Introduction
Approximately one-third of patients with
drug-resistant epilepsy (DRE) suffer from a structural lesion that
can be treated surgically (1,2).
Identifying these lesions is important, as they often represent the
ictal onset zone. Epilepsy surgery targeting these lesions can lead
to a significant reduction in seizure frequency and improve quality
of life. Patients with lesions identified through magnetic
resonance imaging (MRI) were more likely to experience seizure
freedom following resection, highlighting the importance of imaging
in treatment planning (3). Even
when phase I presurgical evaluations yield concordant data, 30-50%
of surgical candidates still experience seizure recurrence
(4,5). Failure of epilepsy surgery for focal
epilepsy may be caused by insufficient excision of the
epileptogenic zone (EZ) or by inadequate disruption of pathogenic
hubs within the epileptogenic network (6).
An intraoperative electrocorticography (ECoG) method
was developed by Penfield and Jasper in the 1930s. This method
involved placing electrodes directly on surgically exposed cortical
surfaces and recording brain activity before and after resection.
This process allowed the identification of the irritative zone as
well as the EZ intraoperatively (7). ECoG is particularly advantageous for
patients with neocortical lesions that cause temporal or
extratemporal epilepsy. This approach has been utilized in patients
with DRE stemming from either temporal or extratemporal tumors,
mesial temporal sclerosis (MTS) and focal cortical dysplasia (FCD).
It can be utilized to prevent the need for long-term extraoperative
invasive electroencephalography (EEG) in carefully selected
individuals (8,9). However, it cannot replace the
presurgical phase I evaluation, as precise lateralization and
localization of the ictal onset zone or a strong hypothesis of the
EZ is necessary before performing ECoG.
ECoG effectiveness has been well documented in
high-resource settings, particularly across academic centers in
North America and Europe (9).
However, evidence from the Eastern Mediterranean Region (EMR),
including countries such as Saudi Arabia, Egypt, Jordan, and
Lebanon remains limited. This lack of data is concerning, as
healthcare systems in the EMR often face distinct challenges such
as restricted access to specialized epilepsy surgery programs,
disparities in diagnostic resources, and delayed referral (10). Moreover, existing studies from the
region are typically limited to small case series and rarely report
long-term seizure outcomes, making it difficult to assess the
broader utility and effectiveness of ECoG-guided surgical
interventions.
To address this gap, a retrospective analysis of
ECoG-guided epilepsy surgeries performed at a tertiary care center
in Saudi Arabia was conducted. The objective of the present study
was to fill this gap by assessing whether postresection ECoG
silence is predictive of Engel Class I outcomes.
Patients and methods
The present retrospective observational study
involved 30 patients with DRE who underwent ECoG-guided epilepsy
surgery as part of the comprehensive epilepsy program at King Fahad
Specialist Hospital in Dammam, Saudi Arabia, between January 2013
and December 2024. Patients with a minimum follow-up duration of 6
months were recruited. Clinical and demographic information, such
as sex, handedness, central nervous system examination, seizure
type, epilepsy duration, MRI findings, age at surgery, and
follow-up duration, were obtained. Participants with incomplete
data were excluded. The present study received ethics approval
(approval no. NEU0402) from the Ethics Committee of King Fahad
Specialist Hospital (Dammam, Saudi Arabia). Given the retrospective
nature of the study and the use of de-identified clinical data, the
requirement for patient consent for participation in the present
study was waived by the Ethics Committee of King Fahad Specialist
Hospital.
All patients underwent phase I presurgical
evaluation in the ABRET-accredited Epilepsy Monitoring Unit. The
patients were monitored with continuous noninvasive video EEG using
a 10-20 electrode placement system with additional left and right
anterior temporal T1 and T2 electrodes. On average, at least two
seizures were recorded. Preoperative imaging studies included 1.5
Tesla or 3 Tesla MRI scans. Lesions were categorized as temporal,
extratemporal, or both temporal and extratemporal. Patients with
normal MRI scans underwent advanced modalities, such as positron
emission tomography (PET) or single photon emission computed
tomography (SPECT) scans to localize or lateralize the EZ. All
patients were reviewed during the weekly multidisciplinary epilepsy
surgery care conference. Both the patients and their relatives were
informed about the need for EcoG, and written informed consent was
obtained.
All patients underwent the procedure under general
anesthesia. Anesthesia was induced with propofol at a dose of 2-3
mg/kg, fentanyl at 2 mcg/kg, and rocuronium at 0.5 mg/kg. After
intubation, arterial and peripheral lines were secured. An infusion
of remifentanil at a rate of 0.1-0.3 mcg/kg/min, in combination
with inhaled sevoflurane at 1 MAC, was used for anesthetic
maintenance. After 2021, due to a nationwide shortage, remifentanil
was replaced by dexmedetomidine infusion at a rate of 0.2-0.5
mcg/kg/h. Rocuronium at a dose of 0.2 mg/kg was given as needed,
especially before ECoG. During ECoG recordings, the sevoflurane
concentration was reduced to 0.2 MAC to decrease anesthesia levels.
The dexmedetomidine infusion at 0.2-0.5 mcg/kg/h and rocuronium at
0.2 mg/kg/dose were continued throughout the ECoG recording.
Sevoflurane was increased back to 1 MAC at the end of each ECoG
recording session. ECoG was performed during surgery to detect the
presence of interictal discharges (IEDs) and to assist in making
surgical decisions regarding the extent of resection of the EZ.
ECoG was conducted both before and after resection to monitor for
IEDs in the cortical areas and to help guide the surgeons in
achieving maximal or complete excision of the epileptogenic
regions. In total, four to six contact strip electrodes were
utilized for recording. Engel's classification method was used to
determine the outcomes of seizures (Class I-IV), with Class I
defined as completely seizure-free (11).
The data were summarized using descriptive
statistics, covering variables such as gender, age at surgery,
duration and type of seizures, lesion type identified on MRI,
surgical approach, pre- and post-resection ECoG findings,
histological results, as well as postoperative MRI and EEG
evaluations. A postoperative CT scan of the brain was performed on
all patients to assess complications such as hemorrhage,
infarction, and hydrocephalus. Post-operative MRI was performed
only if clinically necessary due to logistical constraints at King
Fahad Specialist Hospital (Dammam, Saudi Arabia). Due to the small
sample size, descriptive statistics were used to summarize patient
demographics and clinical outcomes, including means, medians, and
ranges for continuous variables, and frequencies and percentages
for categorical variables. All analyses were performed using IBM
SPSS Statistics for Windows, Version 26.0 (IBM Corp.). No
multivariate analysis was performed due to sample size
limitations.
Results
In the present study, a total of 30 patients were
included, consisting of 16 males and 14 females. Of these, 26
patients were right-handed and 4 patients were left-handed. A total
of 28 patients had a normal neurological examination, while two had
right hemiparesis. In terms of seizure type, 15 patients had focal
to bilateral tonic-clonic seizures, 10 patients had focal unaware
seizures, 3 patients had focal aware seizures, 1 patient had a
generalized tonic-clonic seizure, and 1 patient had epileptic
spasms. The epileptogenic lesions were lateralized to the right
hemisphere in 12 patients and to the left hemisphere in 15
patients. In addition, 1 patient had bilateral lesions, and 2
patients had a normal MRI. In terms of localization, 18 patients
had a temporal lesion, 6 patients had extratemporal lesions, 4
patients had both temporal and extratemporal lesions, and 2
patients had a normal MRI. The mean duration of epilepsy prior to
surgery was 59.5 months. At the time of surgery, the mean age was
13.7 years. Following surgery, the mean follow-up duration was 45.9
months (Table I).
 | Table IDemographic and clinical
characteristics of patients. |
Table I
Demographic and clinical
characteristics of patients.
| Categories | No. of patients
(N=30) | Percentage (%) of
patients |
|---|
| Sex | | |
|
Male | 16 | 53.3 |
|
Female | 14 | 46.6 |
| Handedness | | |
|
Right | 26 | 86.6 |
|
Left | 4 | 13.3 |
| CNS examination | | |
|
Normal | 28 | 93.3 |
|
Right
hemiparesis | 2 | 6.6 |
| Seizure type | | |
|
Focal to
bilateral tonic-clonic | 15 | 50 |
|
Focal
unaware | 10 | 33.3 |
|
Focal
aware | 3 | 10 |
|
Generalized
tonic-clonic | 1 | 3.3 |
|
Epileptic
spasms | 1 | 3.3 |
| Lesion lateralization
on MRI | | |
|
Right | 12 | 40 |
|
Left | 15 | 50 |
|
Bilateral | 1 | 3.3 |
|
Normal | 2 | 6.6 |
| Lesion localization
on MRI | | |
|
Temporal | 18 | 60 |
|
Extratemporal | 6 | 20 |
|
Both | 4 | 13.3 |
|
Normal
MRI | 2 | 6.6 |
| Mean (range) epilepsy
duration before surgery (months) | 59.5 (3-204) | |
| Mean (range) age at
surgery (years) | 13.7 (3-29) | |
| Mean (range)
follow-up duration (months) | 45.9 (7-120) | |
The most commonly observed pathologies were tumors,
MTS, and FCD. Detailed MRI characteristics are presented in
Table II. Of the 30 patients, 9
underwent lesionectomy alone: 6 had extratemporal lesions, 2 had
temporal lesions, and 1 patient had both temporal and extratemporal
lesions. A total of 19 patients underwent lesionectomy with
anterior temporal lobectomy (ATL), 1 patient underwent selective
amygdalohippocampectomy and another patient underwent a complete
right hemispherectomy. The surgical approach varied depending on
the location of the lesion, but the goal in all cases was to
achieve complete or maximal resection of the EZ (Table III). Histopathological analysis
confirmed the presence of tumors in 16 patients, accounting for
53.3% of those examined. The most common tumor types identified
were gangliogliomas (7 patients), dysembryoplastic neuroepithelial
tumors (4 patients) and astrocytomas (3 patients). In addition, 3
patients with tuberous sclerosis complex had cortical tubers. FCD
was identified in 9 patients, representing 30% of the cases, with 5
of them also exhibiting MTS. Gliosis was present in 5 cases, either
as an isolated finding or in combination with other pathologies
(Table SI).
 | Table IIMRI characteristics of patients. |
Table II
MRI characteristics of patients.
| Lesion location | Lesion type | No. of patients |
|---|
| Temporal | MTS + FCD | 4 |
| | MTS + tumor | 1 |
| | MTS + gliosis | 1 |
| | Tumor | 8 |
| | Tumor + FCD | 4 |
| | FCD | 2 |
| | Gliosis | 2 |
| Extra-temporal | FCD | 2 |
| | Tumors | 3 |
| | Gliosis +
calcification | 1 |
| Both temporal
and | Gliosis | 1 |
| extra-temporal | FCD | 1 |
 | Table IIIDetails of surgery. |
Table III
Details of surgery.
| Type of
surgery | Details | No. of
patients |
|---|
| Lesionectomy
alone | | 9 |
| | Temporal | 2 |
| | Extra-temporal | 6 |
| | Both | 1 |
| Lesionectomy
plus | | 21 |
| | Anterior temporal
lobectomy | 19 |
| | Selective
amygdalo-hippocampectomy | 1 |
| | Right
hemispherectomy | 1 |
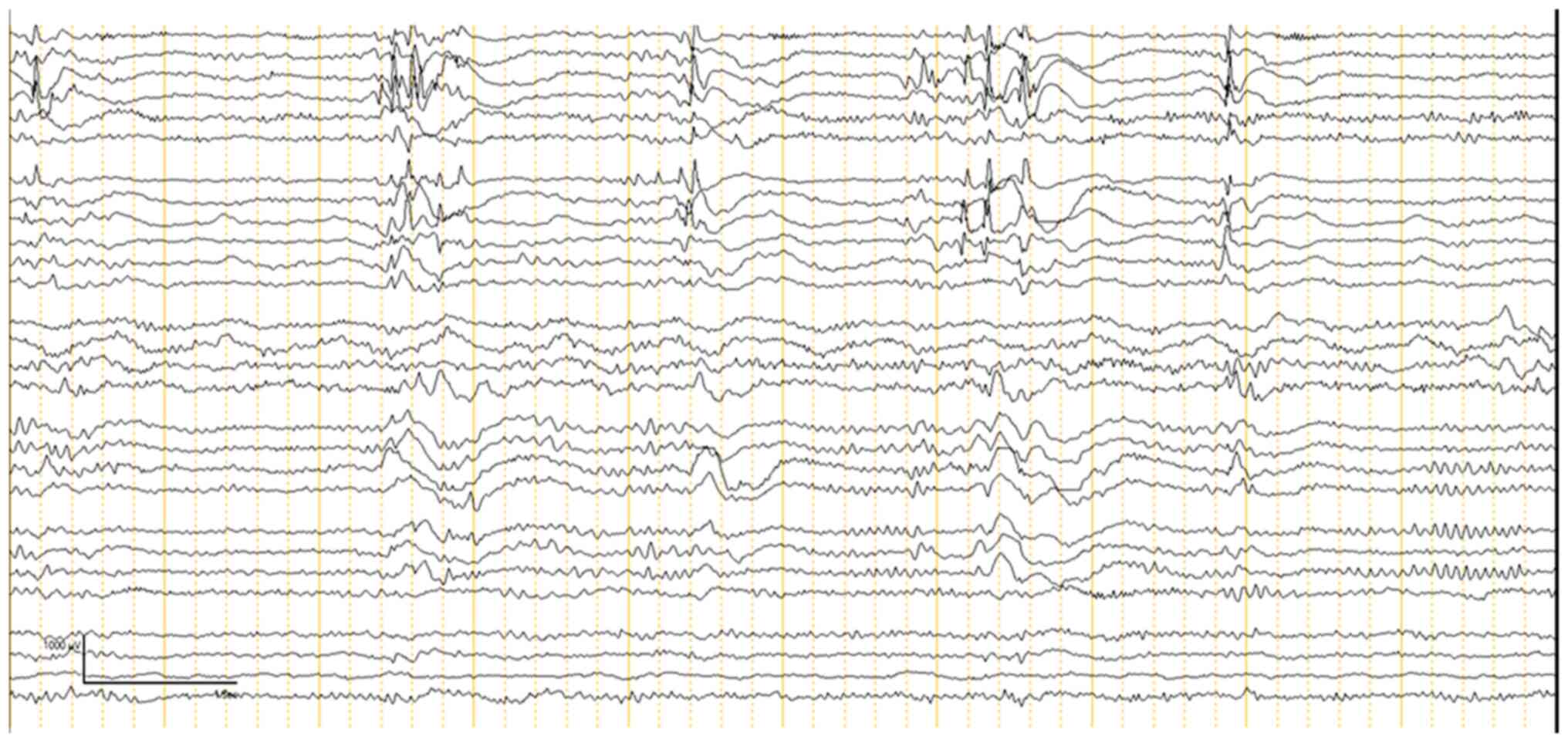
ECoG was performed on all 30 patients to guide the
extent of epileptogenic tissue resection. The ECoG findings were
categorized into two phases: Pre-resection (Fig. 1) and post-resection (Fig. 2). Pre-resection spikes were noted in
all 30 cases, indicating the presence of epileptogenic foci. The
most prominent spikes were found in areas identified by MRI as
locations of seizure focus localization. ECoG confirmed some cases
where more extensive resections were necessary due to the inability
of the previous MRI to show the full extent of the affected area.
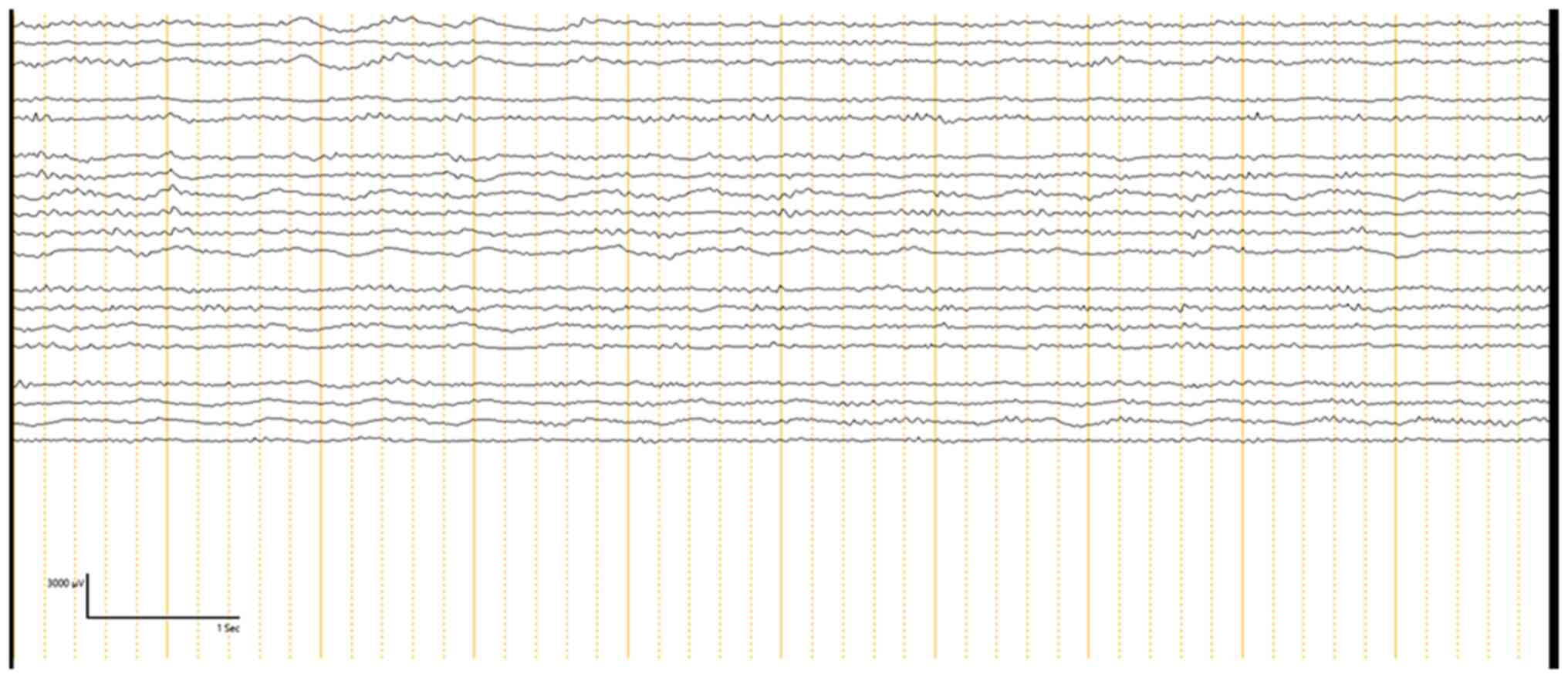
Following lesion resection, post-resection ECoG was conducted to
determine if any epileptogenic activity remained. Out of the 30
patients, 13 patients (43.3%) had no residual IEDs. These patients
were most likely to achieve seizure freedom post-operatively.
However, 17 patients (56.6%) showed attenuated spikes on
post-resection ECoG. In these cases, further resection was not
possible, either due to the proximity of the epileptogenic tissue
to eloquent brain areas or because the decision was made to
minimize the risk of neurological deficits. Among these patients, 5
achieved Engel's Class II or III seizure outcomes, while 3 patients
had Engel's Class IV outcomes, indicating persistent seizure
activity post-operatively (Tables
IV and V). Details of patients
who were seizure-free (Engel Class I) are presented in Table IV, while details of patients who
had Engel Class II-IV outcomes are presented in Table V. Of the patients that were
seizure-free, 5 patients were completely off anti-seizure
medications (ASMs), while 14 patients had their ASMs either tapered
or maintained at the same dose if 2 years of post-operative seizure
freedom had not yet passed (Table
IV). Patients who did not achieve seizure freedom continued on
ASMs with some adjustments.
 | Table IVDetails of patients who achieved
seizure freedom (Engel Class I). |
Table IV
Details of patients who achieved
seizure freedom (Engel Class I).
| Serial no. | MRI | Post-resection IEDs
on iECoG | Histopathology | Type of
surgery | Post-op EEG | Post-op MRI |
|---|
| 1 | LT
occipital-parietal old infarction | Attenuated | Gliosis | Lesionectomy
alone | Abnormal | Not performed |
| 2 | RT frontal FCD | Absent | FCD IIB | Lesionectomy
alone | Not performed | Not performed |
| 3 | LT temporal
lesion | Attenuated | Ganglioglioma | Lesionectomy +
ATL | Normal | Complete
excision |
| 4 | LT temporal lesion
+ MTS | Absent | Ganglioglioma +
MTS | Lesionectomy +
ATL | Normal | Complete
excision |
| 5 | Multiple cortical
tubers (+ RT temporal) | Absent | Tuber + FCD
IIIB | Lesionectomy +
ATL | Normal | Not performed |
| 6 | LT hippocampal
atrophy | Absent | MTS + FCD IIIa | Lesionectomy +
ATL | Abnormal | Not performed |
| 7 | LT temporal
lesion | Absent | Ganglioglioma +
FCD | Lesionectomy +
ATL | Not performed | Complete
excision |
| 8 | LT temporal
lesion | Attenuated | Astrocytoma + FCD
IIIB | Lesionectomy +
ATL | Normal | Complete
excision |
| 9 | RT temporal
lesion | Attenuated | Ganglioglioma +
FCD | Lesionectomy +
ATL | Normal | Complete
excision |
| 10 | RT temporal
lesion | Attenuated | DNET | Lesionectomy
alone | Normal | Residual
lesion |
| 11 | LT temporal lesion
(incomplete previous resection) | Absent | Ganglioglioma | Lesionectomy +
ATL | Not performed | Complete
excision |
| 12 | LT temporal
lesion | Attenuated | Ganglioglioma | Lesionectomy +
ATL | Normal | Complete
excision |
| 13 | RT temporal
lesion | Absent | Ganglioglioma + FCD
IIIB | Lesionectomy +
ATL | Normal | Not performed |
| 14 | Unremarkable | Attenuated | FCD | Lesionectomy +
ATL | Normal | N/A |
| 15 | LT parietal
lesion | Absent | DNET | Lesionectomy
alone | Normal | Complete
excision |
| 16 | RT temporal
MTS | Attenuated | FCD Ia | Lesionectomy +
ATL | Normal | Complete
excision |
| 17 | LT temporal
lesion | Absent | Astrocytoma | Lesionectomy
alone | Normal | Residual
lesion |
| 18 | RT temporal
lesion | Attenuated | Pleomorphic
xanthroastrocytoma | Lesionectomy +
ATL | Normal | Complete
excision |
| 19 | LT temporal
lesion | Absent | MTS + FCD IIIa | Lesionectomy +
ATL | Abnormal | Complete
excision |
 | Table VDetails of patients not achieving
seizure freedom. |
Table V
Details of patients not achieving
seizure freedom.
| No. | MRI | Post-resection IEDs
ECoG | Histopathology | Type of
surgery | Post-op EEG | Post-op MRI | Engel Class |
|---|
| 1 | LT temporal
FCD | Absent | MTS + FCD IIIa | Lesionectomy +
ATL | Abnormal | Complete
excision | Class II |
| 2 | RT frontal gyrus
rectus FCD | Attenuated | MTS + FCD IIIa | Lesionectomy
alone | Abnormal | Residual
lesion | Class IV |
| 3 | LT temporal
MTS | Attenuated | MTS + FCD IIIa | Lesionectomy +
ATL | Abnormal | Not performed | Class III |
| 4 | RT inferior frontal
lesion | Absent | DNET | Lesionectomy
alone | Normal | Complete
excision | Class II |
| 5 | RT TPO
post-ischemic changes | Attenuated | Gliosis | RT
hemispherotomy | Abnormal | Residual
lesion | Class II |
| 6 | RT LV SEGA +
multiple tubers | Attenuated | Tubers | Lesionectomy +
ATL | Normal | Not performed | Class II |
| 7 | Unremarkable | Absent | MTS + gliosis | Lesionectomy +
SAH | Normal | Complete
excision | Class II |
| 8 | LT temporal
atrophy | Attenuated | Gliosis | Lesionectomy +
ATL | Abnormal | Residual
lesion | Class IV |
| 9 | RT temporal
occipital lesion | Attenuated | FCD | Lesionectomy
alone | Abnormal | Residual
lesion | Class IV |
| 10 | RT temporal
lesion | Attenuated | Gliosis | Lesionectomy +
ATL | Normal | Not performed | Class III |
| 11 | LT cingulate gyrus
lesion | Attenuated | DNET | Lesionectomy
alone | Normal | Residual
lesion | Class III |
Post-operative MRIs confirmed complete excision in
14 patients, with 7 patients showing residual lesions.
Post-operative MRIs were not performed for 9 patients. Of the
patients with residual lesions, 4 were classified as Engel's Class
III or IV (Tables IV and V). Post-operative EEG was normal in 18
patients (60%), while EEG abnormalities were associated with poorer
outcomes. In addition, 4 patients experienced transient
contralateral hemiparesis affecting gross motor function,
attributed to a lesion around the motor cortex, which resolved in 3
to 4 weeks. No other neurological deficits were observed in the
remaining patients, indicating overall favorable neurological
outcomes in this cohort.
Discussion
The present study examined the role of ECoG in
guiding epilepsy surgery and predicting seizure outcomes in a
cohort of patients with DRE. The findings demonstrated the utility
of ECoG in aiding resection decisions, especially in achieving
seizure freedom for the majority of patients, with 63.3% achieving
Engel Class I outcomes, indicating complete seizure freedom.
The results are consistent with previous studies
regarding the role of ECoG in identifying EZs and guiding resection
to achieve seizure freedom. The absence of IEDs on post-resection
ECoG was associated with Engel Class I outcomes in 13 patients
(43.3%), aligning with findings from Ravat et al (12) and Greiner et al (13), which highlight the predictive value
of the absence of IEDs for positive postoperative results. ECoG
enabled adjustments to the extent of resection during surgery,
helping to reduce seizure recurrence, particularly for patients
with temporal lesions, which were the most common pathology in the
present cohort.
The high incidence of temporal lobe pathologies,
such as MTS and FCD, highlights the surgical challenge of complete
resection, particularly when lesions are near eloquent areas. In
these instances, a decrease rather than total absence of
post-resection ECoG IEDs often indicates worse outcomes (Engel
Classes II-IV). This is in line with findings from studies by
Sugano et al (14) and
Fernandez and Loddenkemper (15),
which showed that persistent spikes in ECoG were associated with
lower rates of seizure freedom. The pre-resection ECoG identified
epileptogenic activity in all patients, offering valuable insights
into delineating the boundaries of the EZ, particularly when MRI
results are inconclusive (16,17).
Among patients who did not achieve complete absence of IEDs on
ECoG, 56.6% exhibited reduced spikes after resection. This subset
had lower rates of seizure freedom, with some falling into Engel
Class II-IV categories. These findings emphasize the significance
of striving for maximal resection while ensuring safety, as
residual epileptogenic activity tends to correlate with less
favorable seizure outcomes.
According to the present findings, the majority of
lesions were located in the temporal lobe, with MTS, FCD, and
tumors being the most common pathologies. These types of lesions
often require extensive resection, especially in cases of MTS or
FCD where the EZ may extend beyond the visible lesion. Consistent
with other studies, patients with tumors or FCD were more likely to
have epileptogenic activity beyond the lesion itself, highlighting
the importance of performing ECoG to determine the extent of
resection (12,18,19).
In the subgroup of patients who underwent ATL in addition to
lesionectomy, seizure freedom was achieved more frequently than in
patients undergoing lesionectomy alone, demonstrating the
effectiveness of combined surgical methods.
Post-operative MRI findings in the present study
underscored the critical role of imaging in evaluating surgical
success and its impact on seizure outcomes. Complete resection of
epileptogenic lesions, achieved in 14 patients (46%), was strongly
linked to favorable outcomes, with all patients attaining Engel
Class I or II status indicating significant seizure control or
freedom. These results emphasize the importance of MRI in
confirming lesion removal and establishing a foundation for ongoing
care and monitoring. By contrast, residual lesions were identified
in 7 patients, of whom 4 experienced persistent or recurrent
seizures (Engel Class III or IV). This highlights the imperative of
maximizing lesion resection to optimize seizure control, while
carefully managing the risks of neurological deficits, particularly
in cases involving lesions near eloquent brain areas.
The post-operative EEG findings further reinforced
the significance of thorough resection. Among the patients, 18
(60%) exhibited normal EEG patterns post-surgery, strongly
associated with improved seizure outcomes. However, persistent IEDs
were observed in 4 patients, aligning with poorer seizure control.
These findings are consistent with prior research highlighting the
predictive value of EEG in postoperative evaluations (20,21).
By detecting residual epileptogenic activity, EEG serves as a
crucial tool for identifying patients at risk of seizure recurrence
and guiding the need for additional therapeutic strategies.
Together, post-operative MRI and EEG offer a complementary
approach, providing a comprehensive framework to predict and
enhance surgical outcomes in epilepsy care.
Additional research is needed to explore advanced
ECoG techniques, such as high-resolution grid electrodes to improve
the accuracy of localizing EZs. Combining ECoG with novel imaging
tools such as PET or functional MRI in future studies could enhance
the precision of EZ localization, particularly in cases of
extratemporal epilepsy. Establishing a stronger association between
ECoG results and histopathological findings could guide tailored
surgical strategies based on specific lesion types. The present
study provides evidence supporting the effectiveness of
intraoperative ECoG in improving surgical outcomes for patients
with DRE. The observed seizure freedom rates align with those
reported in high-income countries, highlighting the feasibility and
value of ECoG-guided resections even at local healthcare
systems.
This research addresses a significant gap in the
literature by contributing long-term outcome data from the EMR
(Saudi Arabia), where epilepsy remains highly prevalent, yet access
to surgical care is often restricted. Unlike Western centers with
established surgical pathways and monitoring protocols,
institutions in our region commonly face challenges such as limited
specialist availability, low public awareness, and delayed
referrals (22-24).
The retrospective design of the present study
introduces limitations, including potential recall and
documentation bias, as data were collected from existing medical
records rather than prospectively. The single-center setting and
small sample size may also limit the external validity and
generalizability of the findings to broader populations.
Post-operative MRI was not obtained in almost a third of the
patients due to logistical constraints. This missing data may
introduce selection bias and limit the strength of conclusions
drawn regarding the association between the extent of resection and
seizure outcomes. Future research should aim for prospective,
multicenter studies with standardized follow-up protocols and
multidimensional outcome assessments to enhance the robustness and
applicability of findings.
Supplementary Material
Histological details of patients.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by King Salman Center For
Disability Research (grant no. KSRG-2024-307).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
AA and ZMA contributed equally to data collection
and initial manuscript drafting. RAB and AM provided clinical
expertise and interpretation of findings and data. TJ, AN, FA, MF,
BH and SB contributed to data analysis and manuscript revision. FA
and MF assisted with literature review and data verification. AM
conceptualized the study, supervised the project, and finalized the
manuscript. AA and AM confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study received ethics approval (approval
no. NEU0402) from the Ethics Committee of King Fahad Specialist
Hospital (Dammam, Saudi Arabia). Given the retrospective nature of
the study and the use of de-identified clinical data, the
requirement for patient consent for participation was waived by the
Ethics Committee of King Fahad Specialist Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bell GS and Sander JW: The epidemiology of
epilepsy: The size of the problem. Seizure. 10:306–314.
2002.PubMed/NCBI
|
|
2
|
Dwivedi R, Ramanujam B, Chandra PS, Sapra
S, Gulati S, Kalaivani M, Garg A, Bal CS, Tripathi M, Dwivedi SN,
et al: Surgery for drug-resistant epilepsy in children. N Engl J
Med. 377:1639–1647. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Guo J, Guo M, Liu R, Kong Y, Hu X, Yao L,
Lv S, Lv J, Wang X and Kong QX: Seizure Outcome after surgery for
refractory epilepsy diagnosed by 18F-fluorodeoxyglucose positron
emission tomography (18F-FDG PET/MRI): A systematic review and
Meta-analysis. World Neurosurg. 173:34–43. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Engel J Jr, McDermott MP, Wiebe S,
Langfitt JT, Stern JM, Dewar S, Sperling MR, Gardiner I, Erba G,
Fried I, et al: Early surgical therapy for drug-resistant temporal
lobe epilepsy. JAMA. 307:922–930. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Harroud A, Bouthillier A, Weil AG and
Nguyen DK: Temporal lobe epilepsy surgery failures: A review.
Epilepsy Res Treat. 2012(201651)2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nair DR, Mohamed A, Burgess R and Lüders
H: A critical review of the different conceptual hypotheses framing
human focal epilepsy. Epileptic Disord. 6:77–83. 2004.PubMed/NCBI
|
|
7
|
Penfield W and Jasper H: Epilepsy and the
functional anatomy of the human brain. Boston, Little, Brown &
Co., Boston MA, pp363-365, 1954.
|
|
8
|
Tripathi M, Garg A, Gaikwad S, Bal CS,
Chitra S, Prasad K, Dash HH, Sharma BS and Chandra PS:
Intra-operative electrocorticography in lesional epilepsy. Epilepsy
Res. 89:133–141. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang T, Hakimian S and Schwartz TH:
Intraoperative electrocorticography (ECoG): Indications,
techniques, and utility in epilepsy surgery. Epileptic Disord.
16:271–279. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
World Health Organization (WHO): Epilepsy
in the WHO Eastern Mediterranean Region. WHO Regional Office for
the Eastern Mediterranean, Cairo, 2010. https://applications.emro.who.int/dsaf/dsa1039.pdf.
|
|
11
|
Engel J Jr: Outcome with respect to
epileptic seizures. In: Surgical Treatment of the Epilepsies. Engel
J Jr (ed). 2nd edition. Raven Press, New York, NY, pp609-621,
1993).
|
|
12
|
Ravat S, Iyer V, Panchal K, Muzumdar D and
Kulkarni A: Surgical outcomes in patients with intraoperative
Electrocorticography (EcoG) guided epilepsy surgery-experiences of
a tertiary care centre in India. Int J Surg. 36:420–428.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Greiner HM, Horn PS, Tenney JR, Arya R,
Jain SV, Holland KD, Leach JL, Miles L, Rose DF, Fujiwara H and
Mangano FT: Preresection intraoperative electrocorticography (ECoG)
abnormalities predict Seizure-onset zone and outcome in pediatric
epilepsy surgery. Epilepsia. 57:582–589. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sugano H, Shimizu H and Sunaga S: Efficacy
of intraoperative electrocorticography for assessing seizure
outcomes in intractable epilepsy patients with temporal-lobe-mass
lesions. Seizure. 16:120–127. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fernández IS and Loddenkemper T:
Electrocorticography for seizure foci mapping in epilepsy surgery.
J Clin Neurophysiol. 30:554–570. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chiba R, Enatsu R, Kanno A, Tamada T,
Saito T, Sato R and Mikuni N: Usefulness of intraoperative
electrocorticography for the localization of epileptogenic zones.
Neurol Med Chir (Tokyo). 63:65–72. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Peng SJ, Wong TT, Huang CC, Chang H, Hsieh
KL, Tsai ML, Yang YS and Chen CL: Quantitative analysis of
intraoperative electrocorticography mirrors histopathology and
seizure outcome after epileptic surgery in children. J Formos Med
Assoc. 120:1500–1511. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Goel K, Pek V, Shlobin NA, Chen JS, Wang
A, Ibrahim GM, Hadjinicolaou A, Roessler K, Dudley RW, Nguyen DK,
et al: Clinical utility of intraoperative electrocorticography in
epilepsy surgery: A systematic review and meta-analysis. Epilepsia.
64:253–265. 2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pilcher WH, Silbergeld DL, Berger MS and
Ojemann GA: Intraoperative electrocorticography during tumor
resection: Impact on seizure outcome in patients with
gangliogliomas. J Neurosurg. 78:891–902. 1993.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Jehi L, Yardi R, Chagin K, Tassi L, Russo
GL, Worrell G, Hu W, Cendes F, Morita M, Bartolomei F, et al:
Development and validation of nomograms to provide individualised
predictions of seizure outcomes after epilepsy surgery: A
retrospective analysis. Lancet Neurol. 14:283–290. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Téllez-Zenteno JF, Hernández-Ronquillo L,
Moien-Afshari F and Wiebe S: Surgical outcomes in lesional and
non-lesional epilepsy: A systematic review and meta-analysis.
Epilepsy Res. 89:310–318. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Benamer HT and Grosset DG: A systematic
review of the epidemiology of epilepsy in Arab countries.
Epilepsia. 50:2301–2304. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Idris A, Alabdaljabar MS, Almiro A,
Alsuraimi A, Dawalibi A, Abduljawad S and AlKhateeb M: Prevalence,
incidence, and risk factors of epilepsy in Arab countries: A
systematic review. Seizure. 92:40–50. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alfayez SM and Aljafen BN: Epilepsy
services in Saudi Arabia. Quantitative assessment and
identification of challenges. Neurosciences (Riyadh). 21:326–330.
2016.PubMed/NCBI View Article : Google Scholar
|