Introduction
Type 2 diabetes mellitus (T2DM) is characterized by
insulin resistance and dysfunction of pancreatic β cells (1). The inability of pancreatic β cells to
produce sufficient insulin to counteract insulin resistance leads
to hyperglycemia (2). Elevated
blood sugar levels result in excessive production of reactive
oxygen species (ROS), which can damage pancreatic β cells, thereby
decreasing their number and function (3,4).
Hyperglycemia not only increases ROS generation but also
compromises the antioxidant defense system in pancreatic β cells.
Research on antioxidant enzymes, including catalase (CAT),
superoxide dismutase (SOD), glutathione peroxidase and thioredoxin,
has shown that prolonged exposure to high glucose levels diminishes
the antioxidant capacity of pancreatic β cells (5). Additionally, hepatic insulin
resistance disrupts normal glucose metabolism by impairing the
ability to suppress gluconeogenesis and glycogenolysis. This
impairment results from decreased inhibition of key enzymes,
including glucose-6-phosphatase and phosphoenolpyruvate
carboxykinase (PEPCK) via the PI3K/Akt pathway (6,7).
Consequently, hepatic glucose output increases while glycogen
storage decreases (8).
Simultaneously, insulin resistance in adipose tissue enhances
lipolysis, increasing circulating fatty acids that fuel hepatic
gluconeogenesis through β-oxidation, further elevating PEPCK
activity (9). These combined
effects contribute to hyperglycemia and the progression of
T2DM.
Syzygium samarangense [SS; (Blume)] Merr.
& L. M. Perry, commonly known as rose or wax apple, belongs to
the Myrtaceae family and is widely cultivated across Southeast
Asia. Rose apple is rich in polyphenolic compounds, including
phenols, flavonoids, anthocyanins, ellagitannins, carotenoids and
triterpenoids (10,11). In type 1 diabetic rats induced by
streptozotocin (STZ), unprocessed rose apple powder decreases
hyperglycemia while preserving pancreatic β cell mass through
antioxidant and anti-inflammatory mechanisms (12). Our recent study employed
microwave-assisted extraction combined with response surface
technology to extract rose apple fruits, and ultrahigh-performance
liquid chromatography (UHPLC)-microtime of flight (micrOTOF) Q
II-tandem mass spectrometry (MS/MS) analysis was used to identify
the phytochemical profiles of the extract (13). High concentrations of 12 bioactive
compounds, including matairesinol and phenolic substances, were
discovered (13). Furthermore,
antioxidant properties of rose apple extracts can prevent
glucotoxicity in a rat insulinoma cell line (INS-1 cells) (13). However, the effects of rose apple
extracts on type 2 diabetic rats remain unexplored.
Materials and methods
Plant material and preparation of SS
extract (SSE)
SSE was prepared and the major bioactive compounds
were identified as previously described (13).
Animals
A total of 20 adult male Wistar rats (age, 6 weeks;
weight, 180-220 g) were obtained from Nomura Siam International in
Bangkok, Thailand. All rats were housed in a temperature- and
humidity-controlled environment (23±3˚C; humidity, 60±10%) with a
12/12-h light/dark cycle at the Animal Center of Ubon Ratchathani
University, Ubon Ratchathani, Thailand. They had unrestricted
access to rodent diet and water and were allowed 1 week for
acclimatization. The Animal Ethics Committee of Ubon Ratchathani
University approved the protocol for animal experimentation
(approval no. 37/2564 IACUC).
Induction of T2DM using high-fat diet
(HFD) and low-dose STZ
The rats were divided into two dietary groups:
Normal pellet diet (NPD; 12% calories from fat) and HFD; 58% fat,
25% protein and 17% carbohydrate, as percentages of total kcal).
The HFD consisted of powdered NPD, 365 g/kg (National Laboratory
Animal Center, Mahidol University, Nakhon Pathom, Thailand);
casein, 250 g/kg (Difco, Becton Dickinson); cholesterol, 10 g/kg
(Loba Chemie PVT Ltd.); vitamin and mineral mixture, 60 g/kg
(Sigma-Aldrich; Merck KGaA); 2-amino-4-(methylthio) butanoic
acid-methionine, 3 g/kg (Sigma-Aldrich; Merck KGaA); yeast powder,
1 g/kg and sodium chloride, 1 g/kg (14). Following a 2 week dietary
modification period, the rats on HFD received an intraperitoneal
injection of STZ at a dosage of 45 mg/kg body weight, dissolved in
0.1 M sodium citrate buffer (pH 4.5). At 3 days post-STZ injection,
glucose levels were assessed in all rats using blood samples
obtained from the tail tip using a glucometer (Accu-Chek Performa;
Roche Diagnostics). Only rats with fasting blood glucose (FBG)
levels ≥200 mg/dl were included in the diabetic group (DM)
(15). The control rats were
administered an injection of 0.1 M sodium citrate buffer at pH
4.5.
Experimental design
Following the establishment of diabetes, diabetic
rats were orally administered glibenclamide or SSE at a volume of 1
ml/kg vehicle once daily. The rats were randomly assigned into four
groups (n=5/group) as follows: Control and DM rats received only
the vehicle; DM + SSE comprised DM rats treated with SSE at a
dosage of 400 mg/kg body weight (; chosen based on preliminary
findings showing effective antihyperglycemic activity without
detectable toxicity in diabetic rats) and glibenclamide group (DM +
GB) included DM rats receiving glibenclamide [Innova CapTab, Solan
(H.P.)] at a dosage of 5 mg/kg body weight, serving as the positive
control group (16). The treatment
period lasted for 4 weeks, based on a previous study (12), and was considered sufficient for
assessing sub-chronic exposure. The experimental protocol is
illustrated in Fig. 1.
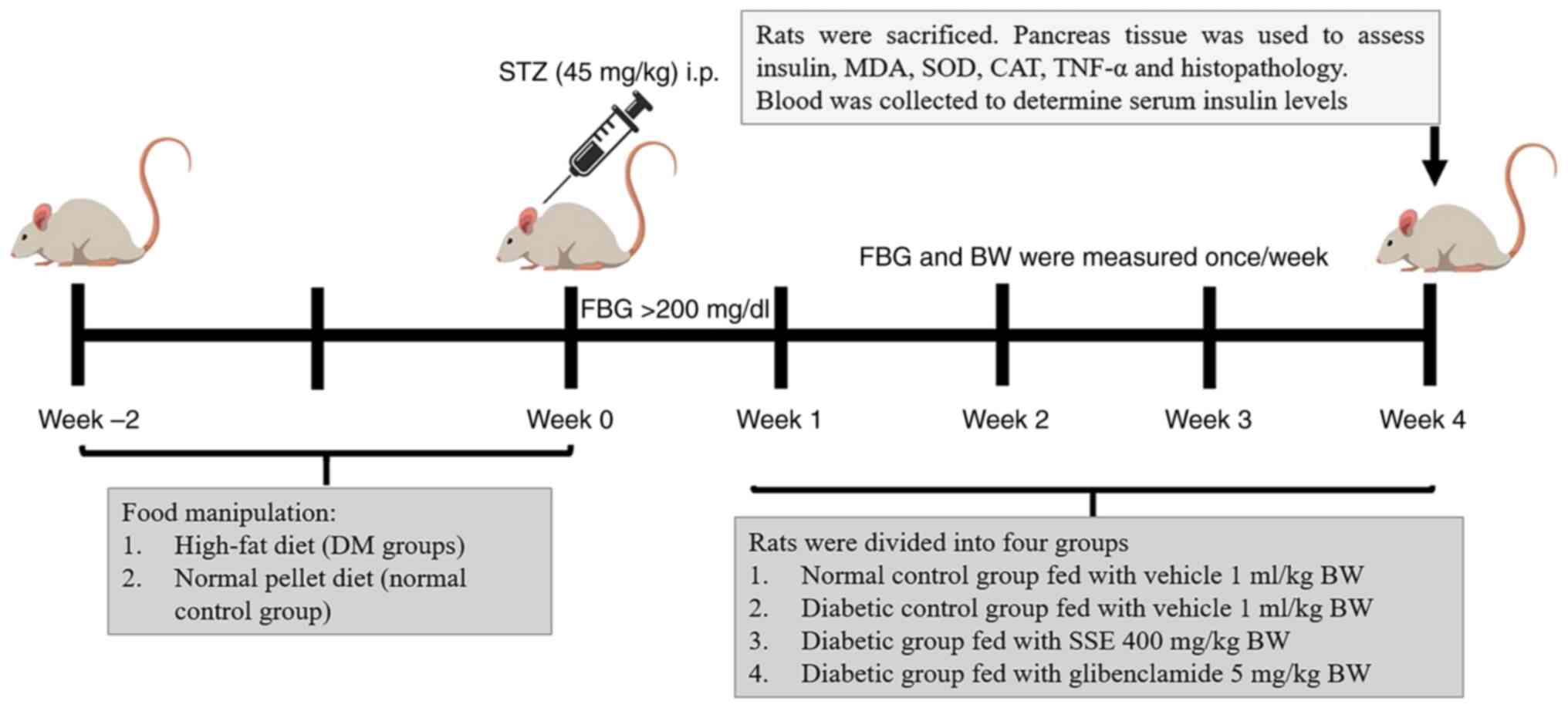 | Figure 1Experimental protocol. STZ,
streptozotocin; DM, diabetes mellitus; MDA, malondialdehyde; SOD,
superoxide dismutase; CAT, catalase; H&E, hematoxylin and
eosin; BW, body weight; SSE, Syzygium samarangense extract;
FBG, fasting blood glucose; i.p., intraperitoneal. |
Collection of blood samples
At the end of the study period, the rats underwent
an overnight fast and were euthanized via intraperitoneal
administration of thiopental sodium (120 mg/kg body weight;
Scott-Edil Pharmacia Limited). Death was confirmed by the absence
of heartbeat, respiration, corneal reflex and response to toe
pinch, in accordance with the American Veterinary Medical
Association guidelines for the euthanasia of animals (2020)
(17). Blood samples were obtained
through heart puncture. The blood collection tubes were immediately
placed on ice, and serum was separated by centrifugation at 3,500 x
g for 15 min at 4˚C. The serum samples were then stored at -80˚C
until further analysis. Fresh anticoagulated blood was transported
to the Pathological Laboratory at Ubon Ratchathani University
Hospital for the measurement of liver enzyme levels, including
alanine aminotransferase (ALT), alkaline phosphatase (ALP) and
aspartate aminotransferase (AST), using kinetic ultraviolet
spectrophotometry with a Beckman Coulter AU680 Analyzer (Beckman
Coulter).
Determination of pancreatic and serum
insulin levels
Pancreatic tissue (80 mg) was homogenized in a
Teflon homogenizer (Glas-Col homogenizer system) using RIPA lysis
buffer with protease inhibitor (Thermo Fisher Scientific, Inc.).
Following sonication (20 kHz; three cycles of 10 sec with 30-sec
intervals), the homogenate was centrifuged at 2,000 x g for 10 min
at 4˚C. The pancreatic protein concentration was measured using a
micro-BCA kit (cat. no. SK3061; Bio Basic). Serum insulin levels
and pancreatic proteins were determined using a Rat Insulin ELISA
kit (cat. no. RAB0904; Sigma-Aldrich; Merck KGaA) according to the
manufacturer's guidelines.
Assay for lipid peroxidation
Malondialdehyde (MDA), a product of lipid
peroxidation, interacts with thiobarbituric acid (TBA) to generate
TBA reactive substances (TBARS), which are used to assess lipid
peroxidation. Lipid peroxidation was evaluated according to the
manufacturer's instructions (cat. no. MAK085, Sigma-Aldrich; Merck
KGaA). To produce TBARS, 600 µl TBA solution was added to each
Eppendorf tube containing either a pancreatic protein sample or an
MDA standard (0-20 mM). The tubes were incubated at 95˚C for 1 h.
Samples and MDA standards were transferred into a 96-well plate at
a volume of 200 µl/well. The concentration of TBARS in pancreatic
protein samples and MDA standards was quantified using a
spectrophotometer at 532 nm (Fluostar Omega, BMG Labtech).
Determination of antioxidant
markers
CAT activity in pancreatic tissue was measured using
a CAT assay kit (cat. no. MAK381; Sigma-Aldrich; Merck KGaA)
following the manufacturer's instructions. CAT activity was
quantified as the amount of enzyme required to degrade 1 µmol
hydrogen peroxide/min. The activity of SOD was evaluated with a SOD
Assay kit (cat. no. 574601; Merck Millipore) according to the
manufacturer's guidelines. SOD activity was quantified as the
amount of enzyme necessary to achieve 50% dismutation of the
superoxide radical.
Determination of inflammatory
markers
Quantitative measurement of TNF-α levels in the
pancreas was conducted using a Rat TNF-α ELISA kit (cat. no.
RAB0479; Sigma-Aldrich, Merck KGaA) in accordance with the
manufacturer's guidelines.
Western blotting
Liver tissue (80 µg) was lysed in 200 µl RIPA
solution containing a protease inhibitor cocktail (Wuhan Servicebio
Technology Co., Ltd.). The tissue homogenates were centrifuged at
14,000 x g for 15 min at 4˚C and the supernatant was collected. The
total protein concentration was measured using a Micro BCA protein
assay kit (Thermo Fisher Scientific, Inc.). Equal amounts of total
protein (150 µg/lane) were resolved using 12% (w/v) SDS-PAGE and
subsequently transferred to a PVDF membrane (Bio-Rad Laboratories,
Inc.). Non-specific binding was blocked using 5% (w/v) skimmed milk
in Tris-buffered saline with 0.1% Tween 20 (TBST) for 60 min at
room temperature. The membranes were incubated overnight at 4˚C
with rat monoclonal anti-PEPCK (cat. no. 12940S; Cell Signaling
Technology, Inc.) and mouse monoclonal anti-β-actin (both 1:1,000;
cat. no. STCSC-47778; Santa Cruz Biotechnology, Inc.). After
washing with TBST buffer, the membranes were incubated with
HRP-conjugated secondary antibodies (PEPCK, 1:3,000; β-actin,
1:5,000; both Cell Signaling Technology, Inc. cat. nos. 7074S and
7076S, respectively) for 1 h at room temperature. Protein bands
were detected using DAB substrate (cat.no. E-IR-R101; Elabscience,
Wuhan, China), and band intensities were assessed with ImageJ
software (version 1.43; National Institutes of Health).
Histopathological examination
The pancreatic tissue was excised and weighed. Half
of each tissue specimen was preserved in 10% neutral buffered
formalin at room temperature for 24 h and processed for embedding
in paraffin blocks. Thin slices (5 µm) of paraffin-embedded tissue
were prepared, deparaffinized and stained with hematoxylin for 8
min and eosin for 1 min at room temperature. Images (x200) were
captured using Nikon ECLIPSE Ni-U light microscope and analyzed
using NIS-Elements software (Nikon Corporation).
Statistical analysis
All data were analyzed using SPSS software (version
23; IBM Corp.). All data are presented as the mean ± SEM. Normality
of the data distribution was assessed using the Shapiro-Wilk test.
Differences were analyzed using one-way ANOVA followed by
Tukey-Kramer's post hoc test. P#x003C;0.05 was considered to
indicate a statistically significant difference.
Results
Bioactive compounds derived from
SSE
The total phenolic compound content was 10.21±0.22
mg GAE/g, as reported in our previous work (13).
Effect of SSE on FBG, body weight and
food intake
FBG levels were elevated in diabetic (DM) compared
with control rats (Table I).
Diabetic rats treated with SSE at 400 mg/kg (DM + SSE) exhibited
decreased FBG levels, however, this reduction was not statistically
significant. By contrast, diabetic rats treated with glibenclamide
at 5 mg/kg (DM + GB) demonstrated a significant decrease in FBG
compared with DM rats. Despite increased food intake, DM rats
showed a marked decrease in body weight compared with control rats.
Following 28 days of SSE treatment, the DM + SSE rats exhibited an
increase in body weight, however, this was not significant compared
with untreated DM rats.
 | Table IEffects of SSE on FBG, body weight
and food intake in diabetic rats. |
Table I
Effects of SSE on FBG, body weight
and food intake in diabetic rats.
| | Mean FBG,
mg/dl | Mean body weight,
g | Mean food intake,
g |
|---|
| Group | Week 1 | Week 4 | Week 1 | Week 4 | Week 1 | Week 4 |
|---|
| Control | 100.33±22.06 | 106.50±24.37 | 255.23±6.54 | 508.12±16.56 | 22.98±3.78 | 17.73±2.01 |
| DM |
389.33±31.20a |
425.00±34.47a | 250.15±8.01 |
334.33±20.29a | 29.87±4.62 |
35.83±2.46a |
| DM + SSE |
401.33±31.20a |
314.67±35.79a | 263.60±7.16 |
372.14±18.14a | 28.20±4.62 |
35.95±2.46a |
| DM + GB |
311.40±24.17a |
278.80±21.55a,b | 249.08±7.16 |
370.62±18.14a | 23.36±4.14 |
36.52±2.20a |
Effect of SSE on serum and pancreatic
insulin levels
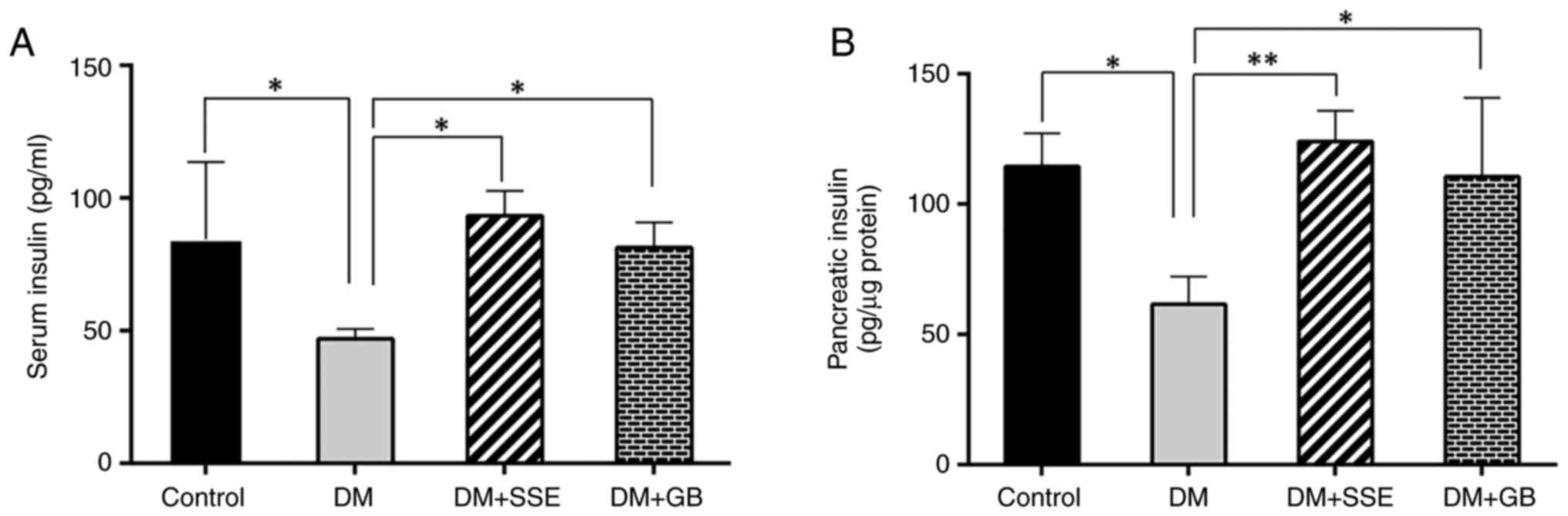
Serum (Fig. 2A) and
pancreatic insulin levels (Fig. 2B)
were significantly decreased in DM compared with control rats.
Notably, DM + SSE or DM + GB rats exhibited elevated serum and
pancreatic insulin levels compared with untreated DM rats. These
findings underscore the potential of SSE to alleviate hyperglycemia
in diabetic rats by preserving pancreatic β cells, as indicated by
the maintenance of islet area and enhancing insulin release.
Effect of SSE on lipid peroxidation in
pancreatic tissue
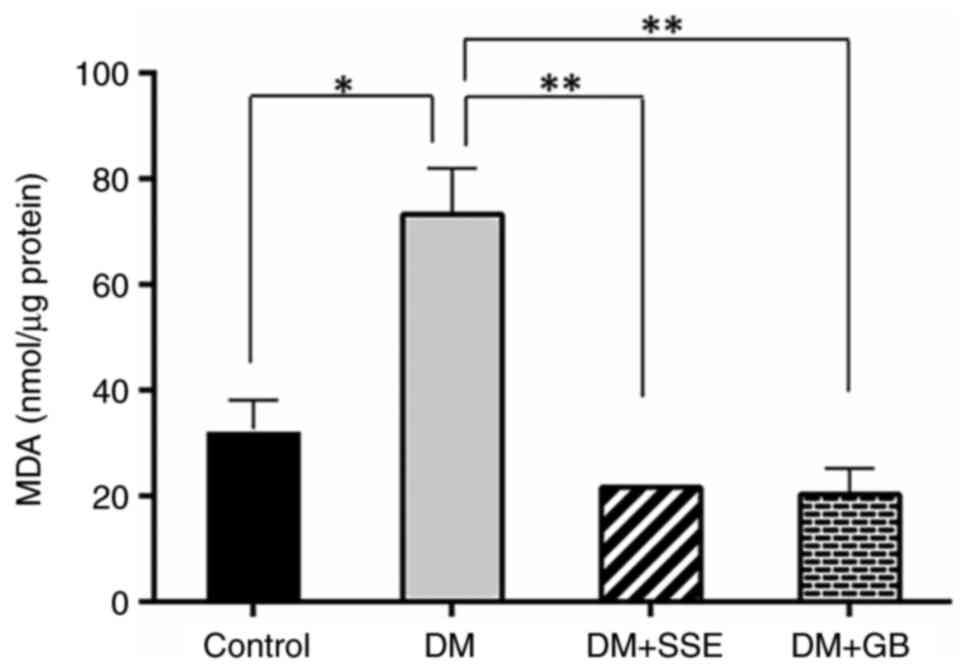
TBARS levels indicate MDA as a byproduct of lipid
peroxidation. Compared with control rats, MDA levels in DM rats
were significantly elevated (Fig.
3). However, after 28 days of treatment with SSE and
glibenclamide, MDA levels in the treated diabetic rats
significantly decreased.
Effect of SSE on CAT and SOD
activities
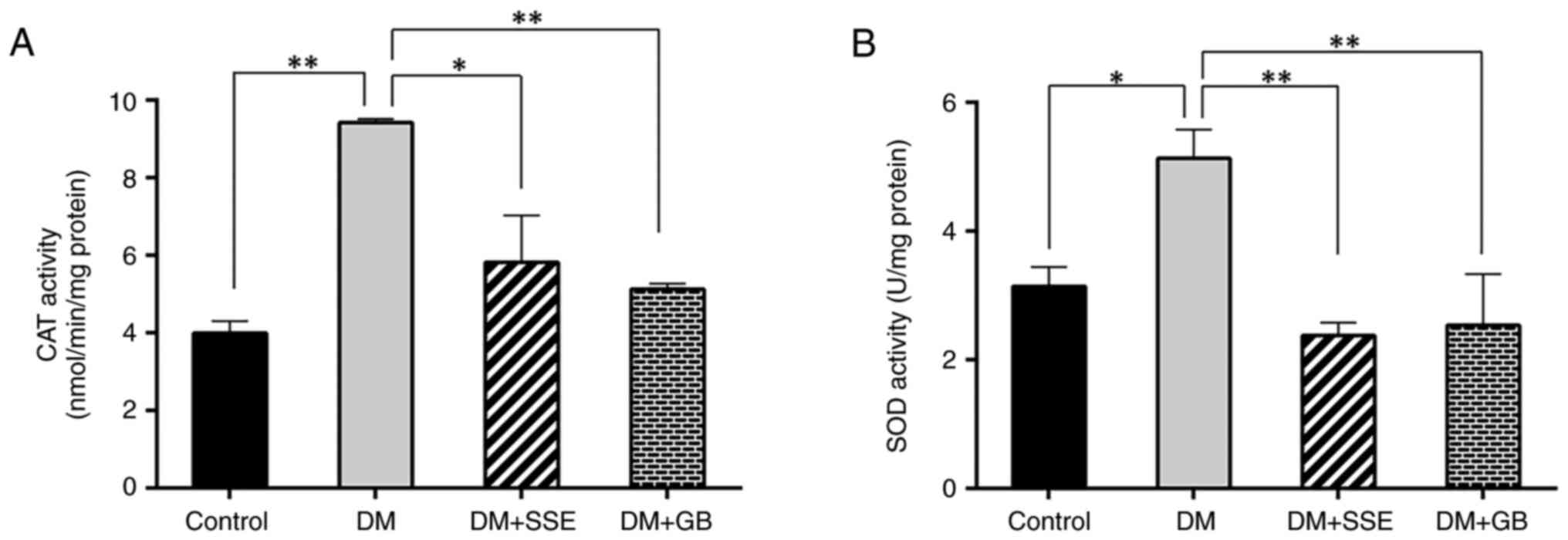
DM rats exhibited increased CAT activity compared
with control rats, indicating heightened oxidative stress (Fig. 4A). SSE significantly decreased CAT
activity compared with untreated DM rats. The amount of SOD
required to eliminate superoxide radicals was elevated in DM rats,
indicating increased SOD activity. SSE treatment led to a
substantial reduction in SOD activity compared with untreated DM
rats (Fig. 4B). These data suggest
that the decrease in antioxidant enzyme activity in SSE-treated
diabetic rats resulted from a reduction of free radicals in
pancreatic tissue.
Effect of SSE on proinflammatory
cytokine markers
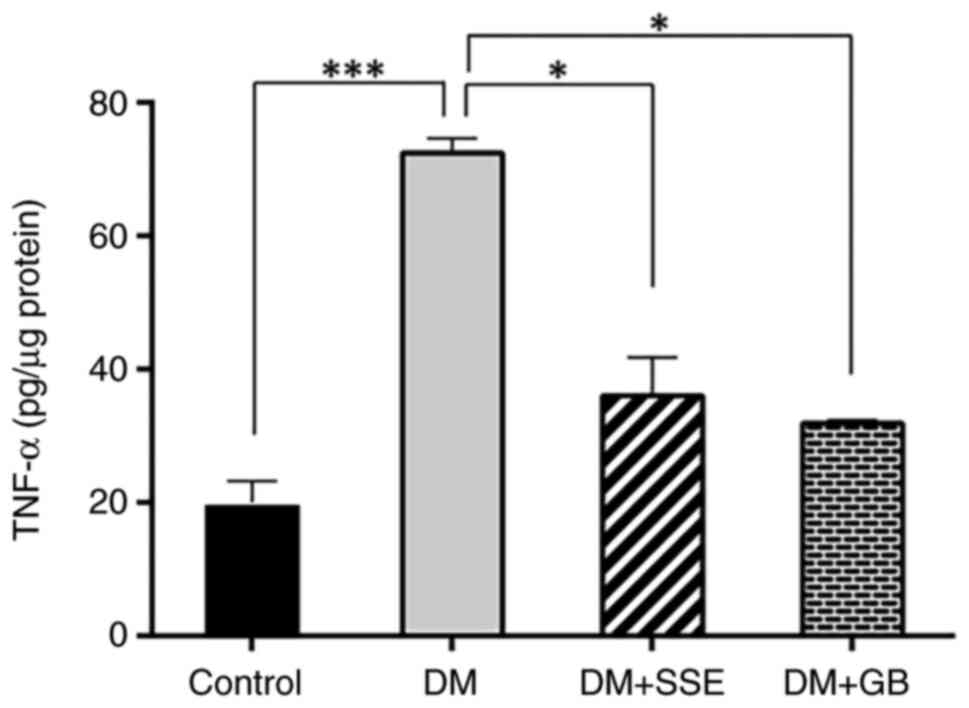
The apoptosis of pancreatic β cells in diabetes is
associated with proinflammatory cytokines (18). Therefore, TNF-α, a key
proinflammatory cytokine, was measured using ELISA to determine if
SSE preserves pancreatic β cells by reducing the synthesis of
proinflammatory cytokines. DM rats had significantly increased
TNF-α levels in pancreatic tissue compared with control rats. SSE
or glibenclamide resulted in a significant decrease in TNF-α levels
in DM rats (Fig. 5).
Effect of SSE on hepatic gluconeogenic
enzymes
The elevation of hepatic glucose synthesis, driven
by the activation of gluconeogenic enzymes, contributes to
hyperglycemia in diabetic conditions (19). The present study evaluated the
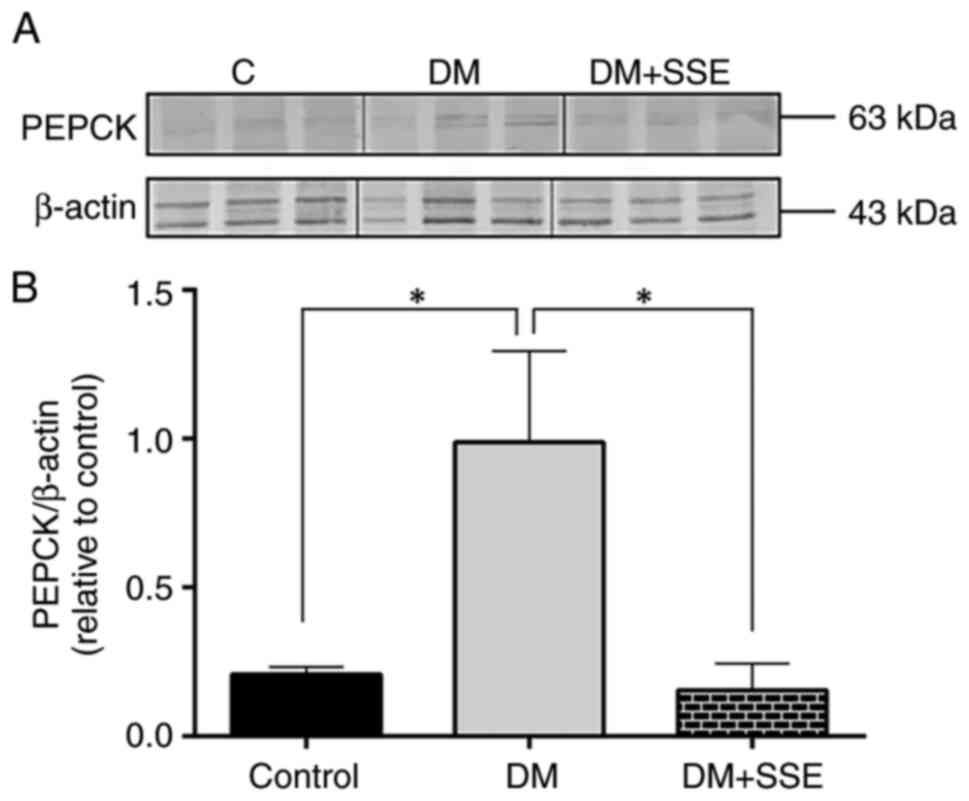
expression of PEPCK, a key gluconeogenic enzyme in the liver, via
western blot analysis. The results demonstrated that hepatic PEPCK
expression was significantly increased in DM rats compared with
controls. However, compared with no treatment, SSE treatment
significantly reduced hepatic PEPCK expression in DM rats (Fig. 6).
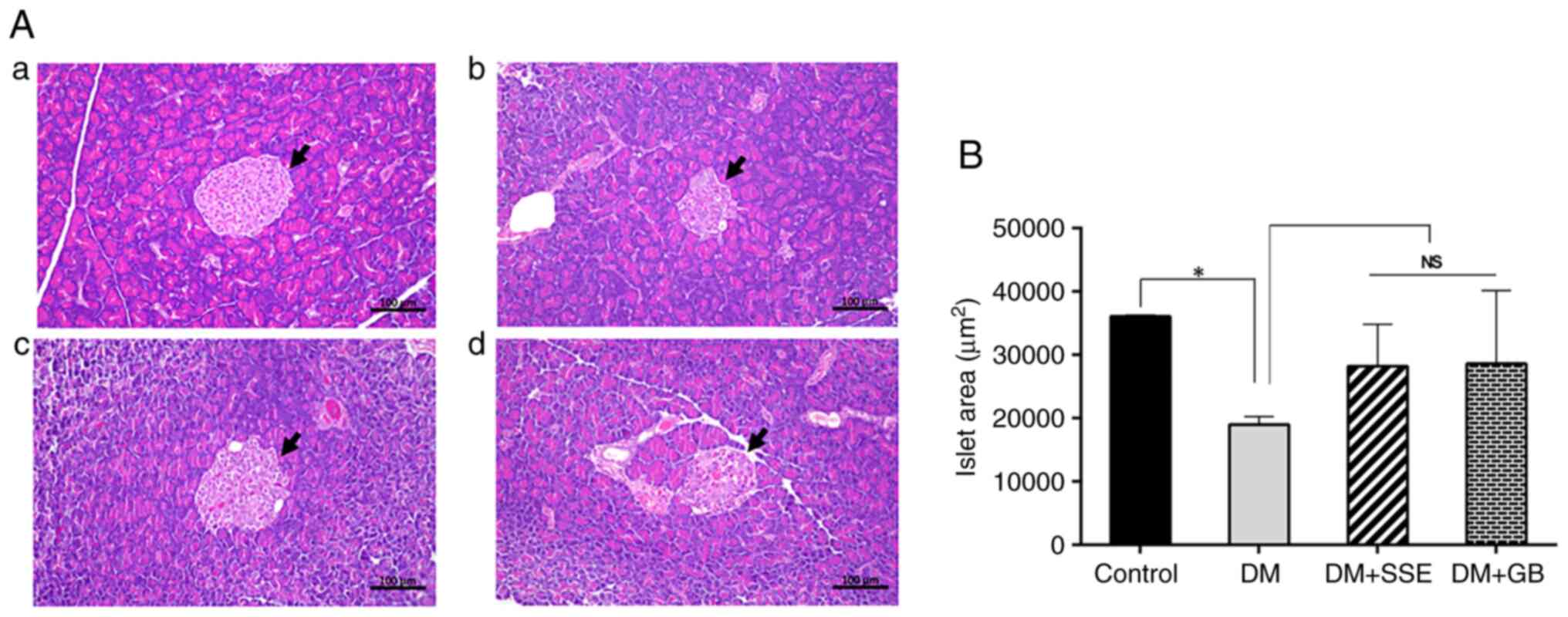
Histopathological changes in
pancreatic islets
Histo-pathological alterations in the pancreatic
islets were observed using H&E staining (Fig. 7). In control rats, the histological
examination revealed the typical structure of islet cells.
Conversely, DM rats exhibited abnormalities, as evidenced by a
notable decrease in islet size and a deviation from the typical
spherical morphology observed in control rats. DM rats administered
SSE, along with those receiving glibenclamide, showed an increase
in pancreatic islet size compared with DM rats; however, this
difference was not statistically significant.
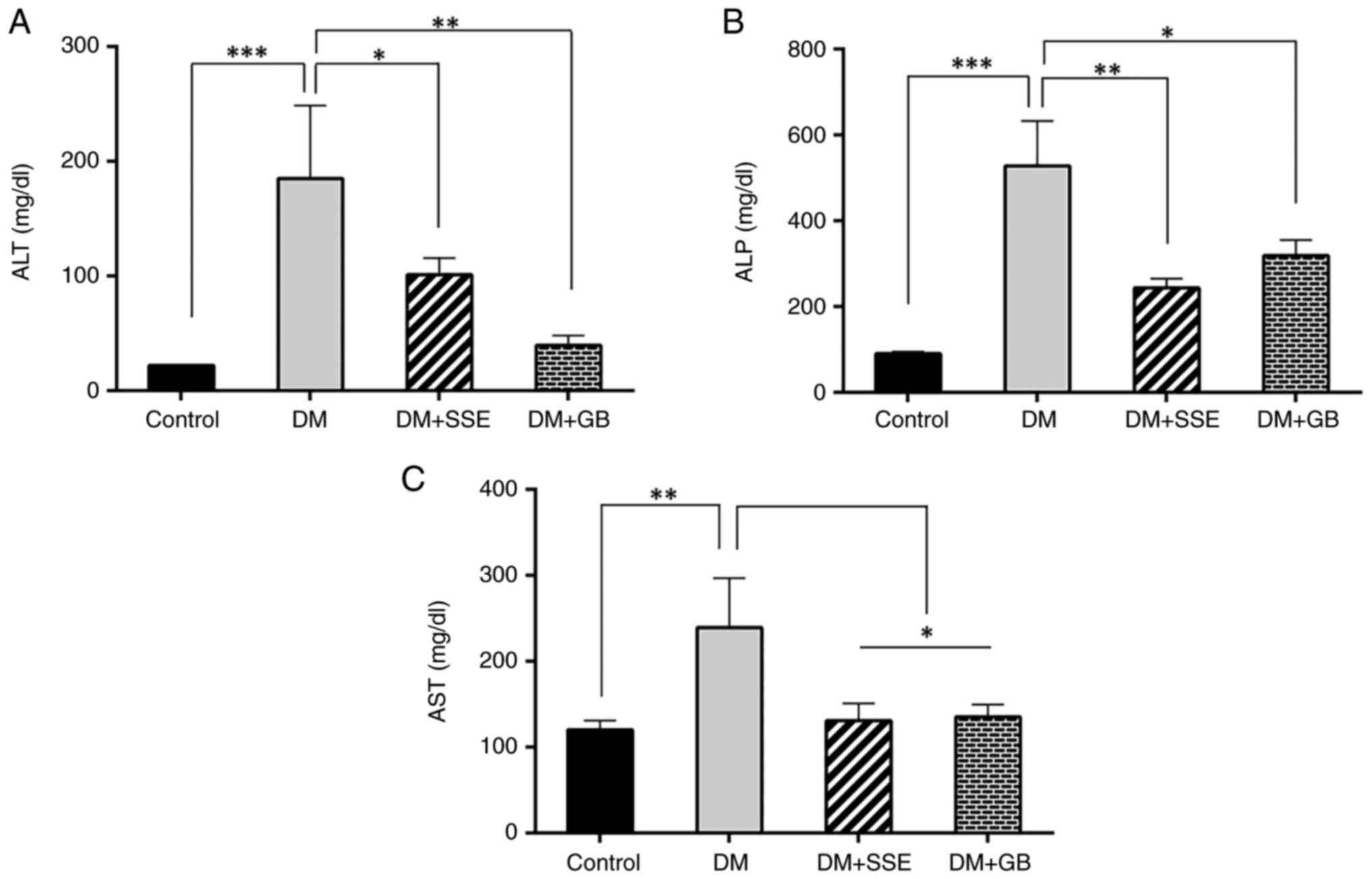
Liver function enzyme levels
Hyperglycemia is recognized as a contributor to
oxidative stress and hepatic damage (20). The present study assessed serum
liver enzyme levels, which serve as indicators of hepatic injury.
DM rats exhibited signs of hepatic injury, as evidenced by
substantial elevations in serum concentrations of alanine
aminotransferase (ALT), alkaline phosphatase (ALP) and aspartate
aminotransferase (AST) (Fig. 8).
SSE or glibenclamide markedly reduced the increased levels of ALT,
ALP, and AST, suggesting a protective effect of SSE against
diabetes-induced hepatic damage.
Discussion
Rats with T2DM induced by a combination of HFD and
low-dose STZ were used to examine the potential antidiabetic
effects of SSE, as well as its impact on pancreatic oxidative
stress and inflammation. Consistent with findings in the literature
(21), the present results
demonstrated a notable increase in glucose levels and food intake,
accompanied by a decrease in body weight in rats subjected to HFD
and low-dose STZ treatment. Additionally, diabetic rats presented
decreased serum and pancreatic insulin levels. The weight loss in
diabetic rats can be attributed to increased protein breakdown and
fat mobilization due to diminished insulin production, which
impairs glucose absorption and utilization (12). Moreover, impaired insulin secretion
contributes to increased appetite, leading to polyphagia in
diabetic rats (22). Treatment with
SSE over a 28-day period resulted in decreased hyperglycemia in
diabetic rats, alongside significantly elevated serum and
pancreatic insulin levels, indicating the potential of SSE to
preserve pancreatic β cell mass and enhance insulin production.
This aligns with a prior study involving STZ-induced type 1
diabetic rats, where administration of SSE fruit powder (100 mg/kg)
attenuated hyperglycemia, increased serum insulin concentrations
and enhanced pancreatic β cell activity, as evidenced by increased
Homeostatic Model Assessment of β cell Function (12). SSE fruit powder contains notable
quantities of phenolics, flavonoids and anthocyanins, which exhibit
considerable antidiabetic properties by improving pancreatic β cell
function and promoting insulin production in individuals with
diabetes (23,24). Furthermore, our previous
investigation revealed that SSE comprises at least 12 compounds,
including cinnamic and glucaric acid, maloyl-hexose, citric,
mono-caffeoylquinic, gallic, 2-methylcitric and ellagic acid,
naringin-O-glucoside, kaempferol deoxyhexose, benzyl-diglycoside
and matairesinol (13). Among these
compounds, naringin has been extensively studied for its diverse
antidiabetic effects (23-26).
Naringin has demonstrated a dose-dependent decrease in blood
glucose levels and an elevation in insulin concentrations through
its antioxidative effects in STZ-induced diabetic rats (25). Additionally, naringin effectively
decreases inflammation, oxidative stress and mitochondrial
apoptosis caused by T2DM-related steatohepatitis via the RAGE/NF-κB
pathway (26). Kaempferol, another
flavonoid, also exhibits antidiabetic properties. The
administration of kaempferol to STZ-induced diabetic rats is
associated with a return to near-normal levels of plasma glucose,
insulin, lipid peroxidation products and both enzyme- and
non-enzyme-dependent antioxidants (27). In diabetic mice, kaempferol restores
hexokinase activity while inhibiting hepatic pyruvate carboxylase
activity and gluconeogenesis, indicating its ability to enhance
skeletal muscle glucose metabolism and diminish liver
gluconeogenesis (28). To the best
of our knowledge, matairesinol, a notable secondary metabolite
found in SSE, has been less extensively studied for its
antidiabetic effects. However, in a rat model of brain sepsis,
matairesinol increases antioxidant enzyme levels in brain tissue
and inhibits neuronal death (29).
Hyperglycemia induces the generation of ROS while
simultaneously impairing the antioxidant defense mechanisms. This
imbalance can lead to oxidative stress, promoting pancreatic β cell
death and a subsequent decline in insulin production over time
(30). The present study
investigated the antioxidant properties of SSE in pancreatic
tissue. SSE in diabetic rats not only enhanced the activity of
antioxidant enzymes but also decreased levels of oxidative stress
markers. In the pancreatic tissue of rats with STZ-induced
diabetes, SSE fruit powder demonstrates antioxidant activity,
associated with an increase in insulin-expressing pancreatic β
cells and pancreatic insulin protein levels (12). Additionally, SSE exerts notable
antioxidant effects in liver damage induced by carbon tetrachloride
(31). These beneficial effects may
be due to the presence of potent antioxidant compounds in SSE,
including phenolics, gallic acid, kaempferol, naringin and
matairesinol.
Oxidative stress and inflammatory responses are
associated with hyperglycemia. Hyperglycemia triggers oxidative
stress and inflammatory signaling pathways, resulting in the
synthesis of inflammatory cytokines within pancreatic β cells,
ultimately leading to cellular damage and dysfunction (32). Consistent with prior studies
(33,34), the present study observed elevated
levels of TNF-α in the pancreatic tissue of diabetic rats, while
treatment with SSE resulted in a decrease in TNF-α levels.
Furthermore, SSE alleviates insulin resistance and mitigates
inflammation in the liver following TNF-α exposure (35).
Dysregulation of glucose metabolism is a common
feature of diabetes and contributes to increased hepatic glucose
production through gluconeogenesis. This upregulation is primarily
driven by the increased expression of key gluconeogenic enzymes
such as PEPCK and glucose-6-phosphatase, which exacerbates
hyperglycemia under diabetic conditions (20). Here, SSE significantly downregulated
the expression of hepatic PEPCK, indicating its potential role in
suppressing gluconeogenesis. This effect may be due to kaempferol,
a major bioactive compound found in SSE. Additionally, other
phytochemicals present in SSE, such as matairesinol and glucaric
acid, suppress PEPCK expression by inhibiting the signaling
pathways of PGC-1α and FOXO1, key transcription factors that
regulate gluconeogenic gene expression (36,37).
SSE exhibited hepatoprotective effects, as evidenced
by a significant decrease in elevated liver enzyme levels,
including AST, ALT and ALP, in diabetic rats. These beneficial
effects may be associated with the antioxidant and
anti-inflammatory properties of the bioactive constituents within
SSE.
To the best of our knowledge, the present study is
the first investigation of the antidiabetic properties of SSE in
rats with diabetes induced by HFD and low-dose STZ. The effects are
attributed to the enhancement of antioxidant defense mechanisms,
reduction in oxidative stress levels and suppression of the
proinflammatory cytokine TNF-α. These findings suggest that SSE may
serve as a supplementary treatment to help preserve β cell function
in individuals with T2DM. However, the present study had
limitations, including the use of a single SSE dose (400 mg/kg), a
relatively small sample size and a short treatment duration (4
weeks). Future investigations should include multiple doses,
extended treatment periods and larger sample sizes to optimize
therapeutic efficacy and assess potential long-term effects and
safety of SSE.
Acknowledgements
The authors would like to thank Associate Professor
Songsak Chumpawadee (Department of Agricultural Technology,
Mahasarakham University, Maha Sarakham, Thailand) for producing the
high-fat diet used to induce T2DM in the rats. The authors would
also like to thank Mr Tanapat Wisapon (Faculty of Pharmaceutical
Sciences, Ubon Ratchathani University, Ubon Ratchathani, Thailand),
who contributed to the design of equipment for pellet production
and drying, facilitating the preparation of high-fat diet for the
experimental rats.
Funding
Funding: The present study was supported by the National
Science, Research, and Innovation Fund (Fundamental Fund 2565-2566)
and the Faculty of Pharmaceutical Sciences, Ubon Ratchathani
University (grant no. phar.ubu.0604-11-3/2567).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
NM and KS conceived the study and wrote the
manuscript. BY designed and performed experiments, constructed
figures and wrote the manuscript. NM, KS, PP, SP, SS, JK, PK, JO,
CP and YB designed and performed experiments and analyzed data. NC
performed experiments and analyzed data. NM and KS confirm the
authenticity of all the raw data. NM edited the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Animal
Ethics Committee of Ubon Ratchathani University (approval no.
37/2564 IACUC).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stumvoll M, Goldstein BJ and van Haeften
TW: Type 2 diabetes: Principles of pathogenesis and therapy.
Lancet. 365:1333–1346. 2005.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Porte D Jr and Kahn SE: Beta-cell
dysfunction and failure in type 2 diabetes: Potential mechanisms.
Diabetes. 50 (Suppl 1):S160–S163. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Park JH, Shim HM, Na AY, Bae KC, Bae JH,
Im SS, Cho HC and Song DK: Melatonin prevents pancreatic β-cell
loss due to glucotoxicity: The relationship between oxidative
stress and endoplasmic reticulum stress. J Pineal Res. 56:143–153.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tang C, Han P, Oprescu AI, Lee SC,
Gyulkhandanyan AV, Chan GNY, Wheeler MB and Giacca A: Evidence for
a role of superoxide generation in glucose-induced beta-cell
dysfunction in vivo. Diabetes. 56:2722–2731. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Coskun O, Kantera M, Korkmaz A and Oter S:
Quercetin, a flavonoid antioxidant, prevents and protects
streptozotocin-induced oxidative stress and beta-cell damage in rat
pancreas. Pharmacol Res. 51:117–123. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Saltiel AR and Kahn CR: Insulin signalling
and the regulation of glucose and lipid metabolism. Nature.
414:799–806. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Lu M, Wan M, Leavens KF, Chu Q, Monks BR,
Fernandez S, Ahima RS, Ueki K, Kahn CR and Birnbaum MJ: Insulin
regulates liver metabolism in vivo in the absence of hepatic Akt
and Foxo1. Nat Med. 18:388–395. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Adeva-Andany MM, Pérez-Felpete N,
Fernández-Fernández C, Donapetry-García C and Pazos-García C: Liver
glucose metabolism in humans. Biosci Rep. 36(e00416)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Mobasheri L, Ahadi M, Beheshti Namdar A,
Alavi MS, Bemidinezhad A, Moshirian Farahi SM, Esmaeilizadeh M,
Nikpasand N, Einafshar E and Ghorbani A: Pathophysiology of
diabetic hepatopathy and molecular mechanisms underlying the
hepatoprotective effects of phytochemicals. Biomed Pharmacother.
167(115502)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shü ZH, Shiesh CC and Lin HL: Wax apple
(Syzygium samarangense (Blume) Merr and L.M. Perry) and
related species. In: Postharvest Biology and Technology of Tropical
and Subtropical Fruits. Vol 4. pp458-473, 2011.
|
|
11
|
Mukaromah AS: Wax apple (Syzygium
samarangense (Blume) Merr & L.M. Perry): A comprehensive
review in phytochemical and physiological perspectives. Perspect J
Biol Appl Biol. 3:40–58. 2020.
|
|
12
|
Khamchan A, Paseephol T and Hanchang W:
Protective effect of wax apple [Syzygium samarangense
(Blume) Merr. & L.M. Perry] against streptozotocin-induced
pancreatic β-cell damage in diabetic rats. Biomed Pharmacother.
108:634–645. 2018.
|
|
13
|
Suksri K, Yingngam B and Muangchan N:
Optimization of phenolic extraction from Syzygium
samarangense fruit and its protective properties against
glucotoxicity-induced pancreatic β-cell death. ScienceAsia.
49:529–540. 2023.
|
|
14
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high-fat diet-fed and low-dose
streptozotocin-treated rat: A model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320.
2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miaffo D, Ntchapda F, Mahamad TA, Maidadi
D and Kamanyi A: Hypoglycemic, antidyslipidemic and antioxydant
effects of Vitellaria paradoxa barks extract on high-fat diet and
streptozotocin-induced type 2 diabetes rats. Metabol Open.
9(100071)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gandhi GR and Sasikumar P: Antidiabetic
effect of Merremia emarginata Burm. F. in streptozotocin induced
diabetic rats. Asian Pac J Trop Biomed. 2:281–286. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
American Veterinary Medical Association.
AVMA Guidelines for the Euthanasia of Animals. AVMA, Schaumburg,
IL, 2020. Available from: https://www.avma.org/resources-tools/avma-policies/avma-guidelines-euthanasia-animals.
|
|
18
|
Russell MA and Morgan NG: The impact of
anti-inflammatory cytokines on the pancreatic β-cell. Islets.
6(e950547)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang S, Young JL, Wang K, Qian Y and Cai
L: Diabetic-induced alterations in hepatic glucose and lipid
metabolism: The role of type 1 and type 2 diabetes mellitus
(review). Mol Med Rep. 22:603–611. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yoopum S, Wongmanee N, Rojanaverawong W,
Rattanapunya S, Sumsakul W and Hanchang W: Mango (Mangifera indica
L.) seed kernel extract suppresses hyperglycemia by modulating
pancreatic β cell apoptosis and dysfunction and hepatic glucose
metabolism in diabetic rats. Environ Sci Pollut Res Int.
30:123286–123308. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hininger-Favier I, Benaraba R, Coves S,
Anderson RA and Roussel AM: Green tea extract decreases oxidative
stress and improves insulin sensitivity in an animal model of
insulin resistance, the fructose-fed rat. J Am Coll Nutr.
28:355–361. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ozougwu JC, Obimba KC, Belonwu CD and
Unakalamba CB: The pathogenesis and pathophysiology of type 1 and
type 2 diabetes mellitus. J Physiol Pathophysiol. 4:46–57.
2013.
|
|
23
|
Lin D, Xiao M, Zhao J, Li Z, Xing B, Li X,
Kong M, Li L, Zhang Q, Liu Y, et al: An overview of plant phenolic
compounds and their importance in human nutrition and management of
type 2 diabetes. Molecules. 21(1374)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dragan S, Andrica F, Maria-Corina S and
Timar R: Polyphenols-rich natural products for treatment of
diabetes. Curr Med Chem. 22:14–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ali MM and Abd El Kader MA: The influence
of naringin on the oxidative state of rats with
streptozotocin-induced acute hyperglycaemia. Z Naturforsch C J
Biosci. 59:726–733. 2004.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Syed AA, Reza MI, Shafiq M, Kumariya S,
Singh P, Husain A, Hanif K and Gayen JR: Naringin ameliorates type
2 diabetes mellitus-induced steatohepatitis by inhibiting
RAGE/NF-κB mediated mitochondrial apoptosis. Life Sci.
257(118118)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Al-Numair KS, Chandramohan G, Veeramani C
and Alsaif MA: Ameliorative effect of kaempferol, a flavonoid, on
oxidative stress in streptozotocin-induced diabetic rats. Redox
Rep. 20:198–209. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Alkhalidy H, Moore W, Wang Y, Luo J,
McMillan RP, Zhen W, Zhou K and Liu D: The flavonoid kaempferol
ameliorates streptozotocin-induced diabetes by suppressing hepatic
glucose production. Molecules. 23(2338)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu Q, Wang Y and Li Q: Matairesinol exerts
anti-inflammatory and antioxidant effects in sepsis-mediated brain
injury by repressing the MAPK and NF-κB pathways through
up-regulating AMPK. Aging (Albany NY). 13:23780–23795.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Banday MZ, Sameer AS and Nissar S:
Pathophysiology of diabetes: An overview. Avicenna J Med.
10:174–188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sobeh M, Youssef FS, Esmat A, Petruk G,
El-Khatib AH, Monti DM, Ashour ML and Wink M: High resolution
UPLC-MS/MS profiling of polyphenolics in the methanol extract of
Syzygium samarangense leaves and its hepatoprotective
activity in rats with CCl4-induced hepatic damage. Food
Chem Toxicol. 113:145–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rehman K and Akash MSH: Mechanism of
generation of oxidative stress and pathophysiology of type 2
diabetes mellitus: How are they interlinked? J Cell Biochem.
118:3577–3585. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rashid K and Sil PC: Curcumin enhances
recovery of pancreatic islets from cellular stress induced
inflammation and apoptosis in diabetic rats. Toxicol Appl
Pharmacol. 282:297–310. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mardiah Zakaria FR, Prangdimurti E and
Damanik R: Anti-inflammatory of purple roselle extract in diabetic
rats induced by streptozotocin. Procedia Food Sci. 3:182–189.
2015.
|
|
35
|
Shen SC, Chang WC and Chang CL: Fraction
from wax apple [Syzygium samarangense (Blume) Merrill and
Perry] fruit extract ameliorates insulin resistance via modulating
insulin signaling and inflammation pathway in tumor necrosis factor
α-treated FL83B mouse hepatocytes. Int J Mol Sci. 13:8562–8577.
2012.
|
|
36
|
Zhang Y: Flavonoids as potential agents
for the treatment of diabetes and its complications. Molecules.
25(5646)2020.
|
|
37
|
Rendell MS: Current and emerging
gluconeogenesis inhibitors for the treatment of type 2 diabetes.
Expert Opin Pharmacother. 22:2167–2179. 2021.PubMed/NCBI View Article : Google Scholar
|