Introduction
Retinopathy of prematurity (ROP) is a proliferative
vascular disease that occurs in areas of the retina where vascular
development is incomplete in preterm infants. It is characterized
by abnormal blood vessel proliferation, which can lead to retinal
detachment and blindness (1). A
meta-analysis involving 121,618 preterm infants reported that the
global incidence of ROP in preterm infants is 31.9%, with the
incidence of severe ROP at 7.5% (2). A cohort study (3) conducted in the United States from 2003
to 2019 involving 125,212 cases of ROP showed that the overall
incidence of ROP in premature infants increased from 4.4 to 8.1%;
the incidence in African-American infants rose from 5.8 to 11.6%
and that in infants from low-income families rose from 4.9 to 9.0%;
and the increase was the greatest in the southern region (3.7 to
8.3%). Therefore, there are certain differences in the incidence of
ROP in various regions and populations, and it is necessary to
develop risk prediction models for different groups. Clinically,
the diagnosis and severity assessment of ROP are based on the
International Classification of Retinopathy of Prematurity, 3rd
Edition (ICROP3), released in 2021. This classification
systematically defines the lesion location, progression extent and
risk level of ROP through indicators including lesion zones I-III,
disease stages 1-5 and plus disease (severe retinal vascular
dilation and tortuosity in the posterior pole), thereby providing a
foundation for clinical diagnosis (4).
Current research suggests that the pathogenesis of
ROP is characterized by a biphasic pattern of retinal vascular
development: An initial phase of reduced retinal vascular growth,
followed by excessive vessel proliferation into the vitreous body
(5). Vascular endothelial growth
factor (VEGF) serves a critical role in retinal neovascularization.
In recent years, therapeutic approaches have shifted from laser
therapy to anti-VEGF therapy (6).
The RAINBOW Extension study confirmed that ranibizumab, when
administered to preterm infants with ROP, significantly decreases
the risk of high myopia (7).
However, follow-up studies (8,9) have
indicated that infants receiving anti-VEGF therapy may have a
higher risk of adverse neurodevelopmental outcomes. Furthermore, in
remote areas, the lack of medical resources and equipment can lead
to worsening conditions in preterm infants, resulting in blindness
(10). Therefore, compared with
single biomarkers or imaging examinations, ROP prediction models
have value in integrating multi-dimensional clinical data to
achieve dynamic risk stratification. Particularly in resource-poor
regions, these models may compensate for the insufficiency of
fundus screening equipment, enabling the rapid identification of
high-risk preterm infants through basic indicators. To obtain more
practical evidence for ROP risk prediction, current research
(11) focuses on early disease risk
prediction, conducting large-scale clinical cohort studies, and
developing new artificial intelligence/machine learning algorithms
to enhance the accuracy and practicality of ROP risk prediction.
However, with the development of computer vision and deep learning
algorithms, despite the continuous emergence of various prediction
models and algorithms, challenges such as overfitting and
insufficient data diversity remain. There is significant
heterogeneity among studies (12,13) in
terms of sample size, predictors, model construction methods and
reported performance metrics, and there are relatively few
systematic reviews on the diagnostic performance of these models
(14,15).
Almutairi et al (10) performed a meta-analysis that focused
on the association between platelet count, thrombocytopenia and
severe ROP, and explored the potential mechanisms linking a single
biomarker to the disease through methods such as Bayesian model
averaging. However, this previous study was limited to the causal
exploration of a single risk factor, failing to involve the
systematic integration and methodological evaluation of existing
multifactorial ROP risk prediction models. It also did not assess
differences in applicability of various predictive tools in
clinical settings, and did not provide a comprehensive
understanding and basis for selecting ROP risk screening tools. The
present study aimed to overcome the limitations of single-factor
association studies by conducting a systematic review and
meta-analysis on risk prediction models for ROP in preterm infants.
It systematically organizes, synthesizes and evaluates the
methodological characteristics and application potential of
existing models, thereby providing a design framework for the
rational selection and optimization of ROP risk prediction models
in clinical practice.
Materials and methods
Study design
The present study adhered to the Transparent
Reporting of a Multivariable Prediction Model for Individual
Prognosis Or Diagnosis statement (16), and followed the critical appraisal
and data extraction checklist of Critical Appraisal and Data
Extraction for Systematic Reviews of Prediction Modelling Studies
(17). The study did not involve
human experiments or direct collection of clinical data, thus no
ethics committee approval was required. The key items of the
systematic review were as follows: People, preterm infants;
intervention model, development and publication of risk prediction
models for ROP in preterm infants; comparator, no competing model;
outcome, incidence of ROP in preterm infants (primary) and
performance metrics of the prediction model (secondary); timing,
from birth of preterm infants until ROP stabilizes or the relevant
observation period ends and setting, healthcare settings, including
neonatal intensive care units, pediatric wards and specialized
ophthalmic clinics in hospitals of various levels.
Search strategy
A comprehensive literature search was conducted
using the PubMed (pubmed.ncbi.nlm.nih.gov/), Cochrane Library
(cochranelibrary.com/), Web of Science
(https://www.webofscience.com/) and
Embase (https://www.embase.com/) databases to
identify studies associated with ROP in preterm infants. The core
search terms used across the four databases are as follows: PubMed:
‘Infant, Premature’ (MeSH), ‘Infant, Extremely Premature’ (MeSH),
‘Retinopathy of Prematurity’ (MeSH), ‘Retinal Diseases’ (MeSH),
‘Area Under Curve’ (MeSH); Web of Science: core topic terms
including ‘Infant, Premature’, ‘Infant, Extremely Premature’,
‘Retinopathy of Prematurity’, ‘Retinal Diseases’, ‘Area Under
Curve’, ‘AUC’; Embase: ‘prematurity’/exp, ‘retrolental
fibroplasia’/exp, ‘retina disease’/exp, ‘area under the curve’/exp;
Cochrane Library: ‘Infant, Premature’ (explode all trees), ‘Infant,
Extremely Premature’ (explode all trees), ‘Retinal Diseases’
(explode all trees), ‘Retinopathy of Prematurity’ (explode all
trees), ‘Area Under Curve’ (explode all trees). A detailed search
strategy is provided in Table SI,
Table SII, Table SIII and Table SIV. To minimize discrepancies from
database updates, searches were conducted from database inception
until April 18, 2025, with all data collection completed by this
date.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i)
Cross-sectional, cohort, retrospective or prospective studies; ii)
Diagnosis of ROP according to ICROP3(4), based on the affected area (zone I, II
or III), disease stage (stage 1-5) and retinal vascular
abnormalities [pre-plus disease (mild retinal vascular dilation and
tortuosity confined to the posterior pole, not meeting the severity
threshold for plus disease) or plus disease (severe retinal
vascular dilation and tortuosity involving the posterior pole,
typically affecting at least two quadrants, as defined by ICROP3 to
indicate advanced disease severity)] to determine disease severity;
iii) studies focusing on the development, validation or evaluation
of ROP prediction models in preterm infants; iv) study subjects
including preterm infants with a birth weight of ≥500 g or
gestational age of <37 weeks; v) prediction model including ≥2
predictors; vi) models associated with the occurrence, screening or
risk prediction of ROP, including traditional statistical models
(such as logistic regression) and machine learning algorithms (such
as decision trees and neural networks); vii) study providing
statistical indicators related to the model, such as the area under
the receiver operating characteristic curve (AUC) and viii) English
literature. The exclusion criteria were as follows: i) Systematic
reviews, meta-analyses or other types of review articles; ii)
studies that did not provide quantitative results for the
prediction model, or where key data were unavailable or there was a
substantial amount of missing data; iii) studies involving children
with severe congenital malformation, complex systemic disease or
other ocular disease; iv) studies lacking a control group or with
unclear control group definitions; v) unclear sample definitions or
a large number of non-preterm infants and vii) conference
abstracts, editorials or studies on radiomics.
Literature screening
The literature screening was conducted using the
EndNote reference management tool (version 21, endnote.com/). Two authors independently screened the
titles and abstracts of the studies, excluding those that did not
meet the inclusion criteria. Full texts of the remaining studies
were reviewed for further screening. In case of disagreements, a
third researcher (was consulted to resolve the discrepancies and
reach a consensus.
Data extraction
The following information was collected from the
included studies: First author, publication year, region, study
design, sample size, predictive variables, model type, validation
methods and model evaluation metrics. All data were independently
extracted by two researchers and cross-checked for accuracy.
Assessment of literature quality
The risk of bias in the predictive models was
assessed using the Prediction Model Risk Of Bias Assessment Tool
(PROBAST) (18). A total of two
authors independently conducted the evaluation and cross-validated
the results; in case of discrepancies, a third author was consulted
for final assessment. PROBAST is designed for evaluating the risk
of bias and applicability of prediction model studies. It has been
used in systematic reviews (19,20)
and is suitable for the development, validation, and updating of
diagnostic or prognostic prediction models (21). The core framework of PROBAST
includes four primary domains and 20 items, aiming to
systematically assess potential biases introduced during the
research design, implementation and analysis phases to ensure the
reliability and clinical applicability of the prediction models.
The evaluation considers whether the outcome definitions align with
clinical needs. If the outcome definitions in the domain match
clinical concerns and provide valuable information for clinical
decision-making, the applicability is classified as ‘low risk’. If
the outcome definitions are disconnected from clinical needs and do
not effectively guide clinical practice, the applicability is
considered ‘high risk’. If the alignment of the outcome definitions
with clinical needs is unclear, the applicability is rated as
‘unclear’.
Subgroup analyses
To explore potential sources of heterogeneity in the
performance of ROP risk prediction models, predefined subgroup
analyses were planned and conducted based on model type and
geographic region.
For subgroup analysis by model type, studies were
stratified into two distinct subgroups according to the modeling
approaches employed. The first subgroup included studies utilizing
traditional statistical models, with logistic regression being the
primary method. The second subgroup comprised studies that adopted
machine learning models, which encompassed various techniques such
as neural networks, support vector machines, long short-term
memory, and deep learning models.
For subgroup analysis by geographic region, studies
were stratified into three regional subgroups based on the
geographical locations of the study populations. The first subgroup
included studies conducted in South America. The second subgroup
consisted of studies performed in Asia. The third subgroup combined
studies from North America and Europe, as these regions share
similarities in healthcare systems and population characteristics,
justifying their integration for comparative analysis.
Statistical analysis
Statistical analysis was performed using R version
4.4.2 (R Foundation for Statistical Computing, 2024; cran.r-project.org/bin/windows/base/old/4.4.2/),
utilizing the ‘meta (https://cran.r-project.org/package=meta)’ and ‘metafor
(https://cran.r-project.org/package=metafor)’ packages
to perform data pooling, heterogeneity testing, subgroup and
sensitivity analyses and publication bias assessment. The AUC and
its 95% confidence intervals (CIs) were extracted from studies as
the primary measure of predictive performance. Cochran's Q test and
I² statistic were used to assess heterogeneity between studies. A
random-effects model was used to pool the data, addressing the
heterogeneity across studies. To assess potential publication bias,
funnel plots were constructed. According to the Cochrane Handbook
(22), when the number of included
studies is ≥10, publication bias risk assessment should not rely on
Egger's or Begg's test. Instead, Peters' test (23) was used to assess the symmetry of the
funnel plot. Sensitivity analysis was performed using the
Leave-One-Out method to evaluate the impact of each individual
study on the overall pooled effect size. P<0.05 was considered
to indicate a statistically significant difference.
Results
Literature screening results
The present study conducted literature screening in
accordance with the PRISMA 2020 guidelines (24). A total of 492 studies were retrieved
through systematic searching; 163 duplicates were removed.
Subsequently, seven meta-analyses, eight reviews, four studies
including animal experiments and 64 conference records, guidelines
and letters were excluded. Additionally, 105 studies that did not
meet the inclusion criteria after title and abstract screening were
excluded. Moreover, 20 studies for which full texts could not be
obtained were excluded, along with eight studies without outcome
indicators and 85 that only focused on risk factor research.
Finally, a total of 28 studies were included (25-52)
(Fig. 1).
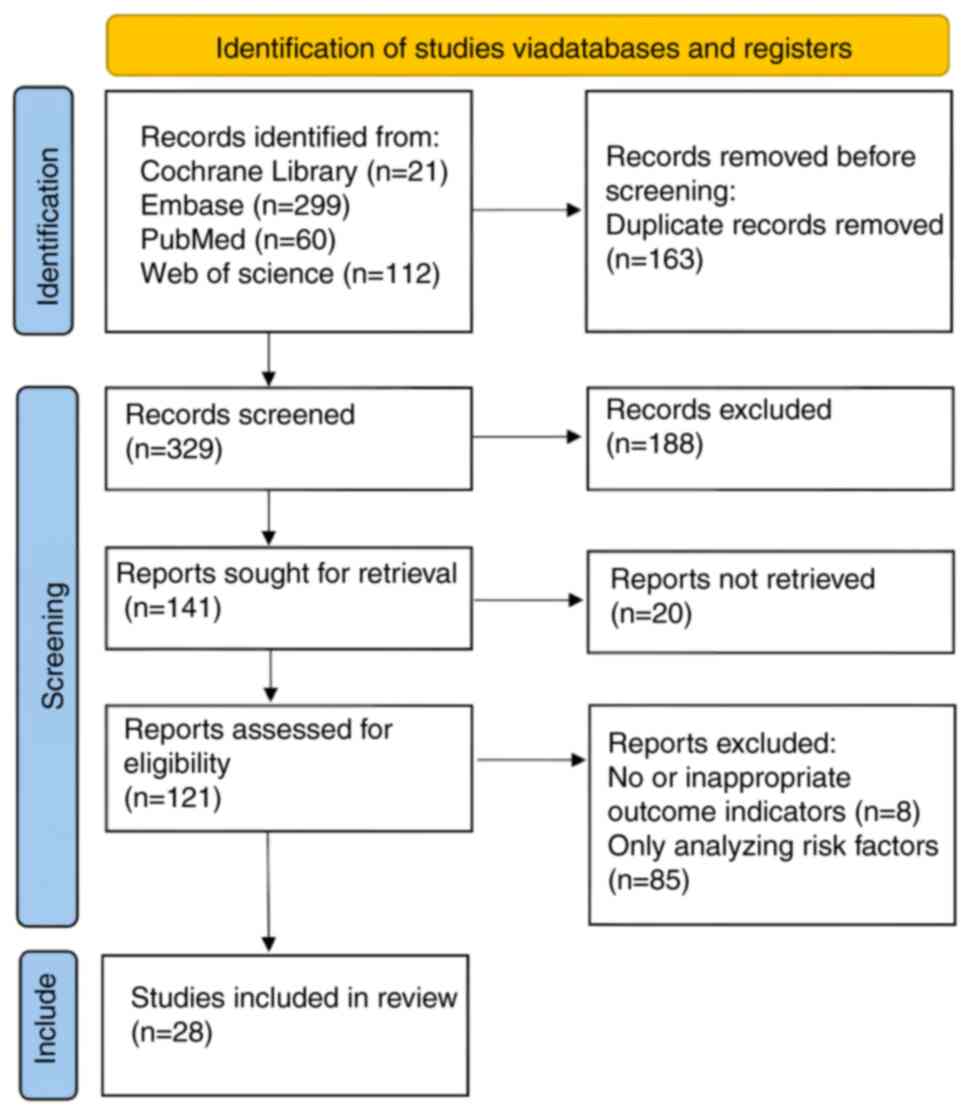 | Figure 1Flowchart of literature screening.
Initial retrieval from PubMed, Cochrane Library, Web of Science and
Embase yielded 492 records. After removing 163 duplicates, 329
records remained. Following the exclusion of 83 ineligible records
(meta-analyses, reviews, animal studies, conference abstracts), 246
full texts were assessed. Further exclusions (n=218) included
studies without prediction models. Ultimately, 28 studies were
included, encompassing 28 retinopathy of prematurity risk
prediction models with a total sample size of 72,991 infants. |
Characteristics of the included
studies
The studies were published between 2009 and 2025. A
total of 14 studies (32,34,36,40,41,45,47-49,51,52)
were conducted in East Asia, eight (28,29,31,33,37,38,46,50) in
North America, two each in Europe (30,39)
and South America (25,26), and one each in West (31) and Southeast Asia (27). A total of 11 studies (28,30,33,36,38,40,41,46-48,52)
were multicenter studies. The sample size ranged from 90 to 22,569
cases, with an overall sample size of 72,991 cases (Table SV). A total of 28 items were
included in the ROP risk prediction model (Table SVI).
Model validation
A total of 16 studies (25-27,29,31-35,39,43,45,49-51)
did not mention the specific validation methods, five (28,30,41,42,48)
conducted external validation, two (40,47)
performed both internal and external validation and five (36,38,44,46,52)
performed internal validation. This lack of rigorous validation
limits the generalizability and clinical applicability of existing
ROP risk prediction models.
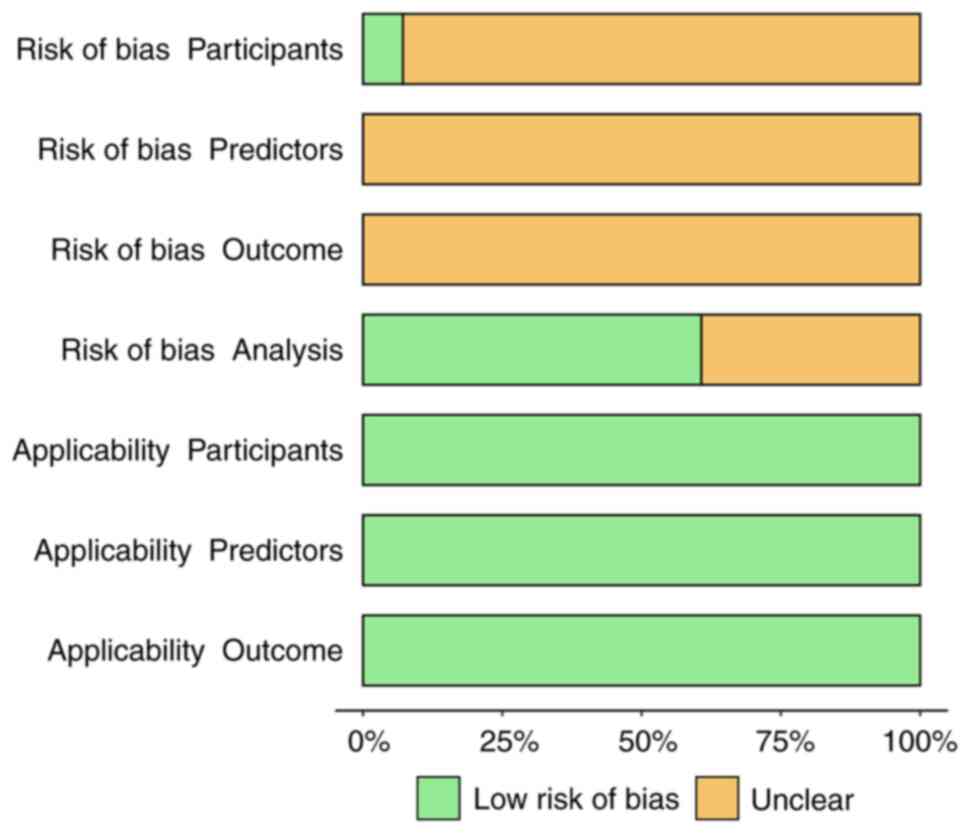
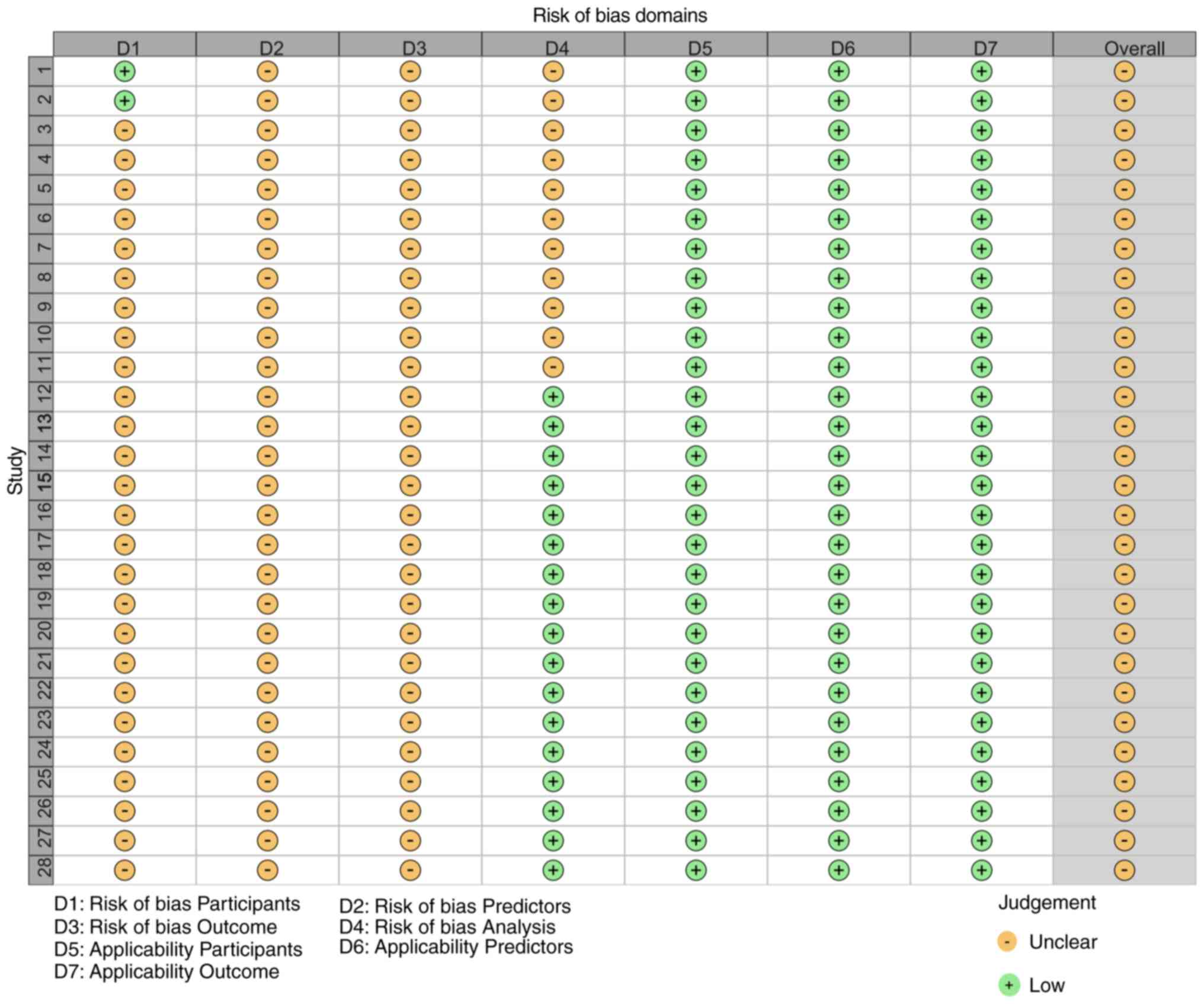
Literature quality assessment
According to the PROBAST assessment tool, all the
models have an unclear risk of bias (Figs. 2 and 3). This was primarily due to the
susceptibility of retrospective studies to information bias,
limited sample selection affecting representativeness, inconsistent
variable definitions and measurement methods, insufficient control
of confounding factors and small sample sizes that may lead to
model instability. In the future model development process, more
rigorous research designs and standardized variable measurement
methods need to be adopted.
Meta-analysis of risk prediction
models
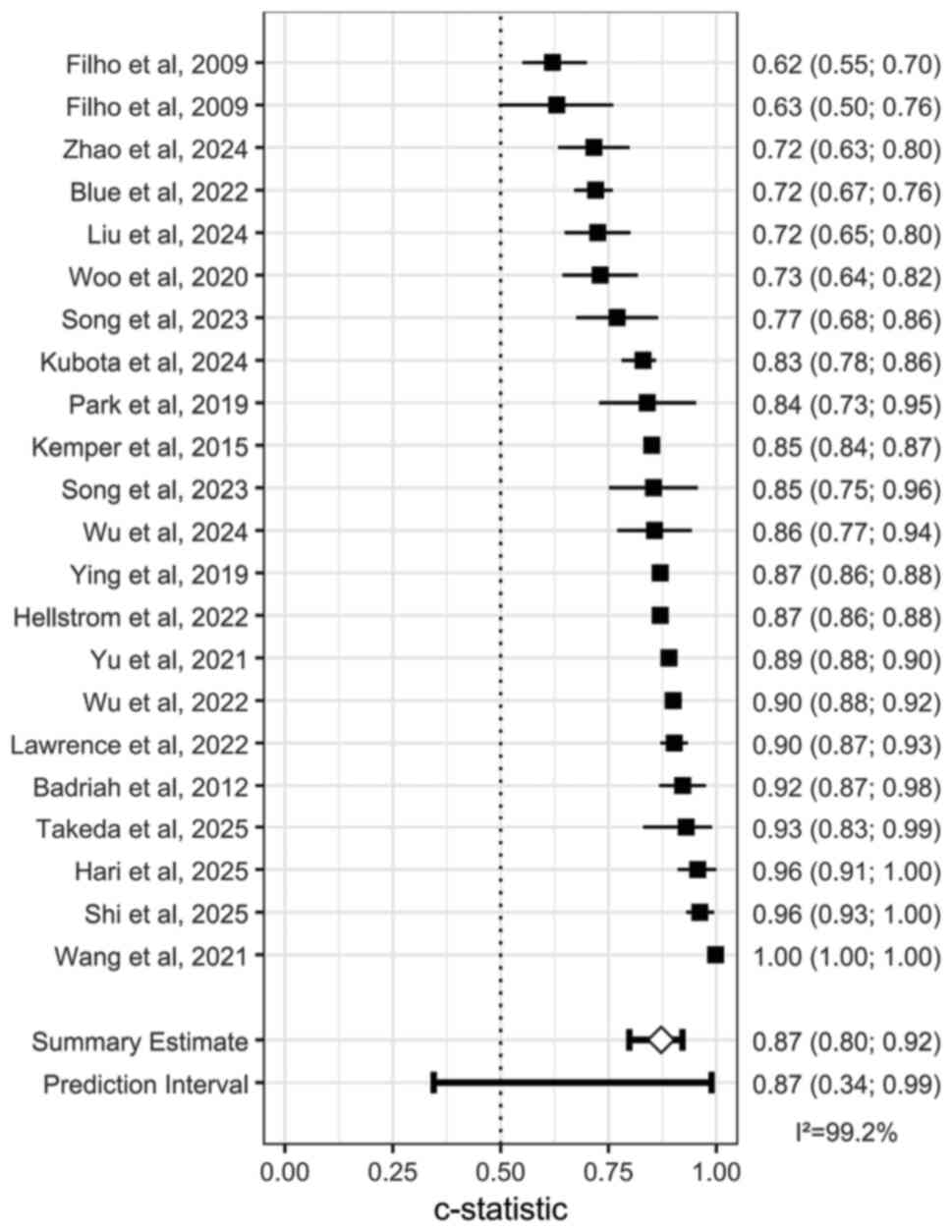
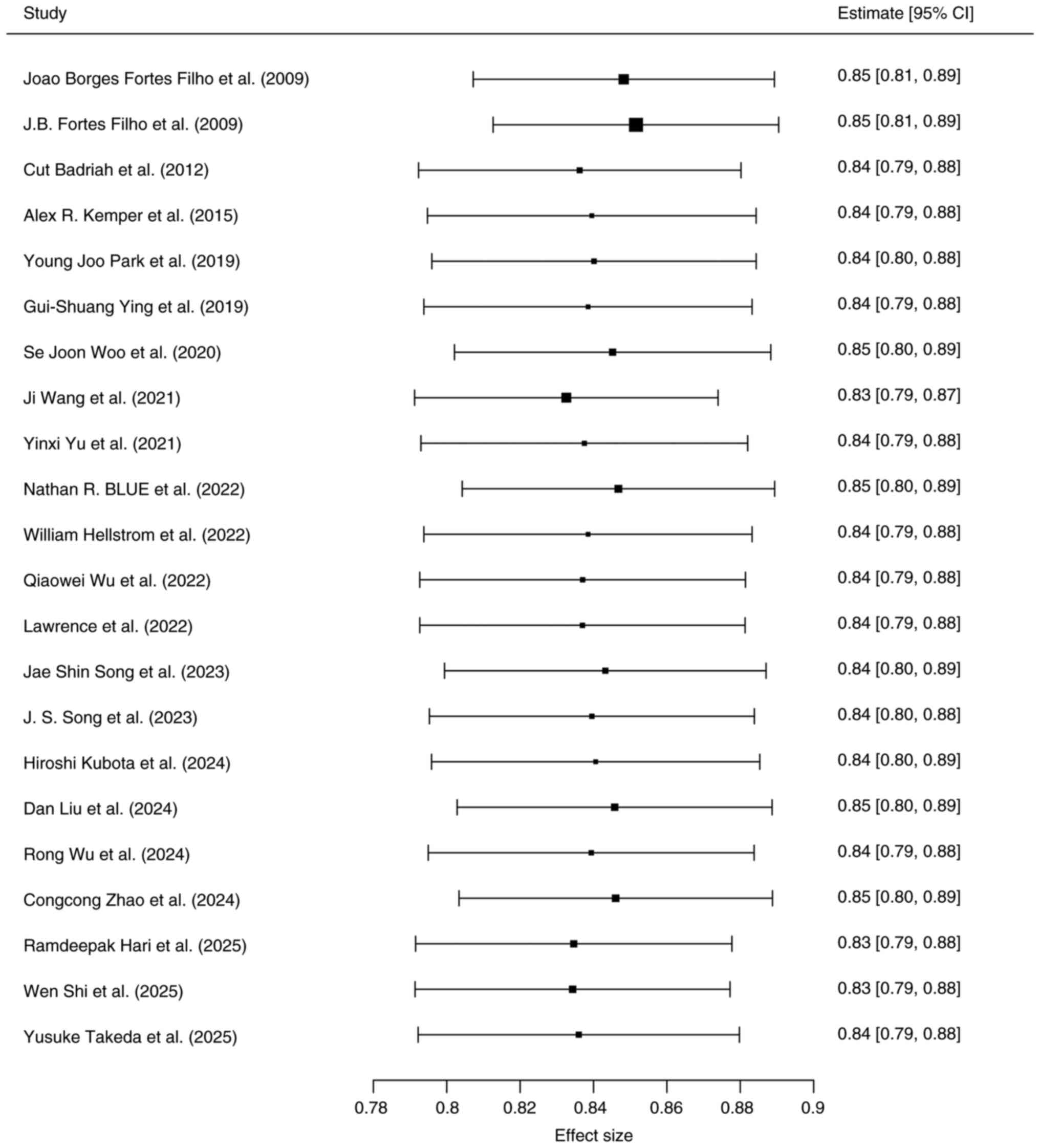
Due to missing model evaluation data, only 22
studies (25-29,32-34,36-44,47-52)
were included in the analysis. The pooled effect size was AUC=0.87
(95% CI: 0.34; 0.99), indicating good discriminative ability of the
models. However, significant heterogeneity was observed among the
studies (I²=99.2%, P<0.05; Fig.
4).
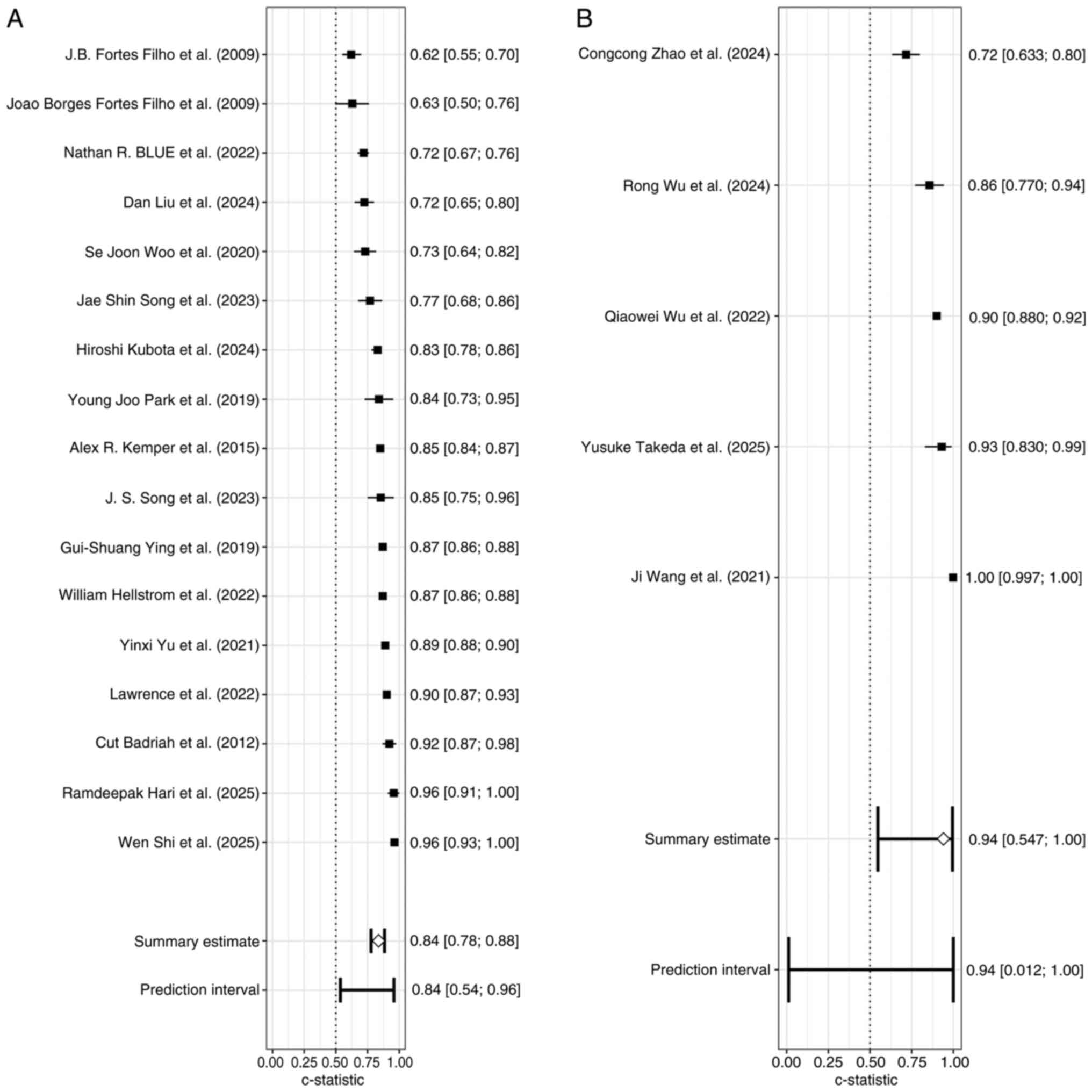
Studies were stratified by modeling approach into
subgroup A [traditional statistical models (logistic regression]
(25-29,32-34,37-39,41-43,45,47,50,51)
and B [machine learning models (neural networks, support vector
machines, long short-term memory, deep learning models] (36,40,48,49,52).
There was significant heterogeneity within both subgroups
(P<0.05), with I²=92.2% in subgroup A and I²=97.3% in subgroup
B. These findings indicated that the differences between studies,
whether using traditional statistical models or machine learning
models were greater than what can be explained by random error,
reflecting substantial methodological or study characteristic
variations (Fig. 5).
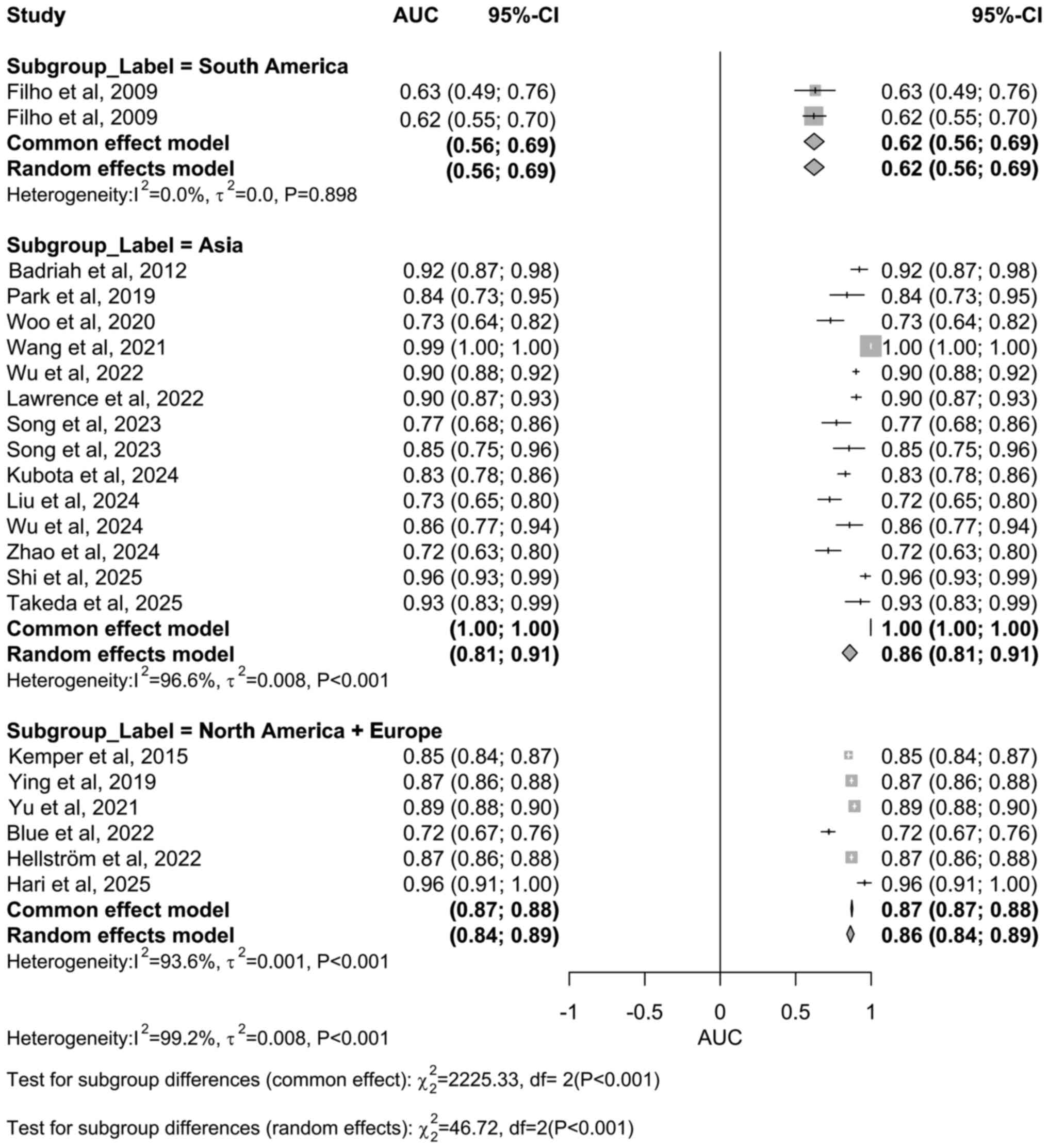
Subgroup analyses were also performed by study
region. The South America subgroup (25,26)
showed I²=0% with P=0.898, indicating highly consistent results and
no statistically significant heterogeneity. By contrast, the Asia
(I²=96.6%) (27,32,36,40-43,45,47-49,51,52)
and North America + Europe subgroup (I²=93.6%) (28,33,37-39,50)
both exhibited very high heterogeneity with P<0.05, suggesting
statistically significant differences in study results within these
regional subgroups (Fig. 6).
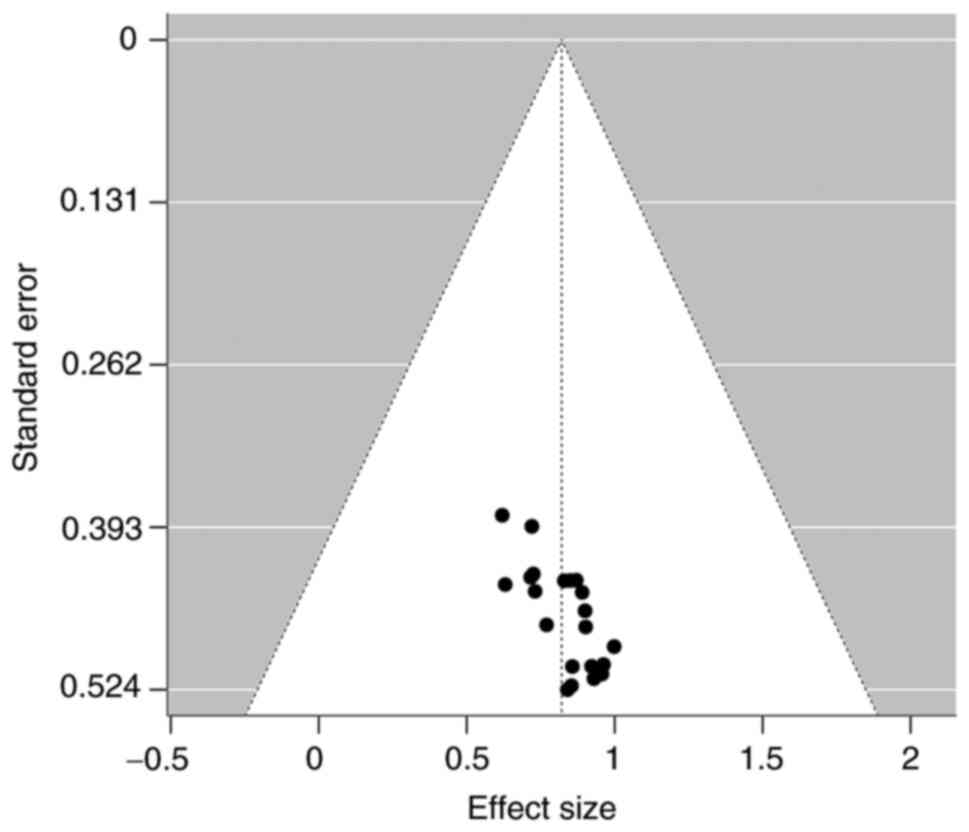
Based on the symmetry of the funnel plot around the
pooled effect size (AUC=0.87), there was no obvious study bias
(Fig. 7). Peters' bias test in
showed t=-0.55, P=0.590, indicating no evidence of significant
bias. This indicated that the findings of the included studies were
not distorted by selective publication, and the overall evidence
chain is highly reliable. From the results of bias assessments, the
overall conclusions of the study are highly credible. The symmetry
of the funnel plot and potential bias issues demonstrated minimal
influence on the results. Sensitivity analysis showed that
excluding any single study did not substantially alter the results,
confirming stability (Fig. 8).
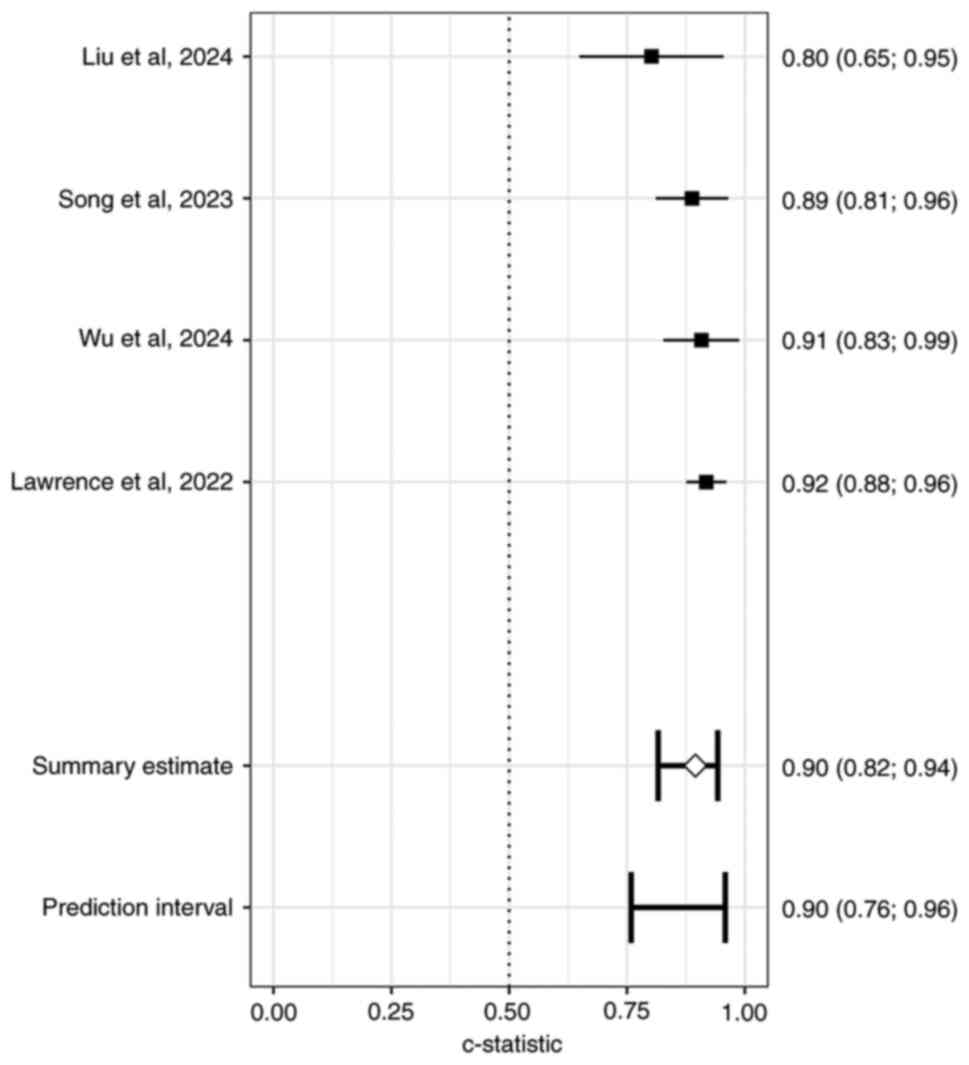
Of the 28 studies, seven reported external
validation; among these, three did not provide 95% CI values for
the external validation AUC. Pooling results from the remaining
four studies (40,41,47,50)
yielded an external validation AUC of 0.90 (0.76; 0.96; Fig. 9). The symmetry of the funnel plot,
non-significant Peters' bias test and stable sensitivity analysis
confirm that the overall findings are not distorted by publication
bias and are robust to individual study exclusion, supporting the
reliability of the meta-analysis conclusions.
Discussion
Effective screening combined with timely
intervention can notably decrease the blindness rate of ROP
(12). Novel imaging technologies
and internet connectivity have transformed the ROP screening model,
with artificial intelligence-supported ROP screening becoming a
research hotspot (53,54). However, existing predictive models
have limitations and are not well-suited for areas with quality
poor neonatal care (55,56). The present study conducted a
meta-analysis to evaluate the performance of current ROP prediction
models and improve ROP risk prediction models.
The present study included 28 ROP risk prediction
models, among which six studies did not provide the 95% CI values
for the AUC. Pooling results from the remaining 22 studies, the AUC
of ROP prediction models was 0.87 (0.34; 0.99), indicating that the
overall performance of ROP risk prediction models is favorable,
with good discriminative ability. However, there was high
heterogeneity between the models. When the models were sub-grouped
into traditional statistical and machine learning models, high
heterogeneity remained within each subgroup. The causes of
heterogeneity were considered to be differences in covariate
selection, sample characteristics and outcome definitions and a
lack of unified standards for data preprocessing and validation
protocols. Regional subgroup analysis showed that studies from
South America had no significant heterogeneity, while those from
Asia, North America and Europe exhibited high heterogeneity. It was
hypothesized that studies in South America showed consistent
results due to the uniformity of population characteristics and
medical standards within the region; by contrast, the high
heterogeneity in Asia, North America and Europe may be attributed
to notable differences in cross-regional population characteristics
and medical systems. Nevertheless, funnel plot symmetry and Peters'
bias test indicated no significant publication bias, and
sensitivity analysis showed stable results, supporting the
reliability of the conclusions. Future studies should reduce
heterogeneity by unifying methodological standards and conducting
multicenter research.
The risk of bias for all the models was unclear.
Badriah et al (27) and Park
et al (32) adopted
retrospective designs, relying on medical record reviews for data
collection. This may introduce information bias due to incomplete
record-keeping or subjective data assignment. The study by Park
et al (32) was a
single-center study, with sample selection limited to a specific
medical setting, which may decrease the generalizability of the
results.
In terms of variable measurement, there were
differences in the definitions and measurement methods of key
predictors across studies. Filho et al (25) used ‘weight gain proportion at 6
weeks after birth’ as an indicator, while Cerda et al
(31) employed changes in z-scores
based on the Fenton growth curve. Such inconsistent standards
limited data comparability. While most studies (25-52)
referenced international classification criteria, Blue et al
(38) and Chen et al
(44) did not explicitly mention
international ROP severity assessment criteria in their relevant
evaluations. In addition, Filho et al (26) and Gerull et al (30) did not explicitly describe the
implementation of assessor blinding, which may introduce subjective
bias, however, the core staging criteria remained consistent.
The multicenter study by Ying et al (33) covered 29 hospitals in North America.
Differences in medical care practices between institutions may have
interfered with the identification of ROP risk factors, but the
study adjusted for key variables such as gestational age and birth
weight through multivariate regression, decreasing confounding
effects to a certain extent. Park et al (32), Hari et al (50), and Shi et al (51) had relatively small sample sizes,
which may pose a risk of model instability. However, owing to the
explicit and rigorous definition of outcome events, the risk of
overfitting (where a model or analytical approach exhibits
excessive adaptation to the original dataset while lacking
generalizability to new data-was kept at a controllable level.
While specific biases were present across multiple dimensions of
the study, they did not substantially compromise the overall
validity of the findings. Consequently, the overall risk of bias
was evaluated as unclear, indicating that although biases exist,
they are not severe enough to invalidate the core conclusions of
the study.
In predictor selection, the included studies did not
report the effectiveness of predictors in practical applications or
clearly specify whether the predictive ability of predictors was
independently evaluated under blinded conditions. In addition,
there were notable differences in terms of predictor selection and
their determination methods. Some studies (45,46)
failed to fully consider potential interactions between predictors.
Multiple included studies consistently identified oxygen therapy as
a key risk factor for ROP; however, the studies did not conduct
in-depth exploration of interaction effects between factors such as
different oxygen therapy modalities and fluctuations in oxygen
concentration. Kubota et al (45) focused on analyzing the association
between oxygen saturation (SpO2) fluctuations and ROP
risk by calculating the total difference in SpO2 values
over the total effective time. On the other hand, Lin et al
(46) mainly focused on relevant
indicators of fraction of inspired oxygen (FiO2),
including the average FiO2 and the coefficient of
variation of FiO2, aggregating daily data to smooth
short-term fluctuations and decrease noise. Unlike other studies
(26,27,30,37,40,50)
that may focus only on oxygen concentration or oxygen duration at a
fixed point in time, this previous study emphasized the impact of
the trend in FiO2 changes over time on ROP. In practical
applications, there may be differences in the frequency and method
of FiO2 monitoring and recording between different
medical institutions.
Future studies should adopt prospective designs and
increase sample sizes. This would allow for more precise control of
influencing factors during data collection, enable tracking and
observation of changes in the target population, provide more
representative study samples, and avoid information and selection
biases inherent in retrospective studies. Regularization methods
should be used to limit model degrees of freedom and prevent
overfitting. When constructing prediction models, potential
confounding factors should be controlled.
Of 28 studies, 16 did not mention model validation,
while five conducted internal validation. The internal validation
sample sizes in the studies by Lin et al (46) and Takeda et al (52) were relatively small, which may not
fully capture the various characteristics and associations in the
data. While the models performed well on the training sets, they
may not perform as well in real-world applications. In the study by
Chen et al (44), five-fold
cross-validation was performed on data from 22,569 patients,
improving the training effect of the model and enhancing its
stability and reliability. However, the occurrence and development
of ROP may be influenced by factors such as medical conditions and
environmental factors in different regions. Therefore, it is
essential to include data from different regions and medical
centers for external validation to ensure the general applicability
of the model. The present study combined the external validation
results from four studies (40,41,47,48)
with an external validation model AUC of 0.90 (0.76; 0.96), showing
good external validation performance. However, in clinical
applications, the practical value of the model should be verified
by incorporating other evaluation indicators.
Additionally, in the statistical analysis, certain
studies (25,26) had issues with improper handling of
missing data and insufficient assessment of collinearity. In the
study by Takeda et al (52),
five machine learning methods (decision trees, random forests,
gradient boosting trees, neural networks and naive Bayes) were used
to construct the models. Random forests and naive Bayes models
performed well. Non-imaging machine learning models demonstrated
high performance in predicting ROP occurrence, providing a feasible
predictive approach for hospitals that lack access to retinal
images or pediatric retinal cameras. However, this previous study
acknowledged the small sample size and issues with variable
collinearity. In the future, combining LASSO regression with
embedded feature selection techniques may help control for
collinearity between variables, select a stable and effective set
of features and improve the robustness of the models.
Of 28 studies, 24 reported (25-27,29-35,37,39,40-48,50-52)
that low birth weight in preterm infants is a risk factor for the
occurrence of ROP. Yildirim et al (57) found that, compared with preterm
infants without ROP, the average weight gain in the third week
after birth was significantly lower in infants with ROP; Preterm
infants with low birth weight have underdeveloped retinal vascular
systems, and their blood vessels are structurally and functionally
fragile, making it difficult to cope with abnormal vascular
proliferation, fibrosis and other pathological changes, thereby
increasing the risk of ROP (58).
Additionally, eight studies (33,34,37,42,47,48,49,52)
used the Apgar score to establish prediction models. The Apgar
score primarily evaluates neonatal health status based on heart
rate, respiration, muscle tone, reflexes and skin color. A low
Apgar score often indicates that the newborn experienced asphyxia
or hypoxia at birth, which affects the normal development of the
retinal blood vessels and increases the risk of ROP (59).
Multiple pregnancies are also a risk factor for ROP.
In multiple pregnancies, the blood supply from the placenta is
distributed unevenly, leading to insufficient fetal nutrition, and
restricted growth and development, which often results in low birth
weight (60)
Furthermore, studies (23,27)
have shown that Caucasian infants have a higher risk of developing
ROP compared with other ethnicities. This is because Caucasian
individuals have less pigmentation, making the retinas more
sensitive to oxygen and light damage. Under oxygen therapy or
high-oxygen exposure conditions, the retina is more prone to
vascular development disorders (61). However, a 2006-2017 study (62) of 41 North American hospitals found
African-American infants had lower birth weight and gestational
ages than white and Asian infants; African-American and Asian
infants had significantly lower daily weight gain than Caucasian
infants 31-40 days after birth and after adjusting for birth weight
and gestational age, African-American infants had a lower incidence
of severe ROP than Caucasian and Asian infants. There were no
differences in the incidence or timing of severe ROP between
different ethnicities; this mechanism requires further
exploration.
Although the aforementioned factors have been
confirmed to be associated with ROP in most studies, certain
research (27,32,34,42,43,46,49,50-52)
still has the limitation of having a relatively small sample size.
Additionally, there are notable differences in medical standards,
preterm infant care and environmental factors across regions, and
the level of care provided to infants, including nutritional
support and oxygen therapy management, varies (27,33,35).
It is recommended that pregnant patients at risk of
preterm birth receive prenatal corticosteroids: these medications
accelerate fetal lung maturation, reducing the incidence and
severity of respiratory distress syndrome in preterm infants
(63). Additionally, unnecessary
postnatal ventilation and oxygen therapy for neonates should be
avoided, as excessive oxygen exposure directly disrupts retinal
vascular development. Neonates should also receive high-quality
care and providing targeted management of comorbidities like
bronchopulmonary dysplasia.
The present study had certain limitations: the
systematic review was not pre-registered on the PROSPERO platform,
which decreases the traceability of the research design and
implementation process, and may affect methodological transparency
and the validation of the standardization of the research
protocol.
Future studies should consider combining fundus
images with clinical data and use deep learning methods such as
convolutional and recurrent neural networks, or ensemble learning
algorithms such as random forest, gradient boosting machine and
XGBoost to construct ROP prediction models (58,64).
Such an approach could potentially handle large volumes of complex
non-linear relationships and enable automated feature extraction,
which might provide a more accurate basis for early
screening-though this remains to be validated in future
research.
In summary, existing ROP prediction models have
potential in discriminative ability and may provide references for
preliminary clinical screening. However, their application is
limited by insufficient external validation and inadequate sample
representativeness, and their generalization ability needs to be
improved. Future model development should prioritize multicenter,
large-sample prospective designs, control confounding factors, and
systematically incorporate data from populations with different
medical resource backgrounds and ethnic groups to enhance the
generalizability of results. Meanwhile, it is necessary to
strengthen external validation processes, explore multimodal fusion
of fundus images and clinical indicators, introduce deep learning
or ensemble learning methods to handle complex data associations
and explore potential biomarkers associated with ROP, thereby
providing more reliable tools for early accurate screening and
intervention for neonatal ROP.
Supplementary Material
PubMed database search strategy.
Web of Science database search
strategy.
Embase database search strategy.
Cochrane Library database search
strategy.
Study characteristics.
Prediction model characteristics.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 82160205) and the Tianjin
Key Medical Discipline (Specialty) Construction Project (grant no.
TJYXZDXK-3-004A-3).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LL and YG confirm the authenticity of all the raw
data. LL and YG designed and performed the experiments and wrote
the manuscript. WC interpreted data. MH conceived the study. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fu Y, Lei C, Qibo R, Huang X, Chen Y, Wang
M and Zhang M: Insulin-like growth factor-1 and retinopathy of
prematurity: A systemic review and meta-analysis. Surv Ophthalmol.
68:1153–1165. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
García H, Villasis-Keever MA,
Zavala-Vargas G, Bravo-Ortiz JC, Pérez-Méndez A and Escamilla-Núñez
A: Global prevalence and severity of retinopathy of prematurity
over the last four decades (1985-2021): A systematic review and
meta-analysis. Arch Med Res. 55(102967)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bhatnagar A, Skrehot HC, Bhatt A, Herce H
and Weng CY: Epidemiology of retinopathy of prematurity in the US
from 2003 to 2019. JAMA Ophthalmol. 141:479–485. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chiang MF, Quinn GE, Fielder AR, Ostmo SR,
Chan RV, Berrocal A, Binenbaum G, Blair M, Campbell JP, Capone A
Jr, et al: International classification of retinopathy of
prematurity, third edition. Ophthalmology. 128:e51–e68.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schmitz AM, Bumbaru SM, Fakhouri LS and
Zhang DQ: Long-term impairment of retinal ganglion cell function
after oxygen-induced retinopathy. Cells. 14(512)2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Beccasio A, Mignini C, Caricato A,
Iaccheri B, Di Cara G, Verrotti A and Cagini C: New trends in
intravitreal anti-VEGF therapy for ROP. Eur J Ophthalmol.
32:1340–1351. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sankar BK, Amin H, Pappa P and Riaz KM:
Risk factors of retinopathy of prematurity: A prospective study.
Indian J Public Health. 69:111–114. 2025.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Karmouta R, Strawbridge JC, Langston S,
Altendahl M, Khitri M, Chu A and Tsui I: Neurodevelopmental
outcomes in infants screened for retinopathy of prematurity. JAMA
Ophthalmol. 141:1125–1132. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tan H, Blasco P, Lewis T, Ostmo S, Chiang
MF and Campbell JP: Neurodevelopmental outcomes in preterm infants
with retinopathy of prematurity. Surv Ophthalmol. 66:877–891.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Almutairi MF, Gulden S, Hundscheid TM,
Bartoš F, Cavallaro G and Villamor E: Platelet counts and risk of
severe retinopathy of prematurity: A bayesian model-averaged
meta-analysis. Children (Basel). 10(1903)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nakayama LF, Mitchell WG, Ribeiro LZ,
Dychiao RG, Phanphruk W, Celi LA, Kalua K, Santiago APD, Regatieri
CVS and Moraes NSB: Fairness and generalisability in deep learning
of retinopathy of prematurity screening algorithms: A literature
review. BMJ Open Ophthalmol. 8(e001216)2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim SJ, Port AD, Swan R, Campbell JP, Chan
RVP and Chiang MF: Retinopathy of prematurity: A review of risk
factors and their clinical significance. Surv Ophthalmol.
63:618–637. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shah S, Slaney E, VerHage E, Chen J, Dias
R, Abdelmalik B, Weaver A and Neu J: Application of artificial
intelligence in the early detection of retinopathy of prematurity:
Review of the literature. Neonatology. 120:558–565. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hellström A and Hård AL: Screening and
novel therapies for retinopathy of prematurity-A review. Early Hum
Dev. 138(104846)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Diggikar S, Gurumoorthy P, Trif P, Mudura
D, Nagesh NK, Galis R, Vinekar A and Kramer BW: Retinopathy of
prematurity and neurodevelopmental outcomes in preterm infants: A
systematic review and meta-analysis. Front Pediatr.
11(1055813)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Collins GS, Moons KGM, Dhiman P, Riley RD,
Beam AL, Van Calster B, Ghassemi M, Liu X, Reitsma JB, van Smeden
M, et al: TRIPOD+AI statement: Updated guidance for reporting
clinical prediction models that use regression or machine learning
methods. BMJ. 385(e078378)2024.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fernandez-Felix BM, López-Alcalde J, Roqué
M, Muriel A and Zamora J: CHARMS and PROBAST at your fingertips: A
template for data extraction and risk of bias assessment in
systematic reviews of predictive models. BMC Med Res Methodol.
23(44)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Moons KGM, Damen JAA, Kaul T, Hooft L,
Navarro CA, Dhiman P, Beam AL, Van Calster B, Celi LA, Denaxas S,
et al: PROBAST+AI: An updated quality, risk of bias, and
applicability assessment tool for prediction models using
regression or artificial intelligence methods. BMJ.
388(e082505)2025.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fu H, Hou D, Xu R, You Q, Li H, Yang Q,
Wang H, Gao J and Bai D: Risk prediction models for deep venous
thrombosis in patients with acute stroke: A systematic review and
meta-analysis. Int J Nurs Stud. 149(104623)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kuo RYL, Harrison C, Curran TA, Jones B,
Freethy A, Cussons D, Stewart M, Collins GS and Furniss D:
Artificial intelligence in fracture detection: A systematic review
and meta-analysis. Radiology. 304:50–62. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
de Jong Y, Ramspek CL, Zoccali C, Jager
KJ, Dekker FW and van Diepen M: Appraising prediction research: A
guide and meta-review on bias and applicability assessment using
the prediction model risk of bias ASsessment tool (PROBAST).
Nephrology (Carlton). 26:939–947. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Furuya-Kanamori L, Barendregt JJ and Doi
SAR: A new improved graphical and quantitative method for detecting
bias in meta-analysis. Int J Evid Based Healthc. 16:195–203.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Filho JB, Bonomo PP, Maia M and Procianoy
RS: Weight gain measured at 6 weeks after birth as a predictor for
severe retinopathy of prematurity: Study with 317 very low birth
weight preterm babies. Graefes Arch Clin Exp Ophthalmol.
247:831–836. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Filho JB, Dill JC, Ishizaki A, Aguiar WW,
Silveira RC and Procianoy RS: Score for neonatal acute physiology
and perinatal extension II as a predictor of retinopathy of
prematurity: Study in 304 very-low-birth-weight preterm infants.
Ophthalmologica. 223:177–182. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Badriah C, Amir I, Elvioza E and Ifran E:
Prevalence and risk factors of retinopathy of prematurity.
Paediatrica Indonesiana. 52:138–144. 2012.
|
|
28
|
Kemper AR, Wade KC, Hornik CP, Ying GS,
Baumritter A and Quinn GE: Telemedicine Approaches to Evaluating
Acute-phase Retinopathy of Prematurity (e-ROP) Study Cooperative
Group. Retinopathy of prematurity risk prediction for infants with
birth weight less than 1251 grams. J Pediatr. 166:257–261.e2.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Owen LA, Morrison MA, Hoffman RO, Yoder BA
and DeAngelis MM: Retinopathy of prematurity: A comprehensive risk
analysis for prevention and prediction of disease. PLoS One.
12(e0171467)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gerull R, Brauer V, Bassler D, Laubscher
B, Pfister RE, Nelle M, Müller B, Roth-Kleiner M, Gerth-Kahlert C
and Adams M: Swiss Neonatal Network & Follow-up Group.
Prediction of ROP treatment and evaluation of screening criteria in
VLBW infants-a population based analysis. Pediatr Res. 84:632–638.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cerda AM, McCourt EA, Thevarajah T, Wymore
E, Lynch AM and Wagner BD: Comparison between weight gain and
Fenton preterm growth z scores in assessing the risk of retinopathy
of prematurity. J AAPOS. 23:281–283. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Park YJ, Woo SJ, Kim YM, Hong S, Lee YE
and Park KH: Immune and inflammatory proteins in cord blood as
predictive biomarkers of retinopathy of prematurity in preterm
infants. Invest Ophthalmol Vis Sci. 60:3813–3820. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ying GS, Bell EF, Donohue P, Tomlinson LA
and Binenbaum G: G-ROP Research Group. Perinatal risk factors for
the retinopathy of prematurity in postnatal growth and rop study.
Ophthalmic Epidemiol. 26:270–278. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Woo SJ, Park JY, Hong S, Kim YM, Park YH,
Lee YE and Park KH: Inflammatory and angiogenic mediators in
amniotic fluid are associated with the development of retinopathy
of prematurity in preterm infants. Invest Ophthalmol Vis Sci.
61(42)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fekri Y, Ojaghi H, Momeni N and Amani F:
Retinopathy of prematurity in Ardabil, North West of Iran:
Prevalence and risk factors. Eur J Transl Myol.
31(10063)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang J, Ji J, Zhang M, Lin JW, Zhang G,
Gong W, Cen LP, Lu Y, Huang X, Huang D, et al: Automated
explainable multidimensional deep learning platform of retinal
images for retinopathy of prematurity screening. JAMA Netw Open.
4(e218758)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu Y, Tomlinson LA, Binenbaum G and Ying
GS: G-Rop Study Group. Incidence, timing and risk factors of type 1
retinopathy of prematurity in a North American cohort. Br J
Ophthalmol. 105:1724–1730. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Blue NR, Allshouse AA, Grobman WA, Day RC,
Haas DM, Simhan HN, Parry S, Saade GR and Silver RM: Developing a
predictive model for perinatal morbidity among small for
gestational age infants. J Matern Fetal Neonatal Med. 35:8462–8471.
2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hellström W, Martinsson T, Morsing E,
Gränse L, Ley D and Hellström A: Low fraction of fetal haemoglobin
is associated with retinopathy of prematurity in the very preterm
infant. Br J Ophthalmol. 106:970–974. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wu Q, Hu Y, Mo Z, Wu R, Zhang X, Yang Y,
Liu B, Xiao Y, Zeng X, Lin Z, et al: Development and validation of
a deep learning model to predict the occurrence and severity of
retinopathy of prematurity. JAMA Netw Open.
5(e2217447)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Iu LPL, Yip WWK, Lok JYC, Fan MCY, Lai
CHY, Ho M and Young AL: Prediction model to predict type 1
retinopathy of prematurity using gestational age and birth weight
(PW-ROP). Br J Ophthalmol. 107:1007–1011. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Song JS, Woo SJ, Park KH, Joo E, Kim H, Oh
E and Lee KN: Cord blood transforming growth factor-β-induced as
predictive biomarker of retinopathy of prematurity in preterm
infants. Graefes Arch Clin Exp Ophthalmol. 261:2477–2488.
2023.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Song JS, Woo SJ, Park KH, Kim H, Lee KN
and Kim YM: Association of inflammatory and angiogenic biomarkers
in maternal plasma with retinopathy of prematurity in preterm
infants. Eye (Lond). 37:1802–1809. 2023.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Chen S, Zhao X, Wu Z, Cao K, Zhang Y, Tan
T, Lam CT, Xu Y, Zhang G and Sun Y: Multi-risk factors joint
prediction model for risk prediction of retinopathy of prematurity.
EPMA J. 15:261–274. 2024.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kubota H, Fukushima Y, Kawasaki R, Endo T,
Hatsukawa Y, Ineyama H, Hirata K, Hirano S, Wada K and Nishida K:
Continuous oxygen saturation and risk of retinopathy of prematurity
in a Japanese cohort. Br J Ophthalmol. 108:1275–1280.
2024.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lin WC, Jordan BK, Scottoline B, Ostmo SR,
Coyner AS, Singh P, Kalpathy-Cramer J, Erdogmus D, Chan RVP, Chiang
MF and Campbell JP: Oxygenation fluctuations associated with severe
retinopathy of prematurity: Insights from a multimodal deep
learning approach. Ophthalmol Sci. 4(100417)2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu D, Li XY, He HW, Jin KL, Zhang LX,
Zhou Y, Zhu ZM, Jiang CC, Wu HJ and Zheng SL: Nomogram to predict
severe retinopathy of prematurity in Southeast China. Int J
Ophthalmol. 17:282–288. 2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wu R, Chen H, Bai Y, Zhang Y, Feng S and
Lu X: Prediction models for retinopathy of prematurity occurrence
based on artificial neural network. BMC Ophthalmol.
24(323)2024.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhao C, Sun Z, Chen H, Li K and Sun H: The
impact of blood lactic acid levels on retinopathy of prematurity
morbidity. BMC Pediatr. 24(152)2024.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hari R, Mellacheruvu P, Nonye OC, Rastogi
A and Mydam J: Severe patent ductus arteriosus is a risk factor for
clinically significant retinopathy of prematurity in very low birth
weight infants. SN Compr Clin Med. 7(60)2025.
|
|
51
|
Shi W, Zhu L, He X, Wang S and Wang C:
Combined indicator assists in early recognition of retinopathy of
prematurity. Sci Rep. 15(8048)2025.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Takeda Y, Kaneko Y, Sugimoto M, Yamashita
H, Sasaki A and Mitsui T: Prediction models for retinopathy of
prematurity using nonimaging machine learning approaches: A
regional multicenter study. Ophthalmol Sci.
5(100715)2025.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wagner SK, Liefers B, Radia M, Zhang G,
Struyven R, Faes L, Than J, Balal S, Hennings C, Kilduff C, et al:
Development and international validation of custom-engineered and
code-free deep-learning models for detection of plus disease in
retinopathy of prematurity: A retrospective study. Lancet Digit
Health. 5:e340–e349. 2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Rashidian P, Karami S and Salehi SA: A
review on retinopathy of prematurity. Med Hypothesis Discov Innov
Ophthalmol. 13:201–212. 2025.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Maitra P, Shah PK, Campbell PJ and Rishi
P: The scope of artificial intelligence in retinopathy of
prematurity (ROP) management. Indian J Ophthalmol. 72:931–934.
2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Xu S, Liang Z, Du Q, Li Z, Tan G, Nie C,
Yang Y, Lv X, Zhang C and Luo X: A systematic study on the
prevention and treatment of retinopathy of prematurity in China.
BMC Ophthalmol. 18(44)2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Yildirim M, Coban A, Bulut O, Mercül NK
and Ince Z: Postnatal weight gain and retinopathy of prematurity in
preterm infants: A population-based retrospective cohort study. J
Matern Fetal Neonatal Med. 37(2337720)2024.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Han G, Lim DH, Kang D, Cho J, Guallar E,
Chang YS, Chung TY, Kim SJ and Park WS: Association between
retinopathy of prematurity in very-low-birth-weight infants and
neurodevelopmental impairment. Am J Ophthalmol. 244:205–215.
2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Dammann O, Hartnett ME and Stahl A:
Retinopathy of prematurity. Dev Med Child Neurol. 65:625–631.
2023.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Engin CD, Ozturk T, Ozkan O, Oztas A,
Selver MA and Tuzun F: Prediction of retinopathy of prematurity
development and treatment need with machine learning models. BMC
Ophthalmol. 25(194)2025.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Gilbert CE: Global perspectives of
retinopathy of prematurity. Indian J Ophthalmol. 71:3431–3433.
2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Wang J, Ying GS, Yu Y, Tomlinson L and
Binenbaum G: Racial differences in retinopathy of prematurity.
Ophthalmic Epidemiol. 30:523–531. 2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kim ES, Calkins KL and Chu A: Retinopathy
of prematurity: The role of nutrition. Pediatr Ann. 52:e303–e308.
2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
He D, Luo X, Ying B, Quinn GE, Baumritter
A, Chen Y, Ying GS and He L: Machine learning models for predicting
treatment-requiring retinopathy of prematurity in the e-ROP study.
Transl Vis Sci Technol. 14(14)2025.PubMed/NCBI View Article : Google Scholar
|