1. Introduction
Chronic obstructive pulmonary disease (COPD) is
defined by the Global Initiative for Obstructive Lung Disease
(GOLD) guidelines as a common preventable and treatable disease
characterized by persistent respiratory symptoms and airflow
limitation due to airway and/or alveolar abnormalities usually
caused by significant exposure to noxious particles or gases
(1). According to a recent
meta-analysis, the pooled prevalence of COPD is 15.47% in male and
8.79% in female patients (2).
Bronchial asthma (BA) is defined by the Global
Initiative for Asthma as a heterogeneous disease usually
characterized by chronic airway inflammation, defined by the
history of respiratory symptoms such as wheezing, shortness of
breath, chest tightness and cough that vary in duration and in
intensity, together with variable expiratory airflow limitation
(3). According to the World Health
Organization, in 2019 asthma affected ~262 million people worldwide
and caused ~461,000 deaths (4).
COPD and BA are highly prevalent, associated with significant
morbidity and socio-economic impact and often overlap, complicating
their clinical management (5).
Although a variety of treatment options exist for
COPD and BA, there remains a pressing need for novel therapies
capable of effectively suppressing chronic inflammation in the
lungs (6,7). Theophylline, a purine-derived
methylxanthine, exhibits smooth muscle relaxant and bronchodilator
effects. It has been used in the management of BA and COPD,
although its use is limited by a narrow therapeutic window and
multiple drug-drug interactions, as reflected in current Global
Initiative for Asthma and GOLD strategies (1,3).
An alternative to theophylline may be doxofylline,
which is characterized by an improved tolerability profile and
fewer notable drug-drug interactions. Doxofylline also exhibits
distinct pharmacological properties compared with theophylline,
characterized by improved safety, reduced central nervous system
(CNS) stimulation, minimal cardiac effects and few drug
interactions. Unlike theophylline, it does not require plasma
concentration monitoring, making it more suitable for routine
clinical use (8). Preliminary data
suggest that doxofylline may demonstrate comparable or superior
efficacy in the management of COPD and BA symptoms with better
tolerability (8,9). Although its mechanisms of action (such
as selective inhibition of phosphodiesterase (PDE), modulation of
inflammatory mediators and adenosine receptor antagonism) have been
described in previous studies, an integrated and up-to-date
overview of its clinical potential, pharmacokinetic advantages and
positioning in current therapeutic strategies is lacking (7,8). In
addition, this therapeutic agent offers an effective and affordable
alternative for patients who may face difficulties in accessing
more expensive therapy, particularly in low- and middle-income
countries where healthcare resources are limited (9).
The present review aimed to explore not only the
established role but also the emerging potential of doxofylline in
the management of COPD and BA. The present study aimed to summarize
its pharmacological properties, therapeutic margin and safety
profile, with emphasis on how these characteristics may
differentiate it from classical methylxanthines and support its
place in contemporary treatment strategies.
2. Materials and methods
A comprehensive literature search for published
clinical trials evaluating the influence of doxofylline in patients
with COPD and BA was conducted. The search terms included
‘doxofylline’ and ‘theophylline’ for the intervention, and ‘chronic
obstructive pulmonary disease’, ‘COPD’ and ‘bronchial asthma’ for
the disease. Databases searched were PubMed (from 1966 to January
2025; pubmed.ncbi.nlm.nih.gov/),
ScienceDirect (from 1997 to January 2025; sciencedirect.com/), Google Scholar (from 2004 to
January 2025; scholar.google.com/), and Web of Science (from 1900 to
January 2025; https://www.webofscience.com/).
Inclusion criteria were as follows: i) Original
clinical trials, including randomized controlled trials (RCTs), as
well as meta-analyses or systematic reviews evaluating doxofylline
in COPD and/or BA; ii) studies published in English and iii)
studies reporting at least one clinical outcome [such as lung
function, quality of life (QoL), exacerbation rate and safety].
Exclusion criteria were as follows: i) Non-clinical studies (in
vitro or animal studies without clinical correlation); ii) case
reports, editorials, letters or conference abstracts without full
data and iii) articles with insufficient data or unavailable full
text. Articles were assessed by two reviewers, with disagreements
resolved by consensus.
The search initially identified 183 records. After
removing 46 duplicates, 137 unique records were screened by title
and abstract. Of these, 71 articles were excluded for irrelevance,
leaving 66 full-text articles assessed for eligibility; 22 were
excluded (non-clinical focus, inadequate outcome or insufficient
data). Following full-text review, 44 studies met all inclusion
criteria and were included in the present review.
3. Mechanisms of action and pharmacological
properties
Chemical properties and
pharmacodynamics
Doxofylline
[7-(1,3-dioxolan-2-ylmethyl)-3,7-dihydro-1,3-dimethyl-1H-purine-2,6-dione]
is a xanthine derivative introduced in 1987. Preclinical studies
suggest that it modulates cAMP-associated pathways, with limited
affinity for adenosine receptors and a less defined role in PDE
inhibition compared with theophylline (10,11).
Structurally, it differs from theophylline by the presence of a
dioxalane group at position 7 (Fig.
1), which contributes to its improved tolerability and reduced
drug-drug interactions (12,13).
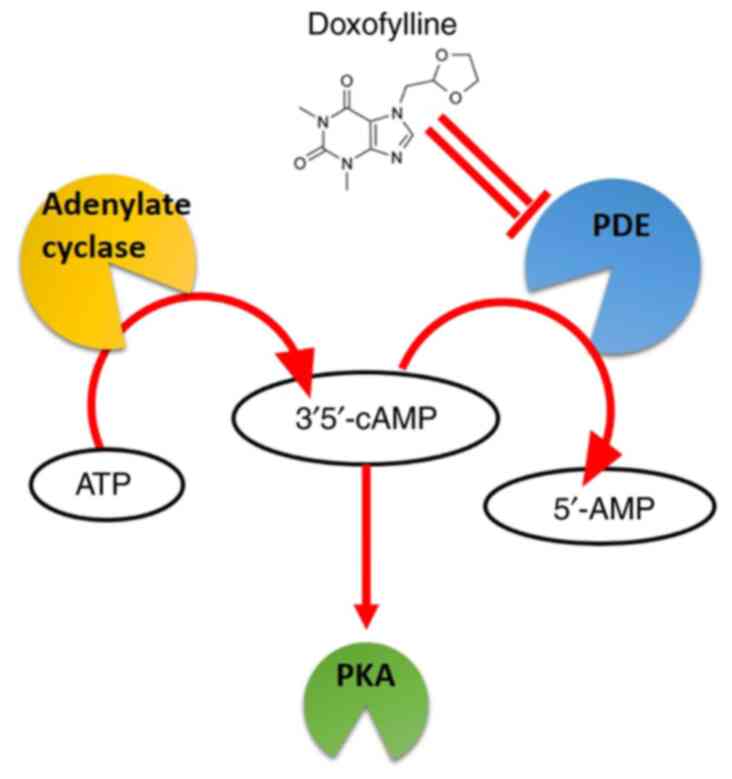
PDE2A and cyclic (c)GMP-stimulated
inhibition
PDE enzymes are key to cell signaling, hydrolyzing
cyclic nucleotides such as cAMP and cGMP. PDE2A hydrolyzes both
cAMP and cGMP, and its activity is further stimulated by cGMP,
leading to preferential hydrolysis of cAMP under conditions of
elevated cGMP (14).
Doxofylline may interfere with PDE activity
(including PDE2A1; Fig. 2),
potentially contributing to its bronchodilator and
anti-inflammatory effects (10).
However, other investigations did not confirm this mechanism,
indicating that the role of PDE inhibition remains uncertain and
requires further clarification (11,15).
In contrast, theophylline exhibits a broader inhibitory profile,
affecting PDE2A1, PDE3A and PDE10A1, which may contribute to its
wider range of side effects, including nausea, vomiting,
gastroesophageal reflux, headache, insomnia, tremor and potentially
severe cardiac arrhythmia (11,16).
These targeted actions enhance its efficacy and safety profile in
managing respiratory disease.
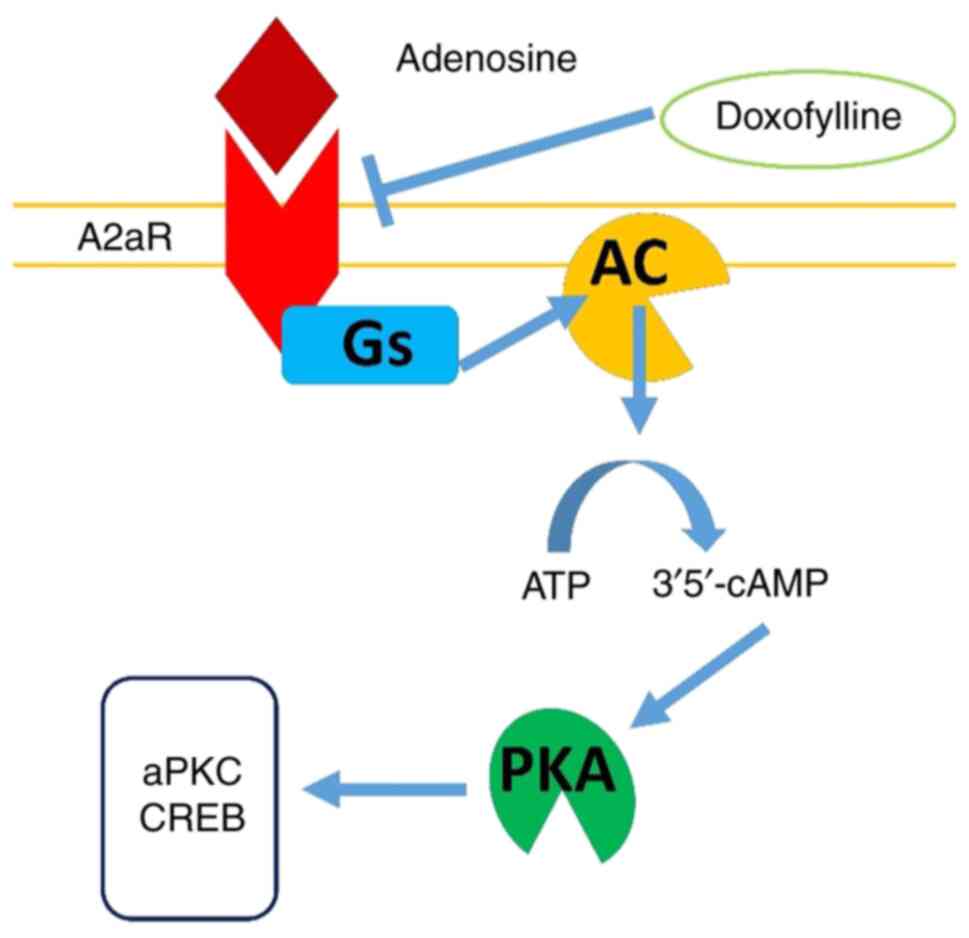
Adenosine receptor A2A
antagonism
In the respiratory system, adenosine mediates
bronchoconstriction and inflammatory responses through receptor
activation. Theophylline serves as a non-selective antagonist of
adenosine receptors, including the A2A subtype,
producing anti-inflammatory and bronchodilator effects (7). However, this non-selective antagonism
is associated with adverse effects, such as CNS stimulation,
cardiac arrhythmias (via A1 receptor blockade), gastric
hypersecretion, gastroesophageal reflux and diuresis. Additionally,
paradoxical inhibition of adenosine A2A receptor
signaling may exacerbate inflammation (17).
Unlike theophylline, doxofylline demonstrates a low
affinity for adenosine A1 and A2 receptors
and does not antagonize calcium channel blockers, which may explain
its decreased cardiac adverse effects (17). Selective inhibition of PDE, along
with its unique chemical structure featuring the dioxalane group,
may contribute to its improved safety profile. Studies indicate
that its effect on the A2A receptor decreases airway
inflammation and promotes bronchial relaxation, thereby supporting
its efficacy in treating BA and COPD (10,18)
(Fig. 3).
Interaction with
β2-adrenergic pathways
β2-adrenergic receptor agonists are
widely used as bronchodilators in BA and COPD due to their role in
G-protein-mediated smooth muscle relaxation in the bronchial tree,
resulting in bronchodilation (19,20).
While doxofylline does not act as a direct β2-agonist,
it may enhance bronchodilation by potentiating the effects of
β2-agonists via increased intracellular cAMP
availability (10).
Theophylline enhances bronchodilation by
potentiating the effects of β2-agonists, increasing
intracellular cAMP levels. This synergistic effect is beneficial in
combination therapies for patients with severe BA or COPD. Inhaled
bronchodilators, including β2-agonists and
antimuscarinics, are critical for managing COPD at all stages and
are essential for BA treatment (21).
Doxofylline also enhances the bronchodilator effects
of β2-agonists. Studies highlight its lower side-effect
profile compared with theophylline, positioning it as a safer
option for long-term management (13,18).
Doxofylline, in conjunction with β2-agonists, achieves
effective bronchodilation without the notable adverse effects
associated with high-dose theophylline (10).
Histone deacetylase (HDAC)
inhibition
HDAC enzymes regulate gene transcription by
remodeling chromatin structure. Decreased HDAC2 activity is
associated with corticosteroid resistance in COPD and BA,
underscoring the clinical relevance of this pathway (22,23).
Low-dose theophylline has been reported to restore
HDAC2 activity, thereby enhancing the transcriptional suppression
of pro-inflammatory genes and improving corticosteroid
responsiveness (7).
Unlike theophylline, the role of doxofylline in HDAC
pathways remains uncertain; its improved safety profile is
attributed to the presence of the dioxalane group, which reduces
interaction with PDEs and adenosine receptors while maintaining
anti-inflammatory activity (10,24).
Protein kinase C (PKC) inhibition
PKC is a family of enzymes that serves an essential
role in cell processes, including proliferation, differentiation
and inflammation. PKC inhibitors interfere with PKC activity,
modulating these processes and offering therapeutic potential in
inflammatory disease (25).
Theophylline has demonstrated anti-inflammatory effects through PKC
inhibition. By modulating inflammatory pathways, it decreases
airway inflammation and improves respiratory function in patients
with BA and COPD (7).
Doxofylline also exhibits PKC inhibitory properties.
PKC inhibition potentiates its anti-inflammatory effects, making it
a more specific and safer option compared with theophylline for the
long-term management of chronic respiratory disease (18,24).
Mechanisms of anti-inflammatory
effects
Doxofylline significantly decreases inflammatory
response by blocking several inflammatory pathways, such as
lipopolysaccharides (LPS)-induced thioredoxin-interacting
protein/NOD-like receptor protein 3 (NLRP3) inflammasome
activation, leading to decreased IL-1β and IL-18 release (26,27).
In addition, it has been shown to reduce the need for steroid use
in patients with bronchial inflammation by decreasing eosinophilic
and neutrophilic infiltration in lung tissue, accompanied by
inhibition of nitric oxide and prostaglandin E2 production
(24,28).
Doxofylline decreases LPS-induced lung inflammation
in mice (24) and inhibits NLRP3
inflammasome activation in human bronchial epithelial cells
(26). Furthermore, rat models
confirm that doxofylline may attenuate leukocyte adhesion to the
vessel wall and migration of vascular endothelial cells via
inhibition of IL-6 and tumor necrosis factor-α release,
highlighting its potential role in managing inflammatory
respiratory conditions (10,17).
Pharmacological and pharmacokinetic
properties of doxofylline
The pharmacological and pharmacokinetic profiles of
doxofylline and theophylline reveal notable differences in their
mechanisms of action, indications and administration (Table I).
 | Table IPharmacological properties of
doxofylline and theophylline. |
Table I
Pharmacological properties of
doxofylline and theophylline.
| Property | Doxofylline | Theophylline |
|---|
| Mechanism of
action | Selective
inhibition of PDE2A1 activity; increases intracellular cAMP by
decreasing its breakdown, promoting bronchodilation and
anti-inflammatory effects | Non-selective PDE
inhibition, adenosine receptor antagonism |
| Indication | Bronchodilator used
in asthma and chronic obstructive pulmonary disease | Asthma, COPD,
chronic bronchitis |
| Dosage and
administration | 400 mg p.o.,
immediate-release tablet, 2-3 times daily | 300-600 mg daily,
administered in 2-3 doses |
| Absorption
bioavailability | 90-100% | 62-96% |
| Therapeutic drug
concentration for chronic bronchitis | 8-20 µg/ml | 5-15 µg/ml |
| Time to peak
concentration | 1.5-2 h | 1-2 h |
| Steady state within
6 h | 9.43 µg/ml | 5-15 µg/ml |
| AUC | 69.5 µg h/ml | 87 µg h/ml |
| Protein
binding | 48% | 40-60% |
| Distribution
half-life | 1.5 h | 8.7 h |
| Volume of
distribution | 0.81 l/kg | 0.3-0.7 l/kg |
| Metabolism sites
and kinetics | Liver (>90% of
the administered dose), primarily CYP3A4 | Liver, primarily
CYP1A2, CYP2E1 |
| Metabolites |
Hydroxyethyltheophylline (inactive) | 1,3-dimethyluric
acid, 1-methylxanthine |
| Renal
excretion | <4% | 10-13% |
| Total body
clearance | 5.4 l/h | 0.65 l/h/kg |
| Elimination
half-life | 6 h | 8 h |
Doxofylline achieves effective bronchodilation at a
dosage of 400 mg administered two to three times daily, whereas
theophylline requires more variable dosing, typically 300-600 mg
daily in 2-3 doses (29). In terms
of pharmacokinetics, doxofylline shows high oral bioavailability of
90-100%, compared with 62-96% for theophylline (30), has been reported to reach
therapeutic plasma concentrations of 8-20 µg/ml in chronic
bronchitis, compared with theophylline's narrower therapeutic range
of 5-15 µg/ml (31). However,
unlike theophylline, doxofylline generally does not require routine
plasma monitoring because of its more favorable pharmacokinetic
stability and wider safety margin.
The time to peak concentration for doxofylline is
1.5-2.0 h, with a distribution half-life of about 1.5 h, compared
with a longer elimination half-life for theophylline (8.7 h).
Doxofylline exhibits moderate protein binding (~48%) and a
relatively high volume of distribution (0.81 l/kg), compared with
theophylline (40-60% and 0.3-0.7 l/kg, respectively) (30). Its metabolism occurs predominantly
in the liver, with >90% of the administered dose metabolized
primarily via cytochrome P450 3A4 (CYP3A4), producing inactive
metabolites (30), whereas
theophylline is metabolized primarily by CYP1A2 and CYP2E1,
yielding active metabolites (31).
Renal excretion of doxofylline is minimal, with
<4% of the dose excreted unchanged in urine, compared with
10-13% for theophylline. Doxofylline has a total body clearance of
5.4 l/h and an elimination half-life of ~6 h, whereas theophylline
shows a clearance of ~0.65 l/h/kg (equivalent to ~2.6-3.0 l/h in
adults) and an elimination half-life of approximately 8 h, which
may be further prolonged in patients with hepatic or cardiac
impairment (30,31).
4. Doxofylline in chronic obstructive airway
disease
Doxofylline in COPD
COPD is a prevalent and debilitating respiratory
condition that imposes a notable global burden. According to the
Global Burden of Disease 2019 study, COPD was the third leading
cause of death worldwide, responsible for 3.23 million deaths and
affecting ~212 million people (32). This highlights the socio-economic
and public health challenges associated with COPD. Effective
management of COPD involves a combination of bronchodilators and
anti-inflammatory medications aimed at improving lung function and
enhancing the quality of life (QoL) in affected patients (32).
Theophylline has been a cornerstone in the treatment
of COPD and BA since its introduction in 1937. However, due to its
narrow therapeutic window and associated safety concerns, the GOLD
Management Strategy guidelines (33) recommend its use only in patients who
do not benefit from other bronchodilators or those unable to afford
alternative treatments. In this context, doxofylline, a newer
methylxanthine derivative, has emerged as a promising alternative
(1). Unlike theophylline,
doxofylline exhibits distinct pharmacological properties, including
minimal effects on PDE isotypes, negligible antagonism at adenosine
receptors and no impact on HDAC pathways. While not a direct
β2-agonist, doxofylline may potentiate
β2-adrenergic bronchodilation via increased
intracellular cAMP (10,13).
In a clinical study involving 154 patients with
COPD, doxofylline was compared with theophylline and demonstrated
improvements in baseline spirometric parameters; doxofylline was
better tolerated, with fewer side effects and lower dropout rates
due to adverse events (34). A
subset analysis of high-quality trials confirmed an improvement in
FEV1 of 239 ml (35).
Grading of Recommendations Assessment, Development, and Evaluation
analysis provided high-quality evidence for the impact of
doxofylline on FEV1 and moderate-quality evidence for
its safety profile in COPD (35).
Furthermore, an analysis of RCTs reported a significant increase in
forced expiratory volume in 1 sec (FEV1) of 8.2% and 324
ml from baseline with doxofylline (35).
Studies evaluating the effects of doxofylline in
severe COPD have demonstrated its efficacy in decreasing
respiratory symptoms such as dyspnea and cough and improving
exercise tolerance (13,36,37).
These findings underscore the rationale for using doxofylline in
the treatment of COPD, with a superior efficacy-to-safety profile
compared with theophylline (13,34,36).
The safety profile of doxofylline is a critical
factor in the management of COPD, particularly given the disease
association with significant declines in health-related (HRQoL).
Improvements in HRQoL are key indicators of treatment success.
Doxofylline is associated with HRQoL improvement (36), consistent with broader evidence on
HRQoL determinants in COPD (38).
Patients receiving 400 and 800 mg doses of doxofylline demonstrated
significant decrease in symptom scores, including cough (77.35 and
97.43%. respectively), shortness of breath (77.60 and 95.90%.
respectively) and chest tightness (86.29 and 98.40%. respectively)
following 4 weeks of treatment (37). Consistent findings were observed in
another study evaluating the combination of doxofylline with
inhaled corticosteroids and long-acting bronchodilators, which
resulted in significant improvements in QoL score and reductions in
the frequency of exacerbation (36).
Doxofylline represents a promising therapeutic
alternative for the long-term treatment of COPD. Its enhanced
efficacy and safety profile, combined with better tolerability and
improvement in lung function and QoL, position it as a safe and
effective alternative to traditional therapies for COPD (34,39).
Doxofylline in BA
The literature supports doxofylline as an effective
and safe methylxanthine for the treatment of BA, with a superior
efficacy-to-safety profile compared with theophylline (13,36).
A meta-analysis demonstrated that doxofylline is
more effective than theophylline in decreasing daily BA events and
preventing adverse reactions (13).
Similar findings were reported in other studies, which highlighted
improvements in spirometric parameters, decreased consumption of
salbutamol and fewer undesirable side effects or treatment dropouts
(13,18). In patients with BA responsive to
salbutamol, particularly those with features overlapping with COPD,
increases in spirometric values-including slow and forced vital
capacity (FVC), FEV1, forced expiratory flow at 25-75%,
and peak expiratory flow rate-have also been reported, consistent
with evidence from bronchodilator response studies in BA and
Asthma-COPD Overlap (ACO) (40).
Although a meta-analysis found no notable difference
between doxofylline and theophylline in terms of FEV1
improvement, doxofylline is superior in reducing the need for
salbutamol as a rescue medication (13). Moreover, the LESDA long-term trial
demonstrated that one year of doxofylline therapy in asthmatic
patients significantly improved FEV1, reduced daily
asthma event rates, and decreased salbutamol usage, with a
favorable safety profile (41).
Doxofylline has also proven to be a rapid and
effective bronchodilator in mechanically ventilated patients with
acute respiratory failure and airflow obstruction. It is associated
with a decrease in respiratory resistance and intrinsic positive
end-expiratory pressure, thereby improving the mechanical
efficiency of respiratory muscles at lower lung volumes (42).
A meta-analysis involving 820 patients from 20 RCTs
demonstrated that doxofylline significantly improves
FEV1, with fewer adverse events (35). Another meta-analysis examining the
efficacy and safety of xanthines in BA reported that while
doxofylline is no more effective than aminophylline or theophylline
in improving baseline FEV1, it is superior in
alleviating dyspnea and significantly safer than both aminophylline
and theophylline (13).
Drug interactions and safety
considerations
As with other methylxanthines, doxofylline is
associated with potential drug interactions. Concomitant use of
doxofylline with certain drugs such as erythromycin,
troleandomycin, lincomycin, clindamycin, allopurinol, cimetidine,
ranitidine, propranolol, is not recommended. These agents may
decrease the hepatic clearance of xanthines, leading to elevated
plasma levels of doxofylline (8,10,17).
Additionally, doxofylline should not be co-administered with other
xanthine derivatives to avoid competitive inhibition at enzymatic
metabolization sites, which may further slow drug clearance
(30).
Adverse effects associated with doxofylline are
typically mild and may include gastrointestinal disturbances such
as nausea and vomiting, as well as CNS symptoms such as headache
and dizziness. Notably, doxofylline has a reduced incidence of
cardiovascular side effects, including tachycardia and
palpitations, compared with other xanthines (30).
In terms of drug interactions, doxofylline safety
profile is favorable due to its limited interaction with CYP450
enzymes, reducing the likelihood of significant interactions with
other medications metabolized via this pathway (8,10,17,30),
although caution is advised when co-administering doxofylline with
other xanthine derivatives to prevent potential additive
effects.
Overall, improved therapeutic window and reduced
adverse effect profile make doxofylline a safer alternative to
traditional methylxanthines in the management of respiratory
conditions.
Cost considerations
COPD continues to represent a significant economic
burden despite therapeutic advances, particularly in
resource-limited regions. Doxofylline is a cost-effective option
compared with other commonly used bronchodilators in the treatment
of COPD and BA (Table II). For
example, the average monthly price of doxofylline at usual doses
(400 mg twice daily) is €5-15 (1,30). In
comparison, tiotropium, a commonly used inhaled anticholinergic,
has an estimated monthly cost of €45-65, while inhaled formoterol
and budesonide-based inhaled combinations are €55-75/month
(1,3). Salmeterol, either as monotherapy or in
combination with fluticasone, incurs a monthly cost of €35-65,
depending on dosage and formulation (1,4).
 | Table IIEstimated monthly costs of therapies
used in bronchial asthma and chronic obstructive pulmonary
disease. |
Table II
Estimated monthly costs of therapies
used in bronchial asthma and chronic obstructive pulmonary
disease.
| Therapy | Estimated monthly
cost, € | Remarks | (Refs.) |
|---|
| Doxofylline (400 mg
twice daily) | 5-15 | Widely available;
generic options | (1,30) |
| Theophylline
(300-400 mg twice daily) | 5 | Narrow therapeutic
window | (1,31) |
| LABA (such as
salmeterol) | 35-65 | Inhaler; moderate
accessibility | (1,3) |
| LAMA (such as
tiotropium) | 45-65 | Inhaler; limited
access in low-income countries | (1,3) |
| ICS/LABA
combination (such as Symbicort) | 55-75 | Moderate to high
cost | (1,3) |
| Biological agents
(such as omalizumab) | 800 | For severe cases
only; hospital-based administration | (3,4) |
Despite being inexpensive (~€5/month), theophylline
is limited by its narrow therapeutic window and increased risk of
side effects, requiring close monitoring. In this context,
doxofylline offers an advantageous balance between efficacy,
superior safety profile and economic sustainability. Therefore,
compared with riskier options (such as theophylline) and costly
modern therapy, doxofylline represents a viable and affordable
therapeutic alternative.
5. Future considerations
The present review has certain limitations. First,
the data presented are mainly from studies published in English,
which may introduce a selection bias. Second, methodological
variability between the included studies in terms of design, study
population, doses administered and treatment duration limits the
possibility to draw generalizable conclusions. To the best of our
knowledge, there is a lack of recent studies on certain
pharmacological aspects of doxofylline. In addition, the lack of
recent meta-analyses and direct comparisons with modern biological
therapies decreases the ability to position doxofylline in relation
to state-of-the-art treatments. Therefore, further multicenter and
independent research is needed to validate the conclusions.
Future research on doxofylline should focus on
long-term safety and efficacy studies across diverse populations,
including pediatric and elderly patients, to ensure its broad
applicability. Comparative studies with newer bronchodilators and
anti-inflammatory agents are needed to define its role in
combination therapy, particularly in severe and overlapping
respiratory conditions. Exploring pharmacogenomic factors,
biomarkers of treatment response and its potential in
non-respiratory indications may provide insights into personalized
medicine. Additionally, economic analyses and cost-effectiveness
studies, especially in low- and middle-income countries, may
support its global adoption. Further mechanistic research into
doxofylline pathways, such as selective PDE inhibition and HDAC
activity, may unlock novel therapeutic potential.
6. Conclusion
Doxofylline represents a notable advancement in the
treatment of chronic respiratory diseases such as COPD and BA. By
contrast with theophylline, whose use has diminished due to its
narrow therapeutic window and associated side effects, doxofylline
offers a more favorable safety profile and wider therapeutic margin
(8,10). Meta-analyses and clinical studies
have demonstrated that doxofylline not only enhances spirometric
parameters and decreases the need for salbutamol administration but
also results in fewer adverse effects, thereby improving patient
tolerance and compliance (13,36,38).
Clinical evidence highlights significant improvements in lung
function, exercise capacity and QoL among patients treated with
doxofylline (17). Its ability to
improve airway function and exert anti-inflammatory effects,
coupled with its rapid bronchodilator activity in acute situations,
underscores its potential as a therapeutic alternative (9,14).
Moreover, doxofylline decreases glucocorticoid dependence and
promotes long-term inflammation control, with a low incidence of
adverse reactions, making it a safer option for clinical use
(10,36). The present review may help clarify
the clinical role of xanthine derivatives in COPD, particularly
regarding the safety concerns and narrow therapeutic window of
theophylline (10,13,38).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SC, ACe and ACo conceived the study. SC, DP, AB,
ACe, ACo, VEG and DAS performed the literature review and wrote the
manuscript SC and ACo revised the manuscript. Data authentication
is not applicable. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tool Chat
GPT was used to improve the readability and language of the
manuscript, and subsequently, the authors revised and edited the
content produced by the AI tool as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Singh D, Agusti A, Anzueto A, Barnes PJ,
Bourbeau J, Celli BR, Criner GJ, Frith P, Halpin DMG, Han M, et al:
Global strategy for the diagnosis, management, and prevention of
chronic obstructive lung disease: The GOLD science committee report
2019. Eur Respir J. 53(100164)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al Wachami N, Guennouni M, Iderdar Y,
Boumendil K, Arraji M, Mourajid Y, Bouchachi FZ, Barkaoui M,
Louerdi ML, Hilali A and Chahboune M: Estimating the global
prevalence of chronic obstructive pulmonary disease (COPD): A
systematic review and meta-analysis. BMC Public Health.
24(297)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Global Initiative for Asthma (GINA):
Global Strategy for Asthma Management and Prevention; GINA,
Fontana, WI, 2024. https://ginasthma.org/. Accessed January 10, 2025.
|
|
4
|
World Health Organization (WHO): Asthma.
WHO, Geneva, 2023. https://www.who.int/news-room/fact-sheets/detail/asthma.
Accessed September 8, 2025.
|
|
5
|
Mart MF and Peebles RS Jr: Asthma-chronic
obstructive pulmonary disease overlap syndrome. Curr Opin Immunol.
66:161–166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Barnes PJ: New therapies for asthma: Is
there any progress? Trends Pharmacol Sci. 31:335–343.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Aprile S, Colombo G, Serafini M, di Paola
R, Pisati F, Bhela IP, Cuzzocrea S, Grosa G and Pirali T: An
unexpected deuterium-induced metabolic switch in doxofylline. ACS
Med Chem Lett. 13:1278–1285. 2022.
|
|
8
|
Page CP: Doxofylline: A ‘novofylline’.
Pulm Pharmacol Ther. 23:231–234. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cazzola M, Page CP, Calzetta L, Rogliani P
and Matera MG: Doxofylline: Advancing and empowering equitable
asthma and COPD management beyond tradition. Adv Therap.
7(2400103)2024.
|
|
10
|
Matera MG, Page C and Cazzola M:
Doxofylline is not just another theophylline! Int J Chron Obstruct
Pulmon. Dis. 12:3487–3493. 2017.
|
|
11
|
van Mastbergen J, Jolas T, Allegra L and
Page CP: The mechanism of action of doxofylline is unrelated to
HDAC inhibition, PDE inhibition or adenosine receptor antagonism.
Pulm Pharmacol Ther. 25:55–61. 2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Singh N, Shreshtha AK, Thakur MS and Patra
S: Xanthine scaffold: Scope and potential in drug development.
Heliyon. 4(e00829)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rogliani P, Calzetta L, Ora J, Cazzola M
and Matera MG: Efficacy and safety profile of doxofylline compared
to theophylline in asthma: A meta-analysis. Multidiscip Respir Med.
14(25)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bender AT and Beavo JA: Cyclic nucleotide
phosphodiesterases: Molecular regulation to clinical use. Pharmacol
Rev. 58:488–520. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Surapisitchat J, Jeon KI, Yan C and Beavo
JA: Differential regulation of endothelial cell permeability by
cGMP via phosphodiesterases 2 and 3. Circ Res. 101:811–818.
2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Billington CK, Ojo OO, Penn RB and Ito S:
cAMP regulation of airway smooth muscle function. Pulm Pharmacol
Ther. 26:112–120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shukla D, Chakraborty S, Singh S and
Mishra B: Doxofylline: A promising methylxanthine derivative for
the treatment of asthma and chronic obstructive pulmonary disease.
Expert Opin Pharmacother. 10:2343–2356. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cazzola M, Calzetta L, Barnes PJ, Criner
GJ, Martinez FJ, Papi A and Gabriella Matera M: Efficacy and safety
profile of xanthines in COPD: A network meta-analysis. Eur Respir
Rev. 27(180010)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Billington CK, Penn RB and Hall IP: β2
agonists. Handb Exp Pharmacol. 237:23–40. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Georgakopoulou VE, Gkoufa A, Garmpis N,
Garmpi A and Damaskos C: Inhaled bronchodilators and
corticosteroids in the management of bronchiolitis obliterans due
to allogenic hematopoietic stem cell transplantation. Oman Med J.
37(e388)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stolz D, Matera MG, Rogliani P, van den
Berge M, Papakonstantinou E, Gosens R, Singh D, Hanania N, Cazzola
M, Maitland-van der Zee AH, et al: Current and future developments
in the pharmacology of asthma and COPD: ERS seminar, Naples 2022.
Breathe (Sheff). 19(220267)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Garmpi A, Damaskos C, Garmpis N,
Kaminiotis VV, Georgakopoulou VE, Spandidos DA, Papalexis P,
Diamantis E, Patsouras A, Kyriakos G, et al: Role of histone
deacetylase inhibitors in diabetic cardiomyopathy in experimental
models (Review). Med Int (Lond). 2(26)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zwinderman MRH, de Weerd S and Dekker FJ:
Targeting HDAC complexes in asthma and COPD. Epigenomes.
3(19)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Riffo-Vasquez Y, Man F and Page CP:
Doxofylline, a novofylline inhibits lung inflammation induced by
lipopolysacharide in the mouse. Pulm Pharmacol Ther. 27:170–178.
2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Leppänen T, Tuominen RK and Moilanen E:
Protein kinase C and its inhibitors in the regulation of
inflammation: Inducible nitric oxide synthase as an example. Basic
Clin Pharmacol Toxicol. 114:37–43. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jiao P, Li W, Shen L, Li Y, Yu L and Liu
Z: The protective effect of doxofylline against lipopolysaccharides
(LPS)-induced activation of NLRP3 inflammasome is mediated by SIRT1
in human pulmonary bronchial epithelial cells. Artif Cells Nanomed
Biotechnol. 48:687–694. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Riffo-Vasquez Y, Venkatasamy R and Page
CP: Steroid sparing effects of doxofylline. Pulm Pharmacol Ther.
48:1–4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Y, Zeng K, Wang J, Gao H, Nan Y and
Zheng X: Identifying the antiasthmatic target of doxofylline using
immobilized β2 -adrenoceptor based high-performance affinity
chromatography and site-directed molecular docking. J Mol Recognit.
29:492–498. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Calzetta L, Hanania NA, Dini FL, Goldstein
MF, Fairweather WR, Howard WW and Cazzola M: Impact of doxofylline
compared to theophylline in asthma: A pooled analysis of functional
and clinical outcomes from two multicentre, double-blind,
randomized studies (DOROTHEO 1 and DOROTHEO 2). Pulm Pharmacol
Ther. 53:20–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
DrugBank: Doxofylline (Internet).
https://go.drugbank.com/drugs/DB09273. Accessed
September 8, 2025.
|
|
31
|
DrugBank: Theophylline (Internet).
https://go.drugbank.com/drugs/DB00277. Accessed
September 8, 2025.
|
|
32
|
GBD 2019 Chronic Respiratory Disease
Collaborators. Global, regional, and national deaths, prevalence,
disability-adjusted life years, and years lived with disability for
chronic respiratory diseases, 1990-2019: A systematic analysis for
the Global Burden of Disease Study 2019. Lancet Respir Med.
8:585–596. 2020.
|
|
33
|
Global Initiative for Chronic Obstructive
Lung Disease (GOLD): Global strategy for the diagnosis, management,
and prevention of chronic obstructive pulmonary disease, 2025
report. GOLD, Fontana, WI, 2025. https://goldcopd.org.
|
|
34
|
Akram MF, Nasiruddin M, Ahmad Z and Ali
Khan R: Doxofylline and theophylline: A comparative clinical study.
J Clin Diagn Res. 6:1681–1684. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cazzola M, Calzetta L, Rogliani P, Page C
and Matera MG: Impact of doxofylline in COPD: A pairwise
meta-analysis. Pulm Pharmacol Ther. 51:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Cazzola M and Matera MG: The effect of
doxofylline in asthma and COPD. Respir Med.
164(105904)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Khandelwal PN, Maaz SH and Borade DM:
Evaluation of efficacy and safety of doxofylline 800mg sustained
release tablet in treatment of patients with COPD: An open label,
prospective and RCT. Int J Basic Clin Pharmacol. 7:644–649.
2018.
|
|
38
|
Corlateanu A, Botnaru V, Covantev S,
Dumitru S and Siafakas N: Predicting health-related quality of life
in patients with chronic obstructive pulmonary disease: The impact
of age. Respiration. 92:229–234. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Safka KA, Wald J, Wang H, McIvor L and
McIvor A: GOLD stage and treatment in COPD: A 500 patient point
prevalence study. Chronic Obstr Pulm Dis. 4:45–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kraemer R, Smith HJ, Gardin F, Barandun J,
Minder S, Kern L and Brutsche MH: Bronchodilator response in
patients with COPD, Asthma-COPD-Overlap (ACO) and asthma, evaluated
by plethysmographic and spirometric z-score target parameters. Int
J Chron Obstruct Pulmon Dis. 16:2487–2500. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Calzetta L, Matera MG, Goldstein MF,
Fairweather WR, Howard WW, Cazzola M and Rogliani P: A long-term
clinical trial on the efficacy and safety profile of doxofylline in
Asthma: the LESDA study. Pulm Pharmacol Ther.
60(101883)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Poggi R, Brandolese R, Bernasconi M,
Manzin E and Rossi A: Doxofylline and respiratory mechanics.
Short-term effects in mechanically ventilated patients with airflow
obstruction and respiratory failure. Chest. 96:772–778.
1989.PubMed/NCBI View Article : Google Scholar
|















![Chemical structure of theophylline
and doxofylline
[7-(1,3-dioxolan-2-ylmethyl)-3,7-dihydro-1,3-dimethyl-1H-purine-2,6-dione].
The presence of a dioxalane group at position 7 in doxofylline
differentiates it structurally from theophylline, contributing to
its improved safety and tolerability.](/article_images/br/23/5/br-23-05-02060-g00.jpg)