1. Introduction
Cervical cancer (CC) is the fourth leading cause of
mortality in women, claiming 341,831 lives worldwide, and is one of
the main types of cancer affecting women of all ages, with an
incidence rate of 604,127 new cases reported in 2020, according to
the International Cancer Genome Atlas (IARC) (1). CC is associated with the persistent
infection of high-risk human papillomaviruses (HR-HPV), where the
expression of its oncoproteins E6 and E7 promotes the dysregulation
of cellular processes characteristically altered in cancer
(2). E7 mediates the degradation of
numerous cellular proteins, including the retinoblastoma protein
(pRB). The direct interaction of E7 with pRB promotes its
degradation. pRB is a crucial regulator of the cell cycle, and its
degradation results in the loss of proliferation control (3). Hyperproliferation induced by E7 would
cause cellular mechanisms to initiate controlled cell death;
however, the virus evades this response by expressing E6. E6
induces the degradation of p53, a key regulator of apoptosis
mechanisms, and p53 degradation inhibits apoptosis mechanisms
leading to cellular immortalization (4,5).
Moreover, the coordinated expression of these proteins dysregulates
angiogenesis and promotes invasion and metastasis, as well as the
decrease in telomerase activity and the decrease in the activity of
growth suppressors related to carcinogenesis (6).
To date, there are prophylactic alternatives to
prevent infections of at least nine types of HR-HPV (types 6, 11,
16, 18, 31, 33, 45, 52, and 58) (7). These alternatives have shown positive
effects on prevention, but it is unclear if these prophylactic
alternatives improve an already established infection. For this
reason, inhibition of specific molecules, such as E6 and E7, could
precisely enhance CC therapies (8).
Moreover, drugs administered in CC therapy that aim to inhibit
pathways related to carcinogenesis have shown positive effects in
CC treatment. However, their high degree of cytotoxicity has also
been documented (9). To address
this, an enhanced approach based on the molecular insights of viral
pathogenesis could be instrumental in developing more effective
prevention and treatment strategies for CC (10-12).
There have been significant advancements in CC
treatment, for which various approaches are available. These
include surgery, radiotherapy, chemotherapy, immunotherapy, and
targeted therapies. Notably, these conventional treatments are
highly invasive, toxic, and cause numerous side effects, including
leukopenia, thrombocytopenia, gastrointestinal damage, metabolic
alterations, and drug resistance, which makes it challenging to
ensure both the quality of life and treatment survival of patients.
For these reasons, it is crucial to prioritize the development of
targeted and personalized therapies that minimize cytotoxicity and
reduce adverse effects (13,14).
For instance, it is possible to develop multimodal treatment
approaches that combine conventional therapies and nanotechnologies
to improve effectiveness in treating CC and reducing side effects.
The lack of effective treatments and their complications have
spurred the development of nanosystems, which offer promising
advancements in improving cancer therapies. Nanosystems engineered
at the molecular level have shown great potential in
revolutionizing cancer treatments, addressing the limitations of
conventional approaches, and enhancing effectiveness while
minimizing side effects (15).
Nanotechnology, a branch of technology that
manipulates and controls matter at the nanoscale, holds immense
potential for various industrial and biomedical applications. At
the heart of nanotechnology are nanoparticles, nano-objects with 3D
external dimensions at the nanoscale. These nanoparticles,
including nanobars and nanoplates, enable groundbreaking
advancements in diverse fields driven by scientific knowledge and
innovation (16). The aim of the
present review was to provide a comprehensive understanding of the
differences between conventional and next-generation
nanosystem-based treatments for CC. Through a detailed analysis,
the importance of developing innovative therapeutic strategies
based on nanotechnologies that can improve treatment outcomes,
overcome the limitations of traditional approaches, and reduce side
effects is highlighted. Thus, the present review focuses on showing
how nanosystems offer transformative potential in the treatment of
CC, overcoming the barriers of conventional therapies.
2. Conventional treatments used in CC
Cervical intraepithelial neoplasia (CIN), closely
associated with HR-HPV infection, can progress to either in
situ or invasive carcinoma (17). CIN is classified into three grades:
CIN 1 (low-grade), CIN 2 (moderate), and CIN 3 (severe) (18). The invasive stage of CC is
correlated with a poor prognosis and entails the spread of cancer
cells to adjacent structures. Although CIN staging pertains to the
precancerous condition, invasive CC is staged using the FIGO
classification, which divides the disease into stages I-IV
(19). In stage I, carcinoma is
strictly confined to the cervix and subdivided into invasive
carcinoma IA which can only be diagnosed by microscopy (IA1 and
IA2), and invasive carcinoma IB (IB1, IB2, and IB3) with invasion
≥5 mm, limited to the uterine cervix. In stage II, subdivided into
IIA (IIA1 and IIA2), the carcinoma invades beyond the uterus,
without affecting the lower third of the vagina and the pelvic
wall, limited to two-thirds of the vagina, while in IIB there is
parametrial involvement but not reaching the pelvic wall. In stage
III, subdivided into IIIA, IIIB, and IIIC (IIIC1 and IIIC2), the
carcinoma affects the lower third of the vagina. In this stage the
carcinoma invades the pelvic wall causing hydronephrosis, involving
pelvic and para-aortic lymph nodes. In stage IV, the carcinoma has
spread beyond the pelvis affecting the bladder or rectum (20).
First-line treatment options include surgery,
radiotherapy, and chemotherapy (Table
I). The International Federation of Gynecology and Obstetrics
(FIGO) recommends surgery as immediate early-stage treatment (IA,
IB1, IB2, and IIA1), and in cases with contraindications for
surgery or anesthesia, radiotherapy is the suggested alternative.
However, in advanced stages (IB3, IIA2, IIB-IVB), platinum-based
chemotherapy combined with external radiation and intracavitary
brachytherapy is recommended. Additionally, the application of
radiotherapy and chemotherapy, in combination, is recommended
(20,21). Nevertheless, second-generation
therapy administration is necessary for patients with
unsatisfactory responses to first-generation treatment or disease
recurrence. These therapies involve a combination of drugs that
collectively enhance the treatment response. Treatment of both
precursor lesions and cancer is highly invasive, causing numerous
side effects that negatively impact the quality of life of patients
(22).
 | Table ITherapies used in the different
stages of cervical cancer. |
Table I
Therapies used in the different
stages of cervical cancer.
| Stage | Treatment |
|---|
| IA1 | • Surgical
conization. |
| | • Total
hysterectomy |
| | • Pelvic
lymphadenectomy |
| IA2 | • Pelvic
lymphadenectomy |
| | • Radical
hysterectomy with removal of lymph nodes |
| | • Radical uterine
cervicectomy |
| IB | • Radical
hysterectomy |
| | • Pelvic
lymphadenectomy |
| | • Radical
trachelectomy |
| IB2 and IIA1 | • Surgery or
radiotherapy |
| | • Radical
hysterectomy and removal of pelvic lymph nodes along with
chemotherapy (cisplatin or carboplatin) |
| IB3 and IIA2 | • Pelvic
irradiation |
| | • Platinum-based
chemoradiation |
| IVA | • Pelvic
exenteration |
| | • Radiotherapy |
| IVB | • Radiotherapy |
| | • Chemotherapy
(bevacizumab, cisplatin, ifosfamide, irinotecan, gemcitabine,
paclitaxel and topotecan), can be administered alone or in
combination |
| Recurrence | • Radiotherapy
combined with immunotherapy (pembrolizumab) and/or chemotherapy
such as cisplatin, carboplatin, ifosfamide, irinotecan,
gemcitabine, paclitaxel, topotecan and vinorelbine |
| | • Pelvic
exenteration |
To enhance the treatment response of patients,
combined alternatives such as immunotherapy, hormonal therapy, stem
cell transplantation, and targeted therapy have been proposed
(23). One of the challenges in
cancer treatment is the occurrence of side effects that can
potentially harm healthy tissues or organs. However, advancements
in targeted cell therapy serve as the cornerstone of precision
medicine, revolutionizing cancer treatment by employing
macromolecular drugs or monoclonal antibodies targeting
intracellular markers. This approach offers immense potential for
personalized and effective cancer management (24). However, these therapeutic strategies
often face significant limitations, including low aqueous
solubility, systemic cytotoxicity, rapid degradation at the
physiological level, and poor gastrointestinal absorption. To
improve these promising therapies, attention has shifted to the
design of efficient drug delivery systems. While conventional
approaches have shown some utility, they have fallen short in
effectively delivering a wide range of drugs (25).
3. Chemotherapy in the treatment of CC
Cisplatin, 5-fluorouracil, and pembrolizumab are
drugs widely recognized as first-line treatments for patients with
CC, as they are fundamental for the effective management of the
disease in its initial phase (22,26).
However, in patients at stage IB2 and beyond, based on the FIGO
classification, treatment primarily relies on the administration of
cisplatin, as it is the most extensively studied and active agent.
In patients where the administration of these drugs does not offer
optimal results, the combined use of cisplatin and topotecan has
shown promising results as a second-line therapy for patients with
advanced, recurrent, and persistent CC (27). In addition, other research has been
conducted on multi-drug combination alternatives to improve
response to CC, such as the combination of paclitaxel, carboplatin,
and bevacizumab, which is safe and effective in cases of advanced
or recurrent CC (28). Scatchard
et al (29) in 2012,
demonstrated that cisplatin-based regimens are the most widely
used, due to their high response rate and low toxicity compared to
regimens in combination with cisplatin and nonplatinum regimens.
Various drugs used in different types of cancer is shown in
Table II.
 | Table IISummary of the types of drugs
administered for various types of cancer. |
Table II
Summary of the types of drugs
administered for various types of cancer.
| Type of drug | Function | Drugs | Type of cancer |
|---|
| Alkylating
agents | | Nitrogen mustard:
Bendamustine, cyclophosphamide and ifosfamide | Lung, breast and
ovarian cancer, as well as leukemia, lymphoma, Hodgkin lymphoma,
multiple myeloma and sarcoma. |
| | | Nitrosoureas:
Carmustine and lomustine | |
| | Inhibition of DNA
replication and transcription | Platinum analogs:
Carboplatin, cisplatin and oxaliplatin | |
| | | Triazenes:
Dacarbazine, procarbazine and temozolamide | |
| | | Alkyl sulfonate:
Busulfan | |
| | | Ethyleneimine:
Thiotepa | |
|
Antimetabolites | Inhibition of DNA
replication | Cytidine analogs:
Azacitidine, decitabine, cytarabine and gemcitabine | Leukemias, breast,
ovarian, pancreatic and bladder cancer, as well as sarcoma, Hodgkin
lymphoma, colorectal, anal and gastric cancer. |
| | | Folate antagonists:
Methotrexate and pemetrexed | |
| | | Purine analogs:
Cladribine, clofarabine and nelarabine | |
| | | Pyrimidine analogs:
5-FU, and capecitabine (prodrug of 5-FU) | |
| Antimicrotubule
agents | Inhibit RNA and DNA
synthesis and disruption of the balance of microtubule
polymerization and depolymerization | Topoisomerase II
inhibitors: Anthracyclines Topoisomerase I inhibitors: Irinotecan
and topotecan Taxanes: Paclitaxel, docetaxel, cabazitaxel Vinca
alkaloids: Vinblastine, vincristine, vinorelbine | ALL, AML, Wilms
tumor, neuroblastoma, sarcomas, breast, ovarian, bladder and
thyroid cancer, Hodgkin lymphoma, non-Hodgkin lymphoma, as well as
colorectal, cervical, esophageal pancreatic, lung and prostate
cancer. |
| Antitumor
antibiotic | Inhibits DNA and
RNA synthesis | Actinomycin D,
bleomycin, daunomycina | Testicular, Hodgkin
lymphoma, as well as head, and neck cancers. |
| Others | Inhibit
ribonucleoside diphosphate reductase; S-phase specific; induces
cell differentiation | Hydroxyurea,
tretinoin, arsenic trioxide and proteasome inhibitors | Leukemias |
As an alternative to standard chemotherapy,
oncologists recommend the use of novel drugs such as pazopanib (an
antiproliferative and anti-angiogenic drug), lapatinib and
temsirolimus (antiproliferative drugs) (30). However, there are significant side
effects associated with the administration of these drugs
individually or in combination (Fig.
1) (31). Lapatinib is a
tyrosine kinase inhibitor that targets and inhibits the human
epidermal growth factor receptor type II and epidermal growth
factor receptor (EGFR), thereby decreasing cell proliferation and
cell migration (32). Furthermore,
temsirolimus exerts its antitumor activity primarily through
selective inhibition of the mTOR pathway, altering multiple
cellular processes involved in tumor progression and angiogenesis
(33). Moreover, pazopanib inhibits
several growth factors such as vascular endothelial growth factor
receptor (VEGFR), platelet-derived growth factor receptor (PDGFR),
and EGFR. By blocking these signaling pathways, which enhance the
growth and survival of tumor cells, pazopanib can help slow cancer
progression (Fig. 1) (34,35).
Similarly, chemotherapeutic drugs such as cisplatin and topotecan
affect cell growth and induce an antiproliferative effect on tumor
cells; both approaches, inhibiting growth factors and inducing
antiproliferative effects, represent complementary strategies in
cancer treatment by interfering with key mechanisms that tumor
cells rely on for growth and survival (24,25).
Cisplatin has no net charge and is not subject to the Born energy
barrier, which prevents small hydrophilic ions from diffusing
through the lipid phase of cell membranes; its internalization into
tumor cells occurs through passive diffusion across the cell lipid
membrane, rendering it one of the most used chemotherapeutic agents
for CC treatment (Fig. 1) (33). Moreover, in patients with advanced,
recurrent, and/or persistent CC, oncologists recommend the use of
combinations of paclitaxel (cell inhibitor), carboplatin (cell
proliferation and division inhibitor), and bevacizumab
(anti-angiogenic inhibitor) (26).
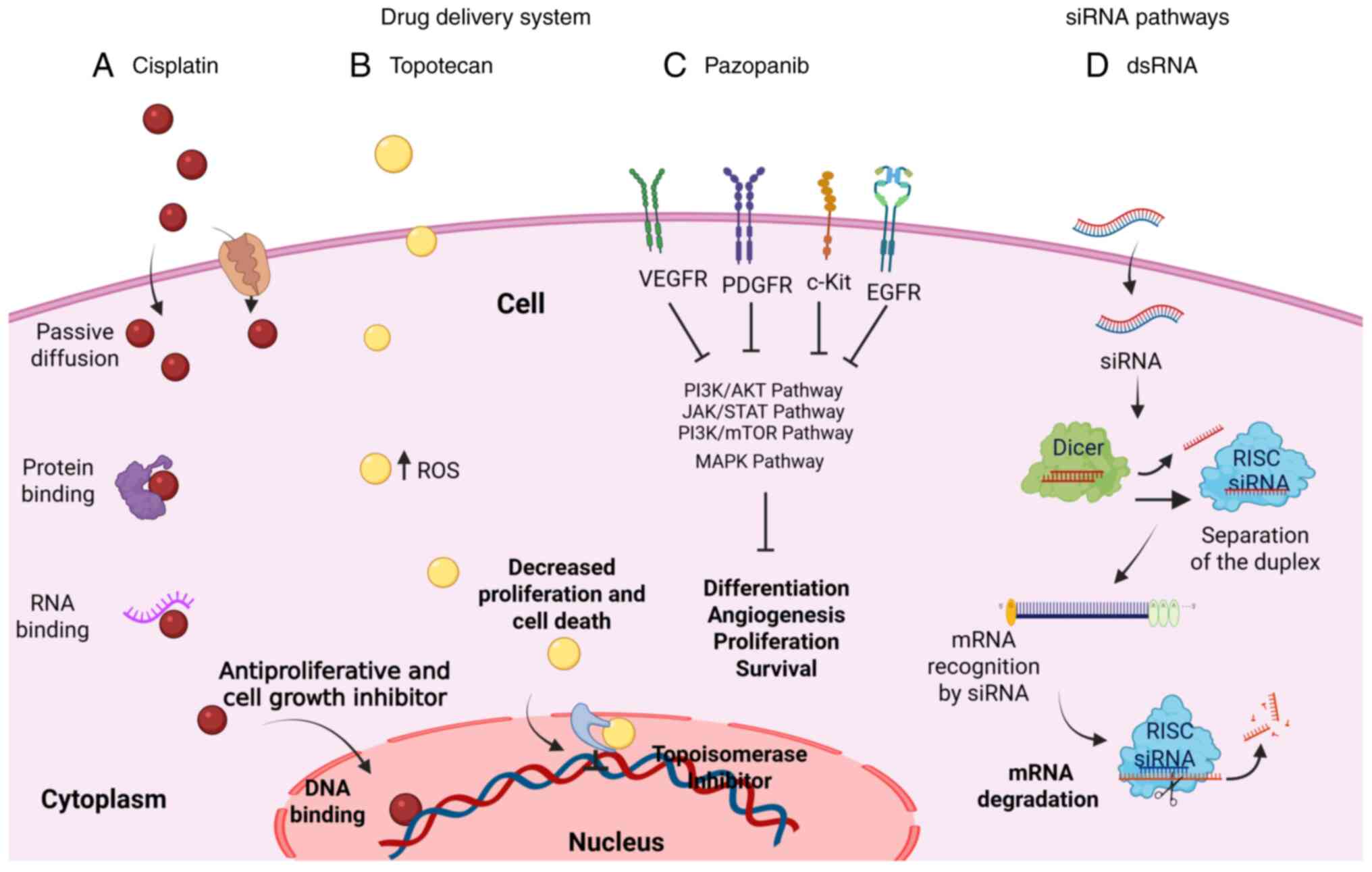 | Figure 1Drug-based therapies and genetic
silencers. This image illustrates the effects of various drugs and
genetic silencers used in cervical cancer therapy, including (A)
cisplatin, (B) topotecan, (C) pazopanib and (D) dsRNA. These agents
act by inhibiting processes such as cell proliferation,
differentiation, and angiogenesis, while promoting cell death. In
the image, activation is represented by arrowheads, whereas
inhibition or blockade is indicated by transverse lines. dsRNA,
double-stranded RNA; ROS, reactive oxygen species; VEGFR, vascular
endothelial growth factor receptor; PDGFR, platelet-derived growth
factor receptor; EGFR, epidermal growth factor receptor; siRNA,
small interfering RNA; RISC, RNA-induced silencing complex. The
figure was created using BioRender software (https://biorender.com/). |
Although chemotherapy is widely used, Scatchard
et al reported that various studies involving patients with
CC have linked chemotherapy to serious adverse outcomes, including
deaths related to neutropenic sepsis, severe thrombocytopenia,
pulmonary toxicity, encephalopathies, sudden cardiac death, and
cerebrovascular accidents (29).
Therefore, significant interest has been placed in developing novel
alternatives to reduce the effects caused by chemotherapy, thereby
improving the survival outcomes of patients with CC.
4. Nucleic acid-based therapy
In recent years, RNA molecular biology has undergone
revolutionary advances that have led to a profound understanding of
the mechanisms of gene regulation (36,37).
The identification and characterization of small non-coding RNAs
was a groundbreaking discovery. Small non-coding RNAs are molecules
composed of 20-30 nucleotides that play an essential role in the
post-transcriptional modulation of genes and genomes (34). This breakthrough has paved the way
for the advancement of nucleic acid-based therapies utilizing DNA
or RNA molecules to target genes associated with pathological
processes. These therapies encompass various approaches, including
plasmid DNA, small interfering RNA, and microRNAs (pDNA, siRNA, and
miRNAs, respectively) (35).
Owing to their marked sensitivity and specificity,
siRNAs have emerged as promising therapeutic agents that can
potentially replace or complement traditional chemotherapy
approaches (37,38). Targeted siRNAs, often considered for
cancer therapy, include genes that promote uncontrolled cell
growth, such as VEGFs, c-Myc, EphA2, Raf-1, Plk1, CDKs, survivin,
and multi-drug resistance genes, to mention a few, that help cancer
cells survive or resist chemotherapy (39-44)
Aberrant expression or activity of these genes causes cells to
escape from well-controlled cell cycles, resulting in malignant
transformation, which involves alterations in cytoskeletal
modulation, cell migration, proliferation, and angiogenesis, among
others (45,46) However, nucleic acid-based therapy is
hindered by several disadvantages, including intrinsic molecule
degradation by cellular nucleases and low cellular uptake (38). These challenges have been
effectively addressed through various strategies, including the
chemical modification of siRNAs and the utilization of nanoparticle
delivery systems based on lipids, polymers, and inorganic
platforms. These approaches serve the dual purpose of protecting
RNA molecules from cellular nucleases and facilitating their
efficient uptake by target cells (46).
5. Nanoparticles: An alternative for the
treatment of CC
Nanoparticles are an important class of nanosystems
used for medical applications. These systems consist of
nanoparticles of different sizes and shapes that are capable of
binding, encapsulating and/or transporting one or more active
substances, including small molecules, proteins, and nucleic acids;
their small size allows them to be administered both systemically
and locally, with efficient cellular internalization and diffusion
(Fig. 2A) (37).
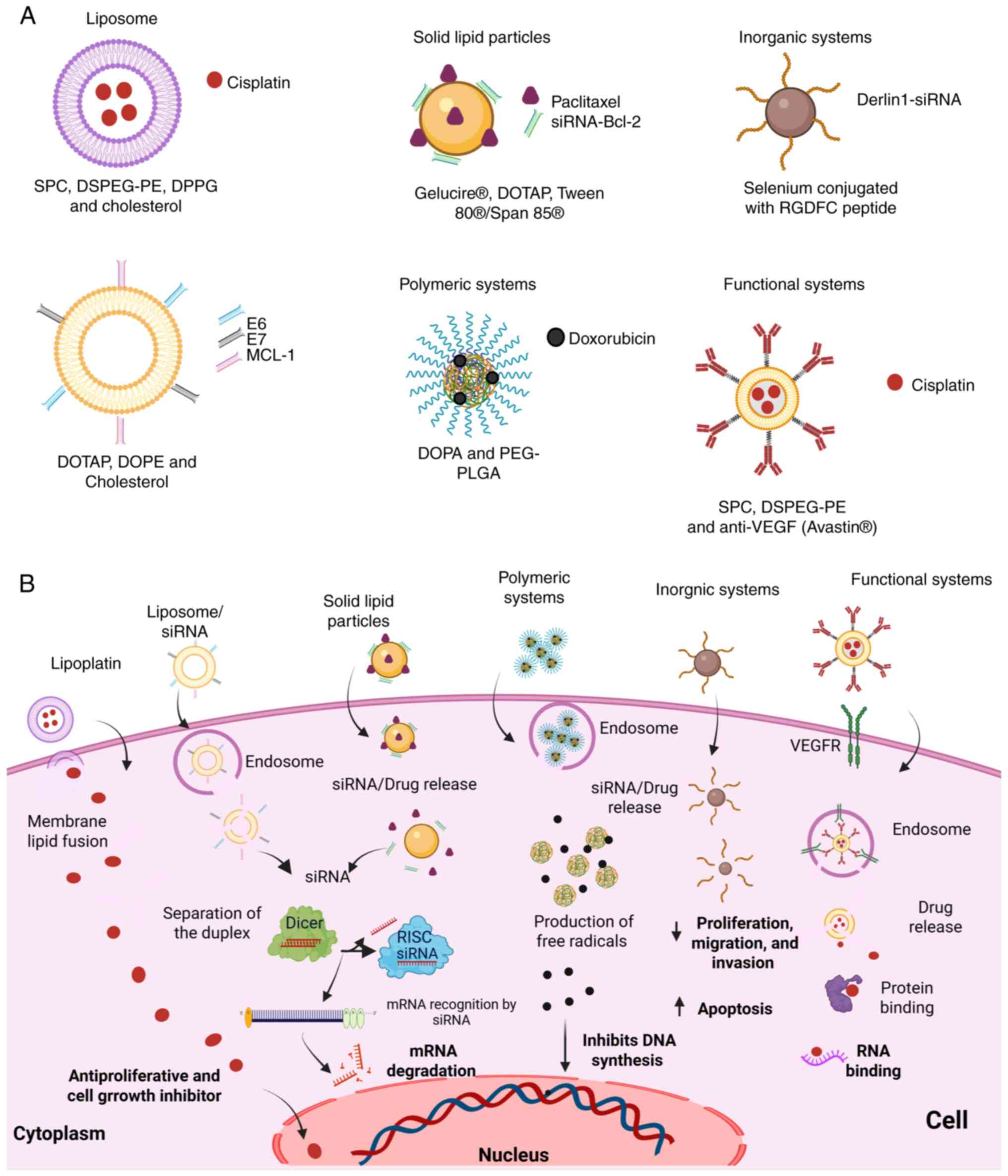 | Figure 2Delivery systems for drugs and gene
silencers. (A) Schematic representation of delivery systems used in
drug delivery and gene silencers. (B) Mechanism of action of
various drug delivery systems and gene silencers. DOPA,
dioleoylphosphatidic acid; DOPE,
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine; DOTAP,
1,2-dioleoyl-3-trimethylammonium propane; DPPG,
1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol; DSPEG-PE,
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene
glycol)-2000]; HSPC, hydrogenated soybean L-α-phosphatidylcholine;
PEG-PLGA polyethylene glycol-polylactic acid-co-glycolic acid;
VEGF, vascular endothelial growth factor; VEGFR, vascular
endothelial growth factor receptor; SPC, soya phosphatidylcholine.
The figure was created using BioRender software (https://biorender.com/). |
Nanotechnology-based delivery systems include viral
systems such as retroviruses and adenoviruses, among other viral
types (47), and nonviral systems:
Biodegradable nanoparticles, dendrimers, polymeric micelles,
liposomes, microcapsules, solid lipid nanoparticles (SLNs), and
solid nanoparticles (48). Polymers
and liposomes of cationic nature are among the most widely used
nanocarriers for the delivery of genetic material in cancer therapy
(Fig. 2A) (49). Engineered nanosystems have been
developped to carry gene-silencing molecules such as E6/E7 siRNA
and Derlin1-siRNA, which inhibit specific signaling pathways
including PI3K/AKT, JAK/STAT, PI3K/mTOR, and MAPK, as shown in
Fig. 1 (50,51).
However, most treatments based on the use of siRNAs are in
experimental (preclinical) phases. By contrast, targeted therapy
drugs, such as Doxil®, Abraxane®,
Lipoplatin® are in advanced clinical stages (52). The formulations of these nanosystems
improve CC therapy and enhance the efficiency of drug delivery for
agents such as cisplatin, topotecan, and pazopanib, along with
their mechanism of action against dsRNA (Fig. 1).
The use of nanoparticles in cancer treatment
overcomes the limitations of conventional chemotherapy by enhancing
the precision and efficacy of therapy. In addition, the use of
nanoparticles can monitor disease progression and adapt to specific
tumor microenvironment conditions, such as hypoxia and acidity,
optimizing drug absorption and concentration within tumor tissue
(53). Due to their
biocompatibility, permeability, and retention properties,
nanoparticles serve as efficient drug carriers, allowing for a
reduction in both dosage and treatment frequency, thereby
minimizing toxicity. Furthermore, the development of
stimulus-responsive systems has improved controlled drug release,
and certain nanoparticles can function as specific markers to
direct therapy precisely to cancer cells (54).
Currently, the nanoparticles used in CC treatment
have several limitations (Table
III). For instance, lipid-based nanocarriers exhibit low
cellular internalization, are prone to the accelerated blood
clearance phenomenon, can cause hemolysis, and present challenges
in achieving industrial-scale production. Polymeric nanoparticles
face limitations such as low drug loading capacity and potential
cytotoxicity. In the case of inorganic nanomaterials, the main
drawbacks include toxic effects on biological systems, low
permeability, and uncertainties regarding the relationship between
particle size and toxicity (55).
Furthermore, most nanoparticles are costly and complex to produce
since their synthesis requires expensive technologies. In addition,
there is a lack of preclinical studies, and their application in
humans remains limited. This is due to the need to evaluate their
safety and effectiveness in the long term before regulatory
approval can be granted (56).
However, despite all these challenges, nanoparticles are a
promising option in the fight against CC, provided these
limitations can be overcome.
 | Table IIIComparison of the various types of
nanoparticle-based therapies. |
Table III
Comparison of the various types of
nanoparticle-based therapies.
| Nanocarriers | Advantages |
Disadvantages/limitations |
|---|
| Lipid systems | -Enhancing oral
absorption | -Drug leakage
during storage |
| | -Eco-friendly
degradation | -Restricted loading
capacity for water-soluble drugs |
| | -Compatibility with
biological systems | -Changes in
crystalline structure |
| | -Efficient
encapsulation capacity | -Increase in
particle size over storage duration |
| | -Superior
structural stability | -Gelation of
lipid-based dispersions |
| Polymeric
systems | -High transfection
efficiency |
-Hydrophobicity |
| |
-Biocompatibility | -Slow
degradation |
| | -Biodegradable | -Existence of
cytotoxicity |
| | -Low toxicity | -Lack of functional
groups |
| | -Efficient
mechanical properties | |
| | -High
elasticity | |
| Inorganic
systems | -Large surface area
relative to volume | -Adverse effects on
biological systems |
| | -Enhanced
structural stability | -Limited
permeability |
| | -Easily
customizable surface properties | -Unclear
correlation between particle size and toxicity |
| | -Compatibility with
biological systems | |
| | -Porous
architecture for efficient functionality | |
| | -No reaction with
drugs | |
| | -Excellent
biocompatibility with minimal toxicity | |
6. Lipid systems in conventional
therapy
Liposomes are lipid-based spherical vesicles
composed of phospholipids that form a lipid bilayer surrounding an
aqueous core (57). Liposomes are
composed of natural or synthetic compounds, and their constituents
are not exclusive to lipids since new-generation liposomes can also
be formed from polymers (occasionally referred to as polymeromas);
some of the main characteristics of liposomes are that they are
biocompatible and biodegradable. In addition, they can
compartmentalize and solubilize in both hydrophilic and hydrophobic
media. These characteristics render them effective transport
vehicles for drug delivery (58).
Among the lipid-based systems for drug delivery,
lipid nanoparticles based on hydrogenated soybean
L-α-phosphatidylcholine (HSPC),
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene
glycol)-2000] (DSPE-mPEG2000), and cholesterol loaded
with cisplatin and mifepristone (L-Cis/MF) have been developed
(Fig. 2A and B). In in vitro experiments, these
nanoparticles have exhibited cytotoxic effects on CC cells.
Moreover, when tested in vivo, these nanoparticles have
decreased tumor growth, demonstrating that this lipid system loaded
with L-Cis/MF enhances the effect of chemotherapy (59). Cationic liposomes are not an
appropriate delivery system in in vivo models because, when
injected systemically, they are quickly degraded from the
bloodstream, which limits their ability to reach their target site.
For this reason, incorporating a flexible polymer such as PEG into
liposomes can significantly prolong circulation time (60).
Liu et al, developed an SNL loaded with both
paclitaxel and TOS-cisplatin and marked it with a signal peptide
(TAT) to specifically target the nanoparticles to CC cells. They
demonstrated that these nanoparticles exhibited enhanced
cytotoxicity against CC cells compared with the individual drug
formulations alone. Co-administration of both drugs using the
nanoparticles resulted in cell growth inhibition, induction of
programmed cell death, and increased antitumor efficacy in mouse
models of CC (61). These findings
suggest that this formulation strategy could improve conventional
therapy in the treatment of CC (62).
7. Advances in targeted therapy with
lipid-based systems
A propensity for nuclease-mediated degradation and
their low cellular uptake are the disadvantages of targeted nucleic
acid therapies (38). These
challenges have been addressed in several ways, including the
chemical modification of siRNA molecules. In addition, nanoparticle
delivery systems based on lipids, polymers, and inorganic
platforms, have been implemented to protect RNAs from cellular
nucleases and facilitate the passage through target cell membranes
(46).
The lipid-based therapy systems have led to
significant advancements, particularly in the local vaginal
administration of siRNAs for the treatment of CC (Fig. 2A). A notable study conducted by
Lechanteur et al (63),
demonstrated the efficacy of liposomes designed with a composition
of 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) as a cationic
lipid, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE),
cholesterol, and ceramide-PEG2000 coupled with a mixture
of E6, E7, and MCL-1 siRNAs. These liposomes targeted the
oncoproteins associated with HPV 16 and HPV 18 carcinogenesis,
confirming successful silencing of these oncoproteins in cell lines
positive for the respective HPV types (Fig. 2B). The coating of lipoplexes with
20% ceramide-PEG2000 has emerged as a significant
advancement in siRNA delivery, particularly for HPV-positive cells.
This coating enables the efficient release of active siRNA into the
cytoplasm of the cell, leading to desirable biological responses.
Furthermore, the inclusion of PEG prevents the aggregation of mucin
proteins within lipoplexes, ensuring that their size remains at
~200 nm (64). Similarly,
DOTAP-based lipoplexes with PEG2000, loaded with E6
siRNA (Lipoplexes-PEG-HPV16 E6), significantly reduced HPV16 E6
protein levels. By contrast, p53 protein expression was restored,
resulting in the inhibition of carcinogenic processes such as
proliferation, migration, and cell invasion in CaSki cells. This
demonstrates that this system is a viable option to complement CC
treatment (38).
Another alternative to improve conventional CC
treatment has been the development of therapies based on the use of
siRNAs (Fig. 2A), such as the
development of vaginal suppositories based on SLNs composed of
Gelucire®, DOTAP, Tween 80®/Span
85® loaded with paclitaxel, siRNA-Bcl-2, and
paclitaxel/siRNA-Bcl-2 (Fig. 2B).
In addition, researchers demonstrated that SLNs loaded with Bcl-2
siRNA have higher toxicity at lower doses than SLNs loaded with
paclitaxel alone. Additionally, SNLs loaded with Bcl-2
siRNA/paclitaxel provided greater apoptosis of paclitaxel-resistant
cells, with advantages such as a reduction in the paclitaxel dose
entering the systemic circulation, cytotoxic reduction, protection
of enzymes present in serum and the possibility of being
self-administered without professional assistance (65).
Javadi et al, presented a compelling strategy
to treat CC by utilizing E6/E7 siRNAs transfected with
Lipofectamine® RNAiMAX (66). This approach successfully led to
decreased cell viability and increased apoptosis in CC cell lines.
Additionally, the study evaluated the synergistic effect of
combining these siRNAs with anti-miR-182. This combination resulted
in a significant increase in apoptosis and a reduction in cell
viability. The combined action of these agents suggests their
potential as promising therapeutic candidates for CC treatment.
Finally, they evaluated the simultaneous use of cisplatin and E6/E7
siRNAs and demonstrated that cell viability was dramatically lower
in CaSki cells than in those treated with cisplatin alone (66). Similarly, it has been documented
that the use of lipid-based systems coupled with HPV16 E6-siRNA
targeting the E6 oncoprotein of HPV 16 significantly decreased the
expression of the E6 oncoprotein and restored the expression of the
p53 protein, leading to a significant reduction in migration and
invasion in CC-positive cells (38). This demonstrates that lipid-based
systems could be considered a viable therapy option.
8. Enhanced therapeutic approaches with
polymeric systems
Advancements in medical treatments have increasingly
turned to polymeric systems to enhance therapeutic efficacy. These
systems, composed of natural, synthetic, or semisynthetic polymers,
offer unique advantages such as improved biocompatibility and
biodegradability (67). Among
natural polymers, hyaluronan, albumin, gelatin, alginate, collagen,
and chitosan have been experimentally tested, and among synthetic
polymers, PEG, polylactic acid (PLA), and polyglycolic acid (PGA)
are mentioned in literature as potential carriers. Occasionally PLA
and PGA are used as copolymers (PLGA) (68,69).
Polymeric-based system, PLGA, has regained significant interest
since it is a biodegradable polymer that has been used as a vehicle
for drug delivery since its approval by the FDA as a copolymer
(39). These polymeric systems
enhance the stability and control the release of therapeutic agents
while minimizing adverse effects, thereby paving the way for more
effective and safer medical interventions.
In 2020, Xu et al (70), developed a PLGA-based nanosystem
(si/PNPs@HeLa) to improve CC
therapy. Using this system, they encapsulated paclitaxel and an
HPV18 siRNA-E7, which was subsequently coated with a HeLa cell
membrane to mimic the membranes of the target cells. The
utilization of HeLa cell membranes not only enhanced the
selectivity of the nanosystem but also inhibited the immune
response, resulting in improved specificity of delivery to the
target tissue, suggesting that E7 siRNA could improve
paclitaxel-induced resistance by inhibiting the activation of the
AKT pathway; thus, the synergistic effect between paclitaxel and E7
siRNA resulted in an improved CC remission and suppression in an
in vivo model (70).
The development of novel drug delivery systems for
cancer treatment represents an opportunity to evaluate the
synergistic effect of different medications, with the potential to
create particles that allow for the specific and efficient delivery
of multiple drugs. Accordingly, nanoparticles based on
dioleoylphosphatidic acid (DOPA) and PEG-PLGA loaded with curcumin
and SN38 have been developed and have demonstrated increased
apoptosis in HeLa and A2780 cells (41), as well as antitumor effects in
xenograft models of colorectal cancer (40). Another polymeric system that has
demonstrated antitumor therapeutic potential due to its high
cellular internalization capacity, easy release of doxorubicin, and
low cytotoxicity is based on poly-γ-glutamic acid (γ-PGA) and
cholesterol-NH2 (γ-PGA/Chol) particles loaded with Dox (Fig. 2A). These advancements underscore the
promise of polymeric systems in revolutionizing cancer treatment by
enabling targeted and efficient drug delivery, thereby enhancing
therapeutic outcomes while minimizing side effects (Fig. 2B) (71).
9. Inorganic-based systems for CC
therapy
Inorganic systems have demonstrated high efficiency
for the transport and delivery of various molecules in cancer
therapy. Inorganic nanoparticles are tiny particles that exhibit
unique and enhanced physical and chemical properties depending on
their size (42). Among inorganic
materials, the use of mesoporous silica nanoparticles, graphene
oxide, black phosphorus, and gold nanoparticles is notable. Their
advantages include high efficiency in drug loading and release,
maintaining their structure intact in the bloodstream, and their
biocompatibility (43,44). Building on these advantages,
specific applications in CC therapy are being explored. For
instance, selenium nanoparticles conjugated with both RGDfC peptide
(which is highly expressed in cancer cells) and Derlin1-siRNA
(Fig. 2A and B), resulting in RGDFC-Se@siRNA are being experimentally
tested. This combination was revealed to suppress proliferation,
migration and invasion, and promote the apoptosis of HeLa cells. In
addition, this nanosystem was highly effective at inhibiting CC
cells; thus, this alternative is a promising strategy for the
treatment of CC (45).
Another novel drug delivery proposal is based on
anionic clay-based systems [layered double hydroxide (LDH)]. The
design of this system was based on the
Mg0.655Al0.344(OH)2[(MTX)0.107CL0.128]·0.1H2O
for the delivery of methotrexate (LDH-MTX), which was assessed in
an orthotopic CC model of C33A cells, and an antitumor effect was
demonstrated; therefore, LDH-MTX has been proposed as a promising
alternative in chemical treatment due to its low toxicity both
in vitro and in vivo (46). To summarize, the advancement of
inorganic systems for drug delivery marks substantial progress in
CC therapy. These systems exhibit high efficacy in targeting and
suppressing cancer cell growth, and the potential to minimize
toxicity, making them promising candidates for future clinical
applications.
10. Functional nanoparticle therapy
Other promising systems for admixing drugs and/or
molecules are nanoparticle systems, which involve the conjugation
of molecules on the surface of the nanoparticles (72). For instance, the optimization of
arsenic trioxide encapsulated in folate-mediated liposomes has
demonstrated enhanced efficacy in the in vitro treatment of
HPV-positive CC cells. The results indicated that small-sized and
negatively charged liposomes, along with the incorporation of
folate as a specific targeting medium exhibit improved cell
internalization capacity and more efficient release of arsenic
trioxide into cancer cells. This enhances the selectivity of the
treatment and augments the inhibitory effects on cell growth.
However, further studies, including in vivo models, are
necessary to validate and assess the feasibility of this approach
in a real clinical setting (47,48).
Organic-inorganic nanohybrid niosomes have been developed as
another alternative for drug delivery in CC; for example,
nanoparticles based on iron oxide, PGLA with PEG, folic acid and
turmeric (F3O4@PGLA-PEG@FA/Cur) have
been shown to increase the efficiency of cell internalization in
vitro and increase the rate of apoptosis in CC cells,
suggesting that folate-conjugated nanoparticles (niosomes) could be
used to identify tumor cell surface receptors, thereby promoting
drug bioavailability and improving the rate of tumor inhibition
(49).
The search for novel and efficient drug alternatives
for the treatment of cancer has allowed the search for natural
extracts as therapeutic agents with the ability to inhibit the
growth of cancer cells specifically but not normal cells; as an
alternative, the use of monomyristin (MM), a monoacylglycerol found
in saw palmetto, has been explored (50). Long-chain monoglyceride derivatives
can induce programmed cell death in cancer cells. Changes in the
expression of genes related to cell proliferation and survival have
also been observed. The findings suggest that these compounds could
have potential as therapeutic agents in the treatment of CC
(51). However, due to their poor
water solubility, functional nanoparticles based on PLA and
hydrophobically modified dextrans, such as DexP26 or DexP26-COOH,
which encapsulate MM coated with transferrin (Tf) ligands, have
been developed for use in treating HeLa cells. These nanoparticles
have shown a cytotoxic effect on HeLa cells that was enhanced by Tf
conjugation, as it increases the efficiency and selectivity of MM
treatment. These results suggest that this encapsulation strategy
could hold promise for the development of more effective treatments
for CC (52). Another system for
the delivery of cisplatin involves the use of PLGA nanoparticles
coated with a lipid layer based on soy phosphatidylcholine,
DSPEG-PE and anti-VEGF (Avastin®)
(L-PLGA-Cis-Avatin®) for enhanced specificity (Fig. 2A), allowing increased
internalization of the particles in vitro and a decrease in
cell viability; moreover, in vivo, this system results in
greater accumulation in the tumor tissue and an inhibition of tumor
growth, demonstrating that this nanosystem could improve the
therapeutic efficacy of cisplatin by reducing its toxicity
(Fig. 2B) (39).
The use of systems for the delivery of different
molecules has enabled the drugs administered in conventional CC
therapy to reduce nephrotoxicity at higher doses compared with the
administration of free drugs such as LipoplatinTM
(57). These drug delivery systems
have reduced adverse effects such as leukopenia, neutropenia,
nausea, and asthenia in patients, providing a longer lifetime of
the drug in circulation, and limiting toxicity in normal cells vs.
the free administration of drugs (58,59).
Similarly, the use of non-lipidic nanoparticles for drug delivery
has been highlighted for its efficacy in inhibiting key cellular
processes related to carcinogenesis, improving the penetration of
the drug into the cellular interior, and considering this type of
nanoparticle as an effective anticancer therapeutic agent (60,62).
The various formulations described in the literature have been
developed specifically according to the molecules to be transported
(Fig. 2A and B); this specificity will allow the
internalization of these molecules to inhibit key cellular
processes in cervical carcinogenesis. This is the reason
nanosystems have been considered as a potential targeted therapy
against cancer (64). Regardless of
their base formulation, this allows a variety of alternatives in
cancer therapy to be considered to improve invasive treatments for
patients.
11. Limitations of nanoparticle use in
therapy
Nanoparticles have had a significant impact as
alternatives in CC therapy; however, they face several limitations
that restrict their use and efficiency. Among the main challenges
are biocompatibility and toxicity risks, difficulties in
large-scale production, stability and shelf-life issues,
inefficiencies in drug delivery, and the precise targeting of
therapies, all of which are addressed were addressed in the present
review. Another noteworthy limitation is that most studies
evaluating the effects of nanoparticles primarily focus on cellular
and animal models, which restricts the understanding and comparison
of their impact on humans. To overcome these limitations, it is
essential to increase the number of clinical studies, identify
biodegradable materials with low or no toxicity, develop efficient
and cost-effective production methods, enhance surface design to
improve biocompatibility and responsiveness to external stimuli,
and establish stricter regulatory protocols.
12. Conclusions and future perspectives
The use of and interest in nanotechnology has
allowed the development of nanoscale systems for the transport of
therapeutic agents in the area of medicine since most of the agents
used in traditional therapy are severely aggressive and have
several limitations both in terms of administration and
assimilation, as well as in terms of performing their functions,
which is the reason great interest has been placed in the design of
novel delivery systems that can be efficient to improve the
administration of molecules with specific targets.
One of the numerous advantages of these nanosystems
is the high degree of control over their physicochemical
characteristics, as well as the ability to transport various
molecules such as drugs, genes or other substances, which allows
them to be administered by various routes and safely internalized
into the target cell. Although there is evidence of toxicity in
some nanosystems, a good balance between this approach and the use
of nanomaterials guarantees an effective transport system with low
immunogenicity, greater safety, low toxicity, enhanced cargo, and
greater specificity for the site of injury.
Future research directions in nanotechnology should
focus on improving the biocompatibility of nanosystems and
mitigating potential toxicities, especially in the long term.
Additionally, strategies for targeted drug delivery should continue
to be refined for complex diseases, such as cancer or neurological
disorders, through approaches such as the use of specific ligands
or receptors. Exploring novel nanomaterials with greater stability,
lower immunogenicity, and enhanced delivery capabilities will be
key to advancing nanotechnology-based therapies. Scalable and
cost-effective manufacturing processes must also be developed to
ensure widespread clinical application. Finally, research should
explore the use of nanosystems in combination therapies to improve
treatment efficacy. These research areas are essential for
maximizing the potential of nanotechnology in enhancing drug
delivery and clinical outcomes.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Consejo Nacional de
Humanidades Ciencia y Tecnología de México (CONAHCYT; grant no.
FORDECYT-PRONACES/1717349/2020). LVSM was the recipient of doctoral
fellowships from CONAHCYT (grant no. 741489/CVU: 700387).
Availability of data and materials
Not applicable.
Authors' contributions
LVSM and JAVD performed the literature research and
preparation of tables. LVSM wrote the first draft and prepared the
figures. CBR, YGG, MALV, LEAA, JON and BIA contributed to the
writing, reviewing, and editing of the manuscript. JON and BIA
supervised the study and acquired the funding. All authors have
read and agreed to the published version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kahue CN, Jerrell RJ and Parekh A:
Expression of human papillomavirus oncoproteins E6 and E7 inhibits
invadopodia activity but promotes cell migration in HPV-positive
head and neck squamous cell carcinoma cells. Cancer Rep (Hoboken).
1(e1125)2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gonzalez SL, Stremlau M, He X, Basile JR
and Munger K: Degradation of the retinoblastoma tumor suppressor by
the human papillomavirus type 16 E7 oncoprotein is important for
functional inactivation and is separable from proteasomal
degradation of E7. J Virol. 75:7583–7591. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Narisawa-Saito M and Kiyono T: Basic
mechanisms of high-risk human papillomavirus-induced
carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci.
98:1505–1511. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Snijders PJ, Steenbergen RD, Heideman DA
and Meijer CJ: HPV-mediated cervical carcinogenesis: Concepts and
clinical implications. J Pathol. 208:152–164. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yugawa T and Kiyono T: Molecular
mechanisms of cervical carcinogenesis by high-risk human
papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev
Med Virol. 19:97–113. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Zhai L and Tumban E: Gardasil-9: A global
survey of projected efficacy. Antiviral Res. 130:101–109.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pal A and Kundu R: Human Papillomavirus E6
and E7: The cervical cancer hallmarks and targets for therapy.
Front Microbiol. 10(3116)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kumar L, Harish P, Malik PS and Khurana S:
Chemotherapy and targeted therapy in the management of cervical
cancer. Curr Probl Cancer. 42:120–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Lizano M, Berumen J and García-Carrancá A:
HPV-related carcinogenesis: Basic concepts, viral types and
variants. Arch Med Res. 40:428–434. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tan S, de Vries EG, van der Zee AG and de
Jong S: Anticancer drugs aimed at E6 and E7 activity in
HPV-positive cervical cancer. Curr Cancer Drug Targets. 12:170–184.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ozols RF, Bundy BN, Greer BE, Fowler JM,
Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM
and Baergen R: Gynecologic Oncology Group. Phase III trial of
carboplatin and paclitaxel compared with cisplatin and paclitaxel
in patients with optimally resected stage III ovarian cancer: A
gynecologic oncology group study. J Clin Oncol. 21:3194–3200.
2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chang JC, Wooten EC, Tsimelzon A,
Hilsenbeck SG, Gutierrez MC, Elledge R, Mohsin S, Osborne CK,
Chamness GC, Allred DC and O'Connell P: Gene expression profiling
for the prediction of therapeutic response to docetaxel in patients
with breast cancer. Lancet. 362:362–369. 2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen J, Gu W, Yang L, Chen C, Shao R, Xu K
and Xu ZP: Nanotechnology in the management of cervical cancer. Rev
Med Virol. 25 (Suppl 1):S72–S83. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nanoparticles. Vocabulary. British
Standards Institution, London, 2011. Available from: https://shop.bsigroup.com/ProductDetail/?pid=000000000030214797.
|
|
17
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN and Gaffney DK: Cervical cancer: A global health
crisis. Cancer. 123:2404–2412. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Balasubramaniam SD, Balakrishnan V, Oon CE
and Kaur G: Key molecular events in cervical cancer development.
Medicina (Kaunas). 55(384)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Burmeister CA, Khan SF, Schäfer G, Mbatani
N, Adams T, Moodley J and Prince S: Cervical cancer therapies:
Current challenges and future perspectives. Tumour Virus Res.
13(200238)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri: 2021 update. Int J
Gynaecol Obstet. 155 (Suppl 1):S28–S44. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pectasides D, Kamposioras K, Papaxoinis G
and Pectasides E: Chemotherapy for recurrent cervical cancer.
Cancer Treat Rev. 34:603–613. 2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gopu P, Antony F, Cyriac S, Karakasis K
and Oza AM: Updates on systemic therapy for cervical cancer. Indian
J Med Res. 154:293–302. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Types of cancer treatment [Internet]. 2021
[cited June 23 th, 2021]. Available from: https://www.cancer.gov/about-cancer/treatment/types.
|
|
24
|
Targeted Cancer Therapies. 2020 [cited
June 23 th, 2021]. Available from: https://www.cancer.gov/about-cancer/treatment/types/targeted-therapies.
|
|
25
|
Madaan K, Kumar S, Poonia N, Lather V and
Pandita D: Dendrimers in drug delivery and targeting:
Drug-dendrimer interactions and toxicity issues. J Pharm Bioallied
Sci. 6:139–150. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Monk BJ, Colombo N, Tewari KS, Dubot C,
Caceres MV, Hasegawa K, Shapira-Frommer R, Salman P, Yañez E, Gümüş
M, et al: First-Line pembrolizumab + chemotherapy versus placebo +
chemotherapy for persistent, recurrent, or metastatic cervical
cancer: Final overall survival results of KEYNOTE-826. J Clin
Oncol. 41:5505–5511. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zighelboim I, Wright JD, Gao F, Case AS,
Massad LS, Mutch DG, Powell MA, Thaker PH, Eisenhauer EL, Cohn DE,
et al: Multicenter phase II trial of topotecan, cisplatin and
bevacizumab for recurrent or persistent cervical cancer. Gynecol
Oncol. 130:64–68. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Suzuki K, Nagao S, Shibutani T, Yamamoto
K, Jimi T, Yano H, Kitai M, Shiozaki T, Matsuoka K and Yamaguchi S:
Phase II trial of paclitaxel, carboplatin, and bevacizumab for
advanced or recurrent cervical cancer. Gynecol Oncol. 154:554–557.
2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Scatchard K, Forrest JL, Flubacher M,
Cornes P and Williams C: Chemotherapy for metastatic and recurrent
cervical cancer. Cochrane Database Syst Rev.
10(Cd006469)2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Moon JY, Song IC, Ko YB and Lee HJ: The
combination of cisplatin and topotecan as a second-line treatment
for patients with advanced/recurrent uterine cervix cancer.
Medicine (Baltimore). 97(e0340)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Monk BJ, Mas Lopez L, Zarba JJ, Oaknin A,
Tarpin C, Termrungruanglert W, Alber JA, Ding J, Stutts MW and
Pandite LN: Phase II, open-label study of pazopanib or lapatinib
monotherapy compared with pazopanib plus lapatinib combination
therapy in patients with advanced and recurrent cervical cancer. J
Clin Oncol. 28:3562–3569. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Segovia-Mendoza M, González-González ME,
Barrera D, Díaz L and García-Becerra R: Efficacy and mechanism of
action of the tyrosine kinase inhibitors gefitinib, lapatinib and
neratinib in the treatment of HER2-positive breast cancer:
preclinical and clinical evidence. Am J Cancer Res. 5:2531–2561.
2015.PubMed/NCBI
|
|
33
|
Rini BI: Temsirolimus, an inhibitor of
mammalian target of rapamycin. Clin Cancer Res. 14:1286–1290.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kasper B and Hohenberger P: Pazopanib: A
promising new agent in the treatment of soft tissue sarcomas.
Future Oncol. 7:1373–1383. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hamberg P, Verweij J and Sleijfer S:
(Pre-)clinical pharmacology and activity of pazopanib, a novel
multikinase angiogenesis inhibitor. Oncologist. 15:539–547.
2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gilbert WV and Nachtergaele S: mRNA
regulation by RNA modifications. Annu Rev Biochem. 92:175–198.
2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gagliardi M: Novel biodegradable
nanocarriers for enhanced drug delivery. Ther Deliv. 7:809–826.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sánchez-Meza LV, Bello-Rios C, Eloy JO,
Gómez-Gómez Y, Leyva-Vázquez MA, Petrilli R, Bernad-Bernad MJ,
Lagunas-Martínez A, Medina LA, Serrano-Bello J, et al: Cationic
liposomes carrying HPV16 E6-siRNA inhibit the proliferation,
migration, and invasion of cervical cancer cells. Pharmaceutics.
16(880)2024.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Dana P, Bunthot S, Suktham K, Surassmo S,
Yata T, Namdee K, Yingmema W, Yimsoo T, Ruktanonchai UR,
Sathornsumetee S and Saengkrit N: Active targeting liposome-PLGA
composite for cisplatin delivery against cervical cancer. Colloids
and Surfaces B: Biointerfaces. 196(111270)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu G, Shi C, Guo D, Wang L, Ling Y, Han X
and Luo J: Functional-segregated coumarin-containing telodendrimer
nanocarriers for efficient delivery of SN-38 for colon cancer
treatment. Acta Biomater. 21:85–98. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li X and Gao Y: Synergistically fabricated
polymeric nanoparticles featuring dual drug delivery system to
enhance the nursing care of cervical cancer. Process Biochem.
98:254–261. 2020.
|
|
42
|
Paul W and Sharma CP: Inorganic
nanoparticles for targeted drug delivery. In: Biointegration of
Medical Implant Materials (2nd edition). Sharma CP (ed). Woodhead
Publishing, Cambridge, pp333-373, 2020.
|
|
43
|
Shi Z, Zhou Y, Fan T, Lin Y, Zhang H and
Mei L: Inorganic nano-carriers based smart drug delivery systems
for tumor therapy. Smart Mater Med. 1:32–47. 2020.
|
|
44
|
Wang W, Wang J and Ding Y: Gold
nanoparticle-conjugated nanomedicine: Design, construction, and
structure-efficacy relationship studies. J Mater Chem B.
8:4813–4830. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xia Y, Tang G, Wang C, Zhong J, Chen Y,
Hua L, Li Y, Liu H and Zhu B: Functionalized selenium nanoparticles
for targeted siRNA delivery silence Derlin1 and promote antitumor
efficacy against cervical cancer. Drug Deliv. 27:15–25.
2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Choi G, Piao H, Alothman ZA, Vinu A, Yun
CO and Choy JH: Anionic clay as the drug delivery vehicle: Tumor
targeting function of layered double hydroxide-methotrexate
nanohybrid in C33A orthotopic cervical cancer model. Int J
Nanomedicine. 20:337–348. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Akhtar A, Wang SX, Ghali L, Bell C and Wen
X: Effective delivery of arsenic trioxide to HPV-positive cervical
cancer cells using optimised liposomes: A size and charge study.
Int J Mol Sci. 4(1081)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Akhtar A, Ghali L, Wang SX, Bell C, Li D
and Wen X: Optimisation of folate-mediated liposomal encapsulated
arsenic trioxide for treating HPV-positive cervical cancer cells in
vitro. Int J Mol Sci. 20(2156)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
You L, Liu X, Fang Z, Xu Q and Zhang Q:
Synthesis of multifunctional Fe3O4@PLGA-PEG nano-niosomes as a
targeting carrier for treatment of cervical cancer. Mater Sci Eng C
Mater Biol Appl. 94:291–302. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shimada H, Tyler VE and McLaughlin JL:
Biologically active acylglycerides from the berries of saw-palmetto
(Serenoa repens). J Nat Prod. 60:417–418. 1997.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rongpan S, Phonnok S, Boondireke S,
Tripinyopap N and Wongsatayanon B: Anti-proliferative effect of
long-chain monoglyceride derivatives on human cervical carcinoma
cell line. J Med Assoc Thai. 100 (Suppl 8):S165–S172. 2017.
|
|
52
|
Boondireke S, Léonard M, Durand A and
Thanomsub Wongsatayanon B: Encapsulation of monomyristin into
polymeric nanoparticles improved its in vitro antiproliferative
activity against cervical cancer cells. Colloids Surf B
Biointerfaces. 176:9–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Song X, Li X, Tan Z and Zhang L: Recent
status and trends of nanotechnology in cervical cancer: A
systematic review and bibliometric analysis. Front Oncol.
14(1327851)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Bakrania A, Zheng G and Bhat M:
Nanomedicine in hepatocellular carcinoma: A new frontier in
targeted cancer treatment. Pharmaceutics. 14(41)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhou X, Lian H, Li H, Fan M, Xu W and Jin
Y: Nanotechnology in cervical cancer immunotherapy: Therapeutic
vaccines and adoptive cell therapy. Front Pharmacol.
13(1065793)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Devi S, Giri J, Makki E and Suryavanshi
MR: Navigating the novel nanoparticles: Current insights,
innovations, and future vistas in detection and treatment of
cervical cancer. Nanocomposites. 10:256–282. 2024.
|
|
57
|
Canta A, Chiorazzi A, Carozzi V, Meregalli
C, Oggioni N, Sala B, Crippa L, Avezza F, Forestieri D, Rotella G,
et al: In vivo comparative study of the cytotoxicity of a liposomal
formulation of cisplatin (lipoplatin™). Cancer Chemother Pharmacol.
68:1001–1008. 2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xu B, Zeng M, Zeng J, Feng J and Yu L:
Meta-analysis of clinical trials comparing the efficacy and safety
of liposomal cisplatin versus conventional nonliposomal cisplatin
in nonsmall cell lung cancer (NSCLC) and squamous cell carcinoma of
the head and neck (SCCHN). Medicina (Baltimore).
97(e13169)2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ledezma-Gallegos F, Jurado R, Mir R,
Medina LA, Mondragon-Fuentes L and Garcia-Lopez P: Liposomes
co-encapsulating cisplatin/mifepristone improve the effect on
cervical cancer: In vitro and in vivo assessment. Pharmaceutics.
12(897)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Menon S, Jayakodi S, Yadav KK, Somu P,
Isaq M, Shanmugam VK, Chaitanyakumar A and Basavegowda N:
Preparation of paclitaxel-encapsulated bio-functionalized selenium
nanoparticles and evaluation of their efficacy against cervical
cancer. Molecules. 27(7290)2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Liu B, Han L, Liu J, Han S, Chen Z and
Jiang L: Co-delivery of paclitaxel and TOS-cisplatin via
TAT-targeted solid lipid nanoparticles with synergistic antitumor
activity against cervical cancer. Int J Nanomedicine. 12:955–968.
2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hernández-Esparza MJ, Fratoddi I, Cerra S,
Juarez-Moreno K and Huirache-Acuña R: Hybrid AuNPs-3MPS-MTX
nanosystem and its evaluation for treating cervical cancer and
melanoma. Nanoscale Adv. 5:7077–7085. 2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Lechanteur A, Furst T, Evrard B, Delvenne
P, Piel G and Hubert P: Promoting vaginal distribution of E7 and
MCL-1 siRNA-silencing nanoparticles for cervical cancer treatment.
Mol Pharm. 14:1706–1717. 2017.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Adeyemi SA, Az-Zamakhshariy Z and Choonara
YE: In vitro prototyping of a nano-organogel for thermo-sonic
intra-cervical delivery of 5-fluorouracil-loaded solid lipid
nanoparticles for cervical cancer. AAPS PharmSciTech.
24(123)2023.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Reddy TL, Garikapati KR, Reddy SG, Reddy
BV, Yadav JS, Bhadra U and Bhadra MP: Simultaneous delivery of
Paclitaxel and Bcl-2 siRNA via pH-Sensitive liposomal nanocarrier
for the synergistic treatment of melanoma. Sci Rep.
6(35223)2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Javadi H, Lotfi AS, Hosseinkhani S,
Mehrani H, Amani J, Soheila SZ, Hojati Z and Kamali M: The
combinational effect of E6/E7 siRNA and anti-miR-182 on apoptosis
induction in HPV16-positive cervical cells. Artif Cells Nanomed
Biotechnol. 46 (Suppl 2):S727–S736. 2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Cervical Cancer Treatment by Stage. 2023
[cited january 27, 2023]. Available from: https://www.cancer.gov/types/cervical/stages.
|
|
68
|
Kokka F, Bryant A, Brockbank E and
Jeyarajah A: Surgical treatment of stage IA2 cervical cancer.
Cochrane Database Syst Rev. 2014(CD010870)2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Guimarães YM, Godoy LR, Longatto-Filho A
and Reis RD: Management of early-stage cervical cancer: A
literature review. Cancers (Basel). 14(575)2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Xu C, Liu W, Hu Y, Li W and Di W:
Bioinspired tumor-homing nanoplatform for co-delivery of paclitaxel
and siRNA-E7 to HPV-related cervical malignancies for synergistic
therapy. Theranostics. 7:3325–3339. 2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cho SH, Hong JH, Noh YW, Lee E, Lee CS and
Lim YT: Raspberry-like poly(γ-glutamic acid) hydrogel particles for
pH-dependent cell membrane passage and controlled cytosolic
delivery of antitumor drugs. Int J Nanomedicine. 11:5621–5632.
2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Thiruppathi R, Mishra S, Ganapathy M,
Padmanabhan P and Gulyás B: Nanoparticle functionalization and its
potentials for molecular imaging. Adv Sci (Weinh).
4(1600279)2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Muhammad T, Amjad AC and Anup K: Cancer
Chemotherapy. StatPearls Publishing, Treasure Island, 2023.
|
|
74
|
How Chemotherapy Drugs Work [Internet].
2019 [cited November 22, 2019]. Available from: https://www.cancer.org/cancer/managing-cancer/treatment-types/chemotherapy/how-chemotherapy-drugs-work.html.
|
|
75
|
Ghasemiyeh P and Mohammadi-Samani S: Solid
lipid nanoparticles and nanostructured lipid carriers as novel drug
delivery systems: Applications, advantages and disadvantages. Res
Pharm Sci. 13:288–303. 2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Amini S, Salehi H, Setayeshmehr M and
Ghorbani M: Natural and synthetic polymeric scaffolds used in
peripheral nerve tissue engineering: Advantages and disadvantages.
Polym Adv Technol. 32:2267–2289. 2021.
|
|
77
|
Degli Esposti L, Carella F and Iafisco M:
Inorganic nanoparticles for theranostic use. In:
Electrofluidodynamic Technologies (EFDTs) for Biomaterials and
Medical Devices. Guarino V and Ambrosio L (eds). Woodhead
Publishing, Cambridge, pp351-376, 2018.
|
















