Introduction
Angiogenesis, defined as the formation of new blood
vessels from pre-existing ones, is a key biological process
critical for supporting growth, development and tissue repair.
However, under certain pathological conditions such as diabetes,
hypotension, thrombosis and atherosclerosis, angiogenesis may
become dysregulated, resulting in pathological outcomes (1,2).
Angiogenesis is a multi-step process that involves endothelial cell
(EC) proliferation, migration and tubular structure formation. This
complex process is regulated by a balance between pro- and
anti-angiogenic factors.
Studies have identified an angiogenic pathway
mediated by non-neuronal α7 nicotinic acetylcholine receptors
(α7-nAChRs) (3-5).
These receptors are pentameric, ligand-gated channels,
characterized by their high permeability to Ca2+ influx
upon agonist binding (5).
Ca2+ influx promotes the activation of kinases,
including phosphatidylinositol 3-kinase (PI3K), which serves a key
role in EC migration (5,6). In EC, functional and molecular studies
have demonstrated that PI3K signaling is essential for angiogenesis
by orchestrating cytoskeletal remodeling, adherens junction
dynamics and directed cell migration (7,8).
To explore the role of α7-nAChRs in angiogenesis,
researchers have developed specific ligands targeting these
receptors (9-11).
Among these ligands, the isoxazole analog of nicotine ABT-418 is a
standard agonist for the α7-nAChR (12). Additionally, compounds such as
N-(4-chlorophenyl)-a-[[(4-chlorophenyl)
amino]methylene]-3-methyl-5-isoxazoleacetamide and PNU-120596 serve
as positive allosteric modulators, enhancing receptor activation by
destabilizing receptor desensitization and modulating channel
currents (11,13,14).
In our previous study, we synthesized a novel isoxazolic compound,
3-(4-nitrophenyl)-5-phenylisoxazole (ISO-1), and demonstrated its
ability to activate α7-nAChRs in human umbilical vein ECs (HUVECs)
(15). Specifically, ISO-1 induces
a dose-dependent increase in intracellular Ca²+
concentration [(Ca²+)i], mediated by the
activation of α7-nAChRs, as previously described (15) This finding highlights the importance
of HUVECs, as they predominantly express α7-nAChRs, which are key
for activating angiogenic processes through the cholinergic pathway
(4,5) Moreover, HUVECs provide a robust model
for investigating other key molecules and pathways involved in EC
migration, proliferation and tube formation (16-18).
The present study investigated the pro-angiogenic
potential of the isoxazole molecule ISO-1 in ECs based on its
ability to promote key processes of angiogenesis, including cell
proliferation and migration and the formation of capillary-like
structures. Given previous evidence linking ISO-1 to α7-nAChR
activation (15), the present study
also explored whether these effects are mediated through this
receptor.
Materials and methods
Reagents and chemicals
M199 (cat. no. 11310882), FBS (cat. no. A5256701)
and DMSO (cat. no. 855190) were purchased from Gibco (Thermo Fisher
Scientific, Inc.). Collagenase type I (cat. no. SCR103), nicotine
(cat. no. N3876), choline (cat. no. C7527), neutral red (cat. no.
N4638), sulforhodamine B (cat. no. S1402) and BSA (cat. no. A9418)
were obtained from Sigma-Aldrich (Merck KGaA). Cultrex®
extracellular matrix (cat. no. 3533-010-02) was acquired from
Trevigen, Inc. (BioTechne). CellTiter 96® Aqueous One
Solution Cell Proliferation Assay (cat. no. G3582) was obtained
from Promega Corporation. ISO-1 was synthesized as described by
Cortés et al (15).
Isolation and primary culture of
HUVECs
All umbilical cords were obtained following delivery
from full-term normal pregnancies from Gynecological-Obstetric Unit
of the Carlos Van Buren Hospital (Valparaiso, Chile) with written
informed consent of the mothers and the approval of the Ethics
Committee of the Faculty of Pharmacy of Universidad de Valparaiso
in Valparaiso, Chile (approval no. 19/2015) and the Ethics
Committee of the Valparaiso-San Antonio Health Service in
Valparaiso, Chile (approval no. 1435), which has responsibility for
ethically approving studies on behalf of Carlos Van Buren
Hospital.
ECs were isolated as described by Jaffe et al
(19) HUVECs were isolated using
collagenase I (0.5 mg/ml) digestion and cells were cultured in M199
supplemented with 2.5 mM L-glutamine, 14 mM HEPES, 200 UI/l
penicillin, 400 UI/l streptomycin and 20% FBS at pH 7.42, at 37˚C
and 5% CO2 atmosphere. Experiments were performed in
confluent cultures of ECs (~5 days of primary culture) or in cells
at passage 1-3.
Cytotoxicity assessment using Neutral
Red Uptake and MTS assays
HUVECs were seeded in 96-well plates at a density of
3x104 cells/well and incubated in M199 supplemented with
5% FBS (negative control) at 37˚C for 72 h. Cells were treated with
solvent (S control, DMSO 0.3%) or ISO-1 in solvent medium at
concentrations of 1x10-3.5 M, 1x10-4 M,
1x10-5, 1x 10-7 M and 1x10-9 M and
incubated for 6 or 24 h at 37˚C and 5% CO2.
Lysosomal activity was evaluated using the neutral
red uptake assay, as described by Repetto et al (20). Each treatment was removed, and the
wells were washed twice with PBS. Subsequently, a control medium
containing neutral red (40 µg/ml) was added, and the plate was
incubated at 37˚C for 2 h. Neutral red was discarded, the plate was
washed with PBS and neutral red destain solution [50% ethanol
(96%), 49% deionized water, 1% glacial acetic acid] was added.
Plates were agitated for 10 min using an orbital shaker and
absorbance was determined at 540 nm using Thermo Fisher Scientific,
Inc. Varioskan® Flash microplate reader. Results were
expressed as percentage of the negative control response.
Mitochondrial activity was assessed using the MTS
assay. Mitochondrial activity, used as a marker of viability, was
evaluated using CellTiter 96® Aqueous One Solution Cell
Proliferation Assay kit (Promega Corporation), following the
manufacturer's instructions. Absorbance at 490 nm was determined in
each well using Thermo Fisher Scientific, Inc. Varioskan Flash
microplate reader. Results were expressed as percentage of the
negative control response.
Cell proliferation assay using
sulforhodamine B (SRB)
The proliferation of HUVECs was determined using the
SRB assay described by Vichai and Kirtikara (21). HUVECs were treated as
aforementioned, 10% trichloroacetic acid (Sigma-Aldrich; Merck
KGaA) was added and the plate was incubated for 1 h at 4˚C. Each
well was washed four times with deionized water and the plate was
allowed to air-dry at room temperature. Then, 0.057% SRB solution
(Sigma-Aldrich) was added to each well at room temperature for 30
min. After that, the plate was rinsed with 1% acetic acid and
allowed to air-dry at room temperature. Finally, 10 mM Tris
solution (pH 10.5) was added to each well and the plate was shaken
on an orbital shaker for 10 min to solubilize the protein-bound
dye. The absorbance at 510 nm of each well was measured using
Thermo Fisher Scientific, Inc. Varioskan® Flash
microplate reader. Results obtained were expressed as percentage of
control medium.
Wound healing assay
The migration of HUVECs was analyzed through the
scratch wound assay (22). HUVECs
were seeded in 96-well plates at confluence (3x104
cells/well) and incubated in M199 supplemented with 20% FBS for 24
h at 37˚C. A confluent monolayer of HUVECs (100% confluence) in
control conditions (M199 supplemented with 5% FBS) or treated with
nicotine (1x10-8 M), ISO-1 (1x10-10 M,
1x10-8 M, 1x10-6 M, 1x10-4 M) or
choline (1x10-5 M), alone or in combination with 100 nM
α-Bungarotoxin (BTX, Sigma-Aldrich), was scratched using a P10
micropipette tip. The monolayer was washed with PBS to remove cell
debris. Treatments were maintained throughout the experiment in
M199 supplemented with 5% FBS, and the plate was incubated at 37˚C
for 24 h. Images were captured at 0 and 24 h using Nikon Eclipse
TE200 microscope (Nikon Corporation). Wound closure was analyzed
using ImageJ version 1.52p (23)
and HUVEC migration was expressed as the percentage of wound
closure relative to the control.
Tubular structure formation
The ability of HUVECs to form capillary-like tubular
structures was examined using the Matrigel-based tube formation
assay (24). HUVEC tube formation
assay was performed in 96-well plates coated with 50 µl
Cultrex® (Trevigen, Inc.; BioTechne), according to the
manufacturer's protocol. Cultrex solution was added to the plates
and allowed to solidify and polymerize at 37˚C for 30 min. HUVECs
at density of 1x104 cells/well were seeded on the top of
the Cultrex matrix and tubular structure formation was monitored
for 4 and 12 h in control conditions (M199 supplemented with 5%
FBS) or in the presence of solvent medium (0.3% DMSO), positive
control (nicotine,1x10-8 M) or ISO-1
(1x10-10, 1x10-8, 1x10-6 or
1x10-4 M). A total of six fields/well were examined
using Nikon Eclipse TE200 microscope (Nikon Corporation) and
results were analyzed using the Angiogenesis Analyzer for ImageJ
(25). Number of branches/field and
total length of tubular structures were calculated relative to the
control.
Statistical analysis
The data analysis was performed using SigmaPlot
version 11.0 Build 11.0.0.77(26).
All data are presented as the mean ± SEM of ≥3 independent
experiments. Comparisons between groups were performed using
unpaired or paired Student's t-test or one-way ANOVA followed by
Holm-Sidak post hoc test as appropriate. P<0.05 was considered
to indicate a statistically significant difference.
Results
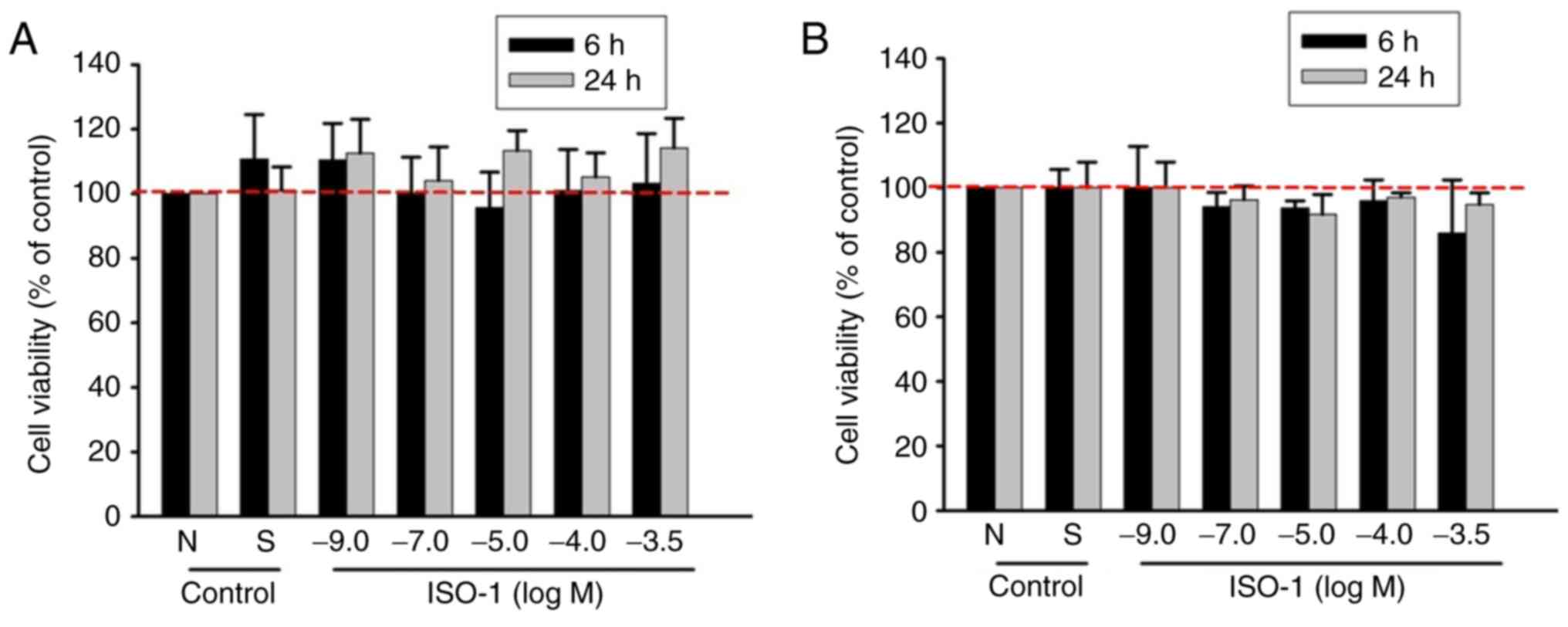
ISO-1 does not exert cytotoxic effects
on ECs
Before evaluating the biological effects of a
synthetic molecule, it is necessary to assess its potential toxic
effects. Viability of HUVECs exposed to increasing concentrations
of ISO-1 (1x10-9-10-3.5 M) was assessed by
measuring lysosomal activity through the neutral red uptake assay
and mitochondrial activity using the MTS assay at 6 and 24 h. The
highest concentration of ISO-1 tested (1x10-3.5 M)
corresponded to the solubility limit of ISO-1 in culture medium
without exceeding non-toxic DMSO concentration, while the lowest
concentration tested (1x10-9 M) was selected due to the
absence of cytotoxic effects at higher concentrations. The
viability of ECs treated with ISO-1 was similar to that of the
negative control (M199 supplemented with 5% FBS), indicating that
ISO-1 did not affect cell viability (Fig. 1). As DMSO was used to dissolve
ISO-1, a solvent control containing the maximum DMSO concentration
(0.3% DMSO) was included; the solvent control did not show any
differences compared with medium control conditions (Fig. 1A and B).
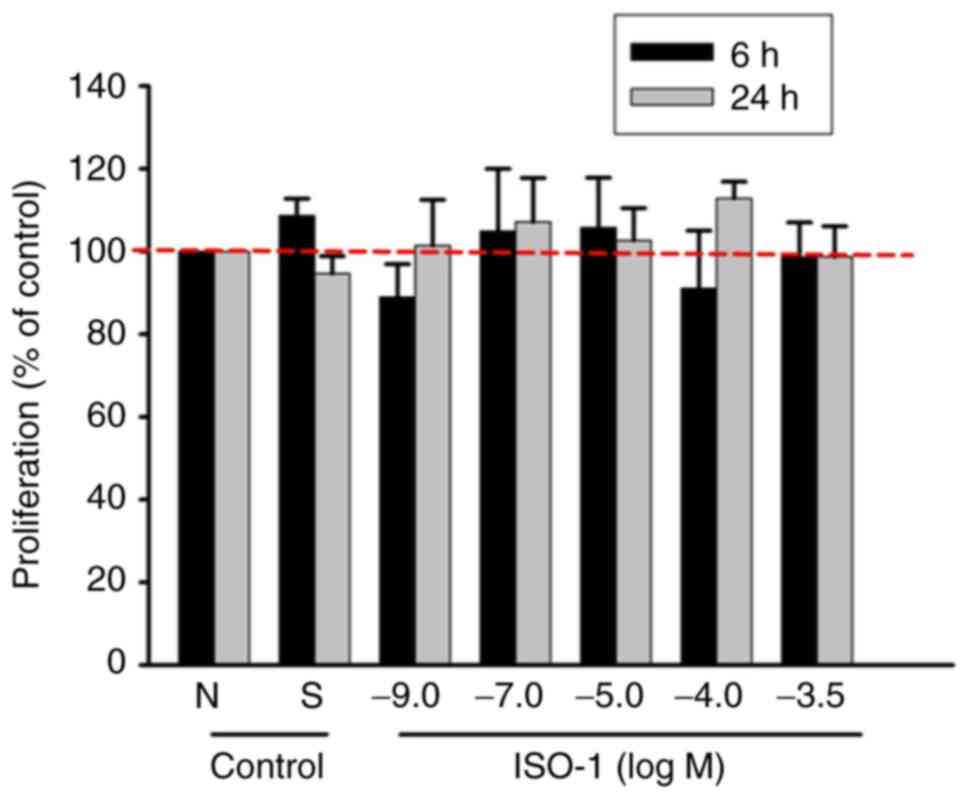
ISO-1 does not induce EC
proliferation
EC proliferation, which is key for the formation of
new blood vessels, was evaluated using the SRB assay. ECs exposed
to ISO-1 at concentrations ranging from 1x10-9 to
1x10-3.5 M. ISO-1 showed no significant differences in
proliferation compared with negative or solvent control (Fig. 2). These results indicate that ISO-1
does not promote EC proliferation under these conditions.
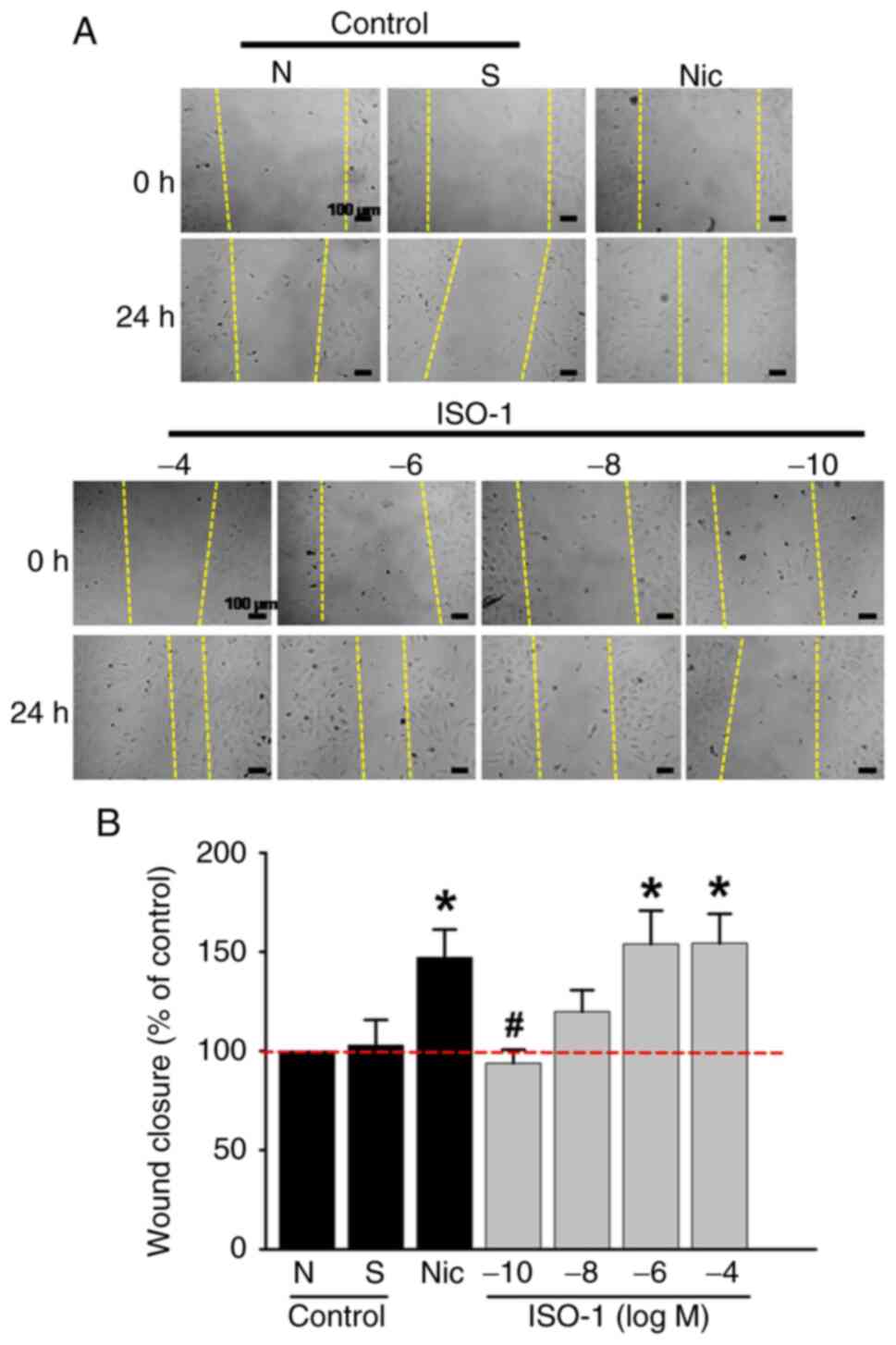
ISO-1-induced endothelial migration
mirrors effects of Nicotine
To investigate the effects of ISO-1 on cell
migration, ECs were treated with ISO-1 at concentrations ranging
from 1x10-10 to 10-4 M. This range was
selected based on previous findings from our group, which
demonstrated that ISO-1 at concentrations of 10-5 and
10-7 M increases intracellular calcium levels
[Ca2+]i via activation of α7 nAChR) (15). Additionally, this concentration
range falls within the non-cytotoxic window established in the
present study (10-9 to 10-³.5 M).
A wound was introduced into the EC monolayer to assess migration.
The results demonstrated a significant increase in EC migration
when cells were treated with ISO-1 at 1x10-6 M and
1x10-4 M compared with the negative control. The effects
of ISO-1 at these concentrations were similar to those observed
with nicotine, a nAChR agonist, at 1x10-8 M (Fig. 3A and B).
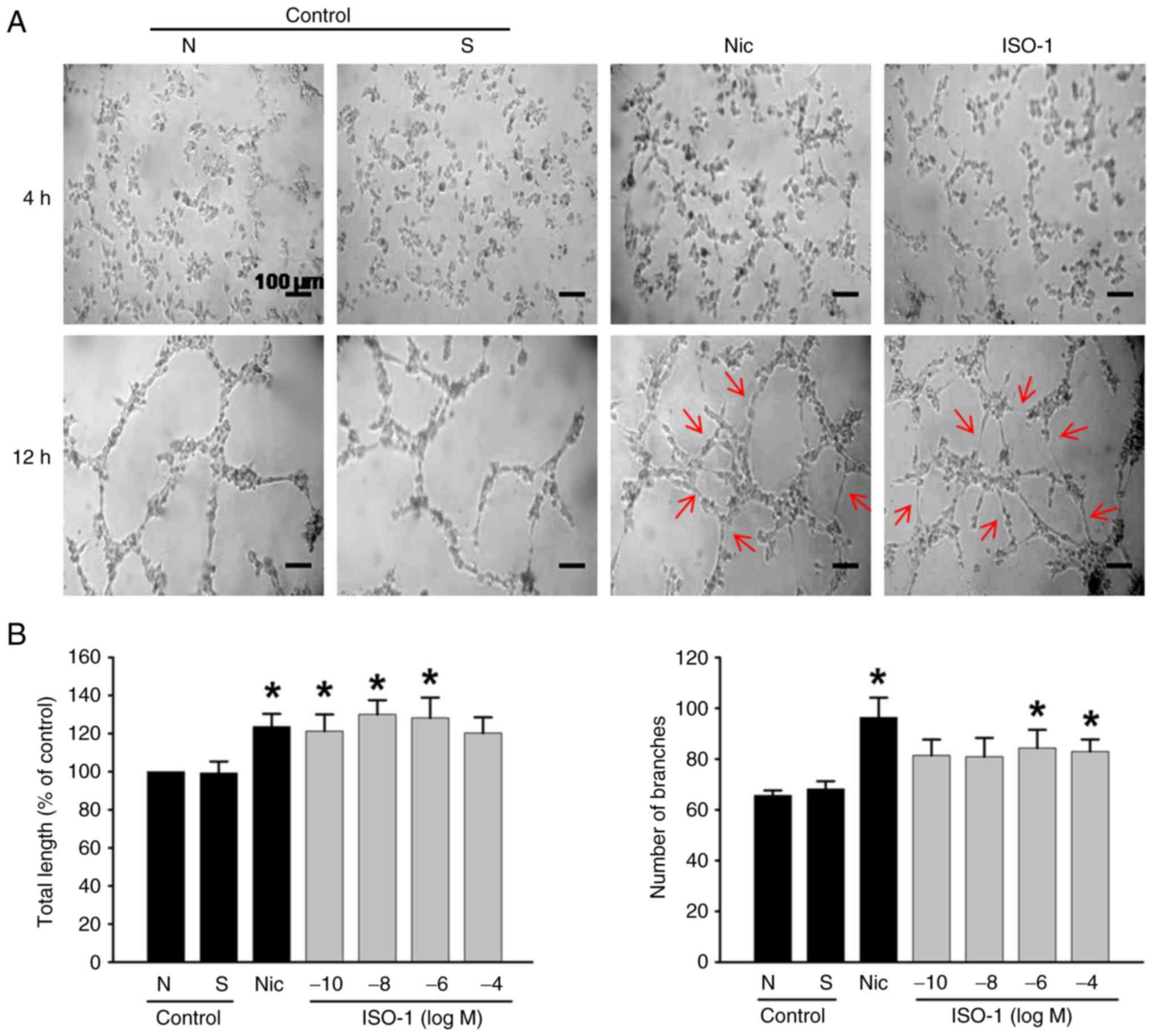
ISO-1 promotes tubular network
formation in ECs
Since new blood vessel formation involves the
formation of tubular structures, this was evaluated using the
Cultrex tube formation assay at 4 and 12 h. Compared with the
negative control, treatment with ISO-1 at concentrations ranging
from 1x10-10 to 1x10-6 M led to an increase
in the total length of the tubular structures (Fig. 4B). Additionally, ISO-1 at
1x10-6 and 1x10-4 M resulted in a significant
increase in the number of branches within these structures
(Fig. 4A and B). Furthermore, no significant differences
were observed between ISO-1 at these concentrations and nicotine
group.
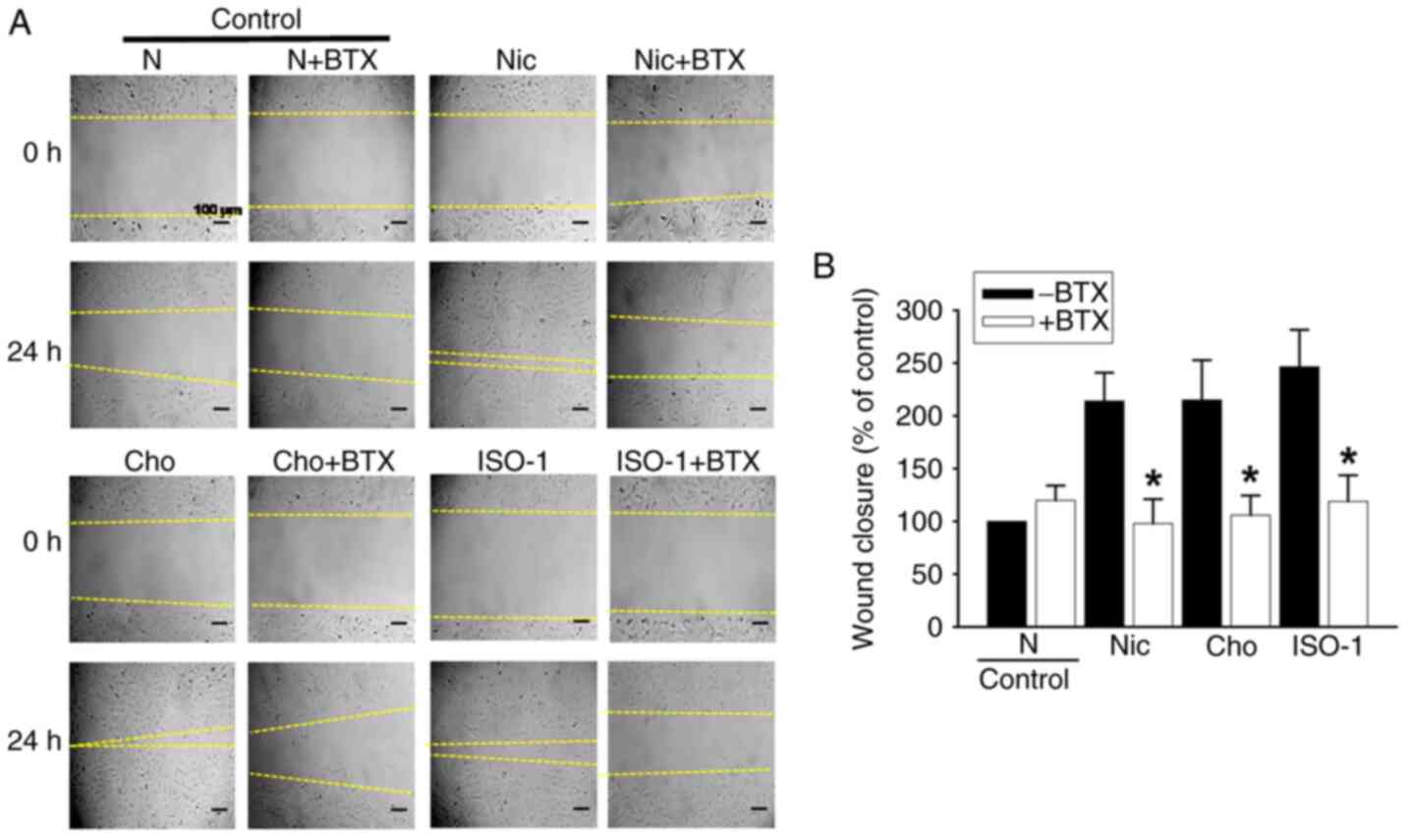
ISO-1 promotes EC migration through
α7-nAChR signaling
To investigate the role of α7-nAChR signaling in the
proangiogenic effects of ISO-1, HUVEC migration was assessed using
the α7-nAChR selective antagonist, BTX. ISO-1-induced increase in
EC migration was completely abolished by BTX. This effect was
similar to the inhibition observed with choline, suggesting that
activation of the α7-nAChR pathway was involved in the
pro-angiogenic response to ISO-1 (Fig.
5A and B).
Discussion
The present study demonstrated that the organic
synthetic molecule ISO-1, featuring an isoxazole core, effectively
promoted two key stages of angiogenesis, migration and tubular
structure formation in HUVECs. These effects were mediated by the
activation of α7-nAChRs, as confirmed using the selective α7-nAChR
antagonist BTX.
A key requirement for nAChR agonists is the presence
of a cationic nitrogen and hydrogen bond acceptor group (27). The orthosteric binding site of the
α7-nAChRs is formed by a series Cys loops on the principal face of
a subunit; key amino acid residues involved in ligand binding
include Tyr93, Lys143, Trp147, Tyr188, Cys189, Cys190 and Tyr195.
On the complementary face, Trp55, Leu118 and Met114 are the primary
residues contributing to ligand interaction (28). The binding of agonists to the
orthosteric site of the receptor primarily involves cation-π
interactions between the aromatic amino acids of the receptor and
the cationic nitrogen of the agonist, along with the formation of
hydrogen bonds with the hydroxyl group of Tyr residues, the indole
group of Trp residues and the amino group of Lys residues (29).
Nitrogen present in the structure of ISO-1 is able
to form cation-π bonds. Although hydrophobic interactions are not
essential for the interaction of the agonist with the orthosteric
site of α7-nAChR, they stabilize the ligand-receptor binding
(30). The ISO-1 molecule, due to
the presence of a phenyl substituent on the isoxazole ring, may
form these types of interactions. The optimal distance between the
cationic nitrogen and the hydrogen bond acceptor is 3.7-3.8 Å,
usually comprising 2-3 carbon atoms (31). These structural features of ISO-1
may not only enhance ligand affinity by lowering the dissociation
constant, but also improve the stability and efficiency of receptor
binding.
The inhibition of ISO-1 activity by BTX could
provide some insights into potential interactions with the receptor
orthosteric site. BTX forms cation-π interactions with Tyr188 and
hydrogen bond interactions with Tyr89, Trp147 and Tyr188 in the
principal site, as well as hydrogen bonds with Tyr53 in the
complementary site (28). This
underscores the importance of these amino acid residues in
ligand-receptor binding and suggests they may be relevant in the
binding of ISO-1 molecules.
The greatest effect on the migration of ISO-1
(1x10-6 M) was observed at lower concentrations compared
with choline (1x10-5 M) (4). This suggests ISO-1 could represent a
better alternative to choline to induce angiogenesis. ISO-1 does
not present cytotoxic effects in the range of 1x10-9 to
1x10-4 M. Therefore, these compounds may serve as
potential pharmacological candidates in angiogenesis.
On the other hand, our previous study demonstrated
that ISO-1 induces a dose-dependent increase in cytosolic calcium,
mediated by the activation of α7-nAChRs (15). Several studies have described
distinct Ca²+ signaling dynamics in ECs, which are
associated with specific functional responses in angiogenesis
(32-35).
Low VEGF concentrations (1-5 ng/ml) elicit rapid and repetitive
increases in [Ca²+]i, associated with EC proliferation,
while high VEGF concentrations (10-50 ng/ml) induce slow but
sustained increases in [Ca²+]i, linked to EC migration
(35). This suggests that in the
response to ISO-1 there could be different concentration-dependent
functional responses, potentially due to different Ca2+
dynamics in response to different concentrations of the compounds,
however future studies are required to determine this.
α7-nAChRs are characterized by rapid desensitization
in response to agonists (36);
therefore, their activation may give rise to transient increases in
[Ca2+]i. The sustained increase in
Ca2+ required for EC migration may be associated with
the VEGF pathway in angiogenesis (37). Stimulation with VEGF produces
sustained increases in endothelial [Ca2+]i
(38). Thus, activation of
α7-nAChRs may stimulate the VEGF pathway leading to the sustained
increase in [Ca2+]i necessary for EC
migration and tubular formation.
To the best of our knowledge, the present study is
the first to demonstrate the involvement of ISO-1in promoting
angiogenesis in vitro. ISO-1 stimulates HUVEC migration and
tubular structure formation through activation of α7-nAChR. The
therapeutic potential of ISO-1 as a pro-angiogenic agent represents
a promising direction for the development of treatments for
conditions associated with impaired angiogenesis, such as
cardiovascular disease.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the University of
Valparaiso (grant nos. UVA1402, PMI UVA 1315, Puente UVA 22991 and
ImpulsaTInES100).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HE performed the experiments and analyzed the data.
GV synthesized the ISO-1. MC directed and supervised the study,
designed the experimental protocols and contributed to data
analysis. ML analyzed and interpreted data. ML, HE and MC confirm
the authenticity of all the raw data. All authors wrote and edited
the manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Collection of umbilical cords from full-term normal
pregnancies from Gynecological-Obstetric Unit of the Carlos Van
Buren Hospital (Valparaiso, Chile, was performed with written
informed consent of the mothers. Isolation of human vein
endothelial cells and experimental protocols were approved by the
Ethics Committee of the Faculty of Pharmacy of Universidad de
Valparaiso, Valparaiso, Chile (approval no. 19/2015) and the Ethics
Committee of the Valparaiso-San Antonio Health Service, Valparaiso,
Chile (approval no. 1435).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dudley AC and Griffioen AW: Pathological
angiogenesis: Mechanisms and therapeutic strategies. Angiogenesis.
26:313–347. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li J, Zhao Y and Zhu W: Targeting
angiogenesis in myocardial infarction: Novel therapeutics (review).
Exp Ther Med. 23(64)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Heeschen C, Weis M, Aicher A, Dimmeler S
and Cooke JP: A novel angiogenic pathway mediated by non-neuronal
nicotinic acetylcholine receptors. J Clin Invest. 110:527–536.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Li X and Wang H: Non-neuronal nicotinic
alpha 7 receptor, a new endothelial target for revascularization.
Life Sci. 78:1863–1870. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu JC, Chruscinski A, De Jesus Perez VA,
Singh H, Pitsiouni M, Rabinovitch M, Utz PJ and Cooke JP:
Cholinergic modulation of angiogenesis: Role of the 7 nicotinic
acetylcholine receptor. J Cell Biochem. 108:433–446.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Espinoza H and Figueroa XF: Opening of
Cx43-formed hemichannels mediates the Ca2+ signaling
associated with endothelial cell migration. Biol Direct.
18(52)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Graupera M, Guillermet-Guibert J, Foukas
LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J,
Cutillas PR, et al: Angiogenesis selectively requires the p110alpha
isoform of PI3K to control endothelial cell migration. Nature.
453:662–666. 2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cain RJ, Vanhaesebroeck B and Ridley AJ:
The PI3K p110alpha isoform regulates endothelial adherens junctions
via Pyk2 and Rac1. J Cell Biol. 188:863–876. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Matera C, Papotto C, Dallanoce C and De
Amici M: Advances in small molecule selective ligands for
heteromeric nicotinic acetylcholine receptors. Pharmacol Res.
194(106813)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gill JK, Chatzidaki A, Ursu D, Sher E and
Millar NS: Contrasting properties of α7-selective orthosteric and
allosteric agonists examined on native nicotinic acetylcholine
receptors. PLoS One. 8(e55047)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Papke RL and Horenstein NA: Therapeutic
targeting of α7 nicotinic acetylcholine receptors. Pharmacol Rev.
73:1118–1149. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Briggs CA, Mckenna DG and Piattina-kaplan
M: Human alpha 7 nicotinic acetylcholine receptor responses to
novel ligands. Neuropharmacology. 34:583–590. 1995.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Grønlien JH, Håkerud M, Ween H,
Thorin-Hagene K, Briggs CA, Gopalakrishnan M and Malysz J: Distinct
profiles of alpha7 nAChR positive allosteric modulation revealed by
structurally diverse chemotypes. Mol Pharmacol. 72:715–724.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hurst RS, Hajós M, Raggenbass M, Wall TM,
Higdon NR, Lawson JA, Rutherford-Root KL, Berkenpas MB, Hoffmann
WE, Piotrowski DW, et al: A novel positive allosteric modulator of
the alpha7 neuronal nicotinic acetylcholine receptor: In vitro and
in vivo characterization. J Neurosci. 25:4396–4405. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cortés M, Alvarez R, Sepúlveda E,
Jiménez-Aspee F, Astudillo L, Vallejos G and Gutiérrez M: A new
isoxazolic compound acts as alpha7 nicotinic receptor agonist in
human umbilical vein endothelial cells. Z Naturforsch C J Biosci.
69:291–299. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yuan W and Wang X: Propranolol
participates in the treatment of infantile hemangioma by inhibiting
HUVECs proliferation, migration, invasion, and tube formation.
Biomed Res Int. 2021(6636891)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Y, Xu Q, Shi M, Gan P, Huang Q, Wang A,
Tan G, Fang Y and Liao H: Low-level laser therapy induces human
umbilical vascular endothelial cell proliferation, migration and
tube formation through activating the PI3K/Akt signaling pathway.
Microvasc Res. 129(103959)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cho YR, Park K, Kang JS, Byun HW, Oh JS,
Seo DW and Ahn EK: Trigonostemon reidioides modulates endothelial
cell proliferation, migration and tube formation via downregulation
of the Akt signaling pathway. Oncol Lett. 14:4677–4683.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jaffe EA, Nachman RL, Becker CG and Minick
CR: Culture of human endothelial cells derived from umbilical
veins. Identification by morphologic and immunologic criteria. J
Clin Invest. 52:2745–2756. 1973.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Repetto G, del Peso A and Zurita JL:
Neutral red uptake assay for the estimation of cell
viability/cytotoxicity. Nat Protoc. 3:1125–1131. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Suarez-Arnedo A, Figueroa FT, Clavijo C,
Arbeláez P, Cruz JC and Muñoz-Camargo C: An image J plugin for the
high throughput image analysis of in vitro scratch wound healing
assays. PLoS One. 15(e0232565)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Arnaoutova I and Kleinman HK: In vitro
angiogenesis: Endothelial cell tube formation on gelled basement
membrane extract. Nat Protoc. 5:628–635. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Carpentier G, Martinelli M, Courty J and
Cascone I: Angiogenesis analyzer for ImageJ. ImageJ Plugin, 2012.
Available from: http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ.
|
|
26
|
Systat Software Inc: SigmaPlot (Version
11.0, Build 11.0.0.77). Systat Software, Inc., Chicago IL, 2008.
Available from: https://systatsoftware.com/products/sigmaplot/.
|
|
27
|
Li Z, Chan K, Nickels J and Cheng X:
Electrostatic contributions to the binding free energy of nicotine
to the acetylcholine binding protein. J Phys Chem B. 126:8669–8679.
2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Huang S, Li SX, Bren N, Cheng K, Gomoto R,
Chen L and Sine SM: Complex between α-bungarotoxin and an α7
nicotinic receptor ligand-binding domain chimaera. Biochem J.
454:303–310. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Blum AP, Lester HA and Dougherty DA:
Nicotinic pharmacophore: The pyridine N of nicotine and carbonyl of
acetylcholine hydrogen bond across a subunit interface to a
backbone NH. Proc Natl Acad Sci USA. 107:13206–13211.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Peng W and Ding F: Biomolecular
recognition of antagonists by α7 nicotinic acetylcholine receptor:
Antagonistic mechanism and structure-activity relationships
studies. Eur J Pharm Sci. 76:119–132. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Arunrungvichian K, Boonyarat C, Fokin VV,
Taylor P and Vajragupta O: Cognitive improvements in a mouse model
with substituted 1,2,3-triazole agonists for nicotinic
acetylcholine receptors. ACS Chem Neurosci. 6:1331–1340.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Moccia F, Negri S, Shekha M, Faris P and
Guerra G: Endothelial Ca2+ signaling, angiogenesis and
vasculogenesis: Just what it takes to make a blood vessel. Int J
Mol Sci. 20(3962)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Moccia F, Berra-Romani R and Tanzi F:
Update on vascular endothelial Ca(2+) signalling: A tale of ion
channels, pumps and transporters. World J Biol Chem. 3:127–158.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Debir B, Meaney C, Kohandel M and Unlu MB:
The role of calcium oscillations in the phenotype selection in
endothelial cells. Sci Rep. 11(23781)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Noren DP, Chou WH, Lee SH, Qutub AA,
Warmflash A, Wagner DS, Popel AS and Levchenko A: Endothelial cells
decode VEGF-mediated Ca2+ signaling patterns to produce distinct
functional responses. Sci Signal. 9(ra20)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao L, Kuo YP, George AA, Peng JH,
Purandare MS, Schroeder KM, Lukas RJ and Wu J: Functional
properties of homomeric, human alpha 7-nicotinic acetylcholine
receptors heterologously expressed in the SH-EP1 human epithelial
cell line. J Pharmacol Exp Ther. 305:1132–1141. 2003.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ng MKC, Wu J, Chang E, Wang BY,
Katzenberg-Clark R, Ishii-Watabe A and Cooke JP: A central role for
nicotinic cholinergic regulation of growth factor-induced
endothelial cell migration. Arterioscler Thromb Vasc Biol.
27:106–112. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Dawson NS, Zawieja DC, Wu MH and Granger
HJ: Signaling pathways mediating VEGF165-induced calcium transients
and membrane depolarization in human endothelial cells. FASEB J.
20:991–993. 2006.PubMed/NCBI View Article : Google Scholar
|