Introduction
Acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS) are serious respiratory conditions
characterized by hypoxemia, bilateral opacities on chest
radiographs and reduced lung compliance (1,2).
ALI/ARDS has a high annual mortality rate among critically ill
patients. The underlying pathology of ALI/ARDS involves the
disruption of the pulmonary vascular endothelial barrier function,
due to endothelial cell death and the loss of endothelial adhesion
connections (3,4). The mechanism of cell death in
pulmonary microvascular endothelial cells (PMVECs) is relatively
complex, with pyroptosis being considered as the primary mode
(5,6).
Pyroptosis is a form of programmed cell death
primarily mediated by gasdermin D (GSDMD), resulting in the release
of numerous pro-inflammatory cytokines (7). In addition, autophagy is a process
essential for maintaining cellular homeostasis (8). Comprehensively, autophagy can
negatively regulate pyroptosis by degrading, pathogen-associated
molecular patterns and other cellular components involved in this
process (9).
SPD, as an autophagic inducer, may exert protective
effects against ALI. In the present study, the role and mechanism
of SPD in LPS-induced ALI was investigated by establishing both an
in vivo ALI mouse model and an in vitro pyroptosis
model using PMVECs. The aim of the study was to demonstrate that
spermidine (SPD) effectively suppresses PMVEC pyroptosis by
promoting autophagic flux, thereby ameliorating LPS-induced
ALI.
Materials and methods
Reagents and antibodies
LPS and SPD were obtained from Sigma-Aldrich (Merck
KGaA). Nigericin (cat. no. HY-100381) and chloroquine (CQ) (cat.
no. HY-17589A) were provided by MedChemExpress. Antibodies against
caspase-1 (cat. no. ab207802), GSDMD (cat. no. ab219800), and
sequestosome 1 (SQSTM1)/p62 (cat. no. ab109012) were supplied by
Abcam. The antibody against microtubule-associated protein light
chain 3 (LC3) I/II (cat. no. 12741) was supplied by Cell Signaling
Technology, Inc. The HRP-conjugated β-actin antibody (cat. no.
ET1702-67) and goat anti-rabbit IgG-HRP antibody (cat. no. HA1001)
were supplied by HUABIO. Calcein/PI Cell Viability and Cytotoxicity
Assay Kit (cat. no. C2015M), RIPA lysis buffer (cat. no. P0013B),
Cell Counting Kit-8 (CCK-8) (cat. no. C0038), BCA Protein
Concentration Assay Kit (cat. no. P0010S) were obtained from
Beyotime Institute of Biotechnology. The ECL chemiluminescent
substrate (cat. no. RM02867) was obtained from ABclonal. The
CheKineTM Micro Lactate Dehydrogenase (LDH) Assay Kit (cat. no.
KTB1110) was provided by Abbkine Scientific Co., Ltd.
LPS-induced ALI model
Male C57BL/6 mice (n=30), weighing 20-25 g and aged
8-10 weeks, were obtained from Jinan Pengyue Experimental Animal
Breeding Center (Jinan, China). The mice were housed in a
controlled environment with regulated temperature (22-24˚C) and
humidity (50-60%), maintained on a 12-h light/dark cycle, and
provided with a standard diet and tap water ad libitum. SPD was
administered at doses of 10 mg/kg once daily, 10 mg/kg twice daily
and 20 mg/kg once daily (10). Mice
were pretreated with different concentrations of SPD for 3 days,
followed by the induction of ALI via intratracheal instillation of
LPS (5 mg/kg) for 24 h (11,12).
Mice were anesthetized by an i.p. injection of 0.3% sodium
pentobarbital (30 mg/kg), and then subjected to intratracheal
instillation of LPS. After 24 h, mice were euthanized via an i.p.
injection of an overdose of sodium pentobarbital (200 mg/kg). The
present study was approved (approval no. KYDWLL-202212) by the
Ethics Committee of Qilu Hospital of Shandong University (Qingdao,
China). All animal experiments were conducted in accordance with
the ARRIVE guidelines (13) and
complied with the NIH Guide for the Care and Use of Laboratory
Animals (14).
Protein concentration determination in
bronchoalveolar lavage fluid (BALF)
The collected BALF was centrifuged at 500 x g for 10
min at 4˚C. The protein concentration of the supernatant was
determined using the BCA Protein Concentration Assay Kit.
Lung tissue examination
The right lung lobe was fixed by soaking in 10%
formalin buffer at room temperature for 48 h. It was then embedded
in paraffin and sliced. The lung tissue sections (4 µm in
thickness) were stained with hematoxylin for 2 min and eosin
for 10 min at room temperature. Images were observed under a light
microscope (Olympus Corporation).
Lung wet-to-dry ratio (W/D)
Following the removal of the right lung, its wet
weight was measured and then dried in an oven at 60˚C for ~48 h.
The W/D was calculated by dividing the wet weight by the dry
weight.
Cell culture, cell cytoxicity assay
and LDH assay
The HULEC-5a (cat. no. C1402) immortalized pulmonary
microvascular endothelial cell line (cat. no. CVCL_0A11) was
purchased from Wuhan SUNNCELL Biotechnology Co., Ltd. To establish
the pyroptosis model of PMVECs, the cells were stimulated with LPS
(1 µg/ml) for 4 h, followed by treatment with nigericin (NIG) (20
µM) for 1 h. In the SPD group, the cells were pretreated with SPD
for 12 h prior to stimulation with LPS and NIG. In the LPS + NIG +
SPD + CQ group the cells were pretreated with CQ (20 µM) for 2 h
prior to stimulation with SPD. Cells were incubated with CCK-8
working solution for 2 h, followed by cell viability detection. The
necrotic cells were detected using the Calcein/PI Cell Viability
and Cytotoxicity Assay Kit, according to the manufacturer's
instructions. The cells were observed using a fluorescence
microscope (Nikon, Ti-E Live Cell Imaging System; Nikon
Corporation). LDH concentrations in cell homogenates were measured
using the CheKine™ Micro Lactate Dehydrogenase (LDH)
Assay Kit. After incubation, the cells were analyzed using an
automatic microplate reader (SpectraMax i3x; Molecular Devices,
LLC).
Western blotting
Lung tissue homogenate and cell lysate were prepared
in RIPA lysis buffer. The protein concentration of the extracted
samples was determined using a BCA assay kit. Equal protein amounts
(20 µg) were analyzed using 12% SDS-PAGE electrophoresis, followed
by transfer to a PVDF membrane. The membrane was blocked with 5%
non-fat milk at room temperature for 2 h and then incubated
overnight at 4˚C with primary antibodies against caspase-1
(1:1,000), GSDMD (1:1,000), SQSTM1/p62 (1:10,000), LC3 I/II
(1:1,000) and β-actin (1:2,000). The membrane was then incubated
with the secondary antibody (1:10,000) at room temperature for 60
min and then developed using ECL chemiluminescent substrate.
Densitometric analysis was performed using ImageJ (version 1.54;
National Institutes of Health).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. The experiments were repeated 3 times. Unpaired
Student's t-tests were used for comparisons between two groups, and
one-way ANOVA was used for comparisons among multiple groups. Post
hoc Tukey's HSD test was applied following ANOVA to identify
specific group differences. All statistical analyses were performed
using GraphPad Prism (version 9.0.0, GraphPad Software; Dotmatics).
P<0.05 was considered to indicate a statistically significant
difference.
Results
SPD alleviates LPS-induced ALI
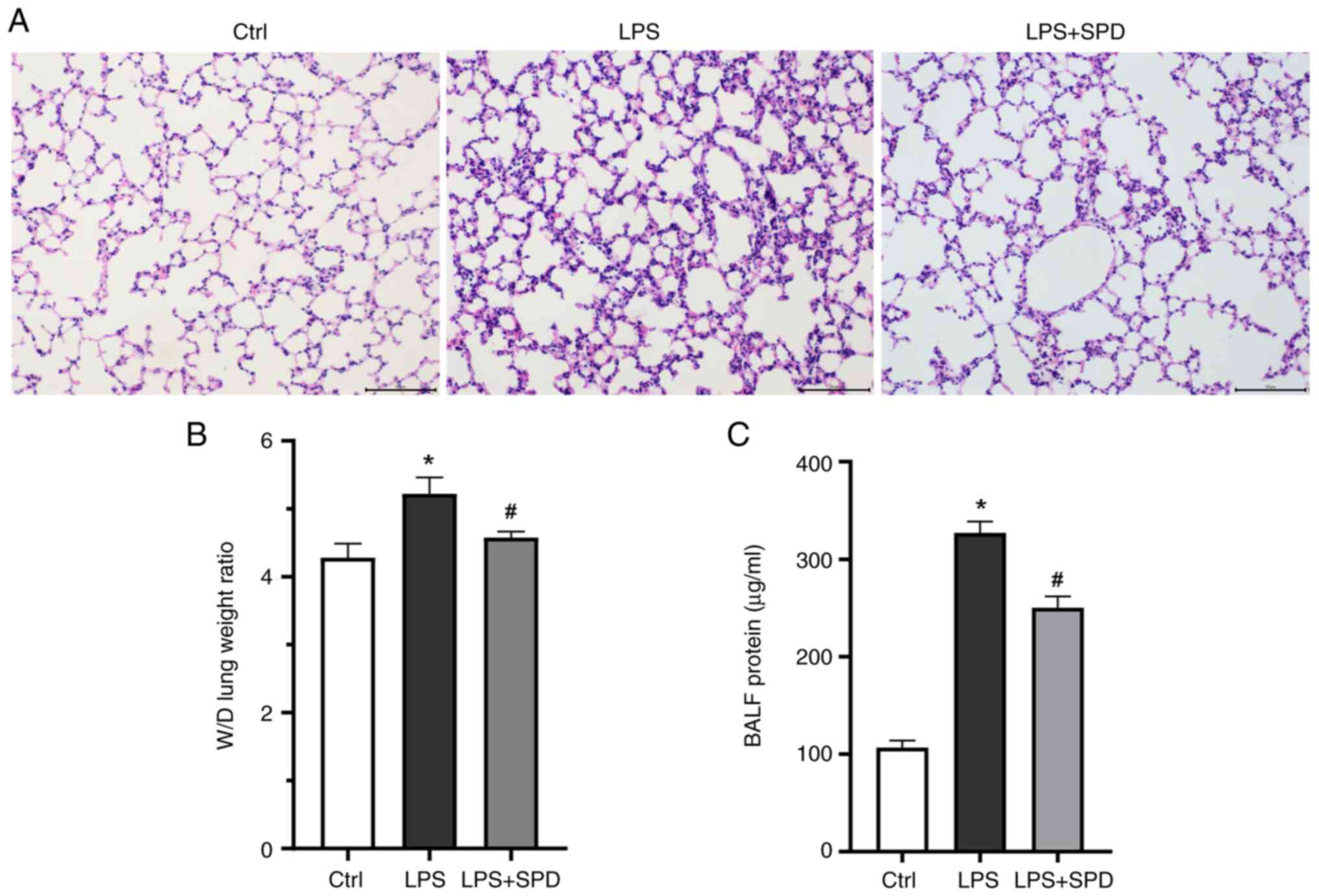
As shown in Fig.
S1, SPD at 10 mg/kg twice daily significantly alleviated lung
injury. Consequently, this dosing regimen (10 mg/kg twice daily)
was selected for all subsequent experiments. Notably, mice treated
with LPS exhibited significant lung damage, including diffuse
alveolar and interstitial edema, when compared with the control
group as revealed in Fig. 1A. SPD
pretreatment significantly reduced alveolar and interstitial edema
and inflammatory cell infiltration. Lung W/D and protein levels in
BALF were also measured (Fig. 1B
and C) to assess pulmonary vascular
endothelial permeability. SPD pretreatment significantly reduced
the lung W/D and protein in BALF. These findings indicated that SPD
pretreatment effectively reduces LPS-induced lung damage.
SPD alleviates lung pyroptosis
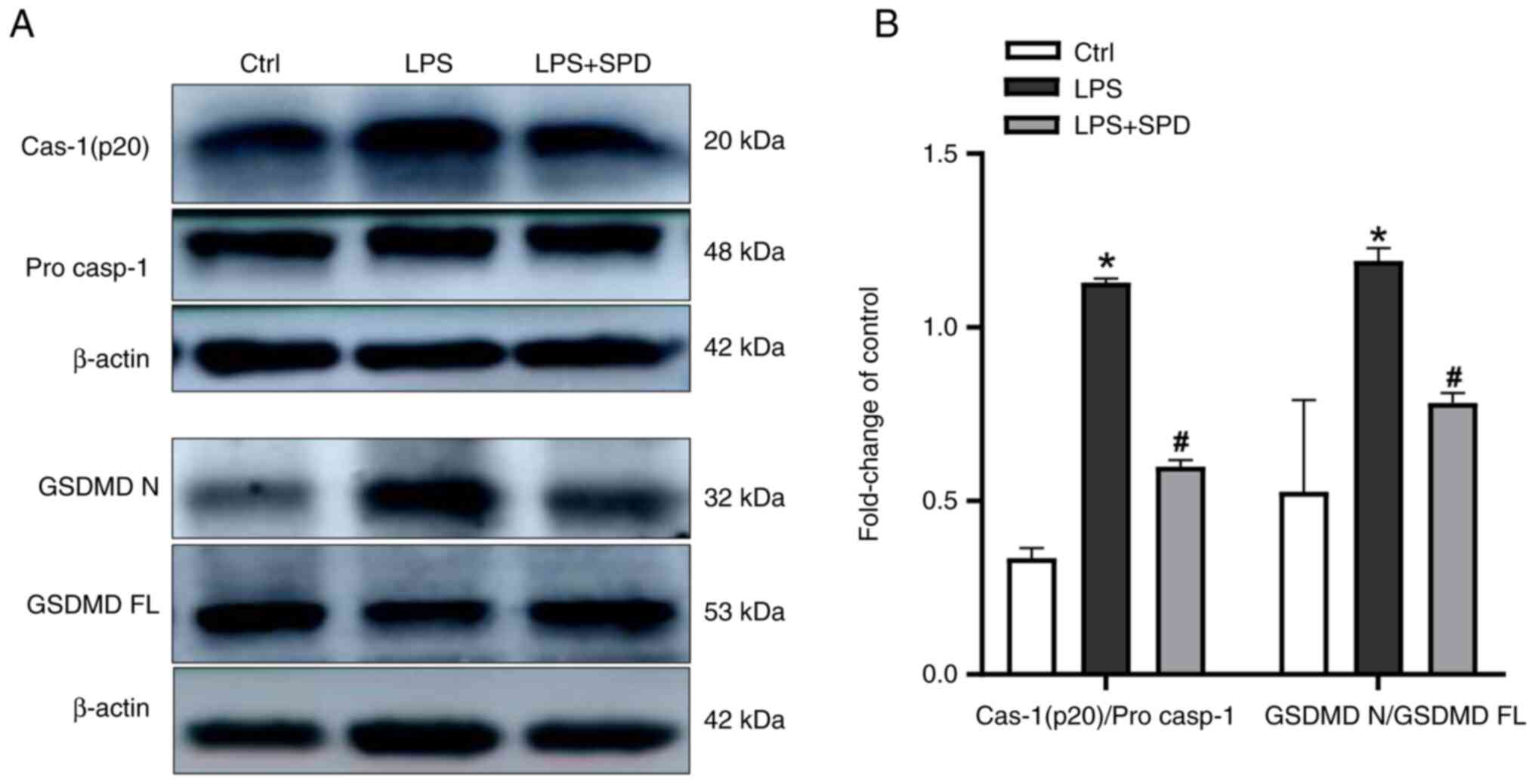
To explore whether SPD alleviates lung damage
through its effect on pyroptosis, the expression levels of
pyroptosis-related proteins were detected in lung tissue. As shown
in Fig. 2A and B, LPS administration significantly
increased the levels of cleaved caspase-1 and the gasdermin D
N-terminal (GSDMDNT) in lung tissue, as compared with
the control group. By contrast, SPD pretreatment decreased the
levels of these proteins. This suggested that SPD reduces
LPS-induced lung damage by inhibiting pyroptosis in lung
tissue.
LPS- and NIG-induced pyroptosis in
PMVECs
A concentration-dependent NIG screening (5, 10, 20,
30 and 40 µM) was performed on LPS-primed (1 µg/ml, 4 h) PMVECs
(15-17).
As shown in Fig. S2A, 20 µM NIG
was identified as the optimal concentration, balancing pyroptosis
induction with preserved assay sensitivity, and was thus employed
in further studies. Following SPD pretreatment (20, 40, 60, 80, 100
and 200 µM) and sequential LPS/NIG challenge, CCK-8 analysis
(Fig. S2B) revealed maximal
viability enhancement at 40 µM. Absence of additional benefit at
higher concentrations (80-200 µM) established 40 µM as the optimal
concentration for subsequent experimental applications.
SPD attenuates endothelial cell
pyroptosis
To investigate the effect of SPD on endothelial cell
pyroptosis, cells were treated with LPS and NIG. Pyroptotic cells
form membrane pores that permit extracellular dyes, such as
propidium iodide (PI), to enter and stain the nucleus (18). As revealed in Fig. 3A and B, a significant increase in the percentage
of PI-positive cells was observed following stimulation with LPS
and NIG. In addition, the stimulation with LPS and NIG led to a
significant increase in the release of LDH from endothelial cells
(Fig. 3C). Following stimulation
with LPS and NIG, the expression levels of cleaved caspase-1 and
GSDMDNT increased in endothelial cells (Fig. 3D and E). However, SPD reduced all LPS- and
NIG-induced effects.
 | Figure 3SPD reduces pyroptosis in PMVECs.
PMVECs were first treated with 40 µm SPD for 12 h, followed by
exposure to LPS and NIG. (A) Pyroptotic cells were identified
through PI staining, with nuclei stained blue using DAPI. Scale
bars, 50 µm. (B) Percentages of PI-positive cells. (C) LDH
concentrations in cell homogenates were measured using the
CheKine™ LDH Assay Kit. (D) Western blotting was
performed to detect cleaved caspase-1 (p20) and GSDMDNT
in PMVECs. (E) Semi-quantification of (D). The results are
presented as the mean ± SD. *P<0.05, the LPS + NIG
group vs. the control group; #P<0.05, the LPS + NIG +
SPD group vs. the LPS + NIG group. SPD, spermidine; PMVECs,
pulmonary microvascular endothelial cells; LPS, lipopolysaccharide;
NIG, nigericin; PI, propidium iodide; DAPI,
4',6-diamidino-2-phenylindole; LDH, lactate dehydrogenase;
GSDMDNT or GSDMD N, gasdermin D N-terminal; GSDMD FL,
gasdermin D full length; Ctrl, control. |
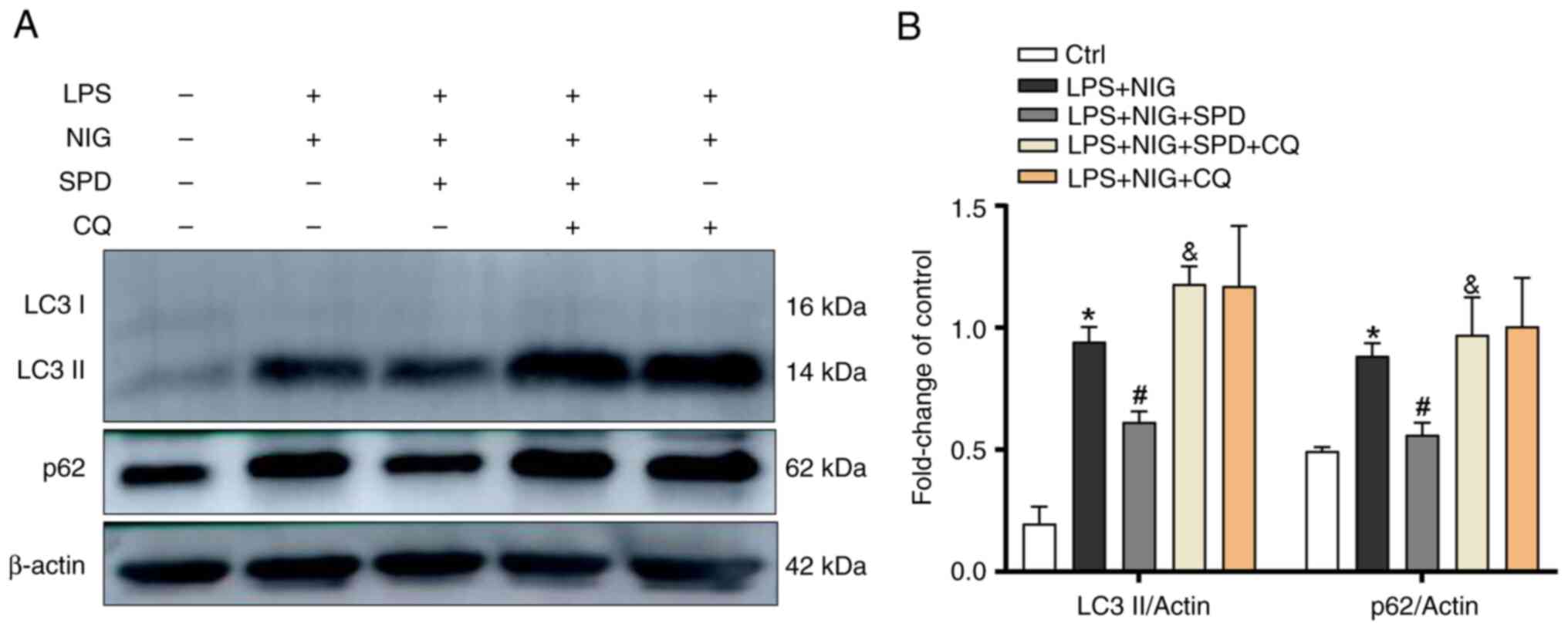
SPD enhances the disrupted autophagic
process in endothelial cells undergoing LPS- and NIG-induced
pyroptosis
SPD promotes autophagy, which can inhibit pyroptosis
through different mechanisms. It was examined whether SPD restores
autophagy in LPS- and NIG-treated endothelial cells. This was
performed by measuring the levels of autophagic proteins LC3-II and
p62. As shown in Fig. 4A and
B, the levels of autophagy markers
LC3-II and p62 increased in LPS- and NIG-treated endothelial cells.
Following SPD treatment, the levels of LC3-II and p62 in
endothelial cells affected by LPS and NIG decreased. Notably, the
addition of CQ to the LPS + NIG + SPD group further elevated LC3-II
and p62 levels, suggesting that the protective effect of SPD was
attenuated by CQ-mediated autophagy blockade. These results
indicated that LPS and NIG inhibited autophagic flux in PMVECs,
while SPD successfully restored this disrupted process.
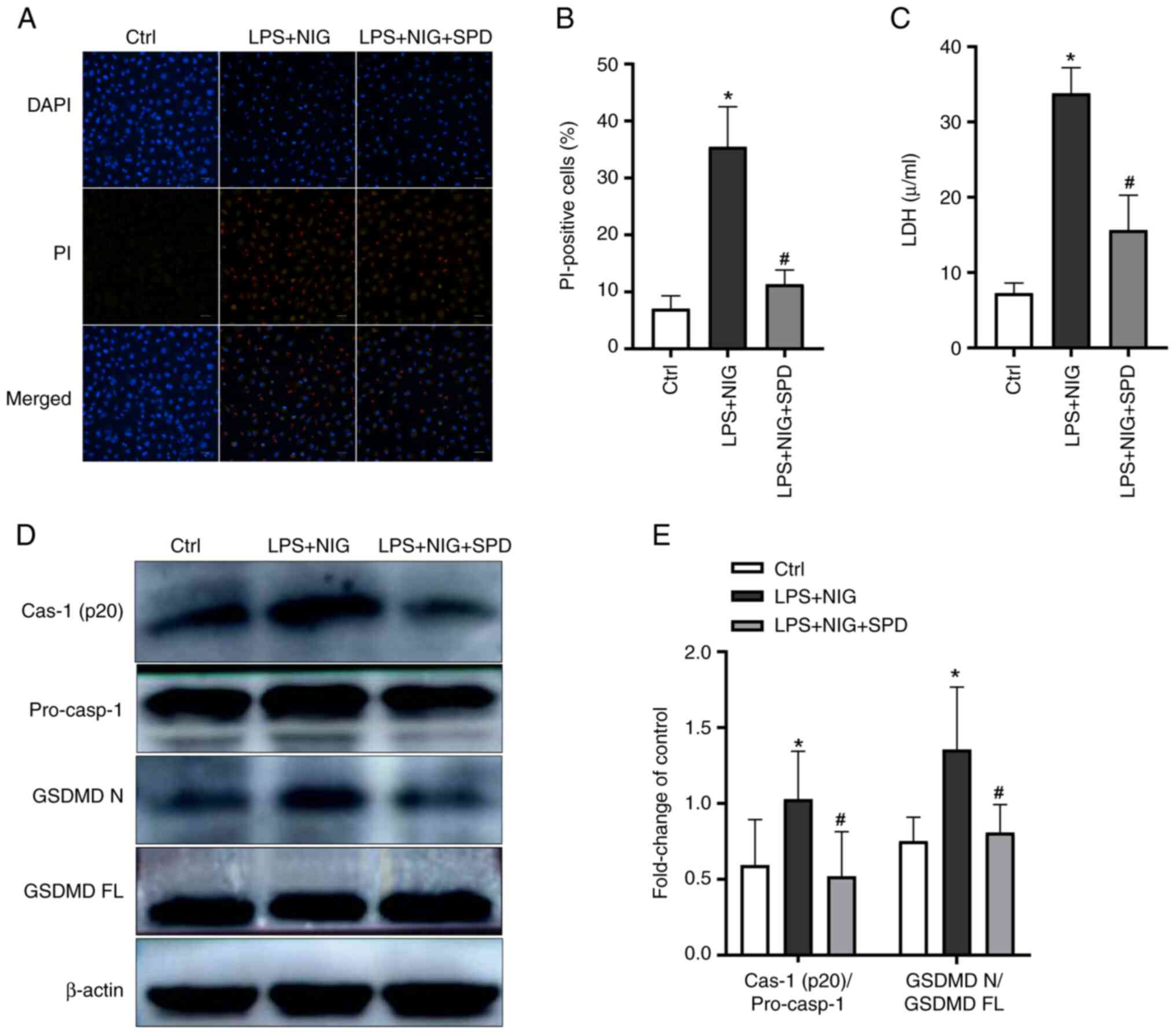
SPD reduces pyroptosis in LPS- and
NIG-treated endothelial cells by restoring the damaged autophagic
flux
To better understand this process, the autophagy
inhibitor CQ was used to block autophagic flux. As revealed in
Fig. 5, the LPS + NIG + SPD + CQ
group had more PI staining-positive cells and higher LDH release
than the LPS + NIG + SPD group. In addition, this group exhibited
increased levels of cleaved caspase-1 and GSDMDNT. These
findings indicated that by restoring autophagic flux, SPD mitigates
pyroptosis in LPS- and NIG-treated PMVECs.
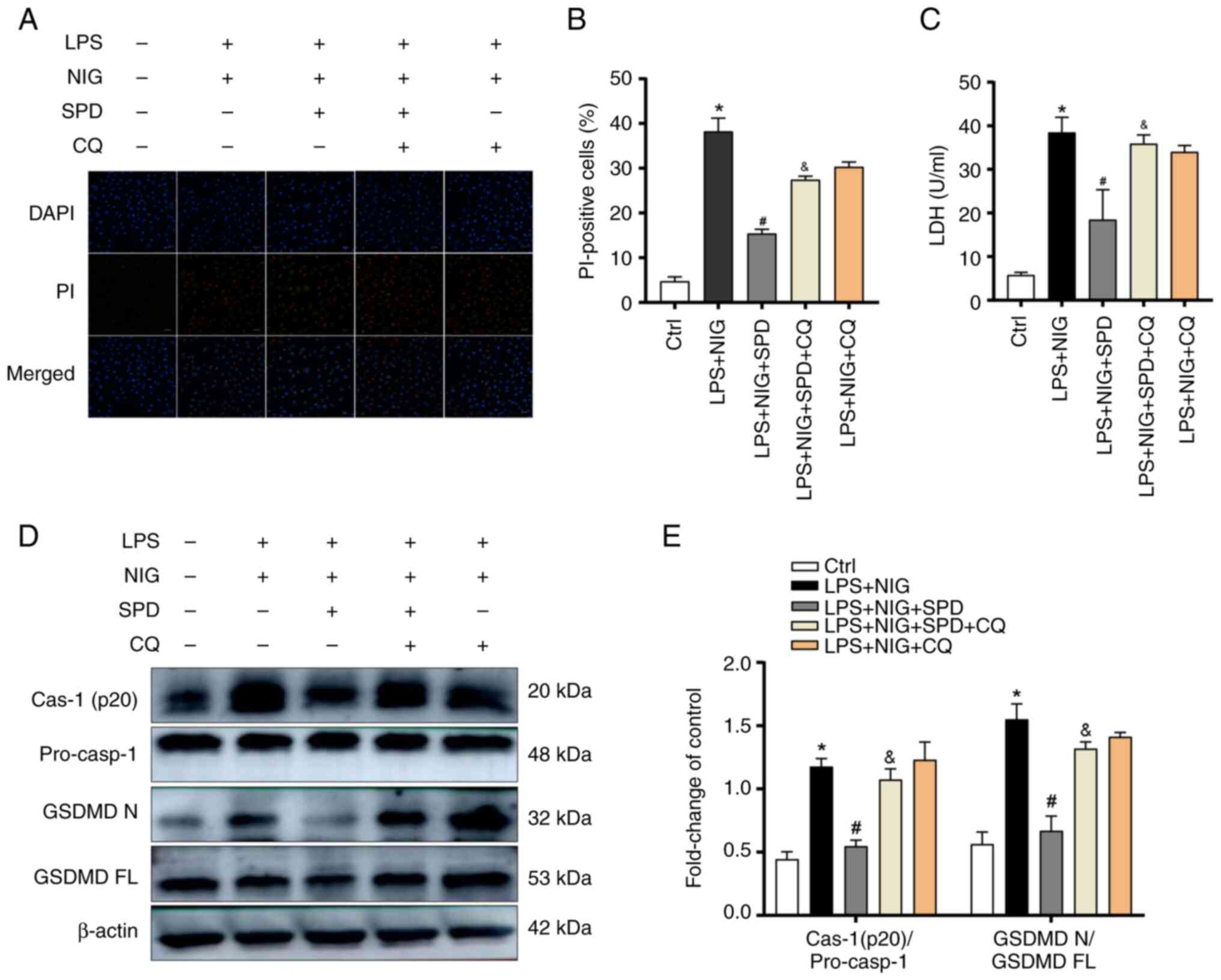 | Figure 5SPD reduces PMVEC pyroptosis caused by
LPS and NIG by restoring the impaired autophagic flux. (A)
Pyroptotic cells were labeled red using PI staining, while the cell
nuclei were stained blue with DAPI. Scale bars, 50 µm. (B)
Percentages of PI-positive cells. (C) LDH concentrations in cell
homogenates were measured using the CheKine™ LDH Assay
Kit. (D) Western blotting was conducted to assess the levels of
cleaved caspase-1 (p20) and GSDMDNT in PMVECs. (E)
Semi-quantification of (D). Results are presented as the mean ± SD.
*P<0.05, the LPS + NIG group vs. the control group;
#P<0.05, the LPS + NIG + SPD group vs. the LPS + NIG
group; &P<0.05, the LPS + NIG + SPD + CQ group
vs. LPS + NIG + SPD group. SPD, spermidine; PMVECs, pulmonary
microvascular endothelial cells; LPS, lipopolysaccharide; NIG,
nigericin; PI, propidium iodide; DAPI,
4',6-diamidino-2-phenylindole; LDH, lactate dehydrogenase;
GSDMDNT or GSDMD N, gasdermin D N-terminal; GSDMD FL,
gasdermin D full length; CQ, chloroquine; Cas-1, caspase-1;
pro-casp-1, pro-caspase-1; Ctrl, control. |
Discussion
SPD is a key metabolic regulator produced from the
breakdown of ornithine in mammals, playing significant roles in
various physiological and pathological processes (19). It has been shown to be a potent
inducer of autophagy, contributing to anti-aging and antioxidant
effects, inflammation reduction and apoptosis inhibition (20-22).
However, the therapeutic potential of SPD for ameliorating ALI has
not been previously reported. Hence, exploration of the therapeutic
effects of SPD in ALI may provide novel insights for clinical
applications.
Pyroptosis is recognized as the main mechanism
through which LPS induces endothelial cell death. Early in the
process of inflammation, endothelial cells undergo pyroptotic death
to remove damaged cells. However, if this process is not
controlled, it can damage the vascular endothelial barrier
(23-25).
Pyroptosis is a distinct form of programmed cell death that depends
on caspases and gasdermins. The key effector, GSDMD, is cleaved by
caspases into two fragments: The N-terminal fragment
(GSDMDNT) and the C-terminal fragment
(GSDMDCT). The GSDMDNT binds to cell
membranes to create pores, resulting in cell destruction (26). The present findings were aligned
with those of previous studies (23-26),
showing an increase in GSDMDNT and pro-caspase-1
expression in the lungs of mice with LPS-induced ALI and pulmonary
vascular cells.
There are three main types of autophagy:
Macroautophagy, microautophagy and chaperone-mediated autophagy.
Macroautophagy is considered the primary form. During this process,
the membrane of the autophagic vesicle encloses cellular
components, leading to the formation of an autophagosome. The
autophagosome then merges with a lysosome to create an
autophagolysosome, where the cellular contents are broken down.
This sequence of events is called autophagic flux (27). Studies have demonstrated that
activating autophagy mitigates sepsis and attenuates organ
dysfunction by suppressing pyroptosis (25,28,29).
To date, therapies targeting both autophagy and pyroptosis for
treating ARDS have not yet been reported in clinical practice.
p62, or SQSTM1, is a key target of autophagy; it
interacts with LC3 on the autophagosome membrane, gets incorporated
into the autophagosome, and is eventually degraded (8). The disruption of autophagy can cause
the buildup of p62 protein. In the pyroptosis of LPS- and
NIG-treated PMVECs, higher levels of p62 and LC3 II were observed,
which suggested that autophagic flux was inhibited in PMVECs.
Previous research has shown that autophagy negatively regulates
cellular pyroptosis. Pu et al (30) found that autophagy inhibition
increased macrophage pyroptosis in a Pseudomonas
aeruginosa-induced sepsis model. In addition, a previous study
showed that activating autophagy reduces neuronal cell pyroptosis
in a mouse model of brain injury (31). To examine the effects of SPD, a
potent autophagy inducer, on pyroptosis and autophagy in PMVECs,
the cells were treated in vitro with SPD. It was observed
that SPD attenuated LPS/nigericin-induced pyroptosis and rescued
the suppressed autophagic flux in PMVECs. CQ has been revealed to
block autophagic flux by inhibiting the fusion of autophagosomes
with lysosomes (32). To
investigate whether the SPD-mediated attenuation of PMVEC
pyroptosis is dependent on the enhancement of autophagic flux,
autophagic flux was inhibited using CQ in PMVECs. When autophagic
flux was pharmacologically blocked by CQ, the protective effect of
SPD against PMVEC pyroptosis was significantly abrogated. These
findings collectively indicated that SPD mitigates PMVEC pyroptosis
through the potentiation of autophagic flux.
In conclusion, in the present study it was
demonstrated that SPD alleviates LPS-induced ALI by suppressing
pyroptosis in PMVECs through an autophagy-dependent mechanism.
These findings provide novel insights for the clinical management
of ARDS. However, it should be noted that the present study has
certain limitations. Interleukin-1β (IL-1β) is a well-established
downstream effector of caspase-1(33), and although it was demonstrated that
SPD suppresses caspase-1 activation, additional validation for this
specific cytokine within the scope of the present study was not
performed. Evidence indicates that SPD can induce autophagy through
the adenosine monophosphate-activated protein kinase
(AMPK)/mammalian target of rapamycin (mTOR) pathway to improve
cardiac function and reverse B-cell senescence by controlling eIF5A
hypusination (21,22). Future research should focus on
elucidating whether SPD exerts its protective effects against ALI
and PMVEC pyroptosis by inducing autophagy through the AMPK/mTOR
signaling pathway, H5A phosphorylation or alternative mechanisms,
along with its effects on IL-1β maturation and secretion. Further
clinical trials are warranted to validate the therapeutic efficacy
of SPD in human patients.
Supplementary Material
Effect of SPD at various
concentrations on LPS-induced acute lung injury. H&E staining
of lung tissue sections showing that SPD (10 mg/kg twice daily)
pretreatment alleviated LPS-induced lung injury. Scale bars, 100
μm. SPD, spermidine; LPS, lipopolysaccharide; H&E,
hematoxylin & eosin; Ctrl, control.
Protective effect of SPD concentration
pretreatment on LPS/NIG-induced pyroptosis. (A) PMVECs were treated
with LPS (1 μg/ml) for 4 h, followed by incubation with
different concentrations of NIG (5, 10, 20, 30 and 40 μM).
Cell viability was measured using the CCK-8 assay 1 h following the
addition of NIG. *P<0.05, the NIG (20 μM)
group vs. the NIG (0 μM) group. (B) Pulmonary vascular
endothelial cells were pretreated with different concentrations of
SPD (20, 40, 60, 80, 100 and 200 μM), followed by sequential
treatment with LPS and NIG. Cell viability was assessed using the
CCK-8 assay 1 h after the final treatment. *P<0.05,
the SPD (40 μM) group vs. the SPD (0 μM) group. LPS,
lipopolysaccharide; NIG, nigericin; PMVECs, pulmonary microvascular
endothelial cells; CCK-8, Cell Counting Kit-8; SPD,
spermidine.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
Scientific Research Foundation of Qilu Hospital (Qingdao) (grant
no. QDKY2020RX05) and Qingdao Science and Technology Project (grant
no. 25-1-5-smjk-9-nsh).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XX designed the study, critically reviewed and
edited the draft, supervised the study, and acquired funding. XZ
conducted the investigation, performed data collection and analysis
and drafted the manuscript. HL worked on partial data collection
and analysis, and participated in manuscript revision. XX and XZ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
KYDWLL-202212) by the Ethics Committee of Qilu Hospital of Shandong
University (Qingdao, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
ARDS Definition Task Force. Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The berlin definition. JAMA. 307:2526–2533. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Fan E, Brodie D and Slutsky AS: Acute
respiratory distress syndrome: Advances in diagnosis and treatment.
JAMA. 319:698–710. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Matthay MA and Zemans RL: The acute
respiratory distress syndrome: Pathogenesis and treatment. Annu Rev
Pathol. 6:147–163. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Wang L, Mehta S, Brock M and Gill SE:
Inhibition of murine pulmonary microvascular endothelial cell
apoptosis promotes recovery of barrier function under septic
conditions. Mediators Inflamm. 2017(3415380)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gill SE, Rohan M and Mehta S: Role of
pulmonary microvascular endothelial cell apoptosis in murine
sepsis-induced lung injury in vivo. Respir Res.
16(109)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Moreno-Gonzalez G, Vandenabeele P and
Krysko DV: Necroptosis: A novel cell death modality and its
potential relevance for critical care medicine. Am J Respir Crit
Care Med. 194:415–428. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guo R, Wang H and Cui N: Autophagy
regulation on pyroptosis: Mechanism and medical implication in
sepsis. Mediators Inflamm. 2021(9925059)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gao M, Zhao W, Li C, Xie X, Li M, Bi Y,
Fang F, Du Y and Liu X: Spermidine ameliorates non-alcoholic fatty
liver disease through regulating lipid metabolism via AMPK. Biochem
Biophys Res Commun. 505:93–98. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ehrentraut H, Weisheit CK, Frede S and
Hilbert T: Inducing acute lung injury in mice by direct
intratracheal lipopolysaccharide instillation. J Vis Exp: Jul 6,
2019 (Epub ahead of print).
|
|
12
|
D'Alessio FR: Mouse models of acute lung
injury and ARDS. Methods Mol Biol. 1809:341–350. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Percie du Sert N, Hurst V, Ahluwalia A,
Alam S, Avey MT, Baker M, Browne WJ, Clark A, Cuthill IC, Dirnagl
U, et al: The ARRIVE guidelines 2.0: Updated guidelines for
reporting animal research. PLoS Biol. 18(e3000410)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
National Research Council: Committee for
the Update of the Guide for the Care and Use of Laboratory Animals:
Guide for the Care and Use of Laboratory Animals. 8th edition.
National Academies Press, Washington, DC, pp1-246, 2011.
|
|
15
|
Yan S, Yu L, Chen Z, Xie D, Huang Z and
Ouyang S: ZBP1 promotes hepatocyte pyroptosis in acute liver injury
by regulating the PGAM5/ROS pathway. Ann Hepatol.
29(101475)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liang Q, Cai W, Zhao Y, Xu H, Tang H, Chen
D, Qian F and Sun L: Lycorine ameliorates bleomycin-induced
pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and
pyroptosis. Pharmacol Res. 158(104884)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li Q, Tan Y, Chen S, Xiao X, Zhang M, Wu Q
and Dong M: Irisin alleviates LPS-induced liver injury and
inflammation through inhibition of NLRP3 inflammasome and NF-κB
signaling. J Recept Signal Transduct Res. 41:294–303.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang X, Zhang P, An L, Sun N, Peng L,
Tang W, Ma D and Chen J: Miltirone induces cell death in
hepatocellular carcinoma cell through GSDME-dependent pyroptosis.
Acta Pharm Sin B. 10:1397–1413. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pegg AE: Mammalian polyamine metabolism
and function. IUBMB Life. 61:880–894. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Madeo F, Eisenberg T, Büttner S,
Ruckenstuhl C and Kroemer G: Spermidine: A novel autophagy inducer
and longevity elixir. Autophagy. 6:160–162. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang H, Alsaleh G, Feltham J, Sun Y,
Napolitano G, Riffelmacher T, Charles P, Frau L, Hublitz P, Yu Z,
et al: Polyamines control eIF5A hypusination, TFEB translation, and
autophagy to reverse B cell senescence. Mol Cell. 76:110–125.e9.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yan J, Yan JY, Wang YX, Ling YN, Song XD,
Wang SY, Liu HQ, Liu QC, Zhang Y, Yang PZ, et al:
Spermidine-enhanced autophagic flux improves cardiac dysfunction
following myocardial infarction by targeting the AMPK/mTOR
signalling pathway. Br J Pharmacol. 176:3126–3142. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi J, Gao W and Shao F: Pyroptosis:
Gasdermin-mediated programmed necrotic cell death. Trends Biochem
Sci. 42:245–254. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Brigham KL and Meyrick B: Endotoxin and
lung injury. Am Rev Respir Dis. 133:913–927. 1986.PubMed/NCBI
|
|
25
|
Singla S and Machado RF: Death of the
endothelium in sepsis: Understanding the crime scene. Am J Respir
Cell Mol Biol. 59:3–4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Broz P, Pelegrín P and Shao F: The
gasdermins, a protein family executing cell death and inflammation.
Nat Rev Immunol. 20:143–157. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu S, Yao S, Yang H, Liu S and Wang Y:
Autophagy: Regulator of cell death. Cell Death Dis.
14(648)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mitra S, Exline M, Habyarimana F, Gavrilin
MA, Baker PJ, Masters SL, Wewers MD and Sarkar A: Microparticulate
caspase 1 regulates gasdermin D and pulmonary vascular endothelial
cell injury. Am J Respir Cell Mol Biol. 59:56–64. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang YC, Liu QX, Zheng Q, Liu T, Xu XE,
Liu XH, Gao W, Bai XJ and Li ZF: Dihydromyricetin alleviates
sepsis-induced acute lung injury through inhibiting NLRP3
inflammasome-dependent pyroptosis in mice model. Inflammation.
42:1301–1310. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pu Q, Gan C, Li R, Li Y, Tan S, Li X, Wei
Y, Lan L, Deng X, Liang H, et al: Atg7 deficiency intensifies
inflammasome activation and pyroptosis in Pseudomonas
sepsis. J Immunol. 198:3205–3213. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gao C, Yan Y, Chen G, Wang T, Luo C, Zhang
M, Chen X and Tao L: Autophagy activation represses pyroptosis
through the IL-13 and JAK1/STAT1 pathways in a mouse model of
moderate traumatic brain injury. ACS Chem Neurosci. 11:4231–4239.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr
M, Hijlkema KJ, Coppes RP, Engedal N, Mari M and Reggiori F:
Chloroquine inhibits autophagic flux by decreasing
autophagosome-lysosome fusion. Autophagy. 14:1435–1455.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu J and Núñez G: The NLRP3 inflammasome:
Activation and regulation. Trends Biochem Sci. 48:331–344.
2023.PubMed/NCBI View Article : Google Scholar
|