Introduction
Cornelia de Lange Syndrome (CdLS; OMIM 122470,
300590, 610759, 614701, 300882, 617116, 618736), also referred to
as Brachmann-de Lange syndrome, is the most common disorder among
the chromatinopathies, with a prevalence estimated between 1 in
10,000 to 1 in 30,000 live births (1). This condition is characterized by a
variety of anomalies that affect the craniofacial region,
musculoskeletal system, gastrointestinal tract and neurodevelopment
(2). CdLS belongs to the group of
disorders known as chromatinopathies, disorders caused by variants
in proteins involved in chromatin remodeling and transcriptional
regulation, resulting in global dysregulation of gene expression
(3). CdLS results from mutations in
any of the seven genes that constitute the cohesin complex:
NIPBL, SMC1A, SMC3, RAD21, HDAC8, ANKRD11, MAU2,
AFF2 and BRD4, each playing a role in the
construction or regulation of the complex (4,5). The
NIPBL gene mutation is most frequently implicated in CdLS
cases, particularly those exhibiting the typical phenotype,
accounting for ~60-70% of instances (6). Most NIPBL mutations associated
with CdLS are located in the coding region, while a few are in the
5'-UTR (untranslated region) (7-9).
Previously, a novel mutation c.-467C>T was identified in the
NIPBL gene, which is associated with CdLS. Cs (10). Although the 5'-UTR mutation in
NIPBL linked to CdLS is recognized for introducing a novel
upstream open reading frame (uORF) (7), the underlying pathogenic mechanisms
remain unclear.
The 5'-UTR includes structural elements and uORFs,
which are essential for regulating translation. The extended length
and intricacy of 5'-UTRs underscore the significance of
post-transcriptional and translational mechanisms in modulating the
expression levels of the proteins they encode, particularly in the
context of dose-sensitive genes (11). The NIPBL protein, with >2,500
amino acids, is crucial for the function of the cohesin complex and
its role in chromatin organization, gene regulation and
developmental processes (12,13).
In total, ~10% of CdLS cases involve mutations in cohesin
regulators and components, indicating that NIPBL mutations
may contribute to CdLS pathogenesis by disrupting cohesin function
(14). The expression levels of the
NIPBL gene are critical, as even minor decreases in them can
lead to CdLS, indicating its dosage sensitivity (14,15).
Cells derived from patients with CdLS with a heterozygous
pathogenic variant in the NIPBL gene, as well as mice
heterozygous for a Nipbl mutation, exhibit decreased overall
or regional cohesin association and impaired three-dimensional
interactions within the chromatin structure, which is consistent
with the role of NIPBL as a component that assists in the
attachment of cohesion (16-19).
The present study established a heterozygous cell
line with a point mutation to investigate the impact of the 5'-UTR
mutation on NIPBL gene expression, cohesin complexes and the
transcription of genes involved in cellular development. The
present study aimed to further elucidate the preliminary mechanisms
by which the 5'-UTR mutation of the NIPBL gene contributes
to CdLS development.
Materials and methods
RNA secondary structure
prediction
The RNA secondary structure was computed, and the
folding energy for RNA sequences was estimated using the RNAfold
tool, which is part of the ViennaRNA package (version 2.6.4), as
previously described (20).
Cell culture and knock-in cell line
construction
The 293t cell line was sourced from the American
Type Culture Collection repository of the Chinese Academy of
Sciences. These cells were maintained in Dulbecco's Modified Eagle
Medium supplemented with 10% fetal bovine serum (Shanghai ExCell
Biology, Inc.) and cultured at 37˚C in a humidified atmosphere with
5% CO2. A variant of the 293t cell line, designated as
NIPBL-mut, was engineered to harbor a 5'-UTR mutation in the
NIPBL gene using the CRISPR/Cas9 system. The sgRNA targeting
the NIPBL 5'-UTR was designed with CRISPOR: web-based design
tool (https://crispor.tefor.net; Kircher Lab,
Max Delbrück Center for Molecular Medicine, Berlin, Germany). The
NIPBL-specific sgRNA sequence (5'-TTTGTTCTGAGAGGGAGAGA-3'),
designed to target the first exon of the NIPBL gene, was
cloned into the PX459 vector, which was acquired from Addgene, Inc.
The sgRNA-PX459 construct (2 µg in 6 well plate) and the homology
repair template (500 ng;
5'-GTCGGCATTTTGTTCTGAGAGGGAGAGATGGAACGAGA-3') were co-transfected
into 293t cells using Lipofectamine 2000 transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 36 h.
Following transfection, the cells were subjected to selection with
2 µg/ml puromycin for 48 h, after which the antibiotic-resistant
cells were single-cell cloned in the same medium. Genomic DNA was
extracted from the edited cells (Tissue DNA Kit; Omega Bio-Tek,
Inc.) and a 517-bp fragment spanning the entire sgRNA insertion
site (chr5: 36,876,519-36,877,036, hg38) was amplified with
PrimeSTAR® HS DNA Polymerase (Takara Biotechnology Co.,
Ltd.) under standard high-fidelity cycling conditions (98˚C for 30
sec; 35 cycle x [98˚C for 10 sec, 63˚C for 20 sec, 72˚C for 30
sec]; 72˚C for 2 min). The purified 517-bp product was then sent to
Tsingke Biological Technology for Sanger sequencing with the
forward PCR primer (5'-GCAAACGTCGCTCTGAAGCAT-3') and a reverse
primer (5'-CGAGAAGACAAATCGTTGCT-3'). Chromatograms were aligned to
the reference gene sequence with SnapGene to confirm 100% identity
and absence of indels. The template was verified on three separate
occasions to confirm sequence identity.
Luciferase reporter assay
The procedures for plasmid construction and
dual-luciferase reporter analysis were conducted as previously
described by the authors (10). The
data were standardized to account for transfection efficiency by
quantifying the activities of firefly and Renilla
luciferases, with the results presented as a ratio of these two
luciferase activities. Most control samples were set to a value of
100% for normalization purposes. The results are depicted as the
mean values ± standard deviations, derived from triplicate
experiments.
Immunofluorescence
WT and mut 293t cells were seeded onto glass
coverslips in their respective growth media. The cells were
cultured for the specified number of days until they reached ~80%
confluence. The cell layers were then fixed with a 4%
paraformaldehyde solution in phosphate-buffered saline (PBS, pH
7.5) at room temperature (RT, 25˚C) for 20 min. Following fixation,
the coverslips were permeabilized with a 0.2% Triton X-100 solution
for 15 min and then were blocked with 3% bovine serum albumin (BSA;
Sangon Biotech Co., Ltd.) for 1 h at RT. Primary antibodies
targeting NIPBL (cat. no. sc-374625; Santa Cruz Biotechnology,
Inc.), diluted to a ratio of 1:100 in 1% BSA, were then applied,
and the solution was incubated overnight (for >18 h) at 4˚C in a
light-protected environment. Subsequently, the Alexa Fluor 488 Goat
Anti-Mouse IgG (H+L) secondary antibodies (cat. no. ab150113;
Abcam) were incubated for 1 h at RT. The cells were then stained at
RT with 0.2 mg/ml DAPI for 7 min to visualize the nuclei. After
staining, the slides were air-dried and mounted with anti-fade
reagents to preserve the fluorescence. Finally, the samples were
analyzed using a fluorescence microscope.
Cell fractionation
This process started with the collection of cells
and their subsequent incubation in lysis buffer I, which is
composed of 20 mM HEPES at a pH of 8.0, 2 mM MgCl2, 10
mM KCl, 0.5% NP-40 and protease inhibitors, for 10 min, under
refrigeration at 4˚C and with the inclusion of protease inhibitors
in all solutions. The mixture was centrifuged at 1,500 x g for 5
min after incubation. The supernatant, which was rich in cytosolic
components, was then carefully separated and set aside. The
remaining pellet was rinsed twice with PBS and then subjected to
lysis using nucleus lysis buffer II, which is prepared by
incorporating 0.5 M NaCl into lysis buffer I. After lysis, the
nuclear lysate was allowed to chill on ice for 5 min before
centrifugation at 15,000 x g for 10 min. The supernatant,
containing the nuclear components, was subsequently collected and
preserved as the nuclear fraction.
Western blot analysis
Cells were subjected to lysis using RIPA buffer
(cat. no. P0013C; Beyotime Institute of Biotechnology) supplemented
with phenylmethylsulfonyl fluoride. The concentration of total
proteins in the lysates was quantified using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology). Aliquots
of 20 µg of protein samples were resolved using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis on a 10% acrylamide gel
and then transferred onto polyvinylidene fluoride membranes. The
membranes were then blocked with a 5% skim milk in TBS-T at RT for
1 h. After blocking, the membranes were incubated overnight at 4˚C
with a panel of primary antibodies targeting NIPBL (cat. no.
sc-374625; Santa Cruz Biotechnology, Inc.), α-Tubulin (cat. no.
ab7291; Abcam), RAD21 (cat. no. DF7520; Abcam), SMC1A (cat. no.
AF6439; Affinity Biosciences), CTNNB1 (cat. no. 8480; Cell
Signaling Technology, Inc.) and GAPDH (cat. no. ab8245; Abcam).
Subsequently, the membranes were treated with HRP-Goat Anti-rabbit
IgG (H+L) or HRP-Goat Anti-mouse IgG (H+L) secondary antibodies
(BK-R050-50 µl or BK-M050-50 µl΄ Hangzhou Baoke Biotech Co., Ltd.),
diluted at 1:5,000, for 1 h. The immunoreactive bands were
visualized using an enhanced chemiluminescence detection system
(Hangzhou Fude Biological Technology Co., Ltd.) and their
intensities were quantified through densitometric analysis using
ImageJ 1.54f (National Institutes of Health). The following primary
antibodies were used: NIPBL (1:500; Santa Cruz Biotechnology,
Inc.); α-Tubulin and GAPDH (1:1,000; both from Abcam); RAD21 and
SMC1A (1:1,000; both from Affinity Biosciences) and CTNNB1(1:1,000;
Cell Signaling Technology, Inc.).
EDU staining
The DNA replication in 293t cells was detected using
an EdU staining kit (Beyotime Institute of Biotechnology),
following the manufacturer's instructions. Initially, 293t cells
were plated in 24-well plates and cultured for 12 h until they
reached a confluency of ~50%. Subsequently, the cells were treated
with a 10 µM concentration of EdU solution, which was incorporated
into the cell medium and left for 2 h. After this incubation
period, the cells were fixed with 4% paraformaldehyde in PBS (pH
7.4) at room temperature for 15 min and then rinsed three times (5
min each) with PBS to remove any unincorporated EdU. A click
reaction solution containing Azide 488 was then added to the cells
and incubated for 30 min to facilitate the detection of EdU
incorporation. To visualize the cell nuclei, 0.2 mg/ml DAPI
staining was performed at RT for 7 min, and the stained cells were
subsequently examined using a fluorescence microscope.
Statistical analyses
The experiments were repeated at least three times
unless otherwise indicated. Statistical analyses were performed
using unpaired two-tailed t-tests in GraphPad Prism 10.1.2
(GraphPad Software; Dotmatics). Most control groups were normalized
to 100%. The data are presented as the mean ± standard error of the
mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
Impact of a 5'-UTR mutation on
uORFs
To assess the impact of the c.-467C>T variant on
post-transcriptional regulation, the 5'-UTR of NIPBL was
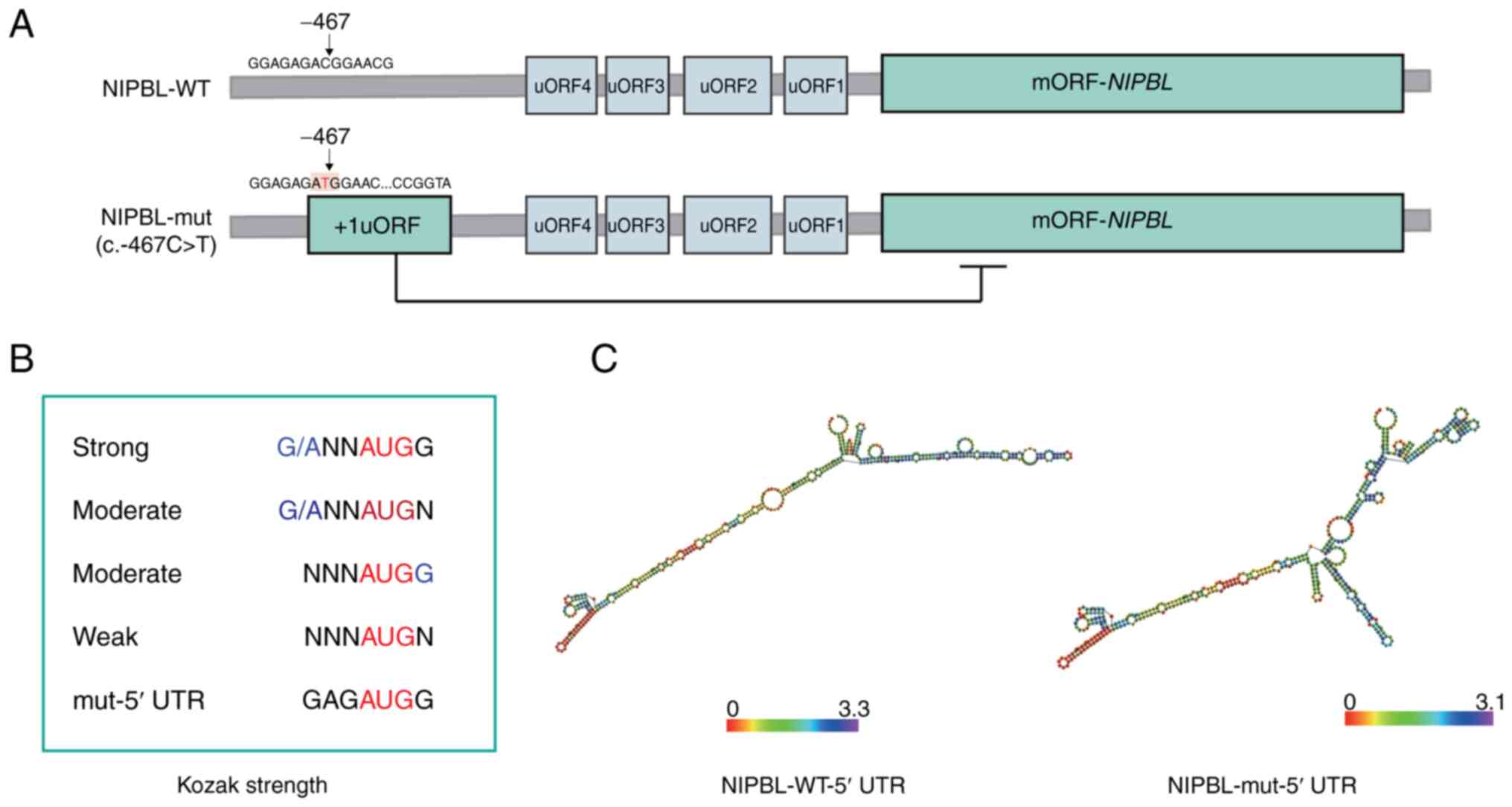
analyzed for uORFs using NCBI ORFfinder. A total of Four uORFs were
identified in the wild-type (WT) sequence, whereas the mutant
harbored an additional uORF, yielding a total of five (Fig. 1A). The de novo uORF initiates
at a strong Kozak sequence (GAGAUGG) (21) and spans 53 codons, terminating
upstream of the NIPBL start codon (Fig. 1B). Secondary structure prediction
with ViennaRNA revealed a complex secondary RNA structure for the
mutated 5'-UTR, characterized by numerous stem-loops (Fig. 1C), with no significant difference in
the lowest free energy between the WT (-2548.10 kcal/mol) and the
mutant (-2546.70 kcal/mol) for the initial 10,000 bases.
Impact of uORF variants on NIPBL
expression
The CRISPR/Cas9 system-mediated editing introduced
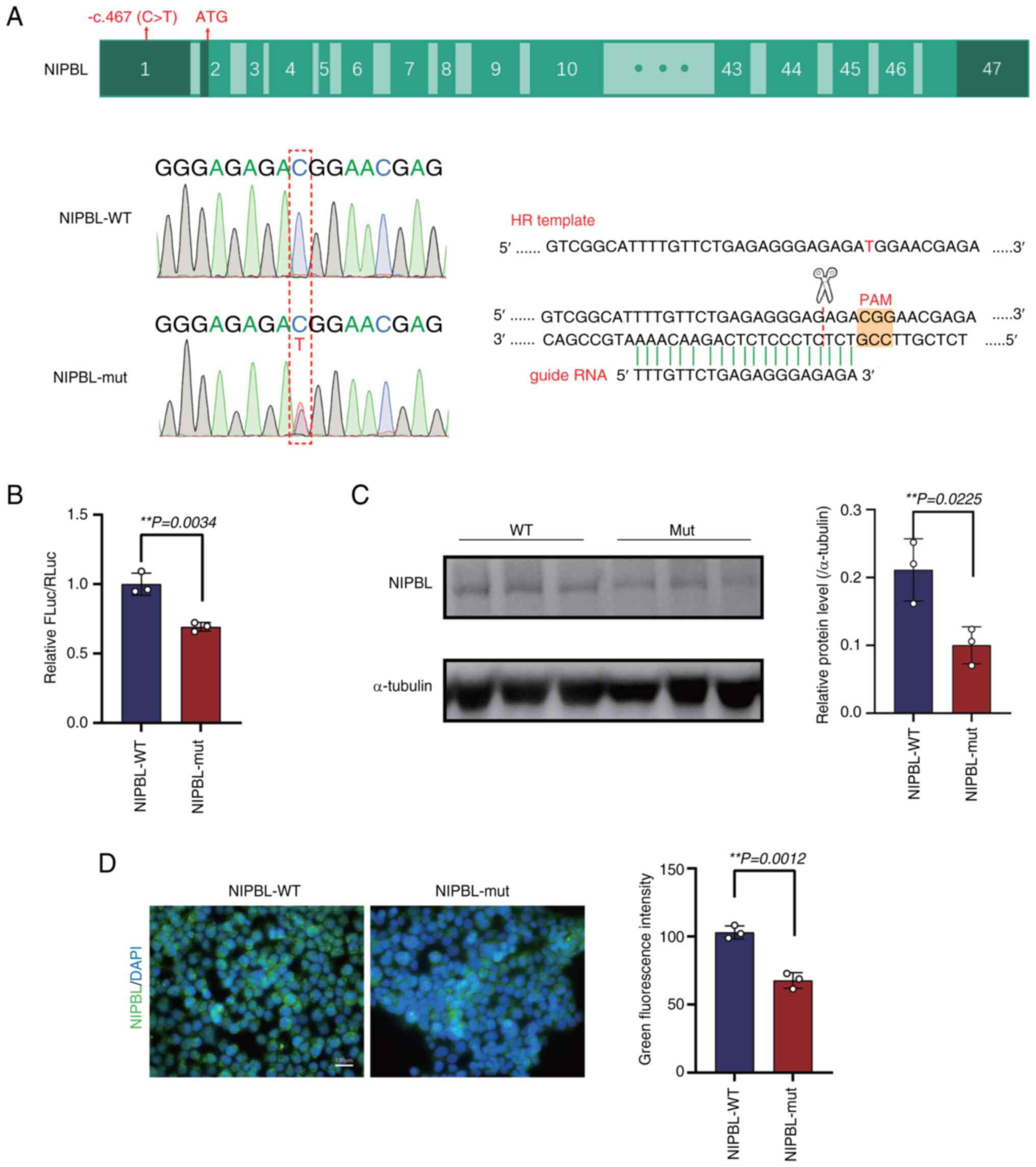
the c.-467C>T variant into the endogenous NIPBL locus,
yielding heterozygous NIPBL-mut cells (Fig. 2A). A dual-luciferase reporter assay
was used to quantify the transcriptional impact of the variant.
Compared with the WT, the mutant (mut) 5'-UTR produced a modest but
significant reduction in reporter activity (1±0.046 vs.
0.693±0.019; P=0.0034; Fig. 2B).
Consistently, immunoblot analysis revealed a ~50% decrease in
endogenous NIPBL protein levels in mutant cells relative to
isogenic controls (0.211±0.027 vs. 0.100±0.016; P=0.0225; Fig. 2C). Immunofluorescence staining
further showed that the subcellular distribution of NIPBL was
unaltered by the mutation. However, the fluorescence intensity in
the mutant cells was significantly reduced by 40% compared with
that in the WT (103.000±2.767 vs. 67.680±3.323; P=0.0012; Fig. 2D).
NIPBL haploinsufficiency downregulates
RAD21
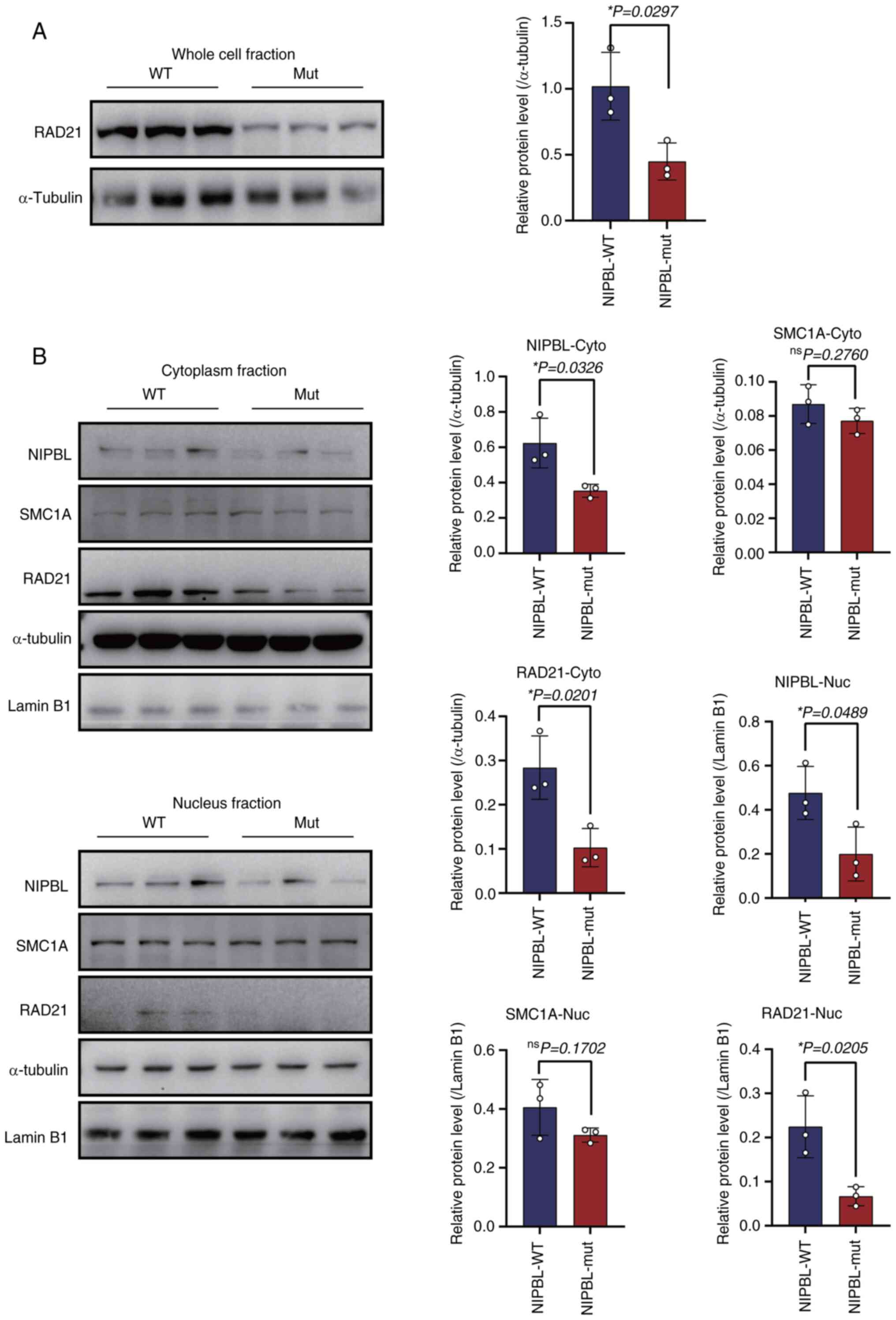
It was next examined whether the NIPBL
mutation affects the steady-state abundance of core cohesin
subunits. Whole-cell lysates revealed a significant reduction in
RAD21 protein (1.020±0.257 vs. 0.449±0.141; P=0.0297; Fig. 3A). To determine compartment-specific
changes, nuclei and cytoplasm were fractionated. Consistent with
our previous findings, NIPBL protein levels decreased in both the
nucleus (0.476±0.070 vs. 0.199±0.070, P=0.0489 Fig. 3B) and cytoplasm (0.623±0.081 vs.
0.354±0.021, P=0.0326, Fig. 3B).
Similarly, RAD21 expression in cellular fractions decreased in the
mut compared with the WT (Cytoplasm, 0.283±0.042 vs. 0.103±0.025;
P=0.0201; nucleus 0.224±0.041 vs. 0.067±0.013; P=0.0205; Fig. 3B). By contrast, SMC1A levels were
comparable between genotypes in both compartments (Fig. 3B).
Reduction in NIPBL expression leads to
decreased β-catenin and impacts cell proliferation
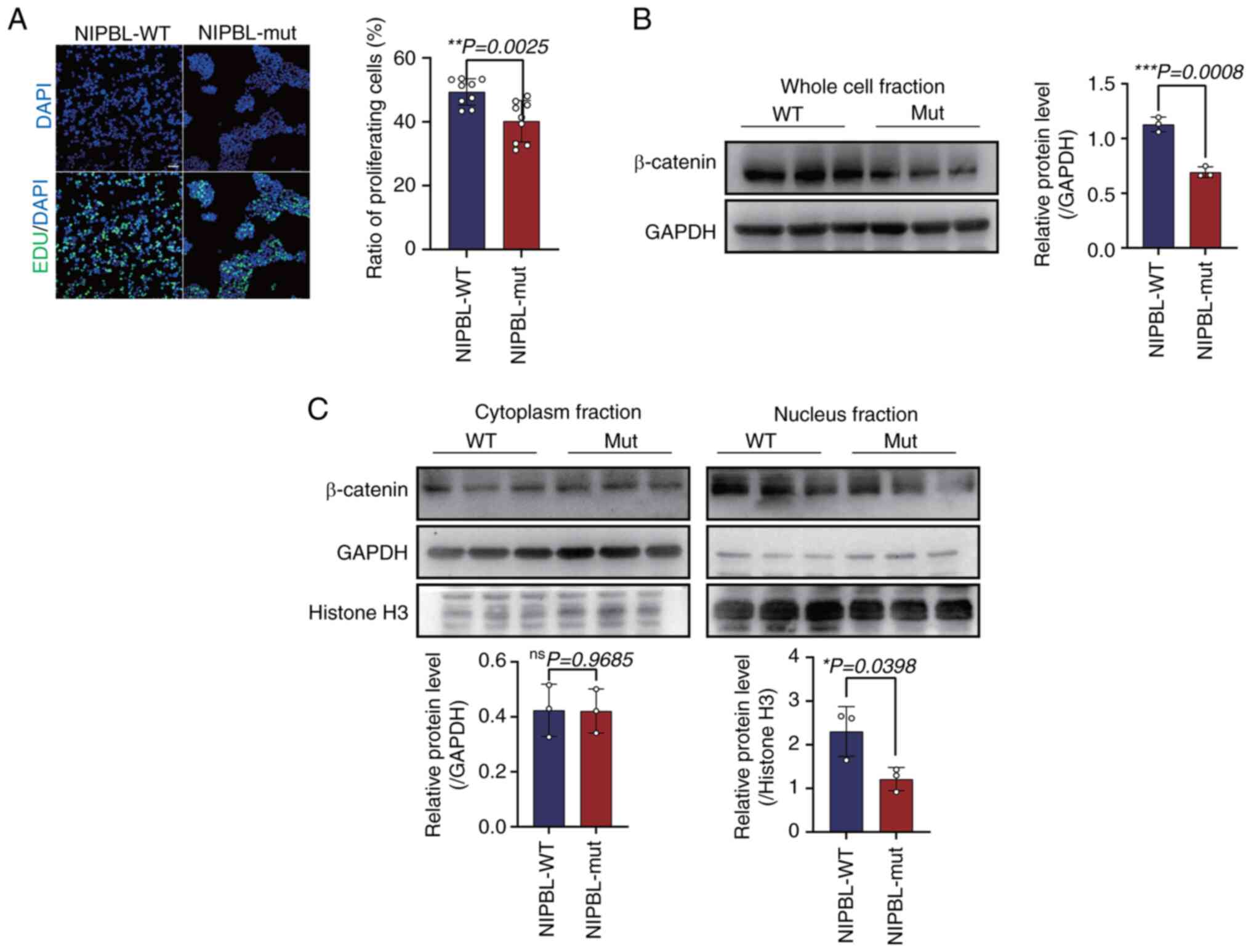
EdU staining assays were conducted to quantify the
proliferative capacity ofNIPBL-mutant cells. Compared with
WT controls, the fraction of EdU-positive cells were significantly
lower in the mutant population (49.360±1.384 vs. 40.200±2.154;
P=0.0025; Fig. 4A). Western blot
analysis revealed that total β-catenin protein levels were
significantly reduced in mut cells compared with WT controls
(1.129±0.039 vs. 0.692±0.028, P=0.0008; Fig. 4B). Since nuclear accumulation of
β-catenin is required for canonical Wnt signaling, β-catenin was
further resolved into nuclear and cytoplasmic fractions. Nuclear
β-catenin was significantly decreased in the mut group (2.301±0.328
vs. 1.213±0.152; P=0.0398; Fig.
4C); cytoplasmic β-catenin levels remained unaffected in both
the WT and mutant groups.
Discussion
In our previous publication, a novel NIPBL
variant (c.-467 C>T) was identified in a Chinese boy affected by
CdLS (10). In the present study,
it was demonstrated that this mutation creates a strong uORF that
represses NIPBL translation without affecting mRNA
stability. Mechanistically, the uORF stalls ribosome scanning,
preventing initiation at the downstream NIPBL start codon
(22). Critically, reduced NIPBL
diminishes cohesin loading onto chromatin, disrupting long-range
chromatin interactions, a core defect in CdLS pathogenesis
(23,24). These data establish uORF-mediated
translational inhibition as a recurrent mechanism underlying NIPBL
haploinsufficiency in CdLS.
Reduced NIPBL dosage, in turn, perturbs the cohesin
complex. Western blotting and subcellular-fractionation analyses
revealed a decrease in RAD21 protein. This finding contrasts with
some studies in which NIPBL mutations altered cohesin
chromosomal occupancy without affecting total cohesin subunit
abundance (25,26). However, it aligns with recent
evidence from small-cell lung cancer showing that NIPBL protein
promoted RAD21 gene transcription by enhancing H3K27
demethylation via recruiting lysine demethylase 6B to the
RAD21 gene promoter (27),
reconciling our findings with the emerging view that NIPBL
dosage can exert both occupancy and abundance-dependent effects on
cohesin. Because cohesin dysfunction underlies the group of
disorders termed chromatinopathies, CdLS being the archetype, the
observed RAD21 downregulation provides a direct mechanistic link
between the NIPBL 5'-UTR mutation and CdLS pathogenesis
(28,29). These findings suggest that
downregulation of RAD21 may be caused by pathogenic mutations in
the NIPBL 5'-UTR. This interpretation is further supported
by clinical genetics: Heterozygous RAD21 variants can
themselves cause CdLS, but the resulting phenotype is typically
mild (30-32),
mirroring the attenuated presentation associated with NIPBL
5'-UTR mutations. Thus, converging molecular and clinical evidence
indicates that reduced NIPBL dosage elicits RAD21-mediated
disruption of cohesin that contributes to the milder end of the
CdLS phenotypic spectrum.
To determine how NIPBL-RAD21 deficiency translates
into cellular pathology, the Wnt/β-catenin pathway was
interrogated. Functional assays revealed modest yet consistent
defects in cell proliferation that were accompanied by a marked
reduction in nuclear β-catenin. This result is consistent with the
downregulation of the classical Wnt signaling pathway observed in
the CdLS zebrafish model (33,34),
and extends it to human cells. Importantly, this finding also
supports the notion that NIPBL or RAD21 knockdown
leads to a decrease in cell proliferation ability, suggesting a
causal rather than correlative relationship (15,33,35).
Supporting this notion, transcriptomic profiling of CdLS-associated
cardiac dysplasia demonstrates that RAD21 depletion alone is
sufficient to derail Wnt signaling (8). Consequently, it was hypothesized that
NIPBL mutations trigger the downregulation of RAD21, leading
to the dysregulation of β-catenin and ultimately to defects in cell
proliferation.
In conclusion, it was demonstrated that a single
5'-UTR variant (c.-467C>T) creates a de novo uORF,
quantitatively reduces NIPBL and cohesin levels, and thereby
disrupts Wnt signalling and proliferation. These findings establish
a direct molecular link between a non-coding mutation and mild
Cornelia de Lange phenotypes.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 82270837).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
CW and CZ conceived and designed the research. QC
and YC performed the experiments and analyzed the data. QC, YC and
CW wrote the manuscript. All authors read and approved the final
version of the manuscript. QC and YC confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Garcia P, Fernandez-Hernandez R, Cuadrado
A, Coca I, Gomez A, Maqueda M, Latorre-Pellicer A, Puisac B, Ramos
FJ, Sandoval J, et al: Disruption of NIPBL/Scc2 in cornelia de
lange syndrome provokes cohesin genome-wide redistribution with an
impact in the transcriptome. Nat Commun. 12(4551)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Caplan IF, Ye M and Pearlman AN:
Management of nasal polyposis in pediatric patients with cornelia
de lange syndrome: A case series and literature review. Ear Nose
Throat J: Sep 24, 2024 (Epub ahead of print).
|
|
3
|
Parenti I and Kaiser FJ: Cornelia de lange
syndrome as paradigm of chromatinopathies. Front Neurosci.
15(774950)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Parenti I, Diab F, Gil SR, Mulugeta E,
Casa V, Berutti R, Brouwer RWW, Dupé V, Eckhold J, Graf E, et al:
MAU2 and NIPBL variants impair the heterodimerization of the
cohesin loader subunits and cause cornelia de lange syndrome. Cell
Rep. 31(107647)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Lucia-Campos C, Parenti I,
Latorre-Pellicer A, Gil-Salvador M, Bestetti I, Finelli P, Larizza
L, Arnedo M, Ayerza-Casas A, Del Rincón J, et al: An intragenic
duplication in the AFF2 gene associated with cornelia de lange
syndrome phenotype. Front Genet. 15(1472543)2024.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shangguan H and Chen R: Phenotypes of
cornelia de lange syndrome caused by non-cohesion genes: Novel
variants and literature review. Front Pediatr.
10(940294)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Coursimault J, Rovelet-Lecrux A, Cassinari
K, Brischoux-Boucher E, Saugier-Veber P, Goldenberg A, Lecoquierre
F, Drouot N, Richard AC, Vera G, et al: uORF-introducing variants
in the 5'UTR of the NIPBL gene as a cause of cornelia de lange
syndrome. Hum Mutat. 43:1239–1248. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Schuster K, Leeke B, Meier M, Wang Y,
Newman T, Burgess S and Horsfield JA: A neural crest origin for
cohesinopathy heart defects. Hum Mol Genet. 24:7005–7016.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Teresa-Rodrigo ME, Eckhold J, Puisac B,
Pozojevic J, Parenti I, Baquero-Montoya C, Gil-Rodríguez MC,
Braunholz D, Dalski A, Hernández-Marcos M, et al: Identification
and functional characterization of two intronic NIPBL mutations in
two patients with cornelia de lange syndrome. Biomed Res Int.
2016(8742939)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen Y, Chen Q, Yuan K, Zhu J, Fang Y, Yan
Q and Wang C: A novel de novo variant in 5' UTR of the NIPBL
associated with cornelia de lange syndrome. Genes (Basel).
13(740)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wieder N, D'Souza EN, Martin-Geary AC,
Lassen FH, Talbot-Martin J, Fernandes M, Chothani SP, Rackham OJL,
Schafer S, Aspden JL, et al: Differences in 5'untranslated regions
highlight the importance of translational regulation of dosage
sensitive genes. Genome Biol. 25(111)2024.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang S, Übelmesser N, Josipovic N, Forte
G, Slotman JA, Chiang M, Gothe HJ, Gusmao EG, Becker C, Altmüller
J, et al: RNA polymerase II is required for spatial chromatin
reorganization following exit from mitosis. Sci Adv.
7(eabg8205)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vitoria M, Landwerlin P, Diebold-Durand
ML, Shaik TB, Durand A, Troesch E, Weber C, Brillet K, Lemée MV,
Decroos C, et al: The cohesin ATPase cycle is mediated by specific
conformational dynamics and interface plasticity of SMC1A and SMC3
ATPase domains. Cell Rep. 43(114656)2024.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Panarotto M, Davidson IF, Litos G,
Schleiffer A and Peters JM: Cornelia de Lange syndrome mutations in
NIPBL can impair cohesin-mediated DNA loop extrusion. Proc Natl
Acad Sci USA. 119(e2201029119)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu W, Ying Y, Shan L, Feng J, Zhang S, Gao
Y, Xu X, Yao Y, Zhu C and Mao W: Enhanced expression of cohesin
loading factor NIPBL confers poor prognosis and chemotherapy
resistance in non-small cell lung cancer. J Transl Med.
13(153)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Higashi TL, Eickhoff P, Sousa JS, Locke J,
Nans A, Flynn HR, Snijders AP, Papageorgiou G, O'Reilly N, Chen ZA,
et al: A structure-based mechanism for DNA entry into the cohesin
ring. Mol Cell. 79:917–933.e9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rinaldi L, Fettweis G, Kim S, Garcia DA,
Fujiwara S, Johnson TA, Tettey TT, Ozbun L, Pegoraro G, Puglia M,
et al: The glucocorticoid receptor associates with the cohesin
loader NIPBL to promote long-range gene regulation. Sci Adv.
8(eabj8360)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Weiss FD, Calderon L, Wang YF, Georgieva
R, Guo Y, Cvetesic N, Kaur M, Dharmalingam G, Krantz ID, Lenhard B,
et al: Neuronal genes deregulated in cornelia de lange syndrome
respond to removal and re-expression of cohesin. Nat Commun.
12(2919)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Luna-Peláez N, March-Díaz R,
Ceballos-Chávez M, Guerrero-Martínez JA, Grazioli P,
García-Gutiérrez P, Vaccari T, Massa V, Reyes JC and
García-Domínguez M: The cornelia de lange syndrome-associated
factor NIPBL interacts with BRD4 ET domain for transcription
control of a common set of genes. Cell Death Dis.
10(548)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fallmann J, Will S, Engelhardt J, Grüning
B, Backofen R and Stadler PF: Recent advances in RNA folding. J
Biotechnol. 261:97–104. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ambrosini C, Destefanis E, Kheir E, Broso
F, Alessandrini F, Longhi S, Battisti N, Pesce I, Dassi E, Petris
G, et al: Translational enhancement by base editing of the Kozak
sequence rescues haploinsufficiency. Nucleic Acids Res.
50:10756–10771. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Whiffin N, Karczewski KJ, Zhang X,
Chothani S, Smith MJ, Evans DJ, Roberts AM, Quaife NM, Schafer S,
Rackham O, et al: Characterising the loss-of-function impact of 5'
untranslated region variants in 15,708 individuals. Nat Commun.
11(2523)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mills JA, Herrera PS, Kaur M, Leo L,
McEldrew D, Tintos-Hernandez JA, Rajagopalan R, Gagne A, Zhang Z,
Ortiz-Gonzalez XR and Krantz ID: NIPBL(+/-) haploinsufficiency
reveals a constellation of transcriptome disruptions in the
pluripotent and cardiac states. Sci Rep. 8(1056)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Alonso-Gil D, Cuadrado A, Giménez-Llorente
D, Rodríguez-Corsino M and Losada A: Different NIPBL requirements
of cohesin-STAG1 and cohesin-STAG2. Nat Commun.
14(1326)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Newkirk DA, Chen YY, Chien R, Zeng W,
Biesinger J, Flowers E, Kawauchi S, Santos R, Calof AL, Lander AD,
et al: The effect of Nipped-B-like (Nipbl) haploinsufficiency on
genome-wide cohesin binding and target gene expression: Modeling
cornelia de lange syndrome. Clin Epigenetics. 9(89)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mannini L, C Lamaze F, Cucco F, Amato C,
Quarantotti V, Rizzo IM, Krantz ID, Bilodeau S and Musio A: Mutant
cohesin affects RNA polymerase II regulation in Cornelia de Lange
syndrome. Sci Rep. 5(16803)2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xu X, Wang D, Xu W, Li H, Chen N, Li N,
Yao Q, Chen W, Zhong J and Mao W: Author Correction: NIPBL-mediated
RAD21 facilitates tumorigenicity by the PI3K pathway in
non-small-cell lung cancer. Commun Biol. 7(397)2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pileggi S, La Vecchia M, Colombo EA,
Fontana L, Colapietro P, Rovina D, Morotti A, Tabano S, Porta G,
Alcalay M, et al: Cohesin mutations induce chromatin conformation
perturbation of the H19/IGF2 imprinted region and gene expression
dysregulation in cornelia de lange syndrome cell lines.
Biomolecules. 11(1622)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Avagliano L, Parenti I, Grazioli P, Di
Fede E, Parodi C, Mariani M, Kaiser FJ, Selicorni A, Gervasini C
and Massa V: Chromatinopathies: A focus on Cornelia de Lange
syndrome. Clin Genet. 97:3–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
De Falco A, De Brasi D, Della Monica M,
Cesario C, Petrocchi S, Novelli A, D'Alterio G, Iolascon A, Capasso
M and Piscopo C: A novel variant in RAD21 in cornelia de lange
syndrome type 4: Case report and bioinformatic analysis. Genes
(Basel). 14(119)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yu R, Roseman S, Siegenfeld AP, Gardner Z,
Nguyen SC, Tran KA, Joyce EF, Jain R, Liau BB, Krantz ID, et al:
CTCF/RAD21 organize the ground state of chromatin-nuclear speckle
association. Nat Struct Mol Biol. 32:1069–1080. 2025.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yue X, Chen M, Ke X, Yang H, Gong F, Wang
L, Duan L, Pan H and Zhu H: Clinical characteristics, genetic
analysis, and literature review of cornelia de lange syndrome type
4 associated with a RAD21 variant. Mol Genet Genomic Med.
12(e70009)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Grazioli P, Parodi C, Mariani M, Bottai D,
Di Fede E, Zulueta A, Avagliano L, Cereda A, Tenconi R, Wierzba J,
et al: Lithium as a possible therapeutic strategy for Cornelia de
Lange syndrome. Cell Death Discov. 7(34)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gruca-Stryjak K, Doda-Nowak E, Dzierla J,
Wróbel K, Szymankiewicz-Bręborowicz M and Mazela J: Advancing the
clinical and molecular understanding of cornelia de lange syndrome:
A multidisciplinary pediatric case series and review of the
literature. J Clin Med. 13(2423)2024.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Pallotta MM, Di Nardo M and Musio A:
Synthetic lethality between cohesin and WNT signaling pathways in
diverse cancer contexts. Cells. 13(608)2024.PubMed/NCBI View Article : Google Scholar
|