1. Introduction
Glioma-related epilepsy (GRE) refers to seizures
that arise as a secondary symptom of gliomas. These seizures are
not only a frequent complication during disease progression but
also serve as the first noticeable sign of the condition (1). GRE is a variable and unpredictable
disease closely related to the progression and recurrence of
gliomas. Both seizures and antiepileptic drug (AED) treatment can
cause cognitive impairment. The preoperative prevalence of anxiety
or depression in adult patients with low-grade gliomas (LGGs) is
markedly higher than that in patients without epilepsy (2). However, the interaction between AEDs
and chemotherapy may have a direct impact on tumours, influencing
the therapeutic effects on tumours, imposing a heavy economic and
psychological burden on patients and their families, and
significantly reducing the quality of life of patients (3,4).
Therefore, understanding the pathogenesis and clinical features of
epilepsy is essential for the clinical management of brain tumours
in affected patients. The present review summarises the findings of
recent studies on this topic.
2. Clinical characteristics of GRE
Incidence of GRE
The occurrence of epilepsy in GRE is influenced by
various factors such as tumour location, histological
characteristics, the peritumoural microenvironment, and specific
genetic changes. Seizures affect 30-90% of patients with glioma
during the course of their disease (5), with approximately two-thirds
manifesting at the onset and one-third emerging during treatment
(6). Patients with diffuse LGGs
often experience GRE in 60-90% of cases, whereas those with
glioblastomas (GBMs) have seizures in 30-70% of cases (5). The cumulative incidence of brain
tumour-related epilepsy (BTRE) has been shown to increase with
tumour progression. Notably, ~50% of patients experience at least
one seizure, with the proportion of cases increasing to 50-70% in
the end-of-life phase (7).
Isocitrate dehydrogenase 1 (IDH1) mutation, p53 overexpression
(>40%), younger age (<38 years), male patients, cortical
involvement, and large tumour volume are associated with a higher
incidence of preoperative GRE in LGGs (3,4,8). A
total of 75% of patients with grade 2 gliomas (astrocytomas or
oligodendrogliomas) with IDH1/2 mutations and 25% of those with IDH
wild-type (IDHwt) GBMs suffer from seizures, and patients with
secondary GBMs carrying IDH1 mutations have an increased likelihood
of seizures (8,9). Over 50% of patients exhibit
preoperative GRE resistance, and postoperative seizure remission
rates range from 43 to 87%, depending on the extent of resection
(EOR). Subtotal resection, older age (>45 years), generalised
seizures, shorter history of epilepsy (<1 year), and low Ki-67
expression are predictors of favourable postoperative seizure
control (3,10). Notably, 70% of patients with GBMs
and preoperative GRE are seizure-free early after tumour resection,
and near-total resection remains a predictor of postoperative
seizure control. In addition, GRE recurrence after resection of
high-grade gliomas (HGGs) is usually associated with tumour
recurrence/progression (3).
Preoperative GRE is generally associated with prolonged overall
survival in patients with LGG and GBM (3).
Clinical behaviour
The clinical manifestations of GRE predominantly
encompass focal awareness seizures, focal impaired awareness
seizures, generalised tonic-clonic seizures, and focal-to-bilateral
tonic-clonic seizures (11).
Research has highlighted the distinct seizure patterns in low- and
high-grade gliomas. In patients with LGGs, 69.7% exhibited
focal-to-bilateral tonic-clonic seizures. By contrast, HGGs were
more commonly associated with focal motor-aware seizures (38%) or
focal-to-bilateral tonic-clonic seizures (40%) (12). Secondary generalised epilepsy is
common in patients with LGGs (40%), whereas simple partial seizures
predominate in patients with HGGs (38.3%) (13). However, research suggests that LGGs
frequently cause functional localisation-related focal seizures
(45-95%) (4). HGGs are more
frequently associated with generalised seizures and status
epilepticus and are often triggered by factors such as medication
non-adherence or infections (14-16).
Tumour-associated status epilepticus typically manifests as complex
focal seizures (74%) (17).
Seizure semiology is location-dependent; for
example, precentral gyrus lesions cause focal motor seizures,
whereas left frontal or right temporal lesions lead to seizures
with or without consciousness disturbances (18). Patients may also experience
additional symptoms such as visual disturbances, changes in mental
status, or signs of elevated intracranial pressure, including
headaches and nausea. Additionally, patients may exhibit postictal
phenomena such as Todd's paralysis and psychosis (19).
Diagnosis
Tumour-related epilepsy is characterised by the
occurrence of at least one seizure resulting from a brain
abnormality, such as a glioma (20). The diagnosis of GRE requires
confirmation of both glioma and epilepsy, as well as evidence of
their correlation (3). Magnetic
resonance imaging (MRI) is crucial for preoperative diagnosis, and
is supplemented by magnetic resonance spectroscopy (MRS), computed
tomography (CT), and positron emission tomography (PET). For
cortical tumours, diffusion tensor imaging and functional MRI can
help localise functional areas and track fibres (3). Notably, seizure history duration is
correlated with intratumoural T1-weighted hyperintensity, styloid
signs, and regional atrophy in angiocentric gliomas (21). Definitive glioma diagnosis requires
surgery/biopsy and pathological evaluation, including
histopathological and molecular analyses, with an emphasis on the
IDH1 mutation status (3,22). Patients with IDH1-mutated gliomas,
which are common in LGGs (>80%) and secondary GBM (73%), are
more prone to preoperative seizures than those with IDH1 wild-type
(IDH1wt) gliomas (22-24).
Previous studies have used MRI-based radiomics to identify the IDH
mutation status (25) and the
occurrence of GRE (26,27). A research team recently explored the
potential connection between the two to develop a novel radiomics
approach that reduces the risk of overfitting, enhances model
performance, and may be used to identify valuable generalised
biomarkers for various clinical issues (28). Seizures are defined as transient
symptoms of abnormal neuronal activity (9). For patients with glioma, epilepsy
history and seizure signs should be documented, with a diagnosis
typically made after a single seizure and classified according to
the 2017 International League Against Epilepsy guidelines (3,9).
3. Underlying mechanisms in GRE
The mechanisms underlying GRE are multifactorial and
involve tumour-related changes and the tumour microenvironment
(TME) (Fig. 1). Seizure mechanisms
vary between LGGs and HGGs and may differ between preoperative and
postoperative seizures, with surgical complications potentially
contributing postoperatively (4,29).
Regardless of the glioma grade, epilepsy onset and tumour
progression may share a dual relationship. Neuronal
hyperexcitability and glutamate release during seizures can promote
tumour growth, indicating common underlying mechanisms (4,30). The
current research on GRE mechanisms are subsequently summarised and
reviewed.
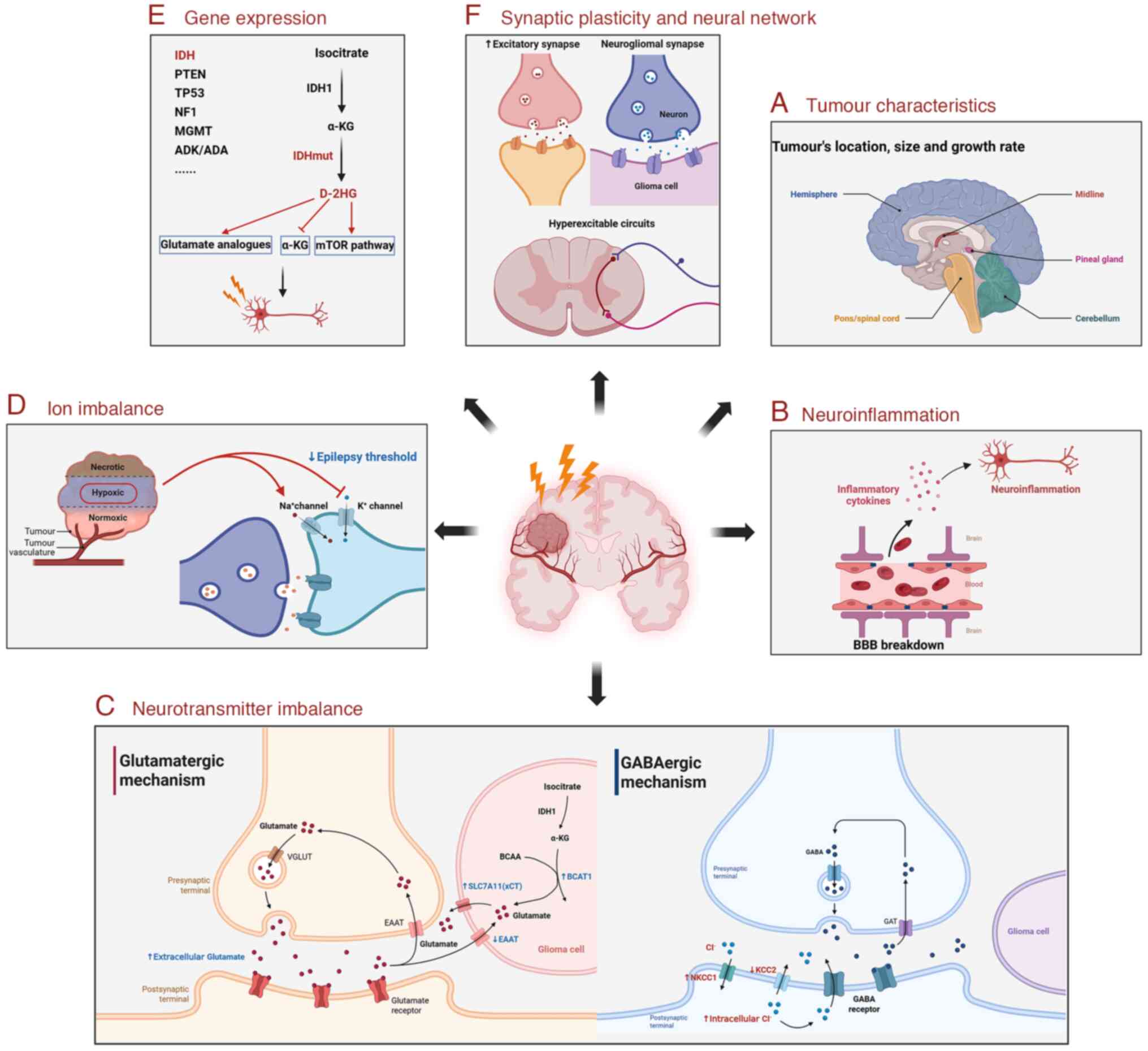 | Figure 1Underlying mechanisms in GRE. The
mechanisms underlying GRE are multifactorial and involve
tumour-related changes and the TME. (A) GRE is affected by the
tumour characteristics, including its location, size, and growth
rate. (B) Gliomas disrupt the BBB and cause neuroinflammation to
induce seizures. (C) Excitation-inhibition imbalance of glutamate
and GABA in the TME induces neuronal excitation and seizures. (D)
Abnormal Na+ and K+ concentrations in the TME
reduce seizure thresholds. (E) The aberrant expression of enzymes
and proteins in the TME drives GRE through changes in the
surrounding neuronal environment. (F) Gliomas can form neurogliomal
synapses, promote excitatory synapses and hyperexcitable circuit
formation, thereby mediating seizures. GRE, glioma-related
epilepsy; TME, tumour microenvironment; BBB, blood-brain barrier;
VGLUT, vesicular glutamate transporter; EAAT, excitatory amino acid
transporter; IDH, isocitrate dehydrogenase; α-KG, α-ketoglutarate;
BCAA, branched-chain amino acids; BCAT1, branched chain amino acid
transaminase 1; SLC7A11/xCT, solute carrier family 7 member 11;
GABA, gamma-aminobutyric acid; GAT, GABA transporter; NKCC1,
Na+-K+-2Cl- co-transporter 1;
KCC2, K+-Cl- co-transporter 2; PTEN,
phosphatase and tensin homologue; TP53, tumour protein 53; NF1,
neurofibromin 1; MGMT, methylguanine methyltransferase; ADK,
adenosine kinase; ADA, adenosine deaminase; IDHmut, IDH-mutated;
D-2HG, D-2-hydroxyglutarate; mTOR, mammalian target of rapamycin.
The figure was created using BioRender (https://www.biorender.com/). |
Tumor characteristics
GRE is affected by the mechanical effects of the
tumour, including its location, size, and growth rate. Cortical
tumours, particularly in the frontal, temporal, and parietal lobes
(18,31), are significantly related to
seizures, while deep-seated or infratentorial tumours are less
frequently associated with epilepsy (4). LGGs involving the neocortex,
especially oligodendrogliomas (10), have a higher seizure risk, while
GBMs are associated with a lower incidence of epilepsy owing to
shorter survival times and fewer epileptogenic origins (32,33).
For subcortical and cortical brain regions, a significantly
decreased risk has been reported in tumours within the left
frontomesial and dorsal voxels (A3C1S1), and an increased seizure
risk has been found in tumours located in the left supramarginal
and posterior insular voxels (A4C2S3) (34). Tumour volume also plays a role:
Smaller HGGs exhibit a higher propensity to present with seizures,
whereas larger LGGs are more epileptogenic. Slow-growing tumours,
such as LGGs, are more prone to epilepsy due to complex cellular
reorganisation and vascularisation, unlike fast-growing HGGs, in
which seizures are often triggered by necrosis or bleeding
(18,33). Additionally, specific glioma cell
subpopulations, such as astrocyte population C in GBM models, may
contribute to epileptogenicity through synaptic gene expression and
tumour progression (35). Another
study showed that patients with lower anaplastic
oligodendroglioma/anaplastic oligoastrocytoma exhibited more
frequent instances of postoperative seizures (36).
Neuroinflammation
Gliomas disrupt the blood-brain barrier (BBB),
causing vasogenic oedema, inflammation, hypoxia, and necrosis.
These changes alter the TME, leading to sodium-calcium imbalances,
abnormal ion concentrations, acidosis, and glutamate pathway
activation, all of which contribute to neuronal hyperexcitability
and seizures (8,18,37,38).
Inflammatory reactions and reactive astrogliosis, which are
characteristic of the GBM microenvironment, play critical roles in
BBB injury-induced epileptogenesis (37). Research using translocator
protein-PET imaging have shown higher contralateral hemisphere
neuroinflammatory signals in individuals with persistent seizures,
which are associated with shorter survival (39). Despite the immunosuppressive TME,
pro-inflammatory cytokines [such as interleukin (IL)-1β, IL-6, and
TNF-α] and chemokines play roles in tumour progression and seizures
(37,38). IL-6, in particular, promotes glioma
cell proliferation and invasion, while contributing to seizure
development (40-44).
Recent findings have suggested that IL-6 levels predict poor
post-resection seizure control in patients with LGGs (45). Over the past 20 years, studies have
shown the presence of cytomegalovirus (CMV) in GBMs (46), and the degree of infection has been
shown to be related to the survival rate of patients (47,48).
Valganciclovir treatment can significantly prolong survival in
patients with GBM (49-51).
Recent research has confirmed that high CMV infection levels can
promote epileptic seizures through a pro-inflammatory
microenvironment (52).
Neurotransmitter imbalance.
Glutamatergic mechanism
Glutamate, the primary excitatory neurotransmitter,
plays a key role in BTRE through aberrant signalling in gliomas and
peritumoural tissues (53).
Elevated glutamate levels (>100 µM) in these regions promote
tumor progression, cognitive impairment, epilepsy, and
neurodegeneration by hyperactivating
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors
(AMPARs)/N-methyl-d-aspartate receptors (NMDARs), causing
neuronal hyperexcitability and excitotoxic cell death, while also
stimulating glioma proliferation and invasion (1,4,6).
Glutamate imbalance arises from disrupted regulation by glial
cells, which normally maintain homeostasis via proteins such as
solute carrier family 7 member 11 (SLC7A11 or xCT), excitatory
amino acid transporter 1 (EAAT1), and excitatory amino acid
transporter 2 (EAAT2). In gliomas, high xCT and low EAAT expression
lead to excessive glutamate release, triggering seizures (1,54). xCT
expression was not elevated in the peri-pain region of human tumour
samples; however, in a mouse xenograft tumour model, primary
central nervous system (CNS) tumours were found to release massive
amounts of glutamate due to high xCT expression, thereby triggering
epileptic activity in the peritumoural region (54). Notably, IDHwt HGGs exhibit
heightened activity of the Xc-cysteine glutamate transporter system
in comparison with IDH-mutated (IDHmut) diffuse low-grade gliomas
(DLGGs) (55). This indicates
distinct mechanisms underlying glutamatergic-mediated
epileptogenicity in these tumour types, wherein glutamate release
through the Xc-cysteine glutamate transporter system from HGGs
plays a key role, whereas in DLGGs, epileptogenicity is driven by
extracellular accumulation of D-2-hydroxyglutarate (D-2HG), which
acts as a glutamate receptor agonist (4). While xCT is highly expressed in tumour
cells, EAAT1 and EAAT2 are significantly downregulated in gliomas
across cell lines, animal models, and human GBMs (54), causing impaired reuptake of
glutamate from the extracellular space (1). Additionally, hypoxia-induced
overexpression of branched chain amino acid transaminase 1 further
elevates glutamate levels by producing glutamate from
branched-chain amino acids, exacerbating extracellular glutamate
accumulation and epileptogenicity (56,57).
Extracellular glutamate activates glutamate
receptors on tumour cells (autocrine) or neighbouring neurones and
astrocytes (paracrine). The glutamate receptors include ionotropic
[AMPARs, kainite receptors (KARs), and NMDARs] and metabotropic
glutamate receptors (mGluR). Ca2+-permeable AMPARs,
which are frequently expressed in gliomas, promote tumour
proliferation and migration (58),
and their antagonist perampanel (PER) may suppress seizures and
tumour growth (1). KARs may also
contribute to GRE, with astrocyte-released glutamate specifically
activating GluK1-containing KARs in interneurons (59). NMDARs, particularly the NR2B
subunits, are highly phosphorylated in periglioma neurones,
enhancing Ca2+ influx and neuronal overexcitation
(60), which may be counteracted by
PER by reversing GluN2B phosphorylation (1). mGluRs 1-8 have been less extensively
studied but may influence glioma progression. For example, mGluR1/5
have been shown to promote tumourigenesis (1); low levels of mGluR3 have been shown to
be correlated with longer survival (61,62);
and mGluR4 signalling may affect tumour growth (63). However, no evidence linking mGluRs
to glioma-associated seizures has been reported to date.
GABAergic mechanisms
Gamma-aminobutyric acid (GABA), a key inhibitory
neurotransmitter, plays an important role in epileptogenesis.
Peritumoural structural and functional changes reduce GABAergic
inhibition and decrease inhibitory interneurones and synapses in
pyramidal cells, leading to excitation-inhibition imbalances and
seizures (4,8). Dysregulated chloride transporters
[Na+-K+-2Cl- co-transporter 1
upregulation and K+-Cl- co-transporter 2
(KCC2) downregulation] in peritumoural neurones increase
intracellular chloride levels, causing GABA receptor activation and
reducing chloride efflux and neuronal excitation (64-66).
Glutamate in the TME further reduces KCC2 expression. Antagonists
such as picrotoxin and gabazine suppress epileptic discharges, thus
confirming this mechanism (66).
Additionally, proteolytic enzymes released by tumours disrupt
neural networks, reducing GABAergic inhibition, whereas an acidic
hypoxic environment further impairs GABA signalling and increases
seizure susceptibility (18,67).
Ion imbalances
Ion imbalances contribute to tumour progression and
seizures. The acidic, hypoxic environment around astrocytomas
activates voltage-gated sodium channels and inhibits
inward-rectifying potassium (KIR) channels, lowering the seizure
threshold (67). BBB disruption and
serum exposure downregulate astrocyte Kir4.1 channels, disrupting
K+ homeostasis (37).
Additionally, immunoglobulin superfamily member 3, a mediator of
glioma progression, interacts with Kir4.1 to impair K+
buffering in peritumoural areas and increase extracellular
K+ levels and neuronal excitability, which can lead to
seizures (68).
Gene expression
The aberrant expression of enzymes and proteins in
the TME drives GRE through changes in the surrounding neuronal
environment. IDH1 mutations (60-72),
1p/19q co-deletion (73), and high
Ki-67 expression have been linked to seizure occurrence (73,74),
whereas 1p/19q loss of heterozygosity, p53 overexpression
(<40%), and Ki-67 loss are correlated with reduced seizure
frequency and remission (8). In
addition, methylation of the methylguanine methyltransferase (MGMT)
repair protein promoter can significantly increase the risk of
postoperative epileptic seizures (75,76).
In the following sections, the changes in gene expression that
influence GRE are detailed.
IDH is a crucial enzyme involved in cellular
metabolism, particularly in the tricarboxylic acid (TCA) cycle. It
catalyses the conversion of isocitrate to α-ketoglutarate (α-KG),
generating NADH or NADPH. IDH has three primary isoforms: IDH1,
IDH2, and IDH3. Mutations in IDH1 and IDH2 are frequently observed
in certain cancers, including GBM (77,78)
and acute myeloid leukaemia (79-81).
These mutations alter the enzyme activity, leading to the
production of the oncometabolite 2-HG, which contributes to
tumourigenesis and progression (14). Notably, IDH mutations are strongly
associated with GRE.
According to the Ivy Glioblastoma Atlas Project
(http://glioblastoma.alleninstitute.org), IDH1
expression is low at the tumour leading edge (LE) but elevated in
the tumour core, whereas IDH2 is upregulated in regions of
microvascular proliferation (82).
Additionally, IDH3A has emerged as a potential risk gene for
epilepsy (83,84). IDH mutations are predictive of
seizure occurrence and prognosis in adults with lower-grade gliomas
(85). While 18-34% of patients
with IDH1wt GBM experience preoperative seizures, this rate
increases to 59-74% in patients with IDH1mut GBM (69). Multiple studies have shown that IDH1
mutations increase the risk of developing GRE before, during, and
after surgery (14,22,69,86,87).
However, some studies have suggested that IDH mutations are not
related to postoperative epilepsy or are negatively correlated with
postoperative seizure control (75,87,88).
Similar predictive effects have been attributed to IDH2 mutations
in studies on preoperative epilepsy (5,23,71).
In WHO grade II and III gliomas, IDH1 mutations were revealed to be
linked to epilepsy; however, this association was absent in
patients with GBMs (14).
Among IDHmut lower-grade gliomas, seizure control
and prognosis vary among subgroups. Grade 3 tumours are associated
with improved seizure control throughout the disease course, and
seizure freedom post-surgery and adjuvant therapy is correlated
with longer progression-free survival (PFS), regardless of tumour
grade (89). Notably, current
evidence suggests that IDHwt and IDHmut genotypes differentially
affect tumour metabolism and consequently have different
pathological mechanisms that cause excitability in peritumoural
neuronal populations. IDHwt tumours upregulate glycolysis and
subsequently release excess glutamate and lactate into the
peritumoural environment, both of which can mediate
hyperexcitability in surrounding neurons (90). The increased seizure risk in IDHmut
gliomas may stem from the accumulation of D-2HG, an abnormal
metabolite detected at elevated levels using MRS in IDHmut gliomas
(91). Mechanistically, IDH1mut
glioma cells produce D-2HG instead of α-KG, with D-2HG
concentrations 100-300 times higher than that in normal tissues
(14). Structurally similar to
glutamate, D-2HG binds NMDARs, impairing glutamate clearance and
increasing neuronal excitability (92,93).
Furthermore, D-2HG competitively inhibits α-KG (94,95),
an antiepileptic metabolite, and induces hypermetabolic changes in
peritumoural neurones, including upregulated lactate dehydrogenase
A, TCA cycle dysfunction, and activation of the mammalian target of
rapamycin (mTOR) pathway, a known pro-epileptogenic mechanism
(96). Preclinical studies have
demonstrated that mTOR inhibitors, such as rapamycin, reduce
neuronal excitability and suppress seizures (30,97,98),
highlighting mTOR as a potential therapeutic target for GRE.
Adenosine, an endogenous regulator of the mammalian
brain, plays a critical role in neuroprotection and seizure
suppression (99,100). Adenosine levels increase during
seizure activity (101), making
the adenosinergic system a promising therapeutic target for
epilepsy (102). Adenosine
homeostasis is maintained through its metabolic clearance by
adenosine kinase (ADK), which converts adenosine into
5'-adenosine-monophosphate, and adenosine deaminase (ADA), which
deaminates adenosine to inosine (103). Notably, ADA levels increase during
pentylenetetrazole-induced seizures, as demonstrated in adult
zebrafish models (104). Studies
have shown that both ADA and ADK are upregulated in the
peritumoural tissues of glioma patients with epilepsy in comparison
with those without epilepsy, suggesting their involvement in glioma
progression and epileptogenesis (105,106). Elevated ADA and ADK expression may
lead to excessive adenosine degradation, reducing its inhibitory
effects and contributing to seizure activity (105,106). Additionally, astrocytomas with
high ADK expression exhibit lower extracellular concentrations of
inhibitory neurotransmitters and increased aquaporin-4 levels,
further decreasing the seizure threshold (107,108). Since ADK inhibition effectively
treats epilepsy in animal models (109), the development of suitable
experimental models for tumour-associated epileptogenesis is
essential for evaluating the potential of adenosine augmentation
therapies in patients with brain tumours and epilepsy.
Mutations in the tumour suppressor genes phosphatase
and tensin homologue (PTEN) (110,111), neurofibromin 1 (NF1)
(112-114),
and tumour protein (TP53) (115-117)
are frequently observed in primary GBM. These mutations disrupt
downstream signalling pathways and play critical roles in
tumourigenesis. These genes are also closely associated with
epilepsy. Previous experimental studies have targeted these genes
for deletion or mutation in rodent models, successfully generating
reliable epileptogenic tumours (118,119).
Physiologically, PTEN inhibits the phosphoinositide
3-kinase (PI3K)/protein kinase B (Akt)/mTOR pathway. However,
mutations or deletions in PTEN in GBM have been shown to lead to
the disinhibition of PI3K/Akt and hyperactivation of mTOR signaling
(120,121). These alterations are associated
with poor GBM prognosis and have been implicated in epilepsy
syndromes. Conditional deletion of PTEN in mice was shown to result
in spontaneous seizures, with post-mortem analyses revealing
features consistent with temporal lobe epilepsy (122-126).
NF1 functions as a critical regulator of the
Ras signalling pathway. It exerts its inhibitory effects by
enhancing the GTPase activity of Ras, thereby converting active
guanosine triphosphate-bound Ras into its inactive guanosine
diphosphate-bound form (112,113). Mutations that impair the function
of the NF1 gene, which are often observed in GBM, disinhibit
the Ras/MAPK pathway, resulting in hyperactivation of the mTOR
pathway and uncontrolled cell proliferation, thereby promoting
cancer development. In mouse models, NF1 knockout was
demonstrated to be associated with a reduced latency period before
the onset of epilepsy and more severe seizures (127). Similarly, in patients with
neurofibromatosis type 1, NF1 mutations are correlated with
increased seizure frequency (128). TP53 (p53) mutations disrupt the
functioning of p53 in promoting cell cycle arrest, senescence, and
apoptosis, enabling the uncontrolled proliferation of damaged cells
(129). The gain-of-function
mutant form of p53 is frequently overexpressed in GBM cells. This
mutant p53 promotes invasive signalling pathways by increasing the
expression of receptor tyrosine kinases (RTKs), including MET and
epidermal growth factor receptor (EGFR) (116). Research has shown that the
amplification of the RTK proto-oncogene MET can best predict
intraoperative epileptic seizures (130). In addition, the high expression of
platelet derived growth factor receptor-α can aggravate epilepsy,
while inhibiting the RTK signalling pathway can reduce epileptic
seizures (131), and
tumour-related changes in EGFR may also increase the risk of
intraoperative epilepsy and postoperative epilepsy (36,132).
One possible mechanism is that activation of the RTK signalling
pathway promotes the release of glutamate and enhances the
excitability of the TME, thereby inducing epilepsy (133,134). Increased p53 levels, particularly
in the hippocampal region, have been detected in both experimental
models and clinical specimens obtained from individuals with
drug-resistant temporal lobe epilepsy (135,136). In the context of seizures, high
p53 expression has been linked to increased apoptosis and neuronal
death, contributing to excitability imbalances (135). Furthermore, elevated p53 levels
were revealed to be associated with epileptogenic GBMs (137). Additionally, the p53 signalling
pathway has been demonstrated to be associated with drug resistance
in epilepsy in diffuse astrocytoma and oligodendroglioma, but
through a mechanism distinct from that of p53-ATRX (138). Notably, TP53 mutations alone are
insufficient to drive GBM formation; they require concurrent
mutations in other genes such as PTEN to promote GBM progression
(139).
As a tumour suppressor gene, promoter methylation of
MGMT is an important molecular feature of gliomas. In the presence
of IDH mutations, the methylation frequency of the MGMT promoter
increases significantly: This phenomenon is particularly common in
oligodendrogliomas and astrocytomas, with approximately 35-45% of
gliomas exhibiting MGMT promoter methylation (140).
An increase in the chromosome 7 arm (7+)
and the loss of the chromosome 10 arm (10-) are typical
features of IDHwt epileptic LGG. This 7+/10-
chromosomal abnormality pattern is associated with the malignant
progression of GBM: 59% of patients with GBMs carry such
variations, and their survival rate is significantly reduced
(141). Subsequent studies have
shown that gene expression on chromosome 10, including the
expression of key genes encoding MGMT, PTEN, and vimentin (Vim), is
generally downregulated in epileptic gliomas (142). Moreover, Vim is also a biomarker
of epilepsy (76). Notably, MGMT
promoter methylation not only affects the biological behaviour of
tumours, but may also significantly increase the risk of
postoperative epileptic seizures through epigenetic regulation
(36,75,76).
However, the specific molecular mechanisms underlying this
phenomenon remain unclear.
One study investigated the spatial distribution and
expression patterns of 358 clinically validated human epilepsy
genes within the GBM transcriptome and compared them with datasets
from non-tumour adults and developing cortices. Nearly half of
these genes, including the dosage-sensitive genes strongly linked
to monogenic epilepsy, were strikingly enriched and aberrantly
regulated at the LE of the tumour. These findings support the
complex epistatic basis of peritumoural epileptogenesis. The
surrounding hyperexcitability, driven by intricate patterns of
proepileptic gene expression, may explain the limited efficacy of
narrowly targeted anti-seizure medications and the persistence of
epilepsy even after tumour resection. This may also clarify why not
all brain tumours provoke seizures (82). Additionally, 52 genes exhibiting
differential expression in patients with lower-grade gliomas and
seizures were identified (104).
These genes span a wide range of biological functions, underscoring
the complexity of the molecular processes underlying
glioma-associated seizures. Differential expression analysis
revealed that gliomas that induce epileptic activity are closely
associated with genes involved in neuronal development. The key
implicated pathways include the RhoGDI, Semaphorin Neuronal
Repulsive, and the Ephrin B signalling pathways (143). Drug-resistant epilepsy (DRE) is
associated with somatic gene mutations. In comparison with patients
showing drug reactivity, patients in the DRE group exhibited
mutations in glutamate receptor genes (GRIA1, GRIK5, GRIN2B, or
GRIN2C), ATRX, and glutamate-S-transferase genes (144). Understanding the genetic and
molecular characteristics of gliomas and their relationship with
seizures can significantly reduce the substantial morbidity and
mortality associated with these conditions.
Synaptic plasticity and neural
network
Gliomas promote axonal branching and synapse
formation, increasing neuronal excitability and seizure risk
(145,146). They form microtubules resembling
neuronal axons and dendrites, enabling functional glial synapses
mediated by AMPARs (38).
Glioma-derived prothrombin also facilitates excitatory synapse
formation in the peritumoural cortex (96). Elevated MMP-9 levels around tumours
convert pro-brain-derived neurotrophic factor (BDNF) to mature
BDNF, activate tropomyosin receptor kinase B, and foster
hyperexcitable circuits (146,147). Chronic NMDAR activation and
increased extracellular glutamate levels further exacerbate these
effects along with abnormal neuronal structures and impaired
synaptic plasticity, thereby contributing to epileptogenesis.
Although it shows major structural and molecular
changes, epilepsy is fundamentally a network disorder. Functional
MRI, electroencephalography (EEG), and magnetoencephalography (MEG)
have revealed disrupted connectivity networks in brain tumours
(148-156),
with preoperative seizures linked to suboptimal network topology
(157). In summary, GRE arises
from diverse mechanisms that reflect pathological heterogeneity and
complex molecular interactions. Understanding these pathways is
crucial for developing targeted therapies.
4. Treatment of GRE
Treatment with AEDs
AED regimens should be promptly initiated upon
seizure diagnosis in patients with gliomas. The guiding principles
of these regimens include avoiding liver enzyme-inducing AEDs in
patients undergoing chemotherapy and adopting individualised
monotherapy with adequate dosing and duration (3,158).
For cases involving preoperative seizures, rapid-acting AEDs
without slow titration are preferred.
Conversely, in postoperative cases, AEDs with
versatile formulations (including injections, tablets, and oral
solutions) and minimal interactions with anti-infectives,
glucocorticoids, and haemostatics are recommended for long-term use
(159). Given the strong
correlation between glioma progression and epileptic seizures
(160), AEDs with antitumour
effects are usually the first choice for GRE.
Usage principles of AEDs
AEDs are commonly used to manage seizures in
patients with glioma. An ideal AED should effectively control
seizures, minimise side effects, and potentially enhance the
efficacy of chemotherapy while protecting healthy brain tissue.
Currently, AED selection is not directly guided by tumour
histology, location, WHO grade, or molecular markers, although
non-enzyme-inducing AEDs are preferred (30,38).
Commonly used non-enzyme-inducing AEDs include lacosamide (LCM),
lamotrigine (LTG), levetiracetam (LEV), topiramate (TPM), valproic
acid (VPA), and zonisamide (38).
Evidence-based guidelines recommend LEV and VPA as first-line
treatments for GRE, with LEV exhibiting superior efficacy and
comparable tolerability in comparison with VPA (3,158).
Phenytoin (PHT) and pregabalin monotherapies may also be used for
GRE, although they exhibit lower efficacy than LEV (161). LEV is particularly effective for
focal and bilateral tonic-clonic seizures and is often combined
with antitumour therapies due to its additional benefits.
Mechanistically, LEV interacts with synaptic vesicle glycoprotein
2A (SV2A), modulating the release of neurotransmitters and
strengthening GABA-mediated inhibitory signaling (96). Its analogue, brivaracetam (BRV), has
a higher SV2A affinity. LEV also enhances p53-mediated MGMT
inhibition, sensitising GBM cells to temozolomide (TMZ), especially
when combined with interferon-α (8,96,162).
Previous research has suggested that LEV may improve overall
survival, potentially by modulating the glutamate-to-GABA ratio
(1).
Similar to LEV, VPA exhibits antitumour effects
mediated through mechanisms such as the upregulation of BDNF and
activation of the ERK/Akt, Akt/mTOR, and Wnt signalling pathways
(96). VPA may enhance the survival
of patients receiving TMZ therapy (8). Although adjuvant VPA with
chemoradiotherapy improves the survival of patients with GBMs, this
benefit has not been observed in patients with grade II gliomas
(1). For patients with inadequate
seizure control after monotherapy, combination therapy with LEV,
VPA, or PHT may be considered (3,161).
However, LEV or VPA should not be used solely for
non-seizure-related purposes in patients with glioma (3). In cases of poor seizure control with
LEV or VPA, TPM, a KAR inhibitor with antitumour effects, is an
alternative option (1).
A previous study on LCM monotherapy reported
seizure control rates of 65% at 3 months and 55% at 6 months. LTG
and LCM exhibited comparable efficacy in reducing seizure frequency
over 1 year (30). However, LTG is
limited by its oral-only formulation, slow titration requirements,
and potential interactions with antineoplastic agents (38).
Talampanel, a noncompetitive AMPAR antagonist, may
reduce seizures and tumour growth. Although its combination with
radiation and TMZ has been shown to improve median survival,
talampanel alone has no significant antitumour effects (1,30).
PER, another noncompetitive AMPAR antagonist, has a longer
half-life and superior BBB penetration. Approved for focal and
generalised epilepsy, PER is effective, safe, and well-tolerated in
BTRE (163,164). It inhibits glioma cell
proliferation, migration, and invasion, while reducing
extracellular glutamate levels, although the precise mechanisms
remain unclear (96). One case
report described a GBM patient achieving 18 months of seizure
freedom and survival with PER treatment (1,6).
Cenobamate, which was recently approved for the treatment of focal
epilepsy, has not yet been evaluated for GRE (38).
Notably, approximately one-third of patients with
glioma continue to experience seizures despite AED monotherapy and
dose escalation, although evidence regarding optimal AEDs for
treatment-resistant cases is lacking (96). Patients with HGGs often require
multiple AEDs for seizure control (18). Studies have suggested that LCM
add-on therapy shows efficacy and tolerability comparable to those
of LTG in patients with glioma (18,30).
LCM, which is available intravenously, allows rapid titration, has
minimal drug interactions, and causes fewer neuropsychiatric side
effects, making it a preferred adjunct (38). Combining AEDs with different
mechanisms of action, such as LEV with LCM, PER, or VPA, is
recommended for multimodal therapy (9). A prior small-scale study reported a
seizure remission rate of 57% with PER, add-on therapy (30). LEV combined with VPA was revealed to
be particularly effective for seizure control (18). In a retrospective study, BRV add-on
therapy reduced monthly seizures from seven to two (30). For status epilepticus in patients
with brain tumours, first- (such as diazepam and midazolam) and
second-line treatments (such as LEV, PHT, and VPA) should be
initiated promptly (30).
Drug-resistant seizures occur in ~15% of patients
with GBM and ~30% of patients with LGG (9,74).
IDHmut gliomas exhibit a significantly higher trend toward
pharmacoresistant seizures, whereas pharmacoresistance is rare in
IDHwt tumours (165). Patients
with IDHmut exhibited a higher 4-year cumulative incidence of DRE
(18%) in comparison with patients with IDHwt (11%), although IDH
mutations are not significantly associated with drug resistance
(9). For patients with GRE who are
resistant to other AEDs, LCM offers improved efficacy and fewer
side effects (3).
Adverse effects of AEDs
Adverse effects are the leading causes of AED
treatment failure and often limit effective dosing and patient
adherence. Both first- and second-generation AEDs exhibit similar
rates of intolerable side effects. Patients with brain tumours are
particularly vulnerable to adverse neurological effects, including
cognitive decline, depression, anxiety, dizziness, headache,
nausea, and somnolence, of which cognitive impairments are more
common with first-generation AEDs (9,30).
First-generation AEDs (such as carbamazepine, PHT, and VPA) often
cause drug interactions such as accelerating dexamethasone
metabolism. VPA is associated with coagulopathy, particularly
thrombocytopenia, but it rarely causes adverse psychiatric effects.
By contrast, second-generation AEDs, such as LEV, have minimal drug
interactions and may improve neurocognitive function. However, LEV
is associated with psychiatric side effects such as depression,
agitation, or psychosis, especially in patients with frontal lobe
tumours (9,30,38).
In patients with glioma undergoing
chemoradiotherapy, the concomitant use of AEDs may have both
beneficial and adverse effects. Although the combination of AEDs
and chemotherapeutic agents can prolong patient survival, they
carry potential risks, including thrombocytopenia and increased
drug toxicity (166,167). Clinical studies have demonstrated
that the combination of VPA and TMZ significantly extends the
median survival in comparison with non-VPA treatment (168,169). Preclinical research has shown that
VPA enhances the anti-glioma efficacy of TMZ in U87 cells, whereas
celecoxib (CXB) combined with TMZ demonstrates optimal inhibitory
effects in C6 and T98G cell lines. The combined use of VPA and CXB
was revealed to synergistically enhance the antitumour effects of
TMZ both in vitro and in vivo, significantly reducing
tumour volume and prolonging survival (170).
In vivo and in vitro studies have
shown that the combined use of PER and TMZ exerts a synergistic
antitumour effect and significantly prolongs survival (171,172). However, the combination of AEDs
and TMZ also has adverse effects. Chemotherapeutic drugs
metabolised by the CYP450 system may reduce the serum levels of
certain AEDs, while VPA, a CYP450 inhibitor, may increase the
toxicity of some chemotherapeutic agents (173). Additionally, AED use during
chemotherapy may induce thrombocytopenia (169,174).
The combination of AEDs and radiotherapy has
primarily demonstrated synergistic effects. VPA, which possesses
histone deacetylase inhibitor activity, exhibits synergistic
anti-glioma effects during radiotherapy and may serve as a
radiosensitiser (173,175). Both in vitro and in
vivo research has shown that the combination of VPA and
radiotherapy effectively inhibits tumour cells, while exerting
minimal impact on normal neurons (176). However, a cohort study of 1,057
patients with GBM revealed that patients concurrently using AEDs
for ≥14 days during chemoradiotherapy (AED group) had a
significantly higher mortality risk than non-AED users. This
adverse effect was dose-dependent, with VPA demonstrating
pronounced detrimental effects (177).
Therefore, when selecting AEDs for GRE, key
considerations include: i) Minimal drug interactions; ii) potential
beneficial side effects (such as anxiety relief and mood
stabilisation); iii) availability of multiple dosage forms (oral or
intravenous); and iv) avoidance of adverse effects (38).
Prophylactic use of AEDs
The preventive administration of AEDs in patients
with glioma is still a topic of debate, since it cannot enhance PFS
or decrease the likelihood of initial seizures occurring within 6
months post-diagnosis. Therefore, AEDs are not recommended for
seizure prevention in newly diagnosed, seizure-free patients with
brain tumours (30). The SNO and
EANO guidelines state that the evidence supporting the use of
prophylactic AEDs on the basis of tumour location, histology, or
grade is currently insufficient (178). Perioperative or postoperative AED
use is not advised in seizure-free patients (9,179),
and a recent study revealed no reduction in postoperative seizures
with preoperative AEDs (180).
Although increased extracellular glutamate is linked to seizures in
patients with glioma, most AEDs, including LEV, do not directly
target the glutamatergic system, limiting their ability to prevent
tumour-associated epilepsy (181).
Nevertheless, 63% of neurosurgeons reported frequent perioperative
AED use to reduce the risk of craniotomy-related epilepsy (181). Guidelines suggest postoperative
AED use for patients with preoperative GRE, whereas prophylactic
AEDs may only be considered for seizure-free patients in the
presence of high-risk factors (3).
AED deactivation time
The discontinuation of AEDs in patients with GRE is
complex due to the significant influence of tumour status and
antineoplastic therapy on seizure risk, unlike idiopathic epilepsy.
The psychosocial impact of seizure recurrence further complicates
accurate risk prediction (3,10).
Notably, 71% of seizure recurrences in patients with glioma occur
within 6 months of post-AED discontinuation (30). Factors such as the adverse effects
of AEDs, financial burden, and psychosocial implications must be
weighed against benefits. Current guidelines recommend
discontinuing prophylactic AEDs 2 weeks post-surgery for patients
seizure-free before and after surgery (3). For patients with a single
postoperative seizure, gradual discontinuation after 3 months is
advised, whereas for patients with recurrent seizures, treatment
should be continued for at least 1 year. In patients with
preoperative GRE with a seizure history of <6 months and
complete tumour resection, AEDs can be stopped after 1 year of
seizure remission. Nevertheless, for individuals with a prolonged
history of epilepsy, partial tumour removal, widespread
epileptiform activity on EEG, preoperative drug-resistant seizures,
or focal seizures accompanied by loss of consciousness, a
seizure-free interval of at least 2 years after surgery is
advisable before contemplating discontinuation of treatment. AED
discontinuation is not recommended for: i) All patients with GBMs;
and ii) other patients with HGG undergoing incomplete tumour
resection or exhibiting postoperative refractory seizures (such as
patients with anaplastic glioma).
Surgical treatment. Tumour resection
extent
In patients with glioma, seizure control is being
increasingly recognised as a critical goal, second only to tumour
control. Maximal tumour resection significantly improves seizure
outcomes, with the EOR being an independent predictor of
postoperative seizure control (182-186).
Surgical resection was shown to achieve seizure control in 36-100%
of patients with LGGs (4), with 80%
of patients with temporal lobe lesions achieving Engel class I
outcomes following maximal resection (187,188). For patients with LGGs with
preoperative epilepsy, resection exceeding 91% significantly
enhanced postoperative seizure control (3,4). Gross
total resection has shown superior seizure reduction over other
resection types (180), with
near-total and subtotal resections achieving 87 and 55% seizure
remission rates, respectively (18).
Maximal safe resection can not only improve seizure
control but also enhance local tumour control and survival. In
insular gliomas, maximal resection was demonstrated to prolong
survival and improve seizure outcomes for both newly diagnosed and
recurrent tumours (189,190). For GBMs, super-total resection,
extending beyond the contrast-enhancing tumour margins, was
revealed to improve overall survival and seizure control in
comparison with near-total resection (9). Maximal resection with functional
preservation is essential for tumours of the cerebral cortex.
Advanced techniques such as ‘sculpting surgery’, which precisely
target epileptic foci, can help reduce postoperative seizures when
near-total resection is not feasible (3).
Location of epileptogenic foci
The epileptogenic zone in BTRE can be located
within, adjacent to, or distant from the tumour. In two-thirds of
patients with BTRE, it is found within or near the tumour (18). A previous electrophysiological study
has revealed that epileptic activity primarily originates in the
superior granular layer of the peritumoural neocortex, which is
infiltrated by glioma cells, rather than in the tumour core
(66). Animal studies have further
indicated that the peritumoural area exhibits heightened
spontaneous epileptiform activity, likely due to increased neuronal
bursting in this region (4,191). In LGGs, epileptic foci are
typically located at the tumour-neocortex interface, with the
glioma-infiltrated peritumoural neocortex playing a key role in
epileptogenesis (4).
Preoperative and intraoperative
electrophysiological techniques, such as MEG, EEG, stereotactic
EEG, and electrocorticography (ECoG), primarily detect epileptic
activity in the peritumoural neocortex (4). While routine EEG can localise
epileptic foci, intracranial EEG is often necessary for precise
localisation and improved treatment outcomes (3,9,18).
Intraoperative ECoG monitoring using strip or grid electrodes is
recommended before and after tumour resection to identify residual
epileptic activity that can be treated with electrocautery
(192). Advances in biomedical
engineering have introduced ‘circular grid’ electrodes, which
enable 360˚ cortical monitoring and demonstrate higher seizure
detection accuracy, tumour resection rates, and postoperative
functional outcomes than conventional electrodes (192).
Awake craniotomy (AC), combined with direct
electrical stimulation (DES) and ECoG, is effective for resecting
gliomas in eloquent areas, reducing postoperative complications,
and improving survival and quality of life (192,193). A previous study revealed no
increase in seizure risk with AC in comparison with general
anaesthesia (180). Recent
research has highlighted the predictive value of transcranial
magnetic stimulation for postoperative neurological outcomes, with
ECoG-guided supratotal resection improving seizure control while
preserving function (194).
Emerging techniques for localising epileptogenic zones include PET
with α-(11C) methyltryptophan, which selectively accumulates in
epileptogenic foci, and proton MRS (1H-MRS), which non-invasively
assesses glutamate and GABA levels in tumour and peritumoural
tissue (195-199).
These advanced methods complement traditional imaging and
electrophysiological tools, offering novel insights into the
management of epilepsy in patients with glioma.
Management of intraoperative and early
postoperative seizures
Near-total resection during glioma surgery can
contribute to intraoperative seizures (158,193), particularly in high-risk patients
with factors such as younger age, frontal lobe involvement
(especially the supplementary motor area), preoperative epilepsy
history, use of multiple AEDs, and IDH1 mutations. Prophylactic
administration of LEV or VPA is recommended for these patients
(3). During AC, DES under real-time
ECoG monitoring, which is essential for functional localisation,
carries a 3.2-15.5% risk of intraoperative seizures, typically
partial seizures. However, this does not increase postoperative
seizure risk (3,4,192).
Minimising the intensity and frequency of electrical stimulation
can reduce the incidence of seizures (193). In the event of a seizure,
immediate cessation of stimulation and cortical irrigation with
ice-cold Ringer's solution or saline is recommended. Persistent
seizures may require benzodiazepine administration, with
intraoperative electromyography facilitating early detection
(3).
Early postoperative seizures that occur within the
first week require prompt airway management and injury prevention
(159). The diagnostic workup
should include electrocardiography, blood tests (glucose,
electrolytes, and liver/kidney function), and neuroimaging (CT/MRI)
to exclude non-epileptic causes such as intracranial haemorrhage or
metabolic disturbances. EEG monitoring for 2 h can help assess
epileptiform discharges related to brain oedema or residual tumour
(3). Seizures lasting over 5 min or
clustered seizures (multiple brief episodes of interictal recovery)
should be treated aggressively with midazolam or other AEDs to
prevent progression to status epilepticus (159). Recurrent seizures warrant
monitoring of AED blood levels and potential medication adjustments
or substitution (3). Prophylactic
AEDs are recommended for 1 week post-surgery, regardless of
preoperative seizure history (158).
Postoperative seizures may result from preoperative
epilepsy, surgical trauma, or metabolic disturbances (such as
electrolyte imbalance and hypoglycaemia). Non-epileptic events
should be distinguished using video EEG and AED-level testing to
identify the underlying cause and guide appropriate management
(159).
Postoperative epilepsy management
Postoperative MRI, including contrast-enhanced
imaging, should be performed within 24-72 h to evaluate the EOR.
MRI findings such as nodules, border blurring, or mass effect on T2
(FLAIR) or T1 sequences can predict postoperative epilepsy risk by
reflecting tumour growth and seizure propensity (3,8). IDH
mutations in gliomas are linked to more severe and refractory
postoperative seizures, although glutamate concentrations in the
LGG microenvironments are not correlated with seizure risk
(4).
Seizure recurrence after a prolonged seizure-free
period may result in tumour recurrence. In cases of tumour
recurrence with drug-resistant seizures, surgery may be considered
after a thorough evaluation. If seizures occur without evidence of
tumour recurrence, management should follow the refractory epilepsy
guidelines. Surgical intervention is recommended for drug-resistant
GRE when frequent seizures significantly impair the quality of life
of a patient (3).
Radiation therapy and
chemotherapy
Radiotherapy and chemotherapy are the cornerstone
treatments for gliomas and are aimed at controlling tumour growth
and improving survival. Their combined use enhances prognosis and
quality of life, with research showing significant seizure
reduction following these therapies in patients with LGGs (183). For instance, focal fractionated
irradiation and TMZ chemotherapy have been associated with a 44-77%
reduction in seizure frequency (200,201). Radiotherapy improves local tumour
control, preserves neurological function, and extends survival
(9). It also significantly reduces
seizures in GRE, with remission rates ranging from 20% after focal
radiotherapy to 80% after brachytherapy (3,9).
Notably, seizure improvement often precedes tumour shrinkage on
MRI, indicating a direct antiepileptic effect (4). For example, 76% of patients with WHO
grade II glioma experienced a 50% reduction in seizure frequency
within 3 months of radiotherapy, despite no apparent tumour changes
on imaging (200,202). Early postoperative radiotherapy is
recommended because earlier intervention is associated with
improved seizure control. Neither seizure duration prior to
radiotherapy nor radiation dose was found to significantly
influence outcomes (3,4). Radiotherapy is also a viable option
for patients with refractory seizures and surgical intolerance,
regardless of tumour recurrence (3).
Common chemotherapy agents for gliomas include TMZ,
procarbazine, lomustine, and vincristine (PCV), and lomustine.
These drugs not only improve survival but also reduce seizures in
30-100% of patients with GRE (3).
In LGGs, seizure remission rates range from 13 to 60% with PCV and
from 13 to 50% with TMZ (9). TMZ,
which is widely used in patients with LGGs and HGGs, was
demonstrated to reduce seizure frequency by 50% in 48% of patients
(203,204). However, evidence of the
antiepileptic efficacy of TMZ remains inconclusive and warrants
further research (205). The
primary goal of TMZ is tumour control; however, its potential
antiepileptic benefits should be leveraged if confirmed.
Emerging therapies. Targeting IDH and
its related downstream pathways
Targeted therapies for IDHmut gliomas have shown
significant promise in both preclinical and clinical settings.
Vorasidenib (AG-881) and ivosidenib (AG-120), which are inhibitors
of mutant IDH1/2 enzymes, specifically target D-2HG (97), a key driver of tumourigenesis and
epileptogenesis. Vorasidenib, which effectively penetrates the BBB,
demonstrated improved PFS in the INDIGO trial, although its direct
impact on seizure control remains unclear (206-208).
Ivosidenib, on the other hand, has shown potential antiepileptic
benefits, as evidenced by reduced seizure frequency in a case of
IDH1mut oligodendroglioma (209).
Additionally, mTOR inhibitors such as rapamycin and everolimus,
which counteract the activation of the mTOR pathway by D-2HG, may
offer therapeutic benefits for patients with IDH1mut glioma with
refractory epilepsy (30). Emerging
therapies, including IDH1-targeted peptide vaccines currently under
development, hold promise for mitigating the seizure risk in GRE
(8). These advancements highlight
the potential of targeted approaches to not only control tumour
growth, but also improve seizure outcomes in patients with IDHmut
glioma.
Targeting metabolic abnormalities
The ketogenic diet has shown promise in inhibiting
glioma proliferation and reducing seizure frequency and severity
(3). Sulfasalazine, which inhibits
glutamate release, has been associated with prolonged seizure-free
survival, although its use is limited by its haematological adverse
reactions (1). PPAR-λ agonists,
such as glitazones, have been demonstrated to enhance glutamate
reuptake, potentially reducing excitotoxicity (38). NMDAR antagonists have been shown to
restore inhibitory GABA signalling in peritumoural neurones,
offering another avenue for seizure control (6). Cannabidiol (CBD), known for its
efficacy in refractory epilepsy, may also benefit patients with
glioma, although its clinical effectiveness in GRE requires further
validation (96).
Immune regulation
Bevacizumab, an anti-VEGF monoclonal antibody, has
been demonstrated to reduce peritumoural oedema and may decrease
the risk of seizures in patients with recurrent GBMs (8). While immune checkpoint inhibitors
enhance antitumour immunity, they may also paradoxically increase
the risk of status epilepticus in patients with brain metastases
(56). Notably, disruption of the
BBB has been shown in both patients with epilepsy and animal models
of epilepsy (210-215).
In patients with temporal lobe epilepsy, CCL2 upregulation was
revealed to be associated with BBB disruption and epileptogenesis
(211,212,216). Therefore, systemic delivery of
immunotherapies may be a viable strategy for these patients,
presenting an advantage over treating other CNS diseases not
accompanied by BBB disruption.
Targeting ion channel
Enrichment analysis revealed conserved pathogenesis
modules between epilepsy and glioma, including calcium-related
pathways (217). Seizures have
also been reported to be controlled by voltage-sensitive calcium
channel antagonists and have been demonstrated in animal models of
epilepsy (218). In addition, a
new generation of calcium channel drugs has emerged for the
treatment of epilepsy and chronic pain. ω-Taro spirotoxin is a
potent blocker of presynaptic calcium channels in neurons. A
synthetic derivative, ziconotide (Prialt), is administered
intrathecally for the control of severe pain in patients with
advanced cancer and other patients suffering from intractable pain
(219). SCN3B is one of the hub
genes involving ion channel regulation, and it is significantly
overexpressed in patients with GRE (220). Upregulated SCN3B may influence
cell excitability and contribute to epileptogenesis (221). Moreover, SCN3B is also a potential
oncogenic factor, as the β3 subunit could promote proliferation and
suppress tumor cell apoptosis by promoting p53 degradation
(222,223). SCN3B appears to have potential as
a shared therapeutic target for both diffuse gliomas and GRE.
These emerging therapies represent innovative
approaches to improving outcomes in GRE by targeting both tumour
biology and epileptogenesis. In conclusion, although radiotherapy
and chemotherapy remain central to glioma treatment, emerging
therapies and targeted agents offer promising avenues for improving
seizure control and overall outcomes in patients with GRE. Further
research is essential to validate their efficacy and optimise their
clinical application.
5. Conclusion and perspectives
Gliomas are the most common primary brain tumours.
Notably, 30-90% of patients with glioma experience epileptic
seizures, especially those with LGGs, who exhibit an even higher
incidence of epilepsy (60-90%). Epilepsy is not only an early
symptom of glioma, but may also affect the quality of life,
cognitive function and prognosis of patients. Its pathogenesis
involves tumour-induced compression, destruction of peripheral
neural tissue, and aberrant excitatory effects of tumour-derived
chemicals. Furthermore, epileptic seizures (a symptom of GBM)
promote tumour progression and exacerbate the increase in
excitability. This establishes a reinforcing feedback loop that
intensifies epileptic seizures (224). However, the detailed molecular,
cellular, and electrophysiological mechanisms underlying seizure
generation remain unclear, necessitating further research to
develop precise therapeutic strategies. Patients with GRE present
with diverse symptoms that complicate its diagnosis and treatment.
Improving early diagnosis, accurate symptom analysis, and the use
of predictive tools based on advanced imaging techniques such as
MRI are crucial for improved patient outcomes. In addition,
accurate and reliable diagnosis of glioma and prediction of glioma
patient survival can provide valuable guidance for the diagnosis,
treatment planning, and prognosis of subsequent complications such
as epilepsy. A recent study proposed a method involving a
standardised workflow of nanoparticle-enhanced laser
desorption/ionisation mass spectrometry and paper-based dried serum
spots to achieve sustainable metabolic diagnosis, which can
diagnose multiple cancers within minutes at an affordable cost,
with environmentally friendly, serum-equivalent precision, and
user-friendly protocols (225).
Another research team established a squeeze-and-excitation deep
learning feature extractor for T1 contrast-enhanced images and
histological sections and explored the significant cyclic
5-hydroxymethylcytosine profile for screening glioma survival
through minimum absolute contraction and Cox regression (226).
Current treatments include surgical resection,
radiotherapy, chemotherapy, and AEDs. However, challenges, such as
surgical limitations, risks to the surrounding brain tissue, and
AED resistance due to long-term use, persist. Future research
should focus on elucidating the pathogenesis of GRE, including its
seizure-initiation mechanisms, neuronal network abnormalities, and
tumour-neuron interactions. Leveraging molecular biology, genomics,
and cell biology techniques to identify seizure biomarkers can
enhance the diagnostic and therapeutic precision. Additionally,
exploring novel therapies, such as neuromodulation, immunotherapy,
and next-generation AEDs, may address the limitations of existing
treatment modalities.
Multidisciplinary collaboration and robust clinical
research are essential to better understand the local and systemic
effects of GRE and to accelerate the development of innovative
therapies. Future research should integrate molecular pathology,
radiomics, and artificial intelligence to promote individualised
treatment and improve the quality of life of patients. Although GRE
research and treatment remain challenging, advancements in these
areas hold promise for improving patient outcomes and quality of
life. Continued exploration and collaboration are the keys to
unlocking novel therapeutic possibilities.
Acknowledgements
Not applicable.
Funding
Funding: This research was supported by the Fundamental Research
Funds for the Central Universities (grant no. 2042025kf0010).
Availability of data and materials
Not applicable.
Authors' contributions
ZQL and FT were involved in the conception and the
design of the review. XC, LYK and ZYL conducted the literature
search and collation. XC and JZY wrote the manuscript. ZQL and FT
reviewed the manuscript. All authors have reviewed the manuscript
and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lange F, Hörnschemeyer J and Kirschstein
T: Glutamatergic mechanisms in glioblastoma and tumor-associated
epilepsy. Cells. 10(1226)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhao K, Gu J and Zhao B: The effect of
epilepsy on anxiety, depression, as well as prognostic value among
adult low-grade gliomas patients. Brain Res.
1863(149737)2025.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liang S, Fan X, Zhao M, Shan X, Li W, Ding
P, You G, Hong Z, Yang X, Luan G, et al: Clinical practice
guidelines for the diagnosis and treatment of adult diffuse
glioma-related epilepsy. Cancer Med. 8:4527–4535. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pallud J and McKhann GM: Diffuse low-grade
glioma-related epilepsy. Neurosurg Clin N Am. 30:43–54.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Du Y, Li R, Fu D, Zhang B, Cui A, Shao Y,
Lai Z, Chen R, Chen B, Wang Z, et al: Multi-omics technologies and
molecular biomarkers in brain tumor-related epilepsy. CNS Neurosci
Ther. 30(e14717)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Radin DP and Tsirka SE: Interactions
between tumor cells, neurons, and microglia in the glioma
microenvironment. Int J Mol Sci. 21(8476)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pallud J, Roux A, Moiraghi A, Aboubakr O,
Elia A, Guinard E, Oppenheim C, Tauziede-Espariat A, Parraga E,
Gavaret M, et al: Characteristics and prognosis of tumor-related
epilepsy during tumor evolution in patients with IDH wild-type
glioblastoma. Neurology. 102(e207902)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goldstein ED and Feyissa AM: Brain tumor
related-epilepsy. Neurol Neurochir Pol. 52:436–447. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
van der Meer PB, Taphoorn MJB and Koekkoek
JAF: Management of epilepsy in brain tumor patients. Curr Opin
Oncol. 34:685–690. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zoccarato M, Nardetto L, Basile AM,
Giometto B, Zagonel V and Lombardi G: Seizures, edema, thrombosis,
and hemorrhages: An update review on the medical management of
gliomas. Front Oncol. 11(617966)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Breemen MS, Wilms EB and Vecht CJ:
Epilepsy in patients with brain tumours: Epidemiology, mechanisms,
and management. Lancet Neurol. 6:421–430. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kerkhof M and Vecht CJ: Seizure
characteristics and prognostic factors of gliomas. Epilepsia. 54
(Suppl 9):S12–S17. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
van Breemen MS, Rijsman RM, Taphoorn MJ,
Walchenbach R, Zwinkels H and Vecht CJ: Efficacy of anti-epileptic
drugs in patients with gliomas and seizures. J Neurol.
256:1519–1526. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang Y, Mao Q, Wang X, Liu Y, Mao Y, Zhou
Q and Luo J: An analysis of 170 glioma patients and systematic
review to investigate the association between IDH-1 mutations and
preoperative glioma-related epilepsy. J Clin Neurosci. 31:56–62.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Knudsen-Baas KM, Power KN, Engelsen BA,
Hegrestad SE, Gilhus NE and Storstein AM: Status epilepticus
secondary to glioma. Seizure. 40:76–80. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fernández-Torre JL, Hernández-Hernández M,
Martino J and Hinojo C: Subclinical focal seizures as a sign of
progression in gliomas. Epileptic Disord. 16:546–553.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mastall M, Wolpert F, Gramatzki D, Imbach
L, Becker D, Schmick A, Hertler C, Roth P, Weller M and Wirsching
HG: Survival of brain tumour patients with epilepsy. Brain.
144:3322–3327. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Debinski W: Gliomas. Exon Publications,
Brisbane, 2021.
|
|
19
|
Cavaliere R, Farace E and Schiff D:
Clinical implications of status epilepticus in patients with
neoplasms. Arch Neurol. 63:1746–1749. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fisher RS, van Emde Boas W, Blume W, Elger
C, Genton P, Lee P and Engel J Jr: Epileptic seizures and epilepsy:
Definitions proposed by the International league against epilepsy
(ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia.
46:470–472. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kurokawa R, Baba A, Emile P, Kurokawa M,
Ota Y, Kim J, Capizzano A, Srinivasan A and Moritani T:
Neuroimaging features of angiocentric glioma: A case series and
systematic review. J Neuroimaging. 32:389–399. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Y, Shan X, Wu Z, Wang Y, Ling M and Fan
X: IDH1 mutation is associated with a higher preoperative seizure
incidence in low-grade glioma: A systematic review and
meta-analysis. Seizure. 55:76–82. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhong Z, Wang Z, Wang Y, You G and Jiang
T: IDH1/2 mutation is associated with seizure as an initial symptom
in low-grade glioma: A report of 311 Chinese adult glioma patients.
Epilepsy Res. 109:100–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nobusawa S, Watanabe T, Kleihues P and
Ohgaki H: IDH1 mutations as molecular signature and predictive
factor of secondary glioblastomas. Clin Cancer Res. 15:6002–6007.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bhandari AP, Liong R, Koppen J, Murthy SV
and Lasocki A: Noninvasive determination of IDH and 1p19q status of
lower-grade gliomas using MRI Radiomics: A systematic review. AJNR
Am J Neuroradiol. 42:94–101. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gao A, Yang H, Wang Y, Zhao G, Wang C,
Wang H, Zhang X, Zhang Y, Cheng J, Yang G and Bai J: Radiomics for
the prediction of epilepsy in patients with frontal glioma. Front
Oncol. 11(725926)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Jie B, Hongxi Y, Ankang G, Yida W, Guohua
Z, Xiaoyue M, Chenglong W, Haijie W, Xiaonan Z, Guang Y, et al:
Radiomics nomogram improves the prediction of epilepsy in patients
with gliomas. Front Oncol. 12(856359)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang Y, Gao A, Yang H, Bai J, Zhao G,
Zhang H, Song Y, Wang C, Zhang Y, Cheng J and Yang G: Using
partially shared radiomics features to simultaneously identify
isocitrate dehydrogenase mutation status and epilepsy in glioma
patients from MRI images. Sci Rep. 15(3591)2025.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Armstrong TS, Grant R, Gilbert MR, Lee JW
and Norden AD: Epilepsy in glioma patients: mechanisms, management,
and impact of anticonvulsant therapy. Neuro Oncol. 18:779–789.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hauff NS and Storstein A: Seizure
management and prophylaxis considerations in patients with brain
tumors. Curr Oncol Rep. 25:787–792. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sirven JI, Wingerchuk DM, Drazkowski JF,
Lyons MK and Zimmerman RS: Seizure prophylaxis in patients with
brain tumors: A meta-analysis. Mayo Clin Proc. 79:1489–1494.
2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Englot DJ, Chang EF and Vecht CJ: Epilepsy
and brain tumors. Handb Clin Neurol. 134:267–285. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen DY, Chen CC, Crawford JR and Wang SG:
Tumor-related epilepsy: Epidemiology, pathogenesis and management.
J Neurooncol. 139:13–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Westermark A, Fahlström M, Mirza S,
Zetterling M, Kumlien E and Latini F: Subcortical brain regions
associated with seizure risk in patients with IDH mutated diffuse
gliomas. Brain Behav. 15(e70477)2025.PubMed/NCBI View Article : Google Scholar
|
|
35
|
John Lin CC, Yu K, Hatcher A, Huang TW,
Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, et al:
Identification of diverse astrocyte populations and their malignant
analogs. Nat Neurosci. 20:396–405. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang P, Liang T, Zhang C, Cai J, Zhang W,
Chen B, Qiu X, Yao K, Li G, Wang H, et al: Clinicopathological
factors predictive of postoperative seizures in patients with
gliomas. Seizure. 35:93–99. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hills KE, Kostarelos K and Wykes RC:
Converging mechanisms of epileptogenesis and their insight in
glioblastoma. Front Mol Neurosci. 15(903115)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Seidel S, Wehner T, Miller D, Wellmer J,
Schlegel U and Grönheit W: Brain tumor related epilepsy:
Pathophysiological approaches and rational management of
antiseizure medication. Neurol Res Pract. 4(45)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bartos LM, Quach S, Zenatti V,
Kirchleitner SV, Blobner J, Wind-Mark K, Kolabas ZI, Ulukaya S,
Holzgreve A, Ruf VC, et al: Remote neuroinflammation in newly
diagnosed glioblastoma correlates with unfavorable clinical
outcome. Clin Cancer Res. 30:4618–4634. 2024.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hong DS, Angelo LS and Kurzrock R:
Interleukin-6 and its receptor in cancer: Implications for
translational therapeutics. Cancer. 110:1911–1128. 2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Shan Y, He X, Song W, Han D, Niu J and
Wang J: Role of IL-6 in the invasiveness and prognosis of glioma.
Int J Clin Exp Med. 8:9114–9120. 2015.PubMed/NCBI
|
|
42
|
Cao F, Zhang Q, Chen W, Han C, He Y, Ran Q
and Yao S: IL-6 increases SDCBP expression, cell proliferation, and
cell invasion by activating JAK2/STAT3 in human glioma cells. Am J
Transl Res. 9:4617–4626. 2017.PubMed/NCBI
|
|
43
|
Alapirtti T, Lehtimäki K, Nieminen R,
Mäkinen R, Raitanen J, Moilanen E, Mäkinen J and Peltola J: The
production of IL-6 in acute epileptic seizure: A video-EEG study. J
Neuroimmunol. 316:50–55. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
M Taalab Y, Mohammed WF, Helmy MA, Othman
AAA, Darwish M, Hassan I and Abbas M: Cannabis influences the
putative cytokines-related pathway of epilepsy among egyptian
epileptic patients. Brain Sci. 9(332)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang Q, Tuerxun N and Tuerxun S: IL-6 is
associated with poor seizure control in low-grade glioma patients
undergoing primary resection. iScience. 27(110267)2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Cobbs CS, Harkins L, Samanta M, Gillespie
GY, Bharara S, King PH, Nabors LB, Cobbs CG and Britt WJ: Human
cytomegalovirus infection and expression in human malignant glioma.
Cancer Res. 62:3347–3350. 2002.PubMed/NCBI
|
|
47
|
Rahbar A, Stragliotto G, Orrego A, Peredo
I, Taher C, Willems J and Söderberg-Naucler C: Low levels of Human
Cytomegalovirus Infection in Glioblastoma multiforme associates
with patient survival; -a case-control study. Herpesviridae.
3(3)2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Rahbar A, Orrego A, Peredo I, Dzabic M,
Wolmer-Solberg N, Strååt K, Stragliotto G and Söderberg-Nauclér C:
Human cytomegalovirus infection levels in glioblastoma multiforme
are of prognostic value for survival. J Clin Virol. 57:36–42.
2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Pantalone MR, Rahbar A, Söderberg-Naucler
C and Stragliotto G: Valganciclovir as Add-on to Second-Line
therapy in patients with recurrent glioblastoma. Cancers (Basel).
14(1958)2022.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Stragliotto G, Pantalone MR, Rahbar A,
Bartek J and Söderberg-Naucler C: Valganciclovir as add-on to
standard therapy in glioblastoma patients. Clin Cancer Res.
26:4031–4039. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Stragliotto G, Pantalone MR, Rahbar A and
Söderberg-Nauclér C: Valganciclovir as add-on to standard therapy
in secondary glioblastoma. Microorganisms. 8(1471)2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
De la Cerda-Vargas MF, Pantalone MR,
Söderberg Nauclér C, Medrano-Guzman R, Jauregui Renaud K, Nettel
Rueda B, Reynoso-Sanchez MJ, Lopez-Quintana B, Rodriguez-Florido
MA, Feria-Romero IA, et al: Focal-to-bilateral tonic-clonic
seizures and High-grade CMV-infection are poor survival predictors
in Tumor-related Epilepsy Adult-type diffuse gliomas-A
single-center study and literature review. Heliyon.
10(e28555)2024.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Yuen TI, Morokoff AP, Bjorksten A, D'Abaco
G, Paradiso L, Finch S, Wong D, Reid CA, Powell KL, Drummond KJ, et
al: Glutamate is associated with a higher risk of seizures in
patients with gliomas. Neurology. 79:883–889. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dahlmanns M, Dahlmanns JK, Savaskan N,
Steiner HH and Yakubov E: Glial Glutamate Transporter-Mediated
Plasticity: System xc-/xCT/SLC7A11 and
EAAT1/2 in Brain Diseases. Front Biosci (Landmark Ed).
28(57)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sørensen MF, Heimisdóttir SB, Sørensen MD,
Mellegaard CS, Wohlleben H, Kristensen BW and Beier CP: High
expression of cystine-glutamate antiporter xCT (SLC7A11) is an
independent biomarker for epileptic seizures at diagnosis in
glioma. J Neurooncol. 138:49–53. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Tönjes M, Barbus S, Park YJ, Wang W,
Schlotter M, Lindroth AM, Pleier SV, Bai AHC, Karra D, Piro RM, et
al: BCAT1 promotes cell proliferation through amino acid catabolism
in gliomas carrying wild-type IDH1. Nat Med. 19:901–908.
2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang B, Chen Y, Shi X, Zhou M, Bao L,
Hatanpaa KJ, Patel T, DeBerardinis RJ, Wang Y and Luo W: Regulation
of branched-chain amino acid metabolism by hypoxia-inducible factor
in glioblastoma. Cell Mol Life Sci. 78:195–206. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ishiuchi S, Tsuzuki K, Yoshida Y, Yamada
N, Hagimura N, Okado H, Miwa A, Kurihara H, Nakazato Y, Tamura M,
et al: Blockage of Ca(2+)-permeable AMPA receptors suppresses
migration and induces apoptosis in human glioblastoma cells. Nat
Med. 8:971–978. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
59
|
Liu QS, Xu Q, Arcuino G, Kang J and
Nedergaard M: Astrocyte-mediated activation of neuronal kainate
receptors. Proc Natl Acad Sci USA. 101:3172–3177. 2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Gao X, Wang H, Cai S, Saadatzadeh MR,
Hanenberg H, Pollok KE, Cohen-Gadol AA and Chen J: Phosphorylation
of NMDA 2B at S1303 in human glioma peritumoral tissue:
implications for glioma epileptogenesis. Neurosurg Focus.
37(E17)2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wirsching HG, Silginer M, Ventura E,
Macnair W, Burghardt I, Claassen M, Gatti S, Wichmann J, Riemer C,
Schneider H and Weller M: Negative allosteric modulators of
metabotropic glutamate receptor 3 target the stem-like phenotype of
glioblastoma. Mol Ther Oncolytics. 20:166–174. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ciceroni C, Bonelli M, Mastrantoni E,
Niccolini C, Laurenza M, Larocca LM, Pallini R, Traficante A,
Spinsanti P, Ricci-Vitiani L, et al: Type-3 metabotropic glutamate
receptors regulate chemoresistance in glioma stem cells, and their
levels are inversely related to survival in patients with malignant
gliomas. Cell Death Differ. 20:396–407. 2013.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Zhang Z, Zheng X, Luan Y and Liu Y, Li X,
Liu C, Lu H, Chen X and Liu Y: Activity of metabotropic glutamate
receptor 4 suppresses proliferation and promotes apoptosis with
inhibition of Gli-1 in human glioblastoma cells. Front Neurosci.
12(320)2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Campbell SL, Robel S, Cuddapah VA, Robert
S, Buckingham SC, Kahle KT and Sontheimer H: GABAergic
disinhibition and impaired KCC2 cotransporter activity underlie
tumor-associated epilepsy. Glia. 63:23–36. 2015.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Conti L, Palma E, Roseti C, Lauro C,
Cipriani R, de Groot M, Aronica E and Limatola C: Anomalous levels
of Cl-transporters cause a decrease of GABAergic inhibition in
human peritumoral epileptic cortex. Epilepsia. 52:1635–1644.
2011.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Pallud J, Le Van Quyen M, Bielle F,
Pellegrino C, Varlet P, Cresto N, Baulac M, Duyckaerts C,
Kourdougli N, Chazal G, et al: Cortical GABAergic excitation
contributes to epileptic activities around human glioma. Sci Transl
Med. 6(244ra89)2014.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Elias AF, Lin BC and Piggott BJ: Ion
Channels in Gliomas-From Molecular Basis to Treatment. Int J Mol
Sci. 24(2530)2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Curry RN, Aiba I, Meyer J, Lozzi B, Ko Y,
McDonald MF, Rosenbaum A, Cervantes A, Huang-Hobbs E, Cocito C, et
al: Glioma epileptiform activity and progression are driven by
IGSF3-mediated potassium dysregulation. Neuron. 111:682–695.e9.
2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Chen H, Judkins J, Thomas C, Wu M, Khoury
L, Benjamin CG, Pacione D, Golfinos JG, Kumthekar P, Ghamsari F, et
al: Mutant IDH1 and seizures in patients with glioma. Neurology.
88:1805–1813. 2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Neal A, Kwan P, O'Brien TJ, Buckland ME,
Gonzales M and Morokoff A: IDH1 and IDH2 mutations in postoperative
diffuse glioma-associated epilepsy. Epilepsy Behav. 78:30–36.
2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Stockhammer F, Misch M, Helms HJ, Lengler
U, Prall F, von Deimling A and Hartmann C: IDH1/2 mutations in WHO
grade II astrocytomas associated with localization and seizure as
the initial symptom. Seizure. 21:194–197. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Snijders TJ, Berendsen S, Seute T and Robe
PA: Glioma-associated epilepsy: Toward mechanism-based treatment.
Transl Cancer Res. 6 (Suppl 2):S337–S341. 2017.
|
|
73
|
You G, Huang L, Yang P, Zhang W, Yan W,
Wang Y, Bao Z, Li S, Li S, Li G and Jiang T: Clinical and molecular
genetic factors affecting postoperative seizure control of 183
Chinese adult patients with low-grade gliomas. Eur J Neurol.
19:298–306. 2012.PubMed/NCBI View Article : Google Scholar
|
|
74
|
You G, Sha ZY, Yan W, Zhang W, Wang YZ, Li
SW, Sang L, Wang Z, Li GL, Li SW, et al: Seizure characteristics
and outcomes in 508 Chinese adult patients undergoing primary
resection of low-grade gliomas: A clinicopathological study. Neuro
Oncol. 14:230–241. 2012.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Feyissa AM, Worrell GA, Tatum WO,
Chaichana KL, Jentoft ME, Guerrero Cazares H, Ertekin-Taner N,
Rosenfeld SS, ReFaey K and Quinones-Hinojosa A: Potential influence
of IDH1 mutation and MGMT gene promoter methylation on
glioma-related preoperative seizures and postoperative seizure
control. Seizure. 69:283–289. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Pan SP, Zheng XL, Zhang N, Lin XM, Li KJ,
Xia XF, Zou CL and Zhang WY: A novel nomogram for predicting the
risk of epilepsy occurrence after operative in gliomas patients
without preoperative epilepsy history. Epilepsy Res.
174(106641)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yan H, Parsons DW, Jin G, McLendon R,
Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ,
et al: IDH1 and IDH2 mutations in gliomas. N Engl J Med.
360:765–773. 2009.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Hartmann C, Meyer J, Balss J, Capper D,
Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, et
al: Type and frequency of IDH1 and IDH2 mutations are related to
astrocytic and oligodendroglial differentiation and age: A study of
1,010 diffuse gliomas. Acta Neuropathol. 118:469–474.
2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Mardis ER, Ding L, Dooling DJ, Larson DE,
McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath
SD, et al: Recurring mutations found by sequencing an acute myeloid
leukemia genome. N Engl J Med. 361:1058–1066. 2009.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Cancer Genome Atlas Research Network. Ley
TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, Hoadley
K, Triche TJ Jr, Laird PW, et al: Genomic and epigenomic landscapes
of adult de novo acute myeloid leukemia. N Engl J Med.
368:2059–2074. 2013.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Marcucci G, Maharry K, Wu YZ, Radmacher
MD, Mrózek K, Margeson D, Holland KB, Whitman SP, Becker H, Schwind
S, et al: IDH1 and IDH2 gene mutations identify novel molecular
subsets within de novo cytogenetically normal acute myeloid
leukemia: A Cancer and Leukemia Group B study. J Clin Oncol.
28:2348–2355. 2010.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Soeung V, Puchalski RB and Noebels JL: The
complex molecular epileptogenesis landscape of glioblastoma. Cell
Rep Med. 5(101691)2024.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Drumm MR, Wang W, Sears TK, Bell-Burdett
K, Javier R, Cotton KY, Webb B, Byrne K, Unruh D, Thirunavu V, et
al: Postoperative risk of IDH-mutant glioma-associated seizures and
their potential management with IDH-mutant inhibitors. J Clin
Invest. 133(e168035)2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
van Opijnen MP, Tesileanu CMS, Dirven L,
van der Meer PB, Wijnenga MMJ, Vincent AJPE, Broekman MLD, Dubbink
HJ, Kros JM, van Duinen SG, et al: IDH1/2 wildtype gliomas grade 2
and 3 with molecular glioblastoma-like profile have a distinct
course of epilepsy compared to IDH1/2 wildtype glioblastomas. Neuro
Oncol. 25:701–709. 2023.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Tang T, Wang Y, Dai Y, Liu Q, Fan X, Cheng
Y, Tang J, Xiao X, Shan Y, Wei P and Zhao G: IDH1 mutation predicts
seizure occurrence and prognosis in lower-grade glioma adults.
Pathol Res Pract. 254(155165)2024.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Skardelly M, Brendle E, Noell S, Behling
F, Wuttke TV, Schittenhelm J, Bisdas S, Meisner C, Rona S, Tatagiba
MS and Tabatabai G: Predictors of preoperative and early
postoperative seizures in patients with intra-axial primary and
metastatic brain tumors: A retrospective observational single
center study. Ann Neurol. 78:917–928. 2015.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Song L, Quan X, Chen C, Chen L and Zhou J:
Correlation between tumor molecular markers and perioperative
epilepsy in patients with glioma: A systematic review and
meta-analysis. Front Neurol. 12(692751)2021.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Shen S, Bai Y, Zhang B, Liu T, Yu X and
Feng S: Correlation of preoperative seizures with a wide range of
tumor molecular markers in gliomas: An analysis of 442 glioma
patients from China. Epilepsy Res. 166(106430)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Bruno F, Pellerino A, Conti Nibali M,
Pronello E, Cofano F, Rossi M, Levis M, Bertero L, Soffietti R,
Cassoni P, et al: Association of clinical, tumor, and treatment
characteristics with seizure control in patients with IDH1/2-Mutant
Lower-Grade Glioma. Neurology. 102(e209352)2024.PubMed/NCBI View Article : Google Scholar
|
|
90
|
McAfee D, Moyer M, Queen J, Mortazavi A,
Boddeti U, Bachani M, Zaghloul K and Ksendzovsky A: Differential
metabolic alterations in IDH1 mutant vs. wildtype glioma cells
promote epileptogenesis through distinctive mechanisms. Front Cell
Neurosci. 17(1288918)2023.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Nagashima H, Tanaka K, Yamanishi S,
Hashiguchi M, Iwahashi H, Uno T, Somiya Y, Komatsu M, Itoh T,
Sasaki R and Sasayama T: Association between accumulation of
2-hydroxyglutarate detected by MR spectroscopy and preoperative
seizure in IDH-mutant glioma. J Neurosurg. 142:712–721.
2024.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Kölker S, Pawlak V, Ahlemeyer B, Okun JG,
Hörster F, Mayatepek E, Krieglstein J, Hoffmann GF and Köhr G: NMDA
receptor activation and respiratory chain complex V inhibition
contribute to neurodegeneration in d-2-hydroxyglutaric aciduria.
Eur J Neurosci. 16:21–28. 2002.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Junqueira D, Brusque AM, Porciúncula LO,
Rotta LN, Frizzo ME, Wyse AT, Wannmacher CM, Souza DO and Wajner M:
In vitro effects of D-2-hydroxyglutaric acid on glutamate binding,
uptake and release in cerebral cortex of rats. J Neurol Sci.
217:189–194. 2004.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Yamamoto HA and Mohanan PV: Effect of
alpha-ketoglutarate and oxaloacetate on brain mitochondrial DNA
damage and seizures induced by kainic acid in mice. Toxicol Lett.
143:115–122. 2003.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim
SH, Ito S, Yang C, Wang P, Xiao MT, et al: Oncometabolite
2-hydroxyglutarate is a competitive inhibitor of
alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 19:17–30.
2011.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Stella M, Baiardi G, Pasquariello S, Sacco
F, Dellacasagrande I, Corsaro A, Mattioli F and Barbieri F:
Antitumor potential of antiepileptic drugs in human glioblastoma:
Pharmacological targets and clinical benefits. Biomedicines.
11(582)2023.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Winkler F: Understanding epilepsy in
IDH-mutated gliomas: Towards a targeted therapy. Neuro Oncol.
24:1436–1437. 2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Mortazavi A, Fayed I, Bachani M, Dowdy T,
Jahanipour J, Khan A, Owotade J, Walbridge S, Inati SK, Steiner J,
et al: IDH-mutated gliomas promote epileptogenesis through
d-2-hydroxyglutarate-dependent mTOR hyperactivation. Neuro Oncol.
24:1423–1435. 2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Boison D: Role of adenosine in status
epilepticus: A potential new target? Epilepsia. 54 (Suppl
6):S20–S22. 2013.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Boison D: Adenosine and seizure
termination: Endogenous mechanisms. Epilepsy Curr. 13:35–37.
2013.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Hargus NJ, Jennings C, Perez-Reyes E,
Bertram EH and Patel MK: Enhanced actions of adenosine in medial
entorhinal cortex layer II stellate neurons in temporal lobe
epilepsy are mediated via A(1)-receptor activation. Epilepsia.
53:168–176. 2012.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Świąder MJ, Kotowski J and Łuszczki JJ:
Modulation of adenosinergic system and its application for the
treatment of epilepsy. Pharmacol Rep. 66:335–342. 2014.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Boison D: Adenosine kinase: Exploitation
for therapeutic gain. Pharmacol Rev. 65:906–943. 2013.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Siebel AM, Piato AL, Schaefer IC, Nery LR,
Bogo MR and Bonan CD: Antiepileptic drugs prevent changes in
adenosine deamination during acute seizure episodes in adult
zebrafish. Pharmacol Biochem Behav. 104:20–26. 2013.PubMed/NCBI View Article : Google Scholar
|
|
105
|
de Groot M, Iyer A, Zurolo E, Anink J,
Heimans JJ, Boison D, Reijneveld JC and Aronica E: Overexpression
of ADK in human astrocytic tumors and peritumoral tissue is related
to tumor-associated epilepsy. Epilepsia. 53:58–66. 2012.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Huang J, He Y, Chen M, Du J, Li G, Li S,
Liu W and Long X: Adenosine deaminase and adenosine kinase
expression in human glioma and their correlation with
glioma-associated epilepsy. Mol Med Rep. 12:6509–6516.
2015.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Pence S, Erkutlu I, Kurtul N, Bosnak M,
Alptekin M and Tan U: Antiepileptogenic effects of glutathione
against increased brain ADA in PTZ-induced epilepsy. Int J
Neurosci. 119:616–629. 2009.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Shen HY, Sun H, Hanthorn MM, Zhi Z, Lan
JQ, Poulsen DJ, Wang RK and Boison D: Overexpression of adenosine
kinase in cortical astrocytes and focal neocortical epilepsy in
mice. J Neurosurg. 120:628–638. 2014.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Boison D: Adenosine dysfunction and
adenosine kinase in epileptogenesis. Open Neurosci J. 4:93–101.
2010.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Wang SI, Puc J, Li J, Bruce JN, Cairns P,
Sidransky D and Parsons R: Somatic mutations of PTEN in
glioblastoma multiforme. Cancer Res. 57:4183–4186. 1997.PubMed/NCBI
|
|
111
|
Han F, Hu R, Yang H, Liu J, Sui J, Xiang
X, Wang F, Chu L and Song S: PTEN gene mutations correlate to poor
prognosis in glioma patients: A meta-analysis. Onco Targets Ther.
9:3485–3492. 2016.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Pearson JRD and Regad T: Targeting
cellular pathways in glioblastoma multiforme. Signal Transduct
Target Ther. 2(17040)2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Soomro SH, Ting LR, Qing YY and Ren M:
Molecular biology of glioblastoma: Classification and mutational
locations. J Pak Med Assoc. 67:1410–1414. 2017.PubMed/NCBI
|
|
114
|
Szopa W, Burley TA, Kramer-Marek G and
Kaspera W: Diagnostic and therapeutic biomarkers in glioblastoma:
Current status and future perspectives. Biomed Res Int.
2017(8013575)2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Dittmer D, Pati S, Zambetti G, Chu S,
Teresky AK, Moore M, Finlay C and Levine AJ: Gain of function
mutations in p53. Nat Genet. 4:42–46. 1993.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Nagpal J, Jamoona A, Gulati ND, Mohan A,
Braun A, Murali R and Jhanwar-Uniyal M: Revisiting the role of p53
in primary and secondary glioblastomas. Anticancer Res. 26
(6C):4633–4639. 2006.PubMed/NCBI
|
|
117
|
Zhang Y, Dube C, Gibert M Jr, Cruickshanks
N, Wang B, Coughlan M, Yang Y, Setiady I, Deveau C, Saoud K, et al:
The p53 Pathway in Glioblastoma. Cancers (Basel).
10(297)2018.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Hatcher A, Yu K, Meyer J, Aiba I, Deneen B
and Noebels JL: Pathogenesis of peritumoral hyperexcitability in an
immunocompetent CRISPR-based glioblastoma model. J Clin Invest.
130:2286–2300. 2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Hu S, Kao HY, Yang T and Wang Y: Early and
Bi-hemispheric seizure onset in a rat glioblastoma Multiforme
model. Neurosci Lett. 766(136351)2022.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Endersby R and Baker SJ: PTEN signaling in
brain: Neuropathology and tumorigenesis. Oncogene. 27:5416–5430.
2008.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Venkatesan S, Lamfers ML, Dirven CM and
Leenstra S: Genetic biomarkers of drug response for small-molecule
therapeutics targeting the RTK/Ras/PI3K, p53 or Rb pathway in
glioblastoma. CNS Oncol. 5:77–90. 2016.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Nishio S, Morioka T, Hisada K and Fukui M:
Temporal lobe epilepsy: A clinicopathological study with special
reference to temporal neocortical changes. Neurosurg Rev. 23:84–89.
2000.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Backman SA, Stambolic V, Suzuki A, Haight
J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO and Mak
TW: Deletion of Pten in mouse brain causes seizures, ataxia and
defects in soma size resembling Lhermitte-Duclos disease. Nat
Genet. 29:396–403. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
124
|
Luikart BW, Schnell E, Washburn EK, Bensen
AL, Tovar KR and Westbrook GL: Pten knockdown in vivo increases
excitatory drive onto dentate granule cells. J Neurosci.
31:4345–4354. 2011.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Williams MR, DeSpenza T Jr, Li M, Gulledge
AT and Luikart BW: Hyperactivity of newborn Pten knock-out neurons
results from increased excitatory synaptic drive. J Neurosci.
35:943–959. 2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Pun RY, Rolle IJ, Lasarge CL, Hosford BE,
Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL,
et al: Excessive activation of mTOR in postnatally generated
granule cells is sufficient to cause epilepsy. Neuron.
75:1022–1034. 2012.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Sabetghadam A, Wu C, Liu J, Zhang L and
Reid AY: Increased epileptogenicity in a mouse model of
neurofibromatosis type 1. Exp Neurol. 331(113373)2020.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Sorrentino U, Bellonzi S, Mozzato C,
Brasson V, Toldo I, Parrozzani R, Clementi M, Cassina M and
Trevisson E: Epilepsy in NF1: Epidemiologic, genetic, and clinical
features. A monocentric retrospective study in a cohort of 784
patients. Cancers (Basel). 13(6336)2021.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Kastenhuber ER and Lowe SW: Putting p53 in
Context. Cell. 170:1062–1078. 2017.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Choi BD, Lee DK, Yang JC, Ayinon CM, Lee
CK, Maus D, Carter BS, Barker FG, Jones PS, Nahed BV, et al:
Receptor tyrosine kinase gene amplification is predictive of
intraoperative seizures during glioma resection with functional
mapping. J Neurosurg. 132:1017–1023. 2020.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Fredriksson L, Stevenson TK, Su EJ,
Ragsdale M, Moore S, Craciun S, Schielke GP, Murphy GG and Lawrence
DA: Identification of a neurovascular signaling pathway regulating
seizures in mice. Ann Clin Transl Neurol. 2:722–738.
2015.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Yang P, You G, Zhang W, Wang Y, Wang Y,
Yao K and Jiang T: Correlation of preoperative seizures with
clinicopathological factors and prognosis in anaplastic gliomas: A
report of 198 patients from China. Seizure. 23:844–851.
2014.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Szelényi A, Joksimovic B and Seifert V:
Intraoperative risk of seizures associated with transient direct
cortical stimulation in patients with symptomatic epilepsy. J Clin
Neurophysiol. 24:39–43. 2007.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Tsuchihashi K, Okazaki S, Ohmura M,
Ishikawa M, Sampetrean O, Onishi N, Wakimoto H, Yoshikawa M,
Seishima R, Iwasaki Y, et al: The EGF receptor promotes the
malignant potential of glioma by regulating amino acid transport
system xc(-). Cancer Res. 76:2954–2963. 2016.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Morrison RS, Wenzel HJ, Kinoshita Y,
Robbins CA, Donehower LA and Schwartzkroin PA: Loss of the p53
tumor suppressor gene protects neurons from kainate-induced cell
death. J Neurosci. 16:1337–1345. 1996.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Engel T, Murphy BM, Schindler CK and
Henshall DC: Elevated p53 and lower MDM2 expression in hippocampus
from patients with intractable temporal lobe epilepsy. Epilepsy
Res. 77:151–156. 2007.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Toledo M, Sarria-Estrada S, Quintana M,
Maldonado X, Martinez-Ricarte F, Rodon J, Auger C, Aizpurua M,
Salas-Puig J, Santamarina E and Martinez-Saez E: Epileptic features
and survival in glioblastomas presenting with seizures. Epilepsy
Res. 130:1–6. 2017.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Suzuki H, Mikuni N, Sugita S, Aoyama T,
Yokoyama R, Suzuki Y, Enatsu R, Akiyama Y, Mikami T, Wanibuchi M
and Hasegawa T: Molecular aberrations associated with seizure
control in diffuse astrocytic and oligodendroglial tumors. Neurol
Med Chir (Tokyo). 60:147–155. 2020.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Zheng H, Ying H, Yan H, Kimmelman AC,
Hiller DJ, Chen AJ, Perry SR, Tonon G, Chu GC, Ding Z, et al: p53
and Pten control neural and glioma stem/progenitor cell renewal and
differentiation. Nature. 455:1129–1133. 2008.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Fontana L, Tabano S, Bonaparte E, Marfia
G, Pesenti C, Falcone R, Augello C, Carlessi N, Silipigni R,
Guerneri S, et al: MGMT-methylated alleles are distributed
heterogeneously within glioma samples irrespective of IDH status
and chromosome 10q deletion. J Neuropathol Exp Neurol. 75:791–800.
2016.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Stichel D, Ebrahimi A, Reuss D, Schrimpf
D, Ono T, Shirahata M, Reifenberger G, Weller M, Hänggi D, Wick W,
et al: Distribution of EGFR amplification, combined chromosome 7
gain and chromosome 10 loss, and TERT promoter mutation in brain
tumors and their potential for the reclassification of IDHwt
astrocytoma to glioblastoma. Acta Neuropathol. 136:793–803.
2018.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Saviuk M, Sleptsova E, Redkin T and
Turubanova V: Unexplained causes of glioma-associated epilepsies: A
review of theories and an area for research. Cancers (Basel).
15(5539)2023.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Mortazavi A, Khan AU, Nieblas-Bedolla E,
Boddeti U, Bachani M, Ksendzovsky A, Johnson K and Zaghloul KA:
Differential gene expression underlying epileptogenicity in
patients with gliomas. Neurooncol Adv. 6(vdae103)2024.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Phabphal K, Kaewborisutsakul A,
Leetanaporn K, Choochuen P, Tunthanathip T, Navakanitworakul R and
Sangkhathat S: Gene mutations linked to drug-resistant epilepsy in
astrocytoma. Front Neurol. 16(1523468)2025.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Hu F, Ku MC, Markovic D, Dzaye O, Lehnardt
S, Synowitz M, Wolf SA and Kettenmann H: Glioma-associated
microglial MMP9 expression is upregulated by TLR2 signaling and
sensitive to minocycline. Int J Cancer. 135:2569–2578.
2014.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Koyama R, Yamada MK, Fujisawa S,
Katoh-Semba R, Matsuki N and Ikegaya Y: Brain-derived neurotrophic
factor induces hyperexcitable reentrant circuits in the dentate
gyrus. J Neurosci. 24:7215–7224. 2004.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Young SZ and Bordey A: GABA's control of
stem and cancer cell proliferation in adult neural and peripheral
niches. Physiology (Bethesda). 24:171–185. 2009.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Rubinov M and Sporns O: Complex network
measures of brain connectivity: Uses and interpretations.
Neuroimage. 52:1059–1069. 2010.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Bullmore E and Sporns O: Complex brain
networks: Graph theoretical analysis of structural and functional
systems. Nat Rev Neurosci. 10:186–198. 2009.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Franaszczuk PJ and Bergey GK: Application
of the directed transfer function method to mesial and lateral
onset temporal lobe seizures. Brain Topogr. 11:13–21.
1998.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Kramer MA, Kolaczyk ED and Kirsch HE:
Emergent network topology at seizure onset in humans. Epilepsy Res.
79:173–186. 2008.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Percha B, Dzakpasu R, Zochowski M and
Parent J: Transition from local to global phase synchrony in small
world neural network and its possible implications for epilepsy.
Phys Rev E Stat Nonlin Soft Matter Phys. 72 (3 Pt
1)(031909)2005.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Morgan RJ and Soltesz I: Nonrandom
connectivity of the epileptic dentate gyrus predicts a major role
for neuronal hubs in seizures. Proc Natl Acad Sci USA.
105:6179–6184. 2008.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Bartolomei F, Bosma I, Klein M, Baayen JC,
Reijneveld JC, Postma TJ, Heimans JJ, van Dijk BW, de Munck JC, de
Jongh A, et al: Disturbed functional connectivity in brain tumour
patients: Evaluation by graph analysis of synchronization matrices.
Clin Neurophysiol. 117:2039–2049. 2006.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Bartolomei F, Bosma I, Klein M, Baayen JC,
Reijneveld JC, Postma TJ, Heimans JJ, van Dijk BW, de Munck JC, de
Jongh A, et al: How do brain tumors alter functional connectivity?
A magnetoencephalography study. Ann Neurol. 59:128–138.
2006.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Douw L, de Groot M, van Dellen E, Aronica
E, Heimans JJ, Klein M, Stam CJ, Reijneveld JC and Hillebrand A:
Local MEG networks: the missing link between protein expression and
epilepsy in glioma patients? Neuroimage. 75:195–203.
2013.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Douw L, van Dellen E, de Groot M, Heimans
JJ, Klein M, Stam CJ and Reijneveld JC: Epilepsy is related to
theta band brain connectivity and network topology in brain tumor
patients. BMC Neurosci. 11(103)2010.PubMed/NCBI View Article : Google Scholar
|
|
158
|
You G, Sha Z and Jiang T: Clinical
diagnosis and perioperative management of glioma-related epilepsy.
Front Oncol. 10(550353)2020.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Liang S, Fan X, Chen F, Liu Y, Qiu B,
Zhang K, Qi S, Zhang G, Liu J, Zhang J, et al: Chinese guideline on
the application of anti-seizure medications in the perioperative
period of supratentorial craniocerebral surgery. Ther Adv Neurol
Disord. 15(17562864221114357)2022.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Ollila L and Roivainen R: Glioma features
and seizure control during long-term follow-up. Epilepsy Behav Rep.
21(100586)2023.PubMed/NCBI View Article : Google Scholar
|
|
161
|
de Bruin ME, van der Meer PB, Dirven L,
Taphoorn MJB and Koekkoek JAF: Efficacy of antiepileptic drugs in
glioma patients with epilepsy: A systematic review. Neurooncol
Pract. 8:501–517. 2021.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Bobustuc GC, Baker CH, Limaye A, Jenkins
WD, Pearl G, Avgeropoulos NG and Konduri SD: Levetiracetam enhances
p53-mediated MGMT inhibition and sensitizes glioblastoma cells to
temozolomide. Neuro Oncol. 12:917–927. 2010.PubMed/NCBI View Article : Google Scholar
|
|
163
|
Tabaee Damavandi P, Pasini F, Fanella G,
Cereda GS, Mainini G, DiFrancesco JC, Trinka E and Lattanzi S:
Perampanel in brain tumor-related epilepsy: A systematic review.
Brain Sci. 13(326)2023.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Rossi J, Cavallieri F, Bassi MC, Biagini
G, Rizzi R, Russo M, Bondavalli M, Iaccarino C, Pavesi G, Cozzi S,
et al: Efficacy and tolerability of perampanel in brain
tumor-related epilepsy: A systematic review. Biomedicines.
11(651)2023.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Correia CE, Umemura Y, Flynn JR, Reiner AS
and Avila EK: Pharmacoresistant seizures and IDH mutation in
low-grade gliomas. Neurooncol Adv. 3(vdab146)2021.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Lim DA, Tarapore P, Chang E, Burt M,
Chakalian L, Barbaro N, Chang S, Lamborn KR and McDermott MW:
Safety and feasibility of switching from phenytoin to levetiracetam
monotherapy for glioma-related seizure control following
craniotomy: A randomized phase II pilot study. J Neurooncol.
93:349–354. 2009.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Iuchi T, Kuwabara K, Matsumoto M, Kawasaki
K, Hasegawa Y and Sakaida T: Levetiracetam versus phenytoin for
seizure prophylaxis during and early after craniotomy for brain
tumours: A phase II prospective, randomised study. J Neurol
Neurosurg Psychiatry. 86:1158–1162. 2015.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Kerkhof M, Dielemans JC, van Breemen MS,
Zwinkels H, Walchenbach R, Taphoorn MJ and Vecht CJ: Effect of
valproic acid on seizure control and on survival in patients with
glioblastoma multiforme. Neuro Oncol. 15:961–967. 2013.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Weller M, Gorlia T, Cairncross JG, van den
Bent MJ, Mason W, Belanger K, Brandes AA, Bogdahn U, Macdonald DR,
Forsyth P, et al: Prolonged survival with valproic acid use in the
EORTC/NCIC temozolomide trial for glioblastoma. Neurology.
77:1156–1164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Pak O, Kosianova A, Zaitsev S, Sharma A,
Sharma H and Bryukhovetskiy I: Valproic acid and celecoxib enhance
the effect of temozolomide on glioblastoma cells. CNS Neurol Disord
Drug Targets. 24:375–381. 2025.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Salmaggi A, Corno C, Maschio M, Donzelli
S, D'Urso A, Perego P and Ciusani E: Synergistic effect of
perampanel and temozolomide in human glioma cell lines. J Pers Med.
11(390)2021.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Nishide T, Yamamuro S, Sano E, Aida Y,
Nara K, Yazama G, Ozawa Y and Yoshino A: Synergistic effect of
perampanel with temozolomide on glioblastoma cells in vivo.
Heliyon. 11(e43167)2025.
|
|
173
|
Vecht C, Royer-Perron L, Houillier C and
Huberfeld G: Seizures and anticonvulsants in brain tumours:
Frequency, mechanisms and anti-epileptic management. Curr Pharm
Des. 23:6464–6487. 2017.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Simó M, Velasco R, Graus F, Verger E, Gil
M, Pineda E, Blasco J and Bruna J: Impact of antiepileptic drugs on
thrombocytopenia in glioblastoma patients treated with standard
chemoradiotherapy. J Neurooncol. 108:451–458. 2012.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Li C, Chen H, Tan Q, Xie C, Zhan W, Sharma
A, Sharma HS and Zhang Z: The therapeutic and neuroprotective
effects of an antiepileptic drug valproic acid in glioma patients.
Prog Brain Res. 258:369–379. 2020.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Thotala D, Karvas RM, Engelbach JA, Garbow
JR, Hallahan AN, DeWees TA, Laszlo A and Hallahan DE: Valproic acid
enhances the efficacy of radiation therapy by protecting normal
hippocampal neurons and sensitizing malignant glioblastoma cells.
Oncotarget. 6:35004–35022. 2015.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Lee PY, Wei YT, Chao KC, Chu CN, Chung WH
and Wang TH: Anti-epileptic drug use during adjuvant
chemo-radiotherapy is associated with poorer survival in patients
with glioblastoma: A nationwide population-based cohort study. J
Cancer Res Ther. 20:555–562. 2024.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Walbert T, Harrison RA, Schiff D, Avila
EK, Chen M, Kandula P, Lee JW, Le Rhun E, Stevens GHJ, Vogelbaum
MA, et al: SNO and EANO practice guideline update: Anticonvulsant
prophylaxis in patients with newly diagnosed brain tumors. Neuro
Oncol. 23:1835–1844. 2021.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Wang X, Zheng X, Hu S, Xing A, Wang Z,
Song Y, Chen J, Tian S, Mao Y and Chi X: Efficacy of perioperative
anticonvulsant prophylaxis in seizure-naïve glioma patients: A
meta-analysis. Clin Neurol Neurosurg. 186(105529)2019.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Youshani AS, Heal C, Lee JX, Younis M,
Mohanraj R, Maye H, Bailey M, Coope D, D'Urso PI and Karabatsou K:
Glioma-related epilepsy following low-grade glioma surgery.
Neurooncol Adv. 6(vdae127)2024.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Stocksdale B, Nagpal S, Hixson JD, Johnson
DR, Rai P, Shivaprasad A and Tremont-Lukats IW: Neuro-oncology
practice clinical debate: Long-term antiepileptic drug prophylaxis
in patients with glioma. Neurooncol Pract. 7:583–588.
2020.PubMed/NCBI View Article : Google Scholar
|
|
182
|
Avila EK, Chamberlain M, Schiff D,
Reijneveld JC, Armstrong TS, Ruda R, Wen PY, Weller M, Koekkoek JA,
Mittal S, et al: Seizure control as a new metric in assessing
efficacy of tumor treatment in low-grade glioma trials. Neuro
Oncol. 19:12–21. 2017.PubMed/NCBI View Article : Google Scholar
|
|
183
|
Koekkoek JA, Kerkhof M, Dirven L, Heimans
JJ, Reijneveld JC and Taphoorn MJ: Seizure outcome after
radiotherapy and chemotherapy in low-grade glioma patients: A
systematic review. Neuro Oncol. 17:924–934. 2015.PubMed/NCBI View Article : Google Scholar
|
|
184
|
Takada S, Iwasaki M, Suzuki H, Nakasato N,
Kumabe T and Tominaga T: Angiocentric glioma and surrounding
cortical dysplasia manifesting as intractable frontal lobe
epilepsy-case report. Neurol Med Chir (Tokyo). 51:522–526.
2011.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Mikuni N, Ikeda A, Takahashi JA, Nozaki K,
Miyamoto S, Taki W and Hashimoto N: A step-by-step resection guided
by electrocorticography for nonmalignant brain tumors associated
with long-term intractable epilepsy. Epilepsy Behav. 8:560–564.
2006.PubMed/NCBI View Article : Google Scholar
|
|
186
|
Dobran M, Nasi D, Chiriatti S, Gladi M,
Somma LD, Iacoangeli M and Scerrati M: Prognostic factors in
glioblastoma: Is there a role for epilepsy? Neurol Med Chir
(Tokyo). 58:110–115. 2018.PubMed/NCBI View Article : Google Scholar
|
|
187
|
Chang EF, Potts MB, Keles GE, Lamborn KR,
Chang SM, Barbaro NM and Berger MS: Seizure characteristics and
control following resection in 332 patients with low-grade gliomas.
J Neurosurg. 108:227–235. 2008.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Englot DJ, Berger MS, Barbaro NM and Chang
EF: Predictors of seizure freedom after resection of supratentorial
low-grade gliomas. A review. J Neurosurg. 115:240–244.
2011.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Hervey-Jumper SL and Berger MS: Insular
glioma surgery: An evolution of thought and practice. J Neurosurg.
130:9–16. 2019.PubMed/NCBI View Article : Google Scholar
|
|
190
|
Zhang JJY, Lee KS, Wang DD, Hervey-Jumper
SL and Berger MS: Seizure outcome after resection of insular
glioma: A systematic review, meta-analysis, and institutional
experience. J Neurosurg. 138:1242–1253. 2022.PubMed/NCBI View Article : Google Scholar
|
|
191
|
Köhling R, Senner V, Paulus W and
Speckmann EJ: Epileptiform activity preferentially arises outside
tumor invasion zone in glioma xenotransplants. Neurobiol Dis.
22:64–75. 2006.PubMed/NCBI View Article : Google Scholar
|
|
192
|
You H and Qiao H: Intraoperative
neuromonitoring during resection of gliomas involving eloquent
areas. Front Neurol. 12(658680)2021.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Saghebdoust S, Dayyani M, Rouhbakhsh
Zahmatkesh MR, Abbasi B, Soltani G and Zare R: Launching awake
craniotomy technique in a resource-limited center: New insights
into the patient experience, costs, and long-term outcomes and a
narrative review of the literature. World Neurosurg.
168:246–257.e4. 2022.PubMed/NCBI View Article : Google Scholar
|
|
194
|
Battista F, Muscas G, Parenti A, Bonaudo
C, Gadda D, Martinelli C, Carrai R, Amadori A, Grippo A and Della
Puppa A: Electrocorticography and navigated transcranial magnetic
stimulation-tailored supratotal resection for epileptogenic
low-grade gliomas. J Neurosurg. 142:918–926. 2024.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Juhász C and Mittal S: Molecular imaging
of brain tumor-associated epilepsy. Diagnostics (Basel).
10(1049)2020.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Ricci R, Bacci A, Tugnoli V, Battaglia S,
Maffei M, Agati R and Leonardi M: Metabolic findings on 3T 1H-MR
spectroscopy in peritumoral brain edema. AJNR Am J Neuroradiol.
28:1287–1291. 2007.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Liubinas SV, Drummond KJ, Desmond PM,
Bjorksten A, Morokoff AP, Kaye AH, O'Brien TJ and Moffat BA:
Glutamate quantification in patients with supratentorial gliomas
using chemical shift imaging. NMR Biomed. 27:570–577.
2014.PubMed/NCBI View Article : Google Scholar
|
|
198
|
Goldenberg JM and Pagel MD: Assessments of
tumor metabolism with CEST MRI. NMR Biomed.
32(e3943)2019.PubMed/NCBI View Article : Google Scholar
|
|
199
|
Simister RJ, McLean MA, Barker GJ and
Duncan JS: Proton MR spectroscopy of metabolite concentrations in
temporal lobe epilepsy and effect of temporal lobe resection.
Epilepsy Res. 83:168–176. 2009.PubMed/NCBI View Article : Google Scholar
|
|
200
|
Rudà R, Magliola U, Bertero L, Trevisan E,
Bosa C, Mantovani C, Ricardi U, Castiglione A, Monagheddu C and
Soffietti R: Seizure control following radiotherapy in patients
with diffuse gliomas: A retrospective study. Neuro Oncol.
15:1739–1749. 2013.PubMed/NCBI View Article : Google Scholar
|
|
201
|
Koekkoek JA, Dirven L, Heimans JJ, Postma
TJ, Vos MJ, Reijneveld JC and Taphoorn MJ: Seizure reduction in a
low-grade glioma: more than a beneficial side effect of
temozolomide. J Neurol Neurosurg Psychiatry. 86:366–373.
2015.PubMed/NCBI View Article : Google Scholar
|
|
202
|
Kahlenberg CA, Fadul CE, Roberts DW,
Thadani VM, Bujarski KA, Scott RC and Jobst BC: Seizure prognosis
of patients with low-grade tumors. Seizure. 21:540–545.
2012.PubMed/NCBI View Article : Google Scholar
|
|
203
|
Pace A, Vidiri A, Galiè E, Carosi M,
Telera S, Cianciulli AM, Canalini P, Giannarelli D, Jandolo B and
Carapella CM: Temozolomide chemotherapy for progressive low-grade
glioma: Clinical benefits and radiological response. Ann Oncol.
14:1722–1726. 2003.PubMed/NCBI View Article : Google Scholar
|
|
204
|
Sherman JH, Moldovan K, Yeoh HK, Starke
RM, Pouratian N, Shaffrey ME and Schiff D: Impact of temozolomide
chemotherapy on seizure frequency in patients with low-grade
gliomas. J Neurosurg. 114:1617–1621. 2011.PubMed/NCBI View Article : Google Scholar
|
|
205
|
Yue J, Yin C, Chen L, Xu R and Zhao D: Is
there a role for temozolomide in glioma related seizures? A
systematic review. Neurol India. 70:864–871. 2022.PubMed/NCBI View Article : Google Scholar
|
|
206
|
Mellinghoff IK, van den Bent MJ,
Blumenthal DT, Touat M, Peters KB, Clarke J, Mendez J, Yust-Katz S,
Welsh L, Mason WP, et al: Vorasidenib in IDH1- or IDH2-Mutant
Low-Grade Glioma. N Engl J Med. 389:589–601. 2023.PubMed/NCBI View Article : Google Scholar
|
|
207
|
Mellinghoff IK, Penas-Prado M, Peters KB,
Burris HA III, Maher EA, Janku F, Cote GM, de la Fuente MI, Clarke
JL, Ellingson BM, et al: Vorasidenib, a dual inhibitor of mutant
IDH1/2, in recurrent or progressive glioma; Results of a
first-in-human phase I trial. Clin Cancer Res. 27:4491–4499.
2021.PubMed/NCBI View Article : Google Scholar
|
|
208
|
Mellinghoff IK, Lu M, Wen PY, Taylor JW,
Maher EA, Arrillaga-Romany I, Peters KB, Ellingson BM, Rosenblum
MK, Chun S, et al: Vorasidenib and ivosidenib in IDH1-mutant
low-grade glioma: A randomized, perioperative phase 1 trial. Nat
Med. 29:615–622. 2023.PubMed/NCBI View Article : Google Scholar
|
|
209
|
Vo AH, Ambady P and Spencer D: The IDH1
inhibitor ivosidenib improved seizures in a patient with
drug-resistant epilepsy from IDH1 mutant oligodendroglioma.
Epilepsy Behav Rep. 18(100526)2022.PubMed/NCBI View Article : Google Scholar
|
|
210
|
Librizzi L, Vila Verde D, Colciaghi F,
Deleo F, Regondi MC, Costanza M, Cipelletti B and de Curtis M:
Peripheral blood mononuclear cell activation sustains seizure
activity. Epilepsia. 62:1715–1728. 2021.PubMed/NCBI View Article : Google Scholar
|
|
211
|
Seiffert E, Dreier JP, Ivens S, Bechmann
I, Tomkins O, Heinemann U and Friedman A: Lasting blood-brain
barrier disruption induces epileptic focus in the rat somatosensory
cortex. J Neurosci. 24:7829–7836. 2004.PubMed/NCBI View Article : Google Scholar
|
|
212
|
Marchi N, Angelov L, Masaryk T, Fazio V,
Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I and
Janigro D: Seizure-promoting effect of blood-brain barrier
disruption. Epilepsia. 48:732–742. 2007.PubMed/NCBI View Article : Google Scholar
|
|
213
|
Xu D, Miller SD and Koh S: Immune
mechanisms in epileptogenesis. Front Cell Neurosci.
7(195)2013.PubMed/NCBI View Article : Google Scholar
|
|
214
|
van Vliet EA, da Costa Araújo S, Redeker
S, van Schaik R, Aronica E and Gorter JA: Blood-brain barrier
leakage may lead to progression of temporal lobe epilepsy. Brain.
130 (Pt 2):521–534. 2007.PubMed/NCBI View Article : Google Scholar
|
|
215
|
Xu D, Robinson AP, Ishii T, Duncan DS,
Alden TD, Goings GE, Ifergan I, Podojil JR, Penaloza-MacMaster P,
Kearney JA, et al: Peripherally derived T regulatory and γδ T cells
have opposing roles in the pathogenesis of intractable pediatric
epilepsy. J Exp Med. 215:1169–1186. 2018.PubMed/NCBI View Article : Google Scholar
|
|
216
|
Broekaart DWM, Anink JJ, Baayen JC, Idema
S, de Vries HE, Aronica E, Gorter JA and van Vliet EA: Activation
of the innate immune system is evident throughout epileptogenesis
and is associated with blood-brain barrier dysfunction and seizure
progression. Epilepsia. 59:1931–1944. 2018.PubMed/NCBI View Article : Google Scholar
|
|
217
|
Zhao K, Bai X, Wang X, Cao Y, Zhang L, Li
W and Wang S: Insight on the hub gene associated signatures and
potential therapeutic agents in epilepsy and glioma. Brain Res
Bull. 199(110666)2023.PubMed/NCBI View Article : Google Scholar
|
|
218
|
McDonough SI, Boland LM, Mintz IM and Bean
BP: Interactions among toxins that inhibit N-type and P-type
calcium channels. J Gen Physiol. 119:313–328. 2002.PubMed/NCBI View Article : Google Scholar
|
|
219
|
Lewis RJ, Dutertre S, Vetter I and
Christie MJ: Conus venom peptide pharmacology. Pharmacol Rev.
64:259–298. 2012.PubMed/NCBI View Article : Google Scholar
|
|
220
|
Li L, Zhang C, Wang Z, Guo Y, Wang Y, Fan
X and Jiang T: Expression changes in ion channel and immunity genes
are associated with glioma-related epilepsy in patients with
diffuse gliomas. J Cancer Res Clin Oncol. 148:2793–2802.
2022.PubMed/NCBI View Article : Google Scholar
|
|
221
|
Jin Y, Zhao C, Chen L, Liu X, Pan S, Ju D,
Ma J, Li J and Wei B: Identification of novel gene and pathway
targets for human epilepsy treatment. Biol Res.
49(3)2016.PubMed/NCBI View Article : Google Scholar
|
|
222
|
Li S, Han J, Guo G, Sun Y, Zhang T, Zhao
M, Xu Y, Cui Y, Liu Y and Zhang J: Voltage-gated sodium channels β3
subunit promotes tumorigenesis in hepatocellular carcinoma by
facilitating p53 degradation. FEBS Lett. 594:497–508.
2020.PubMed/NCBI View Article : Google Scholar
|
|
223
|
Li S, Shao H and Chang L: The important
role of perituberal tissue in epileptic patients with tuberous
sclerosis complex by the transcriptome analysis. Biomed Res Int.
2020(4980609)2020.PubMed/NCBI View Article : Google Scholar
|
|
224
|
Vecht CJ, Kerkhof M and Duran-Pena A:
Seizure prognosis in brain tumors: New insights and evidence-based
management. Oncologist. 19:751–759. 2014.PubMed/NCBI View Article : Google Scholar
|
|
225
|
Wang R, Yang S, Wang M, Zhou Y, Li X, Chen
W, Liu W, Huang Y, Wu J, Cao J, et al: A sustainable approach to
universal metabolic cancer diagnosis. Nature Sustainability.
7:602–615. 2024.
|
|
226
|
Yuan Y, Zhang X, Wang Y, Li H, Qi Z, Du Z,
Chu Y-H, Feng D, Xie Q, Song J, et al: Multimodal data integration
using deep learning predicts overall survival of patients with
glioma. View. 5(20240001)2024.
|















