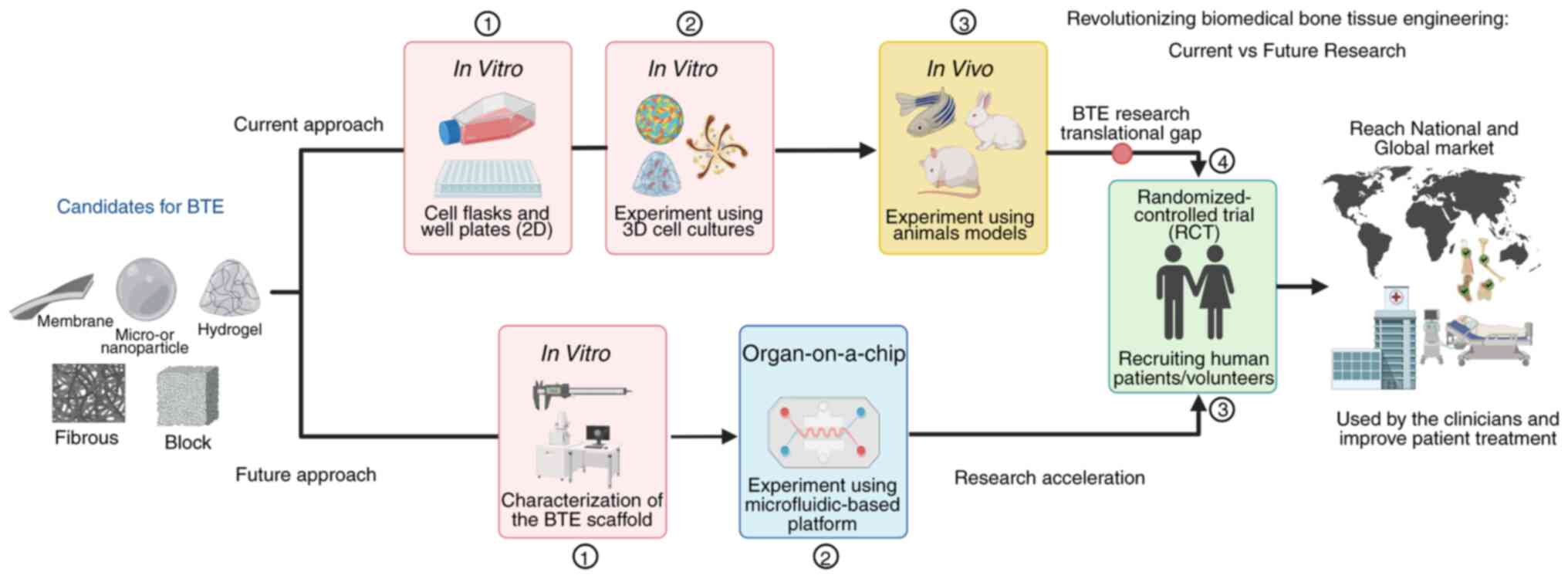
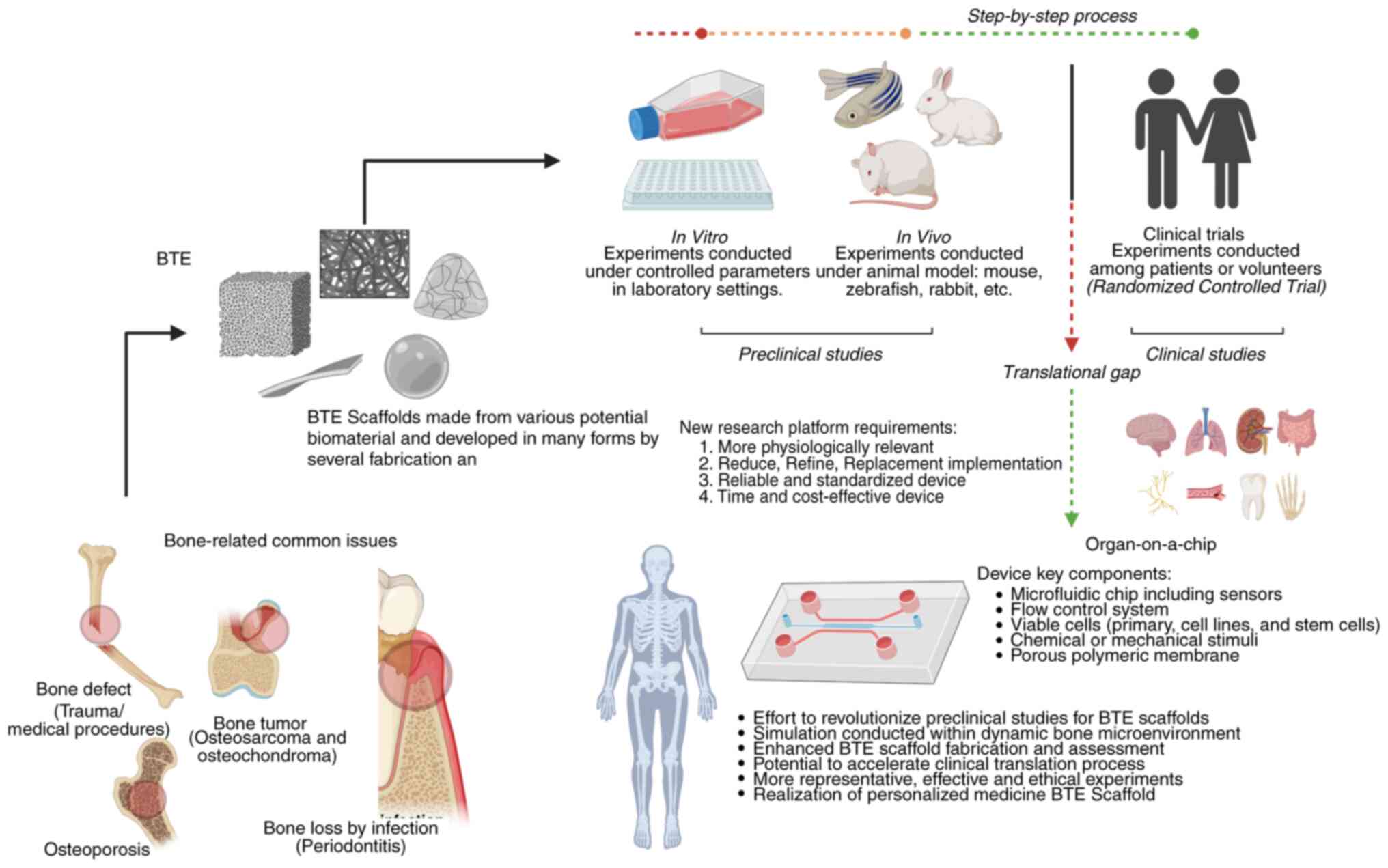
|
1
|
Altyar AE, El-Sayed A, Abdeen A, Piscopo M, Mousa SA, Najda A and Abdel-Daim MM: Future regenerative medicine developments and their therapeutic applications. Biomed Pharmacother. 158(114131)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mao AS and Mooney DJ: Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci USA. 112:14452–14459. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tatullo M, Zavan B and Piattelli A: Critical overview on regenerative medicine: New insights into the role of stem cells and innovative biomaterials. Int J Mol Sci. 24(7936)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Petrosyan A, Martins PN, Solez K, Uygun BE, Gorantla VS and Orlando G: Regenerative medicine applications: An overview of clinical trials. Front Bioeng Biotechnol. 10(942750)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vacanti JP, Otte JB and Wertheim JA: Introduction: Regenerative medicine and solid organ transplantation from a historical perspective. In: Orlando G, Lerut J, Soker S, Stratta RJ, (eds.). Regenerative Medicine Applications in Organ Transplantation, Boston, Academic Press, pp1-15, 2014.
|
|
6
|
Del Barrio Cortés E, Matutano Molina C, Rodríguez-Lorenzo L and Cubo-Mateo N: Generation of controlled micrometric fibers inside printed scaffolds using standard FDM 3D printers. Polymers (Basel). 15(96)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Socci MC, Rodríguez G, Oliva E, Fushimi S, Takabatake K, Nagatsuka H, Felice CJ and Rodríguez AP: Polymeric materials, advances and applications in tissue engineering: A review. Bioengineering (Basel). 10(218)2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ranjan VD, Zeng P, Li B and Zhang Y: In vitro cell culture in hollow microfibers with porous structures. Biomater Sci. 8:2175–2188. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kim MS, Kim JH, Min BH, Chun HJ, Han DK and Lee HB: Polymeric scaffolds for regenerative medicine. Polym Rev. 51:23–52. 2011.
|
|
10
|
Froemel D and Meurer A: Congenital Bone Disorders. In: Rommens P, Hessmann M. (eds). Intramedullary Nailing. Springer, London, 2015.
|
|
11
|
Fassier A: Telescopic rodding in children: Technical progression from Dubow-bailey to Fassier-Duval™. Orthop Traumatol Surg Res. 107(102759)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Toosi S and Behravan J: Osteogenesis and bone remodeling: A focus on growth factors and bioactive peptides. Biofactors. 46:326–340. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Roseti L, Parisi V, Petretta M, Cavallo C, Desando G, Bartolotti I and Grigolo B: Scaffolds for bone tissue engineering: State of the art and new perspectives. Mater Sci Eng C Mater Biol Appl. 78:1246–1262. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Birkhold AI, Razi H, Weinkamer R, Duda GN, Checa S and Willie BM: Monitoring in vivo (re)modeling: A computational approach using 4D microCT data to quantify bone surface movements. Bone. 75:210–221. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Andreasen CM, Delaisse JM, van der Eerden BCJ, van Leeuwen JPTM, Ding M and Andersen TL: Understanding age-induced cortical porosity in women: Is a negative BMU balance in quiescent osteons a major contributor? Bone. 117:70–82. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chocholata P, Kulda V and Babuska V: Fabrication of scaffolds for Bone-tissue regeneration. Materials (Basel). 12(568)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Shang F, Yu Y, Liu S, Ming L, Zhang Y, Zhou Z, Zhao J and Jin Y: Advancing application of mesenchymal stem Cell-based bone tissue regeneration. Bioact Mater. 6:666–683. 2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pereira H, Cengiz IF, Maia FR, Bartolomeu F, Oliveira JM, Reis RL and Silva FS: Physicochemical properties and cytocompatibility assessment of non-degradable scaffolds for bone tissue engineering applications. J Mech Behav Biomed Mater. 112(103997)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Manzini BM, Machado LMR, Noritomi PY and da Silva JVL: Advances in Bone tissue engineering: A fundamental review. J Biosci. 46(17)2021.PubMed/NCBI
|
|
20
|
Thormann U, Ray S, Sommer U, ElKhassawna T, Rehling T, Hundgeburth M, Henß A, Rohnke M, Janek J, Lips KS, et al: Bone formation induced by strontium modified calcium phosphate cement in critical-size metaphyseal fracture defects in ovariectomized rats. Biomaterials. 34:8589–8598. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
O'Brien FJ: Biomaterials & scaffolds for tissue engineering. Mater Today. 14:88–95. 2011.
|
|
22
|
Rohman G, Langueh C, Ramtani S, Lataillade JJ, Lutomski D, Senni K and Changotade S: The use of Platelet-rich plasma to promote cell recruitment into Low-Molecular-Weight fucoidan-functionalized Poly(Ester-Urea-Urethane) scaffolds for soft-tissue engineering. Polymers (Basel). 11(1016)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hunt JA, Chen R, van Veen T and Bryan N: Hydrogels for tissue engineering and regenerative medicine. J Mater Chem B. 2:5319–5338. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Koons GL, Diba M and Mikos AG: Materials design for bone-tissue engineering. Nat Rev Mater. 5:584–603. 2020.
|
|
25
|
Zhao X, Liu S, Yildirimer L, Zhao H, Ding R, Wang H, Cui W and Weitz D: Injectable stem Cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv Funct Mater. 26:2809–2819. 2016.
|
|
26
|
Wu Q, Liu J, Wang X, Feng L, Wu J, Zhu X, Wen W and Gong X: Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed Eng Online. 19(9)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kimura H, Sakai Y and Fujii T: Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab Pharmacokinet. 33:43–48. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Jalili-Firoozinezhad S, Gazzaniga FS, Calamari EL, Camacho DM, Fadel CW, Bein A, Swenor B, Nestor B, Cronce MJ, Tovaglieri A, et al: A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat Biomed Eng. 3:520–531. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chramiec A, Teles D, Yeager K, Marturano-Kruik A, Pak J, Chen T, Hao L, Wang M, Lock R, Tavakol DN, et al: Integrated human organ-on-a-chip model for predictive studies of anti-tumor drug efficacy and cardiac safety. Lab Chip. 20:4357–4372. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Y, Liu Y, Hu C, Chang Q, Deng Q, Yang X and Wu Y: Study of the neurotoxicity of indoor airborne nanoparticles based on a 3D human blood-brain barrier chip. Environ Int. 143(105598)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ma C, Peng Y, Li H and Chen W: Organ-on-a-Chip: A new paradigm for drug development. Trends Pharmacol Sci. 42:119–1133. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Osório LA, Silva E and Mackay RE: A review of biomaterials and scaffold fabrication for organ-on-a-chip (OOAC) systems. Bioeng. 8(113)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zamprogno P, Thoma G, Cencen V, Ferrari D, Putz B, Michler J, Fantner GE and Guenat OT: Mechanical properties of soft biological membranes for Organ-on-a-Chip assessed by bulge test and AFM. ACS Biomater Sci Eng. 7:2990–2997. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Koyilot MC, Natarajan P, Hunt CR, Sivarajkumar S, Roy R, Joglekar S, Pandita S, Tong CW, Marakkar S, Subramanian L, et al: Breakthroughs and Applications of Organ-on-a-Chip technology. Cells. 11(1828)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kwon J and Cho H: Nanomechanical characterization of bone quality depending on tissue age via bimodal atomic force microscopy. Nanomanufacturing Metrol. 6:1–11. 2023.
|
|
36
|
Presbítero G, Gutiérrez D, Lemus-Martínez WR, Vilchez JF, García P and Arizmendi-Morquecho A: Assessment of quality in osteoporotic human trabecular bone and its relationship to mechanical properties. Appl Sci. 11:1–16. 2021.
|
|
37
|
Dec P, Modrzejewski A and Pawlik A: Existing and novel biomaterials for bone tissue engineering. Int J Mol Sci. 24(529)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Qasim M, Chae DS and Lee N: Advancements and frontiers in nano-based 3D and 4D scaffolds for bone and cartilage tissue engineering. Int J Nanomedicine. 14:4333–4351. 2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Morgan EF, Unnikrisnan GU and Hussein AI: Bone mechanical properties in healthy and diseased states. Annu Rev Biomed Eng. 20:119–143. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Legrand E, Chappard D, Pascaretti C, Duquenne M, Krebs S, Rohmer V, Basle MF and Audran M: Trabecular bone microarchitecture, bone mineral density, and vertebral fractures in male osteoporosis. J Bone Miner Res. 15:13–19. 2000.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Van Der Linden JC, Verhaar JA and Weinans H: A three-dimensional simulation of age-related remodeling in trabecular bone. J Bone Miner Res. 16:688–696. 2001.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wang X, Xu S, Zhou S, Xu W, Leary M, Choong P, Qian M, Brandt M and Xie YM: Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials. 83:127–141. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Qu H, Fu H, Han Z and Sun Y: Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 9:26252–26262. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Choi K, Kuhn JL, Ciarelli MJ and Goldstein SA: The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J Biomech. 23:1103–1113. 1990.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Keaveny TM, Morgan EF, Niebur GL and Yeh OC: Biomechanics of trabecular bone. Annu Rev Biomed Eng. 3:307–333. 2001.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Rho JY, Ashman RB and Turner CH: Young's modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J Biomech. 26:111–119. 1993.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Rho JY, Kuhn-Spearing L and Zioupos P: Mechanical properties and the hierarchical structure of bone. Med Eng Phys. 20:92–102. 1998.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sharir A, Barak MM and Shahar R: Whole bone mechanics and mechanical testing. Vet J. 177:8–17. 2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zimmermann EA, Busse B and Ritchie RO: The fracture mechanics of human bone: Influence of disease and treatment. Bonekey Rep. 4(743)2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Takayanagi H: Osteoclast Biology and Bone Resorption. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 9th edition. Wiley, Hoboken, pp46-53, 2018.
|
|
51
|
Reznikov N, Shahar R and Weiner S: Bone hierarchical structure in three dimensions. Acta Biomater. 10:3815–3826. 2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Alford AI, Kozloff KM and Hankenson KD: Extracellular matrix networks in bone remodeling. Int J Biochem Cell Biol. 65:20–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Mackie EJ, Ahmed YA, Tatarczuch L, Chen KS and Mirams M: Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int J Biochem Cell Biol. 40:46–62. 2008.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Oryan A, Monazzah S and Bigham-Sadegh A: Bone injury and fracture healing biology. Biomed Environ Sci. 28:57–71. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Salgado AJ, Coutinho OP and Reis RL: Bone tissue engineering: State of the art and future trends. Macromol Biosci. 4:743–765. 2004.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Florencio-Silva R, Sasso GRDS, Sasso-Cerri E, Simões MJ and Cerri PS: Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed Res Int. 2015(421746)2015.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Nakamura H: Morphology, function, and differentiation of bone cells. J Hard Tissue Biol. 16:15–22. 2007.
|
|
58
|
Capulli M, Paone R and Rucci N: Osteoblast and osteocyte: Games without frontiers. Arch Biochem Biophys. 561:3–12. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Damoulis PD and Hauschka PV: Nitric oxide acts in conjunction with proinflammatory cytokines to promote cell death in osteoblasts. J Bone Miner Res. 12:412–422. 1997.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Marks SCJ and Popoff SN: Bone cell biology: The regulation of development, structure, and function in the skeleton. Am J Anat. 183:1–44. 1988.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Feng X and McDonald JM: Disorders of bone remodeling. Annu Rev Pathol. 6:121–45. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Crockett JC, Mellis DJ, Scott DI and Helfrich MH: New knowledge on critical osteoclast formation and activation pathways from study of rare genetic diseases of osteoclasts: Focus on the RANK/RANKL axis. Osteoporos Int. 22:1–20. 2011.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Su N, Yang J, Xie Y, Du X, Chen H, Zhou H and Chen L: Bone function, dysfunction and its role in diseases including critical illness. Int J Biol Sci. 15:776–787. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Klein-Nulend J, Bacabac RG and Bakker AD: Mechanical loading and how it affects bone cells: The role of the osteocyte cytoskeleton in maintaining our skeleton. Eur Cell Mater. 24:278–2791. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Dallas SL, Prideaux M and Bonewald LF: The osteocyte: An endocrine cell and more. Endocr Rev. 34:658–690. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bonewald LF: Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci. 1116:281–290. 2007.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Plotkin LI: Apoptotic osteocytes and the control of targeted bone resorption. Curr Osteoporos Rep. 12:121–126. 2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Bellido T: Osteocyte-driven bone remodeling. Calcif Tissue Int. 94:25–34. 2014.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Metzger CE, Burr DB and Allen MR: Anatomy and Structural. Considerations. In: Encyclopedia of Bone Biology. Zaidi M (ed). Academic Press, Oxford, pp218-232, 2020. https://doi.org/https://doi.org/10.1016/B978-0-12-801238-3.62234-1.
|
|
70
|
Guo XE, Hu YJ and Dinescu AT: Bone Structure and Function. In: Encyclopedia of Bone Biolog. Zaidi M (ed). Academic Press, Oxford, 233-246, 2020. https://doi.org/https://doi.org/10.1016/B978-0-12-801238-3.11551-X.
|
|
71
|
Aida N, Saito A and Azuma T: Current status of Next-Generation sequencing in bone genetic diseases. Int J Mol Sci. 24(13802)2023.PubMed/NCBI View Article : Google Scholar
|
|
72
|
The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Muscle and Bone Diseases. Health Topics, 2023. Accessed on October 4, 2023. https://www.niams.nih.gov/health-topics/muscle-bone-diseases.
|
|
73
|
Kanis JA, Norton N, Harvey NC, Jacobson T, Johansson H, Lorentzon M, McCloskey EV, Willers C and Borgström F: SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch Osteoporos. 16(82)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Gheita TA and Hammam N: Epidemiology and awareness of osteoporosis: A viewpoint from the Middle East and North Africa. Int J Clin Rheumtol. 13:134–147. 2018.
|
|
75
|
Salari N, Ghasemi H, Mohammadi L, Behzadi MH, Rabieenia E, Shohaimi S and Mohammadi M: The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J Orthop Surg Res. 16(609)2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Gregson CL, Armstrong DJ, Bowden J, Cooper C, Edwards J, Gittoes NJL, Harvey N, Kanis J, Leyland S, Low R, et al: UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 17(58)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Clynes MA, Harvey NC, Curtis EM, Fuggle NR, Dennison EM and Cooper C: The epidemiology of osteoporosis. Br Med Bull. 133:105–117. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, Hoy D, Ashrafi-Asgarabad A, Sepidarkish M, Almasi-Hashiani A, et al: Global, regional and national burden of osteoarthritis 1990-2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 79:819–828. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Allen KD, Thoma LM and Golightly YM: Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 30:184–195. 2022.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, Abbasi-Kangevari M, Abbastabar H, Abd-Allah F, Abdelalim A, et al: Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 396:1204–1222. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Vina ER and Kwoh CK: Epidemiology of osteoarthritis: Literature update. Curr Opin Rheumatol. 30:160–167. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Allen KD and Golightly YM: State of the evidence. Curr Opin Rheumatol. 27:276–283. 2015.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Callahan LF, Cleveland RJ, Allen KD and Golightly Y: Racial/Ethnic, socioeconomic, and geographic disparities in the epidemiology of knee and hip osteoarthritis. Rheum Dis Clin North Am. 47:1–20. 2021.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Courties A, Kouki I, Soliman N, Mathiew S and Sellam J: Osteoarthritis year in review 2024: Epidemiology and therapy. Osteoarthritis Cartilage. 32:1397–1404. 2024.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Yang C, Tian Y, Zhao F, Chen Z, Su P, Li Y and Qian A: Bone microenvironment and osteosarcoma metastasis. Int J Mol Sci. 21(6985)2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Botter SM, Neri D and Fuchs B: Recent advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Cortini M, Avnet S and Baldini N: Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett. 405:90–99. 2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Weiss A, Khoury JD, Hoffer FA, Wu J, Billups CA, Heck RK, Quintana J, Poe D, Rao BN and Daw NC: Telangiectatic osteosarcoma: The St. Jude Children's Research Hospital's experience. Cancer. 109:1627–1637. 2007.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Whelan J, McTiernan A, Cooper N, Wong YK, Francis M, Vernon S and Strauss SJ: Incidence and survival of malignant bone sarcomas in England 1979-2007. Int J Cancer. 131:E508–E517. 2012.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Mirabello L, Troisi RJ and Savage SA: Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer. 115:1531–1543. 2009.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Macedo F, Ladeira K, Pinho F, Saraiva N, Bonito N, Pinto L and Goncalves F: Bone metastases: An overview. Oncol Rev. 11(321)2017.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Sheng G, Gao Y, Yang Y and Wu H: Osteosarcoma and metastasis. Front Oncol. 11(780264)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Urish KL and Cassat JE: Staphylococcus aureus osteomyelitis: Bone, bugs, and surgery. Infect Immun. 88:e00932–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Metsemakers WJ, Smeets B, Nijs S and Hoekstra H: Infection after fracture fixation of the tibia: Analysis of healthcare utilization and related costs. Injury. 48:1204–1210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Peltola H and Pääkkönen M: Acute osteomyelitis in children. N Engl J Med. 370:352–360. 2014.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ III and Huddleston PM III: Trends in the epidemiology of osteomyelitis: A population-based study, 1969 to 2009. J Bone Joint Surg Am. 97:837–845. 2015.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Walter N, Bärtl S, Alt V and Rupp M: The epidemiology of osteomyelitis in children. Children (Basel). 8(1000)2021.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Walter N, Baertl S, Alt V and Rupp M: What is the burden of osteomyelitis in Germany? An analysis of inpatient data from 2008 through 2018. BMC Infect Dis. 21(550)2021.PubMed/NCBI View Article : Google Scholar
|
|
99
|
The Lancet. Global Burden of Disease 2019: Periodontal diseases-Level 4 cause. Global Health Metrics, 3-4, 2019. Accessed on November 7, 2022. https://www.healthdata.org/results/gbd_summaries/2019/periodontal-diseases-level-4-cause.
|
|
100
|
Ferrari AJ, Santomauro DF, Aali A, Abate YH, Abbafati C, Abbastabar H, ElHafeez SA, Abdelmasseh M, Abd-Elsalam S, Abdollahi A, et al: Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet. 403:2133–2161. 2024.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Figueredo CA, Abdelhay N, Figueredo CM, Catunda R and Gibson MP: The impact of vaping on periodontitis: A systematic review. Clin Exp Dent Res. 7:376–384. 2021.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, et al: Periodontitis: Consensus report of workgroup 2 of the 2017 World workshop on the classification of periodontal and Peri-implant diseases and conditions. J Periodontol. 45 (Suppl 20):S162–S170. 2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Birkedal-Hansen H: Role of matrix metalloproteinases in human periodontal diseases. J Periodontol. 64:474–484. 1993.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Mohanty R, Asopa SJ, Joseph MD, Singh B, Rajguru JP, Saidath K and Sharma U: Red complex: Polymicrobial conglomerate in oral flora: A review. J Family Med Prim Care. 8:3480–3486. 2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Page RC, Offenbacher S, Schroeder HE, Seymour GJ and Kornman KS: Advances in the pathogenesis of periodontitis: Summary of developments, clinical implications and future directions. Periodontol. 14:216–248. 1997.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Kwon TH, Lamster IB and Levin L: Current concepts in the management of periodontitis. Int Dent J. 71:462–476. 2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Shin CS, Cabrera FJ, Lee R, Kim J, Ammassam Veettil R, Zaheer M, Adumbumkulath A, Mhatre K, Ajayan PM, Curley SA, et al: 3D-Bioprinted inflammation modulating polymer scaffolds for soft tissue repair. Adv Mater. 33(e2003778)2021.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Murphy MP, Koepke LS, Lopez MT, Tong X, Ambrosi TH, Gulati GS, Marecic O, Wang Y, Ransom RC, Hoover MY, et al: Articular cartilage regeneration by activated skeletal stem cells. Nat Med. 26:1583–1592. 2020.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Hao Q, Wu Y, Wu Y, Wang P and Vadgama JV: Tumor-derived exosomes in Tumor-induced immune suppression. Int J Mol Sci. 23(1461)2022.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Bucher CH, Schlundt C, Wulsten D, Sass FA, Wendler S, Ellinghaus A, Thiele T, Seemann R, Willie BM, Volk HD, et al: Experience in the adaptive immunity impacts bone homeostasis, remodeling, and healing. Front Immunol. 10(797)2019.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Xiong Y, Mi BB, Lin Z, Hu YQ, Yu L, Zha KK, Panayi AC, Yu T, Chen L, Liu ZP, et al: The role of the immune microenvironment in bone, cartilage, and soft tissue regeneration: From mechanism to therapeutic opportunity. Mil Med Res. 9(65)2022.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Ye J, Xie C, Wang C, Huang J, Yin Z, Heng BC, Chen X and Shen W: Promoting musculoskeletal system soft tissue regeneration by biomaterial-mediated modulation of macrophage polarization. Bioact Mater. 6:4096–4109. 2021.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Zhao T, Sun F, Liu J, Ding T, She J, Mao F, Xu W, Qian H and Yan Y: Emerging role of mesenchymal stem Cell-derived exosomes in regenerative medicine. Curr Stem Cell Res Ther. 14:482–494. 2019.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Hao Z, Li H, Wang Y, Hu Y, Chen T, Zhang S, Guo X, Cai L and Li J: Supramolecular peptide nanofiber hydrogels for bone tissue engineering: From multihierarchical fabrications to comprehensive applications. Adv Sci (Weinh). 9(e2103820)2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Salhotra A, Shah HN, Levi B and Longaker MT: Mechanisms of bone development and repair. Nat Rev Mol Cell Biol. 21:696–711. 2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Andrew JG, Andrew SM, Freemont AJ and Marsh DR: Inflammatory cells in normal human fracture healing. Acta Orthop Scand. 65:462–466. 1994.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Claes L, Recknagel S and Ignatius A: Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol. 8:133–143. 2012.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Bolander ME: Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med. 200:165–1670. 1992.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Croes M, Oner FC, Kruyt MC, Blokhuis TJ, Bastian O, Dhert WJA and Alblas J: Proinflammatory mediators enhance the osteogenesis of human mesenchymal stem cells after lineage commitment. PLoS One. 10(e0132781)2015.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Salazar VS, Gamer LW and Rosen V: BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol. 12:203–221. 2016.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Ono T, Okamoto K, Nakashima T, Nitta T, Hori S, Iwakura Y and Takayanagi H: IL-17-producing γδ T cells enhance bone regeneration. Nat Commun. 7(10928)2016.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Bernhardsson M and Aspenberg P: Osteoblast precursors and inflammatory cells arrive simultaneously to sites of a Trabecular-bone injury. Acta Orthop. 89:457–461. 2018.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Lu LY, Loi F, Nathan K, Lin TH, Pajarinen J, Gibon E, Nabeshima A, Cordova L, Jämsen E, Yao Z and Goodman SB: Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res. 35:2378–2385. 2017.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Kusumbe AP, Ramasamy SK and Adams RH: Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 507:323–328. 2014.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Goerke SM, Obermeyer J, Plaha J, Stark GB and Finkenzeller G: Endothelial progenitor cells from peripheral blood support bone regeneration by provoking an angiogenic response. Microvasc Res. 98:40–47. 2015.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Langen UH, Pitulescu ME, Kim JM, Enriquez-Gasca R, Sivaraj KK, Kusumbe AP, Singh A, Di Russo J, Bixel MG, Zhou B, et al: Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat Cell Biol. 19:189–201. 2017.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Ramasamy SK, Kusumbe AP, Wang L and Adams RH: Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 507:376–380. 2014.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Jones RE, Salhotra A, Robertson KS, Ransom RC, Foster DS, Shah HN, Quarto N, Wan DC and Longaker MT: Skeletal stem Cell-schwann cell circuitry in mandibular repair. Cell Rep. 28:2757–2766.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Cao J, Zhang S, Gupta A, Du Z, Lei D, Wang L and Wang X: Sensory nerves affect bone regeneration in rabbit mandibular distraction osteogenesis. Int J Med Sci. 16:831–837. 2019.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Wang L, Zhou S, Liu B, Lei D, Zhao Y, Lu C and Tan A: Locally applied nerve growth factor enhances bone consolidation in a rabbit model of mandibular distraction osteogenesis. J Orthop Res. 24:2238–2245. 2006.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Vermeulen S, Tahmasebi Z and Habibovic P: Biomaterials Biomaterial-induced pathway modulation for bone regeneration. Biomaterials. 283(121431)2022.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Lancaster MA and Huch M: Disease modelling in human organoids. Dis Model Mech. 12(dmm039347)2019.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Clevers H: Modeling development and disease with organoids. Cell. 165:1586–1597. 2016.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Yin X, Mead BE, Safaee H, Langer R, Karp JM and Levy O: Engineering stem cell organoids. Cell Stem Cell. 18:25–38. 2016.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Zhao P, Gu H, Mi H, Rao C, Fu J and Lih-Sheng T: Fabrication of scaffolds in tissue engineering: A review. Front Mechanical Engineering. 13:107–119. 2018.
|
|
136
|
Groen N, Yuan H, Hebels DGAJ, Koçer G, Mbuyi F, LaPointe V, Truckenmüller R, van Blitterswijk CA, Habibović P and de Boer J: Linking the transcriptional landscape of bone induction to biomaterial design parameters. Adv Mater. 29(1603259)2017.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Jeong J, Kim JH, Shim JH, Hwang NS and Heo CY: Bioactive calcium phosphate materials and applications in bone regeneration. Biomater Res. 23(4)2019.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Galván-Chacón VP and Habibovic P: Deconvoluting the bioactivity of calcium Phosphate-based bone graft substitutes: Strategies to understand the role of individual material properties. Adv Healthc Mater. 6(1601478)2017.PubMed/NCBI View Article : Google Scholar
|
|
139
|
Bohner M, Santoni BLG and Döbelin N: β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 113:23–41. 2020.PubMed/NCBI View Article : Google Scholar
|
|
140
|
Schmidlin PR, Nicholls F, Kruse A, Zwahlen RA and Weber FE: Evaluation of moldable, in situ hardening calcium phosphate bone graft substitutes. Clin Oral Implants Res. 24:149–157. 2013.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Kruse A, Jung RE, Nicholls F, Zwahlen RA, Hämmerle CHF and Weber FE: Bone regeneration in the presence of a synthetic hydroxyapatite/silica oxide-based and a xenogenic hydroxyapatite-based bone substitute material. Clin Oral Implants Res. 22:506–511. 2011.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Roberts TT and Rosenbaum AJ: Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis. 8:114–124. 2012.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Cornell CN and Lane JM: Current understanding of osteoconduction in bone regeneration. Clin Orthop Relat Res. 355 (355 Suppl):S267–S273. 1998.PubMed/NCBI View Article : Google Scholar
|
|
144
|
Weber FE: Reconsidering osteoconduction in the era of additive manufacturing. Tissue Eng Part B Rev. 25:375–386. 2019.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Branemark PI, Hansson BO, Adell R, Breine U, Lindström J, Hallén O and Ohman A: Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand J Plast Recons. 16:1–132. 1977.PubMed/NCBI
|
|
146
|
Albrektsson T and Johansson C: Osteoinduction, osteoconduction and osseointegration. Eur Spine J. 10 (Suppl 2):S96–S101. 2001.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Albrektsson T, Branemark P, Hansson H and Lindström J: Osseointegrated titanium implants: Requirements for ensuring a Long-lasting, Direct Bone-to-Implant anchorage in man. Acta Orthop Scand. 52:155–170. 1981.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Brown TD: Techniques for mechanical stimulation of cells in vitro: A review. J Biomech. 33:3–14. 2000.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Walker M, Rizzuto P, Godin M and Pelling AE: Structural and mechanical remodeling of the cytoskeleton maintains tensional homeostasis in 3D microtissues under acute dynamic stretch. Sci Rep. 10(7696)2020.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Zhang X, Wang X, Lee Y, Feng L, Wang B, Pan Q, Meng X, Cao H, Li L, Wang H, et al: Bioactive scaffold fabricated by 3D printing for enhancing osteoporotic bone regeneration. Bioengineering (Basel). 9(525, 525,)2022.PubMed/NCBI View Article : Google Scholar
|
|
151
|
de Wildt BWM, Cramer EEA, de Silva LS, Ito K, Gawlitta D and Hofmann S: Evaluating Material-driven regeneration in a tissue engineered human in vitro bone defect model. Bone. 166(116597)2023.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Langer R and Vacanti JP: Tissue engineering. Science. 260:920–926. 1993.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Koushik TM, Miller CM and Antunes E: Bone tissue engineering scaffolds: Function of Multi-material hierarchically structured scaffolds. Adv. Healthcare Mater. 12(e2202766)2023.PubMed/NCBI View Article : Google Scholar
|
|
154
|
Abdelaziz AG, Nageh H, Abdo SM, Abdalla MS, Amer AA, Abdal-hay A and Barhoum A: A Review of 3D polymeric scaffolds for bone tissue engineering: Principles, fabrication techniques, immunomodulatory roles, and challenges. Bioengineering (Basel). 10(204)2023.PubMed/NCBI View Article : Google Scholar
|
|
155
|
Filip N, Radu I, Veliceasa B, Filip C, Pertea M, Clim A, Pînzariu A, Drochioi LC, Hilitanu RL and Serban IL: Biomaterials in orthopedic devices: Current issues and future perspectives. Coatings. 12(1544)2022.
|
|
156
|
Atala A, Kurtis Kasper F and Mikos AG: Engineering complex tissues. Sci Transl Med. 4(60rv12)2012.
|
|
157
|
Bouet G, Marchat D, Cruel M, Malaval L and Vico L: In vitro Three-dimensional bone tissue models: From cells to controlled and dynamic environment. Tissue Eng Part B Rev. 21:133–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
158
|
Hutmacher DW: Scaffolds in tissue engineering bone and cartilage. Biomaterials. 21:2529–2543. 2000.PubMed/NCBI View Article : Google Scholar
|
|
159
|
Dehghani F and Annabi N: Engineering porous scaffolds using Gas-based techniques. Curr Opin Biotechnol. 22:661–666. 2011.PubMed/NCBI View Article : Google Scholar
|
|
160
|
Hollister SJ: Porous scaffold design for tissue engineering. Nat Mater. 4:518–524. 2005.PubMed/NCBI View Article : Google Scholar
|
|
161
|
Grafahrend D, Heffels KH, Beer MV, Gasteier P, Möller M, Boehm G, Dalton PD and Groll J: Degradable polyester scaffolds with controlled surface chemistry combining minimal protein adsorption with specific bioactivation. Nat Mater. 10:67–73. 2011.PubMed/NCBI View Article : Google Scholar
|
|
162
|
Chernozem RV, Guselnikova O, Surmeneva MA, Postnikov PS, Abalymov AA, Parakhonskiy BV, De Roo N, Depla D, Skirtach AG and Surmenev RA: Diazonium chemistry surface treatment of piezoelectric polyhydroxybutyrate scaffolds for enhanced osteoblastic cell growth. Appl Mater Today. 20(100758)2020.
|
|
163
|
Dave K and Gomes VG: Interactions at scaffold interfaces: Effect of surface chemistry, structural attributes and bioaffinity. Mater Sci Eng C Mater Biol Appl. 105(110078)2019.PubMed/NCBI View Article : Google Scholar
|
|
164
|
Suamte L, Tirkey A, Barman J and Jayasekhar Babu P: Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Materials in Manufacturing. 1(100011)2023.
|
|
165
|
Amini AR, Laurencin CT and Nukavarapu SP: Bone tissue engineering: Recent advances and challenges. Crit Rev Biomed Eng. 40:363–408. 2012.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Gokce C, Gurcan C, Delogu LG and Yilmazer A: 2D materials for cardiac tissue repair and regeneration. Front Cardiovasc Med. 9:1–16. 2022.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Gentile P, Chiono V, Carmagnola I and Hatton PV: An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 15:3640–359. 2014.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Williams DF: There is no such thing as a biocompatible material. Biomaterials. 35:10009–10014. 2014.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Bian N, Chu C, Rung S, Huangphattarakul V, Man Y, Lin J and Hu C: Immunomodulatory biomaterials and emerging analytical techniques for probing the immune Micro-Environment. Tissue Eng Regen Med. 20:11–24. 2023.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Hofmann S and Garcia-Fuentes M: Bioactive scaffolds for the controlled formation of complex skeletal tissues. In: Regenerative Medicine and Tissue Engineering. Eberli D (ed). IntechOpen, Rijeka, pp18, 2011. https://doi.org/10.5772/22061.
|
|
171
|
Sultana N: Mechanical and biological properties of scaffold materials. Functional 3D Tissue Engineering Scaffolds, Materials, Technologies, and Applications, pp1-21, 2018. https://doi.org/10.1016/B978-0-08-100979-6.00001-X.
|
|
172
|
Zhang Y, Xu J, Ruan YC, Yu MK, O'Laughlin M, Wise H, Chen D, Tian L, Shi D, Wang J, et al: Implant-derived magnesium induces local neuronal production of CGRP to improve bone-fracture healing in rats. Nat Med. 22:1160–119. 2016.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Eltom A, Zhong G and Muhammad A: Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv Materials Sci Engineering, Mar 7, 2019 doi: 10.1155/2019/3429527.
|
|
174
|
Yuan N, Rezzadeh KS and Lee JC: Biomimetic scaffolds for osteogenesis. Recept Clin Investig. 2(898)2015.PubMed/NCBI
|
|
175
|
Liu J, Chen G, Xu H, Hu K, Sun J, Liu M, Zhang F and Gu N: Pre-vascularization in fibrin Gel/PLGA microsphere scaffolds designed for bone regeneration. NPG Asia Mater. 10:827–839. 2018.
|
|
176
|
Iviglia G, Kargozar S and Baino F: Biomaterials, current strategies, and novel nano-technological approaches for periodontal regeneration. J Funct Biomater. 10(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
177
|
Nainar SM, Vignesh Vicki W, Begum S and Ansari MNM: A review on bioscaffolds for tissue engineering application. Scholars J Engineering Technol. 2:184–192. 2014.
|
|
178
|
Dalby MJ, Gadegaard N and Oreffo RO: Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 13:558–569. 2014.PubMed/NCBI View Article : Google Scholar
|
|
179
|
Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, et al: Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 14:1269–1277. 2015.PubMed/NCBI View Article : Google Scholar
|
|
180
|
Jiang S, Lyu C, Zhao P, Li W, Kong W, Huang C, Genin GM and Du Y: Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat Commun. 10(3491)2019.PubMed/NCBI View Article : Google Scholar
|
|
181
|
Lee SS, Du X, Kim and Ferguson SJ: Scaffolds for bone-tissue engineering. Matter. 5:2722–2759. 2022.
|
|
182
|
Zhao W, Jin X, Cong Y, Liu Y and Fu J: Degradable natural polymer hydrogels for articular cartilage tissue engineering. J Chem Technol Biotechnol. 88:327–339. 2013.
|
|
183
|
Chanes-Cuevas OA, Perez-Soria A, Cruz-Maya I, Guarino V and Alvarez-Perez MA: Macro-, micro- and mesoporous materials for tissue engineering applications. AIMS Mater Sci. 5:1124–1140. 2018.
|
|
184
|
Silva R, Fabry B and Boccaccini AR: Fibrous protein-based hydrogels for cell encapsulation. Biomaterials. 35:6727–6738. 2014.PubMed/NCBI View Article : Google Scholar
|
|
185
|
Shirazi S, Ravindran S and Cooper LF: Topography-mediated immunomodulation in osseointegration; Ally or Enemy. Biomaterials. 291(121903)2022.PubMed/NCBI View Article : Google Scholar
|
|
186
|
Mitra J, Tripathi G, Sharma A and Basu B: Scaffolds for bone tissue engineering: Role of surface patterning on osteoblast response. RSC Adv. 3:11073–11094. 2013.
|
|
187
|
Wang JR, Ahmed SF, Gadegaard N, Meek RMD, Dalby MJ and Yarwood SJ: Nanotopology potentiates growth hormone signalling and osteogenesis of mesenchymal stem cells. Growth Horm IGF Res. 24:245–250. 2014.PubMed/NCBI View Article : Google Scholar
|
|
188
|
Cabezas MD, Meckes B, Mirkin CA and Mrksich M: Subcellular control over focal adhesion anisotropy, independent of cell morphology, dictates stem cell fate. ACS Nano. 13:11144–11152. 2019.PubMed/NCBI View Article : Google Scholar
|
|
189
|
Lam MT and Wu JC: Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev Cardiovasc Ther. 10:1039–1049. 2012.PubMed/NCBI View Article : Google Scholar
|
|
190
|
Guggenbichler JP, Assadian O, Boeswald M and Kramer A: Incidence and clinical implication of nosocomial infections associated with implantable biomaterials-catheters, ventilator-associated pneumonia, urinary tract infections. GMS Krankenhhyg Interdiszip. (6)2011.PubMed/NCBI View Article : Google Scholar
|
|
191
|
The Food and Drug Administration (FDA). Sterilization for Medical Devices, 2024. Accessed on June 8, 2024. https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/sterilization-medical-devices.
|
|
192
|
Griffin M, Naderi N, Kalaskar DM, Malins E, Becer R, Thornton CA, Whitaker IS, Mosahebi A, Butler PEM and Seifalian AM: Evaluation of sterilisation techniques for regenerative medicine scaffolds fabricated with polyurethane nonbiodegradable and bioabsorbable nanocomposite materials. Int J Biomater. 2018(6565783)2018.PubMed/NCBI View Article : Google Scholar
|
|
193
|
Kumar A and Jacob A: Techniques in scaffold fabrication process for tissue engineering applications: A review. J Appl Biol Biotechnol. 10:163–176. 2022.
|
|
194
|
Nikolova MP and Chavali MS: Recent advances in biomaterials for 3D scaffolds: A review. Bioact Mater. 4:271–292. 2019.PubMed/NCBI View Article : Google Scholar
|
|
195
|
Chan BP and Leong KW: Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur Spine J. 17 (Suppl 4):S467–S479. 2008.PubMed/NCBI View Article : Google Scholar
|
|
196
|
Wei G and Ma PX: Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 25:4749–4757. 2004.PubMed/NCBI View Article : Google Scholar
|
|
197
|
Shruti S, Salinas AJ, Lusvardi G, Malavasi G, Menabue L and Vallet-Regi M: Mesoporous bioactive scaffolds prepared with cerium-, gallium- and zinc-containing glasses. Acta Biomater. 9:4836–4844. 2013.PubMed/NCBI View Article : Google Scholar
|
|
198
|
Boyan BD, Hummert TW, Dean DD and Schwartz Z: Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 17:137–146. 1996.PubMed/NCBI View Article : Google Scholar
|
|
199
|
Le M, Wu BM and Dunn JCY: Effect of scaffold architecture and pore size on smooth muscle cell growth. J Biomed Mater Res A. 87A:1010–1016. 2008.PubMed/NCBI View Article : Google Scholar
|
|
200
|
Hulbert SF, Young FA, Mathews RS, Klawitter JJ, Talbert CD and Stelling FH: Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res. 4:433–456. 1970.PubMed/NCBI View Article : Google Scholar
|
|
201
|
Rampichová M, Buzgo M, Chvojka J, Prosecká E, Kofronová O and Amler E: Cell penetration to nanofibrous scaffolds: Forcespinning®, an alternative approach for fabricating 3D nanofibers. Cell Adh Migr. 8:36–41. 2014.PubMed/NCBI View Article : Google Scholar
|
|
202
|
Ren L, Pandit V, Elkin J, Denman T, Cooper JA and Kotha SP: Large-scale and highly efficient synthesis of micro- and nano-fibers with controlled fiber morphology by centrifugal jet spinning for tissue regeneration. Nanoscale. 5:2337–2345. 2013.PubMed/NCBI View Article : Google Scholar
|
|
203
|
Wang L, Shi J, Liu L, Secret E and Chen Y: Fabrication of polymer fiber scaffolds by centrifugal spinning for cell culture studies. Microelectron Eng. 88:1718–1721. 2011.
|
|
204
|
Loordhuswamy AM, Krishnaswamy VR, Korrapati PS, Thinakaran S and Rengaswami GD: Fabrication of highly aligned fibrous scaffolds for tissue regeneration by centrifugal spinning technology. Mater Sci Eng C Mater Biol Appl. 42:799–807. 2014.PubMed/NCBI View Article : Google Scholar
|
|
205
|
Xu F, Weng B, Materon LA, Kuang A, Trujillo JA and Lozano K: Fabrication of cellulose fine fiber based membranes embedded with silver nanoparticles via Forcespinning. J Polymer Engineering. 36:269–278. 2016.
|
|
206
|
Melke J, Midha S, Ghosh S, Ito K and Hofmann S: Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 31:1–16. 2016.PubMed/NCBI View Article : Google Scholar
|
|
207
|
Xia H, Chen Q, Fang Y, Liu D, Zhong D, Wu H, Xia Y, Yan Y, Tang W and Sun X: Directed neurite growth of rat dorsal root ganglion neurons and increased colocalization with Schwann cells on aligned poly(methyl methacrylate) electrospun nanofibers. Brain Res. 1565:18–27. 2014.PubMed/NCBI View Article : Google Scholar
|
|
208
|
Vasita R and Katti DS: Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 1:15–30. 2006.PubMed/NCBI View Article : Google Scholar
|
|
209
|
Brannon-Peppas L: Recent advances on the use of biodegradable microparticles and nanoparticles in controlled drug delivery. Int J Pharm. 116:1–9. 1995.
|
|
210
|
Stephens D, Li L, Robinson D, Chen S, Chang HC, Liu RM, Tian Y, Ginsburg EJ, Gao X and Stultz T: Investigation of the in vitro release of gentamicin from a polyanhydride matrix. J Control Release. 63:305–317. 2000.PubMed/NCBI View Article : Google Scholar
|
|
211
|
Bergmann NM and Peppas NA: Molecularly imprinted polymers with specific recognition for macromolecules and proteins. Prog Polym Sci. 33:271–288. 2008.
|
|
212
|
Wang S, Chen X, Han X, Hong X, Li X, Zhang H, Li M, Wang Z and Zheng A: A Review of 3D printing technology in pharmaceutics: Technology and applications, now and future. Pharmaceutics. 15(416)2023.PubMed/NCBI View Article : Google Scholar
|
|
213
|
Matai I, Kaur G, Seyedsalehi A, McClinton A and Laurencin CT: Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 226(119536)2020.PubMed/NCBI View Article : Google Scholar
|
|
214
|
Sun W, Starly B, Daly AC, Burdick JA, Groll J, Skeldon G, Shu W, Sakai Y, Shinohara M, Nishikawa M, et al: The bioprinting roadmap. Biofabrication. 12(022002)2020.PubMed/NCBI View Article : Google Scholar
|
|
215
|
Ahmad J, Garg A, Mustafa G, Mohammed AA and Ahmad MZ: 3D printing technology as a promising tool to design Nanomedicine-based solid dosage forms: Contemporary research and future scope. Pharmaceutics. 15(1448)2023.PubMed/NCBI View Article : Google Scholar
|
|
216
|
Marzuka S and Umme Kulsum J: 3D printing: A new avenue in pharmaceuticals. World J Pharm Res. 5:1686–1701. 2016.
|
|
217
|
Samiei N: Recent trends on applications of 3D printing technology on the design and manufacture of pharmaceutical oral formulation: A mini review. Beni-Suef Univ J Basic Appl Sci. 9:1–12. 2020.
|
|
218
|
Gross BC, Erkal JL, Lockwood SY, Chen C and Spence DM: Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 86:3240–3253. 2014.PubMed/NCBI View Article : Google Scholar
|
|
219
|
Perez-Puyana V, Jiménez-Rosado M, Romero A and Guerrero A: Polymer-based scaffolds for Soft-tissue engineering. Polymers (Basel). 12(1566)2020.PubMed/NCBI View Article : Google Scholar
|
|
220
|
Aranaz I, Gutiérrez MC, Ferrer ML and del Monte F: Preparation of chitosan nanocomposites with a macroporous structure by unidirectional freezing and subsequent Freeze-drying. Mar Drugs. 12:5619–5642. 2014.PubMed/NCBI View Article : Google Scholar
|
|
221
|
Sanz-Herrera JA, García-Aznar JM and Doblaré M: On scaffold designing for bone regeneration: A computational multiscale approach. Acta Biomater. 5:219–229. 2009.PubMed/NCBI View Article : Google Scholar
|
|
222
|
Venkatesan J, Bhatnagar I and Kim SK: Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar Drugs. 12:300–316. 2014.PubMed/NCBI View Article : Google Scholar
|
|
223
|
Harris LD, Kim BS and Mooney DJ: Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Sci. 42:396–402. 1998.PubMed/NCBI View Article : Google Scholar
|
|
224
|
Kopp A, Smeets R, Gosau M, Kröger N, Fuest S, Köpf M, Kruse M, Krieger J, Rutkowski R, Henningsen A and Burg S: Effect of process parameters on additive-free electrospinning of regenerated silk fibroin nonwovens. Bioact Mater. 5:241–252. 2020.PubMed/NCBI View Article : Google Scholar
|
|
225
|
Mabrouk M, Beherei HH and Das DB: Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater Sci Eng C Mater Biol Appl. 110(110716)2020.PubMed/NCBI View Article : Google Scholar
|
|
226
|
Agrawal CM and Ray RB: Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mater Res. 55:141–150. 2001.PubMed/NCBI View Article : Google Scholar
|
|
227
|
Du Y, Liu H, Yang Q, Wang S, Wang J, Ma J, Noh I, Mikos AG and Zhang S: Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials. 137:37–48. 2017.PubMed/NCBI View Article : Google Scholar
|
|
228
|
Awad A, Fina F, Goyanes A, Gaisford S and Basit AW: 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int J Pharm. 586(119594)2020.PubMed/NCBI View Article : Google Scholar
|
|
229
|
Madrid APM, Vrech SM, Sanchez MA and Rodriguez AP: Advances in additive manufacturing for bone tissue engineering scaffolds. Mater Sci Eng C Mater Biol Appl. 100:631–644. 2019.PubMed/NCBI View Article : Google Scholar
|
|
230
|
Creff J, Courson R, Mangeat T, Foncy J, Souleille S, Thibault C, Besson A and Malaquin L: Fabrication of 3D scaffolds reproducing intestinal epithelium topography by High-resolution 3D stereolithography. Biomaterials. 221(119404)2019.PubMed/NCBI View Article : Google Scholar
|
|
231
|
Melčová V, Svoradová K, Menčík P, Kontárová S, Rampichová M, Hedvičáková V, Sovková V, Přikryl R and Vojtová L: FDM 3D printed composites for bone tissue engineering based on plasticized Poly(3-hydroxybutyrate)/poly(d,l-lactide) Blends. Polymers (Basel). 12(2806)2020.PubMed/NCBI View Article : Google Scholar
|
|
232
|
Rey F, Barzaghini B, Nardini A, Bordoni M, Zuccotti GV, Cereda C, Raimondi MT and Carelli S: Advances in tissue engineering and innovative fabrication techniques for 3-D-Structures: Translational applications in neurodegenerative diseases. Cells. 9(1636)2020.PubMed/NCBI View Article : Google Scholar
|
|
233
|
Yuan B, Zhou SY and Chen XS: Rapid prototyping technology and its application in bone tissue engineering. J Zhejiang Univ Sci B. 18:303–315. 2017.PubMed/NCBI View Article : Google Scholar
|
|
234
|
Murphy SV and Atala A: 3D bioprinting of tissues and organs. Nat Biotechnol. 32:773–785. 2014.PubMed/NCBI View Article : Google Scholar
|
|
235
|
Mao Q, Wang Y, Li Y, Juengpanich S, Li W, Chen M, Yin J, Fu J and Cai X: Fabrication of liver microtissue with liver decellularized extracellular matrix (dECM) bioink by digital light processing (DLP) bioprinting. Mater Sci Eng C Mater Biol Appl. 109(110625)2020.PubMed/NCBI View Article : Google Scholar
|
|
236
|
Simon CG, Yaszemski MJ, Ratcliffe A, Tomlins P, Luginbuehl R and Tesk JA: ASTM international workshop on standards and measurements for tissue engineering scaffolds. J Biomed Mater Res B Appl Biomater. 103:949–959. 2015.PubMed/NCBI View Article : Google Scholar
|
|
237
|
Pamies D, Ekert J, Zurich MG, Frey O, Werner S, Piergiovanni M, Freedman BS, Keong Teo AK, Erfurth H, Reyes DR, et al: Recommendations on fit-for-purpose criteria to establish quality management for microphysiological systems and for monitoring their reproducibility. Stem Cell Rep. 19:604–617. 2024.PubMed/NCBI View Article : Google Scholar
|
|
238
|
Allwardt V, Ainscough AJ, Viswanathan P, Sherrod SD, McLean JA, Haddrick M and Pensabene V: . Translational Roadmap for the Organs-on-a-Chip Industry toward Broad Adoption. Bioengineering. 7(112)2020.PubMed/NCBI View Article : Google Scholar
|
|
239
|
Lyu Y: Organ-on-chip technology's development and usages: A comprehensive review. Theor Nat Sci 32: pp288-292, 2024. https://doi.org/10.54254/2753-8818/32/20240886.
|
|
240
|
Morais AS, Mendes M, Cordeiro MA, Sousa JJ, Pais AC, Mihăilă SM and Vitorino C: Organ-on-a-Chip: Ubi sumus? Fundamentals and design aspects. Pharmaceutics. 16(615)2024.PubMed/NCBI View Article : Google Scholar
|
|
241
|
Syahruddin MH, Anggraeni R and Ana ID: A microfluidic organ-on-a-chip: Into the next decade of bone tissue engineering applied in dentistry. Future Sci OA. 9(FSO902)2023.PubMed/NCBI View Article : Google Scholar
|
|
242
|
Europe Medicines Agency (EMA): EMA implements new measures to minimise animal testing during medicines development, 2021. Accessed on August 3, 2024. https://www.ema.europa.eu/en/news/ema-implements-new-measures-minimise-animal-testing-during-medicines-development.
|
|
243
|
Food and Drug Administration (FDA): Advancing Alternative Methods at FDA, 2023. Accessed on August 3, 2024. https://www.fda.gov/science-research/about-science-research-fda/advancing-alternative-methods-fda.
|
|
244
|
Cho S, Lee S and Ahn SI: Design and engineering of organ-on-a-chip. Biomed Eng Lett. 13:97–109. 2023.PubMed/NCBI View Article : Google Scholar
|
|
245
|
Mandenius CF: Conceptual design of Micro-Bioreactors and Organ-on-chips for studies of cell cultures. Bioengineering (Basel). 5(56)2018.PubMed/NCBI View Article : Google Scholar
|
|
246
|
Torino S, Corrado B, Iodice M and Coppola G: PDMS-based microfluidic devices for cell culture. Inventions. 3:1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
247
|
Sosa-Hernández JE, Villalba-Rodríguez AM, Romero-Castillo KD, Aguilar-Aguila-Isaías MA, García-Reyes IE, Hernández-Antonio A, Ahmed I, Sharma A, Parra-Saldívar R and Iqbal HMN: Organs-on-a-chip module: A review from the development and applications perspective. Micromachines (Basel). 9(536)2018.PubMed/NCBI View Article : Google Scholar
|
|
248
|
Srinivasan A, Lopez-Ribot JL and Ramasubramanian AK: Microscale microbial culture. Future Microbiol. 10:143–146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
249
|
Whitesides GM: The origins and the future of microfluidics. Nature. 442:368–373. 2006.PubMed/NCBI View Article : Google Scholar
|
|
250
|
Mohanty S, Larsen LB, Trifol J, Szabo P, Burri HVR, Canali C, Dufva M, Emnéus J and Wolff A: Fabrication of scalable and structured tissue engineering scaffolds using water dissolvable sacrificial 3D printed moulds. Mater Sci Eng C. 55:569–578. 2015.PubMed/NCBI View Article : Google Scholar
|
|
251
|
Ahmed I, Iqbal HMN and Akram Z: Microfluidics engineering: Recent trends, valorization, and applications. Arab J Sci Eng. 43:23–32. 2018.
|
|
252
|
Xu Z, Gao Y, Hao Y, Li E, Wang Y, Zhang J, Wang W, Gao Z and Wang Q: Application of a microfluidic chip-based 3D co-culture to test drug sensitivity for individualized treatment of lung cancer. Biomaterials. 34:4109–4117. 2013.PubMed/NCBI View Article : Google Scholar
|
|
253
|
Singh G, Mishra A, Mathur A, Shastri S, Nizam A, Rizwan A, Dadial AS, Firdous A and Hassan H: Advancement of organ-on-chip towards next generation medical technology. Biosens Bioelectron X. 18(100480)2024.
|
|
254
|
Milton LA, Viglione MS, Ong LJY, Nordin GP and Toh YC: Vat photopolymerization 3D printed microfluidic devices for organ-on-a-chip applications. Lab Chip. 23:3537–3560. 2023.PubMed/NCBI View Article : Google Scholar
|
|
255
|
Tajeddin A and Mustafaoglu N: Design and fabrication of organ-on-chips: Promises and challenges. Micromachines (Basel). 12(1443)2021.PubMed/NCBI View Article : Google Scholar
|
|
256
|
Walter FR, Valkai S, Kincses A, Petnehazi A, Czeller T, Veszelka S, Ormos P, Deli MA and Dér A: A versatile lab-on-a-chip tool for modeling biological barriers. Sens Actuators B Chem. 222:1209–1219. 2016.
|
|
257
|
França CM, Tahayeri A, Rodrigues NS, Ferdosian S, Puppin Rontani RM, Sereda G, Ferracane JL and Bertassoni LE: The tooth on-a-chip: A microphysiologic model system mimicking the biologic interface of the tooth with biomaterials. Lab Chip. 20:405–413. 2020.PubMed/NCBI View Article : Google Scholar
|
|
258
|
Shin W, Wu A, Massidda MW, Foster C, Thomas N, Lee DW, Koh H, Ju Y, Kim J and Kim HJ: A robust longitudinal co-culture of obligate anaerobic gut microbiome with human intestinal epithelium in an Anoxic-Oxic Interface-on-a-Chip. Front Bioeng Biotechnol. 7(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
259
|
lebani R, Potla R, Soong M, Bai H, Izadifar Z, Jiang A, Travis RN, Belgur C, Dinis A, Cartwright MJ, et al: Modeling pulmonary cystic fibrosis in a human lung airway-on-a-chip. J Cyst Fibros. 21:606–615. 2021.PubMed/NCBI View Article : Google Scholar
|
|
260
|
Cao T, Shao C, Yu X, Xie R, Yang C, Sun Y, Yang S, He W, Xu Y, Fan Q and Ye F: Biomimetic Alveolus-on-a-Chip for SARS-COV-2 infection recapitulation. Research (Wash D C). 2022(9819154)2022.PubMed/NCBI View Article : Google Scholar
|
|
261
|
Tan J, Zhu L, Shi J, Zhang J, Kuang J, Guo Q, Zhu X, Chen Y, Zhou C and Gao X: Evaluation of drug resistance for EGFR-TKIs in lung cancer via multicellular lung-on-a-chip. Eur J Pharm Sci. 199(106805)2024.PubMed/NCBI View Article : Google Scholar
|
|
262
|
Zakharova M, Palma Do Carmo MA, Van Der Helm MW, Le-The H, De Graaf MNS, Orlova V, van den Berg A, van der Meer AD, Broersen K and Segerink LI: Multiplexed blood-brain barrier organ-on-chip. Lab Chip. 20:3132–3143. 2020.PubMed/NCBI View Article : Google Scholar
|
|
263
|
Zheng F, Fu F, Cheng Y, Wang C, Zhao Y and Gu Z: Organ-on-a-Chip systems: Microengineering to biomimic living systems. Small. 12:2253–2282. 2016.PubMed/NCBI View Article : Google Scholar
|
|
264
|
Vatine GD, Barrile R, Workman MJ, Sances S, Barriga BK, Rahnama M, Barthakur S, Kasendra M, Lucchesi C, Kerns J, et al: Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell. 24:995–1005.e6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
265
|
Park TE, Mustafaoglu N, Herland A, Hasselkus R, Mannix R, FitzGerald EA, Prantil-Baun R, Watters A, Henry O, Benz M, et al: Hypoxia-enhanced Blood-brain barrier chip recapitulates human barrier function and shuttling of drugs and antibodies. Nat Commun. 10(2621)2019.PubMed/NCBI View Article : Google Scholar
|
|
266
|
Wu C, Luo Y, Cuniberti G, Xiao Y and Gelinsky M: Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta Biomater. 7:2644–2650. 2011.PubMed/NCBI View Article : Google Scholar
|
|
267
|
Sachlos E, Czernuszka JT, Gogolewski S and Dalby M: Making tissue engineering scaffolds work. Review on the application ofsolid freeform fabrication technology to the production of tissue engineeringscaffolds. Eur Cells Mater. 5:29–40. 2003.PubMed/NCBI View Article : Google Scholar
|
|
268
|
Grenier J, Duval H, Barou F, Lv P, David B and Letourneur D: Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 94:195–203. 2019.PubMed/NCBI View Article : Google Scholar
|
|
269
|
Doshi J and Reneker DH: Electrospinning process and applications of electrospun fibers. Conf Rec Ind Appl Soc IEEE-IAS Annu Meet. 3:1698–1703. 1993.
|
|
270
|
Dhariwala B, Hunt E and Boland T: Rapid prototyping of Tissue-engineering constructs, using photopolymerizable hydrogels and stereolithography. Tissue Eng. 10:1316–1322. 2004.PubMed/NCBI View Article : Google Scholar
|
|
271
|
Lee DK, Kwon JY and Cho YH: Fabrication of microfluidic channels with various cross-sectional shapes using anisotropic etching of Si and self-alignment. Applied Physics A. 125(291)2019.
|
|
272
|
Kobayashi J, Kikuchi A, Aoyagi T and Okano T: Cell sheet tissue engineering: Cell sheet preparation, harvesting/manipulation, and transplantation. J Biomed Mater Res A. 107:955–967. 2019.PubMed/NCBI View Article : Google Scholar
|
|
273
|
Damiati S, Kompella UB, Damiati SA and Kodzius R: Microfluidic devices for drug delivery systems and drug screening. Genes (Basel). 9(103)2018.PubMed/NCBI View Article : Google Scholar
|
|
274
|
Caballero D, Kaushik S, Correlo VM, Oliveira JM, Reis RL and Kundu SC: Organ-on-chip models of cancer metastasis for future personalized medicine: From chip to the patient. Biomaterials. 149:98–115. 2017.PubMed/NCBI View Article : Google Scholar
|
|
275
|
Imparato G, Urciuolo F and Netti PA: Organ on chip technology to model cancer growth and metastasis. Bioengineering (Basel). 9(28)2022.PubMed/NCBI View Article : Google Scholar
|
|
276
|
Zuchowska A and Skorupska S: Multi-organ-on-chip approach in cancer research. Organs-on-a-Chip. 4(100014)2022.
|
|
277
|
Liu W, Song J, Du X, Zhou Y, Li Y, Li R, Lyu L, He Y, Hao J, Ben J, et al: AKR1B10 (Aldo-keto reductase family 1 B10) promotes brain metastasis of lung cancer cells in a multi-organ microfluidic chip model. Acta Biomater. 91:195–208. 2019.PubMed/NCBI View Article : Google Scholar
|
|
278
|
Kamei K, Mashimo Y, Koyama Y, Fockenberg C, Nakashima M, Nakajima M, Li J and Chen Y: 3D printing of soft lithography mold for rapid production of polydimethylsiloxane-based microfluidic devices for cell stimulation with concentration gradients. Biomed Microdevices. 17(36)2015.PubMed/NCBI View Article : Google Scholar
|
|
279
|
Grant J, Lee E, Almeida M, Kim S, LoGrande N, Goyal G, Sesay AM, Breault DT, Prantil-Baun R and Ingber DE: Establishment of physiologically relevant oxygen gradients in microfluidic organ chips. Lab Chip. 22:1584–1593. 2022.PubMed/NCBI View Article : Google Scholar
|
|
280
|
Shin Y, Jeon JS, Han S, Jung GS, Shin S, Lee SH, Sudo R, Kamm RD and Chung S: In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip. 11:2175–2181. 2011.PubMed/NCBI View Article : Google Scholar
|
|
281
|
Ribas J, Zhang YS, Pitrez PR, Leijten J, Miscuglio M, Rouwkema J, Dokmeci MR, Nissan X, Ferreira L and Khademhosseini A: Biomechanical Strain exacerbates inflammation on a Progeria-on-a-Chip model. Small. 13(1603737)2017.PubMed/NCBI View Article : Google Scholar
|
|
282
|
Wang X, Phan DTT, Zhao D, George SC, Hughes CCW and Lee AP: An on-chip microfluidic pressure regulator that facilitates reproducible loading of cells and hydrogels into microphysiological system platforms. Lab Chip. 16:868–876. 2016.PubMed/NCBI View Article : Google Scholar
|
|
283
|
Feric NT, Pallotta I, Singh R, Bogdanowicz DR, Gustilo MM, Chaudhary KW, Willette RN, Chendrimada TP, Xu X, Graziano MP and Aschar-Sobbi R: Engineered cardiac tissues generated in the Biowire II: A platform for Human-based drug discovery. Toxicol Sci. 172:89–97. 2019.PubMed/NCBI View Article : Google Scholar
|
|
284
|
Lee D, Erickson A, You T, Dudley AT and Ryu S: Pneumatic microfluidic cell compression device for high-throughput study of chondrocyte mechanobiology. Lab Chip. 18:2077–2086. 2018.PubMed/NCBI View Article : Google Scholar
|
|
285
|
Occhetta P, Mainardi A, Votta E, Vallmajo-Martin Q, Ehrbar M, Martin I, Barbero A and Rasponi M: Hyperphysiological compression of articular cartilage induces an osteoarthritic phenotype in a cartilage-on-a-chip model. Nat Biomed Eng. 3:545–557. 2019.PubMed/NCBI View Article : Google Scholar
|
|
286
|
Rosalem GS, Torres LAG, de Las Casas EB, Mathias FAS, Ruiz JC and Carvalho MGR: Microfluidics and organ-on-a-chip technologies: A systematic review of the methods used to mimic bone marrow. PLoS One. 15(e0243840)2020.PubMed/NCBI View Article : Google Scholar
|
|
287
|
Ashammakhi N, Nasiri R, De NR, Tebon P, Thakor J, Goudie M, Shamloo A, Martin MG and Khademhosseini A: Gut-on-a-chip: Current progress and future opportunities. Biomaterials. 255(120196)2020.PubMed/NCBI View Article : Google Scholar
|
|
288
|
Pang L, Shen S, Ma C, Ma T, Zhang R, Tian C, Zhao L, Liu W and Wang J: Deformability and size-based cancer cell separation using an integrated microfluidic device. Analyst. 140:7335–7346. 2015.PubMed/NCBI View Article : Google Scholar
|
|
289
|
Dressaire E and Sauret A: Clogging of microfluidic systems. Soft Matter. 13:37–48. 2017.
|
|
290
|
van der Sman RGM: Simulations of confined suspension flow at multiple length scales. Soft Matter. 5:4376–4387. 2009.
|
|
291
|
He X, Wang B, Meng J, Zhang S and Wang S: How to prevent bubbles in microfluidic channels. Langmuir. 37:2187–2194. 2021.PubMed/NCBI View Article : Google Scholar
|
|
292
|
Busek M, Aizenshtadt A, Amirola-Martinez M, Delon L and Krauss S: Academic user view: Organ-on-a-Chip technology. Biosensors (Basel). 12(126)2022.PubMed/NCBI View Article : Google Scholar
|
|
293
|
Li J, Liang W, Chen Z, Li X, Gu P, Liu A, Chen P, Li Q, Mei X, Yang J, et al: OOCDB: A comprehensive, systematic, and real-time organs-on-a-chip database. Genom Proteom Bioinform. 21:243–58. 2023.PubMed/NCBI View Article : Google Scholar
|
|
294
|
Mauriac H and Casquillas GV: Organ-on-chip companies developing innovative technologies. ELVESYS Microfluidic Innovation Centre. Accessed on August 7, 2024. https://www.elveflow.com/microfluidic-reviews/organs-on-chip-3d-cell-culture/organ-chip-companies/.
|
|
295
|
4Dcell: 4Dcell-Application. 4Dcell, Montreuil, 2024. https://www.4dcell.com/cell-types/#. Accessed August 7, 2024.
|
|
296
|
AIM Biotech: AIM Biotech-Our Products. https://aimbiotech.com/products/. Accessed August 6, 2024.
|
|
297
|
Altis Biosystems: Altis Biosystems-Products. Altis Biosystems, Inc., Durham NC, 2024. https://www.altisbiosystems.com/kits/. Accessed August 6, 2024.
|
|
298
|
ANANDA Devices: ANANDA Devices-Products. Ananda Devices, Quebec, 2024. https://www.anandadevices.com/en/products. Accessed August 7, 2024.
|
|
299
|
AlveoliX: AlveoliX-AXLung-on-Chip System. Alveolix AG, Bern, 2024. https://www.alveolix.com/axlung-on-chip-system/.
|
|
300
|
Aracari Biosciences: Aracari Biosciences-Services. Aracari Biosciences Inc., Irvine CA, 2024. Accessed https://aracaribio.com/services/. Accessed August 7, 2024, 2024.
|
|
301
|
AxoSim: AxoSim-Products. AxoSim, Inc., New Orleans, LA, 2024. https://axosim.com/products/. Accessed August 7, 2024.
|
|
302
|
Beonchip: Beonchip-Products. Beonchip, Zaragoza, 2024. https://beonchip.com/blog. Accessed August 7, 2024.
|
|
303
|
BiomimX: BiomimX-Applications. BiomimX, Milan, 2024. https://www.biomimx.com/our-pipeline/. Accessed August 7, 2024.
|
|
304
|
Bi/ond: Bi/ond-Applications. Bi/ond Solutions, Delft, 2024. https://www.gobiond.com/applications/. Accessed August 7, 2024.
|
|
305
|
Cherry Biotech: Cherry Biotech-CubiX Platform. Cherry Biotech, Montreuil, 2024. https://www.cherrybiotech.com/cubix/. Accessed August 7, 2024.
|
|
306
|
chiron: chiron-Our Science. https://www.chrn.co/science. Accessed August 7, 2024.
|
|
307
|
CN Bio: CN Bio-OOC Single-& Multi-Organ Models. CN Bio, Cambridge, 2024. https://cn-bio.com/organ-models/. Accessed August 7, 2024.
|
|
308
|
DiNABIOS: DiNABIOS-Our Technology. DiNABIOS AG, Zurich, 2024. https://dinabios.com/technology/. Accessed August 7, 2024.
|
|
309
|
Dynamic42: Dynamic42-Organ-on-chip models for research & development. Dynamic42 GmbH, Jena, 2024. https://dynamic42.com/. Accessed on August 7, 2024.
|
|
310
|
Draper Laboratory: Draper-PREDICT96. Draper Laboratory, Massachusetts, 2024. https://www.draper.com/explore-solutions/predict96. Accessed August 7, 2024.
|
|
311
|
Elvesys: Elveflow-Products & Applications. https://www.elveflow.com/microfluidic-products/. Accessed August 7, 2024.
|
|
312
|
Emulate Inc. Emulate-Organ-Chip Products & Services. n.d. https://emulatebio.com/products-services/. Accessed August 7, 2024).
|
|
313
|
Hesperos Inc: Hesperos-Services. Hesperos Inc., Orlando FL, 2024. https://hesperosinc.com/services/. Accessed August 7, 2024.
|
|
314
|
Ibidi: Ibidi-Products and Applications. https://ibidi.com/content/category/37-products. Accessed August 7, 2024.
|
|
315
|
InSphero: InSphero-Technology and solution. https://insphero.com/. Accessed August 7, 2024.
|
|
316
|
Jiksak Bioengineering: Jiksak Bioengineering-Nerve OrganoidTM. Jiksak Bioengineering, Kanagawa, 2024. https://www.jiksak.co.jp/technology/nerve-organoid. Accessed August 7, 2024.
|
|
317
|
Kirkstall Ltd: Kirkstall-Products and Applications. Kirkstall Ltd., York, 2024. https://kirkstall.com/. Accessed August 7, 2024.
|
|
318
|
MesoBioTech: MesoBioTech-Organ on-a-chip. https://mesobiotech.com/organ-chip/. Accessed August 7, 2024.
|
|
319
|
Microbrain Biotech: MICROBRAIN BT-Services. Mocrobrain Biotech, Marly-le-Roi, 2024. https://www.microbrainbiotech.io/our-services. Accessed August 7, 2024.
|
|
320
|
Mimetas: Mimetas-OrganoReady®. Mimetas, Oegstgeest, 2024. https://www.mimetas.com/en/organoready/. Accessed on August 7, 2024.
|
|
321
|
NETRI: NETRI-NeuroFluidics Cultures. NETRI, Lyon, 2025. https://netri.com/netri-products/neurofluidics-cultures/. Accessed on August 7, 2024.
|
|
322
|
Numa Bioscience: NumaBio-NuVivoTM organ chips. Numa Bioscience, Bothell, 2025. https://www.numabio.com/. Accessed August 7, 2024.
|
|
323
|
Quris-AI: Quris-AI-Patients-on-a-Chip. https://www.quris.ai/. Accessed August 7, 2024.
|
|
324
|
REVIVO Biosystems: REVIVO Biosystems-Products. REVIVO Biosystems, Singapore, 2024. https://www.revivobio.com/products. Accessed August 7, 2024.
|
|
325
|
SynVivo: SynVivo-Products. SynVivo, Huntsville, 2024. https://www.synvivobio.com/products/. Accessed August 7, 2024.
|
|
326
|
TissUse GmbH: TissUse-HUMIMIC Products and Services. TissUse GmbH, Berlin, 2024. Accessed on August 7, 2024. https://www.tissuse.com/en/services/.
|
|
327
|
Xona Microfluidics: Xona Microfluidics-XONACHIPS®. Xona Microfluidics, North Carolina, 2025. https://xonamicrofluidics.com/xonachips/. Accessed August 7, 2024.
|
|
328
|
Ching T, Toh YC, Hashimoto M and Zhang YSL: Bridging the academia-to-industry gap: Organ-on-a-chip platforms for safety and toxicology assessment. Trends Pharmacol Sci. 42:715–728. 2021.PubMed/NCBI View Article : Google Scholar
|
|
329
|
Sevinc Ozdemir N, Belyaev D, Castro MN, Balakin S, Opitz J, Wihadmadyatami H, Anggraeni R, Yucel D, Kenar H, Beshchasna N, et al: Advances in in vitro blood-Air barrier models and the use of nanoparticles in COVID-19 research. Tissue Eng Part B Rev. 30:82–96. 2024.PubMed/NCBI View Article : Google Scholar
|
|
330
|
Dasgupta Q, Jiang A, Wen AM, Mannix RJ, Man Y, Hall S, Javorsky E and Ingber DE: A human lung alveolus-on-a-chip model of acute radiation-induced lung injury. Nat Commun. 14(6506)2023.PubMed/NCBI View Article : Google Scholar
|
|
331
|
Pediaditakis I, Kodella KR, Manatakis DV, Le CY, Hinojosa CD, Tien-Street W, Manolakos ES, Vekrellis K, Hamilton GA, Ewart L, et al: Modeling alpha-synuclein pathology in a human brain-chip to assess blood-brain barrier disruption. NatCommun. 12(5907)2021.PubMed/NCBI View Article : Google Scholar
|
|
332
|
Campisi M, Shin Y, Osaki T, Hajal C, Chiono V and Kamm RD: 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 180:117–129. 2018.PubMed/NCBI View Article : Google Scholar
|
|
333
|
Ahn J, Yoon MJ, Hong SH, Cha H, Lee D, Koo HS, Ko JE, Lee J, Oh S, Jeon NL and Kang YJ: Three-dimensional microengineered vascularised endometrium-on-a-chip. Hum Reprod. 36:2720–2731. 2021.PubMed/NCBI View Article : Google Scholar
|
|
334
|
Xie X, Maharjan S, Kelly C, Liu T, Lang RJ, Alperin R, Sebastian S, Bonilla D, Gandolfo S, Boukataya Y, et al: Customizable Microfluidic Origami Liver-on-a-Chip (oLOC). Adv Mater Technol. 7(2100677)2022.PubMed/NCBI View Article : Google Scholar
|
|
335
|
Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, Aleman J, Colosi C, Busignani F, Dell'Erba V, et al: Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 110:45–59. 2016.PubMed/NCBI View Article : Google Scholar
|
|
336
|
Nguyen VVT, Ye S, Gkouzioti V, van Wolferen ME, Yengej FY, Melkert D, Siti S, de Jong B, Besseling PJ, Spee B, et al: A human kidney and liver organoid-based multi-organ-on-a-chip model to study the therapeutic effects and biodistribution of mesenchymal stromal cell-derived extracellular vesicles. J Extracell Vesicles. 11(e12280)2022.PubMed/NCBI View Article : Google Scholar
|
|
337
|
Glaser DE, Curtis MB, Sariano PA, Rollins ZA, Shergill BS, Anand A, Deely AM, Shirure VS, Anderson L, Lowen JM, et al: Organ-on-a-chip model of vascularized human bone marrow niches. Biomaterials. 280(121245)2022.PubMed/NCBI View Article : Google Scholar
|
|
338
|
Shim KY, Lee D, Han J, Nguyen NT, Park S and Sung JH: Microfluidic gut-on-a-chip with three-dimensional villi structure. Biomed Microdevices. 19(37)2017.PubMed/NCBI View Article : Google Scholar
|
|
339
|
Mansoorifar A, Gordon R, Bergan RC and Bertassoni LE: Bone-on-a-Chip: Microfluidic technologies and microphysiologic models of bone tissue. Adv Funct Mater. 31(2006796)2021.PubMed/NCBI View Article : Google Scholar
|
|
340
|
Ng FL, Cen Z, Toh YC and Tan LP: A 3D-printed micro-perfused culture device with embedded 3D fibrous scaffold for enhanced biomimicry. Int J Bioprint. 10:143–159. 2023.
|
|
341
|
Han J, Park S, Kim JE, Park B, Hong Y, Lim JW, Jeong S, Son H, Kim HB, Seonwoo H, et al: Development of a Scaffold-on-a-Chip platform to evaluate cell infiltration and osteogenesis on the 3D-Printed scaffold for bone regeneration. ACS Biomater Sci Eng. 9:968–977. 2023.PubMed/NCBI View Article : Google Scholar
|
|
342
|
Bahmaee H, Owen R, Boyle L, Perrault CM, Garcia-Granada AA, Reilly GC and Claeyssens F: Design and evaluation of an Osteogenesis-on-a-Chip microfluidic device incorporating 3D cell culture. Front Bioeng Biotechnol. 8(557111)2020.PubMed/NCBI View Article : Google Scholar
|
|
343
|
Killinger M, Kratochvilová A, Reihs EI, Matalová E, Klepárník K and Rothbauer M: Microfluidic device for enhancement and analysis of osteoblast differentiation in three-dimensional cell cultures. J Biol Eng. 17(77)2023.PubMed/NCBI View Article : Google Scholar
|
|
344
|
Scheinpflug J, Höfer CT, Schmerbeck SS, Steinfath M, Doka J, Tesfahunegn YA, Violet N, Renko K, Gulich K, John T, et al: A microphysiological system for studying human bone biology under simultaneous control of oxygen tension and mechanical loading. Lab Chip. 23:3405–3423. 2023.PubMed/NCBI View Article : Google Scholar
|
|
345
|
Nguyen M, Tong A, Volosov M, Madhavarapu S, Freeman J and Voronov R: Addressable microfluidics technology for non-sacrificial analysis of biomaterial implants in vivo. Biomicrofluidics. 17(024103)2023.PubMed/NCBI View Article : Google Scholar
|
|
346
|
Salerno E, Orlandi G, Ongaro C, D'Adamo A, Ruffini A, Carnevale G, Zardin B, Bertacchini J and Angeli D: Liquid flow in scaffold derived from natural source: Experimental observations and biological outcome. Regen Biomater. 9(rbac034)2022.PubMed/NCBI View Article : Google Scholar
|
|
347
|
López-Canosa A, Pérez-Amodio S, Engel E and Castaño O: Microfluidic 3D platform to evaluate endothelial progenitor cell recruitment by bioactive materials. Acta Biomater. 151:264–277. 2022.PubMed/NCBI View Article : Google Scholar
|
|
348
|
Peticone C, Thompson DDS, Dimov N, Jevans B, Glass N, Micheletti M, Knowles JC, Kim HW, Cooper-White JJ and Wall IB: Characterisation of osteogenic and vascular responses of hMSCs to Ti-Co doped phosphate glass microspheres using a microfluidic perfusion platform. J Tissue Eng. 11(2041731420954712)2020.PubMed/NCBI View Article : Google Scholar
|
|
349
|
Lyu J, Chen L, Zhang J, Kang X, Wang Y, Wu W, Tang H, Wu J, He Z and Tang K: A microfluidics-derived growth factor gradient in a scaffold regulates stem cell activities for tendon-To-bone interface healing. Biomater Sci. 8:3649–3663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
350
|
Goldman SM and Barabino GA: Spatial engineering of osteochondral tissue constructs through microfluidically directed differentiation of mesenchymal stem cells. Biores Open Access. 5:109–117. 2016.PubMed/NCBI View Article : Google Scholar
|
|
351
|
Jusoh N, Oh S, Kim S, Kim J and Jeon NL: Microfluidic vascularized bone tissue model with hydroxyapatite-incorporated extracellular matrix. Lab Chip. 15:3984–3988. 2015.PubMed/NCBI View Article : Google Scholar
|
|
352
|
Parhizkar M, Sofokleous P, Stride E and Edirisinghe M: Novel preparation of controlled porosity particle/fibre loaded scaffolds using a hybrid micro-fluidic and electrohydrodynamic technique. Biofabrication. 6(045010)2014.PubMed/NCBI View Article : Google Scholar
|
|
353
|
Rashidi N, Slater A, Peregrino G and Santin M: A novel, microfluidic high-throughput single-cell encapsulation of human bone marrow mesenchymal stromal cells. J Mater Sci, Mater Med. 35(19)2024.PubMed/NCBI View Article : Google Scholar
|
|
354
|
An C, Zhou R, Zhang H, Zhang Y, Liu W, Liu J, Bao B, Sun K, Ren C, Zhang Y, et al: Microfluidic-templated cell-laden microgels fabricated using phototriggered imine-crosslinking as injectable and adaptable granular gels for bone regeneration. Acta Biomater. 157:91–107. 2023.PubMed/NCBI View Article : Google Scholar
|
|
355
|
Yang G, Mahadik B, Choi JY, Yu JR, Mollot T, Jiang B, He X and Fisher JP: Fabrication of centimeter-sized 3D constructs with patterned endothelial cells through assembly of cell-laden microbeads as a potential bone graft. Acta Biomater. 121:204–213. 2021.PubMed/NCBI View Article : Google Scholar
|
|
356
|
Rajabnejadkeleshteri A, Basiri H, Mohseni SS, Farokhi M, Mehrizi AA and Moztarzadeh F: Preparation of microfluidic-based pectin microparticles loaded carbon dots conjugated with BMP-2 embedded in gelatin-elastin-hyaluronic acid hydrogel scaffold for bone tissue engineering application. Int J Biol Macromol. 184:29–41. 2021.PubMed/NCBI View Article : Google Scholar
|
|
357
|
Qasim M, Le NXT, Nguyen TPT, Chae DS, Park SJ and Lee NY: Nanohybrid biodegradable scaffolds for TGF-β3 release for the chondrogenic differentiation of human mesenchymal stem cells. Int J Pharm. 581(119248)2020.PubMed/NCBI View Article : Google Scholar
|
|
358
|
Moradikhah F, Doosti-Telgerd M, Shabani I, Soheili S, Dolatyar B and Seyedjafari E: icrofluidic fabrication of alendronate-loaded chitosan nanoparticles for enhanced osteogenic differentiation of stem cells. Life Sci. 254(17768)2020.PubMed/NCBI View Article : Google Scholar
|
|
359
|
Hou Y, Xie W, Achazi K, Cuellar-Camacho JL, Melzig MF, Chen W and Haag R: Injectable degradable PVA microgels prepared by microfluidic technology for controlled osteogenic differentiation of mesenchymal stem cells. Acta Biomater. 77:28–37. 2018.PubMed/NCBI View Article : Google Scholar
|
|
360
|
Lin Z, Wu J, Qiao W, Zhao Y, Wong KHM, Chu PK, Bian L, Wu S, Zheng Y, Cheung KMC, et al: Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials. 174:1–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
361
|
Li F, Truong VX, Thissen H, Frith JE and Forsythe JS: Microfluidic encapsulation of human mesenchymal stem cells for articular cartilage tissue regeneration. ACS Appl Mater Interfaces. 9:8589–8601. 2017.PubMed/NCBI View Article : Google Scholar
|
|
362
|
Angelozzi M, Miotto M, Penolazzi L, Mazzitelli S, Keane T, Badylak SF, Piva R and Nastruzzi C: Composite ECM-alginate microfibers produced by microfluidics as scaffolds with biomineralization potential. Mater Sci Eng C Mater Biol Appl. 56:141–153. 2015.PubMed/NCBI View Article : Google Scholar
|
|
363
|
Ding S, Li L, Liu X, Yang G, Zhou G and Zhou S: A nano-micro alternating multilayer scaffold loading with rBMSCs and BMP-2 for bone tissue engineering. Colloids Surf B Biointerfaces. 133:286–295. 2015.PubMed/NCBI View Article : Google Scholar
|
|
364
|
Blow N: Microfluidics: In search of a killer application. Nat Methods. 4:665–670. 2007.
|
|
365
|
Sackmann EK, Fulton AL and Beebe DJ: The present and future role of microfluidics in biomedical research. Nature. 507:181–189. 2014.PubMed/NCBI View Article : Google Scholar
|
|
366
|
Picollet-D'hahan N, Zuchowska A, Lemeunier I and Le Gac S: Multiorgan-on-a-Chip: A systemic approach to model and decipher Inter-organ communication. Trends Biotechnol. 39:788–810. 2021.PubMed/NCBI View Article : Google Scholar
|
|
367
|
Spagnuolo G, Codispoti B, Marrelli M, Rengo C, Rengo S and Tatullo M: Commitment of oral-derived stem cells in dental and maxillofacial applications. Dent J (Basel). 6(72)2018.PubMed/NCBI View Article : Google Scholar
|
|
368
|
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A and Liu S: oncise reviews: Characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells. 33:627–638. 2015.PubMed/NCBI View Article : Google Scholar
|
|
369
|
Nielsen AV, Beauchamp MJ, Nordin GP and Woolley AT: 3D printed microfluidics. Annu Rev Anal Chem (Palo Alto Calif). 13:45–65. 2020.PubMed/NCBI View Article : Google Scholar
|
|
370
|
Sooriyaarachchi D, Zhou Y, Maharubin S and Tan GZ: Microtube-embedded microfluidic devices for potential applications in blood brain barrier research. Procedia Manuf. 48:294–301. 2020.
|
|
371
|
Zhou Y: The recent development and applications of fluidic channels by 3D printing. J Biomed Sci. (24)2017.PubMed/NCBI View Article : Google Scholar
|
|
372
|
Moore S: Current Global Market of Organ-on-a-Chip, 2023. https://www.azolifesciences.com/article/Global-Market-Overview-Organ-on-a-Chip.aspx (accessed August 8, 2024).
|
|
373
|
Singh D, Mathur A, Arora S, Roy S and Mahindroo N: Journey of organ on a chip technology and its role in future healthcare scenario. Appl Surf Sci Adv. 9(100246)2022.
|
|
374
|
Franzen N, van Harten WH, Retèl VP, Loskill P, van den Eijnden-van Raaij J and IJzerman M: Impact of organ-on-a-chip technology on pharmaceutical R&D costs. Drug Discov Today. 24:1720–1724. 2019.PubMed/NCBI View Article : Google Scholar
|
|
375
|
Hubrecht RC and Carter E: The 3Rs and humane experimental technique: Implementing change. Animals (Basel). 9(754)2019.PubMed/NCBI View Article : Google Scholar
|
|
376
|
Goetz LH and Schork NJ: Personalized medicine: Motivation, challenges, and progress. Fertil Steril. 109:952–963. 2018.PubMed/NCBI View Article : Google Scholar
|
|
377
|
Syama S and Mohanan PV: Microfluidic based human-on-a-chip: A revolutionary technology in scientific research. Trends Food Sci Technol. 110:711–728. 2021.
|
|
378
|
Srivastava SK, Foo GW, Aggarwal N and Chang MW: Organ-on-chip technology: Opportunities and challenges. Biotechnology Notes. 5:8–12. 2024.PubMed/NCBI View Article : Google Scholar
|
|
379
|
Haddrick M and Simpson PB: Organ-on-a-chip technology: Turning its potential for clinical benefit into reality. Drug Discov Today. 24:1217–1223. 2019.PubMed/NCBI View Article : Google Scholar
|
|
380
|
Riehl BD and Lim JY: Macro and microfluidic flows for skeletal regenerative medicine. Cells. 1:1225–1245. 2012.PubMed/NCBI View Article : Google Scholar
|
|
381
|
Abuwatfa WH, Pitt WG and Husseini GA: Scaffold-based 3D cell culture models in cancer research. J Biomed Sci. 31(7)2024.PubMed/NCBI View Article : Google Scholar
|