1. Introduction
Cardiovascular disease (CVD) is the leading cause of
global mortality, and its prevention and treatment thus represent a
major public health challenge. The World Health Organization has
estimated that ~17.9 million deaths worldwide were due to CVD in
2019, with 85% resulting from acute events such as myocardial
infarction and stroke (1). Low- and
middle-income countries account for three-quarters of global
CVD-related mortality, with an estimated one-third of these deaths
occurring in individuals #x003C;70 years of age (1). Pathologically, CVD encompasses a
spectrum of noncommunicable diseases, including coronary artery
disease, cerebrovascular disease, and peripheral arterial disease,
which are driven primarily by modifiable risk factors such as
unhealthy diet, a lack of physical activity, tobacco use, and
obesity (1). Despite
pharmacological advances, current interventions face significant
limitations. For instance, while statins are effective for managing
dyslipidemia, they carry risks of myotoxicity and rhabdomyolysis
(2,3). Similarly, although dual antiplatelet
therapy reduces mortality from myocardial infarction, it is
associated with an increased risk of bleeding complications. These
challenges are compounded by the multifactorial pathogenesis of
CVD, necessitating therapeutic strategies that target multiple
interconnected pathways, such as the use of anti-inflammatory,
antioxidant, and cardioprotective agents (3). The effectiveness of conventional
chemical agents is limited by their single-target mechanisms,
potential for drug resistance, and risk of myotoxicity, while
antiplatelet therapies are also linked to increased hemorrhagic
risk (4). Within this context,
natural products from traditional medicinal formulations have
re-emerged as promising candidates for multimodal intervention.
Honokiol, a hydrophobic allyl biphenol compound
isolated from the traditional Chinese herb Magnolia
officinalis, represents a prime candidate in this quest
(5). As one of the major bioactive
components of Magnolia officinalis, its concentration serves
as a key indicator for evaluating the quality of the herb (6). Beyond its established antimicrobial,
antiviral, anticancer, anti-inflammatory, antioxidative, and
anti-aging properties, honokiol has been found to have significant
pharmacological effects on the cardiovascular system, including
cardioprotection, enhancement of cardiac function, vasodilation,
and antihypertensive effects, inhibition of platelet aggregation,
and anti-atherosclerotic properties (7-9).
These multifaceted properties and effects on cardiovascular health
suggest the potential of developing honokiol as a novel therapeutic
agent, opening new avenues in the treatment of CVD. The present
review focused on the functions and associated mechanisms of
honokiol in CVD, with an anlysis of its clinical applications and
challenges. The aim was to provide new directions for research on
the use of honokiol in clinical practice.
2. Structure and function of honokiol
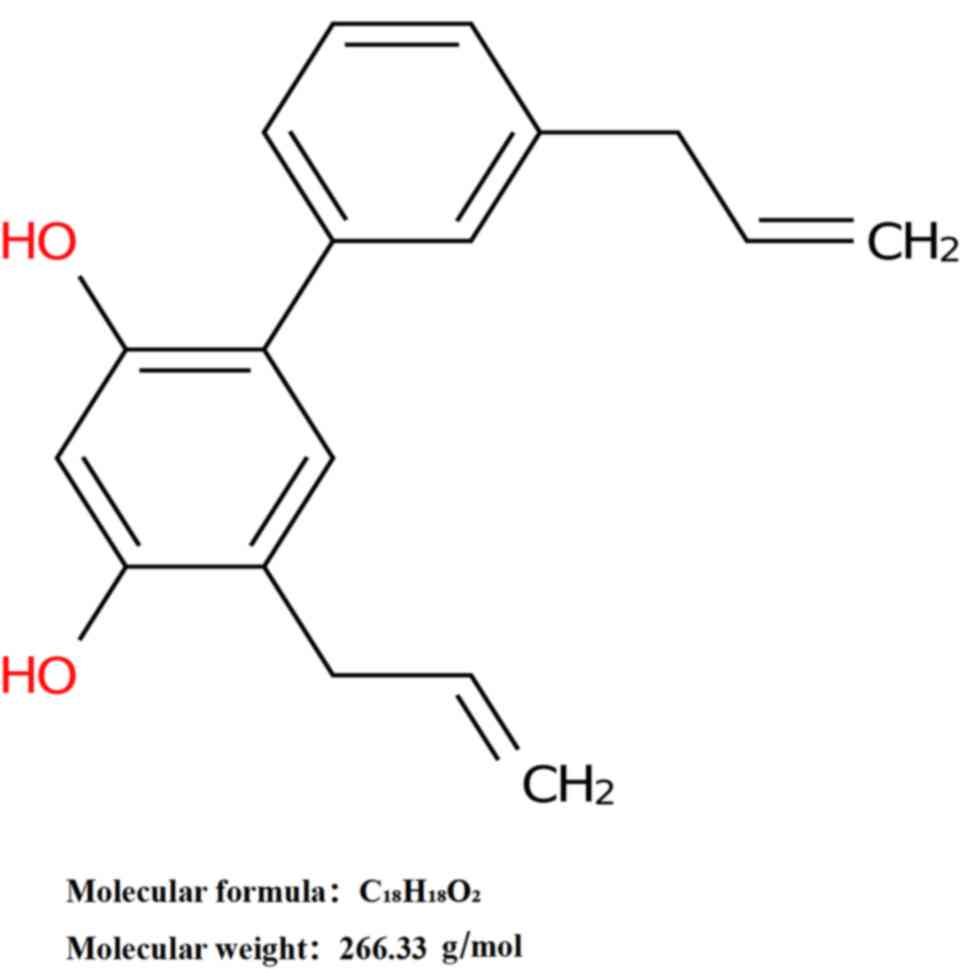
Honokiol, chemically designated as
3',5-di-2-propenyl-1,1'-biphenyl-2,4'-diol, has a molecular formula
of C18H18O2 and a molecular weight
of 266.33 g/mol (Fig. 1). In
appearance, this hydrophobic allyl biphenol compound is a white to
off-white crystalline powder with a melting point of ~87.5˚C
(8). While it has limited
solubility in water, it is highly soluble in organic solvents such
as ethanol, diethyl ether, and chloroform (8). The chemical structure of honokiol is
characterized by the presence of two phenolic hydroxyls and two
allyl groups. This distinctive configuration contributes to the
variety of biological activities of the compound (8). The phenolic hydroxyl moieties act as
hydrogen donors, conferring significant antioxidant activity and
enabling the scavenging of excessive free oxygen radicals and thus
reducing oxidative stress-mediated damage in cells and tissues
(9). Concurrently, the allyl groups
contribute to various chemical reactions involved in antibacterial,
anti-inflammatory, and antitumor activities (9).
Pharmacological analyses of honokiol have
demonstrated its wide range of biological activities (9). Honokiol has antimicrobial properties,
inhibiting the growth of various pathogenic microorganisms,
including Staphylococcus aureus, Escherichia coli, and
Candida albicans. Its antimicrobial effects primarily
involve the disruption of the cell membrane of the microorganism
and interference with transport and energy functions, thereby
reducing both antimicrobial and bactericidal/fungicidal effects
(10). In terms of its
anti-inflammatory properties, honokiol can inhibit the production
of inflammatory mediators such as tumor necrosis factor-alpha
(TNF-α) and interleukin (IL)-6(11). It also regulates
inflammation-related signaling pathways, including that of nuclear
factor kappa B (NF-κB) and reduces inflammatory cell infiltration,
thus inducing anti-inflammatory effects (12). As an effective antioxidant, honokiol
can scavenge and eliminate free radicals, such as superoxide anions
and hydroxyl radicals, directly, and can also upregulate the
activities of intracellular antioxidant enzymes, including
superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px),
thereby enhancing the antioxidant defense mechanisms of the body
(13). Furthermore, honokiol has a
variety of other biological activities, including antitumor,
antiviral, anti-anxiety, and sleep-promoting effects (8,9,14,15).
These biological activities are potentially linked
to pharmacological effects on the cardiovascular system.
Inflammation and oxidative stress play pivotal roles in the onset
and progression of CVD (16,17).
For instance, the formation of atherosclerotic plaques is closely
associated with inflammatory cell infiltration and the production
of oxidized low-density lipoproteins (18). The anti-inflammatory and antioxidant
activities of honokiol can mitigate damage to vascular endothelial
cells, suppress inflammatory responses, and reduce lipid oxidation,
thereby exerting a protective effect on the cardiovascular system.
Additionally, honokiol can block calcium channels, and can thus
modulate calcium ion concentrations in cardiomyocytes (19) and vascular smooth muscle cells
(20), influencing both the
contractile and diastolic functions of the heart, as well as
vascular tension. These findings provide a theoretical basis for
its application in the treatment of CVD.
3. Biological activity of honokiol
Honokiol exhibits a broad spectrum of biological
activities relevant to the therapeutic management of CVDs (21) (Fig.
2). These activities including antioxidant, anti-inflammatory,
vasodilatory, anti-thrombotic, anti-platelet, and cardioprotective
effects, highlight the value of studying honokiol to develop
improved strategies for managing CVDs.
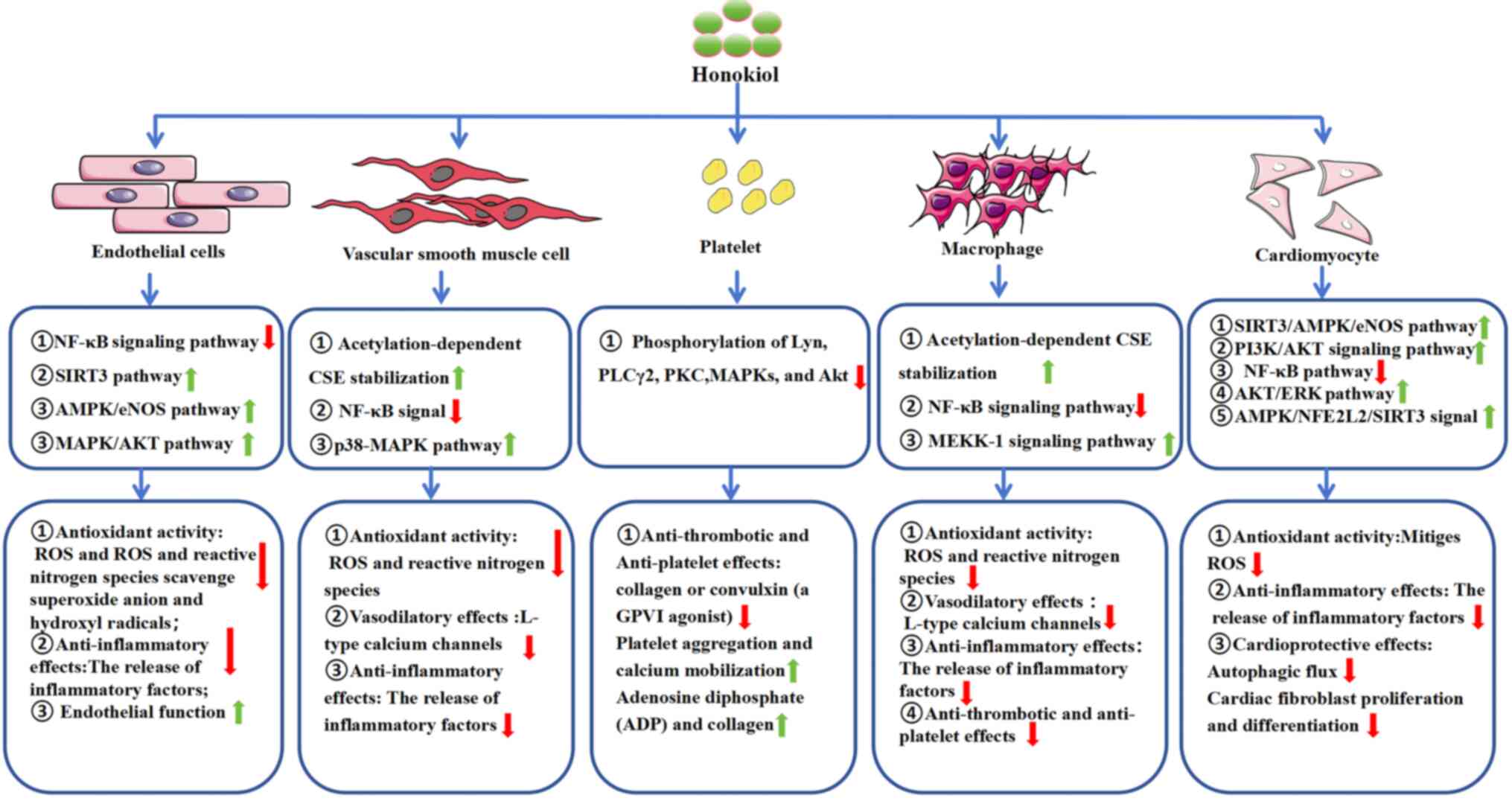 | Figure 2Biological activities of honokiol.
NF-κB, Nuclear factor kappa-B; SIRT3, sirtuin 3; AMPK, adenosine
5'-monophosphate-activated protein kinase; eNOS, endothelial nitric
oxide synthase; CSE, cystathionine γ lyase; MAPK, mitogen-activated
protein kinase; PLCγ2, phospholipase C γ2; PKC, protein kinase C;
AKT, protein kinase B; MEKK-1, mitogen-activated protein kinase
kinase 1; ERK, extracellular regulated protein kinases; NFE2L2,
nuclear factor erythroid-derived 2-like 2. |
Antioxidant activity
A key attribute of honokiol is its potent
antioxidant capacity. Oxidative stress, caused by the loss of
appropriate balance between reactive oxygen species (ROS) and
antioxidant defense systems, is a major contributor to the onset of
CVDs such as atherosclerosis (22),
myocardial infarction (23), and
heart failure (24). Honokiol
exhibits potent antioxidant activity through its ability to
scavenge both ROS and reactive nitrogen species (RNS), including
superoxide anions (O2-), hydroxyl radicals
(·OH), and peroxynitrites (ONOO-). Its unique
ortho-dihydroxy structural motif enables the efficient capture of
free radicals (2.5 radicals per molecule), thereby disrupting lipid
peroxidation. In a linoleic acid emulsion model, honokiol was found
to block lipid peroxidation 15-20% more effectively than under
control conditions, attributed to its ability to stabilize lipid
membranes and suppress free radical propagation. This mechanism
reduces the amount of oxidative damage to cellular organelles,
extracellular matrices, and genomic DNA, as evidenced by decreased
levels of malondialdehyde (MDA) and 8-hydroxy-2'-deoxyguanosine
adducts in treated samples (25).
It can help to scavenge free radicals, including superoxide anions
and hydroxyl radicals, and it suppresses the biogenesis of ROS
within cells (22). Moreover,
honokiol was found to preserve mitochondrial function by inhibiting
Fe(III)-adenosine diphosphate (ADP)/NADPH- and
Fe(III)-ADP/NADH-induced lipid peroxidation, thereby sustaining the
activities of respiratory enzymes and maintaining mitochondrial
redox homeostasis. Honokiol treatment was observed to significantly
reduce MDA levels in myocardial mitochondria in spontaneously
hypertensive rat models while simultaneously increasing plasma
nitrite/nitrate
(NO2-/NO3-) levels,
ultimately improving nitric oxide (NO) metabolism (13). In a model of sodium fluoride
(NaF)-induced neurotoxicity, activation of the AMPK/PGC-1α/SIRT3
signaling axis restored the activity of SOD2, mitochondrial DNA
transcription, and ATP synthesis, thereby reversing synaptic damage
and cognitive deficits (26); these
effects were mediated by honokiol activation of the
AMPK/NFE2L2/SIRT3 signaling pathway, promoting AMPK phosphorylation
and nuclear translocation of nuclear factor erythroid 2-related
factor 2 (Nrf2) (22). This process
led to an upregulation of sirtuin 3 (SIRT3) expression, thereby
enhancing the activities of mitochondrial manganese superoxide
dismutase (MnSOD) and catalase (CAT) and thus reducing ROS
accumulation. Concurrently, the Keap1/Nrf2/ARE axis was found to
promote the expression of the antioxidant enzyme glutathione (GSH),
resulting in further reductions in both ROS accumulation and
apoptosis (27). These data
collectively demonstrate that honokiol enhances the activity of
antioxidant enzymes such as SOD and CAT, strengthening the ability
of the body to defend against oxidative stress. Consistent with
these findings, it is reasonable to infer that these properties are
particularly valuable in combating oxidative damage associated with
endothelial dysfunction, plaque formation, and myocardial
injury.
Anti-inflammatory effects
Chronic inflammation plays a central role in various
cardiovascular conditions, including atherosclerosis, hypertension,
and heart failure (28-30).
Honokiol has been demonstrated to effectively inhibit
pro-inflammatory signaling pathways such as the NF-κB (31), mitogen-activated protein kinase
(MAPK) (32), and silent
information regulator SIRT3(33)
pathways. By suppressing NF-κB activation, honokiol can reduce the
expression of inflammatory cytokines and mediators such as TNF-α,
IL-6, IL-1β, inducible NO synthase (iNOS), cyclooxygenase-2 (COX2),
vascular cell adhesion molecule 1, and intercellular adhesion
molecule-1, all of which are pivotal in vascular inflammation and
atherosclerosis (34).
Additionally, honokiol has been shown to inhibit inflammatory
enzymes, including COX2 and NOS, further mitigating inflammatory
responses (35).
Anti-thrombotic and anti-platelet
effects
Platelet aggregation and thrombus formation are both
central to the pathophysiology of stroke, myocardial infarction,
and other facets of ischemic heart disease. Honokiol has been
demonstrated to inhibit both of these processes (36). It is capable of suppressing platelet
aggregation and the formation of thromboxane A2, which mediates
vasoconstriction and platelet function (37). Honokiol was observed to selectively
inhibit platelet aggregation and calcium mobilization induced by
collagen or convulxin [a glycoprotein VI (GPVI) agonist],
preventing the phosphorylation of Lyn, phosholipase C γ2, protein
kinase C, MAPKs, and AKT following convulxin stimulation, reducing
binding of an anti-GPVI antibody (FITC-JAQ1) to human platelets,
and significantly prolonging closure time in human whole-blood
assays while increasing the occlusion time in murine models of
thrombotic platelet thrombosis. These findings demonstrate the
potential of honokiol as a novel anti-platelet and anti-thrombotic
agent (38). Experimental studies
have shown that honokiol can prevent platelet aggregation by
reducing numerous agonists, such as ADP and collagen, emphasizing
its potential utility as a therapeutic agent for limiting the
incidence of thrombosis-related cardiovascular episodes (36).
Vasodilatory effects and protection of
endothelial function
The maintenance of optimal blood pressure and organ
perfusion hinges on the effective regulation of vascular tonus
(39). Honokiol has been been shown
to enhance vascular function through a variety of mechanisms,
suggesting a promising avenue for the management of hypertension
and ischemic heart disease. By stimulating the release of NO from
endothelial cells, honokiol promotes vasodilation, as NO is a
potent vasodilator that increases cyclic guanosine monophosphate
levels and blocks L-type calcium channels, inhibiting calcium ion
influx and relaxing vascular smooth muscle, thereby reducing
vascular resistance and lowering blood pressure (40). Furthermore, honokiol has been
demonstrated to enhance endothelial relaxation by activation of
SIRT3, particularly under conditions of severe inflammation. As a
specific activator of SIRT3, honokiol was shown to promote the
SIRT3-dependent AMPK/eNOS pathway, restoring phosphorylation of
eNOS at the Ser1177 site (41).
This process not only induced NO generation but also improved
endothelium-dependent vasodilation, ultimately restoring
microcirculatory homeostasis (41).
In addition, honokiol mitigated reduced vasodilation
in the aorta and mesenteric arteries, thereby improving
endothelium-dependent vasodilation in a rat model of type 2
diabetes. Specifically, honokiol maintained the ratio of
phosphorylated to total eNOS and increased the expression of CD31,
which is typically reduced in diabetic rats. These findings suggest
that honokiol may prevent diabetes-induced atherosclerosis by
protecting the vascular endothelium and activating SIRT3, thereby
enhancing the overall health and function of the endothelium
(42). Honokiol has also been found
to play a significant role in the regulation of vascular tonus,
improvements in endothelial function, and the prevention of
vascular dysfunction through multiple mechanisms. Activation of
SIRT3 was shown to promote the generation of NO, and this, together
with the inhibition of inflammation and oxidative stress,
collectively improve vascular function and endothelial health
(42). This multifaceted approach
offers new strategies and hope for the treatment of CVD,
highlighting the potential of honokiol as a valuable therapeutic
agent.
Cardioprotective effects
Honokiol exhibits significant cardioprotective
effects, particularly in ischemic heart diseases such as myocardial
infarction and reperfusion injury (43). Its multifaceted mechanisms,
including the reduction of oxidative stress, inflammation, and
apoptosis, contribute to its efficacy in minimizing myocardial
damage (44,45).
Experimental studies have demonstrated that honokiol
reduces infarct size, prevents cardiomyocyte apoptosis, and
improves cardiac function post-ischemia (44,45).
These effects are mediated primarily through the activation of
survival signaling pathways, inhibition of apoptotic cascades, and
enhancement of mitochondrial function. Honokiol achieves these
benefits by boosting autophagic flux and modulating the PI3K/AKT
signaling pathway (44).
Additionally, it has been shown to reduce the levels of cardiac
fibrosis following ischemia, thereby preserving myocardial
structure and function (45).
The cardioprotective effects of honokiol are further
substantiated by its ability to promote the expression of the
cardiac protective protein uncoupling protein 3 (UCP3), as well as
its maintenance of the mitochondrial membrane potential. These
effects reduce the production and accumulation of ROS following
myocardial infarction, thereby mitigating myocardial fibrosis and
improving heart failure outcomes (24). Honokiol was also found to block
agonist-induced and pressure overload-mediated cardiac hypertrophy
in mice, and these anti-hypertrophic effects were observed to be
dependent on the activation of SIRT3(46). By enhancing the expression and
activity of SIRT3, honokiol treatment resulted in reduced
acetylation of SIRT3 substrates in the mitochondria, such as MnSOD
and oligomycin sensitivity conferring protein (47). This resulted in enhanced
mitochondrial respiration and reduced ROS synthesis in wild-type,
but not in SIRT3-knockout, cells. Moreover, honokiol inhibited the
proliferation of cardiac fibroblasts and their differentiation into
myofibroblasts in a SIRT3-dependent manner (47).
In summary, the comprehensive cardioprotective
functions of honokiol suggest its potential as a promising
therapeutic candidate for ischemic heart diseases. Its ability to
reduce oxidative stress, inflammation, and apoptosis, while
enhancing mitochondrial function and autophagy, underscores its
potential in mitigating myocardial damage and improving cardiac
outcomes. The activation of SIRT3 and modulation of the PI3K/AKT
pathway further consolidate its role in preventing cardiac
hypertrophy and fibrosis.
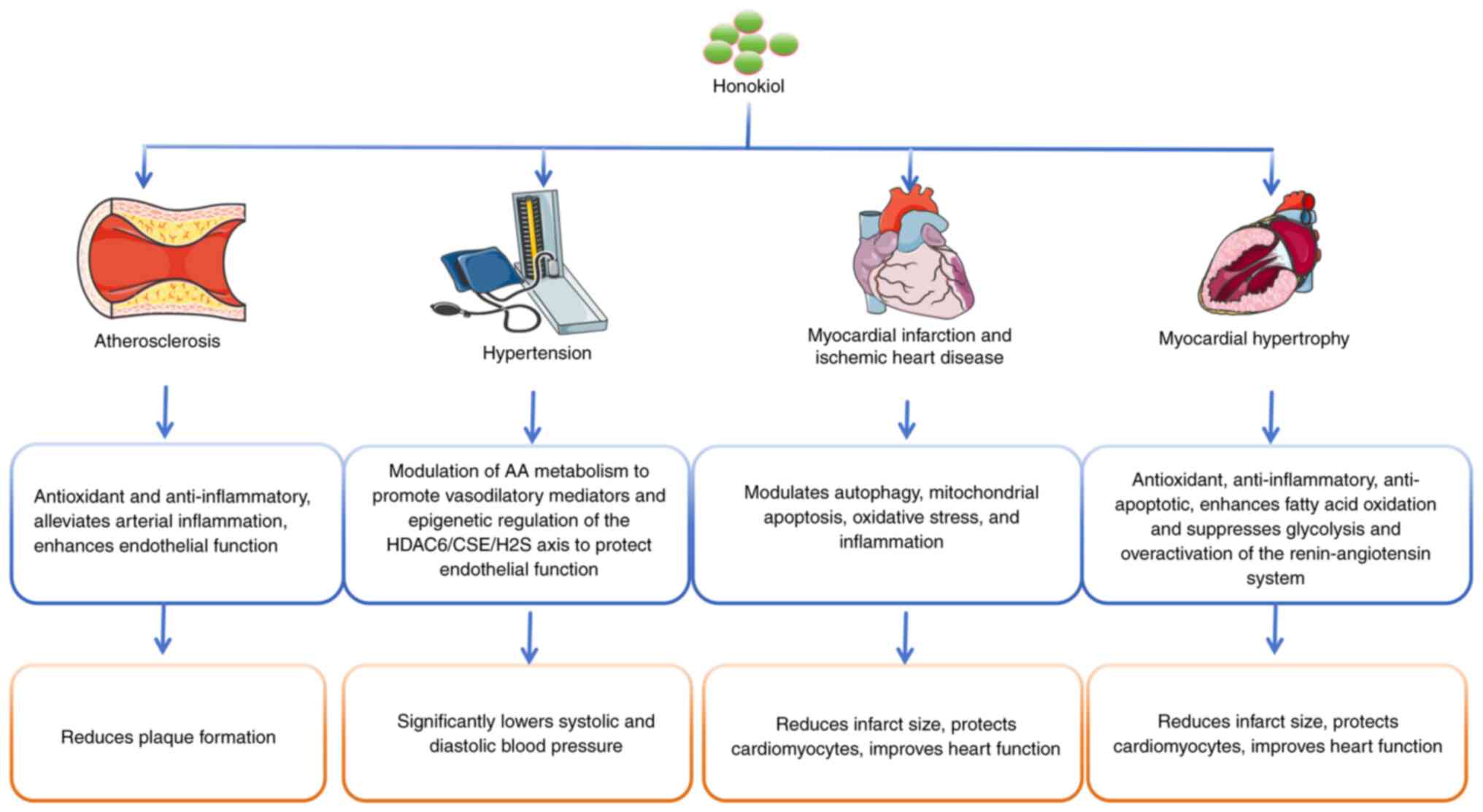
4. Research progress of honokiol in
cardiovascular diseases
Honokiol has garnered increasing attention in
cardiovascular medicine due to its diverse pharmacological
properties. This section offers an overview of the most up-to-date
findings discussing the benefits of honokiol in different
cardiovascular conditions (Fig.
3).
Honokiol in atherosclerosis
Atherosclerosis is characterized by chronic
inflammation, oxidative stress, and endothelial dysfunction, with
honokiol providing a means of targeting all of these deleterious
features (48). Research indicates
that honokiol can limit plaque formation, reduce arterial
inflammation, and enhance endothelial function (11). Zhu et al observed that
honokiol regulated proliferation and migration in rat aortic smooth
muscle cells induced by TNF-α. This involved blocking the
activation of NF-κB through suppression of the ERK signaling
pathway (34). Preclinical
investigations using animal models (such as ApoE-/-
mice) have demonstrated marked reductions in atherosclerotic plaque
size together with improved arterial function, attributed to the
antioxidant and anti-inflammatory properties of honokiol (22).
Honokiol in hypertension
Hypertension management is vital for reducing
cardiovascular morbidity and mortality. Preclinical studies have
shown that honokiol effectively decreases both systolic and
diastolic blood pressure (39,40).
In spontaneously hypertensive rats, oral administration of honokiol
(30 mg/kg/day) significantly reduced systolic blood pressure by 28%
over 4 weeks by the reprogramming of arachidonic acid (AA)
metabolism. This involved the downregulation of COX-2 and
5-lipoxygenase (LOX), critical enzymes in pro-inflammatory
eicosanoid biosynthesis, while upregulating the expression of
12/15-LOX leading to increased production of the vasoprotective
metabolite lipoxin A4. Concurrently, honokiol suppressed
leukotriene B4 generation, thereby reducing inflammatory
vasoconstriction (39).
In a complementary study using angiotensin II (Ang
II)-induced hypertensive mice, honokiol (10 mg/kg/day) was observed
to attenuate systolic hypertension by 22% through a novel
epigenetic mechanism. Honokiol was found to inhibit histone
deacetylase 6 (HDAC6)-mediated ubiquitination and proteasomal
degradation of cystathionine γ-lyase (CSE), rate-limiting enzymes
in the biosynthesis of hydrogen sulfide (H2S). Increased
CSE activity restored H2S bioavailability, which in turn
preserved the phosphorylation of eNOS and reduced oxidative stress,
thereby ameliorating endothelial dysfunction (40).
Collectively, these findings demonstrate that
honokiol exerts antihypertensive effects through several pathways,
namely, the modulation of AA metabolism to promote vasodilatory
mediators and epigenetic regulation of the HDAC6/CSE/H2S
axis to protect endothelial function. These complementary
mechanisms highlight the potential of honokiol as a multi-target
therapeutic agent for hypertension, with implications for both
blood pressure control and vascular protection.
Honokiol in myocardial infarction and
ischemic heart disease
The cardioprotective properties of honokiol have
been extensively explored in both acute and chronic myocardial
infarction contexts. A study by Liu et al (24) investigated the therapeutic potential
of honokiol in ameliorating heart failure following myocardial
infarction. Using a mouse model of myocardial infarction, it was
demonstrated that honokiol treatment led to a marked improvement in
cardiac function, as evidenced by an increased ejection fraction
and reduced left ventricular dilation. Mechanistically, honokiol
upregulated the expression of UCP3, leading to reduced ROS
generation and thus preventing oxidative stress (24). This effect was associated with
preserved mitochondrial integrity and enhanced antioxidant capacity
in cardiomyocytes. Furthermore, honokiol treatment reduced the
levels of pro-inflammatory cytokines (such as TNF-α and IL-6) and
inhibited apoptosis-related pathways, contributing to reduced
myocardial fibrosis and cardiomyocyte death (24). The study highlighted UCP3-mediated
ROS inhibition as a critical mechanism underlying the
cardioprotective effects of honokiol, suggesting its potential as a
therapeutic agent for heart failure after myocardial
infarction.
In addition to its role in myocardial infarction,
honokiol also has cardioprotective effects in ischemic heart
disease. Tan et al (43)
reported that honokiol treatment ameliorated myocardial
ischemia/reperfusion injury by increasing autophagic flux and
reducing ROS production. These protective effects were linked to
the suppression of oxidative stress-related pathways (including
NF-κB), coupled with the preservation of mitochondrial function via
inhibition of mitochondrial permeability transition pore (mPTP)
opening. Furthermore, honokiol alleviated myocardial
ischemia/reperfusion injury by activating the PI3K/AKT signaling
pathway, which inhibits mitochondrial apoptosis. Specifically,
phosphorylation of downstream targets (such as GSK-3β) suppressed
mPTP opening, thereby reducing cardiomyocyte apoptosis and
improving the recovery of cardiac function (44). Moreover, Wang et al (45) described the dual actions of honokiol
in cardioprotection as i) antioxidant effects induced by reducing
lipid peroxidation markers (such as MDA) and ROS generation, and
ii) anti-inflammatory effects induced by downregulation of
pro-inflammatory cytokines (including TNF-α and IL-6).
Additionally, honokiol may enhance myocardial tolerance to hypoxia
by improving both microcirculation and energy metabolism (45). Collectively, these studies
underscore the multifaceted role of honokiol in myocardial
ischemia/reperfusion injury through the modulation of autophagy,
mitochondrial apoptosis, oxidative stress, and inflammation,
offering a promising therapeutic avenue for ischemic heart
disease.
Honokiol in myocardial
hypertrophy
Recent research has demonstrated that honokiol can
effectively mitigate Ang II-induced cardiac hypertrophy by
disrupting the nuclear receptor 77 (Nur77)-liver kinase B1 (LKB1)
complex and activating the AMPK pathway (49). Under Ang II stimulation, Nur77 binds
to LKB1, leading to its sequestration in the nucleus and impairment
of its cytoplasmic role as an upstream activator of AMPKα, a
critical regulator of energy homeostasis and suppressor of
hypertrophy. Honokiol promotes the dissociation of the Nur77-LKB1
complex, facilitating LKB1 translocation to the cytoplasm, where it
phosphorylates AMPKα at Thr172(49). Activated AMPKα inhibits mTOR/p70S6K
signaling, which is a key driver of protein synthesis and
cardiomyocyte growth, while restoring metabolic balance by
enhancing fatty acid oxidation and suppressing glycolysis.
Furthermore, honokiol has also been found to exhibit
multi-target anti-hypertrophic effects by attenuating Ang
II-induced activation of the NF-κB and MAPK pathways, reducing the
levels of inflammatory cytokines (such as TNF-α) and oxidative
stress (40,50). It also downregulates the levels of
angiotensin-converting enzyme and the Ang II receptor type 1
receptor, thereby suppressing overactivation of the
renin-angiotensin system. In vivo studies using Ang
II-infused mice revealed that honokiol could significantly reduce
the thickness of the left ventricular wall, the cross-sectional
area of cardiomyocytes, and the levels of fibrosis markers such as
collagen deposition. In vitro experiments with neonatal
cardiomyocytes confirmed that honokiol reversed Ang II-induced
hypertrophy, an effect abolished by AMPK inhibition or LKB1
knockdown, underscoring the centrality of this pathway (49).
These findings highlight the potential of honokiol
as a dual-target agent that disrupts pathological protein
interactions (Nur77-LKB1) and restores AMPK-mediated metabolic
regulation. In contrast to conventional therapies that primarily
lower blood pressure, honokiol specifically addresses maladaptive
cardiac remodeling without compromising hemodynamics, offering a
safer profile. These findings indicate the advantages of the
clinical translation of natural compounds targeting
energy-sensitive pathways in CVD.
5. Prospects of honokiol in cardiovascular
diseases
Honokiol has emerged as a promising therapeutic
agent for CVD due to its diverse pharmacological properties. Its
ability to exert anti-inflammatory, antioxidant, and cytoprotective
effects makes it a versatile candidate for the management of
various CVD-related pathologies. A key advantage of honokiol is its
cardioprotective ability, particularly in the alleviation of
myocardial ischemia/reperfusion injury. By activating the Nrf2/HO-1
pathway, honokiol promotes cellular resistance to oxidative stress,
a critical contributor to both atherosclerosis and heart failure
(26,27). Additionally, its anti-atherogenic
effects, such as the alleviation of endothelial dysfunction,
inhibition of low-density lipoprotein (LDL) oxidation, and the
prevention of foam cell formation, highlight its potential in
slowing plaque progression (11,48).
Honokiol also has vasoprotective benefits, primarily
through its upregulation of eNOS, which promotes vasodilation and
improves blood pressure regulation (39-42).
This mechanism is particularly relevant for patients with
hypertension and those with metabolic disorders, where endothelial
dysfunction is prevalent. Furthermore, honokiol exhibits
anti-arrhythmic properties through its ability to modulate cardiac
ion channels, thereby stabilizing electrical activity and reducing
the risk of ventricular arrhythmias (45). Its anti-fibrotic effects further aid
in the prevention of adverse myocardial remodeling, making it a
potential therapeutic option for atrial fibrillation and
post-infarction complications. Another notable feature is the
anti-thrombotic activity of honokiol, associated with suppression
of platelet aggregation without increasing the risk of bleeding, a
significant advantage over traditional antiplatelet drugs (36-38).
Its dual role in mitigating inflammation (via NF-κB and NLRP3
inflammasome inhibition) (51,52)
and enhancing metabolic homeostasis (via AMPK/SIRT1 activation)
further underscores its potential in treating complex CVDs
(13). Given its multi-targeted
mechanisms and natural origin, honokiol holds promise as a
complementary or alternative therapy for CVDs, particularly in
patients with multifactorial conditions.
A 2022 patent (patent no. CN114588136A) described
the preparation of honokiol-based medications for the treatment of
myocardial infarction (53). In
mice, honokiol use was found to significantly improve cardiac
function for 4 consecutive weeks (Table
I) (53). When honokiol was
used in ischemic models, it was found that the levels of lactate
dehydrogenase increased while apoptosis decreased. This suggests
that honokiol may be effective for treating ischemia-related injury
(Table I) (53). Moreover, a traditional Chinese
medicinal formulation consisting of honokiol, pachymic acid, and
10-dehydroxygingerdione has been widely utilized in the treatment
of cardiovascular and cerebrovascular diseases (Table I) (54). Additionally, Li et al
(55) synthesized a novel compound
through the cyclization of metformin with honokiol (Table I), which showed significant efficacy
in combating CVD. Notably, it exhibited superior anti-inflammatory
effects in mouse models of early atherosclerosis when compared to
honokiol or metformin individually, indicating enhanced efficacy
and reduced toxicity (55). One
patent describes a class of honokiol derivatives or salts
containing C2 and C4'-phenol hydroxy substitutions for the
prevention and/or treatment of CVD (Table I) (56). In rat models, these derivatives
demonstrated significant cardioprotective effects against
stress-induced cardiomyopathy-related behaviors (Table I) (56). Recently, derivatives of honokiol
that incorporate amine groups on the aromatic ring have been
utilized in the formulation of drugs related to resistance to
myocardial ischemia, as well as in the composition of myocardial
protective medications (Table I)
(57). In rat models of myocardial
ischemia-reperfusion, the administration of compound 16 (1,500
µg/kg, delivered in two intravenous doses) notably decreased the
size of the myocardial infarcts and lowered the levels of serum
creatine kinase muscle-brain fraction and lactate dehydrogenase
(Table I) (57). In conclusion, honokiol holds
promising prospects for application in the prevention and treatment
of CVD.
 | Table IPatents related to enhancing the
therapeutic efficacy of honokiol in the treatment of cardiovascular
diseases. |
Table I
Patents related to enhancing the
therapeutic efficacy of honokiol in the treatment of cardiovascular
diseases.
| Patent number | Patent title | Main findings | (Refs.) |
|---|
| CN114588136A | Application of
honokiol in preparation of medicine for treating myocardial
infarction | Improves heart
function, reduces far-end myocardial fibrosis of an infarction
region and myocardial cell apoptosis of an infarction marginal
region. | (53) |
| CN109045045A | A kind of active
ingredient of Chinese herbs composition for treating cardiovascular
and cerebrovascular disease | This composition
can treat cardiovascular and cerebrovascular diseases, it can
markedly reduce the levels of TC, TG and LDL-C, and increase the
level of HDL-C. | (54) |
| CN108033927A | A kind of compound
and its preparation method and application | It exhibits
beneficial effects in anti-inflammatory, hypoglycemic,
lipid-lowering, antitumor, antibacterial, and cardio- and
cerebrovascular disease-related activities. | (55) |
| CN109771431A | The new application
of honokiol derivative | Preventing and/or
treating cardiovascular and cerebrovascular diseases. | (56) |
| CN115581690A | Application of
magnolol or aromatic ring amino substituted derivative of honokiol
in preparation of anti-myocardial ischemia drugs and pharmaceutical
composition | Myocardial infarct
size, serum CK-MB and LDH levels were significantly decreased. | (57) |
6. Challenges in the clinical application of
honokiol
Despite the therapeutic promise of honokiol, there
are several barriers that impede the clinical adoption of honokiol
in the treatment of CVD.
Limited bioavailability and
pharmacokinetic constraints
One of the primary obstacles is the poor water
solubility of honokiol, which restricts its oral absorption and
systemic bioavailability. In CVDs, where sustained therapeutic
levels are crucial, the rapid metabolism and short half-life of
honokiol further diminish its efficacy. Research has indicated that
honokiol undergoes extensive hepatic glucuronidation, leading to
rapid clearance and suboptimal plasma concentrations (52). While the use of nanoparticle-based
delivery systems and lipid formulations has been explored to
enhance bioavailability, their scalability, stability, and
cost-effectiveness remain unresolved issues for clinical use.
Insufficient clinical evidence in
human CVD
Despite compelling preclinical data showing the
ability of honokiol to reduce oxidative stress, inhibit vascular
smooth muscle proliferation, and improve endothelial function,
there are few clinical trials on its use in humans. Most studies
have been conducted in cultured cells or animal models, which may
not accurately reflect human pathophysiology (22-24).
For instance, while honokiol has shown potential in reducing
myocardial infarction size in rodents, its effects in human
ischemic heart disease remain unverified. Additionally, there have
been no systematic evaluations of optimal dosing, long-term safety,
and potential interactions with standard cardiovascular medications
(such as statins and antiplatelet drugs).
Mechanistic complexity and off-target
effects
Honokiol modulates multiple signaling pathways
relevant to CVDs, including the NF-κB, SIRT3, and PI3K/Akt
pathways, which contribute to its anti-inflammatory and
vasoprotective effects (42,58).
However, this pleiotropic activity raises concerns about potential
unintended effects, such as excessive modulation of blood pressure
or interference with coagulation pathways. For example, the
antiplatelet properties of honokiol could theoretically increase
the risk of bleeding when used in combination with aspirin or
clopidogrel. Further research is required to delineate its precise
mechanisms and identify potential adverse interactions in patients
with complex comorbidities.
Standardization and formulation
challenges
The lack of standardized honokiol extracts
complicates the reproducibility of clinical studies. Variations in
purity, extraction methods, and chemical composition in
preparations from different sources can lead to inconsistent
therapeutic outcomes. Moreover, the development of a stable,
scalable, and patient-friendly formulation (such as oral tablets or
injectables) for CVD management remains a hurdle. While intravenous
administration may bypass absorption issues, it introduces
practical limitations for chronic use in outpatient settings.
Regulatory and commercial hurdles
As a natural product, honokiol faces regulatory
ambiguities in terms of intellectual property and drug approval.
Unlike synthetic drugs, its natural origin complicates its
protection by patents, discouraging pharmaceutical investment.
Additionally, competition with unregulated dietary supplements,
which often lack rigorous quality control, undermines incentives
for its clinical development. To overcome these barriers,
collaborative efforts between academia, industry, and regulatory
agencies are essential to establish standardized protocols and
secure funding for large-scale trials.
7. Conclusion and outlook
Honokiol has demonstrated significant therapeutic
potential in the treatment of CVD through its multifaceted
mechanisms. It exerts cardioprotective effects by modulating
calcium channels, suppressing Ang II-induced myocardial
hypertrophy, and reducing ventricular remodeling. Its
anti-inflammatory and antioxidant properties are mediated through
inhibition of NF-κB signaling and enhancement of SOD/GSH-Px enzyme
activities, effectively mitigating oxidative stress in
atherosclerosis and myocardial ischemia. Furthermore, honokiol can
ameliorate metabolic dysregulation by lowering blood glucose and
lipid levels while inhibiting oxidized LDL formation, thereby
preventing diabetic cardiovascular complications. Additional
benefits include antiplatelet aggregation and vasodilation,
highlighting its utility in thrombosis prevention. Despite these
benefits, its translation to clinical use faces challenges.
Specifically, its hydrophobicity limits oral bioavailability,
necessitating advanced delivery systems such as lipid-based
nanoparticles, its multi-target interactions with pathways, such as
the Nrf2-SLC7A11-GSH and MAPK pathways, require precise mechanistic
elucidation to minimize off-target effects, and the lack of robust
clinical trials hinders validation of safety and efficacy in
humans. Future efforts should prioritize the development of
innovative formulations (such as polymer-stabilized
nanosuspensions), mechanistic exploration using single-cell and
organoid models to map spatiotemporal regulatory networks in
myocardial fibrosis, and investigation of combination therapies
with statins or immunomodulators to enhance synergistic effects.
Expanding its application to pulmonary hypertension and arrhythmia
could further broaden its therapeutic scope. With interdisciplinary
collaboration and evidence-based validation, honokiol is poised to
emerge as a precision treatment in CVD management, bridging the gap
between traditional medicine and modern pharmacotherapy.
Acknowledgements
Not applicable.
Funding
Funding: Funding for the present study was received from the
Natural Science Foundation of Guangdong Province (grant no.
2023A1515030033), and the Guangdong Basic and Applied Basic
Research Foundation (grant no. 2022A1515140185).
Availability of data and materials
Not applicable.
Authors' contributions
YZ and ZL contributed to the conception and design
of the review, and the literature collection. XL prepared the draft
of the manuscript. All authors contributed to manuscript revision,
and read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Podolec P and Matusik PT: New clinical
classification of rare cardiovascular diseases and disorders:
Relevance for cardiovascular research. Cardiovasc Res. 115:e77–e79.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kee PS, Chin PKL, Kennedy MA and Maggo
SDS: Pharmacogenetics of statin-induced myotoxicity. Front Genet.
11(575678)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vinci P, Panizon E, Tosoni LM, Cerrato C,
Pellicori F, Mearelli F, Biasinutto C, Fiotti N, Di Girolamo FG and
Biolo G: Statin-associated myopathy: Emphasis on mechanisms and
targeted therapy. Int J Mol Sci. 22(11687)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang J, Zou J, Shi Y, Zeng N, Guo D, Wang
H, Zhao C, Luan F, Zhang X and Sun J: Traditional Chinese medicine
and mitophagy: A novel approach for cardiovascular disease
management. Phytomedicine. 128(155472)2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen C, Zhang QW, Ye Y and Lin LG:
Honokiol: A naturally occurring lignan with pleiotropic
bioactivities. Chin J Nat Med. 19:481–490. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rauf A, Olatunde A, Imran M, Alhumaydhi
FA, Aljohani ASM, Khan SA, Uddin MS, Mitra S, Emran TB, Khayrullin
M, et al: Honokiol: A review of its pharmacological potential and
therapeutic insights. Phytomedicine. 90(153647)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Prasher P, Fatima R, Sharma M, Tynybekov
B, Alshahrani AM, Ateşşahin DA, Sharifi-Rad J and Calina D:
Honokiol and its analogues as anticancer compounds: Current
mechanistic insights and structure-activity relationship. Chem Biol
Interact. 386(110747)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dai SY, Qin WX, Yu S, Li C, Yang YH and
Pei YH: Honokiol and magnolol: A review of structure-activity
relationships of their derivatives. Phytochemistry.
223(114132)2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li X, Yuan Z, Wang Y, Wang W and Shi J:
Recent advances of honokiol: Pharmacological activities, manmade
derivatives and structure-activity relationship. Eur J Med Chem.
272(116471)2024.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang R, Cui L, Xu S, Zhong Y, Xu T, Liu J,
Lan Z, Qin S and Guo Y: Membrane-targeting amphiphilic honokiol
derivatives containing an oxazole moiety as potential
antibacterials against methicillin-resistant Staphylococcus aureus.
J Med Chem. 67:16858–16872. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu A, Xun S, Zhou G, Zhang Y and Lin L:
Honokiol alleviates sepsis-associated cardiac dysfunction via
attenuating inflammation, apoptosis and oxidative stress. J Pharm
Pharmacol. 75:397–406. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang N, Kong R, Han W, Bao W, Shi Y, Ye L
and Lu J: Honokiol alleviates ulcerative colitis by targeting
PPAR-γ-TLR4-NF-κB signaling and suppressing gasdermin-D-mediated
pyroptosis in vivo and in vitro. Int Immunopharmacol.
111(109058)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou Y, Tang J, Lan J, Zhang Y, Wang H,
Chen Q, Kang Y, Sun Y, Feng X, Wu L, et al: Honokiol alleviated
neurodegeneration by reducing oxidative stress and improving
mitochondrial function in mutant SOD1 cellular and mouse models of
amyotrophic lateral sclerosis. Acta Pharm Sin B. 13:577–597.
2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim H, Lim CY and Chung MS: Magnolia
officinalis and its honokiol and magnolol constituents inhibit
human norovirus surrogates. Foodborne Pathog Dis. 18:24–30.
2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Qu WM, Yue XF, Sun Y, Fan K, Chen CR, Hou
YP, Urade Y and Huang ZL: Honokiol promotes non-rapid eye movement
sleep via the benzodiazepine site of the GABA(A) receptor in mice.
Br J Pharmacol. 167:587–598. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ferrucci L and Fabbri E: Inflammageing:
Chronic inflammation in ageing, cardiovascular disease, and
frailty. Nat Rev Cardiol. 15:505–522. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shaito A, Aramouni K, Assaf R, Parenti A,
Orekhov A, Yazbi AE, Pintus G and Eid AH: Oxidative stress-induced
endothelial dysfunction in cardiovascular diseases. Front Biosci
(Landmark Ed). 27(105)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Marchio P, Guerra-Ojeda S, Vila JM,
Aldasoro M, Victor VM and Mauricio MD: Targeting early
atherosclerosis: A focus on oxidative stress and inflammation. Oxid
Med Cell Longev. 2019(8563845)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Aktay I, Bitirim CV, Olgar Y, Durak A,
Tuncay E, Billur D, Akcali KC and Turan B: Cardioprotective role of
a magnolol and honokiol complex in the prevention of
doxorubicin-mediated cardiotoxicity in adult rats. Mol Cell
Biochem. 479:337–350. 2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fan S, Li X, Lin J, Chen S, Shan J and Qi
G: Honokiol inhibits tumor necrosis factor-alpha-stimulated rat
aortic smooth muscle cell proliferation via caspase- and
mitochondrial-dependent apoptosis. Inflammation. 37:17–26.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yuan Y, Zhou X, Wang Y, Wang Y, Teng X and
Wang S: Cardiovascular modulating effects of magnolol and honokiol,
two polyphenolic compounds from traditional Chinese
medicine-Magnolia officinalis. Curr Drug Target. 21:559–572.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liu Y, Cheng P and Wu AH: Honokiol
inhibits carotid artery atherosclerotic plaque formation by
suppressing inflammation and oxidative stress. Aging (Albany NY).
12:8016–8028. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liou KT, Lin SM, Huang SS, Chih CL and
Tsai SK: Honokiol ameliorates cerebral infarction from
ischemia-reperfusion injury in rats. Planta Med. 69:130–134.
2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Liu J, Tang M, Li T, Su Z, Zhu Z, Dou C,
Liu Y, Pei H, Yang J, Ye H and Chen L: Honokiol ameliorates
post-myocardial infarction heart failure through Ucp3-mediated
reactive oxygen species inhibition. Front Pharmacol.
13(811682)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Zhao C and Liu ZQ: Comparison of
antioxidant abilities of magnolol and honokiol to scavenge radicals
and to protect DNA. Biochimie. 93:1755–1760. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang D, Cao L, Zhou X, Wang G, Ma Y, Hao X
and Fan H: Mitigation of honokiol on fluoride-induced mitochondrial
oxidative stress, mitochondrial dysfunction, and cognitive deficits
through activating AMPK/PGC-1α/Sirt3. J Hazard Mater.
437(129381)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Caballero EP, Mariz-Ponte N, Rigazio CS,
Santamaria MH and Corral RS: Honokiol attenuates oxidative
stress-dependent heart dysfunction in chronic Chagas disease by
targeting AMPK/NFE2L2/SIRT3 signaling pathway. Free Radical Biol
Med. 156:113–124. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin A, Miano JM, Fisher EA and Misra A:
Chronic inflammation and vascular cell plasticity in
atherosclerosis. Nat Cardiovasc Res. 3:1408–1423. 2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guzik TJ, Nosalski R, Maffia P and
Drummond GR: Immune and inflammatory mechanisms in hypertension.
Nat Rev Cardiol. 21:396–416. 2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Boulet J, Sridhar VS, Bouabdallaoui N,
Tardif JC and White M: Inflammation in heart failure:
Pathophysiology and therapeutic strategies. Inflamm Res.
73:709–723. 2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee J, Jung E, Park J, Jung K, Lee S, Hong
S, Park J, Park E, Kim J, Park S and Park D: Anti-inflammatory
effects of magnolol and honokiol are mediated through inhibition of
the downstream pathway of MEKK-1 in NF-kappaB activation signaling.
Planta Med. 71:338–343. 2005.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tang X, Yao K, Zhang L, Yang Y and Yao H:
Honokiol inhibits H2O2-induced apoptosis in
human lens epithelial cells via inhibition of the mitogen-activated
protein kinase and Akt pathways. Eur J Pharmacol. 650:72–78.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ye JS, Chen L, Lu YY, Lei SQ, Peng M and
Xia ZY: SIRT3 activator honokiol ameliorates
surgery/anesthesia-induced cognitive decline in mice through
anti-oxidative stress and anti-inflammatory in hippocampus. CNS
Neurosci Ther. 25:355–366. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhu X, Wang Z, Hu C, Li Z and Hu J: .
Honokiol suppresses TNF-α-induced migration and matrix
metalloproteinase expression by blocking NF-κB activation via the
ERK signaling pathway in rat aortic smooth muscle cells. Acta
Histochem. 116:588–595. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Murakami Y, Kawata A, Seki Y, Koh T,
Yuhara K, Maruyama T, Machino M, Ito S, Kadoma Y and Fujisawa S:
Comparative inhibitory effects of magnolol, honokiol, eugenol and
bis-eugenol on cyclooxygenase-2 expression and nuclear factor-kappa
B activation in RAW264.7 macrophage-like cells stimulated with
fimbriae of Porphyromonas gingivalis. In Vivo. 26:941–950.
2012.PubMed/NCBI
|
|
36
|
Montecino-Garrido H, Méndez D,
Araya-Maturana R, Millas-Vargas JP, Wehinger S and Fuentes E: In
vitro effect of mitochondria-targeted triphenylphosphonium-based
compounds (Honokiol, Lonidamine, and Atovaquone) on the platelet
function and cytotoxic activity. Front Pharmacol.
13(893873)2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Seok YM, Cho HJ, Cha BY, Woo JT and Kim
IK: Honokiol attenuates vascular contraction through the inhibition
of the RhoA/Rho-kinase signalling pathway in rat aortic rings. J
Pharm Pharmacol. 63:1244–1251. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Onselaer MB, Nagy M, Pallini C, Pike JA,
Perrella G, Quintanilla LG, Eble JA, Poulter NS, Heemskerk JWM and
Watson SP: Comparison of the GPVI inhibitors losartan and honokiol.
Platelets. 31:187–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Elbarbry F and Moshirian N: The modulation
of arachidonic acid metabolism and blood pressure-lowering effect
of honokiol in spontaneously hypertensive rats. Molecules.
27(3396)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chi Z, Le TPH, Lee SK, Guo E, Kim D, Lee
S, Seo SY and Lee SY, Kim JH and Lee SY: Honokiol ameliorates
angiotensin II-induced hypertension and endothelial dysfunction by
inhibiting HDAC6-mediated cystathionine γ-lyase degradation. J Cell
Mol Med. 24:10663–10676. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lv D, Luo M, Yan J, Yang X and Luo S:
Protective effect of sirtuin 3 on CLP-induced endothelial
dysfunction of early sepsis by inhibiting NF-κB and NLRP3 signaling
pathways. Inflammation. 44:1782–1792. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
He A, Yu H, Hu Y, Chen H, Li X, Shen J,
Zhuang R, Chen Y, Sasmita BR, Luo M and Lv D: Honokiol improves
endothelial function in type 2 diabetic rats via alleviating
oxidative stress and insulin resistance. Biochem Biophys Res
Commun. 600:109–116. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Tan Z, Liu H, Song X, Ling Y, He S, Yan Y,
Yan J, Wang S, Wang X and Chen A: Honokiol post-treatment
ameliorates myocardial ischemia/reperfusion injury by enhancing
autophagic flux and reducing intracellular ROS production. Chem
Biol Interact. 307:82–90. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Lv L, Kong Q, Li Z and Zhang Y, Chen B, Lv
L and Zhang Y: Honokiol provides cardioprotection from myocardial
ischemia/reperfusion injury (MI/RI) by inhibiting mitochondrial
apoptosis via the PI3K/AKT signaling pathway. Cardiovasc Ther.
2022(1001692)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wang Y, Zhang ZZ, Wu Y, Zhan J, He XH and
Wang YL: Honokiol protects rat hearts against myocardial ischemia
reperfusion injury by reducing oxidative stress and inflammation.
Exp Ther Med. 5:315–319. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pillai VB, Samant S, Sundaresan NR,
Raghuraman H, Kim G, Bonner MY, Arbiser JL, Walker DI, Jones DP,
Gius D and Gupta MP: Honokiol blocks and reverses cardiac
hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun.
6(6656)2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhong C, Wang C, Li W, Li W, Chen X, Guo
J, Feng Y and Wu X: A derivative of honokiol HM568 has an
anti-neuroinflammatory effect in Parkinson's disease. Chem Biol
Interact. 403(111212)2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Qiu L, Xu R, Wang S, Li S, Sheng H, Wu J
and Qu Y: Honokiol ameliorates endothelial dysfunction through
suppression of PTX3 expression, a key mediator of
IKK/IkappaB/NF-ĸB, in atherosclerotic cell model. Exp Mol Med.
47(e171)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lin X, Zhang H, Chu Y, Zhang Y, Xu C, Xie
H, Ruan Q, Lin J, Huang CK and Chai D: Honokiol ameliorates
angiotensin II-induced cardiac hypertrophy by promoting
dissociation of the Nur77-LKB1 complex and activating the AMPK
pathway. J Cell Mol Med. 28(e18028)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhu J, Ning RB, Lin XY, Chai DJ, Xu CS,
Xie H, Zeng JZ and Lin JX: Retinoid X receptor agonists inhibit
hypertension-induced myocardial hypertrophy by modulating
LKB1/AMPK/p70S6K signaling pathway. Am J Hypertens. 27:1112–1124.
2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Cai X, Jiang X, Zhao M, Su K, Tang M, Hong
F, Ye N, Zhang R, Li N, Wang L, et al: Identification of the target
protein and molecular mechanism of honokiol in anti-inflammatory
action. Phytomedicine. 109(154617)2023.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Huang PP, Fu J, Liu LH, Wu KF, Liu HX, Qi
BM, Liu Y and Qi BL: Honokiol antagonizes doxorubicin-induced
cardiomyocyte senescence by inhibiting TXNIP-mediated NLRP3
inflammasome activation. Int J Mol Med. 45:186–194. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Guo Y, Liu N, Zhang J, Yang J, Kang W, Lu
T, Guo Y and Yao Y: Application of honokiol in preparation of
medicine for treating myocardial infarction. China Patent
CN114588136A. Filed April 7, 2022; issued June 7, 2022.
|
|
54
|
Miao GP, Han J, Zhang JF, Tong G and Wu Y:
A kind of active ingredient of Chinese herbs composition for
treating cardiovascular and cerebrovascular disease China Patent
CN109045045A. Filed August 14, 2018; issued December 21, 2018.
|
|
55
|
Li WM, Feng YF, Zhu HN, Yu R and Li Y: A
kind of compound and its preparation method and application. China
Patent CN108033927A. Filed October 27, 2017; issued May 15,
2018.
|
|
56
|
Guang B, Yang T, Dong RH, Peng X, Liu I,
Xie J, Qin L, Xu G and Liao X: The new application of honokiol
derivative. China Patent CN109771431A. Filed January 31, 2019;
issued May 21, 2019.
|
|
57
|
Zhang P, Liu Y, Zhang Y and Gu J:
Application of magnolol or aromatic ring amino substituted
derivative of honokiol in preparation of anti-myocardial ischemia
drugs and pharmaceutical composition. China Patent CN115581690A.
Filed July 5, 2021; issued January 10, 2023.
|
|
58
|
Xian X, Zhao X, Zhou X, Liu H, Li C, Wu X,
Chen Y, Ye K, Yang H, Li M, et al: Honokiol attenuates oxidative
stress and vascular calcification via the upregulation of heme
oxygenase-1 in chronic kidney disease. Toxicol Appl Pharmacol.
499(117318)2025.PubMed/NCBI View Article : Google Scholar
|