Introduction
Acute lymphoblastic leukemia (ALL) is characterized
by the uncontrolled proliferation of lymphoid precursor cells. The
ALL-B subtype constitutes a substantial portion of leukemia cases
in the pediatric population, accounting for ~80% of diagnoses. By
contrast, the T-lymphoid subtype accounts for 10-15% of childhood
ALL cases (1).
The prognosis of leukemia depends on the type and
characteristics of the patient at diagnosis, including cytogenetic
and molecular alterations, the count of white blood cells at
diagnosis, response to primary therapy, and the phenotype of the
blasts (B and T lymphoid precursor cells and myeloid precursor
cells) (2). Current treatments have
led to significant increases in patient survival rates, with
improvements of up to 90% in five years. However, a percentage of
patients do not respond and relapse (3). This event is associated with cancer
stem cells (CSCs), which play a crucial role in resistance to
chemotherapy and radiotherapy, leading to chemo-radioresistant
tumors (4).
The majority of genes associated with hematological
malignancies code for transcription factors, and these play key
roles in regulating the cell cycle, and in the proliferation,
differentiation and survival of lymphoid and myeloid precursors
(5). Notably, CSCs participate in
the origin and progression of cancers (6).
Oct3/4 (also known as Pou5f1, Oct3, OTF3 and OTF4)
is a transcription factor located in stem cells. Its expression is
associated with pluripotency and self-renewal, making it a key
marker for CSCs. The Oct3/4 gene generates three isoforms via
alternative splicing, each with different functions (7). For example, Oct3/4A is located in the
nucleus of embryonic stem cells (ESCs) and CSCs, and functions as a
transcription factor to maintain cell pluripotency; Oct3/4B is
located in the cytoplasm of cancer cells, and cannot sustain the
pluripotency of ESCs (8) and
exhibits anti-apoptotic functions (9). Oct3/4B1 is located in both the
cytoplasm and nucleus of pluripotent cells (10), and participates as an anti-apoptotic
factor in tumorigenesis (11).
Results of previous studies revealed that Oct3/4 is
a prognostic marker of disease, and elevated expression of Oct3/4
has been reported in solid tumors (12-15).
In individuals with acute myeloid leukemia (AML), Oct3/4 expression
is elevated in leukemic stem cells, specifically in
CD34+ CD38- cells (16). In addition, Oct3/4 has been
recognized as a negative prognostic factor associated with lower
survival rates. Results of previous studies also indicated that
elevated Oct3/4 expression levels may act as a prognostic marker in
patients with leukemia (17-19).
However, the expression levels of Oct3/4A, Oct3/4B and Oct3/4B1,
and the specific association with the risk of relapse in patients
with ALL, are yet to be fully elucidated. Thus, the present study
aimed to analyze the expression of Oct4A, Oct4B and Oct4B1, and
determine whether the expression of Oct3/4 isoforms is associated
with relapse in patients with ALL.
Materials and methods
Data source and Gene Expression
Omnibus (GEO) data sets
GSE7638(20),
GSE635(21), GSE9006(22) and GSE5820(23) microarray datasets were downloaded
from the GEO (http://www.ncbi.nlm.nih.gov/geo) and Gene Expression
database of Normal and Tumor tissues 2 (GENT2; http://gent2.appex.kr/gent2/) databases. The platform,
including four microarrays, was GPL96 [(HG-U133A) Affymetrix Human
Genome U133A Array]. Data for non-malignant ALL, based on
morphological criteria, cytochemical staining, and
immunophenotyping of blast cells (reported by hospitals, including
St. Jude Children's Research Hospital and the Hematology/Oncology
Department of Children's Hospital), were used to assess potential
differences in Oct3/4 expression.
Study population
Bone marrow (patients with ALL) and blood (healthy
individuals) samples were collected in the Pediatric Oncology
Service of the State Cancer Institute (SCI) from the South of
Mexico (Acapulco, Mexico), between September 2016 and April 2020.
The samples were placed in tubes with an anticoagulant (EDTA) and
stored in the biobank of the laboratory of Molecular Biomedicine at
the FCQB of Autonomous University of Guerrero. Leukocytes were
purified by selective osmotic lysis, as previously described by
Gómez-Gómez et al (24).
Total RNA was isolated from the bone marrow and blood samples using
the TRIzol® method (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. Total
RNA was stored at -80˚C in the biobank of the laboratory of
Molecular Biomedicine at the FCQB of Autonomous University of
Guerrero.
The present study involved case and control groups:
Cases included samples (total RNA) of patients diagnosed with ALL
(n=51), based on established clinical, cytomorphological, molecular
and immunological criteria. The study also involved 12 healthy
individuals as the control group (4-10x103
leukocytes/mm3) in the absence of a family history of
leukemia. In 2020, RNA samples were received from the biobank of
the laboratory of Molecular Biomedicine at the FCQB of Autonomous
University of Guerrero (Chilpancingo, Mexico). The bone marrow and
blood samples were obtained from patients as part of the samples
taken for clinical diagnostic tests. The present study was approved
by the ethics committee of the Institute of Cancer of the State of
Guerrero, Mexico (approval no. FO-INV-AUT-2018) and by the
committee of the biobank of the laboratory of Molecular Biomedicine
at the FCQB of Autonomous University of Guerrero (Chilpancingo,
Mexico) (approval no. BB-LBM-03-2020). All participants or their
guardians provided written informed consent following a thorough
explanation of the study's objectives Relapse is characterized by
the emergence of blast cells in the marrow or the presence of
localized leukemic infiltrates at any site following the conclusion
of induction chemotherapy, according to protocols previously
established (24,25).
Reverse-transcription quantitative
(RT-q) PCR
A total of 500 ng of RNA was reverse-transcribed
into cDNA using the SuperScript II First-Strand Synthesis System
(Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. qPCR was performed using the following thermocycling
conditions: 65˚C for 10 min, 22˚C for 10 min, 42˚C for 90 min, and
75˚C for 5 min. cDNA was stored at -20˚C for subsequent
experiments. qPCR was performed using a total volume of 15 µl,
consisting of 7.5 µl of 2X TaqMan Universal PCR Master Mix II, 0.5
µM of each primer, 200 ng of template per reaction, and ultrapure
water. Primer sequences and product sizes are outlined in Table SI, and the efficiency and
specificity of these primers were plotted by Asadi et al
(26) and are outlined in Table SI. mRNA expression levels were
quantified using the 2-ΔΔCq method (27). and normalized to the internal
reference gene, GAPDH.
Statistical analysis
Continuous data are presented as the mean ± standard
deviation (SD) and/or median with the 25th and 75th percentiles.
Categorical data comparisons were conducted using Chi-squared or
Fisher's exact tests. The Mann-Whitney test was employed to assess
differences in the expression levels of Oct3/4A, Oct3/4B and
Oct3/4B1 between groups. The association between Oct3/4A, Oct3/4B
and Oct3/4B1 expression and the risk of relapse in patients with
ALL was evaluated using odds ratios (ORs) and 95% confidence
intervals (CIs) derived from univariate and multivariate logistic
regression analyses. Statistical analysis was performed using SPSS
(version 20.0; IBM Corp.) and GraphPad Prism (version 5.0; GraphPad
Software, Inc.; Dotmatics). P<0.05 was considered to indicate a
statistically significant difference.
Results
Oct3/4 is expressed in patients with
ALL obtained from the GEO data sets
Analysis of mRNA expression levels in patients with
ALL and control samples obtained from the GSE datasets in GEO and
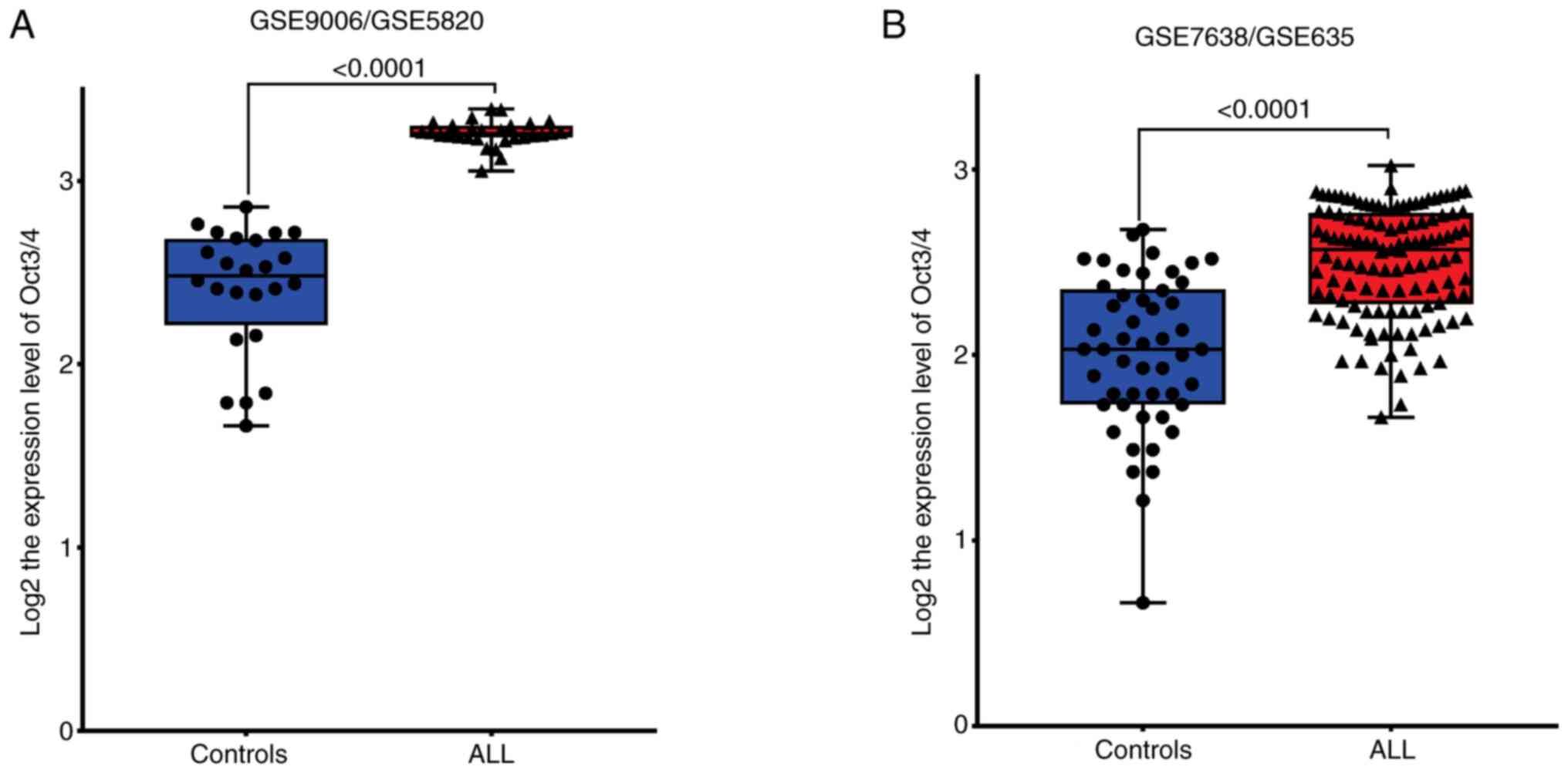
GENT2 databases indicated that Oct3/4 expression in patients with
ALL was higher than in healthy individuals [Fig. 1A (GSE9006/GSE5820) and Fig. 1B (GSE7638/GSE635)]. To the best of
our knowledge, there are no previous literature describing the
presence of the Oct3/4 isoforms in patients with ALL.
Population characteristics
mRNA samples of patients with ALL were obtained from
the biobank of the laboratory of Molecular Biomedicine at the FCQB
of Autonomous University of Guerrero. The present included
participants with a mean age of 8.32±3.99 years, and the median
leukocyte count at diagnosis was 16,300 leukocytes/mm3.
Among patients with ALL, 49.02% were categorized as high-risk, and
58.82% experienced a relapse during treatment. By contrast, healthy
controls exhibited a mean age of 10.58±7.20 years, with a normal
leukocyte count ranging from 4 to 10x103
leukocytes/mm3, and a median of 6,700
leukocytes/mm3. The characteristics of all participants
are presented in Table I.
 | Table IGeneral characteristics and clinicals
of the individuals. |
Table I
General characteristics and clinicals
of the individuals.
| Clinicopathological
characteristics | ALL (n=51) | Controls
(n=12) |
|---|
| Age, years (mean +
SD) | 8.321+3.99 | 10.58+7.20 |
| Leukocyte count,
/mm3 | 16,300
(6,700-37,500)a | 6,700
(6,250-8,450)a |
| Sex, n (%) | | |
|
Female | 26 (50.98) | 7 (58.33) |
|
Male | 25 (49.02) | 5 (41.67) |
| Status of
individuals | | |
|
Alive | 31 (60.78) | 12 (100.00) |
|
Decreased | 20 (39.22) | - |
| Relapse | | |
|
No | 21 (41.18) | - |
|
Yes | 30 (58.82) | - |
| Risk by age and
leukocytes at diagnosis | | |
|
Low-risk | 26 (50.98) | - |
|
High-risk | 25 (49.02) | - |
|
Inmunophenotype | | |
|
B-lineage | 51 (100.00) | - |
| Chromosomal
translocation | | |
|
BCR-ABL
[t(9;22)] | 1 (1.96) | - |
|
ETV6-RUNX1
[t(12;21)] | 4 (7.84) | - |
|
del1(p32)
(STIL-TAL1) | 2 (3.92) | - |
|
None | 44 (86.28) | - |
| CD34
expression | | |
|
CD34- | 24 (47.06) | - |
|
CD34+ | 22 (43.14) | - |
|
no data | 5 (9.80) | - |
| CD34/CD38
expression | | |
|
CD34+CD38+ | 10 (45.45) | - |
|
CD34+CD38- | 12 (54.55) | - |
Oct3/4A, Oct3/4B and Oct3/4B1 are
expressed at high levels in patients with ALL
To determine whether the expression levels of Oct3/4
isoforms differ between patients with ALL and healthy individuals,
samples from 51 patients with ALL and 12 healthy controls, the
expression of Oct3/4A, Oct3/4B and Oct3/4B1 was analyzed in samples
from 51 patients with ALL and 12 healthy controls. The results of
the present study indicated a significant increase in Oct3/4
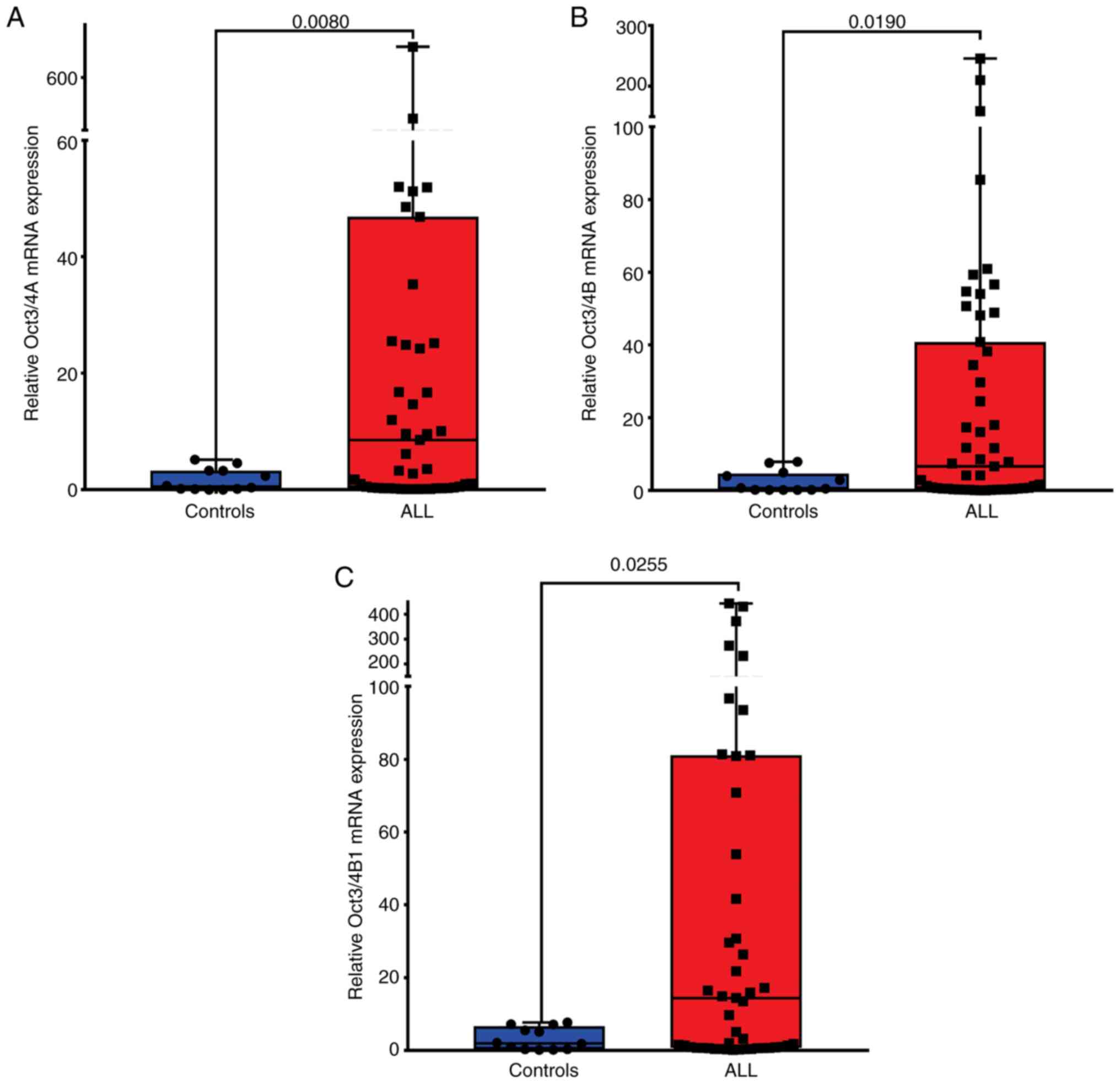
isoforms expression in patients with ALL (Fig. 2). In patients with ALL, the
expression levels of Oct3/4A (median, 8.55; P=0.008; Fig. 2A and Table II), Oct3/4B (median, 6.62; P=0.019;
Fig. 2B and Table II) and Oct3/4B1 (median, 14.42;
P=0.025; Fig. 2C and Table II) were significantly elevated,
compared with those in healthy individuals.
 | Table IIOct3/4 isoforms mRNA expression in
childhood ALL and healthy individuals. |
Table II
Oct3/4 isoforms mRNA expression in
childhood ALL and healthy individuals.
| | | Mean | Std. deviation | Std. error of the
mean | 95% CI of mean | Median | 25-75%
percentiles | P-value |
|---|
| Oct3/4A | Controls | 1.67 | 1.94 | 0.56 | 0.44-2.90 | 0.52 | 0.11-3.27 | 0.008 |
| | ALL | 45.42 | 118.50 | 16.60 | 12.08-78.75 | 8.55 | 0.32-46.86 | |
| Oct3/4B | Controls | 2.44 | 2.97 | 0.86 | 0.56-4.33 | 0.61 | 0.18-4.70 | 0.019 |
| | ALL | 28.02 | 50.59 | 7.08 | 13.79-42.25 | 6.62 | 0.52-40.83 | |
| Oct3/4B1 | Controls | 3.22 | 3.06 | 0.88 | 1.27-5.16 | 1.97 | 0.33-6.73 | 0.025 |
| | ALL | 60.99 | 107.80 | 15.09 | 30.68-91.31 | 14.42 | 0.71-81.09 | |
Oct3/4 isoforms expression is
associated with relapse in patients with ALL
Patients with ALL were categorized into two groups:
namely, those with relapse (n=30) and those without relapse (n=21).
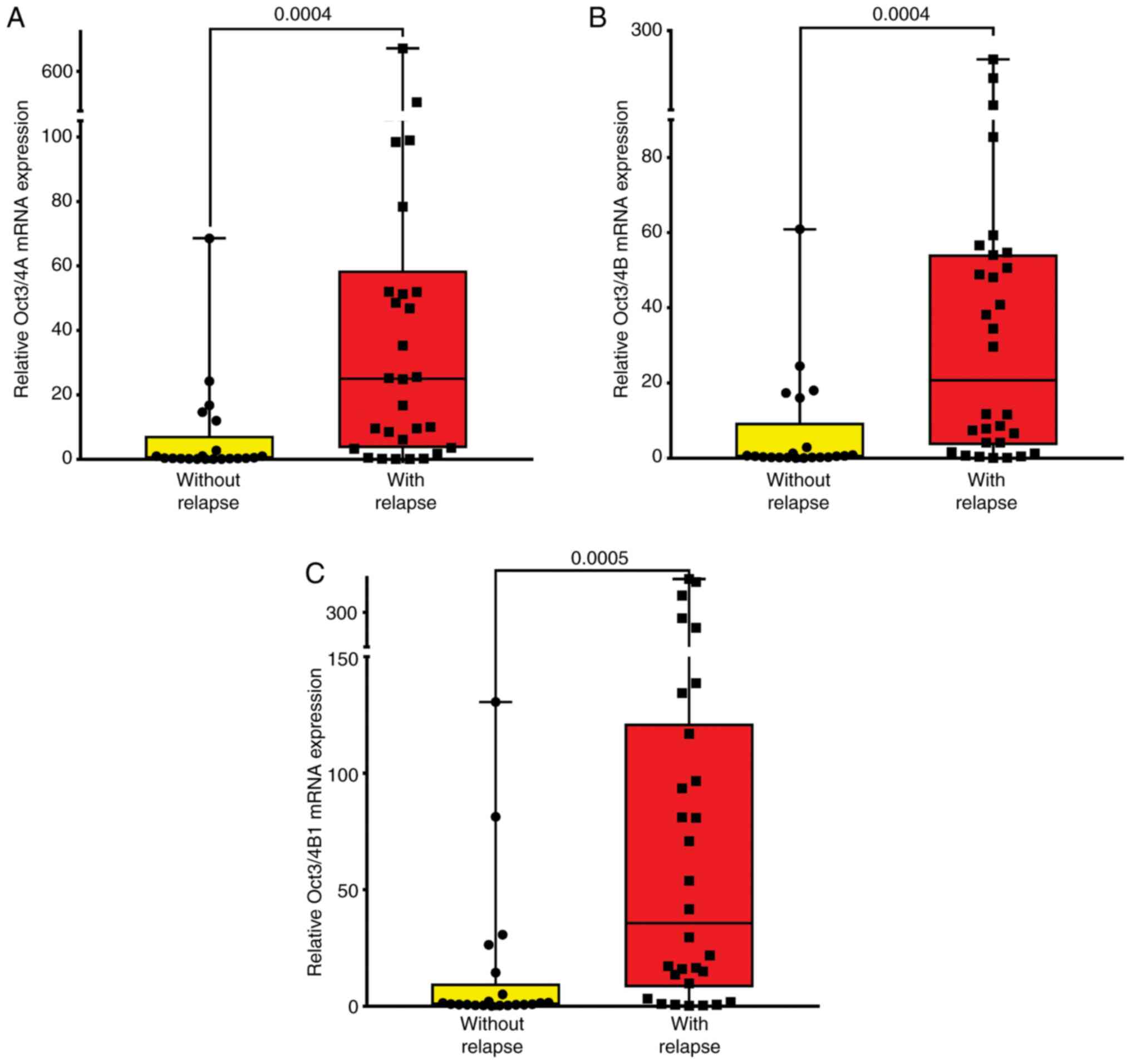
The results of the present study indicated that the expression
levels of Oct3/4A (P=0.0004; Fig.
3A and Table III), Oct3/4B
(P=0.0004; Fig. 3B and Table III), and Oct3/4B1 (P=0.0005;
Fig. 3C and Table III) were significantly higher in
patients with ALL with relapse compared with those in patients
without relapse. To assess the association between Oct3/4 isoforms
expression and the risk of ALL relapse, a logistic regression
analysis was performed. Patients were categorized into two groups
based on their expression levels (low- and high-expression). The
mean expression level of Oct3/4A (mean, 45.42; Fig. 3A and Table II), Oct3/4B (mean, 28.02; Fig. 3B and Table II), and Oct3/4B1 (mean, 60.99;
Fig. 3C and Table II) was used as the cut-off point to
divide all 51 patients with ALL into two groups (Table II). Those who expressed Oct3/4
isoforms at levels less than the cut-off value were assigned to the
low expression group [Oct3/4A (n=38); Oct3/4B (n=35); Oct3/4B1
(n=36)], and those with expression levels above the cut-off value
were assigned to the high expression group [Oct3/4A (n=13); Oct3/4B
(n=16); Oct3/4B1 (n=15)].
 | Table IIIOct3/4 isoforms mRNA expression in
childhood AL with and without relapse. |
Table III
Oct3/4 isoforms mRNA expression in
childhood AL with and without relapse.
| | | Mean | Std. deviation | Std. error of the
mean | 95% CI of the
mean | Median | 25-75%
percentiles | P-value |
|---|
| Oct3/4A | Without
relapse | 6.90 | 15.71 | 3.43 | -0.24-14.05 | 0.38 | 0.22-7.35 | 0.0004 |
| | With relapse | 72.38 | 149.10 | 27.22 | 16.70-128.00 | 25.02 | 3.49-716.60 | |
| Oct3/4B | Without
relapse | 6.96 | 14.47 | 3.16 | 0.37-13.55 | 0.53 | 0.24-9.47 | 0.0004 |
| | With relapse | 42.76 | 61.02 | 11.14 | 19.98-65.55 | 20.72 | 3.52-245.80 | |
| Oct3/4B1 | Without
relapse | 14.34 | 32.59 | 7.11 | -0.50-29.18 | 0.94 | 0.52-9.76 | 0.0005 |
| | With relapse | 93.65 | 128.90 | 23.54 | 45.51-141.80 | 35.64 | 8.12-121.40 | |
The results of the present study revealed an
association between the expression of Oct3/4 isoforms (Oct3/4A,
Oct3/4B and Oct3/4B1) and relapse risk in patients with ALL
(P<0.05). Patients with ALL exhibiting high expression levels of
Oct3/4 isoforms exhibited a notable increase in relapse risk:
Oct3/4A (OR=13.33; 95% CI: 1.57-112.99; P=0.018), Oct3/4B
(OR=20.00; 95% CI: 2.37-168.64; P=0.006) and Oct3/4B1 (OR=7.26; 95%
CI: 1.43-36.93; P=0.017).
Age, leukocyte count at diagnosis, and the
expression of Oct3/4A, Oct3/4B and Oct3/4B1 were included in a
multivariate analysis to assess whether the aforementioned Oct3/4
isoforms are independent predictors of relapse risk. The results of
the present study revealed that Oct3/4A (OR=18.24; 95% CI:
2.03-164.29; P=0.010), Oct3/4B (OR=42.86; 95% CI: 4.25-431.31;
P=0.001) and Oct3/4B1 (OR=14.08; 95% CI: 2.31-85.67; P=0.004)
expression levels serve as independent prognostic indicators for
patients with ALL (Table IV).
Collectively, these results suggested that the expression of these
Oct3/4 isoforms may play a role in the relapse of patients with
ALL.
 | Table IVAssociation of Oct3/4 isoforms mRNA
expression with the risk of relapse to ALL. |
Table IV
Association of Oct3/4 isoforms mRNA
expression with the risk of relapse to ALL.
| | |
Unadjustedb |
Adjustedc |
|---|
| | Without
relapse | With relapse |
P-valuea | OR | CI 95% | P-value | OR | CI 95% | P-value |
|---|
| Risk by age | | | | | | | | | |
| Low-risk | 15 (71.43) | 16 (53.33) | 0.250 | 1.00 | | | | | |
| High-risk | 6 (28.57) | 14 (46.67) | | 1.09 | 0.94-1.26 | 0.239 | | | |
| Risk by leukocytes
at diagnosis | | | | | | | | | |
|
Low-risk | 18 (85.71) | 22 (73.33) | 0.490 | 1.00 | | | | | |
|
High-risk | 3 (14.29) | 8 (26.67) | | 2.18 | 0.50-9.45 | 0.297 | | | |
| Oct3/4A | | | | | | | | | |
|
Low-levels | 20 (95.24) | 18 (60.00) | 0.007 | 1.00 | | | | | |
|
High-levels | 1 (4.76) | 12 (40.00) | | 13.33 | 1.57-112.99 | 0.018 | 18.24 | 2.03-164.29 | 0.010 |
| Oct3/4B | | | | | | | | | |
|
Low-levels | 20 (95.24) | 15 (50.00) | 0.001 | 1.00 | | | | | |
|
High-levels | 1 (4.76) | 15 (50.00) | | 20.00 | 2.37-168.64 | 0.006 | 42.86 | 4.25-431.31 | 0.001 |
| Oct3/4B1 | | | | | | | | | |
|
Low-levels | 19 (90.48) | 17 (56.67) | 0.012 | 1.00 | | | | | |
|
High-levels | 2 (9.52) | 13 (43.33) | | 7.26 | 1.43-36.93 | 0.017 | 14.08 | 2.31-85.67 | 0.004 |
Overall survival based on the
expression levels of Oct3/4 isoforms
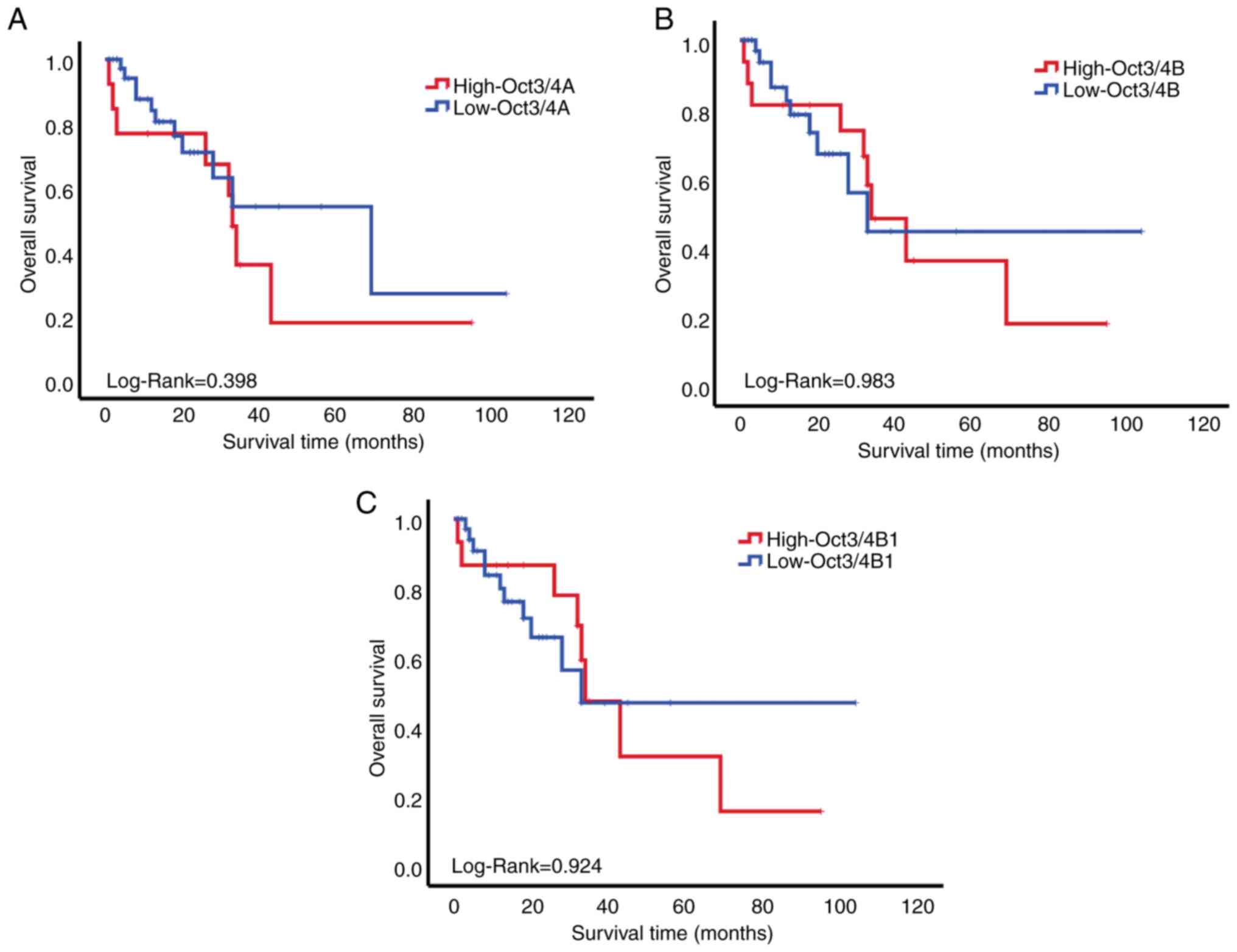
The association between the expression levels of
Oct3/4 isoforms and the overall survival of patients with ALL was
investigated in the present study. In patients with ALL,
Kaplan-Meier analysis revealed no significant difference in overall
survival or the expression levels of Oct3/4 isoforms (log-rank
test; P>0.05; Fig. 4).
Expression levels of Oct3/4A, Oct3/4B
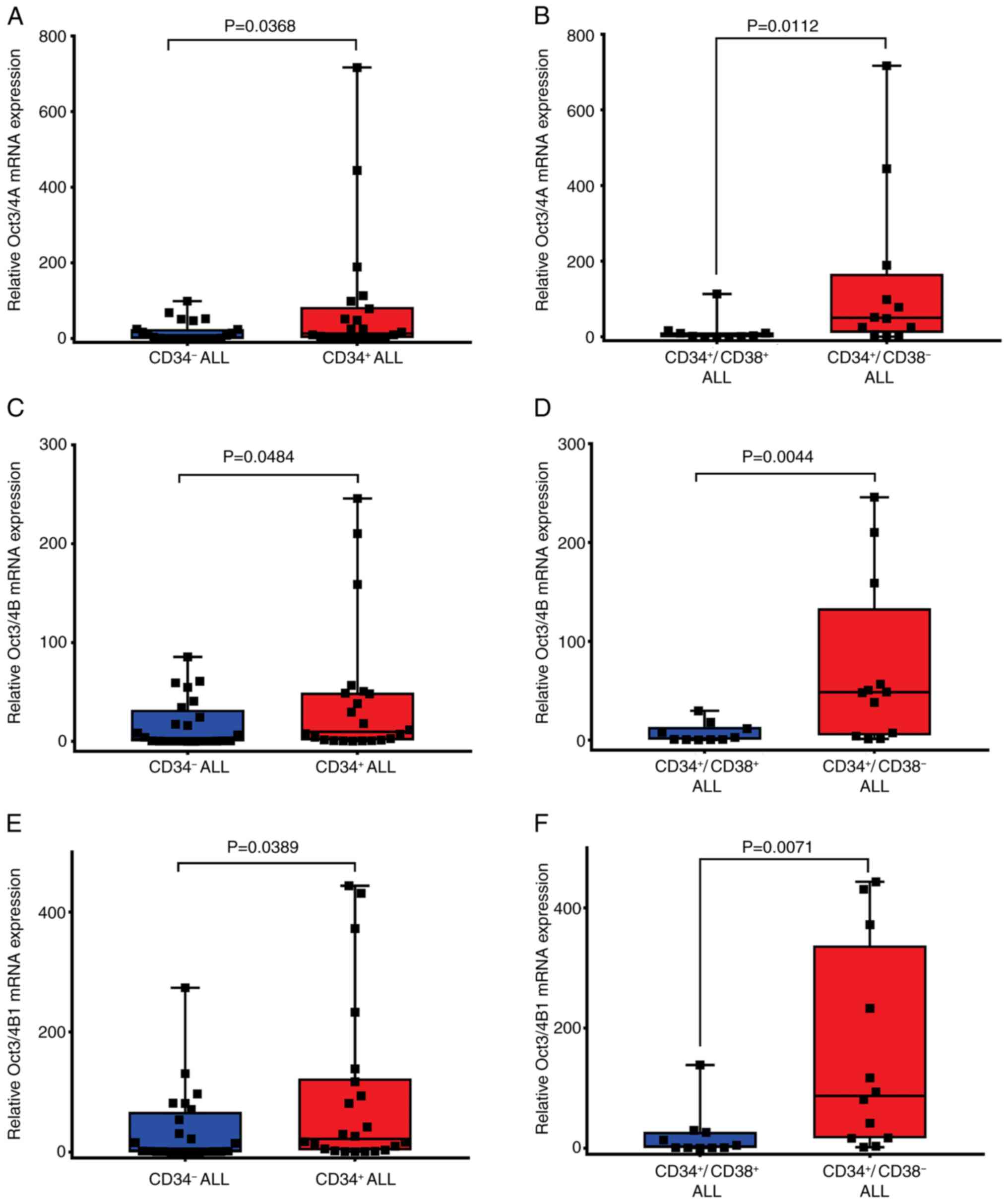
and Oct3/4B1 are high in CD34+ patients with ALL
Surface marker CD34 is used to identify and isolate
hematopoietic stem/progenitor cells (HSC/HPC) (28). CD34+ B-ALL cells are used
in the diagnosis and prognosis of patients with ALL (29). To determine whether Oct3/4 isoforms
are expressed differently between CD34+- and
CD34-- patients with ALL, samples from 51 patients with
ALL that were positive for Oct3/4A, Oct3/4B and Oct3/4B1 were
analyzed. The results of the present study indicated a significant
increase in Oct3/4 isoforms expression in CD34+-patients
with ALL [Oct3/4A (median, 13.41; P=0.0368; Fig. 5A and Table SII); Oct3/4B (median, 9.76;
P=0.0484; Fig. 5C and Table SII); Oct3/4B1 (median, 21.79;
P=0.0389; Fig. 5E and Table SII). CD34+ patients with
ALL were subdivided into two groups: namely,
CD34+/CD38+ (n=10) and
CD34+/CD38- (n=12), based on the expression
of the CD34 and CD38 cell antigens in leukemia cells. In
CD34+/CD38- patients with ALL, the expression
levels of Oct3/4A (median, 50.25; P=0.0112; Fig. 5B and Table SII), Oct3/4B (median, 48.49;
P=0.0044; Fig. 5D and Table SII) and Oct3/4B1 (median, 87.33;
P=0.071; Fig. 5F and Table SII) were significantly elevated
compared with those in CD34+/CD38+ patients
with ALL.
Discussion
ALL is characterized by the uncontrolled
proliferation of blast cells, representing 80% of pediatric ALL
cases (1). This population of
blasts is sustained by rare leukemia-inducing cells known as
leukemic stem cells (LSCs) (30,31).
LSCs (CD34+CD38- cells) represent an immature
leukemic compartment characterized by the expression of the Oct3/4
protein (16). The human Pou5f1
(Oct3/4) gene produces at least three transcripts: Oct3/4A, Oct3/4B
and Oct3/4B1; and four protein isoforms: Oct3/4A, Oct3/4B-190,
Oct3/4B-265 and Oct3/4B-164, through alternative splicing and
initiation of translation (32).
Oct3/4A is located in the nucleus and acts as a transcription
factor, while OCT4B primarily resides in the cytoplasm, providing
cells with resistance to apoptotic death as well as stress from
heat shock or genotoxic factors (33,34).
Moreover, Oct3/4B1 regulates and maintains the undifferentiated
state of stem cells (35). The
present study aimed to investigate the mRNA expression levels of
Oct3/4A, Oct3/4B and Oct3/4B1, and determine the association
between expression and relapse risk in ALL. Oct3/4 acts as a
transcription factor, playing a role in tumorigenesis and
metastasis. Notably, this transcription factor may be associated
with unfavorable outcomes for patients with cancer (36). Previous studies have outlined the
role of Oct3/4 in various tumors. However, research on the
expression, characteristics and functions of Oct3/4 isoforms in
children with ALL remains limited.
The results of the present study demonstrated that
Oct3/4 isoforms were expressed at higher levels in patients with
ALL compared with those without ALL (P<0.05). These findings are
comparable with those of previous studies that also reported
elevated Oct3/4 expression in patients with AML (17,18)
and cervical cancer (37,38). Zhao et al (39) studied Oct3/4 mRNA expression in
individuals with acute and chronic leukemia and revealed that
expression was predominantly higher in patients with acute leukemia
compared with those with chronic leukemia and the control group. By
contrast, Yin et al (18)
revealed that the expression of Oct3/4 was significantly higher in
patients with AML compared with controls (18). Picot et al (16) and Xiang et al (19) reported significantly elevated levels
of Oct3/4 mRNA in leukemic cell lines and patients with AML
compared with their respective controls (16,19).
Collectively, these findings suggested that increased expression of
Oct3/4 may promote the proliferation and survival of leukemic
cells. Moreover, Oct3/4 may play a significant role in the
pathogenesis of leukemia and exhibits potential as a molecular
target for novel treatment strategies (39).
Oct3/4A is the primary isoform associated with
stemness in cancer cells. The results of the present study
demonstrated that Oct3/4A and Oct3/4B mRNA expression levels were
elevated in children diagnosed with ALL, compared with those
without ALL. In addition, Oct3/4B may play a role in the cellular
stress response (32). Li et
al (9) revealed that the
Oct3/4B isoforms enhances angiogenesis in cervical cancer through
the upregulation of vascular endothelial growth factor,
highlighting the oncogenic role of OCT4B (9). A previous study demonstrated that
hypoxia activates OCT4B through a HIF2α-dependent pathway in lung
cancer cells, promoting epithelial-mesenchymal transition,
ultimately leading to cell invasion, migration and metastasis
(40).
Oct3/4B1 plays a role in both pluripotency and
tumorigenesis, through inhibition of apoptosis and dysregulation of
the cell cycle (22). The results
of the present study revealed that OCT4B1 mRNA expression was
significantly higher in children with ALL, compared with those
without ALL. Notably, the findings of the present study are
comparable with those of previous studies, which highlighted the
positive regulation of OCT4B1 transcription across various cancers,
including gastric, colorectal and bladder cancer. A previous study
revealed that OCT4B1 inhibits apoptosis in gastric cancer,
highlighting its crucial role in tumor initiation and progression
(41).
To assess the clinical significance of Oct3/4
isoforms expression in ALL, logistic regression analysis was
conducted to explore the potential association between the clinical
characteristics of patients with ALL and their relapse risk. The
results of the present study revealed that patients who experienced
relapse exhibited a higher mRNA expression of Oct3/4 isoforms than
those who did not experience relapse.
The results of the present study also demonstrated a
notable association between Oct3/4 isoforms expression levels and
the risk of leukemia relapse. Similarly, Aref et al
(42) reported that adults with
acute leukemia who experienced relapse exhibited significantly
elevated Oct3/4 levels compared with those in remission (42). Further previous studies revealed
that elevated levels Oct3/4 mRNA or protein expression levels were
associated with unfavorable clinical outcomes and chemoresistance
across various cancers, including bladder cancer (43), ovarian (44), osteosarcoma (45), liver (46), melanoma (47), prostate, rectal, glioma,
medulloblastoma, hepatocellular carcinoma and esophageal squamous
cell carcinoma (4). High levels of
Oct3/4 are proposed as crucial oncogenic factors in cancer
development, significantly contributing to tumorigenesis,
metastasis and invasion, while also suppressing apoptosis through
the activation of various signaling pathways, such as WNT/β-catenin
and PI3K/Akt pathways (48,49). Collectively, these results indicated
that the expression levels of Oct3/4 isoforms could play a crucial
role in ALL.
Oct3/4 isoforms were not significantly correlated
with overall survival; however, it was observed that patients with
high expression of Oct3/4 isoforms had a worse survival rate,
consistent with other studies (19,42,50).
However, results of the multivariate analysis revealed that
patients with high expression of Oct3/4 isoforms exhibited
significant OR estimates. This significance persisted alongside
other prognostic factors, including age, sex and leukocyte count at
diagnosis, indicating that Oct3/4 isoforms expression is an
independent prognostic marker for ALL.
In addition, the results of previous studies
revealed that CD34+ cells are characterized by the
expression of the Oc3/4 protein (16,17).
The results of the present study revealed that levels of Oct3/4A,
Oct3/4B and Oct3/4B1 were significantly elevated in
CD34+ patients with ALL, compared with those in
CD34- patients with ALL. The results of the present
study are comparable with those observed by Gaafar et al
(51), who reported that the
HSC/HPC subsets expressed pluripotency or stemness genes (SOX2,
Nanog and OCT4). Additionally, it has been previously reported that
CD34+ B-ALL cells can be used in the diagnosis and
prognosis of patients with ALL (25). A recent study also demonstrated that
CD34+/CD38- leukemia cells are associated
with a poor prognosis in patients with ALL (52).
Notably, Oct3/4 may play a role in relapse and
therapy response in patients with ALL via numerous mechanisms: (i)
Oct3/4 may directly promote the expression of miR-125b, which
inhibits its direct target BCL2 Antagonist/Killer 1, resulting in
the reduced apoptosis of cancer cells (37); (ii) Oct3/4 may modulate the
survivin/STAT3 signaling pathway (53), or this transcription factor may
enhance; and (iii) Oct3/4 enhances ATP-binding cassette protein
activity in chemotherapy-resistant cancer cells (54).
The present study exhibits limitations. For example,
the sample size was small, comprising only 51 patients, and more
investigations are required to verify the obtained results.
Furthermore, additional investigations involving larger sample
sizes are necessary. Collectively, the results of the present study
indicated that Oct3/4 isoforms may be associated with a poor
prognosis in children with ALL. To the best of our knowledge, the
present study is the first to demonstrate that Oct3/4 isoforms may
act as independent markers for predicting clinical outcomes in
these patients. In addition, the results highlighted the potential
role of Oct3/4 isoforms expression in the risk and relapse of
childhood ALL; however, future studies are needed to explain the
underlying molecular mechanism of the Oct3/4 isoforms in ALL and to
reveal new perspectives on the potential role of the Oct3/4
isoforms regulation in patients with ALL. In conclusion, Oct3/4 may
exhibit potential as a therapeutic target, particularly in
childhood ALL.
Supplementary Material
Oligonucleotide sequences and probes
used for reverse-transcription quantitative PCR.
Oct3/4 isoforms mRNA expression in
patients with CD34+ ALL.
Acknowledgements
CYGS received mastery fellowships from CONAHCYT
(grant no. 923138). The authors extend their gratitude to Q.B.P.
Natividad Sales Linares and Q.B.P. Josué Feliciano Ortiz from the
Laboratory of Molecular Biomedicine, Faculty of Chemical Biological
Sciences, Autonomous University of Guerrero for their technical
support.
Funding
Funding: The present study was supported by Universidad Autónoma
de Guerrero.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JON and YGG developed methodology. YGG and MALV
conceptualized the study. JON, YGG, CYGS, EGSB, MIZG, ABRR, MVSH,
OAP, FITR, LCAR and BIA conducted investigation. ABRR, MVSH, OAP,
BIA and MALV provided resources. JON, YGG, CYGS and BIA validated
data. JON, YGG, CYGS and EGSB conducted formal analysis. YGG and
MALV supervised the study, performed project administration,
acquired funding, and wrote, reviewed and edited the manuscript.
JON wrote the original draft. JON, YGG, CYGS and MALV confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Institute of Cancer of the State of Guerrero,
Mexico (approval no. FO-INV-AUT-2018) and by the committee of the
biobank of the laboratory of Molecular Biomedicine at the FCQB of
Autonomous University of Guerrero (Chilpancingo, Mexico) (approval
no. BB-LBM-03-2020). All participants or their guardians provided
written informed consent following a thorough explanation of the
study's objectives.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Friedmann AM and Weinstein HJ: The role of
prognostic features in the treatment of childhood acute
lymphoblastic leukemia. Oncologist. 5:321–328. 2000.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hunger SP and Mullighan CG: Acute
lymphoblastic leukemia in children. N Engl J Med. 373:1541–1552.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mohiuddin IS, Wei SJ and Kang MH: Role of
OCT4 in cancer stem-like cells and chemotherapy resistance. Biochim
Biophys Acta Mol Basis Dis. 1866(165432)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Terwilliger T and Abdul-Hay M: Acute
lymphoblastic leukemia: A comprehensive review and 2017 update.
Blood Cancer J. 7(e577)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Di J, Duiveman-de Boer T, Zusterzeel PL,
Figdor CG, Massuger LF and Torensma R: The stem cell markers Oct4A,
Nanog and c-Myc are expressed in ascites cells and tumor tissue of
ovarian cancer patients. Cell Oncol (Dordr). 36:363–374.
2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jerabek S, Merino F, Schöler HR and
Cojocaru V: OCT4: dynamic DNA binding pioneers stem cell
pluripotency. Biochim Biophys Acta. 1839:138–154. 2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shen L, Qin K, Wang D, Zhang Y, Bai N,
Yang S, Luo Y, Xiang R and Tan X: Overexpression of Oct4 suppresses
the metastatic potential of breast cancer cells via Rnd1
downregulation. Biochim Biophys Acta. 1842:2087–2095.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li SW, Wu XL, Dong CL, Xie XY, Wu JF and
Zhang X: The differential expression of OCT4 isoforms in cervical
carcinoma. PLoS One. 10(e0118033)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Panagopoulos I, Möller E, Collin A and
Mertens F: The POU5F1P1 pseudogene encodes a putative protein
similar to POU5F1 isoform 1. Oncol Rep. 20:1029–1033.
2008.PubMed/NCBI
|
|
11
|
Mirzaei MR, Kazemi Arababadi M, Asadi MH
and Mowla SJ: Altered expression of high molecular weight heat
shock proteins after OCT4B1 suppression in human tumor cell lines.
Cell J. 17:608–616. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yasuda H, Tanaka K, Okita Y, Araki T,
Saigusa S, Toiyama Y, Yokoe T, Yoshiyama S, Kawamoto A, Inoue Y, et
al: CD133, OCT4, and NANOG in ulcerative colitis-associated
colorectal cancer. Oncol Lett. 2:1065–1071. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Guzel E, Karatas OF, Duz MB, Solak M,
Ittmann M and Ozen M: Differential expression of stem cell markers
and ABCG2 in recurrent prostate cancer. Prostate. 74:1498–1505.
2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee SH, Koo BS, Kim JM, Huang S, Rho YS,
Bae WJ, Kang HJ, Kim YS, Moon JH and Lim YC: Wnt/β-catenin
signalling maintains self-renewal and tumourigenicity of head and
neck squamous cell carcinoma stem-like cells by activating Oct4. J
Pathol. 234:99–107. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kong D, Su G, Zha L, Zhang H, Xiang J, Xu
W, Tang Y and Wang Z: Coexpression of HMGA2 and Oct4 predicts an
unfavorable prognosis in human gastric cancer. Med Oncol.
31(130)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Picot T, Aanei CM, Fayard A,
Flandrin-Gresta P, Tondeur S, Gouttenoire M, Tavernier-Tardy E,
Wattel E, Guyotat D and Campos L: Expression of embryonic stem cell
markers in acute myeloid leukemia. Tumour Biol.
39(1010428317716629)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Picot T, Kesr S, Wu Y, Aanei CM,
Flandrin-Gresta P, Tondeur S, Tavernier E, Wattel E, Guyotat D and
Campos L: Potential role of OCT4 in leukemogenesis. Stem Cells Dev.
26:1637–1647. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yin JY, Tang Q, Zhai LL, Zhou LY, Qian J,
Lin J, Wen XM, Zhou JD, Zhang YY, Zhu XW and Deng ZQ: High
expression of OCT4 is frequent and may cause undesirable treatment
outcomes in patients with acute myeloid leukemia. Tumour Biol.
36:9711–9716. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xiang Y and Zhou X: Octamer-binding
transcription factor 4 correlates with complex karyotype, FLT3-ITD
mutation and poorer risk stratification, and predicts unfavourable
prognosis in patients with acute myeloid leukaemia. Hematology.
23:721–728. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Meier P, Antonov J, Zbinden R, Kuhn A,
Gloekler S, Delorenzi M, Jaggi R and Seiller C: Non-invasive
gene-expression-based detection of well-developed collateral
function in individuals with and without coronary artery disease.
Heart. 95:900–908. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Holleman A, Cheok MH, den Boer ML, Yang W,
Veerman AJ, Kazemier KM, Pei D, Cheng C, Pui CH, Relling MV, et al:
Gene-expression patterns in drug-resistant acute lymphoblastic
leukemia cells and response to treatment. N Engl J Med.
351:533–542. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaizer EC, Glaser CL, Chaussabel D,
Bancereau J, Pascual V and White PC: Gene expression in peripheral
blood mononuclear cells from children with diabetes. J Clin
Endocrinol Metab. 92:3705–3711. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wei G, Tworney D, Lamb J, Schlis K,
Agarwal J, Stam RW, Opferman JT, Sallan SE, den Boer ML, Golub TR
and Armstrong SA: Gene expression-based chemical genomics
identifies rapamycin as a modulator of MCL1 and glucocorticoid
resistance. Cancer Cell. 10:331–342. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gómez-Gómez Y, Organista-Nava J,
Saavedra-Herrera MV, Rivera-Ramírez AB, Terán-Porcayo MA, Del
Carmen Alarcón-Romero L, Illades-Aguiar B and Leyva-Vázquez MA:
Survival and risk of relapse of acute lymphoblastic leukemia in a
Mexican population is affected by dihydrofolate reductase gene
polymorphisms. Exp Ther Med. 3:665–672. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Buchmann S, Schrappe M, Baruchel A, Biondi
A, Borowitz M, Campbell M, Cario G, Cazzaniga G, Escherich G and
Harrison CJ: , et al: Remission, treatment failure, and
relapse in pediatric ALL: An international consensus of the
Ponte-di-Legno Consortium. Blood. 139:1785–1793. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Asadi MH, Mowla SJ, Fathi F, Aleyasin A,
Asadzadeh J and Atlasi Y: OCT4B1, a novel spliced variant of OCT4,
is highly expressed in gastric cancer and acts as an antiapoptotic
factor. Int J Cancer. 128:2645–2652. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nolan T, Hands RE and Bustin SA:
Quantification of mRNA using real-time RT-PCR. Nat Protoc.
1:1559–1582. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
DiGiusto D, Chen S, Combs J, Webb S,
Namikawa R, Tsukamoto A, Chen BP and Galy AH: Human fetal bone
marrow early progenitors for T, B, and myeloid cells are found
exclusively in the population expressing high levels of CD34.
Blood. 84:421–432. 1994.PubMed/NCBI
|
|
29
|
Jiang Z, Wu D, Lin S and Li P: CD34 and
CD38 are prognostic biomarkers for acute B lymphoblastic leukemia.
Biomark Res. 4(23)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
O'Reilly E, Zeinabad HA and Szegezdi E:
Hematopoietic versus leukemic stem cell quiescence: Challenges and
therapeutic opportunities. Blood Rev. 50(100850)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lapidot T, Sirard C, Vormoor J, Murdoch B,
Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA and
Dick JE: A cell initiating human acute myeloid leukaemia after
transplantation into SCID mice. Nature. 367:645–648.
1994.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X and Dai J: Concise review: Isoforms
of OCT4 contribute to the confusing diversity in stem cell biology.
Stem Cells. 28:885–893. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee J, Kim HK, Rho JY, Han YM and Kim J:
The human OCT-4 isoforms differ in their ability to confer
self-renewal. J Biol Chem. 281:33554–33565. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Atlasi Y, Mowla SJ, Ziaee SA and Bahrami
AR: OCT-4, an embryonic stem cell marker, is highly expressed in
bladder cancer. Int J Cancer. 120:1598–1602. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ
and Andrews PW: OCT4 spliced variants are differentially expressed
in human pluripotent and nonpluripotent cells. Stem Cells.
26:3068–3074. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Clemente-Periván SI, Gómez-Gómez Y,
Leyva-Vázquez MA, Lagunas-Martínez A, Organista-Nava J and
Illades-Aguiar B: Role of Oct3/4 in cervical cancer tumorigenesis.
Front Oncol. 10(247)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wang YD, Cai N, Wu XL, Cao HZ, Xie LL and
Zheng PS: OCT4 promotes tumorigenesis and inhibits apoptosis of
cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis.
4(e760)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gómez-Gómez Y, Organista-Nava J,
Clemente-Periván SI, Lagunas-Martínez A, Salmerón-Bárcenas EG,
Villanueva-Morales D, Ayala-Reyna DY, Del Carmen Alarcón-Romero L,
Ortiz-Ortiz J, Jiménez-López MA, et al: The expression of Oct3/4A
mRNA and not its isoforms is upregulated by the HPV16 E7
oncoprotein. Mol Biol Rep. 50:981–991. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhao Q, Ren H, Feng S, Chi Y, He Y, Yang
D, Ma F, Li J, Lu S, Chen F, et al: Aberrant expression and
significance of OCT-4A transcription factor in leukemia cells.
Blood Cells Mol Dis. 54:90–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lin SC, Chung CH, Chung CH, Kuo MH, Hsieh
CH, Chiu YF, Shieh YS, Chou YT and Wu CW: OCT4B mediates
hypoxia-induced cancer dissemination. Oncogene. 38:1093–1105.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Farashahi Yazd E, Rafiee MR, Soleimani M,
Tavallaei M, Salmani MK and Mowla SJ: OCT4B1, a novel spliced
variant of OCT4, generates a stable truncated protein with a
potential role in stress response. Cancer Lett. 309:170–175.
2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Aref S, Khaled O, Menshawy NE, Azmy E,
Aref M, Salama O and Khaled N: Significance of OCT3/4 and SOX2
antigens expression by leukemic blast cells in adult acute
leukemia. J Egypt Natl Canc Inst. 36(5)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lu CS, Shieh GS, Wang CT, Su BH, Su YC,
Chen YC, Su WC, Wu P, Yang WH, Shiau AL and Wu CL:
Chemotherapeutics-induced Oct4 expression contributes to drug
resistance and tumor recurrence in bladder cancer. Oncotarget.
8:30844–30858. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gwak JM, Kim M, Kim HJ, Jang MH and Park
SY: Expression of embryonal stem cell transcription factors in
breast cancer: Oct4 as an indicator for poor clinical outcome and
tamoxifen resistance. Oncotarget. 8:36305–36318. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gatti M, Solari A, Pattarozzi A,
Campanella C, Thellung S, Maniscalco L, De Maria R, Würth R,
Corsaro A, Bajetto A, et al: In vitro and in vivo characterization
of stem-like cells from canine osteosarcoma and assessment of drug
sensitivity. Exp Cell Res. 363:48–64. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang XQ, Ongkeko WM, Chen L, Yang ZF, Lu
P, Chen KK, Lopez JP, Poon RT and Fan ST: Octamer 4 (Oct4) mediates
chemotherapeutic drug resistance in liver cancer cells through a
potential Oct4-AKT-ATP-binding cassette G2 pathway. Hepatology.
52:528–539. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kumar SM, Liu S, Lu H, Zhang H, Zhang PJ,
Gimotty PA, Guerra M, Guo W and Xu X: Acquired cancer stem cell
phenotypes through Oct4-mediated dedifferentiation. Oncogene.
31:4898–4911. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chai S, Ng KY, Tong M, Lau EY, Lee TK,
Chan KW, Yuan YF, Cheung TT, Cheung ST, Wang XQ, et al: Octamer
4/microRNA-1246 signaling axis drives Wnt/β-catenin activation in
liver cancer stem cells. Hepatology. 64:2062–2076. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Singh RK, Dhadve A, Sakpal A, De A and Ray
P: An active IGF-1R-AKT signaling imparts functional heterogeneity
in ovarian CSC population. Sci Rep. 6(36612)2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Chen Z, Wang T, Cai L, Su C, Zhong B, Lei
Y and Xiang AP: Clinicopathological significance of non-small cell
lung cancer with high prevalence of Oct-4 tumor cells. J Exp Clin
Cancer Res. 31(10)2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gaafar A, Hamza FN, Yousif R, Shinwari Z,
Alotaibi AG, Iqniebi A, Al-Hussein K, Al-Mazrou A, Manogaran PS,
Elhassan T, et al: Distinct phenotypic and molecular
characteristics of CD34- and CD34+ hematopoietic stem/progenitor
cell subsets in cord blood and bone marrow samples: Implications
for clinical applications. Diagnostics (Basel).
15(447)2025.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhao J, Wang Y, Zhou M, Gao J and Yuan Y:
The prognostic effect on childhood acute lymphoblastic leukemia of
CD34(+)CD38(-) expressed in leukemia cells. Hematology. 27:706–713.
2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang G, Zhou H, Gu Z, Gao Q and Shen G:
Oct4 promotes cancer cell proliferation and migration and leads to
poor prognosis associated with the survivin/STAT3 pathway in
hepatocellular carcinoma. Oncol Rep. 40:979–987. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Cioffi M, D'Alterio C, Camerlingo R,
Tirino V, Consales C, Riccio A, Ieranò C, Cecere SC, Losito NS,
Greggi S, et al: Identification of a distinct population of
CD133(+)CXCR4(+) cancer stem cells in ovarian cancer. Sci Rep.
5(10357)2015.PubMed/NCBI View Article : Google Scholar
|