1. Introduction
Ankylosing spondylitis (AS), one of the
spondyloarthropathy (SpA) groups, is a type of autoimmune disease
involved in sacroiliac joints and cartilage, eventually causing
spine ankylosis (1,2). It commonly arises in the second decade
of life and varies in symptom by geographic location and sex
(3,4). The progression of inflammation in AS
is associated with bone erosion, abnormal bone formation, and
ankylosis, leading to pain and reduced mobility (5). Additionally, AS is reported to be
accompanied by extra-articular manifestations, including anterior
uveitis, psoriasis, and chronic inflammatory bowel disease (IBD)
(6).
Although the pathological mechanisms of AS have not
been fully elucidated, genetic background and environmental factors
have been shown to influence its pathogenesis through complex
interactions (7,8). Among them, human leukocyte antigen B27
(HLA-B27) was the first factor investigated and has the strongest
association with AS (9,10), reflecting prevalence differences due
to race-specific genetic variations (11). In addition, it has been reported
that AS is a complex disease in which autoinflammatory and
autoimmune systems are correlated (1). Aberrant HLA-B27, gut microbiota and
biomechanical stress such as orthograde posture trigger
inflammation in collaboration with natural killer (NK) cells and
helper T (Th) 17 cells, resulting in inflammation of the spine
joints and sacroiliac joints with their adjacent soft tissues,
tendons and ligaments (6). Through
those responses, fibrosis and calcification ensue, subsequently
leading to bone erosion and new bone formation (12). Consequently, patients with AS suffer
from chronic back and spine pain (13).
Over the past few years, with the development of
technology, there have been a variety of changes in pathology,
diagnosis, and treatment. Especially, in terms of treatment, the
therapy has been revolutionized due to the introduction of
biotechnology medicine (14).
Currently, nonsteroidal anti-inflammatory drugs (NSAIDs),
disease-modifying antirheumatic drugs (DMARDs), and tumor necrosis
factor (TNF)-α inhibitors are used to relieve pain, restore
physical function, and slow the progression of structural damage
(15,16). Unfortunately, because the mechanism
of AS pathogenesis has not been fully elucidated, the present
treatment mainly focuses on the alleviation of symptoms (17). Moreover, these drugs can cause
several adverse effects from long-term utilization (18). Thus, novel medicine with more
advanced effectiveness and fewer side effects should be explored
for treating AS. Considering the complexity of AS mechanisms, the
multi-targeting traditional Chinese medicines may be promising
alternative treatments for AS. In the present review, the
pathology, symptoms, diagnosis, and current remedies of AS are
described. In addition, recent herbal medicines with their effects
and underlying mechanisms in the treatment of AS, are
presented.
2. Symptoms
The main signs of AS involve back pain,
sacroiliitis, morning stiffness, extra-articular manifestations
such as acute anterior uveitis and IBD (19). Pain in the sacroiliac joint is a
common symptom in the early phase of AS (16). As the disease progresses, patients
with AS may experience mild discomfort or pain (15). At this stage, magnetic resonance
imaging (MRI) can show modest alteration and mild inflammation
(20). As it progresses further,
patients develop severe inflammation and pain along with
abnormalities at the sacroiliac joint, which could emerge in the
imaging (21). The excessive
inflammation causes back pain and morning stiffness, resulting in
restriction of spinal mobility (22). Morning stiffness lasting longer than
30 min is also an important clinical feature of AS (23). Eventually, bone erosion and
formation can lead to the development of syndesmophytes, which
connect adjacent vertebrae (24).
This process can result in fusion of the sacroiliac joints and
spine, leading to loss of spinal mobility, alterations in lumbar
lordosis, and kyphosis, which can be observed on radiographs as the
characteristic ‘bamboo spine’ (24).
3. Pathogenesis
HLA-B27
Several factors are associated with the development
and progression of AS, including genetic predisposition, immune
responses and environmental influences (1). HLA-B27 is known as one of the most
critical genes in AS. HLA is the human version of the major
histocompatibility complex (MHC), which encodes cell surface
proteins essential for adaptive immunity (10). HLA-B27 presents antigenic peptides
to CD8+ T cells, and has been strongly associated with
inflammatory diseases affecting the cartilage and joints (25). The MHC class I encodes HLA-B27,
providing epitopes to T cells and activating cytotoxic T
lymphocytes (CTL) (26). Among the
HLA-B27 subtypes, B*2702, B*2703, B*2704, B*2705 and B*2710 are
associated with a significantly increased susceptibility to AS
(27,28). There are four theories explaining
the mechanism through which HLA influences the development of AS:
The arthritogenic peptide theory, the misfolding theory, the
homodimer theory, and the mimicry theory.
i) The arthritogenic peptide theory postulates that
some microbial peptides similar to self-antigens induce AS
(29). When HLA-B27 presents a
microbial antigen resembling a self-peptide, T cells recognize the
MHC-peptide complexes, leading to autoreactivity and
auto-inflammatory disease (30).
ii) The misfolding theory posits that the unfolded protein response
(UPR) in the endoplasmic reticulum (ER) is a major factor in AS
(31). The quaternary structure of
HLA-B27, composed of three components needs proper folding in the
ER for its correct function (32).
If HLA-B27 misfolds due to abnormalities in its cysteine residues,
it can accumulate in the ER, triggering ER stress (33,34).
Additionally, it can activate the UPR and nuclear factor kappa B
(NF-κB) which leads to the production of pro-inflammatory cytokines
such as interleukin (IL)-23(35).
iii) The homodimer effect theory is also associated with the
structure of HLA-B27. Disulfide bonds in the cysteine residues
facilitate the formation of α heavy-chain homodimers following
their dissociation from the β light chain (36). The homodimer exhibits a stronger
affinity for killer cell immunoglobulin-like receptors (KIRs),
which are expressed on NK cells and Th17 cells that release IL-17,
compared with the heterodimer (37). iv) Finally, the mimicry theory
suggests that the homologous amino acid structures between HLA and
some bacterial antigens can stimulate CTL. CTL would recognize HLA
itself or the peptide directly produced by HLA-B27(38). Notably, certain components of
Klebsiella pneumoniae exhibit genetic sequences similar to
those found in humans, displaying mimicry in AS (39). Although HLA-B27 is regarded as a
critical gene in AS, the pathogenesis of AS remains unclear.
Research indicates that AS may develop in 1-2% of HLA-B27-positive
individuals; however, 5-10% of patients with AS lack HLA-B27
positivity (1). This finding
indicates that HLA-B27 is not directly linked to the manifestation
of AS, suggesting that factors beyond HLA contribute to disease
progression (40).
Endoplasmic reticulum aminopeptidase 1
(ERAP1)
ERAP1 is considered to be the second relevant risk
factor for AS (41). In order to
bind to HLA class I molecules, peptides must be cleaved to an
optimal length. In this process, ERAP1 is involved in trimming
precursors to 8-9 amino acids (42). As regards its function, ERAP1 may be
associated with the presentation of aberrant peptides, contributing
to AS, as reported by the Australo-Anglo-American Spondyloarthritis
Consortium (43). Additionally,
loss of ERAP1 function affects HLA-B27 dimerization or misfolding,
leading to the accumulation of abnormally formed HLA-B27 in the ER
(44).
KIR
Immune cells, such as NK cells and Th17 cells,
express killer-cell immunoglobulin-like receptor, three Ig domains
and long cytoplasmic tail 2 (KIR3DL2) receptor that has a stronger
affinity with the HLA-B27 homodimer (45). Interaction between KIR3DL2 and
HLA-B27 homodimers leads to the production of IL-17, which is known
to have a crucial role in the cytokine network and contributes to
the pathogenesis of AS (22,46).
However, it can be challenging to demonstrate differences between
specific groups, as KIR-mediated responses vary among individuals
(47).
Immune response
AS is a chronic inflammatory SpA, mainly
characterized by the inflammation of the spine and sacroiliac joint
(48). In addition, the tendons and
ligaments attached to the bones contribute to the pathogenesis of
AS, as they are particularly susceptible to inflammatory responses
(49). There are various complex
immune cells and cytokines involved in the pathogenesis of AS
(50). Th17 cells differentiated by
IL-23 are major triggers of inflammation in numerous immune
diseases (51). IL-17 can promote
osteoclastogenesis directly or indirectly through receptor
activator of nuclear factor kappa B (RANK) pathway in conjunction
with TNF-α, thereby inhibiting bone regeneration in SpA (52-54).
It can also stimulate immune cells to release IL-6, TNF-α, and
other cytokines and produce IL-17(55). Autoimmune diseases, including SpA,
IBD, and rheumatoid arthritis, generally arise from dysregulation
of the IL-23/IL-17 pathway (56).
Research has shown that the serum levels of IL-17 and IL-23 are
higher in patients with AS (57).
Nevertheless, the role of IL-23 in AS remains controversial, as its
inhibition has shown limited success (58).
Apart from the IL-23/IL-17 axis, IL-22, IL-32γ, and
IL-37 are known to contribute to the initiation of AS development.
IL-22 was reported to participate in osteogenesis, stimulating
osteoproliferation when exposed to IL-23 in an inflammatory state
(54,59). IL-32γ was shown to be increased in
specific regions such as the joints and tissues in patients with
AS, to generate osteoblast differentiation and abnormal new bone
formation (60). Moreover, IL-37
was also demonstrated to be elevated in patients with AS, with its
increase associated with disease severity and bone mineral density
(61).
Gut microbiome
Recently, growing evidence suggests that the
microbiome, a collection of microorganisms in specific organs of
the body, contributes to the development of AS (62). The gut microbiome exists in
intestinal mucosal surfaces acting as a safeguard against pathogens
(63). Occurrence of gut dysbiosis
may alter the permeability of the intestinal mucosa, inducing
penetration of microbial components (64). This disruption leads to damage in
the mucosal barrier, subsequently activating both innate immunity
and adaptive immunity (65). As a
result, bacterial antigens may enter sacroiliac joints and the
spine through lymph nodes, inducing inflammatory responses
(66). Research has shown that gut
dysbiosis may increase the risk of AS, as nearly 70% of patients
with AS exhibit gut inflammation (67).
4. Diagnosis
The Rome criteria was first established in 1961 by
the Council for International Organizations of Medical Sciences
(68). It included radiographic
findings and clinical presentation. Later, the same council added
the sacroiliitis grading system in the original criteria which is
called the New York criteria. Although the New York criteria has
been modified over the past two decades to be more inclusive, it
still has limitations due to the low sensitivity of X-rays
(69,70). The development of MRI has made it
possible to detect inflammatory changes in the sacroiliac joints at
early stages, as well as structural alterations associated with
advanced AS, with high precision (70). Due to the advent of MRI technology,
the new criteria can classify patients who exhibit active
sacroiliitis on the MRI with one clinical feature as patients with
SpA. This differs from the previous modified New York criteria
which diagnosed patients based on bilateral moderate or unilateral
severe sacroiliitis, which often led to a delay in diagnosis of
approximately 7 to 10 years (70).
The criteria are called Ankylosing Spondylitis Disease Activity
Score (ASDAS) which was determined by the Assessment of
Spondyloarthritis International Society (ASAS) in 2009. ASDAS is a
measure of disease activity using patient global assessment,
clinical pain, and morning stiffness duration (15). The ASDAS score can be categorized
into various levels indicating the disease activity. Additionally,
the Bath Ankylosing Disease Activity Index (BASDAI), a
patient-reported questionnaire, was developed to assess fatigue,
pain, and morning stiffness. It relies on patient self-reporting
and is therefore considered less objective than the ASDAS (15). The ASDAS with the BASDAI now serve
as the principal criteria in AS. However, ASDAS and BASDAI alone
are insufficient for an accurate diagnosis of AS; therefore,
radiographic evaluation and biomarker analysis should also be
included (15).
5. Treatment
The treatment of AS focuses on alleviating back
pain, morning stiffness, and loss of flexibility, as well as
reducing inflammation and preventing complications (1). To date, anti-inflammatory drugs are
the first-line treatment, according to the 2010 recommendations of
the ASAS (71).
Physical therapy (exercise)
Regarding daily activity and overall well-being,
exercise is recommended in clinical guidelines for managing AS to
alleviate pain, improve joint mobility, and maintain muscle
strength (72). Research has shown
that the combination of appropriate medication and physical
activities could be effective (73). In this context, patient education
and maintaining proper posture are important for achieving optimal
treatment effectiveness (74).
Non-steroid anti-inflammatory drugs
(NSAIDs)
NSAIDs are the first-line treatment for patients
with active AS (75). They block
the formation of prostaglandin by inhibiting cyclooxygenase (COX)
enzymes (76). Particularly,
prostaglandin E2 (PGE-2) promotes the activation of Th17 cells,
leading to the production of IL-23 and IL-17, which are strongly
implicated in inflammation in AS (77). However, prescribing the appropriate
NSAID can be challenging, as treatment response rates vary among
individuals. Therefore, NSAIDs should be selected based on the
prior response of the patient to these drugs, and their risk
factors for adverse effects (78).
In clinical practice, daily administration is more effective in
slowing the progression of AS than on-demand use (79). Prolonged use of these medications
can lead to gastrointestinal (GI) or cardiovascular events
(18).
Disease-modifying antirheumatic drugs
(DMARDs)
DMARDs are prescribed for patients with AS who are
unresponsive to NSAIDs (80).
Sulfasalazine is a typical DMARD for acute anterior uveitis in AS
by inhibiting the formation of prostaglandins. Additionally,
methotrexate, which blocks dihydrofolate reductase and,
subsequently inhibits deoxyribonucleic acid (DNA) synthesis, can be
another solution (81). However,
the efficacy of DMARDs on AS is unclear. Most studies have shown
that DMARDs mainly target peripheral joints and certain
extra-articular manifestations, but have limited efficacy for axial
involvement, such as back pain (13,17).
TNF-α inhibitors
TNF-α is a cytokine that is produced by macrophages
and lymphocytes (82). Patients
with AS tend to have elevated levels of TNF-α, indicating its
crucial role in the pathogenicity of AS (83). TNF-α inhibitors are an effective
treatment option for patients with AS, particularly those with an
inadequate response to NSAIDs (84). If the combination of more than 2
types of NSAIDs is ineffective even after 3 months of treatment,
the ASAS guidelines recommend the use of TNF-α inhibitors (71). TNF-α inhibitors are beneficial for
alleviating back pain, peripheral arthritis, morning stiffness, and
inflammatory activity and improving overall daily functioning
(85). Infliximab, the first
developed TNF-α inhibitor, is a monoclonal immunoglobulin G1
antibody consisting of 75% human and 25% mouse sequence, which
binds to the dissolved and receptor-bound forms of TNF-α (86,87).
Adalimumab is a 100% human monoclonal antibody against TNF-α,
blocking inflammatory processes (88). It is recommended to use it
subcutaneously at 40 mg once every 2 weeks (89). Golimumab is also a fully human
monoclonal antibody binding to TNF-α (90). The Food and Drug Administration
(FDA) approved its use for patients with AS by subcutaneous
injection of 50 mg once a month (91). Certolizumab pegol is a fragment
crystallizable (Fc)-free monoclonal antibody that binds to TNF-α
and neutralizes it. However, its long-term efficacy and potential
adverse effects require further investigation (86). Etanercept is a recombinant protein
fused with the Fc portion of immunoglobulin G1 and the TNF
receptor. It has an affinity with the soluble form of TNF-α, thus
blocking interaction with cell receptors. It is administered at 50
mg once or 25 mg twice per week subcutaneously (92). However, there are several challenges
associated with the use of TNF-α inhibitors in the treatment of AS.
Notably, ~40% of patients with AS have intolerance or inactivity to
medications (93). In addition,
recurrence of infections, such as tuberculosis (TB) and
candidiasis, can appear by inhibiting immune responses (94). Furthermore, long-term use does not
guarantee sustained remission (95).
IL-17 inhibitors
In recent years, research has found that the level
of IL-17 is regularly higher in patients with AS, which means it
has a key role in the onset of AS. Therefore, IL-17 has emerged as
one of the important targets in developing drugs for treating AS
(96). IL-17 inhibitors can serve
as a second-line treatment in patients who fail to respond to TNF-α
inhibitors (97). Secukinumab is
the first accepted monoclonal antibody and it is effective in
rapidly and sustainably relieving pain, as well as reducing bone
marrow edema in the sacroiliac joints (98-100).
It is considered to be advantageous in patients with TB, as there
is no evidence of TB occurrence or reactivation associated with its
use (101). Moreover, the efficacy
of secukinumab is maintained long-term, reportedly up to ~5 years
(100). Ixekizumab, an
immunoglobulin G4 monoclonal antibody, was recently approved as an
IL-17A inhibitor (102). It
improves joint and skin symptoms but remains less effective for
gastrointestinal manifestations, which may contribute to the
development of IBD (103).
Bimekizumab, another monoclonal antibody, can neutralize both
cytokines, IL-17A and IL-17F, concurrently (104). It can reduce disease activity,
C-reactive protein (CRP) levels, and bone marrow edema as observed
on MRI, thereby improving scores on the ASDAS (105). Brodalumab is a monoclonal antibody
targeting IL-17 receptor A, unlike bimekizumab. Its efficacy was
validated for psoriatic arthritis, but adverse events were also
reported, including infections, and GI disorders (106). However, a 3-year follow-up of
patients with psoriasis treated with brodalumab revealed
conflicting reports regarding suicidal ideation (107). Therefore, it needs to be further
investigated with continuous monitoring.
Janus kinase (JAK) inhibitors
The JAK pathway involves the expression of
cytokines, which are related to cell proliferation and
inflammation, eventually leading to autoimmune disorders (108). The IL-23/IL-17 axis, a major
regulator in the SpA immune system, could be partially controlled
by the JAK signaling pathway (109). Tofacitinib is a first-in-class JAK
inhibitor that targets JAK1, JAK3, and to a lesser degree
JAK2(110). It interferes with
cytokine-induced signal transduction, leading to abnormal immune
responses (111). Tofacitinib is
generally effective in AS due to its targeting of other JAK
proteins (112). Nevertheless,
clinical trials for JAK2-specific inhibitors are also required
(113). Upadacitinib is also a JAK
inhibitor that targets JAK1 selectively (114). It has recently been studied in
patients with AS who are unresponsive or intolerant to NSAIDs, as
part of the SELECT-AXIS 1 clinical trial (115). However, there was a report that
JAK inhibitors increase the possibility of thrombosis; therefore,
further data to assess the risk is needed (116).
6. Alternative and complementary
treatment
To date, drug therapy has been considered as a main
treatment for AS; however, there have been several adverse effects
and inconveniences to using these medications due to their
long-term use and cost (18,117).
In particular, long-term use of these drugs can lead to GI,
cardiovascular, and renal complications (118,119). In addition, their high cost is a
critical obstacle, as these treatments are generally unaffordable
for most patients (117). For that
reason, alternative and complementary therapies may represent a
viable option due to their safety and cost-effectiveness in
treating AS. However, they are not included in the official
standard treatment guidelines (120). International articles regarding
alternative and complementary treatments for AS were collected from
the PubMed (https://pubmed.ncbi.nlm.nih.gov) and Google Scholar
(https://scholar.google.com). The
keywords included ‘ankylosing spondylitis’, ‘spondyloarthropathy’,
‘oriental medicine’, ‘herbs’ and ‘therapy’. In this section,
efficacious remedies for treating AS are introduced.
Moxibustion
Moxibustion is a heat therapy that puts burned
‘moxa’, dried Artemisia genus leaves (common name: mugwort),
on acupoints of the skin stimulating thermal sensory receptors.
When receptors are activated by stimulation, therapeutic effects
occur when the nerve fibers transmit signals to the central nervous
system (121). In clinical
practice, patients treated with moxibustion, either in combination
with Western medicine or alone, demonstrated greater clinical
efficacy and lower CRP and erythrocyte sedimentation rate (ESR)
levels compared with Western medicine treatment alone. CRP and ESR
are two general indicators in monitoring the activity of
inflammation. However, no significant differences were observed
between the treatment with moxibustion alone and Western medicine
alone, suggesting that the combination therapy with moxibustion and
Western medicine may be an important approach in managing AS
(121). In another study with a
collagen-induced arthritis mouse model, the IL-6 level was lower in
the moxibustion-treated group than the untreated group (122). Moxibustion may also be associated
with regulating Treg cell numbers and altering NF-κB expression
(123). Additionally, moxibustion
can be used with warm acupuncture. After a sterilized needle is
inserted at a specific point, a moxa stick is placed on the needle
and ignited, delivering heat directly to the needle site. This
treatment could control the blood circulation, and improve the
immune system by reducing inflammatory cytokines (124). However, large-scale randomized
controlled trials are needed to confirm the efficacy of
moxibustion.
Herbal medicines
Herbal medicines are effective in alleviating
clinical symptoms and improving the quality of life of patients
(125). Because the constituents
of herbal medicines are complex and variable, elucidating their
exact mechanisms remains challenging (126). Currently, network pharmacology
serves as an effective tool bridging the gap between modern science
and traditional medicine, providing a foundation for further
studies aimed at identifying active compounds and elucidating the
mechanisms of herbal medicines (127). The present review summarizes the
efficacy and underlying mechanisms of these therapies for the
management of AS, as reported in experimental studies (Table I, Table
II and Table III).
 | Table IList of formulations for AS. |
Table I
List of formulations for AS.
| First author,
year | Formulation | Experimental
design | Efficacy | (Refs.) |
|---|
| Huang et al,
2014 |
Bushen-Qiangdu-Zhilv decoction | M1-polarized Raw
264.7 macrophage-like cells with 100 ng/ml interferon-γ | Suppresses TNF-α
and IL-1 mRNA expression levels | (128) |
| Li et al,
2016 | Kunxian
capsule | RCT | Decreases the
disease activity of patients with AS assessed by international
indicators ASAS 20, BASDAI 50, ASDAS-CRP, and serum CRP as well as
by patient global assessment of the disease activity, total back
pain, level of morning stiffness, tender joints, and BASFI
score. | (129) |
| Xie et al,
2022 | Fengshi Gutong
capsule | RCT | Decreases disease
activity of active patients with AS/ASDAS-CRP, BASDAI, BASFI,
BASMI, morning stiffness scores, PGA, nocturnal pain, total back
pain, CRP | (130) |
| Xie et al,
2017 |
Yun-Pi-Yi-Shen-Tong-Du-Tang | Network
analysis | Reduces the
symptoms of morning stiffness, fatigue, pain and decreases the
level of BASDAI, ASDAS-CRP and ASDAS-ESR Associated with the TLR
signaling pathway, the AMPK signaling pathway, the T-cell receptor
signaling pathway, and the TNF signaling pathway | (131) |
| Li et al,
2022 | Xinfeng
capsule | Network
analysis | Improves PLT, ESR,
and hs-CRP Associated with the NF-κB signaling pathway, the TNF
signaling pathway, and the IL-17 signaling pathway | (133) |
| Zhang et al,
2024 | Qiangji Jianpi
decoction | Network
analysis | Associated with
lipids and atherosclerosis, the IL-17 signaling pathway, the TNF
signaling pathway, chemical carcinogenesis-receptor activation, and
the AGE RAGE signaling pathway | (134) |
 | Table IIList of herbs for AS. |
Table II
List of herbs for AS.
| First author,
year | Herb | Experimental
design | Efficacy | (Refs.) |
|---|
| Wang et al,
2024 |
Epimedium | Network
analysis | Modulates signaling
pathways such as the AGE-RAGE, TNF, NF-κB/MAPK, and Toll-like
receptor signaling pathways | (139) |
| Li et al,
2022 | Scutellaria
baicalensis Georgi | Network
analysis | Involved in the
IL-17 pathway, TNF pathway, and NF-κB pathway | (141) |
| Fang et al,
2022 | Salvia
miltiorrhiza Bunge | Network analysis
and PBMCs from patients with AS | Inhibits the
expression levels of PTGS2, IL-6, and TNF-α Reduces ESR and CRP
Associated with the TNF, HIF-1, NF-κB, JAK-STAT, TLR, TGF-β, FoxO,
cytokine receptor interaction, PI3K-Akt, and the MAPK signaling
pathway | (143) |
| Dong et al,
2017 |
Chrysanthemum indicum
Linne | 2 mg Human
proteoglycan extract dissolved in 2 mg DDA induced AS mice | Delays the
progression of peripheral disease (paw swelling and stiffness of
rear leg joints in mice) Alleviates the spondylitis score Decreases
the serum levels of TNF-α, IL-1β, and IL-6 Upregulates the serum
levels of SOD, CAT, and GSH-Px and downregulates the serum levels
of MDA Decreases NF-κB p65 protein Increases the expression level
of SOST and DKK-1 in AS tissues | (147) |
 | Table IIIList of compounds derived from herbs
for AS. |
Table III
List of compounds derived from herbs
for AS.
| First author,
year | Compound | Experimental
design | Efficacy | (Refs.) |
|---|
| Zou et al,
2016 | Celastrol | Hip synovial
fibroblasts from 6 patients with AS | Reduces the cell
viability and EdU-positive AS fibroblasts Decreases ALP activity
Inhibits PEG-2-induced osteogenesis Inhibits the mRNA expression of
BMP2, type I collagen, RUNX2 | (151) |
| Liu et al,
2016 | Naringin | Zygotes from human
chorionic gonadotropin hormone-injected mice and HLAB2704 gene
fragment-injected pseudocyesis mice | Increases
osteocalcin and ALP Decreases the concentration of triglycerides
Attenuates the NF-κB p65, TNF-α, IL-1β and IL-6 activity values
Downregulates the level of MDA and upregulates the expression of
SOD, CAT and GSH-Px Decreases STAT3 and JAK2 | (154) |
| Feng et al,
2020 | Punicalagin | 2 mg of Human
proteoglycan extract dissolved in 2 mg DDA-induced AS mice | Reduces peripheral
disease progression scored for signs and symptoms of arthritis
Reduces IVD damage progression Downregulates the levels of ROS and
MDA and upregulates the levels of SOD, CAT and GPx in the
connective tissues excised from the vertebra Decreases the serum
levels of IL-1β, IL-6, TNF-α, IL-17A, IL-23 and NO Downregulates
the activation of NF-κB and the phosphorylation levels of JAK2 and
STAT3 | (155) |
| Dong, 2018 | Sinomenine | 2 mg Human
proteoglycan extract dissolved in 2 mg DDA-induced AS mice | Decreases the
levels of TNF-α, IL-1β, and IL-6 Increases the levels of SOD, CAT,
and GSH-Px Decreases NF-κBp65 and p-p38 Increases the level of IκB
Decreases the level of COX-2 | (157) |
Formulations. Bushen-Qiangdu-Zhilv
(BQZ) decoction
BQZ decoction is a traditional Chinese medicine that
is used in AS. It is composed of Drynariae Rhizoma,
Psoraleae Fructus, Rehmanniae Praeparata Radix,
Epimedii Herba, Notopterygii Rhizoma et Radix,
Cibotii Rhizoma, Angelicae Pubescentis Radix,
Dipsaci Radix, Eucommiae Cortex, Cyathulae Radix,
Lycopi Herba, Cinnamomi Ramulus, Anemarrhenae
Rhizoma, Aconiti carmichaeli Radix, Ephedrae
Herba, Zingiberis Rhizoma, Atractylodis Rhizoma
Alba, Clematidis Radix, Saposhnikoviae Radix,
Coicis Semen, Paeoniae Radix, and Paeoniae Radix
Alba. In a previous study, BQZ decoction was extracted with
petroleum ether, ethyl acetate, n-butanol (BU) and water. BQZ was
incubated in M1-polarized RAW264 cells that were first induced by
interferon (IFN)-γ. The BQZ water extract significantly decreased
the mRNA level of TNF-α, while the BQZ BU extracts suppressed that
of IL-1. Moreover, neither the water nor the BU extracts induced
cell death. These findings indicate that the BQZ decoction is
beneficial in reducing inflammation and has low cytotoxicity
(128).
Kunxian (KX) capsule
The KX capsule is used as an anti-inflammatory
regulator in autoimmune diseases in China. KX has been reported to
reduce back pain and morning stiffness in AS. It is composed of
four main herbs, namely Tripterygium wildfordii Hook. f.,
Epimedii Herba, Cuscutae Semen, and Lycii
Fructus. A previous randomized, double-blind, controlled trial
involving 80 patients with AS was conducted to evaluate the
efficacy of KX. The study used various indices to assess the
effects of KX in patients with AS. The KX-treated group showed
improvement in indicators, including the ASAS 20, BASDAI 50,
ASDAS-CRP, serum CRP, and Bath Ankylosing Spondylitis Functional
Index when compared to the placebo group. Moreover, 37% of the
patients in the KX group achieved an ASAS 20 at week 12, and marked
improvements in the BASDAI 50 were observed in 40% of the patients
at week 6(129).
Fengshi Gutong capsule (FSGTC)
FSGTC is a traditional Chinese medicine used for
patients suffering from joint pain in China. It contains seven
herbs including Aconiti Radix Cocta, Aconiti Kusnezoffii
Radix Cocta, Carthami Flos, Glycyrrhizae Radix Et
Rhizoma, Chaenomelis Fructus, Mume Fructus, and
Ephedrae Herba. A previous study recruited 180 patients with
AS and randomized them into three groups by treatment type: The
combination group, the FSGTC group, and the imrecoxib group. The
ASAS20 response rate was measured as the primary endpoint. The
results showed that the ASAS20 rate in the FSGTC group was higher
than that in the imrecoxib group. Moreover, the other indicators,
including the ASDAS-CRP, patient's global assessment of disease
activity, morning stiffness, and BASDAI, were improved in the
combination and FSGTC groups compared with the imrecoxib group. In
the safety test, the FSGTC group exhibited the lowest adverse
effects, especially in GI tolerability. The findings indicate that
FSGTC alone or combined with NSAIDs may be another viable option
for patients with GI intolerance (130).
Yun-Pi-Yi-Shen-Tong-Du-Tang (ΥΥΤ)
YYT is a traditional formula that consists of 11
medicinal herbs: Dioscoreae Rhizoma, Atractylodis
Rhizoma, Smilacis Glabrae Rhizoma, Lonicerae
Japonicae Flos, Achyranthis Bidentatae Radix,
Myrrha, Aconiti Praeparata Radix, Astragali
Radix, Glycyrrhizae Radix et Rhizoma, Hirudo, and
Coptidis Rhizoma. A previous study used network pharmacology
to compare YYT targets with those of FDA-approved drugs and
AS-related proteins. A total of 34 proteins overlapped between YYT
targets and drug targets, including TNF and COX-2. Additionally,
YYT targets and AS-related proteins formed 3,732 protein-protein
interaction (PPI) pairs, highlighting two key targets: JAK2 and
signal transducer and activator of transcription 3 (STAT3). These
results indicate the YYT might enhance the effects of western
medicine and exert therapeutic effects on AS-related inflammation
(131).
Xinfeng capsule (XFC)
XFC has been used to treat AS for >10 years and
is associated with fewer adverse effects (132). It primarily consists of four
herbs: Astragali Radix, Coicis Semen, Tripterygium
wilfordii Hook. f., and Scolopendra. In a previous
network analysis, the 103 active compounds and 212 potential
targets of XFC were compared with 1,961 AS-related targets,
resulting in 59 overlapping targets. PPI analysis and core target
screening identified 13 key targets, including IL-4, IL-6, TNF,
IL-1β, vascular endothelial growth factor A (VEGFA), IL-10, C-C
motif chemokine ligand 2 (CCL2), COX-2, C-X-C motif chemokine
ligand 8 (CXCL8), epidermal growth factor, STAT3, NF-κB inhibitor
alpha, and IFN-γ. Kyoto Encyclopedia of Genes and Genomes (KEGG)
enrichment identified 20 important pathways including the NF-κB,
TNF, and IL-17 signaling pathways, which involve inflammatory
responses. In addition, among the 103 active compounds analyzed,
the top four active ingredients that have a strong connection to AS
targets were as follows: Formononetin, triptolide, quercetin, and
kaempferol. Molecular docking of the components with the core
targets IL-6, CCL2, TNF, and IL-4 suggested that formononetin,
triptolide, quercetin, and kaempferol may have important roles in
the treatment of AS (133).
Qiangji Jianpi (QJJP) decoction
QJJP decoction is a modified traditional Chinese
medicine which includes Astragali Radix, Codonopsis
Pilosulae Radix, Atractylodes Rhizome, Angelicae
Sinensis Radix, Cimicifugae Rhizoma, Bupleuri
Radix, Citri Reticulatae Pericarpium and Glycyrrhizae
Radix et Rhizoma. A previous study used network pharmacology,
molecular docking, and Mendelian randomization to analyze the
interactions among the decoction, IBD, and AS. The results showed
that, among 105 targets of the QJJP decoction, 85 targets
overlapped with targets associated with both AS and IBD. In the
Gene Ontology (GO) and KEGG pathways, the targets were associated
with oxidative stress, which is thought to be one of the main
features of IBD. Molecular docking indicated IL-1α, IFN-γ, TGF-β1,
and endothelin-1 as important targets for the treatment of AS with
IBD. IFN-γ is a cytokine that is released in the innate and
adaptive immune systems (134).
IFN-γ is known to be strongly produced in patients with AS
following stimulation with Mycoplasma arthritis (135). Additionally, IFN-γ was revealed to
be closely related to the HLA-B27-associated unfolded protein
responses in SpA, indicating that IFN-γ could be one of the
critical biomarkers for diagnosing and treating AS (136).
Herbs. Epimedium (EP)
EP, the largest herbaceous genus of the
Berberidaceae family, has various components including flavonoids,
alkaloids, and other compounds (137). It has been used as an
antirheumatic agent with its anti-inflammatory effects (138). Using network pharmacology, 16
active compounds were identified in EP, and the corresponding EP
targets were subsequently matched with disease targets, yielding 80
overlapping targets. The core active compounds were
8-(3-methylbut-2-enyl)-2-phenylchromone, anhydroicaritin, and
luteolin, and the top-ranked overlapped targets were TNF, IL-6,
IL-1β, matrix metalloproteinase-9, and COX-2. Through molecular
docking and molecular dynamic simulation analysis, it was found
that the core compounds of EP have a strong binding activity and
stable interactions with five core targets. These findings
indicated that EP has the potential to be used in the treatment of
AS with the intersecting target genes (139).
Scutellaria baicalensis Georgi
(SBG)
SBG is a species of the Lamiaceae family, and its
root is mainly used in various treatments. It is usually used in
immune disorders and inflammatory diseases (140). The acquired active components and
targets of SBG were analyzed by network analysis. The main
components of SBG were baicalein, wogonin, and oroxylin A. Among
them, 29 targets overlapped with AS targets. In the PPI analysis,
TNF, IL-6, CXCL8, COX-2, and VEGFA were found to be associated with
SBG in AS. Moreover, the core targets and main compounds exhibited
strong connections in the molecular docking. As mentioned for
NSAIDs, COX-2 mediates inflammatory pathways by inducing PG
production. PG promotes the proliferation of synoviocytes and
inflammation. Therefore, these findings indicated that SBG may have
a therapeutic role in AS similar to that of NSAIDs (141).
Salvia miltiorrhiza Bunge
Salvia miltiorrhiza Radix (SMR) is from the
root of Salvia miltiorrhiza Bunge, a plant of the Lamiaceae
family. It has been used to treat various diseases, including
cardiovascular and immune diseases (142). A previous study conducted data
mining, network pharmacology, and in vitro assays. In
clinical trials, immunology indices, including ESR, CRP, and
complement component 3, were significantly reduced in 2,079
patients compared with baseline measurements. To identify the
therapeutic targets against AS, the study used network
pharmacology. The study identified COX-2, IL-6, TNF, STAT3, and
VEGFA as key targets of SMR in the treatment of AS. Furthermore,
the TNF signaling pathway appeared to be the most enriched pathway
in the KEGG enrichment analysis. Moreover, cryptotanshinone and
tanshinone IIA exhibited higher affinities with key targets,
including TNF-α, IL-6, and COX-2 through molecular docking. In
in vitro assays using peripheral blood mononuclear cells
from patients with AS, it was demonstrated that cryptotanshinone
and tanshinone IIA, the main compounds of SMR, significantly
reduced the protein expression levels of COX-2, IL-6, and TNF-α.
Consequently, the results indicated that SMR may inhibit the TNF
signaling pathway by modulating the expression of COX-2, IL-6, and
TNF-α (143).
Chrysanthemum indicum (C. indicum)
Linne
C. indicum Linne has been commonly used in
Korean, Chinese, and Japanese medicine for the treatment of
autoimmune diseases. Various studies have concluded that C.
indicum possesses antimicrobial, antioxidant, and
immuneregulatory properties (144-146).
A previous study assessed disease severity in the intervertebral
joints by comparing the quantitative changes in the C.
indicum-treated group with the control AS group. The treatment
group that received the C. indicum extract exhibited
decreased levels of TNF-α, IL-1β, IL-6, and NF-κB p65 protein.
Antioxidant enzymes including catalase, superoxide dismutase, and
glutathione peroxidase were regulated in the AS mice compared with
the control group. Moreover, the levels of sclerostin and
dickkopf-1, which inhibit the wingless and Int-1 (Wnt) pathway were
increased in the AS mice. These findings indicated that C.
indicum can inhibit the Wnt pathway, which plays a key role in
the production, growth, and maturation of osteoblastic cells.
Overall, C. indicum may have a beneficial role in oxidative
stress, inflammation, and osteogenesis in the treatment of AS
(147).
Compounds. Celastrol
Celastrol,
(2R,4aS,6aS,12bR,14aS,14bR)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylic
acid), is one of the compounds in T. wilfordii Hook. f.,
revealed to have effects on decreasing inflammation and reducing
arthritis (148). Fibroblasts
obtained from hip synovial tissues of patients with AS were
incubated with PGE-2, which promotes proliferation and
osteogenesis, and celastrol. Notably, 1.0 µM celastrol had an
inhibitory effect against alkaline phosphatase (ALP) and
mineralization. In addition, it significantly reduced the gene
expression levels of bone morphogenetic protein 2 (BMP2) and the
regulation of runt-related transcription factor 2 (RUNX2) in the
fibroblasts. BMP2, a key inducer of osteogenic activity in AS,
upregulates RUNX2 expression, a transcription factor for ALP that
promotes osteoblast differentiation (149,150). In further investigation with 1.0
µM celastrol, the levels of PGE-2, protein kinase B (AKT) and
phosphatidylinositol 3-kinase (PI3K) were decreased, and the Wnt
pathway was inhibited. Consequently, celastrol may inhibit the
formation of abnormal new bone by blocking PGE-2 and the Wnt
signaling pathway (151).
Naringin
Naringin is a natural flavonoid that can be found
in citrus fruits. It has been suggested to affect oxidation and
inflammation (152). Additionally,
naringin has been reported to possess osteogenic effects (153). A study established an AS-induced
mouse model and treated the mice with naringin at doses of 20, 40
and 80 mg/kg. Following treatment, the expression values of
osteocalcin, ALP, and triglyceride activity became similar to those
of the healthy group. The naringin-treated groups exhibited
significant anti-inflammatory effects by modulating TNF-α, IL-1β,
and IL-6, as well as improving oxidative stress markers.
Furthermore, the JAK2/STAT3 signaling pathways were suppressed by
naringin treatment (154).
Punicalagin
Punicalagin
(2,3-hexahydroxydiphenoyl-gallagyl-D-glucose), a water-soluble
compound usually present in Punica granatum Linné, is
considered to have anti-inflammatory effects in AS. In a previous
study, AS-induced mice injected with human proteoglycan extract
were treated with punicalagin. As a result, the punicalagin
treatment significantly improved antioxidant enzyme activities. It
decreased the levels of reactive oxygen species and
malondialdehyde, indicating that punicalagin may have a direct
effect on oxidative stress. Regarding the anti-inflammatory effects
of punicalagin, serum levels of cytokines, including IL-1β, IL-6,
TNF-α, IL-17A, and IL-23, were reduced. The reduction of IL-1β,
IL-6, and TNF-α suggests that punicalagin may inhibit the NF-κB
pathway, which is generally associated with oxidative stress.
Moreover, the JAK2 and STAT3 phosphorylation levels were decreased
in the punicalagin-treated groups (155).
Sinomenine
Sinomenine
(7,8-didehydro-4-hydroxy3,7-dimethoxy-17-methyl-9a, 13a,
14a-morphinan-6-one) is derived from Sinomenium acutum
Rehder et Wilson, and widely used for rheumatoid arthritis in China
(156). Previous research has
focused on the NF-κB pathway, the mitogen-activated protein kinase
(MAPK) p38 pathway, and COX-2, as these have been reported to
modulate inflammatory cytokines and oxidative stress. In this
study, AS mice were treated with different doses of sinomenine (10,
30, and 50 mg/kg), and in the sinomenine-treated AS mouse groups,
the levels of TNF-α, IL-1β, and IL-6 were dose-dependently reduced.
Moreover, antioxidant enzymes such as catalase, glutathione
peroxidase and superoxide dismutase were increased compared with
the control AS group. Furthermore, the mRNA expression levels of
NF-κBp65 and COX-2 were decreased, while the sinomenine treatment
increased the expression level of the inhibitor of NF-κB. Overall,
sinomenine ameliorated AS via inhibition of the MAPKp38 and NF-κB
pathways, and COX-2 expression (157).
7. Conclusion
AS is a progressive chronic disease that typically
occurs around the second decade of life. Patients with AS
experience pain in the sacroiliac joint, morning stiffness, bone
erosion, and bone formation, which can lead to bone fusion known as
syndesmophytes. In diagnosing AS, criteria from the ASAS,
patient-reported questionnaires such as the BASDAI, and radiographs
are used to assess disease progression. Although the pathogenesis
of AS has yet to be fully elucidated, HLA-B27 is considered to be
an important factor. There are four hypotheses regarding
HLA-related pathogenesis in AS: The arthritogenic peptide theory,
the misfolding theory, the homodimer theory, and the mimicry
theory. The arthritogenic peptide and mimicry theories suggest that
the presentation of an abnormal peptide to HLA activates
CD8+ T cells. The misfolding theory and ERAP1, a
secondary risk factor, are associated. When ERAP1 dysfunction leads
to abnormal peptide trimming, HLA molecules fail to fold properly
and accumulate in the ER, causing ER stress and UPR. This in turn
activates autophagy, promoting IL-23 production. The homodimer
theory suggests that dissociation of the β light chain in HLA
enhances its affinity for KIRs, which are expressed on the surface
of NK cells and Th17 cells. Th17 cells activated by IL-23 and the
homodimer promote the release of IL-17 and IL-22. IL-17 induces
inflammation and bone erosion. IL-22 stimulates new bone formation.
Thus, the bone erosion and new bone formation develop into bone
fusion (Fig. 1).
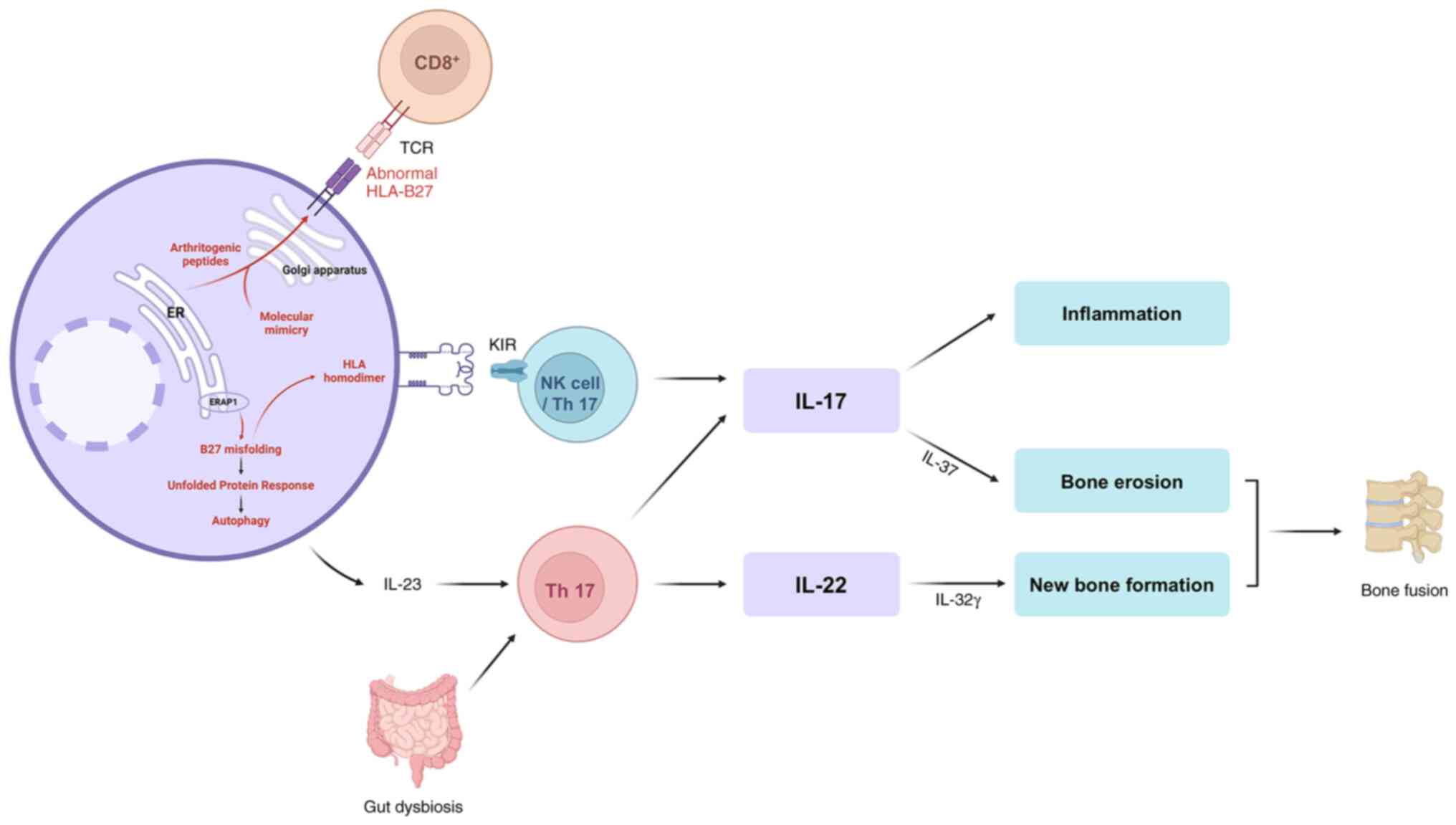 | Figure 1Diagram of possible pathogenesis of
ankylosing spondylitis. The four hypotheses of HLA-related
pathogenesis: The arthritogenic peptide theory, the misfolding
theory, the homodimer theory, and the mimicry theory. The
arthritogenic peptide theory suggests that presentation of abnormal
or self-like peptides by HLA-B27 activates autoreactive
CD8+ T cells. The misfolding theory emphasizes that
ERAP1 variants generate aberrant peptides that fail to stabilize
HLA molecules, resulting in misfolded HLA accumulation in the ER.
This induces ER stress and the UPR, subsequently activating
autophagy and promoting IL-23 production. The homodimer theory
suggests that dissociation of the β2-microglobulin light
chain allows HLA-B27 heavy chains to form homodimers, which
strongly interact with KIRs on NK and Th17 cells. Th17 activation
by IL-23 and homodimer signaling promotes release of IL-17 and
IL-22, leading to inflammation, bone erosion, and new bone
formation. The mimicry theory proposes that microbial peptides
structurally resemble self-peptides, leading to cross-reactive
immune responses when presented by HLA-B27. The interplay of bone
erosion and aberrant new bone formation ultimately contributes to
pathological bone fusion. HLA, human leukocyte antigen, ERAP1,
endoplasmic reticulum aminopeptidase 1; ER, endoplasmic reticulum;
UPR, unfolded protein response; IL, interleukin; KIRs, killer
immunoglobulin-like receptors; NK, natural killer. |
Treatment for AS includes physical exercise and
pharmacological approaches. The main drugs currently in use are
NSAIDs, DMARDs, and TNF-α inhibitors, and IL-17 inhibitors and JAK
inhibitors are currently in development. These drugs primarily
target the control of inflammation. However, Western medicine can
cause side effects depending on treatment duration and is
associated with a significant cost burden. Therefore, alternative
and complementary therapies may be another option for treating AS.
Commonly used alternative remedies include moxibustion and herbal
medicines. Moxibustion is a heat therapy that transmits signals by
stimulating acupoints. Herbal medicines encompass various forms,
including decoctions, formulations, herbal extracts, and isolated
compounds, demonstrating a multi-targeted approach.
In the present review, potential natural medicines
for the treatment of AS, along with their efficacy and underlying
mechanisms were discussed. The underlying mechanisms of herbal
medicines, including formulations, herbs, and compounds derived
from herbs, have been identified as the reduction of inflammation,
oxidative stress, and abnormal osteogenesis in AS. Given that most
known drugs for AS exert anti-inflammatory effects, particularly
through the Th17 cell-mediated IL-23/IL-17 axis and TNF-α, various
responses to herbal medicines may contribute to inhibiting AS
progression. Furthermore, as identified in the present review, the
fact that most herbal medicines have a multi-targeting mechanism
suggests that this could be a key factor in treating AS, a disease
characterized by complex pathogenic mechanisms. In addition,
investigating the functional mechanisms of herbal medicines in
preclinical and clinical research is crucial for the development of
novel treatments for AS. Although numerous studies have explored
effective herbal medicines for AS, most are based on network
analyses, which only provide predictive results. One of the
difficulties in investigating AS is that it is not easy to collect
spinal specimens from patients. For this reason, AS animal models
are considered critical in research aimed at developing novel
treatments. Future studies are expected to focus on the discovery
of new herbal medicines for AS through large-scale preclinical
research, followed by clinical trials to validate their
efficacy.
Acknowledgements
Not applicable.
Funding
Funding: The present review article was supported by Woosuk
University.
Availability of data and materials
Not applicable.
Authors' contributions
DYK and MHK conceived the study and supervised the
review. SYP, MSK, SS and LYC performed the literature search and
data collection. SYP and MSK wrote the first draft of the
manuscript, and all authors commented on previous versions of the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu W, He X, Cheng K, Zhang L, Chen D,
Wang X, Qiu G, Cao X and Weng X: Ankylosing spondylitis: Etiology,
pathogenesis, and treatments. Bone Res. 7(22)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Van Royen BJ and Dijkmans BAC (eds):
Ankylosing Spondylitis Diagnosis and Management. CRC Press,
2006.
|
|
3
|
Gouveia EB, Elmann D and de Ávila Morales
MS: Ankylosing spondylitis and uveitis: Overview. Rev Bras
Reumatol. 52:742–756. 2012.PubMed/NCBI(In English, Portuguese).
|
|
4
|
Zink A, Braun J, Listing J and Wollenhaupt
J: Disability and handicap in rheumatoid arthritis and ankylosing
spondylitis-results from the German rheumatological database.
German collaborative arthritis centers. J Rheumatol. 27:613–622.
2000.PubMed/NCBI
|
|
5
|
Landewe R, Dougados M, Mielants H, van der
Tempel H and van der Heijde D: Physical function in ankylosing
spondylitis is independently determined by both disease activity
and radiographic damage of the spine. Ann Rheum Dis. 68:863–867.
2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kim SH and Lee SH: Updates on ankylosing
spondylitis: Pathogenesis and therapeutic agents. J Rheum Dis.
30:220–233. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pedersen SJ and Maksymowych WP: Beyond the
TNF-α inhibitors: New and emerging targeted therapies for patients
with axial spondyloarthritis and their relation to pathophysiology.
Drugs. 78:1397–1418. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Fiorillo MT, Haroon N, Ciccia F and Breban
M: Editorial: Ankylosing spondylitis and related immune-mediated
disorders. Front Immunol. 10(1232)2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Arévalo M, Gratacós Masmitjà J, Moreno M,
Calvet J, Orellana C, Ruiz D, Castro C, Carreto P, Larrosa M,
Collantes E, et al: Influence of HLA-B27 on the ankylosing
spondylitis phenotype: Results from the REGISPONSER database.
Arthritis Res Ther. 20(221)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen B, Li J, He C, Li D, Tong W, Zou Y
and Xu W: Role of HLA-B27 in the pathogenesis of ankylosing
spondylitis (Review). Mol Med Rep. 15:1943–1951. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Reveille JD: An update on the contribution
of the MHC to AS susceptibility. Clin Rheumatol. 33:749–757.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Baraliakos X, Heldmann F, Callhoff J,
Listing J, Appelboom T, Brandt J, Van den Bosch F, Breban M,
Burmester G, Dougados M, et al: Which spinal lesions are associated
with new bone formation in patients with ankylosing spondylitis
treated with anti-TNF agents? A long-term observational study using
MRI and conventional radiography. Ann Rheum Dis. 73:1819–1825.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Akkoc N, van der Linden S and Khan MA:
Ankylosing spondylitis and symptom-modifying vs disease-modifying
therapy. Best Pract Res Clin Rheumatol. 20:539–557. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Perrotta FM, Scriffignano S, Ciccia F and
Lubrano E: Therapeutic targets for ankylosing spondylitis-recent
insights and future prospects. Open Access Rheumatol. 14:57–66.
2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Agrawal P, Tote S and Sapkale B: Diagnosis
and treatment of ankylosing spondylitis. Cureus.
16(e52559)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moon KH and Kim YT: Medical treatment of
ankylosing spondylitis. Hip Pelvis. 26:129–135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dougados M and Baeten D:
Spondyloarthritis. Lancet. 377:2127–2137. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vonkeman HE and van de Laar MAFJ:
Nonsteroidal anti-inflammatory drugs: Adverse effects and their
prevention. Semin Arthritis Rheum. 39:294–312. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lindström U, Olofsson T, Wedrén S, Qirjazo
I and Askling J: Impact of extra-articular spondyloarthritis
manifestations and comorbidities on drug retention of a first
TNF-inhibitor in ankylosing spondylitis: A population-based
nationwide study. RMD Open. 4(e000762)2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
van der Heijde D, Song IH, Pangan AL,
Deodhar A, van den Bosch F, Maksymowych WP, Kim TH, Kishimoto M,
Everding A, Sui Y, et al: Efficacy and safety of upadacitinib in
patients with active ankylosing spondylitis (SELECT-AXIS 1): A
multicentre, randomised, double-blind, placebo-controlled, phase
2/3 trial. Lancet. 394:2108–2117. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Maksymowych WP: The role of imaging in the
diagnosis and management of axial spondyloarthritis. Nat Rev
Rheumatol. 15:657–672. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sieper J and Poddubnyy D: Axial
spondyloarthritis. Lancet. 390:73–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Taurog JD, Chhabra A and Colbert RA:
Ankylosing spondylitis and axial spondyloarthritis. N Engl J Med.
374:2563–2574. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wordsworth BP, Cohen CJ, Davidson C and
Vecellio M: Perspectives on the genetic associations of ankylosing
spondylitis. Front Immunol. 12(603726)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Simone D, Al Mossawi MH and Bowness P:
Progress in our understanding of the pathogenesis of ankylosing
spondylitis. Rheumatology (Oxford). 57 (Suppl 6):vi4–vi9.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Madden DR: The three-dimensional structure
of peptide-MHC complexes. Annu Rev Immunol. 13:587–622.
1995.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bowness P: Hla-B27. Annu Rev Immunol.
33:29–48. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin H and Gong YZ: Association of HLA-B27
with ankylosing spondylitis and clinical features of the
HLA-B27-associated ankylosing spondylitis: A meta-analysis.
Rheumatol Int. 37:1267–1280. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chatzikyriakidou A, Voulgari PV and Drosos
AA: What is the role of HLA-B27 in spondyloarthropathies? Autoimmun
Rev. 10:464–468. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
López de Castro JA: The HLA-B27 peptidome:
Building on the cornerstone. Arthritis Rheum. 62:316–319.
2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Colbert RA, Tran TM and Layh-Schmitt G:
HLA-B27 misfolding and ankylosing spondylitis. Mol Immunol.
57:44–51. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Colbert RA, DeLay ML, Layh-Schmitt G and
Sowders DP: HLA-B27 misfolding and spondyloarthropathies. Prion.
3:15–26. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lenart I, Guiliano DB, Burn G, Campbell
EC, Morley KD, Fussell H, Powis SJ and Antoniou AN: The MHC class I
heavy chain structurally conserved cysteines 101 and 164
participate in HLA-B27 dimer formation. Antioxid Redox Signal.
16:33–43. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Turner MJ, Sowders DP, DeLay ML, Mohapatra
R, Bai S, Smith JA, Brandewie JR, Taurog JD and Colbert RA: HLA-B27
misfolding in transgenic rats is associated with activation of the
unfolded protein response. J Immunol. 175:2438–2448.
2005.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Smith JA: Regulation of cytokine
production by the unfolded protein response; implications for
infection and autoimmunity. Front Immunol. 9(422)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kavadichanda CG, Geng J, Bulusu SN, Negi
VS and Raghavan M: Spondyloarthritis and the human leukocyte
antigen (HLA)-B*27 connection. Front Immunol.
12(601518)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Payeli SK, Kollnberger S, Marroquin
Belaunzaran O, Thiel M, McHugh K, Giles J, Shaw J, Kleber S, Ridley
A, Wong-Baeza I, et al: Inhibiting HLA-B27 homodimer-driven immune
cell inflammation in spondylarthritis. Arthritis Rheum.
64:3139–3149. 2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Antoniou AN, Lenart I and Guiliano DB:
Pathogenicity of misfolded and dimeric HLA-B27 molecules. Int J
Rheumatol. 2011(486856)2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhang L, Zhang YJ, Chen J, Huang XL, Fang
GS, Yang LJ, Duan Y and Wang J: The association of HLA-B27 and
Klebsiella pneumoniae in ankylosing spondylitis: A
systematic review. Microb Pathog. 117:49–54. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Reveille JD: The genetic basis of
ankylosing spondylitis. Curr Opin Rheumatol. 18:332–341.
2006.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tsui FW, Haroon N, Reveille JD, Rahman P,
Chiu B, Tsui HW and Inman RD: Association of an ERAP1 ERAP2
haplotype with familial ankylosing spondylitis. Ann Rheum Dis.
69:733–736. 2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen B, Li D and Xu W: Association of
ankylosing spondylitis with HLA-B27 and ERAP1: Pathogenic role of
antigenic peptide. Med Hypotheses. 80:36–38. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Evans DM, Spencer CC, Pointon JJ, Su Z,
Harvey D, Kochan G, Oppermann U, Dilthey A, Pirinen M, Stone MA, et
al: Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis
implicates peptide handling in the mechanism for HLA-B27 in disease
susceptibility. Nat Genet. 43:761–767. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
44
|
Campbell EC, Fettke F, Bhat S, Morley KD
and Powis SJ: Expression of MHC class I dimers and ERAP1 in an
ankylosing spondylitis patient cohort. Immunology. 133:379–385.
2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bowness P, Ridley A, Shaw J, Chan AT,
Wong-Baeza I, Fleming M, Cummings F, McMichael A and Kollnberger S:
Th17 cells expressing KIR3DL2+ and responsive to HLA-B27 homodimers
are increased in ankylosing spondylitis. J Immunol. 186:2672–2680.
2011.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Li Z and Brown MA: Progress of genome-wide
association studies of ankylosing spondylitis. Clin Transl
Immunology. 6(e163)2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rajalingam R: Human diversity of killer
cell immunoglobulin-like receptors and disease. Korean J Hematol.
46:216–228. 2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yin Y, Wang M, Liu M, Zhou E, Ren T, Chang
X, He M, Zeng K, Guo Y and Wu J: Efficacy and safety of IL-17
inhibitors for the treatment of ankylosing spondylitis: A
systematic review and meta-analysis. Arthritis Res Ther.
22(111)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Schett G, Lories RJ, D'Agostino MA,
Elewaut D, Kirkham B, Soriano ER and McGonagle D: Enthesitis: From
pathophysiology to treatment. Nat Rev Rheumatol. 13:731–741.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rezaiemanesh A, Abdolmaleki M,
Abdolmohammadi K, Aghaei H, Pakdel FD, Fatahi Y, Soleimanifar N,
Zavvar M and Nicknam MH: Immune cells involved in the pathogenesis
of ankylosing spondylitis. Biomed Pharmacother. 100:198–204.
2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jain R, Chen Y, Kanno Y, Joyce-Shaikh B,
Vahedi G, Hirahara K, Blumenschein WM, Sukumar S, Haines CJ,
Sadekova S, et al: Interleukin-23-induced transcription factor
blimp-1 promotes pathogenicity of T helper 17 cells. Immunity.
44:131–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Garcia-Montoya L, Gul H and Emery P:
Recent advances in ankylosing spondylitis: Understanding the
disease and management. F1000Res. 7(F1000 Faculty
Rev-1512)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Sato K, Suematsu A, Okamoto K, Yamaguchi
A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y,
et al: Th17 functions as an osteoclastogenic helper T cell subset
that links T cell activation and bone destruction. J Exp Med.
203:2673–2682. 2006.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Babaie F, Hasankhani M, Mohammadi H,
Safarzadeh E, Rezaiemanesh A, Salimi R, Baradaran B and Babaloo Z:
The role of gut microbiota and IL-23/IL-17 pathway in ankylosing
spondylitis immunopathogenesis: New insights and updates. Immunol
Lett. 196:52–62. 2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Dong C: TH17 cells in development: An
updated view of their molecular identity and genetic programming.
Nat Rev Immunol. 8:337–348. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mahmoudi M, Aslani S, Nicknam MH, Karami J
and Jamshidi AR: New insights toward the pathogenesis of ankylosing
spondylitis; genetic variations and epigenetic modifications. Mod
Rheumatol. 27:198–209. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mei Y, Pan F, Gao J, Ge R, Duan Z, Zeng Z,
Liao F, Xia G, Wang S, Xu S, et al: Increased serum IL-17 and IL-23
in the patient with ankylosing spondylitis. Clin Rheumatol.
30:269–273. 2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Baeten D and Adamopoulos IE: IL-23
inhibition in ankylosing spondylitis: Where did it go wrong? Front
Immunol. 11(623874)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
El-Zayadi AA, Jones EA, Churchman SM,
Baboolal TG, Cuthbert RJ, El-Jawhari JJ, Badawy AM, Alase AA,
El-Sherbiny YM and McGonagle D: Interleukin-22 drives the
proliferation, migration and osteogenic differentiation of
mesenchymal stem cells: A novel cytokine that could contribute to
new bone formation in spondyloarthropathies. Rheumatology (Oxford).
56:488–493. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lee EJ, Lee EJ, Chung YH, Song DH, Hong S,
Lee CK, Yoo B, Kim TH, Park YS, Kim SH, et al: High level of
interleukin-32 gamma in the joint of ankylosing spondylitis is
associated with osteoblast differentiation. Arthritis Res Ther.
17(350)2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Chen B, Huang K, Ye L, Li Y, Zhang J,
Zhang J, Fan X, Liu X, Li L, Sun J, et al: Interleukin-37 is
increased in ankylosing spondylitis patients and associated with
disease activity. J Transl Med. 13(36)2015.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Lin P, Bach M, Asquith M, Lee AY,
Akileswaran L, Stauffer P, Davin S, Pan Y, Cambronne ED, Dorris M,
et al: HLA-B27 and human β2-microglobulin affect the gut microbiota
of transgenic rats. PLoS One. 9(e105684)2014.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Xu YY, Tan X, He YT, Zhou YY, He XH and
Huang RY: Role of gut microbiome in ankylosing spondylitis: an
analysis of studies in literature. Discov Med. 22:361–370.
2016.PubMed/NCBI
|
|
64
|
Di Vincenzo F, Del Gaudio A, Petito V,
Lopetuso LR and Scaldaferri F: Gut microbiota, intestinal
permeability, and systemic inflammation: A narrative review. Intern
Emerg Med. 19:275–293. 2024.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Thaiss CA, Zmora N, Levy M and Elinav E:
The microbiome and innate immunity. Nature. 535:65–74.
2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Berthelot JM and Claudepierre P:
Trafficking of antigens from gut to sacroiliac joints and spine in
reactive arthritis and spondyloarthropathies: Mainly through
lymphatics? Joint Bone Spine. 83:485–490. 2016.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Li C, Zhang Y, Yan Q, Guo R, Chen C, Li S,
Zhang Y, Meng J, Ma J, You W, et al: Alterations in the gut virome
in patients with ankylosing spondylitis. Front Immunol.
14(1154380)2023.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Symposium on Population Studies in
Relation to Chronic Rheumatic Diseases. Rome Ball J, Jeffrey MR and
Kellgren JH: Council for International Organizations of Medical
sciences, University of Manchester Department of Rheumatology: The
epidemiology of chronic rheumatism; Volume 2: Atlas of standard
radiographs of arthritis. Blackwell Scientific Publications,
Oxford, 1963.
|
|
69
|
Goie The HS, Steven MM, van der Linden SM
and Cats A: Evaluation of diagnostic criteria for ankylosing
spondylitis: A comparison of the Rome, New York and modified New
York criteria in patients with a positive clinical history
screening test for ankylosing spondylitis. Br J Rheumatol.
24:242–249. 1985.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ostergaard M and Lambert RG: Imaging in
ankylosing spondylitis. Ther Adv Musculoskelet Dis. 4:301–311.
2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Braun J, van den Berg R, Baraliakos X,
Boehm H, Burgos-Vargas R, Collantes-Estevez E, Dagfinrud H,
Dijkmans B, Dougados M, Emery P, et al: 2010 update of the
ASAS/EULAR recommendations for the management of ankylosing
spondylitis. Ann Rheum Dis. 70:896–904. 2011.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Uhrin Z, Kuzis S and Ward MM: Exercise and
changes in health status in patients with ankylosing spondylitis.
Arch Intern Med. 160:2969–2975. 2000.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Regnaux JP, Davergne T, Palazzo C, Roren
A, Rannou F, Boutron I and Lefevre-Colau MM: Exercise programmes
for ankylosing spondylitis. Cochrane Database Syst Rev.
10(CD011321)2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Kasapoglu Aksoy M, Birtane M, Taştekin N
and Ekuklu G: The effectiveness of structured group education on
ankylosing spondylitis patients. J Clin Rheumatol. 23:138–143.
2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Song IH, Poddubnyy DA, Rudwaleit M and
Sieper J: Benefits and risks of ankylosing spondylitis treatment
with nonsteroidal antiinflammatory drugs. Arthritis Rheum.
58:929–938. 2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bacchi S, Palumbo P, Sponta A and
Coppolino MF: Clinical pharmacology of non-steroidal
anti-inflammatory drugs: A review. Antiinflamm Antiallergy Agents
Med Chem. 11:52–64. 2012.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Gagliardi MC, Teloni R, Mariotti S,
Bromuro C, Chiani P, Romagnoli G, Giannoni F, Torosantucci A and
Nisini R: Endogenous PGE2 promotes the induction of human Th17
responses by fungal ß-glucan. J Leukoc Biol. 88:947–954.
2010.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Ward MM, Deodhar A, Akl EA, Lui A, Ermann
J, Gensler LS, Smith JA, Borenstein D, Hiratzka J, Weiss PF, et al:
American college of rheumatology/spondylitis association of
america/spondyloarthritis research and treatment network 2015
recommendations for the treatment of ankylosing spondylitis and
nonradiographic axial spondyloarthritis. Arthritis Care Res
(Hoboken). 68:151–166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Wanders A, van der Heijde D, Landewé R,
Béhier JM, Calin A, Olivieri I, Zeidler H and Dougados M:
Nonsteroidal antiinflammatory drugs reduce radiographic progression
in patients with ankylosing spondylitis: A randomized clinical
trial. Arthritis Rheum. 52:1756–1765. 2005.PubMed/NCBI View Article : Google Scholar
|
|
80
|
van der Heijde D, Ramiro S, Landewé R,
Baraliakos X, Van den Bosch F, Sepriano A, Regel A, Ciurea A,
Dagfinrud H, Dougados M, et al: 2016 update of the ASAS-EULAR
management recommendations for axial spondyloarthritis. Ann Rheum
Dis. 76:978–991. 2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ebrahimiadib N, Berijani S, Ghahari M and
Pahlaviani FG: Ankylosing spondylitis. J Ophthalmic Vis Res.
16:462–469. 2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Henderson C and Davis JC: Drug insight:
Anti-tumor-necrosis-factor therapy for ankylosing spondylitis. Nat
Clin Pract Rheumatol. 2:211–218. 2006.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Schulz M, Dotzlaw H and Neeck G:
Ankylosing spondylitis and rheumatoid arthritis: Serum levels of
TNF-α and Its soluble receptors during the course of therapy with
etanercept and infliximab. Biomed Res Int.
2014(675108)2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Gorman JD, Sack KE and Davis JC Jr:
Treatment of ankylosing spondylitis by inhibition of tumor necrosis
factor alpha. N Engl J Med. 346:1349–1356. 2002.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Toussirot É: Current therapeutics for
spondyloarthritis. Expert Opin Pharmacother. 12:2469–2477.
2011.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Ranatunga S and Miller AV: Active axial
spondyloarthritis: Potential role of certolizumab pegol. Ther Clin
Risk Manag. 10:87–94. 2014.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Baraliakos X, Listing J, Fritz C, Haibel
H, Alten R, Burmester GR, Krause A, Schewe S, Schneider M, Sörensen
H, et al: Persistent clinical efficacy and safety of infliximab in
ankylosing spondylitis after 8 years-early clinical response
predicts long-term outcome. Rheumatology (Oxford). 50:1690–1699.
2011.PubMed/NCBI View Article : Google Scholar
|
|
88
|
van der Heijde D, Kivitz A, Schiff MH,
Sieper J, Dijkmans BA, Braun J, Dougados M, Reveille JD, Wong RL,
Kupper H, et al: Efficacy and safety of adalimumab in patients with
ankylosing spondylitis: Results of a multicenter, randomized,
double-blind, placebo-controlled trial. Arthritis Rheum.
54:2136–2146. 2006.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Sieper J, van der Heijde D, Dougados M,
Brown LS, Lavie F and Pangan AL: Early response to adalimumab
predicts long-term remission through 5 years of treatment in
patients with ankylosing spondylitis. Ann Rheum Dis. 71:700–706.
2012.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Steeland S, Libert C and Vandenbroucke RE:
A new venue of TNF targeting. Int J Mol Sci.
19(1442)2018.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Mazumdar S and Greenwald D: Golimumab.
MAbs. 1:422–431. 2009.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zhou H: Clinical pharmacokinetics of
etanercept: A fully humanized soluble recombinant tumor necrosis
factor receptor fusion protein. J Clin Pharmacol. 45:490–497.
2005.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Jethwa H and Bowness P: The interleukin
(IL)-23/IL-17 axis in ankylosing spondylitis: New advances and
potentials for treatment. Clin Exp Immunol. 183:30–36.
2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Miller EA and Ernst JD: Anti-TNF
immunotherapy and tuberculosis reactivation: Another mechanism
revealed. J Clin Invest. 119:1079–1082. 2009.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Baraliakos X, Listing J, Brandt J, Zink A,
Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M,
Sörensen H, et al: Clinical response to discontinuation of anti-TNF
therapy in patients with ankylosing spondylitis after 3 years of
continuous treatment with infliximab. Arthritis Res Ther.
7:R439–R444. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
96
|
Klavdianou K, Tsiami S and Baraliakos X:
New developments in ankylosing spondylitis-status in 2021.
Rheumatology (Oxford). 60:vi29–vi37. 2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Wendling D, Verhoeven F and Prati C:
Anti-IL-17 monoclonal antibodies for the treatment of ankylosing
spondylitis. Expert Opin Biol Ther. 19:55–64. 2019.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Baeten D, Sieper J, Braun J, Baraliakos X,
Dougados M, Emery P, Deodhar A, Porter B, Martin R, Andersson M, et
al: Secukinumab, an interleukin-17A inhibitor, in ankylosing
spondylitis. N Engl J Med. 373:2534–2548. 2015.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Baraliakos X, Braun J, Deodhar A,
Poddubnyy D, Kivitz A, Tahir H, Van den Bosch F, Delicha EM,
Talloczy Z and Fierlinger A: Long-term efficacy and safety of
secukinumab 150 mg in ankylosing spondylitis: 5-Year results from
the phase III MEASURE 1 extension study. RMD Open.
5(e001005)2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Deodhar A, Conaghan PG, Kvien TK, Strand
V, Sherif B, Porter B, Jugl SM and Gandhi KK: MEASURE 2 study
group. Secukinumab provides rapid and persistent relief in pain and
fatigue symptoms in patients with ankylosing spondylitis
irrespective of baseline C-reactive protein levels or prior tumour
necrosis factor inhibitor therapy: 2-Year data from the MEASURE 2
study. Clin Exp Rheumatol. 37:260–269. 2019.PubMed/NCBI
|
|
101
|
Kammüller M, Tsai TF, Griffiths CE, Kapoor
N, Kolattukudy PE, Brees D, Chibout SD, Safi J Jr and Fox T:
Inhibition of IL-17A by secukinumab shows no evidence of increased
Mycobacterium tuberculosis infections. Clin Transl Immunology.
6(e152)2017.PubMed/NCBI View Article : Google Scholar
|
|
102
|
van der Heijde D, Cheng-Chung Wei J,
Dougados M, Mease P, Deodhar A, Maksymowych WP, Van den Bosch F,
Sieper J, Tomita T, Landewé R, et al: Ixekizumab, an
interleukin-17A antagonist in the treatment of ankylosing
spondylitis or radiographic axial spondyloarthritis in patients
previously untreated with biological disease-modifying
anti-rheumatic drugs (COAST-V): 16 Week results of a phase 3
randomised, double-blind, active-controlled and placebo-controlled
trial. Lancet. 392:2441–2451. 2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Hohenberger M, Cardwell LA, Oussedik E and
Feldman SR: Interleukin-17 inhibition: Role in psoriasis and
inflammatory bowel disease. J Dermatolog Treat. 29:13–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Reis J, Vender R and Torres T:
Bimekizumab: The first dual inhibitor of interleukin (IL)-17A and
IL-17F for the treatment of psoriatic disease and ankylosing
spondylitis. BioDrugs. 33:391–399. 2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
van der Heijde D, Gensler LS, Deodhar A,
Baraliakos X, Poddubnyy D, Kivitz A, Farmer MK, Baeten D, Goldammer
N, Coarse J, et al: Dual neutralisation of interleukin-17A and
interleukin-17F with bimekizumab in patients with active ankylosing
spondylitis: Results from a 48-week phase IIb, randomised,
double-blind, placebo-controlled, dose-ranging study. Ann Rheum
Dis. 79:595–604. 2020.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Wei JC, Kim TH, Kishimoto M, Ogusu N,
Jeong H and Kobayashi S: 4827-006 study group. Efficacy and safety
of brodalumab, an anti-IL17RA monoclonal antibody, in patients with
axial spondyloarthritis: 16-Week results from a randomised,
placebo-controlled, phase 3 trial. Ann Rheum Dis. 80:1014–1021.
2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Danesh MJ and Kimball AB: Brodalumab and
suicidal ideation in the context of a recent economic crisis in the
United States. J Am Acad Dermatol. 74:190–192. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Vaddi K and Luchi M: JAK inhibition for
the treatment of rheumatoid arthritis: A new era in oral DMARD
therapy. Expert Opin Investig Drugs. 21:961–973. 2012.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Toussirot E: The use of janus kinase
inhibitors in axial spondyloarthritis: Current insights.
Pharmaceuticals (Basel). 15(270)2022.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Schwartz DM, Kanno Y, Villarino A, Ward M,
Gadina M and O'Shea JJ: JAK inhibition as a therapeutic strategy
for immune and inflammatory diseases. Nat Rev Drug Discov.
16:843–862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Morelli M, Scarponi C, Mercurio L,
Facchiano F, Pallotta S, Madonna S, Girolomoni G and Albanesi C:
Selective immunomodulation of inflammatory pathways in
keratinocytes by the janus kinase (JAK) inhibitor tofacitinib:
implications for the employment of JAK-targeting drugs in
psoriasis. J Immunol Res. 2018(7897263)2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
van der Heijde D, Deodhar A, Wei JC,
Drescher E, Fleishaker D, Hendrikx T, Li D, Menon S and Kanik KS:
Tofacitinib in patients with ankylosing spondylitis: A phase II,
16-week, randomised, placebo-controlled, dose-ranging study. Ann
Rheum Dis. 76:1340–1347. 2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Hanson A and Brown MA: Genetics and the
causes of ankylosing spondylitis. Rheum Dis Clin North Am.
43:401–414. 2017.PubMed/NCBI View Article : Google Scholar
|
|
114
|
van der Heijde D, Braun J, Deodhar A,
Baraliakos X, Landewé R, Richards HB, Porter B and Readie A:
Modified stoke ankylosing spondylitis spinal score as an outcome
measure to assess the impact of treatment on structural progression
in ankylosing spondylitis. Rheumatology (Oxford). 58:388–400.
2019.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Deodhar A, van der Heijde D, Sieper J, Van
den Bosch F, Maksymowych WP, Kim TH, Kishimoto M, Ostor A, Combe B,
Sui Y, et al: Safety and efficacy of upadacitinib in patients with
active ankylosing spondylitis and an inadequate response to
nonsteroidal antiinflammatory drug therapy: One-year results of a
double-blind, placebo-controlled study and open-label extension.
Arthritis Rheumatol. 74:70–80. 2022.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Akkoc N and Khan MA: JAK inhibitors for
axial spondyloarthritis: What does the future hold? Curr Rheumatol
Rep. 23(34)2021.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Le QA, Kang JH, Lee S and Delevry D:
Cost-effectiveness of treatment strategies with biologics in
accordance with treatment guidelines for ankylosing spondylitis: A
patient-level model. J Manag Care Spec Pharm. 26:1219–1231.
2020.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Atzeni F, Nucera V, Galloway J, Zoltan S
and Nurmohamed M: Cardiovascular risk in ankylosing spondylitis and
the effect of anti-TNF drugs: A narrative review. Expert Opin Biol
Ther. 20:517–524. 2020.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Stovall R, Peloquin C, Felson D, Neogi T
and Dubreuil M: Relation of NSAIDs, DMARDs, and TNF inhibitors for
ankylosing spondylitis and psoriatic arthritis to risk of total hip
and knee arthroplasty. J Rheumatol. 48:1007–1013. 2021.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Shao T, Wang K, Liao X and Tang W:
Traditional Chinese medicine in treating Corona Virus Disease 2019:
A systematic review and cost-effectiveness analysis. Health Decis.
1:1–6. 2023.
|
|
121
|
Deng H and Shen X: The mechanism of
moxibustion: Ancient theory and modern research. Evid Based
Complement Alternat Med. 2013(379291)2013.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Kogure M, Mimura N, Ikemoto H, Ishikawa S,
Nakanishi-Ueda T, Sunagawa M and Hisamitsu T: Moxibustion at
mingmen reduces inflammation and decreases IL-6 in a
collagen-induced arthritis mouse model. J Acupunct Meridian Stud.
5:29–33. 2012.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Liu Z, Li X, Zhao C, Chen C, Li M, Tan Q,
Zhang L and Liang W: Effects of moxibustion on Treg/Th17 cell and
its signal pathway in mice with rheumatoid arthritis. Zhongguo Zhen
Jiu. 37:1083–1091. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
124
|
Zhang Y and Song A: Clinical research
progress of acupuncture therapy in the treatment of ankylosing
spondylitis. Med Theory Hypothesis. 5(4)2022.
|
|
125
|
Zhang J, Hu K, Di L, Wang P, Liu Z, Zhang
J, Yue P, Song W, Zhang J, Chen T, et al: Traditional herbal
medicine and nanomedicine: Converging disciplines to improve
therapeutic efficacy and human health. Adv Drug Deliv Rev.
178(113964)2021.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Huang Y, Wu Z, Su R, Ruan G, Du F and Li
G: Current application of chemometrics in traditional Chinese
herbal medicine research. J Chromatogr B Analyt Technol Biomed Life
Sci. 1026:27–35. 2016.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Yuan H, Ma Q, Cui H, Liu G, Zhao X, Li W
and Piao G: How can synergism of traditional medicines benefit from
network pharmacology? Molecules. 22(1135)2017.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Huang RY, Lin JH, He XH, Li X, Lu CL, Zhou
YY, Cai J and He YT: Anti-inflammatory activity of extracts of
Bushen-Qiangdu-Zhilv decoction, a Chinese medicinal formula, in
M1-polarized RAW264.7. BMC Complement Altern Med.
14(268)2014.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Li Q, Li L, Bi L, Xiao C, Lin Z, Cao S,
Liao Z and Gu J: Kunxian capsules in the treatment of patients with
ankylosing spondylitis: A randomized placebo-controlled clinical
trial. Trials. 17(337)2016.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Xie Y, Tu L, Zhang Y, Yu Q, Wu H, Ye S, Li
H, Chen Z, Wu J, Cao S, et al: Efficacy and safety of Fengshi
Gutong Capsule in patients with active ankylosing spondylitis: A
4-week randomized controlled, double-blinded, double-dummy trial. J
Ethnopharmacol. 285(114731)2022.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Xie D, Huang L, Zhao G, Yu Y, Gao J, Li H
and Wen C: Dissecting the underlying pharmaceutical mechanism of
Chinese traditional medicine Yun-Pi-Yi-Shen-Tong-Du-Tang acting on
ankylosing spondylitis through systems biology approaches. Sci Rep.
7(13436)2017.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Ye WF, Liu J, Wan L, Cao YX, Wang SH, Wang
YL and Ruan LP: Effect of xinfeng capsule on AS patients and their
serum immunoglobulin subtypes and peripheral lymphocyte autophagy.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 36:310–316. 2016.PubMed/NCBI(In Chinese).
|
|
133
|
Li X, Liu J, Fang Y, He M, Wang F and Han
Q: Mechanism of xinfeng capsule in the treatment of hypercoagulable
state of ankylosing spondylitis based on data mining and network
pharmacology. Biomed Res Int. 2022(8796980)2022.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Zhang X, Zhou L and Qian X: The mechanism
of ‘treating different diseases with the same treatment’ by qiangji
jianpi decoction in ankylosing spondylitis combined with
inflammatory bowel disease: A comprehensive analysis of multiple
methods. Gastroenterol Res Pract. 2024(9709260)2024.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Seitz M, Lemmel EM, Homfeld J and Kirchner
H: Enhanced interferon-gamma production by lymphocytes induced by a
mitogen from mycoplasma arthritidis in patients with ankylosing
spondylitis. Rheumatol Int. 9:85–90. 1989.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Feng Y, Ding J, Fan CM and Zhu P:
Interferon-γ contributes to HLA-B27-associated unfolded protein
response in spondyloarthropathies. J Rheumatol. 39:574–582.
2012.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Zhuang W, Sun N, Gu C, Liu S, Zheng Y,
Wang H, Tong X and Song J: A literature review on Epimedium,
a medicinal plant with promising slow aging properties. Heliyon.
9(e21226)2023.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Tong D, Chen L, Jiang Z, Ye X, Ma M, Ye A
and Xu J: Progress in the application of Epimedium and its
major bioactive components in the treatment of orthopedic diseases.
Front Pharmacol. 16(1628602)2025.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Wang X, Wu L, Yu M, Wang H, He L, Hu Y, Li
Z, Zheng Y and Peng B: Exploring the molecular mechanism of
Epimedium for the treatment of ankylosing spondylitis based
on network pharmacology, molecular docking, and molecular dynamics
simulations. Mol Mol Divers. 29:591–606. 2025.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Ma W, Liu T, Ogaji OD, Li J, Du K and
Chang Y: Recent advances in Scutellariae radix: A comprehensive
review on ethnobotanical uses, processing, phytochemistry,
pharmacological effects, quality control and influence factors of
biosynthesis. Heliyon. 10(e36146)2024.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Li X, Liu J, Fang Y, Huang D, He M, Wang F
and Han Q: Potential therapeutic mechanism of scutellaria
baicalensis georgi against ankylosing spondylitis based on a
comprehensive pharmacological model. Biomed Res Int.
2022(9887012)2022.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Wang L, Ma R, Liu C, Liu H, Zhu R, Guo S,
Tang M, Li Y, Niu J, Fu M, et al: Salvia miltiorrhiza: A
potential red light to the development of cardiovascular diseases.
Curr Pharm Des. 23:1077–1097. 2017.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Fang Y, Liu J, Xin L, Jiang H, Guo J, Li
X, Wang F, He M, Han Q and Huang D: Radix Salvia
miltiorrhiza for ankylosing spondylitis: determining potential
inflammatory molecular targets and mechanism using network
pharmacology. Biomed Res Int. 2022(3816258)2022.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Youssef FS, Eid SY, Alshammari E, Ashour
ML, Wink M and El-Readi MZ: Chrysanthemum indicum and
Chrysanthemum morifolium: Chemical composition of their
essential oils and their potential use as natural preservatives
with antimicrobial and antioxidant activities. Foods.
14(1460)2020.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Liang Y, Liu T, Wang D and Liu Q:
Exploring the antimicrobial, anti-inflammatory, antioxidant, and
immunomodulatory properties of Chrysanthemum morifolium and
Chrysanthemum indicum: A narrow review. Front Pharmacol.
16(1538311)2025.PubMed/NCBI View Article : Google Scholar
|
|
146
|
Cheng W, Li J, You T and Hu C:
Anti-inflammatory and immunomodulatory activities of the extracts
from the inflorescence of Chrysanthemum indicum Linné. J
Ethnopharmacol. 101:334–337. 2005.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Dong M, Yu D, Duraipandiyan V and Abdullah
Al-Dhabi N: The protective effect of Chrysanthemum indicum
extract against ankylosing spondylitis in mouse models. Biomed Res
Int. 2017(8206281)2017.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Xie Y, Kuan H, Wei Q, Gianoncelli A,
Ribaudo G and Coghi P:
(2R,4aS,6aS,12bR,14aS,14bR)10-Hydroxy-N-(4-((6-methoxyquinolin-8-yl)amino)pentyl)-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxamide.
Molbank. 2023(M1716)2023.
|
|
149
|
Jo S, Han J, Lee YL, Yoon S, Lee J, Wang
SE and Kim TH: Regulation of osteoblasts by alkaline phosphatase in
ankylosing spondylitis. Int J Rheum Dis. 22:252–261.
2019.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Jo S, Lee SH, Jeon C, Jo HR, Ko E, Whangbo
M, Kim TJ, Park YS and Kim TH: Elevated BMPR2 expression amplifies
osteoblast differentiation in ankylosing spondylitis. J Rheum Dis.
30:243–250. 2023.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Zou YC, Yang XW, Yuan SG, Zhang P and Li
YK: Celastrol inhibits prostaglandin E2-induced proliferation and
osteogenic differentiation of fibroblasts isolated from ankylosing
spondylitis hip tissues in vitro. Drug Des Devel Ther. 10:933–948.
2016.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Chen R, Qi QL, Wang MT and Li QY:
Therapeutic potential of naringin: An overview. Pharm Biol.
54:3203–3210. 2016.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Yu KE, Alder KD, Morris MT, Munger AM, Lee
I, Cahill SV, Kwon HK, Back J and Lee FY: Re-appraising the
potential of naringin for natural, novel orthopedic biotherapies.
Ther Adv Musculoskelet Dis. 12(1759720X20966135)2020.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Liu K, Wu L, Shi X and Wu F: Protective
effect of naringin against ankylosing spondylitis via ossification,
inflammation and oxidative stress in mice. Exp Ther Med.
12:1153–1158. 2016.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Feng X, Yang Q, Wang C, Tong W and Xu W:
Punicalagin exerts protective effects against ankylosing
spondylitis by regulating NF-κB-TH17/JAK2/STAT3 signaling and
oxidative stress. Biomed Res Int. 2020(4918239)2020.PubMed/NCBI View Article : Google Scholar
|
|
156
|
Liu W, Zhang Y, Zhu W, Ma C, Ruan J, Long
H and Wang Y: Sinomenine inhibits the progression of rheumatoid
arthritis by regulating the secretion of inflammatory cytokines and
monocyte/macrophage subsets. Front Immunol. 9(2228)2018.PubMed/NCBI View Article : Google Scholar
|
|
157
|
Dong B: Protective effects of sinomenine
against ankylosing spondylitis and the underlying molecular
mechanisms. Med Sci Monit. 24:3631–3636. 2018.PubMed/NCBI View Article : Google Scholar
|















