Prostate cancer (PCa) is a leading cause of
cancer-related deaths in men worldwide (1). As medical technology evolves,
approaches for treating PCa are being regularly refined (2). Metastatic castration-resistant PCa
(mCRPC) poses significant therapeutic challenge (3). Current treatment modalities, including
hormone therapy, chemotherapy, and radiotherapy, show limited
effectiveness against mCRPC and frequently cause considerable
adverse effects (4). The
progression of metastatic PCa is influenced by multiple factors
such as tumor load, molecular traits, and resistance to therapies
(5).
Proteins located on the cell surface serve as
specific markers for tumors and are increasingly recognized as key
targets for targeted therapies in PCa (6). Research indicates that proteins such
as prostate-specific membrane antigen (PSMA) are significantly
overexpressed in PCa cells, making them promising options for
targeted treatment (7). These
proteins play a role in tumor proliferation and spread, as well as
in mechanisms that allow tumors to evade the immune system
(8). Focusing on these surface
proteins can enhance treatment efficacy and minimize adverse
effects (9).
The shortcomings of conventional treatment
strategies have led to the development of targeted therapies.
Although chemotherapy and radiation therapy continue to play
crucial roles in PCa management, their effectiveness is frequently
hindered by variations within tumors and resistance to medications
(10). Recently, therapies that
focus on cell surface proteins have gained attention as innovative
treatment options (11). This
strategy not only targets particular cancer cells but also enhances
the precision of drug delivery and increases the availability of
medications in the body (12).
Consequently, the management of mCRPC must differ from that of
hormone-sensitive diseases (13,14),
necessitating personalized strategies that target specific cell
surface markers [such as PSMA and six-transmembrane epithelial
antigen 1 (STEAP1)] and often employ combination therapies to
overcome resistance (15-17).
This review aims to comprehensively outline the
advancements in targeted treatments associated with PCa cell
surface proteins, focusing on the underlying molecular processes,
therapeutic approaches, and their clinical relevance. By examining
various strategies for targeted therapy and their implementation in
clinical settings, it seeks to offer improved treatment
alternatives for patients with PCa and inform future research
pathways.
mCRPC represents a distinct and advanced stage of
PCa characterized by disease progression despite androgen
deprivation therapy (18). The
management of mCRPC necessitates a differentiated approach compared
to hormone-sensitive PCa due to its unique biological behavior,
resistance mechanisms, and therapeutic vulnerabilities (19). Key challenges in mCRPC include
persistent androgen receptor (AR) signaling, intratumoral
heterogeneity, adaptive immune evasion, and the emergence of
treatment-resistant clones (20).
Current standard therapies for mCRPC, including
novel hormonal agents (such as enzalutamide and abiraterone),
chemotherapies (for example docetaxel), and radiopharmaceuticals
(such as 177Lu-PSMA-617), have demonstrated survival
benefits (21). However, their
efficacy is often limited by primary or acquired resistance, which
is driven by AR alterations, neuroendocrine differentiation, and an
immunosuppressive tumor microenvironment (TME) (22).
Therapeutic differentiation in mCRPC involves
several key approaches: i) Molecular stratification based on
genomic alterations (for example BRCA1/2 and PTEN), AR variant
expression, and PSMA avidity (23);
ii) sequential and combination therapies that leverage synergistic
effects between AR-directed agents, radioligands, immunotherapies,
and targeted agents (24); and iii)
liquid biopsy and biomarker monitoring utilizing circulating tumor
cells (CTCs) and cell-free DNA to track clonal evolution and
treatment response (25). Emerging
evidence supports the integration of multi-omics profiling and
artificial intelligence (AI) to guide personalized therapy in
mCRPC, emphasizing the necessity for dynamic treatment adaptation
in response to evolving tumor biology (26).
PSMA is a glycoprotein that spans the cell membrane
and is predominantly found in PCa cells, especially in aggressive
forms, such as metastatic, poorly differentiated, and
castration-resistant variants, where its elevated presence often
correlates with increased tumor severity (27). Although normal tissues, including
parts of the urinary system and certain nerve tissues, exhibit
lower PSMA levels, PSMA is markedly overexpressed on cancer cell
surfaces (6). This overexpression
plays a crucial role in tumor cell growth and survival within the
TME, and possibly in promoting new blood vessel formation in
tumors. The distinct expression pattern of PSMA, characterized by
high levels in tumors and minimal expression in non-tumor tissues,
makes it a promising therapeutic target (6). Treatment with radiopharmaceuticals,
such as Lu-PSMA-617, can effectively destroy cancer cells while
sparing healthy tissue (28).
Additionally, PSMA is not exclusive to PCa cells; it is also
present in some newly formed blood vessels associated with tumors
(29). The use of radiolabeled
compounds, such as 68Ga-PSMA-11, enhances the diagnostic precision
for detecting metastatic cancer (30). However, PSMA expression shows
intra-and intertumoral heterogeneity, which may lead to varying
treatment responses (31). In
addition, its low expression in normal tissues, such as the
salivary glands and kidneys, may cause off-target toxicity,
requiring close monitoring during treatment (32).
STEAP1 is frequently overexpressed in PCa,
especially in advanced and metastatic forms, with >85% of
prostate tumors exhibiting this expression (33). By contrast, normal tissues show
minimal expression, highlighting their potential as significant
targets for therapy (34). This is
further supported by the link with biomarkers found in
STEAP1-positive extracellular vesicles, which can assist in both
diagnosis and prognosis (35). As a
cell surface antigen, STEAP1 plays a role in various aspects of
tumor development, including cell growth, migration, survival,
intercellular communication, and metal metabolism (36). It also enhances tumor cell functions
through pathways such as PI3K/Akt and is linked to aggressive
characteristics such as invasion and metastasis (37). Previous research on PCa models have
demonstrated that STEAP1 exerts antitumor effects when used in
chimeric antigen receptor T (CAR-T) cell therapy (38). When combined with localized IL-12
therapy, it was shown to improve treatment effectiveness by
modifying the TME and countering antigen escape (29). Moreover, clinical trials are being
conducted using STEAP1-targeted T-cell inducers and antibody-drug
conjugates (ADCs) (39). In
summary, STEAP1 is a promising target in PCa; however, its
expression dynamics in advanced cancer and association with metal
metabolism need to be further elucidated.
Additional important proteins on cell surfaces
include trophoblast cell-surface antigen 2 (TROP-2), a
transmembrane protein frequently found at elevated levels in
various cancers, including PCa, making it a promising candidate for
ADCs (40). Ongoing clinical trials
are assessing the effectiveness of sacituzumab-govitecan in
treating urothelial carcinoma and PCa, with supportive evidence of
its anticancer properties emerging from animal studies (41). Likewise, prostate stem cell antigen
(PSCA) is present in ~90% of PCa cases and is associated with tumor
aggressiveness, leading to the development of targeted
immunotherapies such as CAR-T cells and ADCs (42). Nonetheless, obstacles such as the
immunosuppressive nature of the TME, potential off-target effects,
and variability in expression levels may pose challenges (43). Furthermore, delta-like ligand 3
(DLL3) is overexpressed in neuroendocrine PCa (NEPC), linking it to
tumor severity and patient prognosis (44). Monoclonal antibodies and
radioimmunotherapy, including 177Lu-labeled antibodies, have
demonstrated preclinical promise. However, concerns regarding their
expression in non-neuroendocrine tumors and patient variability
must be resolved (45). In
addition, emerging targets, such as cadherin EGF LAG seven-pass
G-type receptor 3 (CELSR3) and glypican-3 (GPC3), have also
attracted attention. CELSR3 is highly expressed in NEPC and is
involved in the regulation of cell polarity and migration through
the Wnt/PCP signaling pathway; its overexpression is associated
with tumor invasiveness. Research has explored its potential as a
target for CAR-T cells and ADC (46). GPC3 is upregulated in
castration-resistant PCa and promotes tumor growth by regulating
the Hh signaling pathway. Preclinical studies have shown that
GPC3-targeting antibodies can inhibit tumor progression, and phase
I clinical trials are underway (47) (Table
I).
The drug 177Lu-PSMA-617 is a radioligand used for
mCRPC that targets PSMA to deliver the radioactive isotope 177Lu
directly to cancer cells, leading to their destruction (48). Findings from the VISION trial
indicated that it notably enhanced both overall survival and
quality of life in patients, and its favorable tolerability has
established it as a standard treatment for mCRPC (49). Currently, there is growing interest
in exploring novel radioactive isotopes, such as 161Tb-PSMA, which
may offer promising avenues for combination therapies (50). Clinical studies have demonstrated
the safety and effectiveness of 161Tb-PSMA, suggesting that its use
along with 177Lu-PSMA-617 could potentially amplify therapeutic
outcomes (51). Overall,
radioligand therapy (RLT), such as 177Lu-PSMA-617, has been
demonstrated to significantly improve survival in patients with
mCRPC; however, drug resistance and heterogeneous expression remain
major challenges.
ADCs are designed to transport cytotoxic medications
directly to cancer cells using antibodies that specifically attach
to tumor antigens (52). This
targeted approach enhances the therapeutic effectiveness while
minimizing harm to healthy tissues (53). PSMA is recognized as a
well-established therapeutic target that facilitates efficient
delivery of ADCs. Clinical studies have indicated favorable results
and manageable side effects in patients with mCRPC, including a 50%
or more reduction in prostate-specific antigen levels during
second-line treatments, along with some instances of complete
responses (54). Additionally,
TROP-2, which is prominently expressed in metastatic PCa and linked
to poor prognosis, represents another significant target for ADCs,
showing encouraging clinical results (40). In contrast to conventional
chemotherapy, the targeted nature of ADCs mitigates widespread
toxicity and drug resistance (55).
ADCs achieve precise delivery; however, the internalization
efficiency of the target, linker stability, and off-target toxicity
limit their widespread applications.
BiTEs are designed to bind both tumor-specific
antigens and CD3 receptors on T cells, thereby effectively linking
T cells to cancer cells (56). This
connection not only activates T cells to kill tumor cells but also
facilitates the direct destruction of the tumor. For instance, AMG
160, which targets PSMA, elicits cytotoxic effects in PCa, whereas
BiTEs that target STEAP1 further improve therapeutic outcomes
(57). In addition to their direct
effects on tumors, BiTEs enhance the immune response against cancer
by promoting the release of cytokines from activated T cells,
which, in turn, activate nearby immune cells (58). Although the combination of BiTEs
with immune checkpoint inhibitors has the potential to address the
immunosuppressive environments found in ‘cold tumors’, such as PCa,
their application in clinical settings is hindered by issues such
as cytokine release syndrome (CRS) and other immune-related adverse
effects (irAEs) (59). BiTEs
activate T cells with strong cytotoxic effects; however, CRS and
neurotoxicity limit their clinical application.
CAR-T cell therapy is a groundbreaking
immunotherapeutic approach. Preclinical research has demonstrated
that CAR-T cells targeting the STEAP1 antigen in PCa cells can
effectively identify and eliminate tumor cells (60). Additionally, CAR-T therapy targeting
PSMA has been proven effective in patients with metastatic disease.
Studies conducted in vitro and in animal models revealed
enhanced anti-tumor properties, particularly when combined with
chemotherapy agents (60,61). Nonetheless, obstacles, such as the
immunosuppressive nature of the TME and the mechanisms of antigen
escape, hinder their overall effectiveness, leading to the
exploration of dual CAR-T cells paired with IL-23 antibodies to
enhance their proliferation and functionality (62). NEPC is a particularly aggressive
variant that poses distinct treatment challenges owing to the
presence of specific antigens. CAR-T cells targeting
carcinoembryonic antigen-related cell adhesion molecule 5 have
shown significant cytotoxic potential in preclinical studies,
thereby addressing some of the shortcomings of traditional
therapies (63). Although CAR-T has
made breakthroughs in hematologic tumors, its efficacy is limited
in solid tumors such as PCa, mainly due to: i) Immunosuppressive
cells [such as myeloid-derived suppressor cells (MDSCs)] and
factors (such as TGF-β) in the TME inhibiting T-cell function
(64); ii) physical barriers such
as fibrotic stroma hindering T-cell infiltration (65); and iii) antigen heterogeneity
leading to antigen escape (66). By
contrast, hematological tumors often exhibit more uniform antigen
expression and a less immunosuppressive microenvironment, which
facilitates the efficacy of CAR-T therapy (67). CAR-T cells exhibit potential in PCa,
however the obstacles presented by the solid TME and antigen escape
limit their efficacy, requiring combined strategies to overcome
this bottleneck.
BiTEs and CAR-T cells are both T cell-directed
therapies; however, each has advantages and disadvantages. BiTEs
are easy to prepare, act quickly, and can be easily combined with
immune checkpoint inhibitors (57);
however, they have a short half-life and require continuous
infusion, and the risk of CRS is high (59). Although CAR-T is durable and can
expand in vivo, it is complex to prepare, costly, and easily
inhibited by the TME in solid tumors (62,65).
In summary, BiTEs offer a cost-effective, rapid response option for
short-term intervention, whereas CAR-T therapy provides durable,
personalized control, but faces manufacturing and TME challenges in
PCa. The choice should be guided by treatment goals and
patient-specific factors.
The use of nanoparticle-based drug delivery systems,
which include functionalized nanoparticles, such as gold and
polymeric carriers, allows for the precise targeting of cancer
cells that express PSMA (68). This
approach significantly improves drug bioavailability and can
decrease systemic toxicity by 50-70% (68). These systems enhance the solubility
and circulation duration of drugs, encourage tumor-specific
accumulation to enhance effectiveness, and minimize side effects,
while enabling treatment response monitoring through imaging
methods (69). Furthermore,
ultrasound-responsive nanocarriers are effective in delivering
small interfering RNA to suppress gene expression in PCa cells
(70). Nonetheless, the transition
to clinical applications encounters obstacles, such as
biocompatibility, immune reactions, and mechanisms of drug
resistance. Nanocarriers enhance drug targeting and
bioavailability; however, biocompatibility, immunogenicity, and
large-scale production are challenges for translation.
Different treatment strategies have distinct
advantages and disadvantages. RLT (such as 177Lu-PSMA-617) has a
high response rate in PSMA-high-expressing mCRPC but may cause
myelosuppression (71); ADCs have
strong targeting but carry the risk of off-target toxicity
(72); BiTEs activate T cells
rapidly but can easily lead to cytokine release syndrome (73); and CAR-T therapy has been successful
in hematologic neoplasms but is limited in solid tumors such as PCa
owing to TME suppression, resulting in limited efficacy (74). Longitudinal comparisons showed that
both BiTEs and CAR-T cells are T cell-directed therapies, but BiTEs
are cost-effective, quick to deploy, and suitable for short-term
interventions, while CAR-T modifications are durable but complex to
manufacture, making them more suitable for individualized long-term
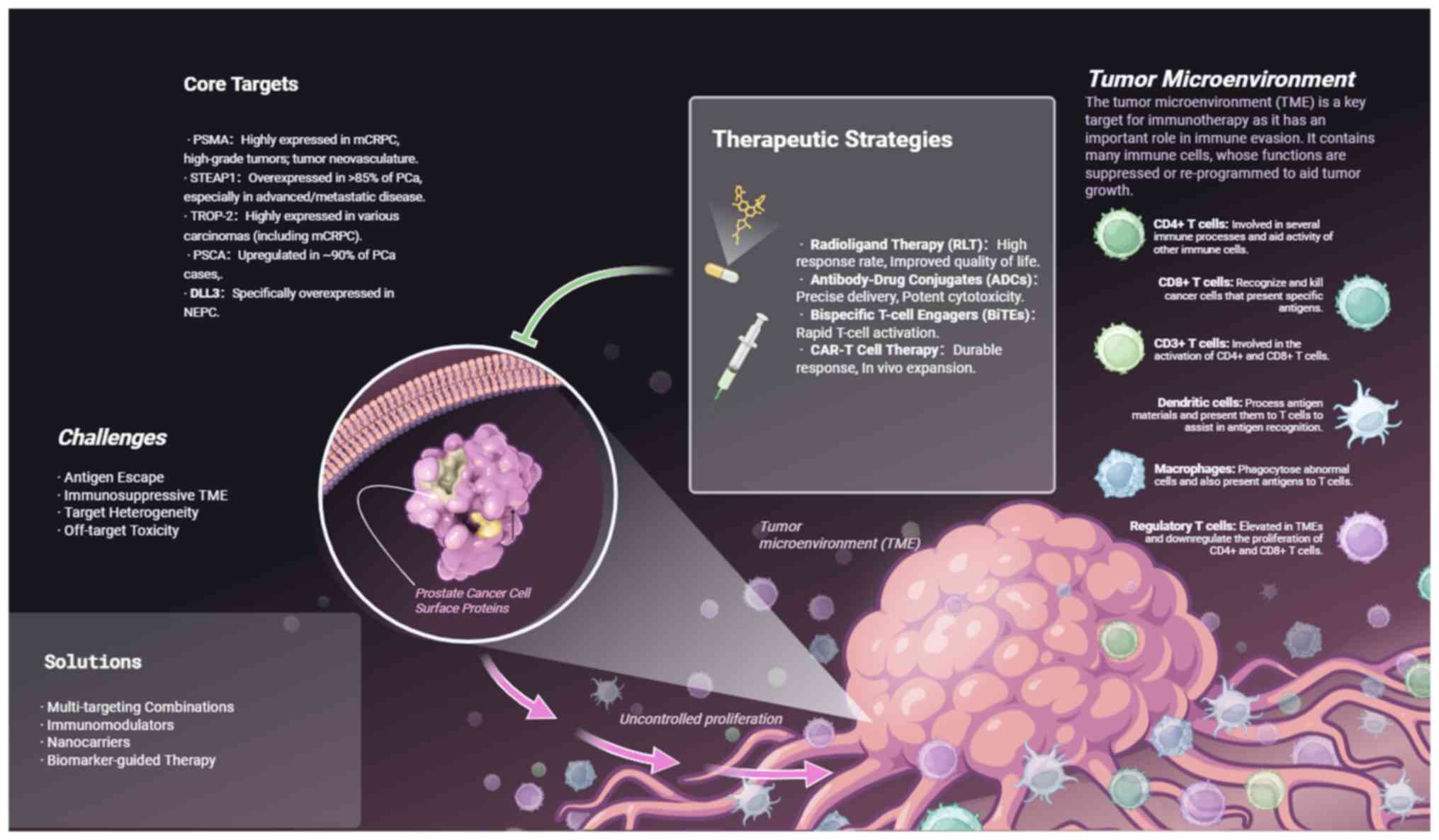
treatment (75) (Fig. 1).
The reduction in antigen expression and the
phenomenon of antigen escape are significant factors in how tumor
cells avoid detection by the immune system (76). This downregulation is often
associated with various strategies employed by tumor cells to
elicit immune responses. In PCa cells, the levels of PSMA and
similar antigens frequently decrease due to factors such as genetic
alterations, epigenetic modifications, and the effects of the TME
(77). Lower PSMA expression can
lead to a diminished response to immunotherapy, allowing tumor
cells to evade immune monitoring (78). Furthermore, decreased antigen
expression can hinder T-cell activation, thus impairing the overall
immune response (79). Research
indicates that antigen escape is intricately linked to the
heterogeneity of tumor cells, where variations in antigen
expression among different tumor cells contribute to the complexity
and variability of immune responses against tumors (80). To cope with antigen downregulation,
multitarget CAR-T cells (such as PSMA and STEAP1) or combined
epigenetic regulators (such as HDAC inhibitors) can be used to
stabilize antigen expression (81,82).
The diversity of PCa tumors significantly
contributes to their ability to evade the immune system (83). Within PCa, various tumor cell
subtypes may exhibit resistance to immunotherapy, whereas others
respond positively to such treatments (84). This variability leads to differences
in antigen expression, enabling some tumor cells to avoid immune
detection by reducing or completely losing expression of crucial
antigens (85). Additionally,
elements within the TME that inhibit immune function, including
tumor-associated macrophages and MDSCs, further facilitate the
immune evasion of these tumor cells, intensifying the heterogeneity
of the tumors (86-88).
Consequently, investigating combination therapies aimed at
enhancing immune responses and improving treatment effectiveness
against tumors is critically important (89).
Management of the immunosuppressive tumor
environment is a crucial focus in PCa research. Factors associated
with tumor-induced immunosuppression, such as S100A4, play a
significant role in the initiation and progression of PCa (90). This protein is elevated in numerous
tumors and is associated with unfavorable outcomes in patients with
PCa (91). The use of anti-S100A4
antibodies could potentially counteract this immunosuppressive
effect in PCa, leading to improved treatment responses (92). Targeting S100A4 with anti-S100A4
antibodies may effectively reverse the immunosuppressive state of
PCa, thereby improving patient response rates to treatment
(90). The combination of
immunomodulatory approaches and targeted therapies has great
potential. For example, YY001, a novel EP4 antagonist, is vital for
shaping the immune landscape of PCa (93). Research indicates that YY001 can
reduce the differentiation and activity of MDSCs, while promoting
T-cell growth and antitumor functions (93). By modulating cytokine and chemokine
levels, YY001 can effectively restore a more favorable immune
environment within tumors, functioning synergistically with
anti-PD-1 antibodies to significantly enhance antitumor immune
activity (93). The effectiveness
of this combined approach highlights the potential of integrating
immunomodulatory and targeted therapies for PCa treatment.
The combination and evaluation of multi-omics
information, including genomics, transcriptomics, and proteomics,
have enabled the discovery of novel biomarkers and treatment
targets for PCa (101). Notably,
mutations in genes such as BRCA1/2 are significantly linked to
patient outcomes. Treatments targeting these mutations, such as
PARP, have demonstrated efficacy (102). Molecular subtyping allows for the
identification of high-risk patients and enhances the likelihood of
successful treatment outcomes. Advanced AI techniques, such as deep
and machine learning, aid in the accurate analysis of biomarkers
and refinement of personalized treatment strategies (103). In addition, tracking CTCs is
essential. The number of CTCs and the expression of surface
proteins (such as PSMA and STEAP1) can indicate tumor severity,
prognosis, and response to therapy (104), whereas liquid biopsy methods can
efficiently isolate CTCs for ongoing clinical assessment (105). The management of mCRPC should be
individualized based on several key factors, including i) tumor
burden and metastatic sites; ii) expression levels of cell surface
targets (such as PSMA PET/CT standardized uptake value); iii)
genome characteristics (such as DNA repair defects); and iv) prior
treatment history (106-108).
Studies have shown that PSMA-targeted therapy can achieve a
response rate of 60-70% in patients with mCRPC and high PSMA
expression, while the effect is limited in patients with low
expression, emphasizing the importance of target assessment before
treatment (109-111).
Investigation of innovative surface proteins,
including CELSR3, which is notably overexpressed in NEPC, is
crucial for controlling cell growth and movement (46). Additionally, GPC3, which is linked
to tumor aggressiveness, opens up new possibilities for the
targeted treatment of PCa. CAR-T cells targeting CELSR3 have
demonstrated significant tumor-suppressive activity in
patient-derived xenograft models (37), while GPC3-targeted ADCs showed
promising results in phase I trials for liver cancer, and its trial
for PCa has been initiated (112).
Concurrently, gene therapy-utilizing carriers, such as
poly(lactic-co-glycolic acid) (PLGA) nanoparticles, can effectively
transport nucleic acid medications aimed at PSMA (47). Treatment efficacy can be enhanced by
employing a multifaceted approach that influences tumor dynamics
and encourages immune cell infiltration (113). Advances in nanotechnology have
enabled the development of immunotherapies. Functionalized
nanoparticles can transport immunomodulatory agents and checkpoint
inhibitors directly to tumor locations, enhancing biodistribution
and facilitating synergistic combination therapies through the
simultaneous delivery of various therapeutic compounds (114). Multi-omics and AI technologies
drive individualized therapy; however, target validation, biomarker
standardization, and clinical trial design remain key to achieving
precision medicine.
In the context of targeted therapy for PCa, a major
challenge in choosing PSMA is the heterogeneity of the targets
(115). Differences in PSMA
expression across various tumor types and their microenvironments
result in notable variations in treatment effectiveness among
individuals (116). To address
this heterogeneity, multimodal imaging (such as PSMA-PET/MRI) can
be used to assess target expression and develop bispecific
antibodies that simultaneously target both PSMA and STEAP1. Beyond
this heterogeneity, there is the potential for unintended toxicity
that can harm non-target tissues (117). This issue can be mitigated by the
use of functionalized nanoparticles and targeted ligands, which
improve the precision of therapeutic agents (118). By utilizing tumor-targeting
peptides in conjunction with nanoparticles, selective absorption of
cancer drugs can be achieved, whereas biocompatible carriers help
minimize systemic toxicity and ensure accurate delivery to PCa
cells (119).
Immunotherapy can result in a range of irAEs when
treating PCa and other urological malignancies, including mild
symptoms such as rashes and fatigue as well as serious conditions
such as endocrine dysfunction and potentially fatal pneumonia
(120). Consequently, it is
crucial to promptly recognize these issues through consistent
monitoring of symptoms and patient feedback (121). Management approaches involve
providing symptomatic relief for less severe cases and
administering immunosuppressive medications, including steroids, to
patients with moderate-to-severe irAEs, alongside patient education
(122,123). Similarly, CAR-T cell therapy
demonstrates promising effectiveness but requires rigorous safety
oversight to monitor adverse reactions such as CRS and
neurotoxicity (124). This
requires ongoing evaluation of vital signs and neurological health,
offering symptomatic care and tocilizumab when appropriate and a
thorough pretreatment assessment to identify risk factors (125). In future, priority should be given
to exploring the dynamic expression patterns of targets, the
mechanisms of immunotoxicity, and the establishment of predictive
models to optimize the treatment window.
Advancements in targeted therapies for PCa cell
surface proteins, including PSMA, STEAP1, and TROP-2, have
significantly improved the management of mCRPC. Innovative
approaches such as RLT (including 177Lu-PSMA-617), ADCs, bispecific
antibodies, and CAR-T cell therapy have demonstrated promising
results. Nonetheless, challenges, such as antigen escape,
immunosuppressive environments, and drug resistance, persist.
Addressing these issues requires the development of combination
therapies, including targeted drug pairings and the integration of
RLT with immunotherapy, alongside personalized treatments informed
by biological markers, such as genomic characteristics and CTC
analysis. Future research should focus on the following directions:
i) Elucidating the molecular mechanisms of antigen escape,
particularly the roles of epigenetic regulation and TME-mediated
immune suppression. ii) Prioritizing the clinical evaluation of
combination strategies, such as RLT (for example 177Lu-PSMA-617)
and immune checkpoint inhibitors (including anti-PD-1). iii)
Accelerating the translational development of therapies against
emerging targets (such as CELSR3- and GPC3-directed ADCs or CAR-T
cells). iv) Developing integrated biomarker panels (for example
combining ctDNA, CTCs, and radiomics) and standardizing toxicity
management guidelines to improve patient quality of life. By
fostering interdisciplinary collaboration to expedite the clinical
application of novel therapies, targeted PCa treatment can advance
to a more precise and effective era.
Not applicable.
Funding: The present review was funded by the Medical Research
Project of Sichuan Medical Association (grant no. S2024047).
Not applicable.
HL conceived and designed the review, drafted the
manuscript, as well as read and approved the final mansucript. Data
authentication is not applicable.
Not applicable.
Not applicable.
The author declares that he has no competing
interests.
|
1
|
Pullen RL Jr and Holter V: Nursing
management of a patient with prostate cancer. Nursing. 55:25–35.
2025.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Campodonico F, Ennas M, Zanardi S, Zigoura
E, Piccardo A, Foppiani L, Schiavone C, Squillace L, Benelli A, De
Censi A, et al: Management of prostate cancer with systemic
therapy: A prostate cancer unit perspective. Curr Cancer Drug
Targets. 21:107–116. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Henriques V, Wenzel M, Demes MC and
Köllermann J: Metastatic castration-resistant prostate cancer.
Pathologe. 42:431–441. 2021.(In German).
|
|
4
|
Heck MM, Tauber R, Schwaiger S, Retz M,
D'Alessandria C, Maurer T, Gafita A, Wester H, Gschwend JE, Weber
WA, et al: Treatment outcome, toxicity, and predictive factors for
radioligand therapy with 177Lu-PSMA-I&T in metastatic
castration-resistant prostate cancer. Eur Urol. 75:920–926.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
von Amsberg G, Busenbender T, Coym A,
Kaune M, Strewinsky N, Ekrutt J, Tilki D and Dyshlovoy S:
Management of metastatic prostate cancer. Oncol Res Treat. 8:1–12.
2025.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nezir AE, Khalily MP, Gulyuz S, Ozcubukcu
S, Küçükgüzel SG, Yilmaz O and Telci D: Synthesis and evaluation of
tumor-homing peptides for targeting prostate cancer. Amino Acids.
53:645–652. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kumar BV, Sachan R, Garad P, Srivastava N,
Saraf SA and Meher N: Dual targeting of prostate-specific membrane
antigen and fibroblast activation protein: Bridging prostate cancer
theranostics with precision. ACS Appl Bio Mater. 8:962–979.
2025.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu C, Chen S, Zhang Y, Zhou X, Wang H,
Wang Q and Lan X: Mechanisms of Rho GTPases in regulating tumor
proliferation, migration and invasion. Cytokine Growth Factor Rev.
80:168–174. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang J, Zhu J, Meng J, Qiu T, Wang W, Wang
R and Liu J: Baicalin inhibits biofilm formation by influencing
primary adhesion and aggregation phases in Staphylococcus
saprophyticus. Vet Microbiol. 262(109242)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Du X, Zhang Y, Jia Y and Gao B: The
Development of Al18F-NOTA-FAP-2286 as an FAP-Targeted PET tracer
and the translational application in the diagnosis of acquired drug
resistance in progressive prostate cancer. Pharmaceutics.
17(552)2025.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Grewal K, Dorff TB, Mukhida SS, Agarwal N
and Hahn AW: Advances in targeted therapy for metastatic prostate
cancer. Curr Treat Options Oncol. 26:465–475. 2025.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao Q, Mo Z, Zeng L, Yuan Y and Wang Y
and Wang Y: Construction and evaluation of hepatic targeted drug
delivery system with hydroxycamptothecin in stem cell-derived
exosomes. Molecules. 29(5174)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fan L, Gong Y, He Y, Gao W, Dong X, Dong
B, Zhu HH and Xue W: TRIM59 is suppressed by androgen receptor and
acts to promote lineage plasticity and treatment-induced
neuroendocrine differentiation in prostate cancer. Oncogene.
42:559–571. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Archer M, Lin KM, Kolanukuduru KP, Zhang
J, Ben-David R, Kotula L and Kyprianou N: Impact of cell plasticity
on prostate tumor heterogeneity and therapeutic response. Am J Clin
Exp Urol. 12:331–351. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Abida W, Beltran H and Raychaudhuri R:
State of the Art: Personalizing treatment for patients with
metastatic castration-resistant prostate cancer. Am Soc Clin Oncol
Educ Book. 245(e473636)2025.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu J, Ma Y, Hu P, Yao J, Chen H and Ma Q:
Recent advances in antibody-drug conjugates for metastatic
castration-resistant prostate cancer. Zhejiang Da Xue Xue Bao Yi
Xue Ban. 54:685–693. 2025.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
17
|
Mulati Y, Shen Q, Tian Y, Chen Y, Yao K,
Yu W, Cui Y, Shi X, He Z, Zhang Q and Fan Y: Characterizing PSMA
heterogeneity in prostate cancer and identifying clinically
actionable tumor associated antigens in PSMA negative cases. Sci
Rep. 15(23902)2025.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Horak J, Petrausch U and Omlin A:
Metastatic castration-resistant prostate cancer-what are rational
sequential treatment options? Urologie. 62:1295–1301.
2023.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
19
|
Davis ID: Combination therapy in
metastatic hormone-sensitive prostate cancer: Is three a crowd?
Ther Adv Med Oncol. 14(17588359221086827)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Reichert ZR and Hussain M: Androgen
receptor and beyond, targeting androgen signaling in
castration-resistant prostate cancer. Cancer J. 22:326–329.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Li PY, Lu YH and Chen CY: Comparative
effectiveness of abiraterone and enzalutamide in patients with
metastatic castration-resistant prostate cancer in Taiwan. Front
Oncol. 12(822375)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Blanc C, Moktefi A, Jolly A, de la Grange
P, Gay D, Nicolaiew N, Semprez F, Maillé P, Soyeux P, Firlej V, et
al: The Neuropilin-1/PKC axis promotes neuroendocrine
differentiation and drug resistance of prostate cancer. Br J
Cancer. 128:918–927. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Russnes HG, Lingjærde OC, Børresen-Dale AL
and Caldas C: Breast cancer molecular stratification: From
intrinsic subtypes to integrative clusters. Am J Pathol.
187:2152–2162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saeed F, Berchuck JE, Bilen MA, Gandhi JS,
Nazha B, Brown JT, Schuster DM, Jani AB, Yu J and Harik LR:
Optimizing treatment for metastatic castration-resistant prostate
cancer: Food and Drug Administration-approved therapies, emerging
strategies, and biomarker-driven approaches. Cancer.
131(e70037)2025.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hollanda CN, Gualberto ACM, Motoyama AB
and Pittella-Silva F: Advancing leukemia management through liquid
biopsy: Insights into biomarkers and clinical utility. Cancers
(Basel). 17(1438)2025.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Liu HH, Tsai YS, Lai CL, Tang CH, Lai CH,
Wu HC, Hsieh JT and Yang CR: Evolving personalized therapy for
castration-resistant prostate cancer. Biomedicine (Taipei).
4(2)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Adnan A and Basu S: PSMA Receptor-Based
PET-CT: The basics and current status in clinical and research
applications. Diagnostics (Basel). 13(158)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ramnaraign B and Sartor O: PSMA-targeted
radiopharmaceuticals in prostate cancer: Current data and new
trials. Oncologist. 28:392–401. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rowe SP, Gorin MA and Pomper MG: Imaging
of prostate-specific membrane antigen with small-molecule PET
radiotracers: From the bench to advanced clinical applications.
Annu Rev Med. 70:461–477. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ali H, Rashid Ul Amin S, Hai A and Nizar
N: Bony lesion analysis in carcinoma prostate: Methylene
diphosphonate bone scan vs. Gallium-68 psma-11 pet/ct. J Ayub Med
Coll Abbottabad. 35:415–418. 2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang M, Li Y, Quan Z, Zhou X, Meng X, Ye
J, Wang Y, Wang J, Qin W, Wang J and Kang F: Value of [68Ga]
Ga-PSMA-11 PET/CT in reflecting the intra- and intertumor
heterogeneity of neovascularization in clear cell renal cell
carcinoma. Mol Pharm. 22:1529–1538. 2025.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alaskarov A, Barel S, Bakavayev S, Kahn J
and Israelson A: MIF homolog d-dopachrome tautomerase (D-DT/MIF-2)
does not inhibit accumulation and toxicity of misfolded SOD1. Sci
Rep. 12(9570)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ihlaseh-Catalano SM, Drigo SA, de Jesus
CM, Domingues MA, Trindade Filho JC, de Camargo JL and Rogatto SR:
STEAP1 protein overexpression is an independent marker for
biochemical recurrence in prostate carcinoma. Histopathology.
63:678–685. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Uemura H, Nakagawa Y, Yoshida K, Saga S,
Yoshikawa K, Hirao Y and Oosterwijk Y: MN/CA I X/G250 as a
potential target for immunotherapy of renal cell carcinomas. Br J
Cancer. 81:741–746. 1999.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Khanna K, Salmond N, Lynn KS, Leong HS and
Williams KC: Clinical significance of STEAP1 extracellular vesicles
in prostate cancer. Prostate Cancer Prostatic Dis. 24:802–811.
2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Oosterheert W and Gros P: Cryo-electron
microscopy structure and potential enzymatic function of human
six-transmembrane epithelial antigen of the prostate 1 (STEAP1). J
Biol Chem. 295:9502–9512. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Li YJ, Wang Y and Wang YY: MicroRNA-99b
suppresses human cervical cancer cell activity by inhibiting the
PI3K/AKT/mTOR signaling pathway. J Cell Physiol. 234:9577–9591.
2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bhatia V, Kamat NV, Pariva TE, Wu LT, Tsao
A, Sasaki K, Sun H, Javier G, Nutt S, Coleman I, et al: Targeting
advanced prostate cancer with STEAP1 chimeric antigen receptor T
cell and tumor-localized IL-12 immunotherapy. Nat Commun.
14(2041)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Thorne AH, Malo KN, Wong AJ, Nguyen TT,
Cooch N, Reed C, Yan J, Broderick KE, Smith TRF, Masteller EL and
Humeau L: Adjuvant screen identifies synthetic DNA-Encoding Flt3L
and CD80 immunotherapeutics as candidates for enhancing anti-tumor
T cell responses. Front Immunol. 11(327)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sperger JM, Helzer KT, Stahlfeld CN, Jiang
D, Singh A, Kaufmann KR, Niles DJ, Heninger E, Rydzewski NR, Wang
L, et al: Expression and therapeutic targeting of TROP-2 in
treatment-resistant prostate cancer. Clin Cancer Res. 29:2324–2335.
2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bardia A, Messersmith WA, Kio EA, Berlin
JD, Vahdat L, Masters GA, Moroose R, Santin AD, Kalinsky K, Picozzi
V, et al: Sacituzumab govitecan, a Trop-2-directed antibody-drug
conjugate, for patients with epithelial cancer: Final safety and
efficacy results from the phase I/II IMMU-132-01 basket trial. Ann
Oncol. 32:746–756. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Matera L: The choice of the antigen in the
dendritic cell-based vaccine therapy for prostate cancer. Cancer
Treat Rev. 36:131–41. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen L, Chen F, Niu H, Li J, Pu Y, Yang C,
Wang Y, Huang R, Li K, Lei Y and Huang Y: Chimeric antigen receptor
(CAR)-T cell immunotherapy against thoracic malignancies:
Challenges and opportunities. Front Immunol.
13(871661)2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Puca L, Gavyert K, Sailer V, Conteduca V,
Dardenne E, Sigouros M, Isse K, Kearney M, Vosoughi A, Fernandez L,
et al: Delta-like protein 3 expression and therapeutic targeting in
neuroendocrine prostate cancer. Sci Transl Med.
11(eaav0891)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Mohsin H, Jia F, Sivaguru G, Hudson MJ,
Shelton TD, Hoffman TJ, Cutler CS, Ketring AR, Athey PS, Simón J,
et al: Radiolanthanide-labeled monoclonal antibody CC49 for
radioimmunotherapy of cancer: Biological comparison of DOTA
conjugates and 149Pm, 166Ho, and 177Lu. Bioconjug Chem. 17:485–492.
2006.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Van Emmenis L, Ku SY, Gayvert K, Branch
JR, Brady NJ, Basu S, Russell M, Cyrta J, Vosoughi A, Sailer V, et
al: The identification of CELSR3 and other potential cell surface
targets in neuroendocrine prostate cancer. Cancer Res Commun.
3:1447–1459. 2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hong S, Jeong SH, Han JH, Yuk HD, Jeong
CW, Ku JH and Kwak C: Highly efficient nucleic acid encapsulation
method for targeted gene therapy using antibody conjugation system.
Mol Ther Nucleic Acids. 35(102322)2024.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Patell K, Kurian M, Garcia JA, Mendiratta
P, Barata PC, Jia AY, Spratt DE and Brown JR: Lutetium-177 PSMA for
the treatment of metastatic castrate resistant prostate cancer: A
systematic review. Expert Rev Anticancer Ther. 23:731–744.
2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Fallah J, Agrawal S, Gittleman H, Fiero
MH, Subramaniam S, John C, Chen W, Ricks TK, Niu G, Fotenos A, et
al: FDA approval summary: Lutetium Lu 177 Vipivotide tetraxetan for
patients with metastatic castration-resistant prostate cancer. Clin
Cancer Res. 29:1651–1657. 2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tschan VJ, Busslinger SD, Bernhardt P,
Grundler PV, Zeevaart JR, Köster U, van der Meulen NP, Schibli R
and Müller C: Albumin-binding and conventional PSMA ligands in
combination with 161Tb: Biodistribution, dosimetry, and preclinical
therapy. J Nucl Med. 64:1625–1631. 2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Buteau JP, Kostos L, Jackson PA, Xie J,
Haskali MB, Alipour R, McIntosh LE, Emmerson B, MacFarlane L,
Martin CA, et al: First-in-human results of terbium-161 [161Tb]
Tb-PSMA-I&T dual beta-Auger radioligand therapy in patients
with metastatic castration-resistant prostate cancer (VIOLET): A
single-centre, single-arm, phase 1/2 study. Lancet Oncol.
26:1009–1017. 2025.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mark C, Lee JS, Cui X and Yuan Y:
Antibody-drug conjugates in breast cancer: Current status and
future directions. Int J Mol Sci. 24(13726)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang Y, Li G, Wang H, Qi Q, Wang X and Lu
H: Targeted therapeutic strategies for Nectin-4 in breast cancer:
Recent advances and future prospects. Breast.
79(103838)2025.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Nguyen H, Hird K, Cardaci J, Smith S and
Lenzo NP: Lutetium-177 Labelled Anti-PSMA monoclonal antibody
(Lu-TLX591) Therapy for metastatic prostate cancer: Treatment
toxicity and outcomes. Mol Diagn Ther. 28:291–299. 2024.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Yassin MT, Al-Otibi FO, Al-Sahli SA,
El-Wetidy MS and Mohamed S: Metal oxide nanoparticles as efficient
nanocarriers for targeted cancer therapy: Addressing
chemotherapy-induced disabilities. Cancers (Basel).
16(4234)2024.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Huang C, Duan X, Wang J, Tian Q, Ren Y,
Chen K, Zhang Z, Li Y, Feng Y, Zhong K, et al: Lipid nanoparticle
delivery system for mRNA encoding B7H3-redirected bispecific
antibody displays potent antitumor effects on malignant tumors. Adv
Sci (Weinh). 10(e2205532)2023.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Deegen P, Thomas O, Nolan-Stevaux O, Li S,
Wahl J, Bogner P, Aeffner F, Friedrich M, Liao MZ, Matthes K, Rau
D, et al: The PSMA-targeting half-life extended BiTE therapy AMG
160 has potent antitumor activity in preclinical models of
metastatic castration-resistant prostate cancer. Clin Cancer Res.
27:2928–2937. 2021.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wei M, Zuo S, Chen Z, Qian P, Zhang Y,
Kong L, Gao H, Wei J and Dong J: Oncolytic vaccinia virus
expressing a bispecific T-cell engager enhances immune responses in
EpCAM positive solid tumors. Front Immunol.
13(1017574)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ayzman A, Pachynski RK and Reimers MA:
PSMA-based therapies and novel therapies in advanced prostate
cancer: The now and the future. Curr Treat Options Oncol.
26:375–384. 2025.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Jin Y, Lorvik KB, Jin Y, Beck C, Sike A,
Persiconi I, Kvaløy E, Saatcioglu F, Dunn C and Kyte JA:
Development of STEAP1 targeting chimeric antigen receptor for
adoptive cell therapy against cancer. Mol Ther Oncolytics.
26:189–206. 2022.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Si M, Xia Y, Cong M, Wang D, Hou Y and Ma
H: In situ co-delivery of doxorubicin and cisplatin by Injectable
thermosensitive hydrogels for enhanced osteosarcoma treatment. Int
J Nanomedicine. 17:1309–1322. 2022.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tu Z, Chen Y, Zhang Z, Meng W and Li L:
Barriers and solutions for CAR-T therapy in solid tumors. Cancer
Gene Ther. 32:923–934. 2025.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kim YJ, Li W, Zhelev DV, Mellors JW,
Dimitrov DS and Baek DS: Chimeric antigen receptor-T cells are
effective against CEACAM5 expressing non-small cell lung cancer
cells resistant to antibody-drug conjugates. Front Oncol.
13(1124039)2023.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Jiang Y, Wen W, Yang F, Han D, Zhang W and
Qin W: Prospect of prostate cancer treatment: Armed CAR-T or
combination therapy. Cancers (Basel). 14(967)2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Wala JA and Hanna GJ: Chimeric antigen
receptor T-cell therapy for solid tumors. Hematol Oncol Clin North
Am. 37:1149–1168. 2023.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Bains RS, Raju TG, Semaan LC, Block A,
Yamaguchi Y, Priceman SJ, George SC and Shirure VS: Vascularized
tumor-on-a-chip to investigate immunosuppression of CAR-T cells.
Lab Chip. 25:2390–2400. 2025.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Norde WJ, Hobo W, van der Voort R and
Dolstra H: Coinhibitory molecules in hematologic malignancies:
Targets for therapeutic intervention. Blood. 120:728–736.
2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Alavi SE, Ebrahimi Shahmabadi H, Sharma LA
and Sharma A: Nanoparticle-based drug delivery systems for
non-surgical periodontal therapy: Innovations and clinical
applications. 3 Biotech. 15(269)2025.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Guo F, Luo S, Wang L, Wang M, Wu F, Wang
Y, Jiao Y, Du Y, Yang Q, Yang X and Yang G: Protein corona,
influence on drug delivery system and its improvement strategy: A
review. Int J Biol Macromol. 256 (Pt 2)(128513)2024.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Guo L, Shi D, Shang M, Sun X, Meng D, Liu
X, Zhou X and Li J: Utilizing RNA nanotechnology to construct
negatively charged and ultrasound-responsive nanodroplets for
targeted delivery of siRNA. Drug Deliv. 29:316–327. 2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Seifert R, Kessel K, Schlack K, Weckesser
M, Kersting D, Seitzer KE, Weber M, Bögemann M and Rahbar K: Total
tumor volume reduction and low PSMA expression in patients
receiving Lu-PSMA therapy. Theranostics. 11:8143–8151.
2021.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Shih CH, Hsieh TY and Sung WW:
Prostate-specific membrane antigen-targeted antibody-drug
conjugates: A promising approach for metastatic
castration-resistant prostate cancer. Cells. 14(513)2025.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Liu D, Bao L, Zhu H, Yue Y, Tian J, Gao X
and Yin J: Microenvironment-responsive anti-PD-L1 x CD3 bispecific
T-cell engager for solid tumor immunotherapy. J Control Release.
354:606–614. 2023.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Tan B, Tu C, Xiong H, Xu Y, Shi X, Zhang
X, Yang R, Zhang N, Lin B, Liu M, et al: GITRL enhances
cytotoxicity and persistence of CAR-T cells in cancer therapy. Mol
Ther. 33:2789–2800. 2025.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Techaapornkun P, Rojpalakorn W, Mejun N,
Khaniya A, Thammahong A, Thu MS, Hirankarn N and Pitakkitnukun P:
Comparative efficacy and safety of BCMA-targeted CAR T cells and
BiTEs in relapsed/refractory multiple myeloma: A meta-analysis of
interventional and real-world studies. Ann Hematol. 104:4791–4809.
2025.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Bolsée J, Violle B, Jacques-Hespel C,
Nguyen T, Lonez C and Breman E: Tandem CAR T-cells targeting CD19
and NKG2DL can overcome CD19 antigen escape in B-ALL. Front
Immunol. 16(1557405)2025.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yamamichi G, Kato T, Watabe T, Hatano K,
Uemura M and Nonomura N: Current status of prostate-specific
membrane antigen-targeted alpha radioligand therapy in prostate
cancer. Anticancer Res. 44:879–888. 2024.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Zhang Q, Helfand BT, Carneiro BA, Qin W,
Yang XJ, Lee C, Zhang W, Giles FJ, Cristofanilli M and Kuzel TM:
Efficacy against human prostate cancer by prostate-specific
membrane antigen-specific, transforming growth Factor-β insensitive
genetically targeted CD8+ T-cells derived from patients with
metastatic castrate-resistant disease. Eur Urol. 73:648–652.
2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Guedan S and Delgado J: Immobilizing a
moving target: CAR T cells hit CD22. Clin Cancer Res. 25:5188–5190.
2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Song EZ, Wang X, Philipson BI, Zhang Q,
Thokala R, Zhang L, Assenmacher CA, Binder ZA, Ming GL, O'Rourke
DM, et al: The IAP antagonist birinapant enhances chimeric antigen
receptor T cell therapy for glioblastoma by overcoming antigen
heterogeneity. Mol Ther Oncolytics. 27:288–304. 2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Peter J, Toppeta F, Trubert A, Danhof S,
Hudecek M and Däullary T: Multi-Targeting CAR-T cell strategies to
overcome immune evasion in lymphoid and myeloid malignancies. Oncol
Res Treat. 48:265–279. 2025.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Borcoman E, Kamal M, Marret G, Dupain C,
Castel-Ajgal Z and Le Tourneau C: HDAC inhibition to prime immune
checkpoint inhibitors. Cancers (Basel). 14(66)2021.PubMed/NCBI View Article : Google Scholar
|
|
83
|
López-Collazo E and Hurtado-Navarro L:
Cell fusion as a driver of metastasis: Re-evaluating an old
hypothesis in the age of cancer heterogeneity. Front Immunol.
16(1524781)2025.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Derderian S, Vesval Q, Wissing MD, Hamel
L, Côté N, Vanhuyse M, Ferrario C, Bladou F, Aprikian A and
Chevalier S: Liquid biopsy-based targeted gene screening highlights
tumor cell subtypes in patients with advanced prostate cancer. Clin
Transl Sci. 15:2597–2612. 2022.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Tserunyan V and Finley SD: Modelling
predicts differences in chimeric antigen receptor T-cell signalling
due to biological variability. R Soc Open Sci.
9(220137)2022.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Fan J, Zhu J, Zhu H and Xu H: Potential
therapeutic targets in myeloid cell therapy for overcoming
chemoresistance and immune suppression in gastrointestinal tumors.
Crit Rev Oncol Hematol. 198(104362)2024.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wang H, Liu S, Zhan J, Liang Y and Zeng X:
Shaping the immune-suppressive microenvironment on tumor-associated
myeloid cells through tumor-derived exosomes. Int J Cancer.
154:2031–2042. 2024.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Kemp SB, Pasca di Magliano M and Crawford
HC: Myeloid cell mediated immune suppression in pancreatic cancer.
Cell Mol Gastroenterol Hepatol. 12:1531–1542. 2021.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kerr MD, McBride DA, Chumber AK and Shah
NJ: Combining therapeutic vaccines with chemo- and immunotherapies
in the treatment of cancer. Expert Opin Drug Discov. 16:89–99.
2021.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ganaie AA, Mansini AP, Hussain T, Rao A,
Siddique HR, Shabaneh A, Ferrari MG, Murugan P, Klingelhöfer J,
Wang J, et al: Anti-S100A4 antibody therapy is efficient in
treating aggressive prostate cancer and reversing
immunosuppression: Serum and biopsy S100A4 as a clinical predictor.
Mol Cancer Ther. 19:2598–2611. 2020.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Wang K, Hao Z, Fu X, Li W, Jiao A and Hua
X: Involvement of elevated ASF1B in the poor prognosis and
tumorigenesis in pancreatic cancer. Mol Cell Biochem.
477:1947–1957. 2022.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Kim B, Jung S, Kim H, Kwon JO, Song MK,
Kim MK, Kim HJ and Kim HH: The role of S100A4 for bone metastasis
in prostate cancer cells. BMC Cancer. 21(137)2021.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Peng S, Hu P, Xiao YT, Lu W, Guo D, Hu S,
Xie J, Wang M, Yu W, Yang J, et al: Single-cell analysis reveals
EP4 as a target for restoring T-cell infiltration and sensitizing
prostate cancer to immunotherapy. Clin Cancer Res. 28:552–567.
2022.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Yan J, Wang Y, Jia Y, Liu S, Tian C, Pan
W, Liu X and Wang H: Co-delivery of docetaxel and curcumin prodrug
via dual-targeted nanoparticles with synergistic antitumor activity
against prostate cancer. Biomed Pharmacother. 88:374–383.
2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhuang R, Xie R, Peng S, Zhou Q, Lin W, Ou
Y, Chen B, Su T, Li Z, Huang H, et al: An anti-androgen
resistance-related gene signature acts as a prognostic marker and
increases enzalutamide efficacy via PLK1 inhibition in prostate
cancer. J Transl Med. 23(480)2025.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Sartor O, de Bono J, Chi KN, Fizazi K,
Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N,
El-Haddad G, et al: Lutetium-177-PSMA-617 for metastatic
castration-resistant prostate cancer. N Engl J Med. 385:1091–1103.
2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Vakil V and Trappe W: Drug-resistant
cancer treatment strategies based on the dynamics of clonal
evolution and PKPD modeling of drug combinations. IEEE/ACM Trans
Comput Biol Bioinform. 19:1603–1614. 2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Xu J, Yang X, Deshmukh D, Chen H, Fang S
and Qiu Y: The role of crosstalk between AR3 and E2F1 in drug
resistance in prostate cancer cells. Cells. 9(1094)2020.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Chatzilygeroudi T, Karantanos T and Pappa
V: Unraveling venetoclax resistance: Navigating the future of
HMA/Venetoclax-Refractory AML in the molecular era. Cancers
(Basel). 17(1586)2025.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Saad F: Should only patients with BRCA
mutation be treated with a combination of an androgen receptor
pathway inhibitor and a PARP inhibitor for metastatic
castration-resistant prostate cancer? The answer is no. Eur Urol
Focus. 10:506–508. 2024.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Hachem S, Yehya A, El Masri J, Mavingire
N, Johnson JR, Dwead AM, Kattour N, Bouchi Y, Kobeissy F,
Rais-Bahrami S, et al: Contemporary update on clinical and
experimental prostate cancer biomarkers: A multi-omics-focused
approach to detection and risk stratification. Biology (Basel).
13(762)2024.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Chen Y, Fan X, Lu R, Zeng S and Gan P:
PARP inhibitor and immune checkpoint inhibitor have synergism
efficacy in gallbladder cancer. Genes Immun. 25:307–316.
2024.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Kumar M, Nguyen TPN, Kaur J, Singh TG,
Soni D, Singh R and Kumar P: Opportunities and challenges in
application of artificial intelligence in pharmacology. Pharmacol
Rep. 75:3–18. 2023.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Danila DC, Szmulewitz RZ, Vaishampayan U,
Higano CS, Baron AD, Gilbert HN, Brunstein F, Milojic-Blair M, Wang
B, Kabbarah O, et al: Phase I study of DSTP3086S, an antibody-drug
conjugate targeting six-transmembrane epithelial antigen of
prostate 1, in metastatic castration-resistant prostate cancer. J
Clin Oncol. 37:3518–3527. 2019.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Paoletti C and Hayes DF: Circulating tumor
cells. Adv Exp Med Biol. 882:235–258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Vis DJ, Palit SAL, Corradi M, Cuppen E,
Mehra N, Lolkema MP, Wessels LFA, van der Heijden MS, Zwart W and
Bergman AM: Whole genome sequencing of 378 prostate cancer
metastases reveals tissue selectivity for mismatch deficiency with
potential therapeutic implications. Genome Med.
17(24)2025.PubMed/NCBI View Article : Google Scholar
|
|
107
|
van der Sar ECA, Kühr AJS, Ebbers SC,
Henderson AM, de Keizer B, Lam MGEH and Braat AJAT: Baseline
imaging derived predictive factors of response following [177Lu]
Lu-PSMA-617 therapy in salvage metastatic castration-resistant
prostate cancer: A lesion- and patient-based analysis.
Biomedicines. 10(1575)2022.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Hwang C, Henderson NC, Chu SC, Holland B,
Cackowski FC, Pilling A, Jang A, Rothstein S, Labriola M, Park JJ,
et al: Biomarker-directed therapy in black and white men with
metastatic castration-resistant prostate cancer. JAMA Netw Open.
6(e2334208)2023.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Parghane RV and Basu S: PSMA-targeted
radioligand therapy in prostate cancer: Current status and future
prospects. Expert Rev Anticancer Ther. 23:959–975. 2023.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Petrylak DP, Vogelzang NJ, Chatta K,
Fleming MT, Smith DC, Appleman LJ, Hussain A, Modiano M, Singh P,
Tagawa ST, et al: PSMA ADC monotherapy in patients with progressive
metastatic castration-resistant prostate cancer following
abiraterone and/or enzalutamide: Efficacy and safety in open-label
single-arm phase 2 study. Prostate. 80:99–108. 2020.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Thang SP, Violet J, Sandhu S, Iravani A,
Akhurst T, Kong G, Ravi Kumar A, Murphy DG, Williams SG, Hicks RJ
and Hofman MS: Poor outcomes for patients with metastatic
castration-resistant prostate cancer with low prostate-specific
membrane antigen (PSMA) expression deemed ineligible for
177Lu-labelled PSMA radioligand therapy. Eur Urol Oncol. 2:670–676.
2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Fu Y, Urban DJ, Nani RR, Zhang YF, Li N,
Fu H, Shah H, Gorka AP, Guha R, Chen L, et al: Glypican-3-specific
antibody drug conjugates targeting hepatocellular carcinoma.
Hepatology. 70:563–576. 2019.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Xu J, Liu W, Fan F, Zhang B, Sun C and Hu
Y: Advances in nano-immunotherapy for hematological malignancies.
Exp Hematol Oncol. 13(57)2024.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Cao P and Bae Y: Polymer nanoparticulate
drug delivery and combination cancer therapy. Future Oncol.
8:1471–1480. 2012.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Paschalis A, Sheehan B, Riisnaes R,
Rodrigues DN, Gurel B, Bertan C, Ferreira A, Lambros MBK, Seed G,
Yuan W, et al: Prostate-specific membrane antigen heterogeneity and
DNA repair defects in prostate cancer. Eur Urol. 76:469–478.
2019.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Hong J, Bae S, Cavinato L, Seifert R,
Ryhiner M, Rominger A, Erlandsson K, Wilks M, Normandin M,
El-Fakhri G, et al: Deciphering the effects of radiopharmaceutical
therapy in the tumor microenvironment of prostate cancer: An
in-silico exploration with spatial transcriptomics. Theranostics.
14:7122–7139. 2024.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Narayana RVL and Gupta R: Exploring the
therapeutic use and outcome of antibody-drug conjugates in ovarian
cancer treatment. Oncogene. 44:2343–2356. 2025.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Grosso C, Silva A, Delerue-Matos C and
Barroso MF: Single and multitarget systems for drug delivery and
detection: Up-to-date strategies for brain disorders.
Pharmaceuticals (Basel). 16(1721)2023.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Yeh CY, Hsiao JK, Wang YP, Lan CH and Wu
HC: Peptide-conjugated nanoparticles for targeted imaging and
therapy of prostate cancer. Biomaterials. 99:1–15. 2016.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Bimbatti D, Maruzzo M, Pierantoni F,
Diminutto A, Dionese M, Deppieri FM, Lai E, Zagonel V and Basso U:
Immune checkpoint inhibitors rechallenge in urological tumors: An
extensive review of the literature. Crit Rev Oncol Hematol.
170(103579)2022.PubMed/NCBI View Article : Google Scholar
|
|
121
|
McMullan C, Turner G, Retzer A, Belli A,
Davies EH, Nice L, Flavell L, Flavell J and Calvert M: Testing an
electronic patient-reported outcome platform in the context of
traumatic brain injury: PRiORiTy usability study. JMIR Form Res.
9(e58128)2025.PubMed/NCBI View
Article : Google Scholar
|
|
122
|
Ramos-Casals M and Sisó-Almirall A:
Immune-related adverse events of immune checkpoint inhibitors. Ann
Intern Med. 177:ITC17–ITC32. 2024.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Naing A, Hajjar J, Gulley JL, Atkins MB,
Ciliberto G, Meric-Bernstam F and Hwu P: Strategies for improving
the management of immune-related adverse events. J Immunother
Cancer. 8(e001754)2020.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Ong SY and Baird JH: A primer on chimeric
antigen receptor T-cell therapy-related toxicities for the
intensivist. J Intensive Care Med. 39:929–938. 2024.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Ciano-Petersen NL, Muñiz-Castrillo S,
Vogrig A, Joubert B and Honnorat J: Immunomodulation in the acute
phase of autoimmune encephalitis. Rev Neurol (Paris). 178:34–47.
2022.PubMed/NCBI View Article : Google Scholar
|