Introduction
Thyroid cancer (THCA) is one of the most common head
and neck malignant tumors, and its incidence is increasing in China
(19.42/100,000). According to the assessment report released by the
International Agency for Research on Cancer of the World Health
Organization, there were >820 000 new cases of thyroid cancer
worldwide in 2022, ranking the seventh in the incidence of cancer
(1). THCA is a common malignancy in
women, characterized by high rates of recurrence, poor prognosis
and mortality due to metastasis and invasion (2). However, the molecular mechanisms
driving THCA progression remain poorly understood (3), highlighting the need for more
comprehensive research. Understanding these processes is crucial
for improving diagnostic and therapeutic strategies.
Rho GTPase activating protein 36 (ARHGAP36) has a
significant role in cell migration, cytoskeletal remodeling and
tumor progression (4). Rho GTPase
family members such as ARHGAP4 and ARHGAP9 have been shown to
promote tumor growth and metastasis via pathways such as mTOR and
hypoxia-inducible factor-1α (5,6).
Evidence has indicated that ARHGAP36 plays a crucial role in
tumorigenesis and progression in medulloblastoma and
pheochromocytoma (7). Although
ARHGAP36 contributes to tumorigenesis and progression, its role in
THCA is still unknown. Therefore, the present study aimed to
explore the role of ARHGAP36 in tumorigenicity in THCA.
In the present study, public databases [such as
GEPIA, The Cancer Genome Atlas (TCGA), TIMER and Metascape] were
utilized to explore the biological functions of ARHGAP36.
Functional in vitro assays were also conducted to evaluate
cell proliferation, migration and apoptosis. The findings of the
present study position ARHGAP36 as a potential therapeutic target
by mediating immune escape and promoting metastasis. Additionally,
the present study underscores the importance of further
investigation into the role of ARHGAP36 in THCA, potentially
leading to novel diagnostic markers and therapeutic strategies.
Materials and methods
Public database analyses
The differential expression of ARHGAP36 mRNA between
thyroid carcinoma and adjacent normal tissues was analyzed using
TCGA-THCA tumor data (8) and GTEx
normal tissue data (9) (accession
no. phs000424.v8.p2) through the GSEA website [www.gsea-msigdb.org (10)]. The limma R package was used for
statistical analysis and the Benjamini-Hochberg false discovery
rate (FDR) correction (significance threshold: |log2(fold
change)|>1 and FDR-adjusted P<0.05) was used as the
significance cut-off. The UALCAN database [http://ualcan.path.uab.edu (11)] was used to evaluate the expression
of ARHGAP36 in TCGA-THCA cohort across various factors, including
sample types, cancer stages, patient demographics, histological
characteristics and nodal metastasis status, applying one-way ANOVA
with Tukey's post-hoc test for multi-group comparisons and unpaired
Student's t-test for binary classifications. The LinkedOmics
database [www.linkedomics.org (12)] was used to identify genes correlated
with ARHGAP36 using Pearson's correlation coefficient, with |r|
>0.3 and FDR <0.5 defined as significant; the top 50
positively/negatively correlated genes were visualized in heatmaps.
The Metascape database [https://metascape.org (13)] was used to perform Gene Ontology
(GO) process enrichment analysis for ARHGAP36 and its associated
genes, highlighting key biological processes, via hypergeometric
testing with Benjamini-Hochberg correction, requiring a minimum
enrichment factor of 1.5, P<0.01 and FDR <0.05. The TIMER
database [https://cistrome.shinyapps.io/timer/ (14)] was used to investigate the
relationships between ARHGAP36 and tumor immune components,
including purity, B cells, CD8+ T cells, CD4+
T cells, macrophages, neutrophils and dendritic cells based on
TCGA-THCA RNA-seq data, employing a deconvoluted gene expression
algorithm with tumor purity-adjusted partial correlation.
Protein-protein interaction (PPI) analyses were performed by the
Metascape analysis tool. The TISCH database (http://tisch.comp-genomics.org/), a publicly available
resource that provides integrated single-cell RNA sequencing data
from multiple tumor types, including THCA. This platform allows for
cell-type-specific gene expression analysis and immune landscape
profiling.
Cell culture
BHT101 and BCPAP cells (obtained from The Cell Bank
of Type Culture Collection of The Chinese Academy of Sciences) were
cultured in DMEM [Ubi Biotech (Shanghai) Co., Ltd.] supplemented
with 10% fetal bovine serum [FBS; cat. no. U11-020A; Ubi Biotech
(Shanghai) Co., Ltd.]. The culture medium was further enriched with
1% penicillin-streptomycin solution and 0.25 µg/ml amphotericin B
to prevent contamination. Cells were maintained at 37˚C in a
humidified incubator with 5% CO2. When the cell density
reached 80-90% confluency, cells were detached using 0.25%
trypsin-EDTA solution and centrifuged at 111 x g at 37˚C for 5 min
to collect the cell pellet.
Small interfering (si)RNA
transfection
The specific siRNA targeting ARHGAP36 were designed
and synthesized (Shanghai Jima Pharmaceutical Technology Co., Ltd.)
to optimize knockdown efficiency. Prior to transfection, cells were
seeded into 6-well plates at a density of 5x105 cells/ml
with 1.5 ml of culture medium per well and incubated at 37˚C with
5% CO2 overnight to achieve 70-80% confluency. A total
of 16 h after seeding, cells were divided into two groups: A
negative control (NC) group and a si-ARHGAP36 group. Each siRNA was
diluted in Opti-MEM (Thermo Fisher Scientific, Inc) to a final
concentration of 100 nM in a 250 µl volume and incubated at room
temperature for 5 min. In a separate tube, 5 µl of Lipofectamine
2000 transfection reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) was added to 250 µl of Opti-MEM and incubated at room
temperature for 5 min. The diluted siRNA solution was then gently
combined with the Lipofectamine 2000 mixture and incubated at room
temperature for 20 min to allow for siRNA-lipid complex formation.
Next, 500 µl of this siRNA-lipid complex was carefully added to
each well containing the cells, followed by gentle rocking of the
plate to ensure even distribution. A total of 6 h
post-transfection, the medium was aspirated and 1.5 ml of fresh
complete medium was added to each well. The cells were collected 48
h after transfection for western blot analysis to assess the
ARHGAP36 protein expression levels and evaluate knockdown
efficiency. All procedures were conducted under sterile conditions
to prevent contamination, freshly prepared reagents were used to
ensure optimal transfection efficiency and strict adherence to
timing at each step was maintained to ensure reproducibility. The
siRNA sequences were as follows: ARHGAP36 siRNA, sense
5'-GCGGGUCAGCUCCGAGAAA-3' and antisense 5'-UUUUCGGUCAGGGGCCGC-3';
NC siRNA, sense 5'-UUCUCCGAACGUGUCACGU-3' and antisense,
5'-ACGUGACACGUUCGGAGAA-3'.
Western blot analysis
Cells were lysed using RIPA buffer (Thermo Fisher
Scientific, Inc.) supplemented with protease inhibitors to ensure
complete protein extraction. Protein concentrations were determined
using the BCA Protein Assay Kit (Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. For SDS-PAGE,
100 µg of total protein from each sample was separated on a 10%
polyacrylamide gel at a constant voltage of 120 V for 2 h. Proteins
were then transferred onto PVDF membranes (MilliporeSigma) using a
semi-dry transfer system at 25 V for 1 h. The membranes were then
blocked with 5% non-fat milk in 1X TBST (0.1% Tween) for 2 h at
room temperature to reduce non-specific binding. Subsequently, the
membranes were incubated overnight at 4˚C with primary antibodies
against ARHGAP36 (1:2,000; cat. no. PA5-31619; Thermo Fisher
Scientific, Inc.) and GAPDH (loading control; 1:2,000; cat. no.
ab8245; Abcam). After washing with TBST, the membranes were
incubated with horseradish peroxidase-conjugated anti-mouse
(1:5,000; cat. no. 58802; Cell Signaling Technology, Inc.) and
anti-rabbit (1:5,000; cat. no. 7074; Cell Signaling Technology,
Inc.) secondary antibodies for 1 h at room temperature. Protein
bands were visualized using an enhanced chemiluminescence detection
kit (Thermo Fisher Scientific, Inc.) and imaged with a Tanon 5200
system. Densitometric analysis of the blots was performed using
ImageJ software (v1.53; National Institutes of Health) to
semi-quantify the protein expression levels.
Plate colony formation assay
For the plate colony formation assay, BHT101 and
BCPAP cells (1x10³ per well) were seeded into 6-well plates and
allowed to adhere overnight. The next day, the medium was replaced
with DMEM containing 10% FBS and 1% penicillin-streptomycin. Cells
were cultured for 1 month at 37˚C in a humidified 5% CO2
incubator, with media changes every 3 days. At the end of the
culture period, colonies were fixed with ice-cold 100% methanol for
10 min, stained with 0.5% crystal violet solution for 20 min at
37˚C and then washed with distilled water. The plates were
air-dried and colonies consisting of >50 cells in each well were
manually counted.
Wound healing assay
To assess the migratory capacity of BHT101 and BCPAP
cells, a scratch wound healing assay was performed. Cells were
seeded at a density of 1x106 cells per well in 6-well
plates and cultured to confluency in DMEM supplemented with 10% FBS
and 1% penicillin-streptomycin. A uniform scratch was created using
a sterile 200 µl pipette tip and debris was removed by washing with
PBS. The medium was then replaced with DMEM containing 1% FBS to
minimize proliferation. Images of the scratch wounds were captured
immediately (0 h) and again at 24 h post-scratch using an inverted
light microscope. Wound closure was quantified by measuring the
distance between the edges of the scratch at multiple points, and
image analysis software (version 2.3; Olympus Corporation) was used
to calculate the average wound width and percentage of closure.
Migration and invasion assays
To evaluate cell migration and invasion, BHT101 and
BCPAP cells (2x105 in 200 µl serum-free DMEM) were
seeded into the top chamber of 24-well Transwell inserts with 8 µm
pores. The lower chamber contained 600 µl of DMEM with 10% FBS as a
chemoattractant. For invasion assays, the top chamber was
pre-coated with Matrigel at 4˚C overnight. After a 24-h incubation
at 37˚C in 5% CO2, the cells that migrated or invaded
through the membrane were fixed with 4% paraformaldehyde at room
temperature for 15 min. After fixation, the cells were stained with
0.5% crystal violet at 37˚C for 20 min. Images were captured under
an Olympus BX50 light microscope (Olympus Corporation). Migrated or
invaded cells were quantified by counting five random fields per
membrane.
Statistical analysis
Data were analyzed using Pearson's or Spearman's
tests for correlation analysis, and group differences were assessed
using an unpaired Student's t-test (GraphPad Prism v6.0;
Dotmatics). Results are presented as the mean ± SD, and P<0.05
was considered to indicate a statistically significant
difference.
Results
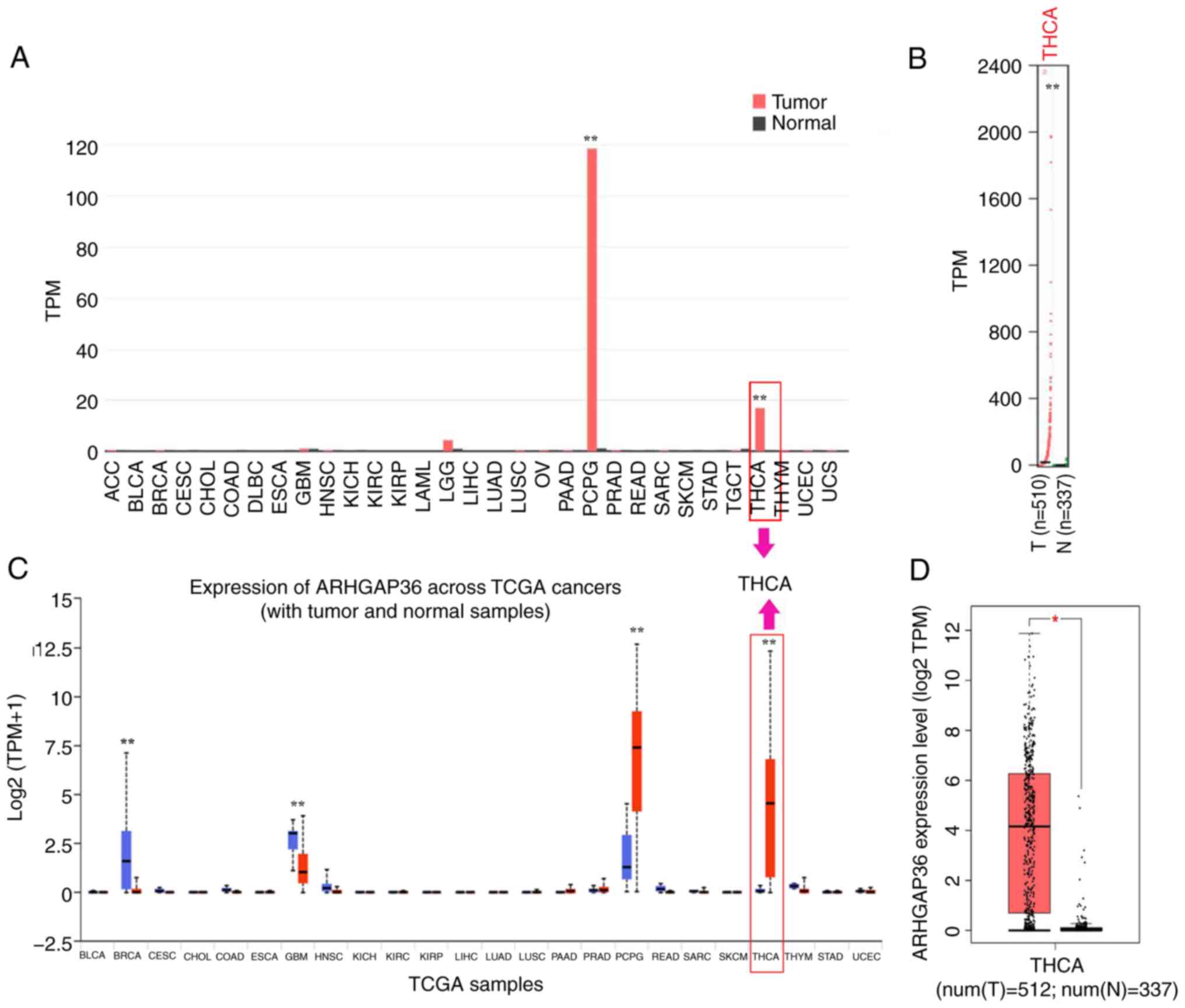
ARHGAP36 expression levels are
elevated in THCA samples compared with adjacent normal tissues
ARHGAP36 expression was analyzed across multiple
databases to assess its potential role in THCA progression. The
GEPIA results showed higher ARHGAP36 mRNA levels in TCGA tumor
tissues versus normal tissues (Fig.
1A and B; P<0.01). The
UALCAN results also confirmed elevated ARHGAP36 expression in
TCGA-THCA samples (Fig. 1C;
P<0.01). GEPIA further revealed similar findings in the THCA
dataset (Fig. 1D; P<0.05). These
consistent results across multiple datasets strongly indicate that
ARHGAP36 levels are markedly elevated in THCA samples. This
suggests a potential role for ARHGAP36 in the progression and
pathogenesis of THCA, positioning it as a candidate biomarker for
further investigation.
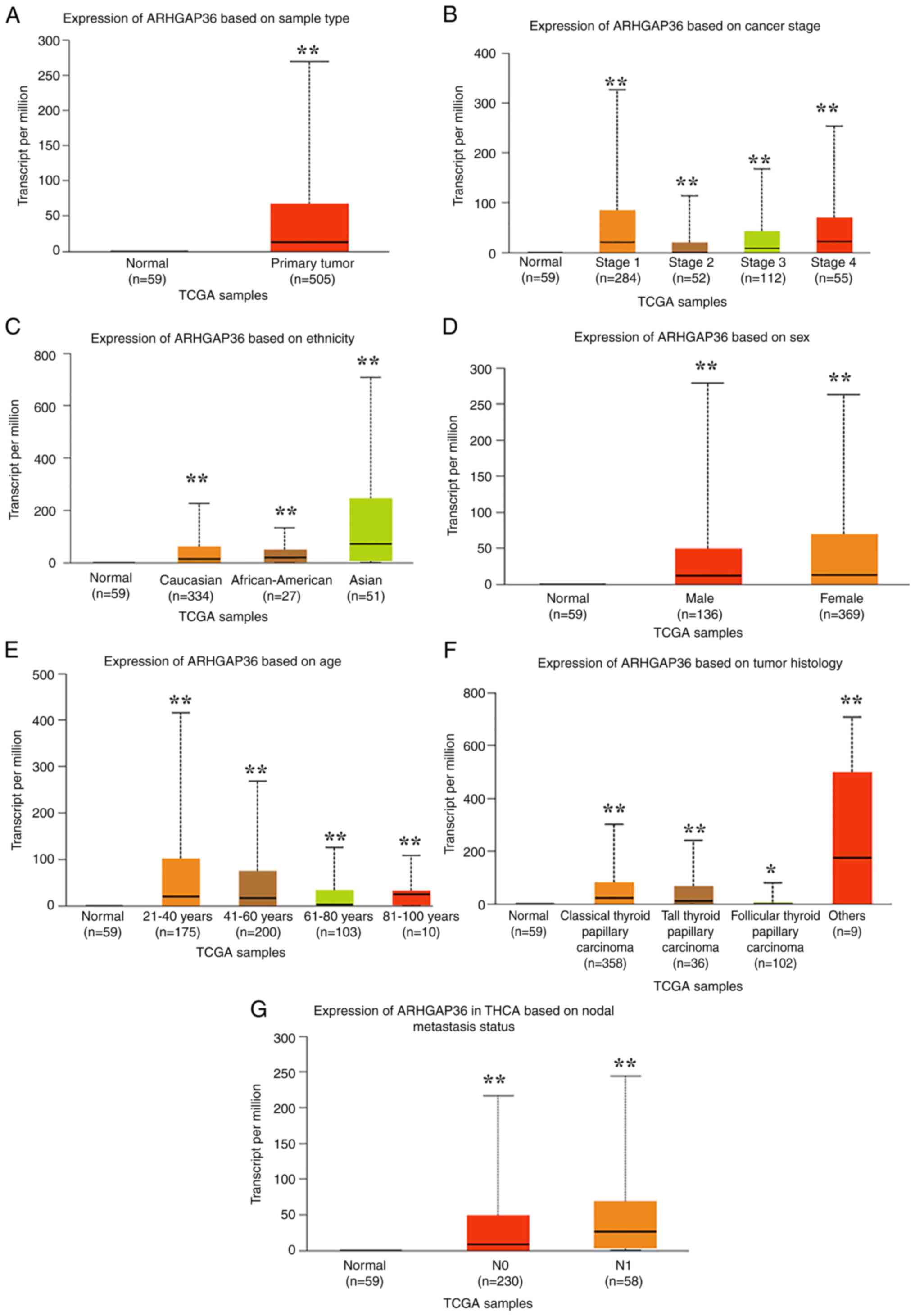
Higher ARHGAP36 mRNA expression is
associated with the TNM stage in patients with THCA
UALCAN data revealed a comprehensive association
between ARHGAP36 expression and various clinical parameters in
THCA. THCA tumor tissues exhibited significantly higher ARHGAP36
levels compared with normal tissues (Fig. 2A; P<0.001). ARHGAP36 expression
progressively increased from stage 1 to 4 disease (Fig. 2B; P<0.001), indicating a
potential role in disease progression. Elevated ARHGAP36 expression
was also observed across different demographic and histological
subtypes. Specifically, significant increases were noted in various
ethnic groups (Fig. 2C;
P<0.001), female patients (Fig.
2D; P<0.001), younger patients (Fig. 2E; P<0.001) and distinct THCA
subtypes (Fig. 2F; P<0.001).
Additionally, lymph node metastasis was associated with higher
ARHGAP36 expression (Fig. 2G;
P<0.001), suggesting its involvement in aggressive tumor
behavior. These findings highlight the broad relevance of ARHGAP36
in THCA, spanning multiple clinical and demographic factors. The
consistent elevation of ARHGAP36 across these diverse parameters
underscores its potential as a biomarker for disease
progression.
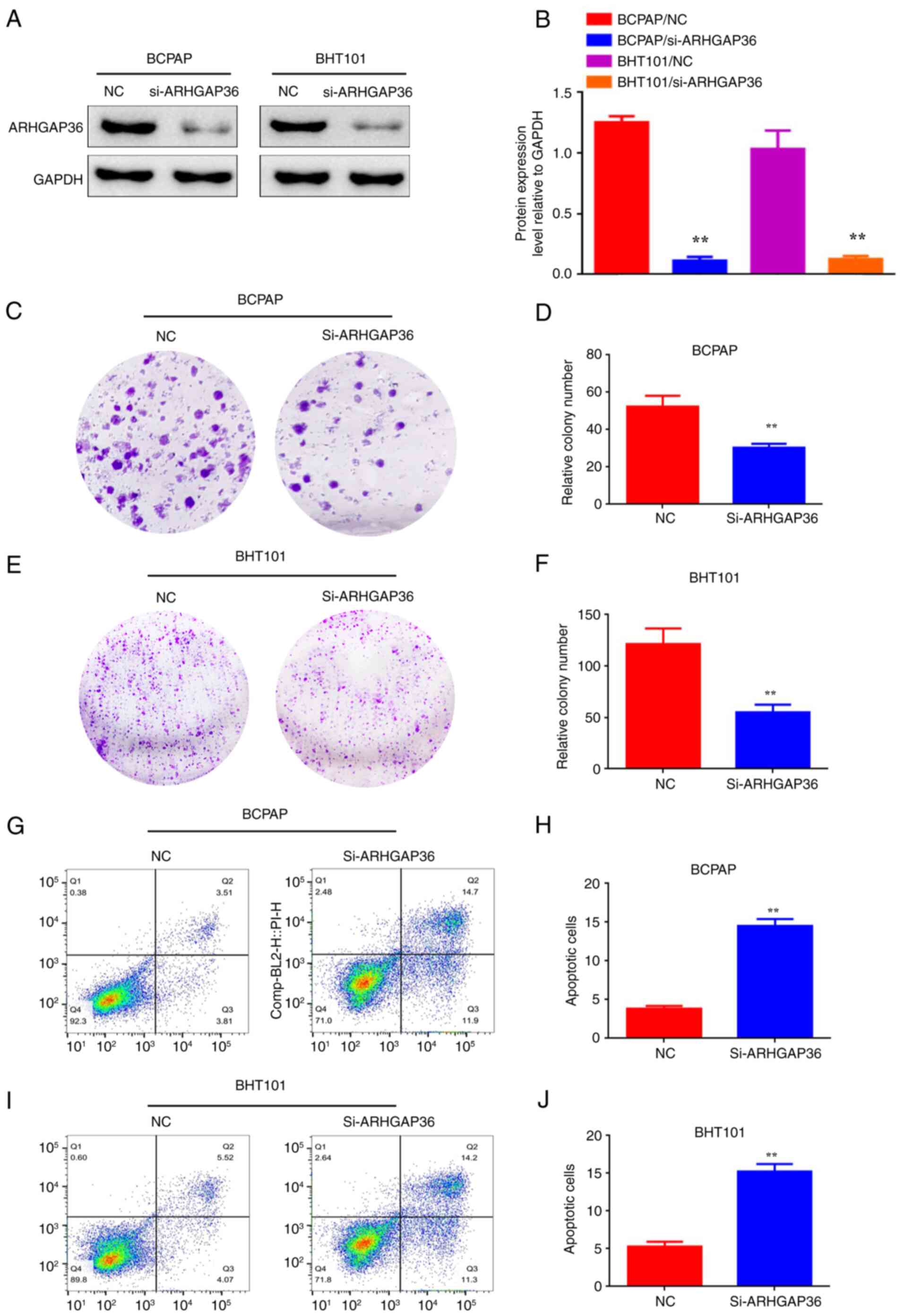
ARHGAP36 downregulation reduces
proliferation and induces apoptosis in THCA cells
To investigate the functional role of ARHGAP36 in
THCA, ARHGAP36 expression was knocked down in BCPAP and BHT101
cells using siRNA (designated as BCPAP/si-ARHGAP36 and
BHT101/si-ARHGAP36). Western blot assays showed the effectiveness
of the siRNA transfection and confirmed the significant decrease in
ARHGAP36 protein levels compared with the control (Fig. 3A and B; P<0.01). Plate colony formation
assays revealed significantly reduced proliferation in these cells
compared with controls (Fig. 3C and
F; P<0.01). Apoptosis assays
also showed increased cell death upon ARHGAP36 knockdown (Fig. 3G-J; P<0.01). These findings
highlight the critical role of ARHGAP36 in promoting THCA cell
survival and suggest that its downregulation can significantly
inhibit tumor cell proliferation while enhancing apoptosis.
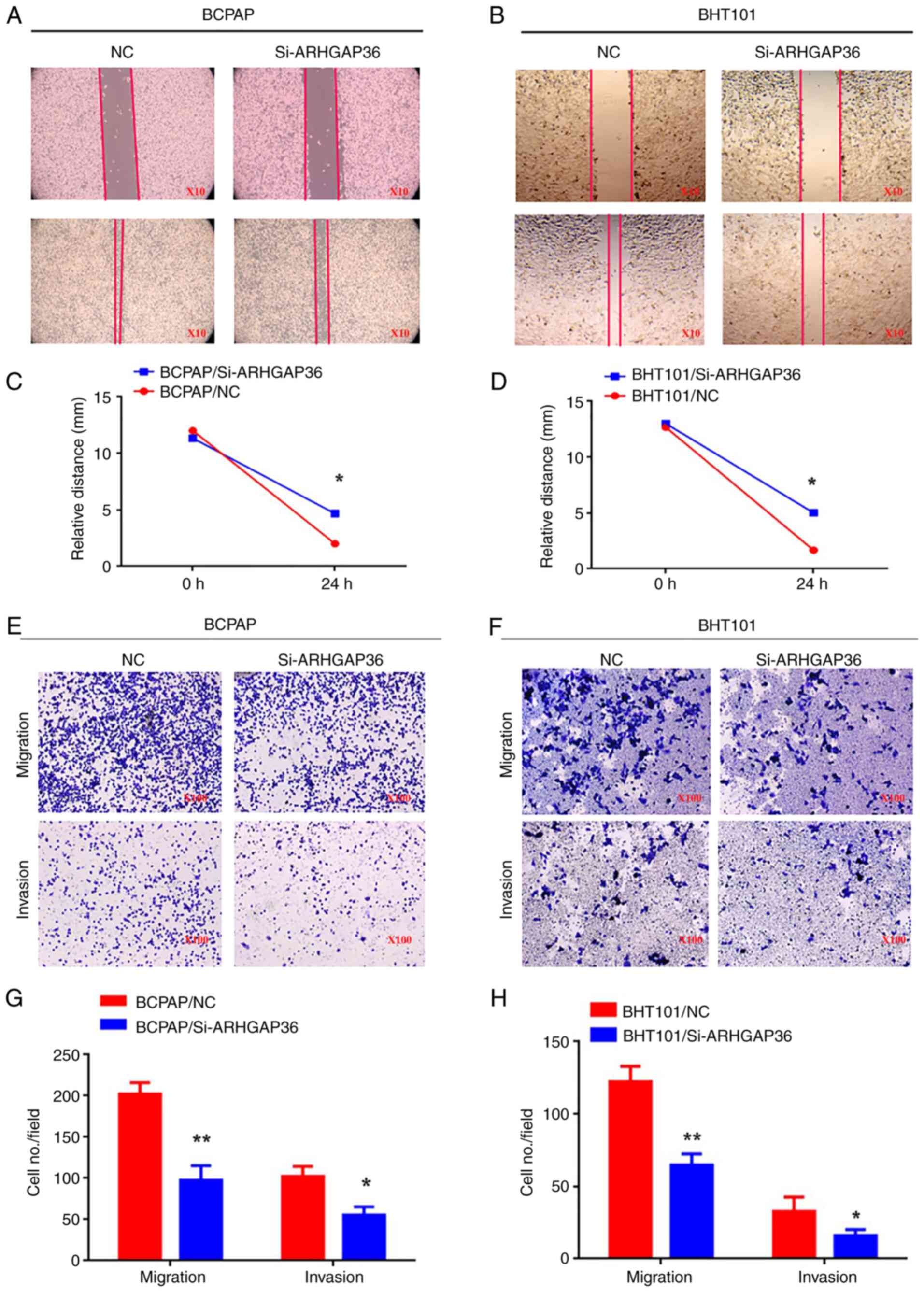
ARHGAP36 downregulation inhibits
migration and invasion
Wound healing assays demonstrated a reduced
migration in ARHGAP36 knockdown cells (Fig. 4A-D; P<0.05). Transwell migration
assays also showed significantly fewer si-ARHGAP36 cells traversing
through Transwell inserts (Fig.
4E-H; P<0.05). Invasion assays revealed similar results,
with significantly decreased invasive behavior (Fig. 4E-H; P<0.05). These findings
collectively indicate that the downregulation of ARHGAP36
expression impairs THCA cell migration and invasion, highlighting
its critical role in facilitating these processes. This evidence
supports the potential of ARHGAP36 as a therapeutic target to
inhibit tumor metastasis in THCA.
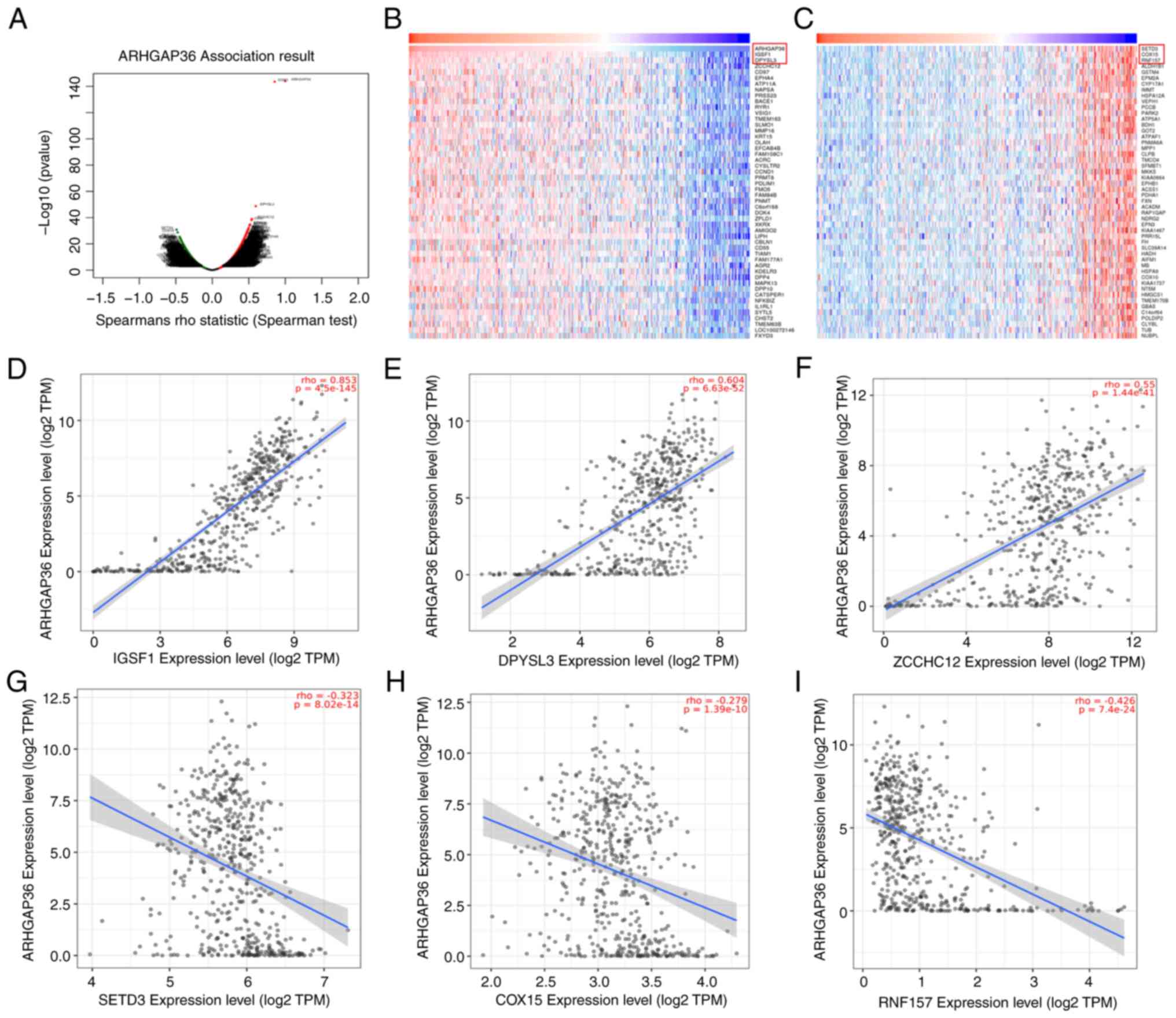
ARHGAP36 gene co-expression network in
THCA
To explore the genes co-expressed with ARHGAP36, the
LinkedOmics database was utilized. A total of 2,436 genes were
found to be positively correlated and 1,764 genes were negatively
correlated with ARHGAP36 (Fig. 5A).
Heatmaps identified the top 50 genes most strongly correlated with
ARHGAP36 expression (Fig. 5B and
C). Key positively correlated genes
included immunoglobulin superfamily member 1 (IGSF1; Fig. 5D; ρ=0.853, P=4.5x10-145),
dihydropyrimidinase like 3 (DPYSL3; Fig. 5E; ρ=0.604, P=6.63x10-52)
and zinc finger CCHC-type containing 12 (ZCCHC12; Fig. 5F; ρ=0.55, P=1.44x10-41),
indicating strong associations between these genes and ARHGAP36.
Negatively correlated genes included SET domain containing 3 actin
N3(tau)-histidine methyltransferase, COX15 and ring finger protein
157 (Fig. 5G-I), highlighting
potential antagonistic relationships in THCA pathogenesis. These
findings provide insights into the molecular network surrounding
ARHGAP36, suggesting its involvement in complex regulatory pathways
that influence THCA progression. This co-expression analysis
supports the hypothesis that ARHGAP36 plays a role in THCA by
interacting with key regulatory genes.
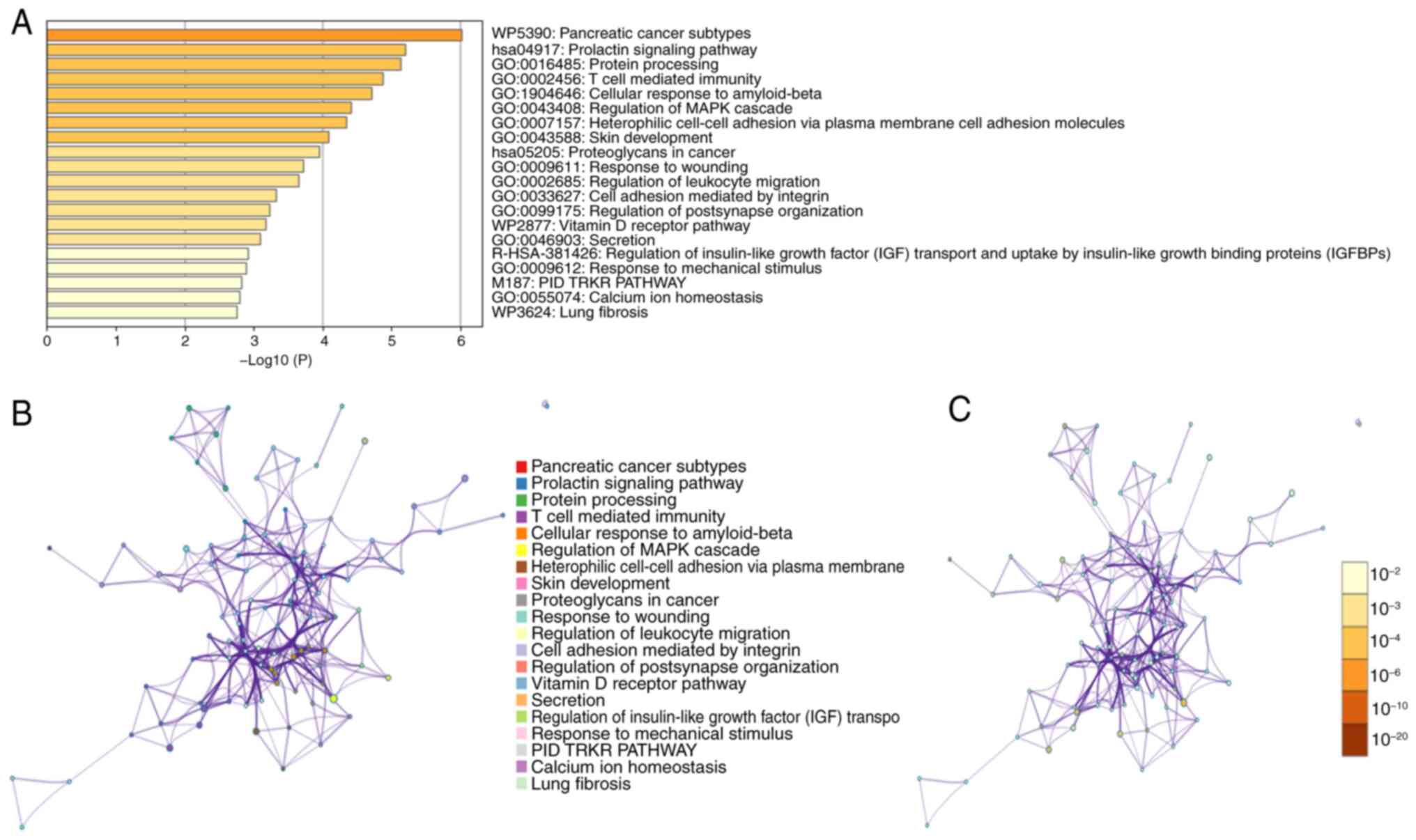
T cell-mediated immunity is a key GO
biological process linked to ARHGAP36
To gain deeper insights into the biological
processes associated with ARHGAP36, a Metascape analysis was
conducted. This analysis revealed that ARHGAP36-associated genes
are significantly enriched in processes such as ‘Pancreatic cancer
subtypes,’ ‘Prolactin signaling pathway’ and ‘T cell mediated
immunity’ (Fig. 6A). PPI networks
further supported these findings, illustrating the
interconnectivity between ARHGAP36 and its associated genes
(Fig. 6B and C). These networks highlight key pathways
and interactions that may underline the functional role of ARHGAP36
in THCA. The analysis revealed that ARHGAP36 may be intricately
involved in multiple signaling pathways critical for THCA
development and progression. Notably, it appears to interact with
components of the MAPK pathway, which is known to play a pivotal
role in cell proliferation, differentiation and survival (15). These results suggest that ARHGAP36
is involved in diverse biological processes beyond THCA,
potentially influencing pathways related to other cancer types and
immune responses. The enrichment in T cell-mediated immunity is
particularly noteworthy, indicating a potential role for ARHGAP36
in modulating the tumor microenvironment and immune evasion
mechanisms.
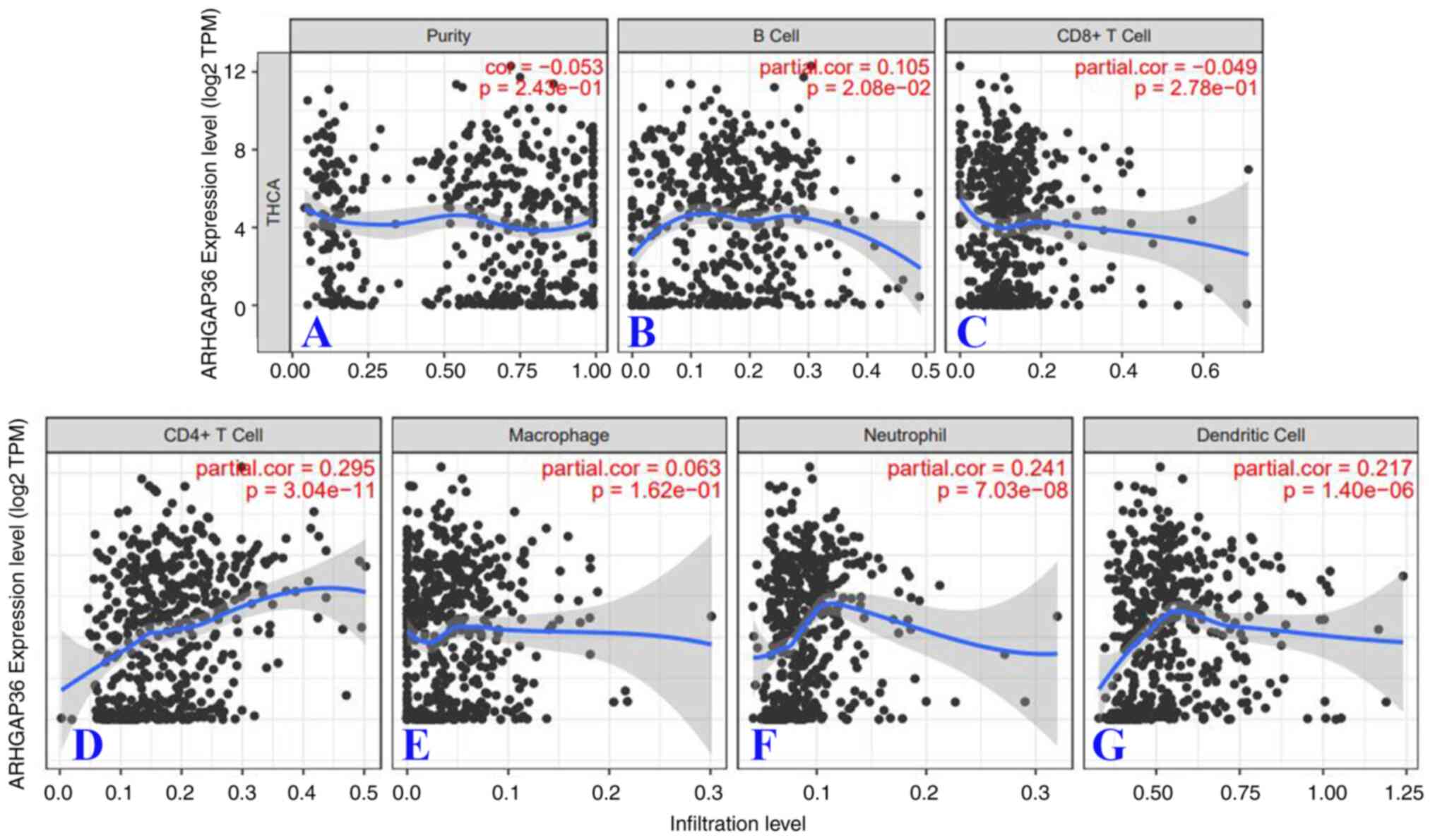
ARHGAP36 is associated with
CD4+ T cell infiltration
To investigate the relationship between ARHGAP36
expression and immune cell infiltration in THCA, an analysis using
the TIMER database was conducted. TIMER analysis indicated a
significant but weak correlation between ARHGAP36 expression and
CD4+ T cell infiltration (Fig. 7D; cor =0.295,
P=3.04x10-11). Additional weak correlations were
observed with neutrophils (Fig. 7F)
and dendritic cells (Fig. 7G),
further supporting the possible role of ARHGAP36 in modulating
immune cell infiltration. These findings suggest that ARHGAP36 may
influence the tumor microenvironment by affecting the recruitment
and activity of various immune cells. The positive correlation with
CD4+ T cells is particularly noteworthy, as it
highlights the potential involvement of ARHGAP36 in adaptive
immunity and immune evasion mechanisms in THCA. These results
collectively underscore the importance of ARHGAP36 in shaping the
immune landscape of THCA, positioning it as a potential target for
immunotherapeutic strategies.
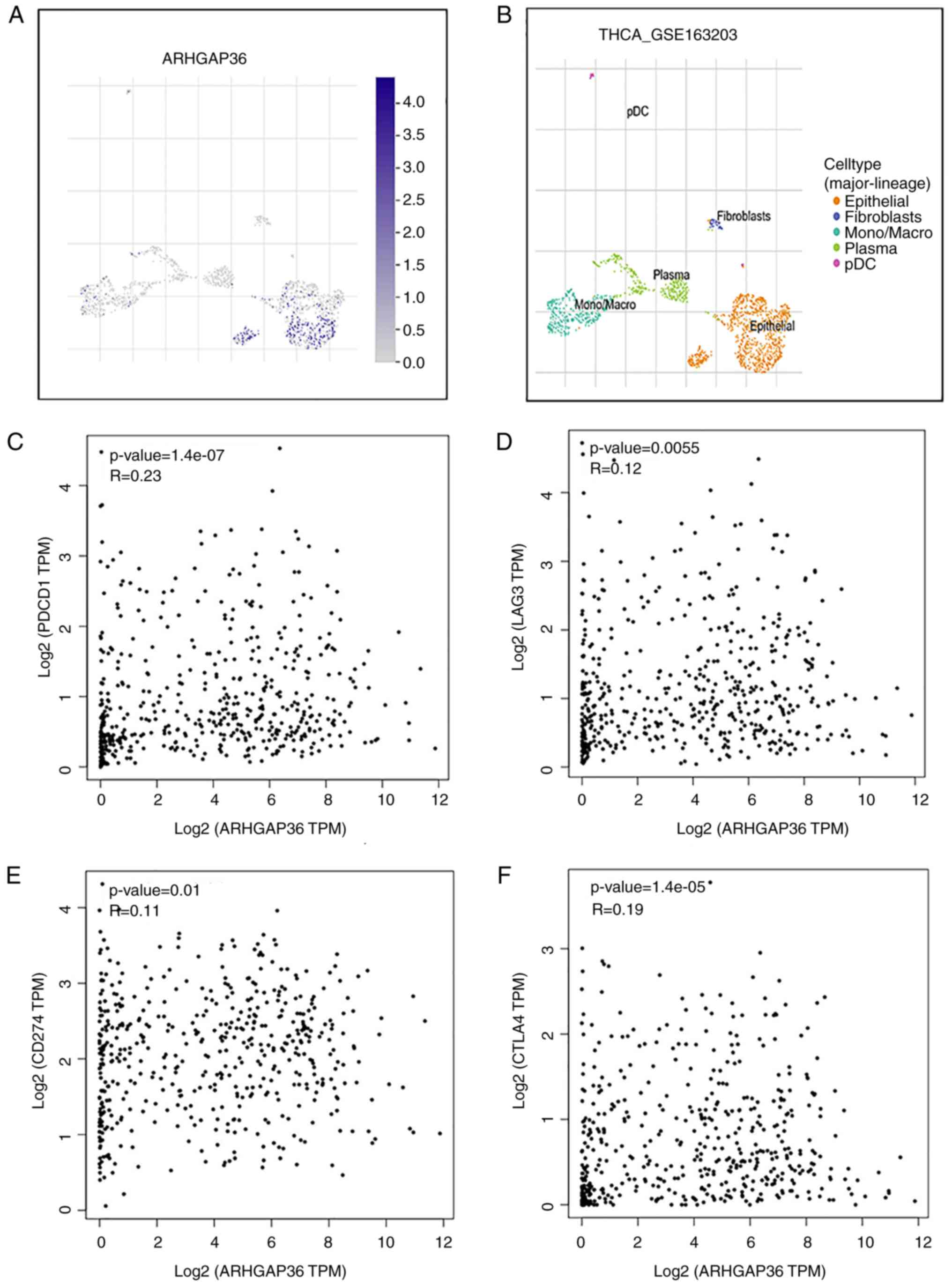
ARHGAP36 is linked to immune
escape
To further explore the role of ARHGAP36 in the tumor
microenvironment, its expression was analyzed using the TISCH
database. This analysis revealed that ARHGAP36 is expressed in
epithelial cells within THCA tissues and exhibits a weak positive
correlation with several immune checkpoint molecules, including
PDCD-1 (programmed cell death protein 1; PD-1),
lymphocyte-activation gene 3 (LAG3), CD274 (programmed death-ligand
1; PD-L1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4)
(Fig. 8A-F). These findings suggest
that ARHGAP36 may facilitate immune escape in THCA by interacting
with these key immune checkpoints. The observed weak correlations
imply that ARHGAP36 could contribute to the suppression of immune
surveillance and the establishment of an immunosuppressive tumor
microenvironment. The positive weak correlations with PD-1, LAG3,
PD-L1 and CTLA4 highlight the potential of ARHGAP36 as a mediator
of immune evasion mechanisms in THCA. This evidence supports the
hypothesis that targeting ARHGAP36 might enhance the efficacy of
immunotherapies aimed at overcoming immune resistance in THCA.
Discussion
The metastasis of THCA is driven by a complex
interplay of mechanisms involving genetic diversity,
epithelial-mesenchymal transition and the tumor microenvironment
(16-18).
Thyroid carcinoma, accounting for ~2.5% of all malignancies, is
exhibiting an increasing incidence rate and represents the fifth
most common cancer in women in the USA (19). Lymph nodal involvement in THCA is
very common and lymph node micrometastases are observed in up to
90% of cases (20). Despite
extensive research, the biological processes underlying THCA
metastasis remain poorly understood, necessitating further
exploration to provide a comprehensive understanding of its
mechanisms.
ARHGAP36, an atypical member of the Rho
GTPase-activating protein family, has roles in spinal cord
development and tumorigenesis by suppressing protein kinase A and
activating Gli transcription factors (21). ARHGAP36 features unique structural
domains, but its role in THCA metastasis has not been extensively
reported. Investigating ARHGAP36 in this context could have
significant clinical value. The present study revealed that
ARHGAP36 expression is significantly higher in THCA tissues
compared with normal tissues, as demonstrated through analyses
using the GEPIA and UALCAN databases. Higher ARHGAP36 levels were
associated with sample types, cancer stages and patient
demographics. In vitro experiments validated these findings,
showing that knocking down ARHGAP36 expression significantly
reduced THCA cell proliferation, migration and invasion, while
inducing apoptosis. These results confirm that ARHGAP36
downregulation impairs the aggressive properties of THCA cells.
In the present study, 2,436 genes positively
correlated and 1,764 genes negatively correlated with ARHGAP36 were
identified using the LinkedOmics database. Notably, ARHGAP36
expression was strongly correlated with genes such as IGSF1, DPYSL3
and ZCCHC12. IGSF1, a plasma membrane glycoprotein associated with
conditions such as hypothyroidism and delayed puberty, is linked to
THCA cell growth, metastasis and apoptosis (22-24).
The immune-targeting potential of ARHGAP36 complements existing
cancer immunotherapies, suggesting that ARHGAP36 may act as a
biomarker influencing immune infiltration in THCA.
In the present study, Metascape analysis identified
key GO biological processes associated with ARHGAP36, including
prolactin signaling, protein processing and T cell-mediated
immunity. TIMER database analyses demonstrated weak correlations
between ARHGAP36 expression and CD4+ T cell infiltration
in THCA. TISCH data further showed ARHGAP36 expression in
epithelial cells and weak correlations with immune checkpoints such
as PDCD-1, LAG3, CD274 and CTLA4, suggesting a possible role in
immune escape. Tumor immune escape allows cancer cells to evade
immune detection, enabling survival and metastasis. Immune cells,
such as macrophages and T cells, are suppressed in the tumor
microenvironment by cytokines and tumor interactions, facilitating
cancer progression (25,26). The positive correlation between
ARHGAP36 and immune checkpoint molecules highlights its potential
involvement in modulating the tumor immune microenvironment.
While the present study provides valuable insights
into the functional impact and underlying mechanisms of ARHGAP36 in
THCA cells, it is important to acknowledge limitations regarding
long-term follow-up data. The present research primarily focused on
short-term cellular effects and did not include extensive
longitudinal patient data. This lack of long-term follow-up data
makes it challenging to fully assess the role of ARHGAP36 in the
prognosis of patients with THCA over extended periods. Longitudinal
studies are essential for understanding how changes in ARHGAP36
expression influence patient outcomes, survival rates and disease
progression in the long term. Future research should aim to address
this gap by conducting comprehensive longitudinal studies and
aggregating multicenter data to better elucidate the prognostic
significance of ARHGAP36 in THCA.
The present study primarily focused on the impact of
downregulating ARHGAP36 expression on THCA cells, providing
detailed insights into its functional consequences and underlying
mechanisms. The experimental data demonstrated that reduced
ARHGAP36 expression significantly affected cell proliferation,
migration and invasion, highlighting its potential as a therapeutic
target in THCA. However, the present investigation has limitations
in comparing and discussing other potential treatment methods.
Therefore, reviewing relevant literature to explore whether changes
in ARHGAP36 expression might influence the efficacy of radiotherapy
or interact with other treatment strategies, may provide
theoretical foundations for future research directions. In future
studies, we aim to conduct experiments using THCA cells or tissues
to explore whether the suppression of ARHGAP36 impacts the
expression of immune checkpoint molecules. This will help elucidate
the mechanisms by which ARHGAP36 influences immune responses.
In conclusion, the results of the present study
indicate that ARHGAP36 may promote THCA metastasis by mediating
immune escape, making it a potential prognostic biomarker and
therapeutic target for THCA. However, further studies are needed to
elucidate the precise mechanisms through which ARHGAP36 regulates
immune escape and interacts with other components of the tumor
microenvironment.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Shanghai Pudong New
Area Public Health Discipline Construction Project (grant no.
20234Y0055), Natural Science Foundation of Fujian Province (grant
no. 2023J011342), Pudong New Area Clinical Characteristic
Discipline (grant no. PWYts2021-15), Pudong Health Commission
Subject Construction Project (grant no. PWZy2020-06), Gongli
Hospital National Fund Cultivation Project (grant no. 2022GPY-B04),
Key Specialty Construction Project of Health Bureau of Shanghai
(grant no. ZX2019C06) and the Pudong New Area Clinical
Characteristic Discipline (grant no. PWYts2021-15).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LY and JL performed the experiments and edited,
drafted and wrote the manuscript; LY, SH, GW and YG conducted the
data analysis; study design and general supervision were led by SH
and GW; data interpretation was performed by LY and YG. LY and JL
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang W, Jin F, Song L, Yang J, Ye Y, Liu
J, Xu L and An P: Prediction of peripheral lymph node metastasis
(LNM) in thyroid cancer using delta radiomics derived from enhanced
CT combined with multiple machine learning algorithms. Eur J Med
Res. 30(164)2025.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Peng X, Zhu Y, Lin S, Yu W, Zhang C, Tan
L, Long M, Luo D and Ji C: Circular RNA_0057209 acts as ceRNA to
inhibit thyroid cancer progression by promoting the STK4-mediated
hippo pathway via sponging MicroRNA-183. Oxid Med Cell Longev.
2022(9974639)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Veschi V, Verona F, Lo Iacono M, D'Accardo
C, Porcelli G, Turdo A, Gaggianesi M, Forte S, Giuffrida D, Memeo L
and Todaro M: cancer stem cells in thyroid tumors: From the origin
to metastasis. Front Endocrinol (Lausanne). 11(566)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Croisé P, Houy S, Gand M, Lanoix J, Calco
V, Tóth P, Brunaud L, Lomazzi S, Paramithiotis E, Chelsky D, et al:
Cdc42 and Rac1 activity is reduced in human pheochromocytoma and
correlates with FARP1 and ARHGEF1 expression. Endocr Relat Cancer.
23:281–293. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shen Y, Chen G, Zhuang L, Xu L, Lin J and
Liu L: ARHGAP4 mediates the Warburg effect in pancreatic cancer
through the mTOR and HIF-1α signaling pathways. Onco Targets Ther.
12:5003–5012. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang TY and Ha MW: Silencing ARHGAP9
correlates with the risk of breast cancer and inhibits the
proliferation, migration, and invasion of breast cancer. J Cell
Biochem. 119:7747–7756. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yan T, Qiu W, Song J, Fan Y and Yang Z:
ARHGAP36 regulates proliferation and migration in papillary thyroid
carcinoma cells. J Mol Endocrinol. 66:1–10. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cancer Genome Atlas Research Network.
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lonsdale J, Thomas J, Salvatore M,
Phillips R, Lo E, Shad S, Hasz R, Walters G, Garcia F, Young N, et
al: The genotype-tissue expression (GTEx) project. Nat Genet.
45:580–585. 2013.
|
|
10
|
Zhao R, Chen Q, Qiao P, Lu Y and Chen X: A
signature of four ferroptosis-related genes in laryngeal squamous
cell carcinoma. Transl Cancer Res. 13:2938–2949. 2024.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yao L, Li Y, Li S, Wang M, Cao H, Xu L and
Xu Y: ARHGAP39 is a prognostic biomarker involved in immune
infiltration in breast cancer. BMC Cancer. 23(440)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vasaikar SV, Straub P, Wang J and Zhang B:
LinkedOmics: Analyzing multi-omics data within and across 32 cancer
types. Nucleic Acids Res. 46(D1):D956–D963. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou Y, Zhou B, Pache L, Chang M,
Khodabakhshi AH, Tanaseichuk O, Benner C and Chanda SK: Metascape
provides a biologist-oriented resource for the analysis of
systems-level datasets. Nat Commun. 10(1523)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Csordas A, Sipos B, Kurucova T, Volfova A,
Zamola F, Tichy B and Hicks DG: Cell tree rings: The structure of
somatic evolution as a human aging timer. Geroscience.
46:3005–3019. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tang L, Shi Y, Liao Q, Wang F, Wu H, Ren
H, Wang X, Fu W, Shou J, Wang WE, et al: Reversing metabolic
reprogramming by CPT1 inhibition with etomoxir promotes
cardiomyocyte proliferation and heart regeneration via DUSP1
ADP-ribosylation-mediated p38 MAPK phosphorylation. Acta Pharm Sin
B. 15:256–277. 2025.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yan HC and Xiang C: Aberrant expression of
BUB1B contributes to the progression of thyroid carcinoma and
predicts poor outcomes for patients. J Cancer. 13:2336–2351.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Qi F, Tang J, Cai Z, Wang G and Wang Z:
Long non-coding RNA CATIP antisense RNA 1 (lncRNA CATIP-AS1)
downregulation contributes to the progression and metastasis of
thyroid cancer via epithelial–mesenchymal transition (EMT) pathway.
Bioengineered. 13:7592–7606. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hong K, Cen K, Chen Q, Dai Y, Mai Y and
Guo Y: Identification and validation of a novel senescence-related
biomarker for thyroid cancer to predict the prognosis and
immunotherapy. Front Immunol. 14(1128390)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Conzo G, Docimo G, Mauriello C,
Gambardella C, Esposito D, Cavallo F, Tartaglia E, Napolitano S and
Santini L: The current status of lymph node dissection in the
treatment of papillary thyroid cancer. A literature review. Clin
Ter. 164:e343–e346. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Conzo G, Mauriello C, Docimo G,
Gambardella C, Thomas G, Cavallo F, Tartaglia E, Napolitano S,
Varriale R, Rossetti G, et al: Clinicopathological pattern of lymph
node recurrence of papillary thyroid cancer. Implications for
surgery. Int J Surg. 12 (Suppl 1):S194–S197. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nano PR, Johnson TK, Kudo T, Mooney NA, Ni
J, Demeter J, Jackson PK and Chen J: Structure-activity mapping of
ARHGAP36 reveals regulatory roles for its GAP homology and
C-terminal domains. PLoS One. 16(e0251684)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Joustra SD, Heinen CA, Schoenmakers N,
Bonomi M, Ballieux BE, Turgeon MO, Bernard DJ, Fliers E, van
Trotsenburg AS, Losekoot M, et al: IGSF1 deficiency: lessons from
an extensive case series and recommendations for clinical
management. J Clin Endocrinol Metab. 101:1627–1636. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Joustra SD, Andela CD, Oostdijk W, van
Trotsenburg AS, Fliers E, Wit JM, Pereira AM, Middelkoop HA and
Biermasz NR: Mild deficits in attentional control in patients with
the IGSF1 deficiency syndrome. Clin Endocrinol (Oxf). 84:896–903.
2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Guan Y, Wang Y, Bhandari A, Xia E and Wang
O: IGSF1: A novel oncogene regulates the thyroid cancer
progression. Cell Biochem Funct. 37:516–524. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Koh DI, Lee M, Park YS, Shin JS, Kim J,
Ryu YS, Lee JH, Bae S, Lee MS, Hong JK, et al: The Immune
Suppressor IGSF1 as a Potential Target for Cancer Immunotherapy.
Cancer Immunol Res. 12:491–507. 2024.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu Q, You L, Nepovimova E, Heger Z, Wu W,
Kuca K and Adam V: Hypoxia-inducible factors: Master regulators of
hypoxic tumor immune escape. J Hematol Oncol. 15(77)2022.PubMed/NCBI View Article : Google Scholar
|