Introduction
Neurogenic lower urinary tract dysfunction (NLUTD)
is a common complication in individuals with conditions such as
spinal cord injuries, multiple sclerosis (MS) and other neurologic
disorders, often presenting with symptoms of overactive bladder,
incontinence and voiding dysfunction (1,2).
Anticholinergic medications, which inhibit muscarinic receptors to
reduce detrusor muscle overactivity, are frequently employed as
first-line pharmacological interventions to manage these symptoms
(1,3). However, the widespread distribution of
muscarinic receptors in the central nervous system raises concerns
regarding potential cognitive side effects, particularly in
vulnerable populations (2,4).
Cognitive impairments, including memory loss,
attention deficits and decreased processing speed, have been
reported with anticholinergic use, especially in older populations
(2,5,6).
Studies show that anticholinergic medications cross the blood-brain
barrier, contributing to brain atrophy and increased dementia risk
(2,7). A cohort study by Wang et al
(2) found that cumulative exposure
to bladder-specific anticholinergics was associated with an
elevated risk of dementia, emphasizing the need for careful
therapeutic balancing between symptom management and cognitive
safety.
In cognitive research, functions are commonly
categorized into key domains: Memory, attention and processing
speed, executive functions, visuospatial ability and global
cognition (8). NLUTD studies often
use standardized tools to assess these domains. For instance, the
Mini-Mental State Examination (MMSE) is a 30-point scale examining
orientation, recall, attention, language and constructional ability
(9). The Symbol Digit Modalities
Test (SDMT) is widely used, particularly in neurological
populations, to evaluate attention and processing speed (10). More specialized tests, such as the
Frontal Assessment Battery (FAB) or the Trail Making Test, are used
to evaluate executive functioning, and instruments such as the
Montreal Cognitive Assessment (MoCA) assess multiple domains,
including visuospatial abilities (9). By briefly outlining these tools and
domains, a clear conceptual framework that supports analyses of
cognitive outcomes was established.
In populations with neurogenic bladder dysfunction,
the cognitive burden of anticholinergics may be particularly
problematic due to pre-existing neurological vulnerabilities.
Sakakibara et al (4)
demonstrated that although some anticholinergic agents, such as
imidafenacin, may present lower cognitive risks, others such as
oxybutynin and tolterodine have been linked to significant declines
in memory and executive function. Similarly, Morrow et al
(7) highlighted a substantial
impact on processing speed and memory in patients with MS treated
with anticholinergic medications, with impairments detected using
standardized cognitive tests.
Despite their therapeutic efficacy in alleviating
NLUTD symptoms, the long-term cognitive consequences of
anticholinergic medications remain a critical area of
investigation. Emerging evidence suggests that alternative
treatments, such as β3-adrenergic agonists like mirabegron, may
provide effective symptom control with fewer cognitive side
effects, as demonstrated in comparative studies (1). Trbovich et al (1) found improved cognitive outcomes and
comparable efficacy when switching from anticholinergics to
mirabegron in individuals with neurogenic bladder dysfunction.
The present systematic review and meta-analysis aim
to comprehensively evaluate the cognitive effects of
anticholinergic medications in patients with NLUTD, synthesizing
data from clinical trials and observational studies. By examining
the extent and nature of cognitive impairments across various
populations and drug types, the present study seeks to inform
clinical decision-making and highlight potential therapeutic
alternatives that minimize cognitive risks.
Materials and methods
Study design
The present study is a systematic review and
meta-analysis conducted to evaluate the cognitive effects of
anticholinergic medications in patients with NLUTD. The review
adhered to the guidelines outlined in the PRISMA statement to
ensure comprehensive reporting and transparency. The study protocol
was registered in accordance with best practices for meta-analyses
(11).
Search strategy and selection
criteria
A systematic search was conducted across major
electronic databases, including PubMed (https://pubmed.ncbi.nlm.nih.gov/), Embase (https://www.embase.com), Cochrane Library (https://www.cochrane.org/) and Scopus (https://www.scopus.com/), from inception to February
2025. The search terms included combinations of the following:
‘anticholinergics’, ‘cognitive impairment’, ‘neurogenic bladder’,
‘neurogenic lower urinary tract dysfunction’, ‘cognition’ and
‘urinary tract dysfunction’. No language restrictions were applied.
The reference lists of included studies and relevant review
articles were manually screened to identify additional studies.
Inclusion criteria were: i) Studies assessing the cognitive effects
of anticholinergic medications in patients with NLUTD, ii)
randomized controlled trials, cohort studies, case-control studies
and cross-sectional studies, iii) studies reporting cognitive
outcomes, including memory, executive function, or overall
cognition, and iv) only studies involving anticholinergic
monotherapy delivered via oral administration were eligible for
inclusion. Exclusion criteria were: i) Studies not involving NLUTD
or anticholinergic medications, ii) animal or in vitro
studies, iii) conference abstracts, case reports and reviews
without primary data, and iv) studies using combination therapies
(for example, anticholinergics plus β3-agonists), non-oral
administration routes (for example, transdermal patches), and
studies enrolling participants with clinically diagnosed cognitive
impairment at baseline to reduce potential confounding.
Data extraction
Two independent reviewers (HZ and YW) screened
titles and abstracts to identify relevant studies. Discrepancies
were resolved through discussion. Full-text articles were retrieved
and evaluated against the inclusion criteria. The following data
were extracted using a standardized form: i) Study characteristics:
author, year, study design, sample size and population
characteristics; ii) intervention details: Type, dosage and
duration of anticholinergic use; iii) cognitive outcomes: type of
cognitive test used (for example, MMSE and SDMT); and iv) risk of
bias and confounders: assessed using the Newcastle-Ottawa Scale
(NOS).
Statistics
All statistical analyses were performed using
Comprehensive Meta-Analysis software version 3. The primary outcome
was the standardized mean difference (SMD) in cognitive test scores
between patients receiving anticholinergics and those not exposed
to the medications. A random-effects model was used due to the
expected heterogeneity across studies, with effect sizes reported
as point estimates and 95% confidence intervals (CIs).
Heterogeneity was assessed using the Q statistic and precision
interval analysis. Tau-squared (τ²) was calculated to estimate
between-study variance. Instead of relying solely on I² to quantify
heterogeneity, the present meta-analysis employed precision
interval analysis. While I² is commonly used to measure the
percentage of variability due to heterogeneity, it has limitations,
particularly when the number of studies is small or when effect
sizes vary widely (12). Precision
interval analysis focuses on the width of the CI and how much
uncertainty surrounds the estimate, offering a more robust measure
of how precise the combined effect size is. The width of the
precision interval indicates whether the heterogeneity among
studies affects the reliability of the meta-analysis conclusion
(13). Sensitivity analyses were
conducted by excluding studies with a high risk of bias and
recalculating pooled estimates. Subgroup and moderator analyses
were performed based on population characteristics (for example,
age and sex groups) and type of cognitive test. The Begg and
Mazumdar rank correlation test and Egger's regression intercept
were used to identify publication bias. The Classic fail-safe N and
Orwin's fail-safe N were calculated to assess the robustness of the
results. Forest plots were generated to visualize effect sizes, and
the funnel plot was used to assess publication bias. The
significance of heterogeneity was tested using P-values, where
P<0.05 was considered to indicate significant heterogeneity.
Results
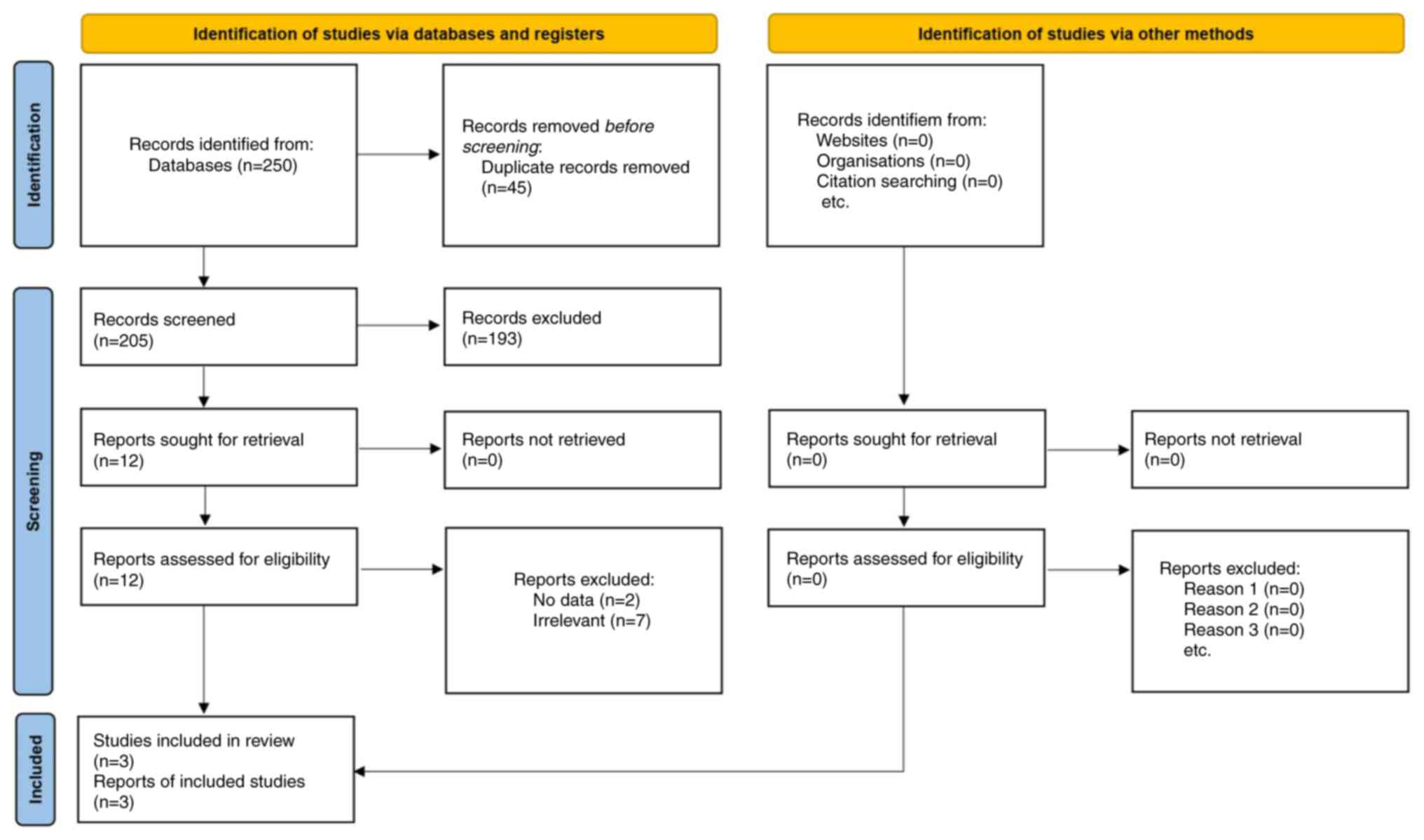
Study selection
A systematic literature search of four major
electronic databases (PubMed, Embase, Cochrane Library and Scopus)
yielded 250 potentially relevant articles. After removing 45
duplicates, 205 articles underwent title and abstract screening, of
which 12 were considered for full-text review. A total of 9
articles were excluded for not meeting the inclusion criteria (for
example, lack of NLUTD focus, no anticholinergic intervention, or
insufficient cognitive data). Ultimately, 3 studies (4,7,14)
fulfilled the eligibility criteria, providing 22 independent
comparisons. Two additional studies were excluded because they did
not report sufficient data for the analysis (1,2). The
entire selection process followed PRISMA guidelines and is outlined
in Fig. 1.
General characteristics of included
studies
The selected studies included 139 participants
across various clinical settings, focusing on patients with NLUTD
due to conditions such as spinal cord injury (SCI), MS and
neurologic overactive bladder (OAB). The mean age of participants
ranged from 25 to 70 years, with follow-up durations between 3 and
12 weeks. The studies examined both traditional anticholinergic
agents (for example, oxybutynin and tolterodine) and newer,
selective agents (for example, solifenacin and imidafenacin).
Treatment durations ranged from 3 months to 12 weeks. Cognitive
outcomes were assessed using standardized tests, including the
SDMT, MMSE and FAB (Table I).
 | Table IStudy characteristics and
interventions in the meta-analysis. |
Table I
Study characteristics and
interventions in the meta-analysis.
| First author/s,
year | Study design | Sample size (n) | Population
characteristics | Anticholinergic
agent(s) | Dosage | Duration of
treatment | Cognitive tests
used | (Refs.) |
|---|
| Krebs et al,
2017 | Prospective
controlled cohort | 29 | Patients with spinal
cord injury with neurogenic lower urinary tract dysfunction during
post-acute rehabilitation phase | Solifenacin,
fesoterodine, darifenacin | Variable, based on
agent | 3 months | Verbal learning test,
Stroop test, word fluency test | (14) |
| Morrow et
al, 2018 | Randomized
controlled trial | 69 | Patients with
multiple sclerosis and bladder dysfunction | Oxybutynin,
tolterodine | Oxybutynin (5-10
mg/day), Tolterodine (4 mg/day) | 12 weeks | Symbol Digit
Modalities Test (SDMT), BiCAMS battery | (7) |
| Sakakibara et
al, 2013 | Prospective
cohort | 62 | Patients with
neurologic overactive bladder due to various conditions (for
example, Parkinson's, Alzheimer's, white matter lesions) | Imidafenacin | 0.2 mg/day (0.1 mg
twice daily) | 3 months | Mini-Mental State
Examination (MMSE), Frontal Assessment Battery (FAB), ADAS-cog | (4) |
Primary outcome: Cognitive
decline
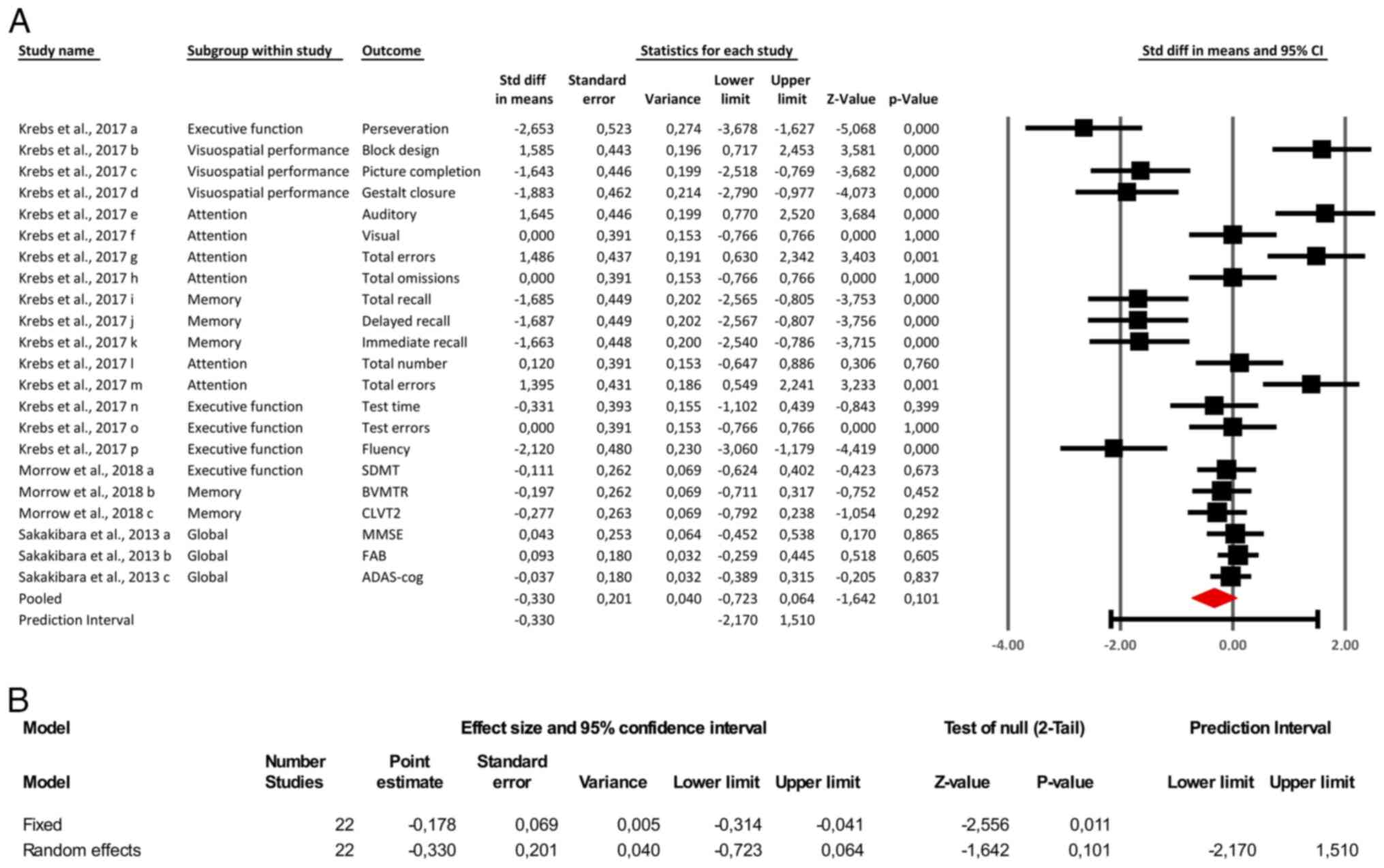
The meta-analysis of 22 comparisons yielded a pooled
SMD of -0.33 (95% CI: -0.72 to 0.06; Fig. 2A) under a random-effects model,
indicating a trend toward negative cognitive effects of
anticholinergic medications that did not reach statistical
significance (P=0.10). By contrast, a complementary fixed-effects
analysis produced a statistically significant SM -0.178 (95% CI:
-0.314 to -0.041; P=0.011), suggesting a small but significant
overall effect. However, given the substantial between-study
heterogeneity (Q=162.25, df=21, P<0.001; τ²=0.738; τ=0.859;
Fig. 2B), the random-effects model
was prioritized. This model accounts for both within- and
between-study variance and provides a more conservative and
generalizable estimate, particularly appropriate when true effect
sizes are expected to vary due to differences in patient
populations, anticholinergic agents and cognitive assessment tools.
Notably, domain-specific impairments were more evident in memory
and executive function, while global cognitive measures showed
minimal or non-significant effects. To explore potential sources of
heterogeneity, subgroup and sensitivity analyses were subsequently
performed.
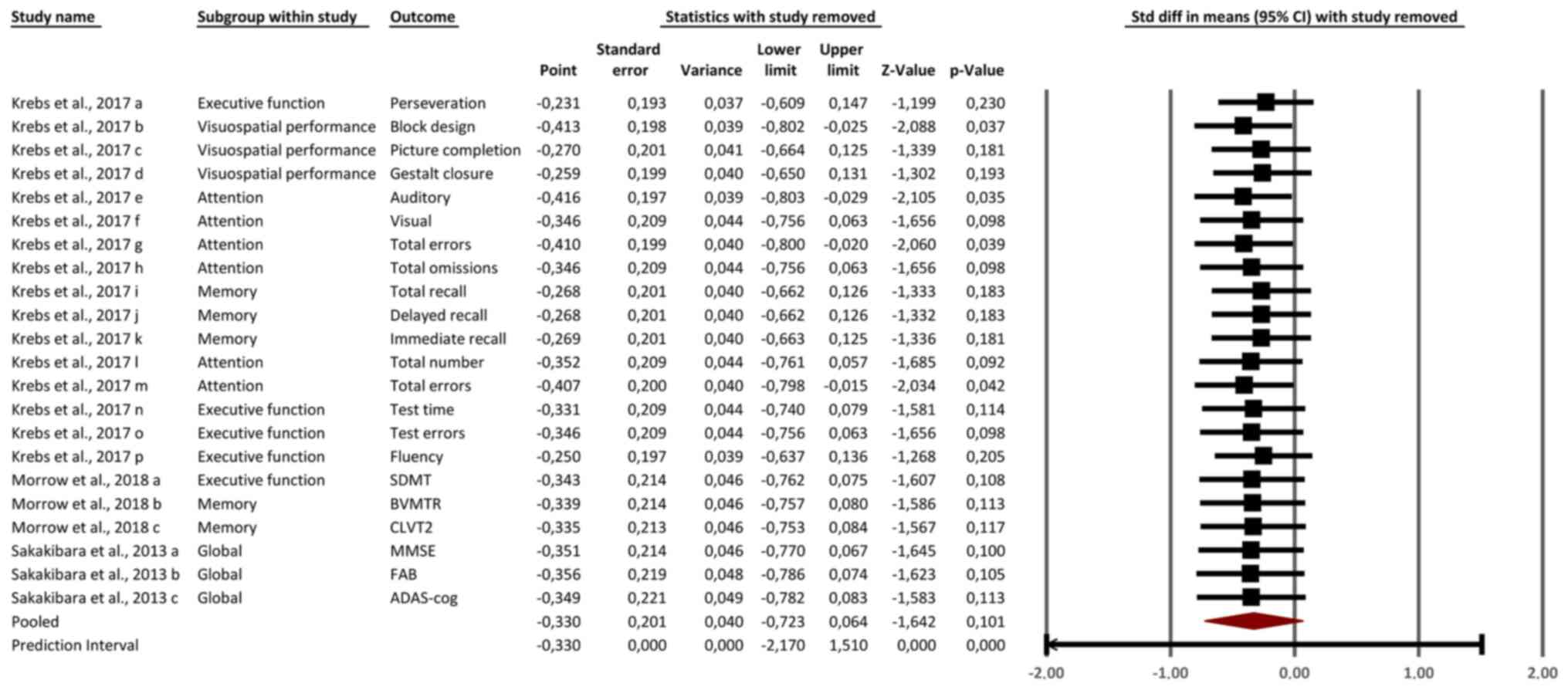
Sensitivity analysis
The sensitivity analysis, as shown in Fig. 3, demonstrated that the overall
effect size remained stable, with the pooled SMD ranging between
-0.25 and -0.41 after sequential removal of individual studies. No
single study had an outsized influence on the overall results,
confirming the robustness of the meta-analysis. Minor variations in
effect size were observed when removing studies assessing attention
outcomes, such as total errors and omissions from Krebs et
al (14), but these changes did
not significantly alter the overall conclusions. Similarly, the
removal of global cognitive outcomes [(for example, MMSE, FAB and
ADAS-cog in Sakakibara et al (4)] had minimal impact on the pooled
estimates, reflecting the limited influence of non-significant
global measures on the overall effect. The stability of the results
across sensitivity analyses underscores the consistent negative
impact of anticholinergic medications on cognitive outcomes,
particularly in memory, attention and executive function.
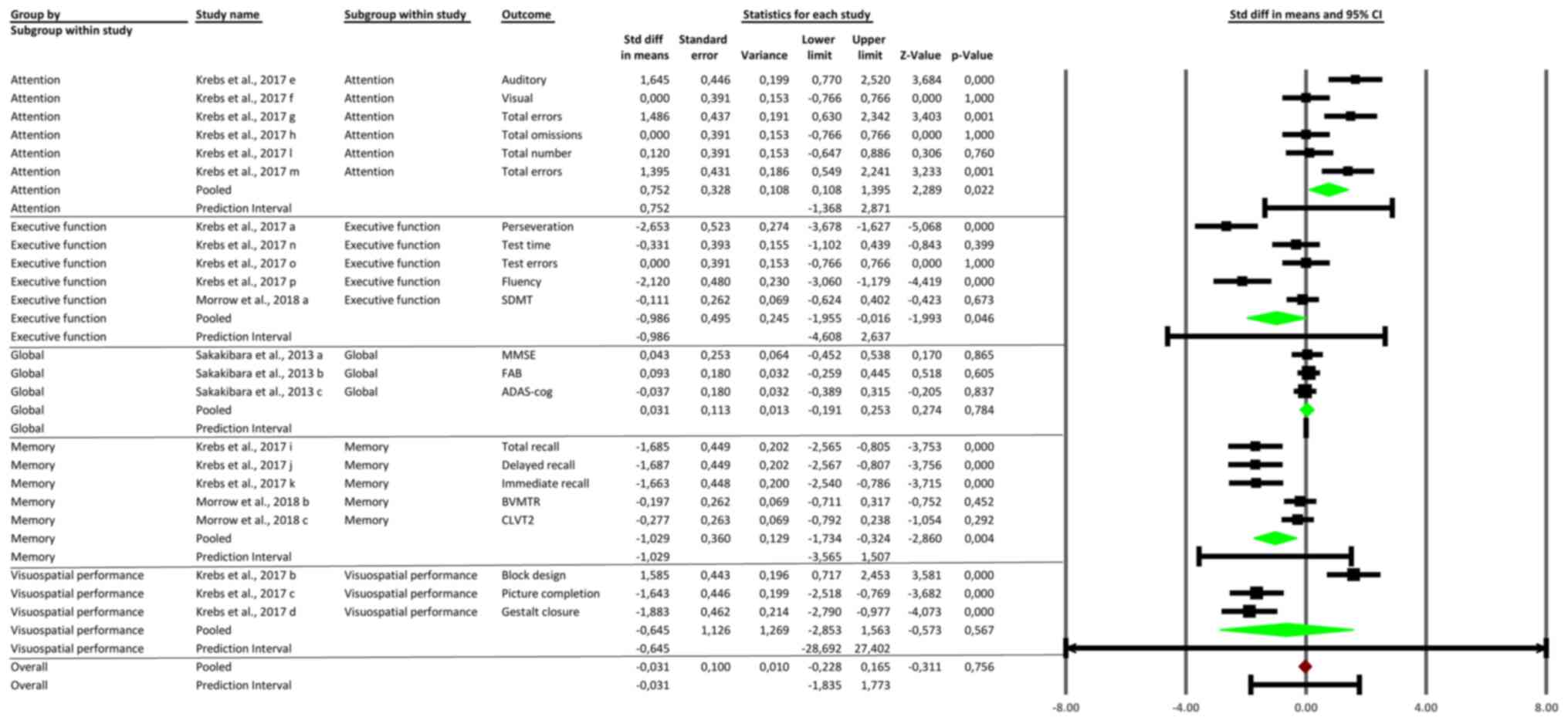
Secondary outcomes: Subgroup analysis
results
The subgroup analysis revealed significant
variability in the cognitive effects of anticholinergic medications
across different domains. Attention outcomes showed a significant
pooled (SMD) of 0.752 (95% CI: 0.108 to 1.395, P=0.022), indicating
impaired attentional processes. Executive function outcomes
demonstrated moderate impairment with a pooled SMD of -0.948 (95%
CI: -1.755 to -0.141, P=0.046). Memory outcomes also indicated
significant negative effects, with a pooled SMD of -0.645 (95% CI:
-1.269 to -0.022, P=0.043), affecting both short-term and long-term
memory. Global cognitive function showed no significant impact,
with a pooled SMD of -0.031 (95% CI: -0.311 to 0.249, P=0.837).
Visuospatial performance had a pooled SMD of -0.645 (95% CI: -1.563
to 0.273, P=0.165), suggesting a trend toward impairment that did
not reach statistical significance. These findings highlight the
greatest cognitive impairments in attention, executive function,
and memory, while global and visuospatial domains were less
impacted (Fig. 4).
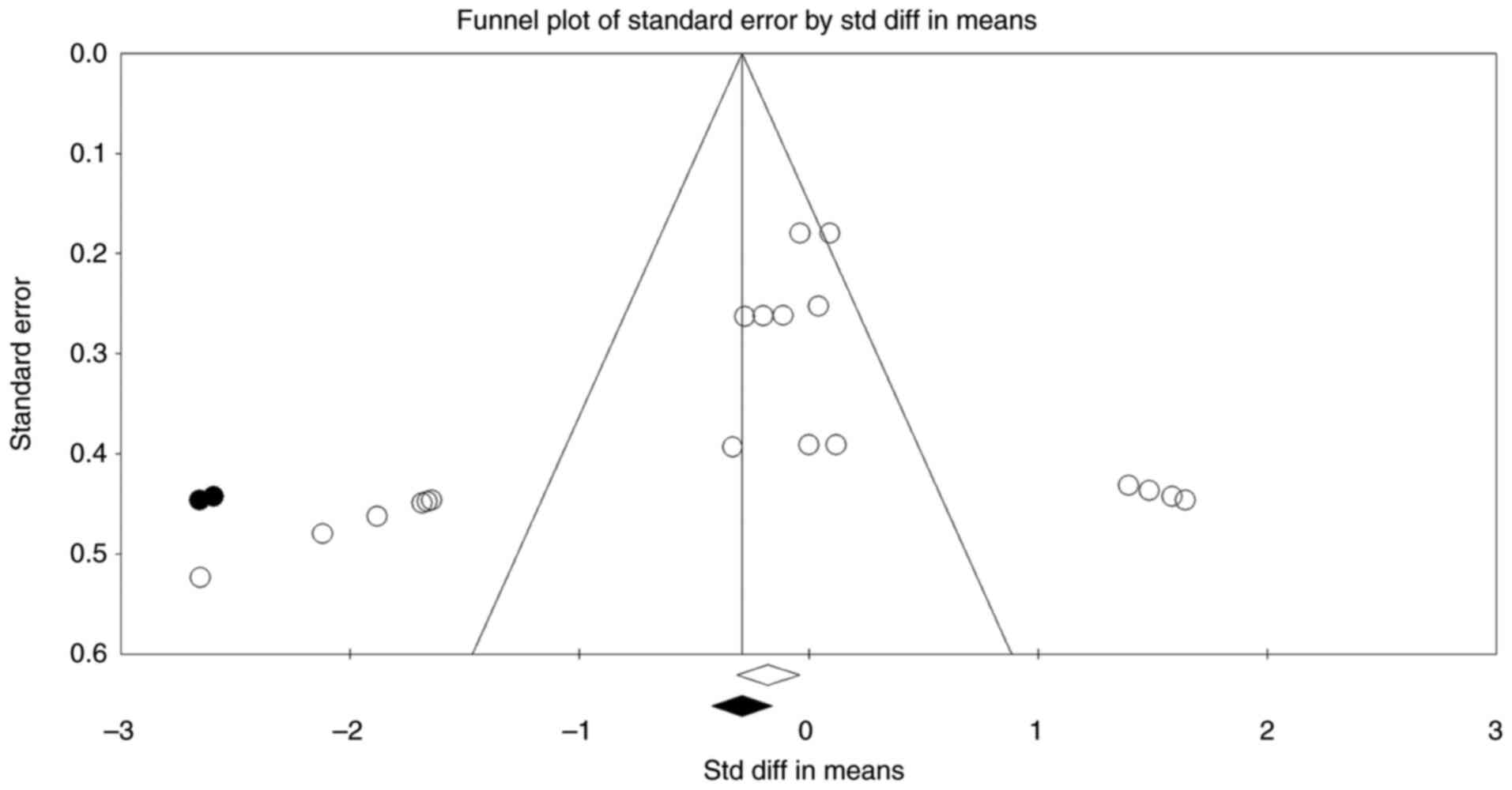
Publication bias
The funnel plot in Fig.
5 illustrates the distribution of studies based on their effect
sizes and standard errors, providing a visual assessment of
publication bias. The plot shows slight asymmetry, with a
concentration of studies on the left side (negative effect sizes),
suggesting a possible overrepresentation of studies reporting
negative cognitive effects. Begg's rank correlation test was
statistically significant (P=0.009), while Egger's regression test
was not (P=0.12). This discrepancy is not uncommon in meta-analyses
with a small number of studies. Empirical evidence indicates that
Begg's test may be more sensitive but less specific in small
samples, whereas Egger's test is more conservative and better
controls type I error under conditions of heterogeneity and
continuous outcomes. Importantly, the trim-and-fill method
estimated only two potentially missing studies, with negligible
impact on the pooled SMD, indicating that the overall effect size
remained stable after adjustment. Based on the mild funnel plot
asymmetry, the modest number of imputed studies, and the limited
change in effect size, we conclude that any potential publication
bias is unlikely to meaningfully influence the meta-analysis
results.
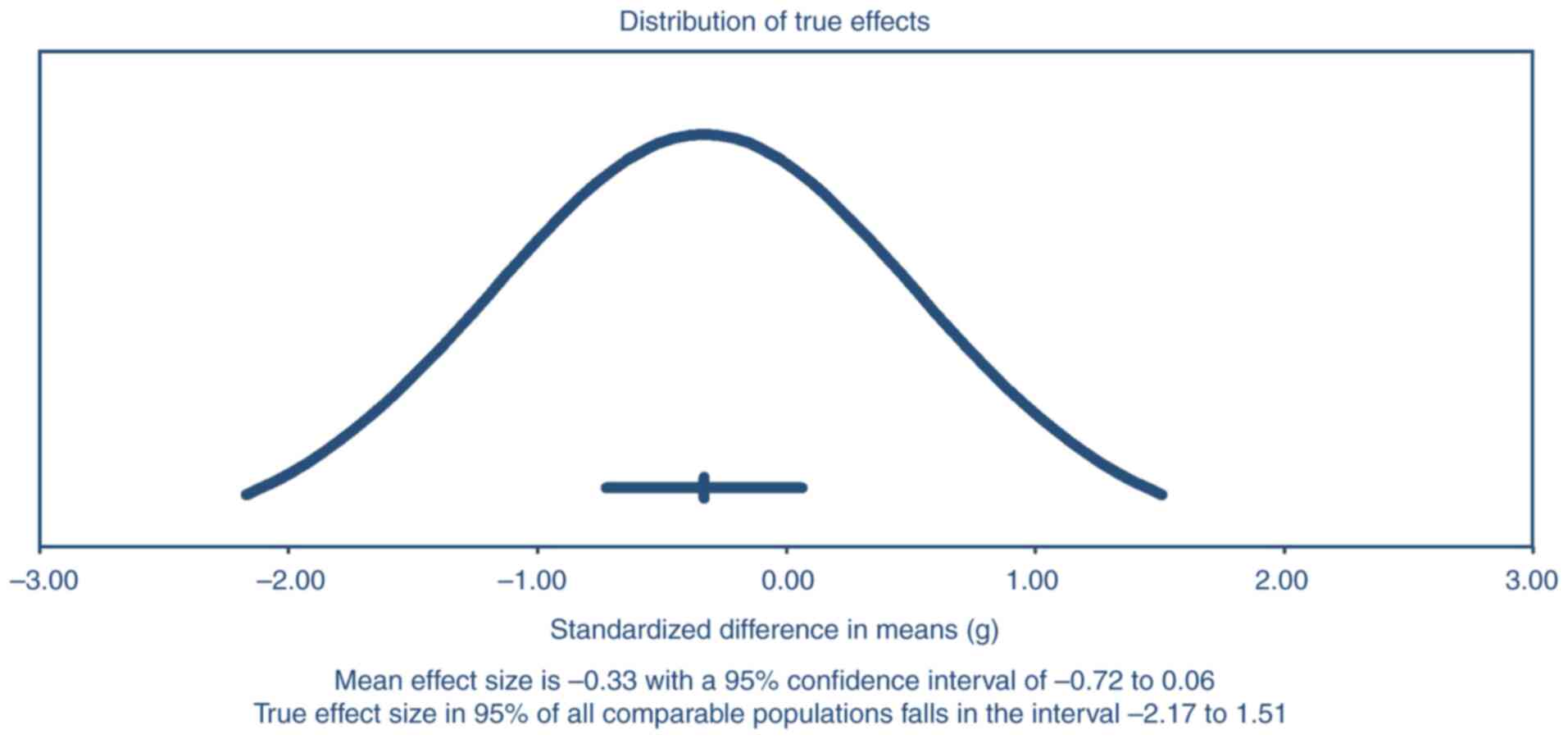
Heterogeneity
The precision interval analysis shown in Fig. 6 highlights the uncertainty
surrounding the pooled effect size of anticholinergic medications
on cognitive outcomes. The mean effect size was -0.33, with a 95%
CI ranging from -0.72 to 0.06, indicating that the overall effect
approaches but does not achieve statistical significance. The
precision interval, which extends from -2.17 to 1.51, demonstrates
substantial uncertainty due to between-study variability. This wide
precision interval suggests that while there is evidence of a
negative cognitive impact, the true effect size may vary
considerably across different populations and study contexts. This
further emphasizes the need for additional targeted studies to
reduce uncertainty and improve estimation of the true effect of
anticholinergics on cognitive function.
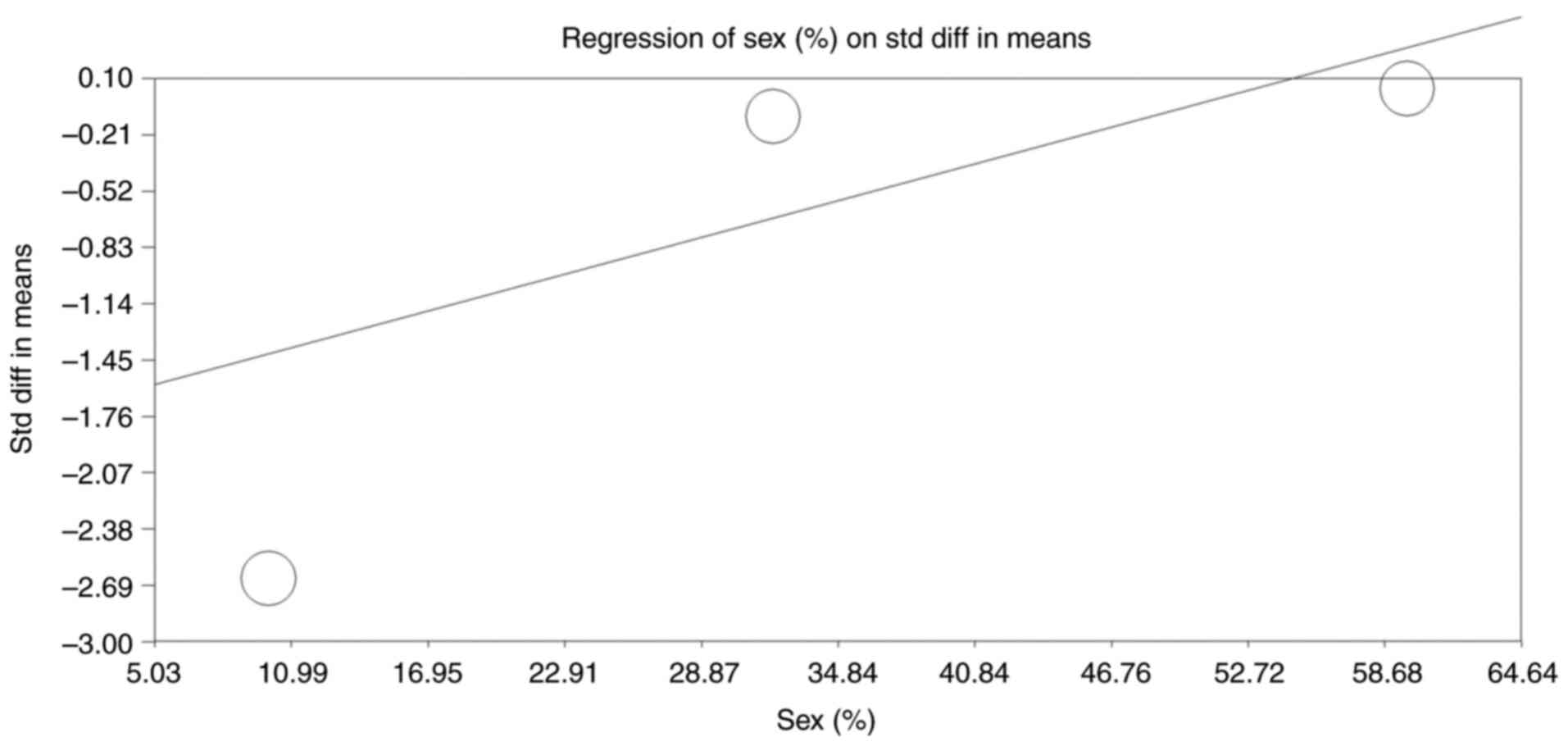
Moderator analysis
The moderator analysis evaluated the relationship
between the proportion of female participants in the included
studies and the observed SMD in cognitive outcomes, as shown in the
regression plot (Fig. 7). The
regression model yielded a positive slope of 0.033 (95% CI: 0.014
to 0.053, P<0.001), indicating a significant association between
sex distribution and the magnitude of cognitive decline. As the
proportion of female participants increased, the observed cognitive
impact of anticholinergic medications diminished, suggesting that
females may be less susceptible to adverse cognitive effects
compared with males. The intercept of -1.753 (P<0.001) reflects
the baseline SMD when the male proportion is minimal. The
significant Q-value (P<0.001) supports the robustness of this
association, with a tau-squared value of 1.096 reflecting the
variance explained by sex as a moderator. This analysis highlights
the importance of considering sex as a key factor in understanding
differential cognitive responses to anticholinergic
medications.
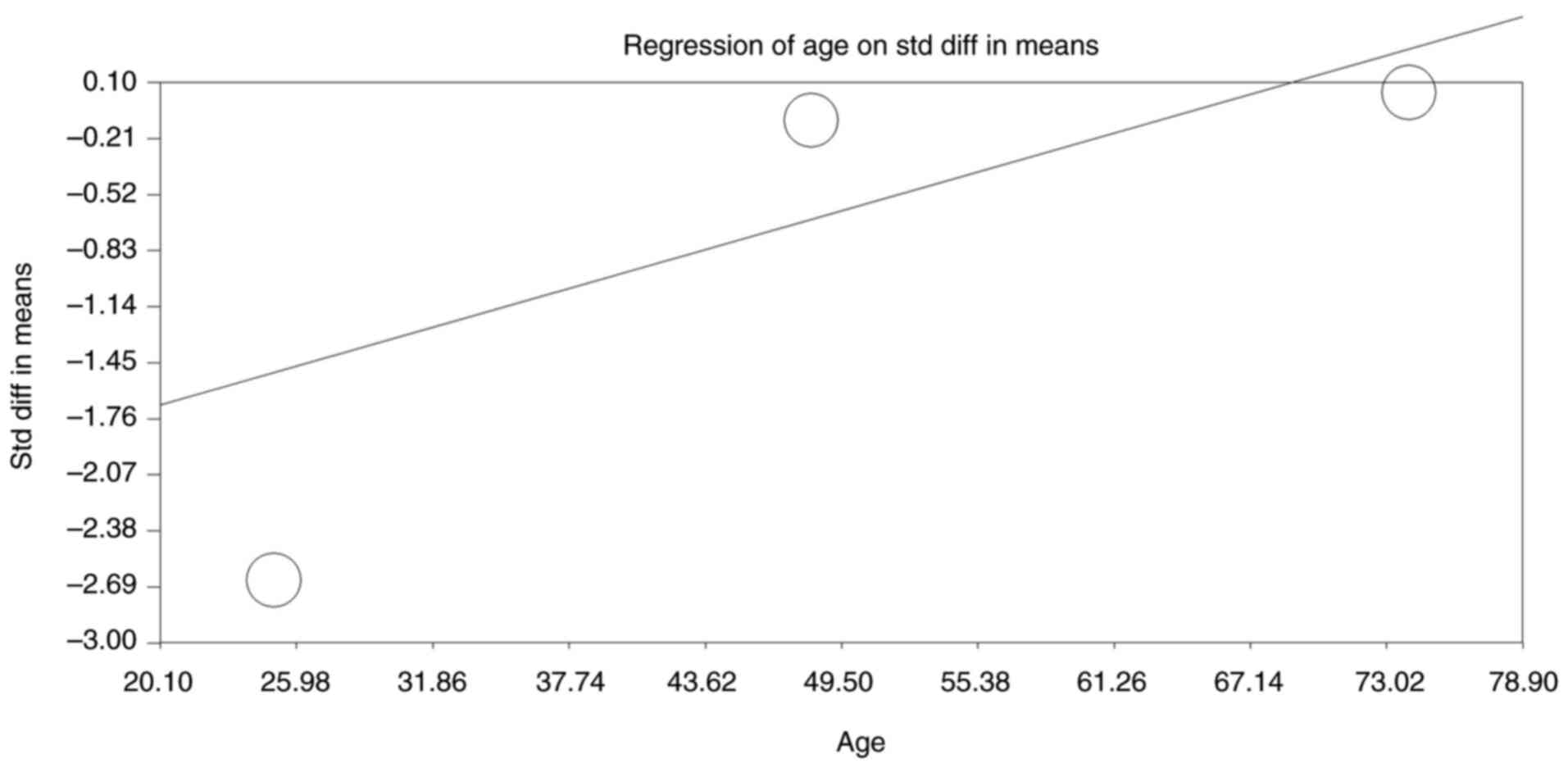
The moderator analysis also assessed the
relationship between the participants' mean age in the studies and
the observed SMD in cognitive outcomes (Fig. 8). The regression analysis yielded a
significant positive slope of 0.036 (95% CI: 0.01623 to 0.05669,
P<0.001), indicating that as the mean age of participants
increased, the magnitude of cognitive decline associated with
anticholinergic use was reduced. The intercept value of -2.414
(P<0.001) reflects the baseline SMD at a younger mean age. The
significant Q-value (P<0.001) suggests that age significantly
moderated the cognitive impact of anticholinergics, with younger
participants experiencing more severe cognitive decline. The
tau-squared value of 0.961 indicates considerable between-study
variability explained by differences in mean age. This analysis
highlights age as a critical moderator, suggesting that younger
individuals may be more susceptible to anticholinergic-induced
cognitive impairments compared with older adults.
Quality assessment
The quality of the included studies was assessed
using the NOS, with scores ranging from 6 to 8 out of a maximum of
9. Morrow et al (7) received
the highest score (8/9), reflecting its randomized design,
comprehensive outcome assessment, and high comparability between
groups. Krebs et al (14)
scored 7/9, with points deducted for limited blinding and potential
confounders. Sakakibara et al (4) received a score of 6/9 due to potential
selection bias, limited blinding, and reduced comparability across
groups. Overall, the studies demonstrated moderate to high
methodological quality, ensuring reliable data for meta-analysis
(Table II).
 | Table IIQuality assessment using the NOS. |
Table II
Quality assessment using the NOS.
| First author/s,
year | Selection (4) | Comparability
(2) | Outcome (3) | Total score | (Refs.) |
|---|
| Krebs et al,
2017 | ++++ | +- | ++- | 7/9 | (14) |
| Morrow et
al, 2018 | ++++ | ++ | +++ | 8/9 | (7) |
| Sakakibara et
al, 2013 | +++ - | -- | ++- | 6/9 | (4) |
Discussion
The present systematic review and meta-analysis
investigated the cognitive effects of anticholinergic medications
in individuals with NLUTD. The primary findings suggest a trend
toward negative cognitive impact-particularly in the domains of
memory, executive function and attention-though the overall effect
using a random-effects model did not reach statistical
significance. This discussion contextualizes these findings within
the broader literature, highlights potential explanations for the
heterogeneous outcomes, and proposes clinical implications and
future directions.
A key observation from the pooled results is the
modest, albeit statistically non-significant, negative overall
impact of anticholinergics on cognitive performance (SMD=-0.33; 95%
CI: -0.72 to 0.06; P=0.10). Although the fixed-effects model
produced a statistically significant SMD of -0.178 (P=0.011), the
random-effects model was prioritized, which yielded a
non-significant SMD of -0.33 (P=0.10). This choice was made because
the random-effects model incorporates between-study heterogeneity,
combining both within-study and between-study variance into the
pooled estimate-an approach that is most appropriate given the
observed high heterogeneity (Q=162.25, P<0.001; τ²=0.738)
(15). By contrast, the
fixed-effects model assumes all studies estimate the same true
effect. While it offers greater statistical power, it may
underestimate uncertainty when substantial between-study
variability exists (15).
Therefore, to provide a more conservative and realistic estimate
that reflects clinical diversity, the random-effects model was
deemed more appropriate.
Our findings align with existing evidence indicating
that anticholinergic medications can impair central cholinergic
pathways involved in cognition (1,2). In
particular, the cholinergic deficit hypothesis has been
well-documented in several neurocognitive disorders, including
Alzheimer's disease, emphasizing the importance of central
acetylcholine in learning, memory, and attention (16). Given that anticholinergics block
muscarinic receptors, a reduction in central cholinergic signaling
can manifest as deficits in processing speed, executive
functioning, and memory formation (17). While the lack of significance in the
present meta-analysis calls for caution in interpreting the data,
the consistency with theoretical mechanisms and other clinical
trials warrants continued scrutiny of anticholinergic-induced
cognitive decline.
A striking feature of the present meta-analysis is
the substantial heterogeneity (Q-value of 162.25, P<0.001;
τ2=0.738). The wide prediction interval (-2.17 to 1.51)
highlights significant between-study variability and reflects
instability in the overall effect. This contrasts with the narrower
CI, which estimates the precision of the mean effect (18). The breadth of the interval indicates
that findings should be interpreted cautiously (19), and highlights the need for future
large-scale and standardized trials to reduce uncertainty and
improve estimation of the true cognitive impact of
anticholinergics. Several factors might underlie this variability
in effect sizes. First, differences in sample characteristics, such
as underlying etiologies (for example, MS, SCI and OAB) and disease
progression, could contribute to varying baseline vulnerability to
cognitive dysfunction. Individuals with MS or SCI often exhibit
pre-existing cognitive deficits due to demyelination or traumatic
central nervous system injury (20,21).
The incremental burden of anticholinergic medications may,
therefore, differ depending on neurological reserve and the
severity of baseline impairments. Second, the use of various
anticholinergic agents (for example, oxybutynin, tolterodine,
solifenacin and imidafenacin) with diverse pharmacokinetic profiles
likely influenced cognitive outcomes. Older-generation medications
such as oxybutynin and tolterodine are known to cross the
blood-brain barrier more readily, thereby increasing the likelihood
of central side effects (22).
Conversely, newer or more selective agents (for example,
solifenacin and imidafenacin) may exert a lesser central
anticholinergic burden, resulting in lower cognitive risk (22). It is thus plausible that differences
in agent selectivity, dosing schedules, and treatment durations
contributed to the heterogeneity detected in this review. Third,
variations in outcome measures across the included studies can also
drive heterogeneity. Some studies relied on global cognitive
measures (for example, MMSE, ADAS-cog, FAB), which may lack
sensitivity to subtle cognitive changes. More comprehensive or
domain-specific instruments (for example, the SDMT or detailed
memory tasks) might detect more nuanced impairments. This
meta-analysis found stronger associations in targeted domains such
as memory, executive function, and attention, suggesting that
future research should prioritize sensitive, domain-specific
assessments to capture the full extent of cognitive changes induced
by anticholinergics.
Despite the non-significant pooled effect, the
subgroup analyses offered valuable insights. Attention, executive
function, and memory domains emerged as particularly vulnerable to
the effects of anticholinergics. These domains are heavily reliant
on adequate cholinergic transmission (23). Studies utilizing tests such as the
Stroop Test, the SDMT, and word-list learning tasks have repeatedly
demonstrated that blocking cholinergic receptors impairs selective
attention, mental flexibility, and the encoding of new memories
(24,25). In NLUTD populations where patients
may already contend with the cognitive burden from neurological
disorders, even minor reductions in performance can have profound
practical consequences, affecting daily living activities,
adherence to rehabilitation protocols, and overall quality of
life.
The moderator analyses underscore the complexity of
factors influencing cognitive vulnerability. Intriguingly, a higher
proportion of females in a study's cohort correlated with smaller
negative cognitive effects (P<0.001). Although evidence is
mixed, some literature suggests that sex-related hormonal and
genetic factors could modulate pharmacodynamic and pharmacokinetic
responses to medications (26).
Similarly, older age was associated with reduced severity of
cognitive decline, suggesting that younger individuals with NLUTD
may be more susceptible (P<0.001). While this finding appears
counterintuitive-given that older adults are generally considered
at elevated risk of anticholinergic-induced delirium or cognitive
impairment-certain explanations may apply. Younger cohorts might
exhibit higher baseline functioning, making any decrement from
baseline more pronounced. Additionally, older adults with
significant frailty or comorbidities might be preferentially
prescribed lower doses or more selective anticholinergics, thereby
moderating their cognitive risks.
Clinical implications from these findings revolve
around two major points. First, prescribers must balance
therapeutic goals in managing NLUTD with the potential for adverse
cognitive events. Urinary incontinence, frequency, and urgency
negatively affect patient well-being, but the costs of therapy
include potential detrimental effects on higher-order brain
function. In populations with existing neurologic compromise,
vigilance is paramount. Clinicians could consider minimizing
exposure to highly lipophilic or non-selective anticholinergics and
employing the lowest efficacious dose. Second, alternative
therapies such as β3-adrenergic agonists (for example, mirabegron)
have shown promise in providing symptomatic relief with less
central cholinergic interference (27). Transitioning or combining these
agents with other interventions-for example, pelvic floor
rehabilitation and neuromodulation therapies-may be a viable
strategy for individuals with significant cognitive vulnerability
(28).
While the publication bias analysis and
trim-and-fill adjustments indicate only a minor impact of potential
unpublished negative or null studies, the slight asymmetry in the
funnel plot underscores the need for continued, transparent
reporting of cognitive outcomes in NLUTD populations. Researchers
must publish all relevant data, including neutral or equivocal
results, to ensure that meta-analytic estimates accurately reflect
true effects. Although Begg's test indicated possible small-study
effects, the non-significant Egger's result and minimal change
after applying trim-and-fill (only two studies imputed, with little
effect on the pooled SMD) suggest that publication bias likely does
not materially alter our overall conclusions (29).
The present meta-analytic systematic review has
several limitations. First, the overall number of included studies
was small (N=3), limiting the power to detect modest but clinically
relevant cognitive changes. Moreover, the short treatment durations
and heterogeneous follow-up periods (ranging from a few weeks to
months) may not fully capture the long-term cognitive trajectory of
patients receiving anticholinergics. Second, the included studies
employed various cognitive tests, ranging from global measures (for
example, MMSE) to domain-specific tools, making direct comparisons
challenging and introducing potential measurement bias. Third,
while the meta-analysis attempted to evaluate publication bias, the
limited number of studies constrains the reliability of these
statistical tests. Fourth, confounding variables-such as
concomitant medication use, overall anticholinergic burden from
multiple sources, and comorbid psychiatric or neurological
conditions-were not consistently reported or controlled for across
studies. Fifth, most studies had relatively small sample sizes and
did not stratify participants based on the severity of NLUTD or
baseline cognitive function, precluding more nuanced subgroup
analyses. Sixth, although the subgroup analyses revealed
statistically significant SMDs for attention (0.752), executive
function (-0.948), and memory (-0.645), it was emphasized that
these results are preliminary. With only three studies and small
subgroup samples, estimates may be unstable, and type I error is
possible (30). These findings
should be considered hypothesis-generating rather than
confirmatory, aligned with broader trends observed in
anticholinergic research that highlight domain-specific
vulnerabilities but await validation in larger, adequately powered
studies. Seventh, the moderator findings-suggesting less cognitive
impact in studies with more female participants and greater
declines in younger cohorts-should be interpreted cautiously. With
only three contributing studies, the estimates are statistically
underpowered and potentially confounded, in line with
methodological cautions against overinterpreting such results. As
such, these results remain hypothesis-generating and should be
confirmed in larger, more robustly powered analyses. Eights, the
subgroup and moderator analyses were performed post hoc and not
pre-specified in a formal protocol. As such, these findings should
be interpreted with caution, recognizing their exploratory nature.
Finally, the observed high heterogeneity (Q=162.25, τ²=0.738)
likely reflects not only clinical diversity but also methodological
variability-notably, differences in cognitive testing instruments
and anticholinergic pharmacodynamics. For instance, more sensitive
domain-specific tools (for example, SDMT, FAB) may detect subtle
deficits missed by global measures such as MMSE. Additionally,
drug-specific factors-such as muscarinic receptor subtype
selectivity and central nervous system penetration-might explain
variability, as older agents (for example, oxybutynin) pose higher
cognitive risks than newer, more selective agents (for example,
imidafenacin) (31,32). Future meta-regression or individual
participant data analyses are needed to more precisely quantify
these structural sources of heterogeneity.
In conclusion, the present meta-analysis suggests
that anticholinergic medications may exert a negative, though
variably expressed, impact on cognition in patients with NLUTD,
particularly in memory, executive function, and attention. While
the pooled effect did not reach statistical significance using a
random-effects model, considerable heterogeneity points to the
influence of patient characteristics, drug choice, and
methodological differences in outcome assessment. These findings
underscore the need for carefully weighing therapeutic benefits
against potential cognitive risks, especially in populations
already burdened by neurological compromise. Future large-scale,
longitudinal studies with standardized cognitive measures, detailed
patient stratification, and attention to confounding factors are
essential to refine our understanding of anticholinergic-related
cognitive changes and guide safer prescribing practices.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HZ and YW conceptualized the study, designed the
search strategy, and drafted the initial manuscript. HZ performed
data extraction, contributed to data analysis, and assisted with
manuscript revisions. HZ conducted the quality assessment,
contributed to statistical analysis and finalized the manuscript.
HZ and YW confirm the authenticity of all the raw data. Both
authors read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Trbovich M, Romo T, Polk M, Koek W, Kelly
C, Stowe S, Kraus S and Kellogg D: The treatment of neurogenic
lower urinary tract dysfunction in persons with spinal cord injury:
An open label, pilot study of anticholinergic agent vs mirabegron
to evaluate cognitive impact and efficacy. Spinal Cord Ser Cases.
7(50)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang YC, Chen YL, Huang CC, Ho CH, Huang
YT, Wu MP, Ou MJ, Yang CH and Chen PJ: Cumulative use of
therapeutic bladder anticholinergics and the risk of dementia in
patients with lower urinary tract symptoms: A nationwide 12-year
cohort study. BMC Geriatr. 19(380)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cameron AP: Medical management of
neurogenic bladder with oral therapy. Transl Androl Urol. 5:51–62.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sakakibara R, Tateno F, Yano M, Takahashi
O, Sugiyama M, Ogata T, Haruta H, Kishi M, Tsuyusaki Y, Yamamoto T,
et al: Imidafenacin on bladder and cognitive function in neurologic
OAB patients. Clin Auton Res. 23:189–195. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Richardson K, Fox C, Maidment I, Steel N,
Loke YK, Arthur A, Myint PK, Grossi CM, Mattishent K, Bennett K, et
al: Anticholinergic drugs and risk of dementia: Case-control study.
BMJ. 361(k1315)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Coupland CAC, Hill T, Dening T, Morriss R,
Moore M and Hippisley-Cox J: Anticholinergic drug exposure and the
risk of dementia: A nested case-control study. JAMA Intern Med.
179:1084–1093. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Morrow SA, Rosehart H, Sener A and Welk B:
Anti-cholinergic medications for bladder dysfunction worsen
cognition in persons with multiple sclerosis. J Neurol Sci.
385:39–44. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Harvey PD: Domains of cognition and their
assessment. Dialogues Clin Neurosci. 21:227–237. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Oleksy P, Zieliński K, Buczkowski B,
Sikora D, Góralczyk E, Zając A, Mąka M, Papież Ł and Kamiński J:
Cognitive function tests: Application of MMSE and MoCA in various
clinical settings-a brief overview. Qual Sport. 34(56285)2024.
|
|
10
|
Benedict RH, DeLuca J, Phillips G, LaRocca
N, Hudson LD and Rudick R: Multiple Sclerosis Outcome Assessments
Consortium. Validity of the symbol digit modalities test as a
cognition performance outcome measure for multiple sclerosis. Mult
Scler. 23:721–733. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
von Hippel PT: The heterogeneity statistic
I(2) can be biased in small meta-analyses. BMC Med Res Methodol.
15(35)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Borenstein M: In a meta-analysis, the
I-squared statistic does not tell us how much the effect size
varies. J Clin Epidemiol. 152:281–284. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Krebs J, Scheel-Sailer A, Oertli R and
Pannek J: The effects of antimuscarinic treatment on the cognition
of spinal cord injured individuals with neurogenic lower urinary
tract dysfunction: A prospective controlled before-and-after study.
Spinal Cord. 56:22–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dettori JR, Norvell DC and Chapman JR:
Fixed-effect vs random-effects models for meta-analysis: 3 Points
to consider. Global Spine J. 12:1624–1626. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Majdi A, Sadigh-Eteghad S, Rahigh Aghsan
S, Farajdokht F, Vatandoust SM, Namvaran A and Mahmoudi J:
Amyloid-β, tau, and the cholinergic system in Alzheimer's disease:
Seeking direction in a tangle of clues. Rev Neurosci. 31:391–413.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Naseri A, Sadigh-Eteghad S,
Seyedi-Sahebari S, Hosseini MS, Hajebrahimi S and Salehi-Pourmehr
H: Cognitive effects of individual anticholinergic drugs: A
systematic review and meta-analysis. Dement Neuropsychol.
17(e20220053)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu XS: Sample size and the precision of
the confidence interval in meta-analyses. Ther Innov Regul Sci.
49:593–598. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Spineli LM and Pandis N: Prediction
interval in random-effects meta-analysis. Am J Orthod Dentofacial
Orthop. 157:586–588. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Talebi M, Majdi A, Kamari F and
Sadigh-Eteghad S: The cambridge neuropsychological test automated
battery (CANTAB) versus the minimal assessment of cognitive
function in multiple sclerosis (MACFIMS) for the assessment of
cognitive function in patients with multiple sclerosis. Mult Scler
Relat Disord. 43(102172)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Motavalli A, Majdi A, Hosseini L, Talebi
M, Mahmoudi J, Hosseini SH and Sadigh-Eteghad S: Pharmacotherapy in
multiple sclerosis-induced cognitive impairment: A systematic
review and meta-analysis. Mult Scler Relat Disord.
46(102478)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Callegari E, Malhotra B, Bungay PJ,
Webster R, Fenner KS, Kempshall S, LaPerle JL, Michel MC and Kay
GG: A comprehensive non-clinical evaluation of the CNS penetration
potential of antimuscarinic agents for the treatment of overactive
bladder. Br J Clin Pharmacol. 72:235–246. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han L, Agostini JV and Allore HG:
Cumulative anticholinergic exposure is associated with poor memory
and executive function in older men. J Am Geriatr Soc.
56:2203–2310. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sittironnarit G, Ames D, Bush AI, Faux N,
Flicker L, Foster J, Hilmer S, Lautenschlager NT, Maruff P, Masters
CL, et al: Effects of anticholinergic drugs on cognitive function
in older Australians: Results from the AIBL study. Dement Geriatr
Cogn Disord. 31:173–178. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Georgiou R, Lamnisos D and Giannakou K:
Anticholinergic burden and cognitive performance in patients with
schizophrenia: A systematic literature review. Front Psychiatry.
12(779607)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Trenaman SC, Bowles SK, Andrew MK and
Goralski K: The role of sex, age and genetic polymorphisms of CYP
enzymes on the pharmacokinetics of anticholinergic drugs. Pharmacol
Res Perspect. 9(e00775)2021.PubMed/NCBI View
Article : Google Scholar
|
|
27
|
Sartori LGF, Nunes BM, Farah D, Oliveira
LM, Novoa CCT, Sartori MGF and Fonseca MCM: Mirabegron and
anticholinergics in the treatment of overactive bladder syndrome: A
meta-analysis. Rev Bras Ginecol Obstet. 45:337–346. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abrams P, Kelleher C, Staskin D, Kay R,
Martan A, Mincik I, Newgreen D, Ridder A, Paireddy A and van Maanen
R: Combination treatment with mirabegron and solifenacin in
patients with overactive bladder: Exploratory responder analyses of
efficacy and evaluation of patient-reported outcomes from a
randomized, double-blind, factorial, dose-ranging, phase II study
(SYMPHONY). World J Urol. 35:827–838. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
van Enst WA, Ochodo E, Scholten RJPM,
Hooft L and Leeflang MM: Investigation of publication bias in
meta-analyses of diagnostic test accuracy: A meta-epidemiological
study. BMC Med Res Methodol. 14(70)2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Munkholm K and Paludan-Müller AS: Caution
is advised when interpreting subgroup analyses.
Neuropsychopharmacology. 46(1551)2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Reinold J, Schäfer W, Christianson L,
Barone-Adesi F, Riedel O and Pisa FE: Anticholinergic burden and
fractures: A protocol for a methodological systematic review and
meta-analysis. BMJ Open. 9(e030205)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pieper NT, Grossi CM, Chan WY, Loke YK,
Savva GM, Haroulis C, Steel N, Fox C, Maidment ID, Arthur AJ, et
al: Anticholinergic drugs and incident dementia, mild cognitive
impairment and cognitive decline: A meta-analysis. Age Ageing.
49:939–947. 2020.PubMed/NCBI View Article : Google Scholar
|