143). In this study, it was found that
the use of nanoemulsion-based hydrogel systems provided higher and
more regular collagen density compared with the use of the standard
gel form TQ and the gold standard therapy, 1% silver sulfadiazine.
All research summaries supporting the potential of N. sativa
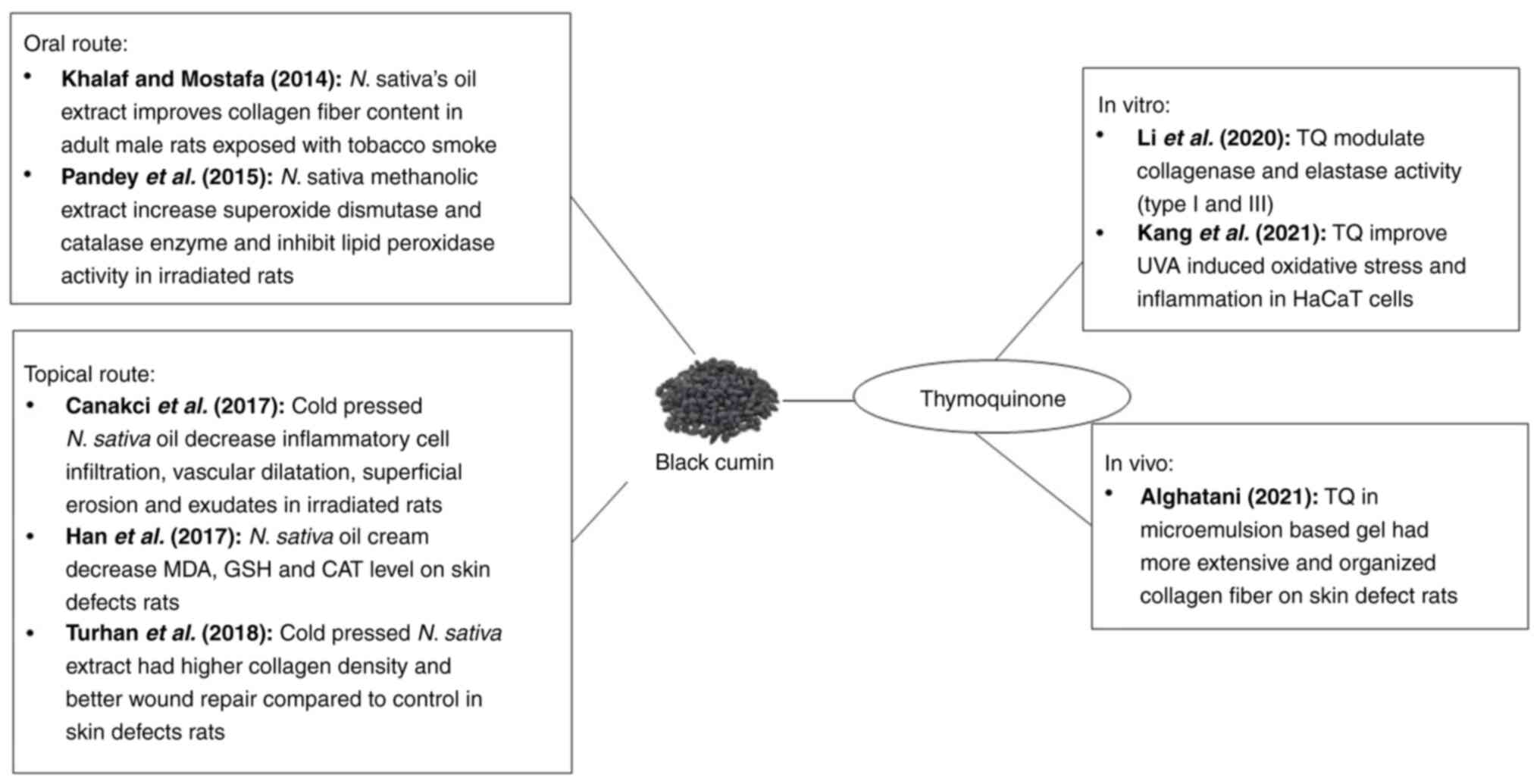
in fighting photoaging are summarized in Table II.
 | Table IIStudies that support the potential
role of Nigella sativa against photoaging. |
Table II
Studies that support the potential
role of Nigella sativa against photoaging.
| First author,
year | Subjects | Methods | Results | (Refs.) |
|---|
| Li et al,
2020 | In
vitro | Analysis of
molecular process within glycation, collagen cross-linking,
collagenase and elastase activities in murine melanoma B16F10 cells
measured with collagenase activity assay kit. |
Thymocid® (50, 100 and 300
µg/ml) interfered with the production of advanced glycation
end-products, collagen cross-linking, collagenase activity, and
elastase activities (type I and III) | (2) |
| Liang et al,
2021 | In
vitro | Analysis of
oxidative stress and mitochondrial function on HaCaT cells
irradiated by UVA using colorimetry, spectrophotometry,
bioluminescence and dual-luciferase reporter assay. Cell viability
was also evaluated using MTT and ELISA assay. | TQ pure extract (6
and 12 µM) not only significantly improved the UVA-induced
cytotoxicity, oxidative stress and inflammation but also promoted
mitochondrial dysregulation in HaCaT cells. | (10) |
| Pandey et
al, 2015 | In vivo, in
rats | Radio-protective
effect of oral Nigella sativa methanolic extract (200 mg/100
g bw/day) in albino rats 2 h before Total Body Irradiation at 4, 6,
10 Gy and continued until 7 consecutive days. They evaluated for
histology and antioxidant parameters | Oral Nigella
sativa methanolic extract (200 mg/100 g bw/day) significantly
increased superoxide dismutase and catalase enzymes and inhibited
lipid peroxidation activities. | (131) |
| Khalaf and Mostafa,
2014 | In vivo, in
rats | Twenty adult male
rats were exposed to side and main stream smoke for 10 min twice
daily for 4 weeks and treated orally with Nigella sativa oil
10 ml/Kgbw. Histological examination was performed. | Oral Nigella
sativa extract 10 ml/kg bw/day had a protective role against
tobacco smoking with collagen fiber content almost the same as the
nonsmoking group (negative control). | (140) |
| Canakci et
al, 2017 | In vivo, in
rats | Radio-protective
effect of topical cold pressed Nigella sativa oil (0.05 ml
at 100% concentration) given on days 1 to 3 to each nostril of
female rats (n=18) after radiotherapy with a single dose of 40 Gy.
Histopathological evaluation was measured. | Decrease of
inflammatory cell infiltration, vascular dilatation, superficial
erosion, and exudates in Nigella sativa group compared with
the control. | (132) |
| Turhan et
al, 2019 | In vivo, in
rats | Collagen density
and wound closure were measured with histological morphometric
analysis on skin defects in 20 albino rats. The wounds were treated
with 2 ml cold-pressed Nigella sativa extracts twice daily
for 15 days. | Higher collagen
density was achieved in Nigella sativa group compared with
the nano silver solution and control group. In addition, the
combination of Nigella sativa and nano silver solution (1:1)
had the highest collagen density among the groups. | (133) |
| Han et al,
2017 | In vivo, in
rats | A total of 42
female albino Wistar rats with skin defects were randomly treated
with 50% Nigella sativa oil cream, 50% Hypericum
perforatum oil cream, and placebo cream twice daily for 14
days. Histological and biochemical evaluations were performed. | A significant
decrease in the MDA, GSH and skin CAT levels and an increase in GPx
and SOD levels were found in the Nigella sativa group when
compared with the other two groups on the 14th day of
treatment. | (142) |
| Algahtani et
al, 2021 | In vivo, in
rats | Collagen fiber was
measured with histopathological analysis on 16 Wistar rats with
skin defects treated with 0.5% TQ nanoemulsion-based hydrogel twice
a day for 20 consecutive days. | Nanoemulsion-based
hydrogel of TQ 0.5% (w/w) group had significantly more extensive
and organized collagen fiber than the 1% silver sulfadiazine
group. | (134) |
Various studies, both in vitro and in
vivo, have shown that the use of N. sativa extract and
its active substance, TQ, has a potential role against photoaging
because of their antioxidant and anti-inflammatory effects
(10,135,139,141). The antioxidant effect was
demonstrated by improvements in parameters such as MDA, CAT and GSH
levels and increased levels of the SOD enzyme (135,141). In several studies, the
anti-inflammatory effect improved inflammatory parameters and
histopathological features in irradiated mice (2,10,139).
These two effects are thought to modulate collagenase and elastase
activity, which in turn improves collagen density and skin
hydration, especially in skin exposed to sunlight (2,137,140,142). A summary of the protective effects
of N. sativa against photoaging is presented in Fig. 2.
These studies have several limitations that require
evaluation. First, the use of N. sativa extract has not been
standardized in terms of ingredients, extraction methods,
ingredient content, oral and topical preparation and concentration
of active ingredients contained therein; second, research on the
effects of N. sativa against UV rays and photoaging is
limited; third, the outcome assessment criteria are not yet
standardized; and fourth, a number of studies use relatively small
sample sizes, animal studies and have a high potential for bias.
Further studies are needed, especially using human samples with
large sample sizes and randomized control trial methods, to clarify
the role of N. sativa as a photoaging protective agent.
Nevertheless, from all available sources, it can be summarized that
N. sativa extract and its active components may have some
potential to counteract the detrimental effects of UV
radiation.
6. Conclusion
N. sativa seeds contain a number of active
ingredients and play a potential role in photoaging therapy. The
mechanism underlying the accumulation of free radicals caused by UV
rays is unclear. Essential active ingredients, including TQ,
flavonoids, saponins, fatty acids and minerals, can participate in
this effect. Their mechanism possibly not only inhibits the MAPK
signaling and NF-κB pathways but also decreases the expression of
proinflammatory cytokines in the inflammatory state. Blocking this
pathway will reduce MMP1 production and collagen degradation.
Several studies have shown that N. sativa seed extract and
TQ have potential as antiaging agents. However, further research is
needed to demonstrate its protective ability against photoaging.
The use of N. sativa extract is still limited because of its
preventive effects on photoaging, so further research is needed,
especially in clinical trials, to determine the optimal dose or its
use as a combination therapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Maranatha Christian
University, Bandung, Indonesia.
Availability of data and materials
Not applicable.
Authors' contributions
AE collected articles and wrote the first draft of
manuscript, JWG initiated the study and revised the manuscript and
TLW finalized the manuscript. Data authentication is not
applicable. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohiuddin AK: Skin aging & modern age
anti-aging strategies. Glob J Med Res. 7:15–60. 2019.
|
|
2
|
Li H, DaSilva NA, Liu W, Xu J, Dombi GW,
Dain JA, Li D, Chamcheu JC, Seeram NP and Ma H:
Thymocid®, a standardized black cumin (Nigella
sativa) seed extract, modulates collagen cross-linking,
collagenase and elastase activities, and melanogenesis in murine
B16F10 melanoma cells. Nutrients. 12(2146)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hwang E, Lee TH, Park SY, Yi TH and Kim
SY: Enzyme-modified Panax ginseng inhibits UVB-induced skin aging
through the regulation of procollagen type I and MMP-1 expression.
Food Funct. 5:265–274. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tu Y and Quan T: Oxidative stress and
human skin connective tissue aging. Cosmetics. 3(28)2016.
|
|
5
|
Ahmed OM and Mohammed MT: Oxidative
stress: The role of reactive oxygen species (ROS) and antioxidants
in human diseases. Plant Arch. 20:4089–4095. 2020.
|
|
6
|
Ardiana M, Pikir BS, Santoso A, Hermawan
HO and Al-Farabi MJ: Effect of Nigella sativa
supplementation on oxidative stress and antioxidant parameters: A
meta-analysis of randomized controlled trials.
ScientificWorldJournal. 2020(2390706)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sudhir SP, Deshmukh VO and Verma HN:
Nigella sativa seed, a novel beauty care ingredient: A
review. Int J Pharm Sci Res. 7:3185–3196. 2016.
|
|
8
|
Adam GO and Shuaib YA: Antioxidant and
anti-tyrosinase potentials of extracts of Nigella sativa and
Senna alexandrina from sudan. J Appl Vet Sci. 7:41–45. 2022.
|
|
9
|
Shahroudi MJ, Mehri S and Hosseinzadeh H:
Anti-aging effect of Nigella sativa fixed oil on
D-galactose-induced aging in mice. J Pharmacopuncture. 20:29–35.
2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Liang J, Lian L, Wang X and Li L:
Thymoquinone, extract from Nigella sativa seeds, protects
human skin keratinocytes against UVA-irradiated oxidative stress,
inflammation and mitochondrial dysfunction. Mol Immunol. 135:21–27.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rizaoglu T: Effects of ozonated oils
(sesame oil, Nigella sativa oil, and hypericium perforatum
oil) on wound healing process in rats. Gov Polit Contemp Middle
East Contin Chang, pp77-116, 2018.
|
|
12
|
Huang AH and Chien AL: Photoaging: A
review of current literature. Curr Dermatol Rep. 9:22–29.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Krutmann J, Schikowski T, Morita A and
Berneburg M: Environmentally-induced (extrinsic) skin aging:
Exposomal factors and underlying mechanisms. J Invest Dermatol.
141:1096–1103. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee LY and Liu SX: Pathogenesis of
photoaging in human dermal fibroblasts. Int J Dermatol Venereol.
3:37–42. 2022.
|
|
15
|
Venkatesh S, Maymone MBC and Vashi NA:
Aging in skin of color. Clin Dermatol. 37:351–357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Karim PL, Aryani IA and Nopriyati :
Anatomy and histologic of intrinsic aging skin. Biosci Med J Biomed
Transl Res. 5:1065–1077. 2021.
|
|
17
|
Tobin DJ: Introduction to skin aging. J
Tissue Viability. 26:37–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sachs DL, Varani J, Chubb H, Fligiel SEG,
Cui Y, Calderone K, Helfrich Y, Fisher GJ and Voorhees JJ: Atrophic
and hypertrophic photoaging: Clinical, histologic, and molecular
features of 2 distinct phenotypes of photoaged skin. J Am Acad
Dermatol. 81:480–488. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tanveer MA, Rashid H and Tasduq SA:
Molecular basis of skin photoaging and therapeutic interventions by
plant-derived natural product ingredients: A comprehensive review.
Heliyon. 9(e13580)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cao C, Xiao Z, Wu Y and Ge C: Diet and
skin aging-from the perspective of food nutrition. Nutrients.
12(870)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cho S: Pathogenesis and prevention of skin
aging. J Korean Med Assoc. 64:438–446. 2021.
|
|
22
|
Mohania D, Chandel S, Kumar P, Verma V,
Digvijay K, Tripathi D, Choudhury K, Mitten SK and Shah D:
Ultraviolet radiations: Skin defense-damage mechanism. Adv Exp Med
Biol. 996:71–87. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roy C and Gies P: Ultraviolet light and
short-term hazards to the skin and eyes. Wiley Online Libr.
3:47–66. 2017.
|
|
24
|
Chen X, Yang C and Jiang G: Research
progress on skin photoaging and oxidative stress. Postepy Dermatol
Alergol. 38:931–936. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Teng Y, Yu Y, Li S, Huang Y, Xu D, Tao X
and Fan Y: Ultraviolet radiation and basal cell carcinoma: An
environmental perspective. Front Public Health.
9(666528)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Furukawa JY, Martinez RM, Morocho-Jácome
AL, Castillo-Gómez TS, Pereda-Contreras VJ, Rosado C, Velasco MVR
and Baby AR: Skin impacts from exposure to ultraviolet, visible,
infrared, and artificial lights-a review. J Cosmet Laser Ther.
23:1–7. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci.
17(868)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Bernard JJ, Gallo RL and Krutmann J:
Photoimmunology: How ultraviolet radiation affects the immune
system. Nat Rev Immunol. 19:688–701. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chambers ES and Vukmanovic-Stejic M: Skin
barrier immunity and ageing. Immunology. 160:116–125.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hart PH, Norval M, Byrne SN and Rhodes LE:
Exposure to ultraviolet radiation in the modulation of human
diseases. Annu Rev Pathol. 14:55–81. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gromkowska-Kępka KJ, Puścion-Jakubik A,
Markiewicz-Żukowska R and Socha K: The impact of ultraviolet
radiation on skin photoaging-review of in vitro studies. J Cosmet
Dermatol. 20:3427–3431. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mirończuk-Chodakowska I, Witkowska AM and
Zujko ME: Endogenous non-enzymatic antioxidants in the human body.
Adv Med Sci. 63:68–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Costantini D: Understanding diversity in
oxidative status and oxidative stress: The opportunities and
challenges ahead. J Exp Biol. 222(jeb194688)2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
De Jager TL, Cockrell AE and Du Plessis
SS: Ultraviolet light induced generation of reactive oxygen
species. Adv Exp Med Biol. 996:15–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cavinato M and Jansen-Dürr P: Molecular
mechanisms of UVB-induced senescence of dermal fibroblasts and its
relevance for photoaging of the human skin. Exp Gerontol. 94:78–82.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Rajendran P, Alzahrani AM, Hanieh HN,
Kumar SA, Ben Ammar R, Rengarajan T and Alhoot MA: Autophagy and
senescence: A new insight in selected human diseases. J Cell
Physiol. 234:21485–21492. 2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Evas O: The role of reactive oxygen
species and antioxidants in oxidative stress. Int J Res Pharm
Biosci. 56:433–437. 2016.
|
|
38
|
Madkour LH: Function of reactive oxygen
species (ROS) inside the living organisms and sources of oxidants.
Pharm Sci Anal Res J. 2(180023)2019.
|
|
39
|
Ighodaro OM and Akinloye OA: First line
defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and
glutathione peroxidase (GPX): Their fundamental role in the entire
antioxidant defence grid. Alexandria J Med. 54:287–293. 2018.
|
|
40
|
Galaris D, Barbouti A and Pantopoulos K:
Iron homeostasis and oxidative stress: An intimate relationship.
Biochim Biophys Acta Mol Cell Res. 1866(118535)2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gu Y, Han J, Jiang C and Zhang Y:
Biomarkers, oxidative stress and autophagy in skin aging. Ageing
Res Rev. 59(101036)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Davinelli S, Bertoglio JC, Polimeni A and
Scapagnini G: Cytoprotective polyphenols against chronological skin
aging and cutaneous photodamage. Curr Pharm Des. 24:99–105.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cho BA, Yoo SK and Seo JS: Signatures of
photo-aging and intrinsic aging in skin were revealed by
transcriptome network analysis. Aging (Albany NY). 10:1609–1626.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shukla A and Mossman BT: Chapter 9 cell
signaling by oxidants: Pathways leading to activation of
mitogen-activated protein kinases (MAPK) and activator protein-1
(AP-1). Curr Top Membr. 61:191–209. 2008.
|
|
45
|
Singh D, Rai V and K Agrawal DK:
Regulation of collagen I and collagen III in tissue injury and
regeneration. Cardiol Cardiovasc Med. 7:5–16. 2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Taniguchi K and Karin M: NF-κB,
inflammation, immunity and cancer: Coming of age. Nat Rev Immunol.
18:309–324. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang Y, Wang L, Wen X, Hao D, Zhang N, He
G and Jiang X: NF-κB signaling in skin aging. Mech Ageing Dev.
184(111160)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Pourzand C, Albieri-Borges A and Raczek
NN: Shedding a new light on skin aging, iron-and redox-homeostasis
and emerging natural antioxidants. Antioxidants (Basel).
11(471)2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Gendrisch F, Esser PR, Schempp CM and
Wölfle U: Luteolin as a modulator of skin aging and inflammation.
Biofactors. 47:170–180. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ke Y and Wang XJ: TGFβ signaling in
photoaging and UV-induced skin cancer. J Invest Dermatol.
141:1104–1110. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ansary TM, Hossain MR, Kamiya K, Komine M
and Ohtsuki M: Inflammatory molecules associated with ultraviolet
radiation-mediated skin aging. Int J Mol Sci.
22(3974)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. 1st edition
Elsevier Inc., 2017.
|
|
53
|
Laronha H and Caldeira J: Structure and
function of human matrix metalloproteinases. Cells.
9(1076)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zorina A, Zorin V, Kudlay D and Kopnin P:
Molecular mechanisms of changes in homeostasis of the dermal
extracellular matrix: Both involutional and mediated by ultraviolet
radiation. Int J Mol Sci. 23(6655)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Riihilä P, Nissinen L and Kähäri VM:
Matrix metalloproteinases in keratinocyte carcinomas. Exp Dermatol.
30:50–61. 2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Shin JW, Kwon SH, Choi JY, Na JI, Huh CH,
Choi HR and Park KC: Molecular mechanisms of dermal aging and
antiaging approaches. Int J Mol Sci. 20(2126)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yue J and López JM: Understanding MAPK
signaling pathways in apoptosis. Int J Mol Sci.
21(2346)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cole MA, Quan T, Voorhees JJ and Fisher
GJ: Extracellular matrix regulation of fibroblast function:
Redefining our perspective on skin aging. J Cell Commun Signal.
12:35–43. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Rogowski-Tylman M, Narbutt J, Woźniacka A
and Lesiak A: Molecular aspects of skin aging. Literature review.
Przegl Dermatol. 103:139–142. 2016.
|
|
60
|
Kanigur Sultuybek G, Soydas T and Yenmis
G: NF-κB as the mediator of metformin's effect on ageing and
ageing-related diseases. Clin Exp Pharmacol Physiol. 46:413–422.
2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Cheng W, Yan-Hua R, Fang-Gang N and Guo-An
Z: The content and ratio of type I and III collagen in skin differ
with age and injury. Afr J Biotechnol. 10:2524–2529. 2011.
|
|
62
|
Hwang SJ, Kim SH, Seo WY, Jeong Y, Shin
MC, Ryu D, Lee SB, Choi YJ and Kim KJ: Effects of human collagen
α-1 type I-derived proteins on collagen synthesis and elastin
production in human dermal fibroblasts. BMB Rep. 54:329–334.
2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Naomi R, Ridzuan PM and Bahari H: Current
insights into collagen type I. Polymers (Basel).
13(2642)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fligiel SEG, Varani J, Datta SC, Kang S,
Fisher GJ and Voorhees JJ: Collagen degradation in
aged/photodamaged skin in vivo and after exposure to matrix
metalloproteinase-1 in vitro. J Invest Dermatol. 120:842–848.
2003.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Yilmaz ÖÖ, Polat T, Tacal Aslan B and
Ulucan K: Can skin aging be reversible by anti-aging treatments
with genetic analysis? İstanbul Gelişim Üniversitesi Sağlık Bilim
Derg. 21:1242–1250. 2023.
|
|
66
|
Devos H, Zoidakis J, Roubelakis MG,
Latosinska A and Vlahou A: Reviewing the regulators of COL1A1. Int
J Mol Sci. 24(10004)2023.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Yamaba H, Haba M, Kunita M, Sakaida T,
Tanaka H, Yashiro Y and Nakata S: Morphological change of skin
fibroblasts induced by UV Irradiation is involved in photoaging.
Exp Dermatol. 25 (Suppl 3):S45–S51. 2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Balasubramanian P, Prabhakaran MP,
Sireesha M and Ramakrishna S: Collagen in human tissues: Structure,
function, and biomedical implications from a tissue engineering
perspective. Adv Polym Sci. 251(173)2013.
|
|
69
|
Derby B and Akhtar R: Mechanical
properties of aging soft tissues. Eng Mater Process. 10:237–263.
2015.
|
|
70
|
Biskanaki F, Kefala V, Lazaris AC and
Rallis E: Aging and the impact of solar ultraviolet radiation on
the expression of type I and type VI collagen. Cosmetics.
10(48)2023.
|
|
71
|
Liu H, Dong J, Du R, Gao Y and Zhao P:
Collagen study advances for photoaging skin. Photodermatol
Photoimmunol Photomed. 40(e12931)2024.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Watson RE, Gibbs NK, Griffiths CE and
Sherratt MJ: Damage to skin extracellular matrix induced by UV
exposure. Antioxid Redox Signal. 21:1063–1077. 2014.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Svobodova A, Walterova D and Vostalova J:
Ultraviolet light induced alteration to the skin. Biomed Pap Med
Fac Univ Palacky Olomouc Czech Repub. 150:25–38. 2006.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kamata H, Honda SI, Maeda S, Chang L,
Hirata H and Karin M: Reactive oxygen species promote
TNFalpha-induced death and sustained JNK activation by inhibiting
MAP kinase phosphatases. Cell. 120:649–661. 2005.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Calvo MJ, Navarro C, Durán P, Galan-Freyle
NJ, Parra Hernández LA, Pacheco-Londoño LC, Castelanich D, Bermúdez
V and Chacin M: Antioxidants in photoaging: From molecular insights
to clinical applications. Int J Mol Sci. 25(2403)2024.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Liu Z, Li Y, Song H, He J, Li G, Zheng Y
and Li B: Collagen peptides promote photoaging skin cell repair by
activating the TGF-β/Smad pathway and depressing collagen
degradation. Food Funct. 10:6121–6134. 2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Ahmad MF, Ahmad FA, Ashraf SA, Saad HH,
Wahab S, Khan MI, Ali M, Mohan S, Hakeem KR and Athar MT: An
updated knowledge of black seed (Nigella sativa Linn.):
Review of phytochemical constituents and pharmacological
properties. Elsevier GmbH, 2021.
|
|
78
|
Ahmad M, Khan A, Marwat K, Zafar M, Khan
MA, Hassan U and Sultana S: Useful medicinal flora enlisted in holy
quran and ahadith. Am J Agric Environ Sci. 5:126–140. 2009.
|
|
79
|
Hannan MA, Rahman MA, Sohag AAM, Uddin MJ,
Dash R, Sikder MH, Rahman MS, Timalsina B, Munni YA, Sarker PP, et
al: Black cumin (Nigella sativa L.): A comprehensive review
on phytochemistry, health benefits, molecular pharmacology, and
safety. Nutrients. 13(1784)2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Dalli M, Bekkouch O, Azizi SE, Azghar A,
Gseyra N and Kim B: Nigella sativa l. phytochemistry and
pharmacological activities: A review (2019-2021). Biomolecules.
12(20)2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Eisenman SW, Zaurov DE and Struwe L:
Medicinal plants of central asia: Uzbekistan and kyrgyzstan.
Springer New York, pp1-340, 2013.
|
|
82
|
Ramadan MF: Black cumin (Nigella
sativa) oils. Elsevier Inc., 2015.
|
|
83
|
Yimer E, Tuem KB, Karim A, Rehan N, Mariod
AA, Saeed Mirghani ME and Hussein I: Nigella sativa L.
(black cumin): A promising natural remedy for wide range of
illnesses. Hindawi Publ Corp. 2019:73–80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Javed S, Shahid AA, Haider MS, Umeera A,
Ahmad R and Mushtaq S: Nutritional, phytochemical potential and
pharmacological evaluation of Nigella sativa (kalonji) and
trachyspermum ammi (Ajwain). J Med Plants Res. 6:768–775. 2012.
|
|
85
|
Majeed A, Muhammad Z, Ahmad H, Rehmanullah
Hayat SSS, Inayat N and Siyyar S: Nigella sativa L.: Uses in
traditional and contemporary medicines-an overview. Acta Ecol Sin.
41:253–258. 2021.
|
|
86
|
Hossain MS, Sharfaraz A, Dutta A, Ahsan A,
Masud MA, Ahmed IA, Goh BH, Urbi Z, Sarker MMR and Ming LC: A
review of ethnobotany, phytochemistry, antimicrobial pharmacology
and toxicology of Nigella sativa L. Biomed Pharmacother.
143(112182)2021.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Fatima Shad K, Soubra W and Cordato DJ:
The role of thymoquinone, a major constituent of Nigella
sativa, in the treatment of inflammatory and infectious
diseases. Clin Exp Pharmacol Physiol. 48:1445–1453. 2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Rajabian A and Hosseinzadeh H:
Dermatological effects of Nigella sativa and its
constituent, thymoquinone: A review. Elsevier Inc., 2020.
|
|
89
|
Benazzouz-Smail L, Achat S, Brahmi F,
Bachir-Bey M, Arab R, Lorenzo JM, Benbouriche A, Boudiab K,
Hauchard D, Boulekbache L and Madani K: Biological properties,
phenolic profile, and botanical aspect of Nigella sativa L.
and Nigella damascena L. seeds: A comparative study. Molecules.
28(571)2023.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Hwang JR, Cartron AM and Khachemoune A: A
review of Nigella sativa plant-based therapy in dermatology.
Int J Dermatol. 60:e493–e499. 2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Eid AM, Elmarzugi NA, Abu Ayyash LM,
Sawafta MN and Daana HI: A review on the cosmeceutical and external
applications of Nigella sativa. J Trop Med.
2017(7092514)2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Santoso ARB, Huwae TECJ, Kristianto Y and
Putera MA: Effect of thymoquinone: the extract of Nigella
sativa in accelerating soft callus formation in fracture. Int J
Res Med Sci. 7:4068–4072. 2019.
|
|
93
|
Nyemb JN, Shaheen H, Wasef L, Nyamota R,
Segueni N and El-Saber Batiha G: Black Cumin: A review of its
pharmacological effects and its main active constituent. Pharmacogn
Rev. 16:107–125. 2022.
|
|
94
|
Ali BH and Blunden G: Pharmacological and
toxicological properties of Nigella sativa. Phyther Res.
14:299–305. 2003.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Mashayekhi-Sardoo H, Rezaee R and Karimi
G: An overview of in vivo toxicological profile of thymoquinone.
Toxin Rev. 39:115–122. 2020.
|
|
96
|
Thakur S, Kaurav H and Chaudhary G:
Nigella sativa (kalonji): A black seed of miracle. Int J Res
Rev. 8:342–357. 2021.
|
|
97
|
Rahim MA, Shoukat A, Khalid W, Ejaz A,
Itrat N, Majeed I, Koraqi H, Imran M, Nisa MU, Nazir A, et al: A
narrative review on various oil extraction methods, encapsulation
processes, fatty acid profiles, oxidative stability, and medicinal
properties of black seed (Nigella sativa). Foods.
11(2826)2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Nasiri N, Ilaghi Nezhad M, Sharififar F,
Khazaneha M, Najafzadeh MJ and Mohamadi N: The therapeutic effects
of Nigella sativa on skin disease: A systematic review and
meta-analysis of randomized controlled trials. Evid Based
Complement Alternat Med. 2022(7993579)2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Kalus U, Pruss A, Bystron J, Jurecka M,
Smekalova A, Lichius JJ and Kiesewetter H: Effect of Nigella
sativa (black seed) on subjective feeling in patients with
allergic diseases. Phyther Res. 17:1209–1214. 2003.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Srinivasan K: Cumin (cuminum cyminum) and
black cumin (Nigella sativa) seeds: Traditional uses,
chemical constituents, and nutraceutical effects. Food Qual Saf.
2:1–16. 2018.
|
|
101
|
Darakhshan S, Bidmeshki Pour A,
Hosseinzadeh Colagar A and Sisakhtnezhad S: Thymoquinone and its
therapeutic potentials. Pharmacol Res. 95-96:138–158.
2015.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Modarresi Chahardehi A, Ojaghi HR,
Motedayyen H and Arefnezhad R: Nano-based formulations of
thymoquinone are new approaches for psoriasis treatment: A
literature review. Front Immunol. 15(1416842)2024.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Chen WP, Tang JL, Bao JP and Wu LD:
Thymoquinone inhibits matrix metalloproteinase expression in rabbit
chondrocytes and cartilage in experimental osteoarthritis. Exp Biol
Med (Maywood). 235:1425–1431. 2010.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Rasheeda K, Samyuktha D and Fathima NN:
Self-association of type I collagen directed by thymoquinone
through alteration of molecular forces. Int J Biol Macromol.
140:614–620. 2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Ghorbanibirgani A, Khalili A and
Rokhafrooz D: Comparing Nigella sativa oil and fish oil in
treatment of vitiligo. Iran Red Crescent Med J.
16(e4515)2014.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Yousefi M, Barikbin B, Kamalinejad M,
Abolhasani E, Ebadi A, Younespour S, Manouchehrian M and Hejazi S:
Comparison of therapeutic effect of topical Nigella with
Betamethasone and Eucerin in hand eczema. J Eur Acad Dermatol
Venereol. 27:1498–1504. 2013.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Michalak M: Plant-derived antioxidants:
Significance in skin health and the ageing process. Int J Mol Sci.
23(585)2022.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Rivera-Yañez CR, Ruiz-Hurtado PA,
Mendoza-Ramos MI, Reyes-Reali J, García-Romo GS, Pozo-Molina G,
Reséndiz-Albor AA, Nieto-Yañez O, René Méndez-Cruz A, Méndez-Catalá
CF and Rivera-Yañez N: Flavonoids present in propolis in the battle
against photoaging and psoriasis. Antioxidants (Basel).
10(2014)2021.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Singh Joshan D and Singh SK:
Investigational study of juglans regia extract and quercetin
against photoaging. Biomed Aging Pathol. 3:193–200. 2013.
|
|
110
|
Sim GS, Lee BC, Cho HS, Lee JW, Kim JH,
Lee DH, Kim JH, Pyo HB, Moon DC, Oh KW, et al: Structure activity
relationship of antioxidative property of flavonoids and inhibitory
effect on matrix metalloproteinase activity in UVA-irradiated human
dermal fibroblast. Arch Pharm Res. 30:290–298. 2007.PubMed/NCBI View Article : Google Scholar
|
|
111
|
He X, Wan F, Su W and Xie W: Research
progress on skin aging and active ingredients. Molecules.
28(5556)2023.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Hatahet T, Morille M, Hommoss A,
Devoisselle JM, Müller RH and Bégu S: Quercetin topical
application, from conventional dosage forms to nanodosage forms.
Eur J Pharm Biopharm. 108:41–53. 2016.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Choi HJ, Alam MB, Baek ME, Kwon YG, Lim JY
and Lee SH: Protection against UVB-induced photoaging by Nypa
fruticans via inhibition of MAPK/AP-1/MMP-1 signaling. Oxid Med
Cell Longev. 2020(2905362)2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Maghsoudloo M, Aliakbari RBS and Velisdeh
ZJ: Pharmaceutical, nutritional, and cosmetic potentials of
saponins and their derivatives. Nano Micro Biosyst. 2:1–6.
2023.
|
|
115
|
Cavinato M, Waltenberger B, Baraldo G,
Grade CVC, Stuppner H and Jansen-Dürr P: Plant extracts and natural
compounds used against UVB-induced photoaging. Biogerontology.
18:499–516. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Keskin Çavdar H: Active compounds, health
effects, and extraction of unconventional plant seed oils. In:
Plant and Human Health. Ozturk M and Hakeem K (eds). Vol 2.
Springer, Cham, pp245-285, 2019.
|
|
117
|
Sarkhail P, Esmaily H, Baghaei A, Shafiee
A, Abdollahi M, Khademi Y, Madandar M and Sarkheil P: Burn healing
potential of Nigella sativa seed oil in rats. Int J Pharm
Sci Res. 2:34–40. 2011.
|
|
118
|
Pavlou P, Siamidi A, Varvaresou A and
Vlachou M: Skin care formulations and lipid carriers as skin
moisturizing agents. Cosmetics. 8(89)2021.
|
|
119
|
Choi HJ, Song BR, Kim JE, Bae SJ, Choi YJ,
Lee SJ, Gong JE, Lee HS, Lee CY, Kim BH and Hwang DY: Therapeutic
effects of cold-pressed perilla oil mainly consisting of linolenic
acid, oleic acid and linoleic acid on UV-induced photoaging in NHDF
cells and SKH-1 hairless mice. Molecules. 25(989)2020.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Borkow G: Using copper to improve the
well-being of the skin. Curr Chem Biol. 8:89–102. 2014.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Prasad AS: Zinc is an antioxidant and
anti-inflammatory agent: Its role in human health. Front Nutr.
1(14)2014.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Polefka TG, Bianchini RJ and Shapiro S:
Interaction of mineral salts with the skin: A literature survey.
Int J Cosmet Sci. 34:416–423. 2012.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Azab AE, Adwas A, Elsayed ASI, Adwas AA,
Elsayed ASI, Azab AE and Quwaydir FA: Oxidative stress and
antioxidant mechanisms in human body. J Appl Biotechnol Bioeng.
6:43–47. 2019.
|
|
124
|
Kaymak E, Ceylan T, Akın T, Kuloğlu N,
Sayan M, Değer N, Önal E, Yildirim AB and Karabulut D: Effect of
thymoquinone on NRF2/NF-kB/MAPK pathway in methotrexate-induced rat
testis injury. Iran J Basic Med Sci. 27:1410–1416. 2024.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Nguyen CH, Senfter D, Basilio J, Holzner
S, Stadler S, Krieger S, Huttary N, Milovanovic D, Viola K,
Simonitsch-Klupp I, et al: NF-κB contributes to MMP1 expression in
breast cancer spheroids causing paracrine PAR1 activation and
disintegrations in the lymph endothelial barrier in vitro.
Oncotarget. 6:39262–39275. 2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Pardo A and Selman M: MMP-1: The elder of
the family. Int J Biochem Cell Biol. 37:283–288. 2005.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Stratigos AJ and Katsambas AD: The role of
topical retinoids in the treatment of photoaging. Drugs.
65:1061–1072. 2005.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Farris PK: Topical vitamin C: A useful
agent for treating photoaging and other dermatologic conditions.
Dermatol Surg. 31:814–818. 2005.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Correia G and Magina S: Efficacy of
topical vitamin C in melasma and photoaging: A systematic review. J
Cosmet Dermatol. 22:1938–1945. 2023.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Thielitz A and Gollnick H: Topical
retinoids in acne vulgaris: Update on efficacy and safety. Am J
Clin Dermatol. 9:369–381. 2008.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Pandey N, Kumar M, Pradhan S, Mandal A and
Tripathi YB: Effect of methanolic fraction of the seeds of
Nigella sativa linn on radiation induced Gi damage in rats.
Sciforschen. 10:1–5. 2015.
|
|
132
|
Çanakci H, Yilmaz AAŞ, Canpolat MS,
Şeneldir H, Kir G, Eriş AH, Mayadagli A and Oysu Ç: Evaluation of
the effect of topical application of Nigella sativa on acute
radiation-induced nasal mucositis. J Craniofac Surg. 29:e279–e282.
2018.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Turhan Y, Arican M, Karaduman ZO, Turhal
O, Gamsizkan M, Aydin D and Ozkan K: Comparison of the effects of
Nigella sativa oil and nano-silver on wound healing in an
experimental rat model. Iran Red Cresent Med J. 21(e84650)2019.
|
|
134
|
Algahtani MS, Ahmad MZ, Shaikh IA,
Abdel-Wahab BA, Nourein IH and Ahmad J: Thymoquinone loaded topical
nanoemulgel for wound healing: Formulation design and in-vivo
evaluation. Molecules. 26(3863)2021.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Khatoon M, Kushwaha P, Usmani S and Madan
K: Dermaceutical utilization of Nigella sativa seeds:
Applications and opportunities. Drug Res (Stuttg). 74:5–17.
2024.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Balyan P, Khan J and Ali A: Therapeutic
potential of Nigella sativa in the prevention of aggregation
and glycation of proteins. In: Black Seeds (Nigella sativa).
Khan A and Rehman M (eds). Elsevier, pp313-336, 2022.
|
|
137
|
Sun Z, Park SY, Hwang E, Zhang M, Seo SA,
Lin P and Yi TH: Thymus vulgaris alleviates UVB irradiation induced
skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE
antioxidant system. J Cell Mol Med. 21:336–348. 2017.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Yadav DK, Kumar S, Saloni Misra S, Yadav
L, Teli M, Sharma P, Chaudhary S, Kumar N, Choi EH, et al:
Molecular insights into the interaction of RONS and
thieno[3,2-C]pyran analogs with SIRT6/COX-2: A molecular dynamics
study. Sci Rep. 8(4777)2018.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Shabeeb D, Musa AE, Ali HSA and Najafi M:
Curcumin protects against radiotherapy-induced oxidative injury to
the skin. Drug Des Devel Ther. 14:3159–3163. 2020.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Khalaf G and Mostafa HKK: Histological and
immunohistochemical study on the effect of passive smoking on the
skin of adult male albino rats and the possible protective role of
Nigella sativa oil. Egypt J Histol. 35:87–94. 2012.
|
|
141
|
Lee H, Hong Y and Kim M: Structural and
functional changes and possible molecular mechanisms in aged skin.
Int J Mol Sci. 22(12489)2021.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Han MC, Durmuş AS, Sağliyan A, Günay C,
Özkaraca M, Kandemir FM, Çomakli S and Firat Öztopalan D: Effects
of Nigella sativa and hypericum perforatum on wound healing.
Turkish J Vet Anim Sci. 41:99–105. 2017.
|
|
143
|
Khatoon K, Ali A, Ahmad FJ, Hafeez Z,
Rizvi MMA, Akhter S and Beg S: Novel nanoemulsion gel containing
triple natural bio-actives combination of curcumin, thymoquinone,
and resveratrol improves psoriasis therapy: In vitro and in vivo
studies. Drug Deliv Transl Res. 11:1245–1260. 2021.PubMed/NCBI View Article : Google Scholar
|